Resveratrol Alleviates Acute Campylobacter jejuni Induced Enterocolitis in a Preclinical Murine Intervention Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Determination of the Minimal Inhibitory Concentration of Resveratrol
2.3. Generation of Secondary Abiotic IL-10−/− Mice
2.4. Resveratrol Treatment
2.5. C. jejuni Infection and Gastrointestinal Colonization
2.6. Clinical Conditions
2.7. Sampling Procedures
2.8. Immunohistochemistry
2.9. Measurements of Intestinal, Extra-Intestinal and Systemic Inflammatory Mediators
2.10. Electrophysiological Measurements
2.11. Statistical Analysis
3. Results
3.1. Antimicrobial Properties of Resveratrol against C. jejuni In Vitro
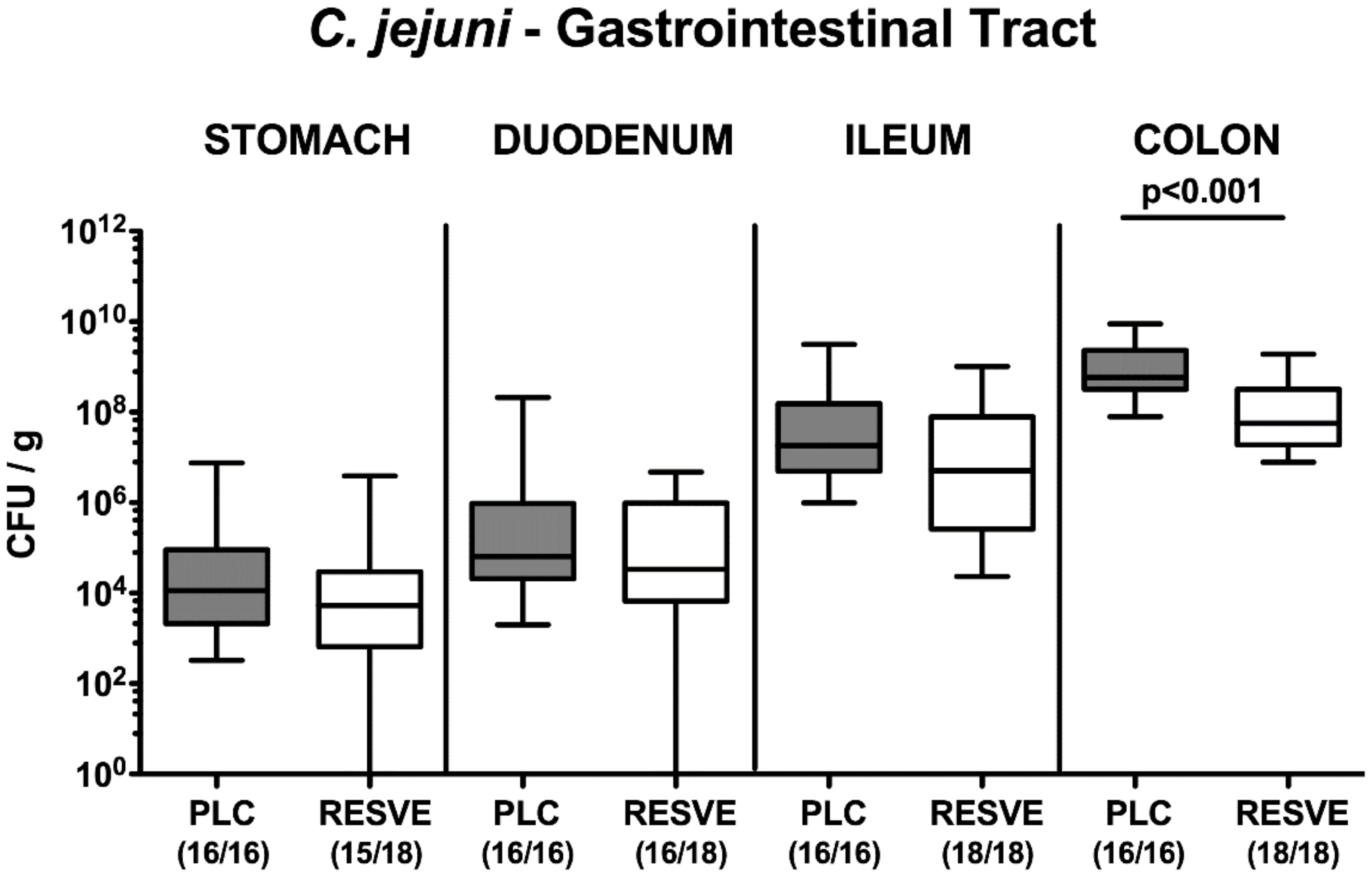
3.2. Gastrointestinal Pathogen Loads Following Resveratrol Treatment of Mice Suffering from C. jejuni Induced Enterocolitis
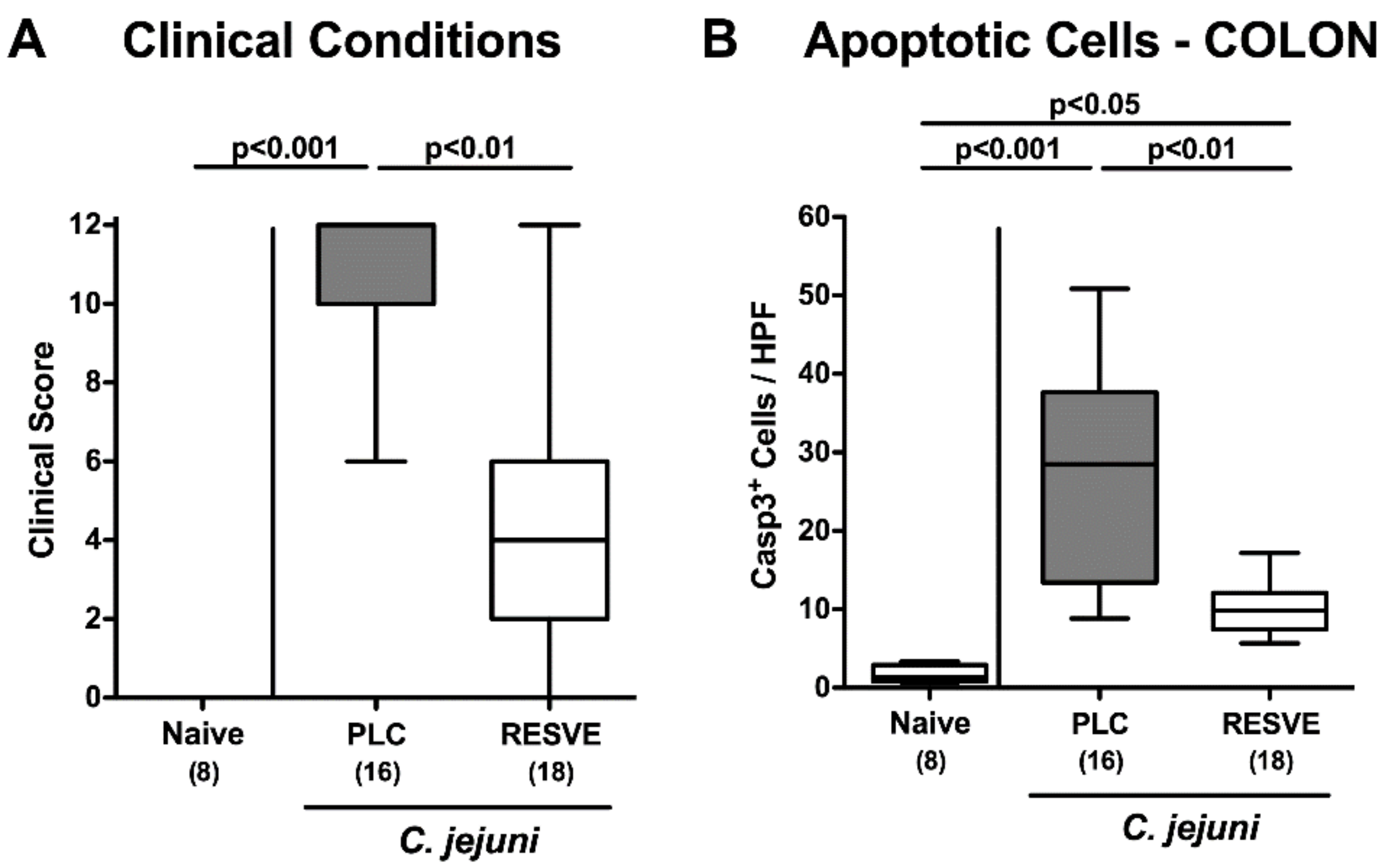
3.3. Clinical Conditions of Resveratrol-Treated Mice Suffering from C. jejuni Induced Enterocolitis
3.4. Apoptotic Responses in Large Intestinal Epithelia of Resveratrol-Treated Mice Suffering from C. jejuni Induced Enterocolitis
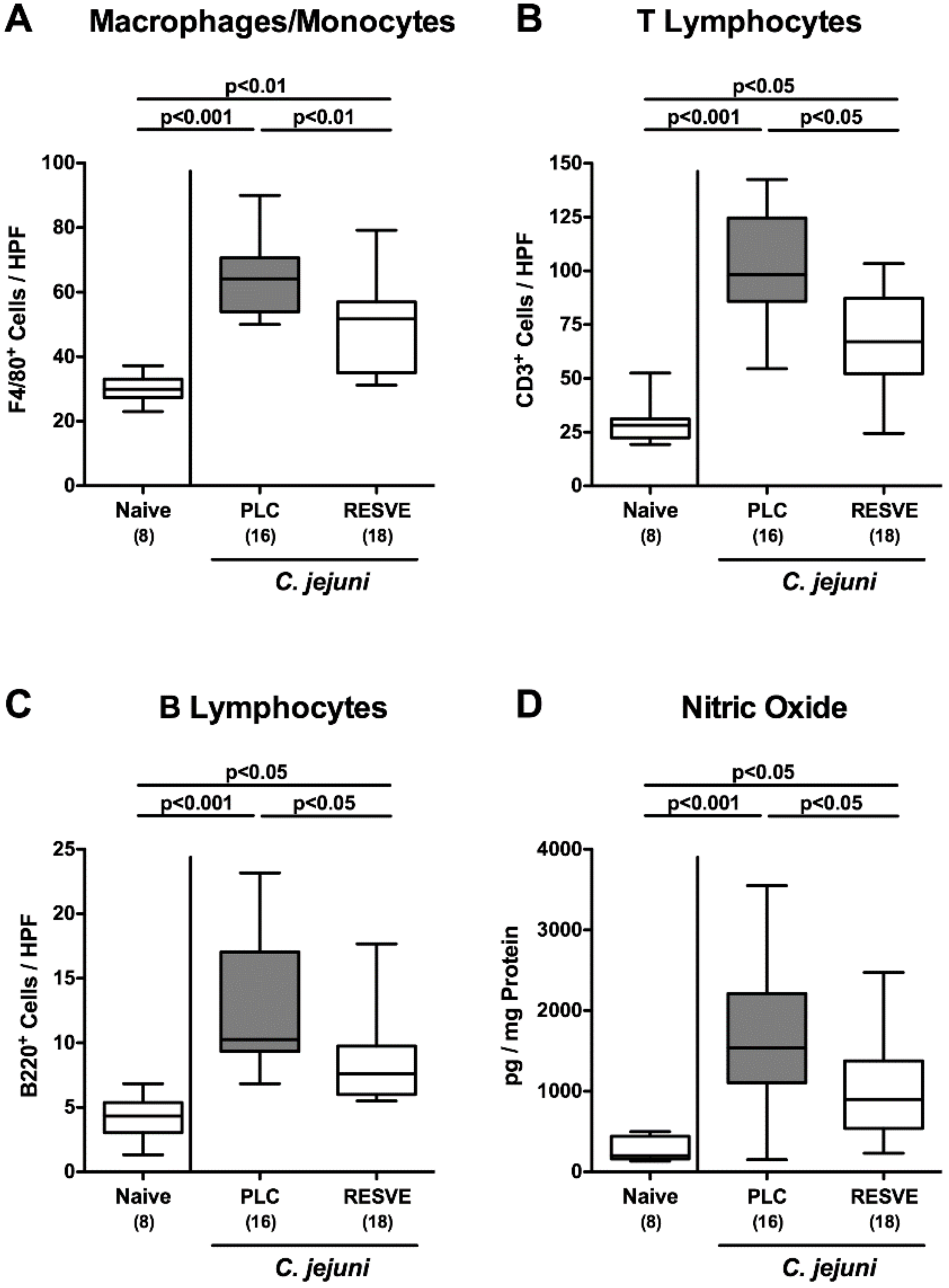
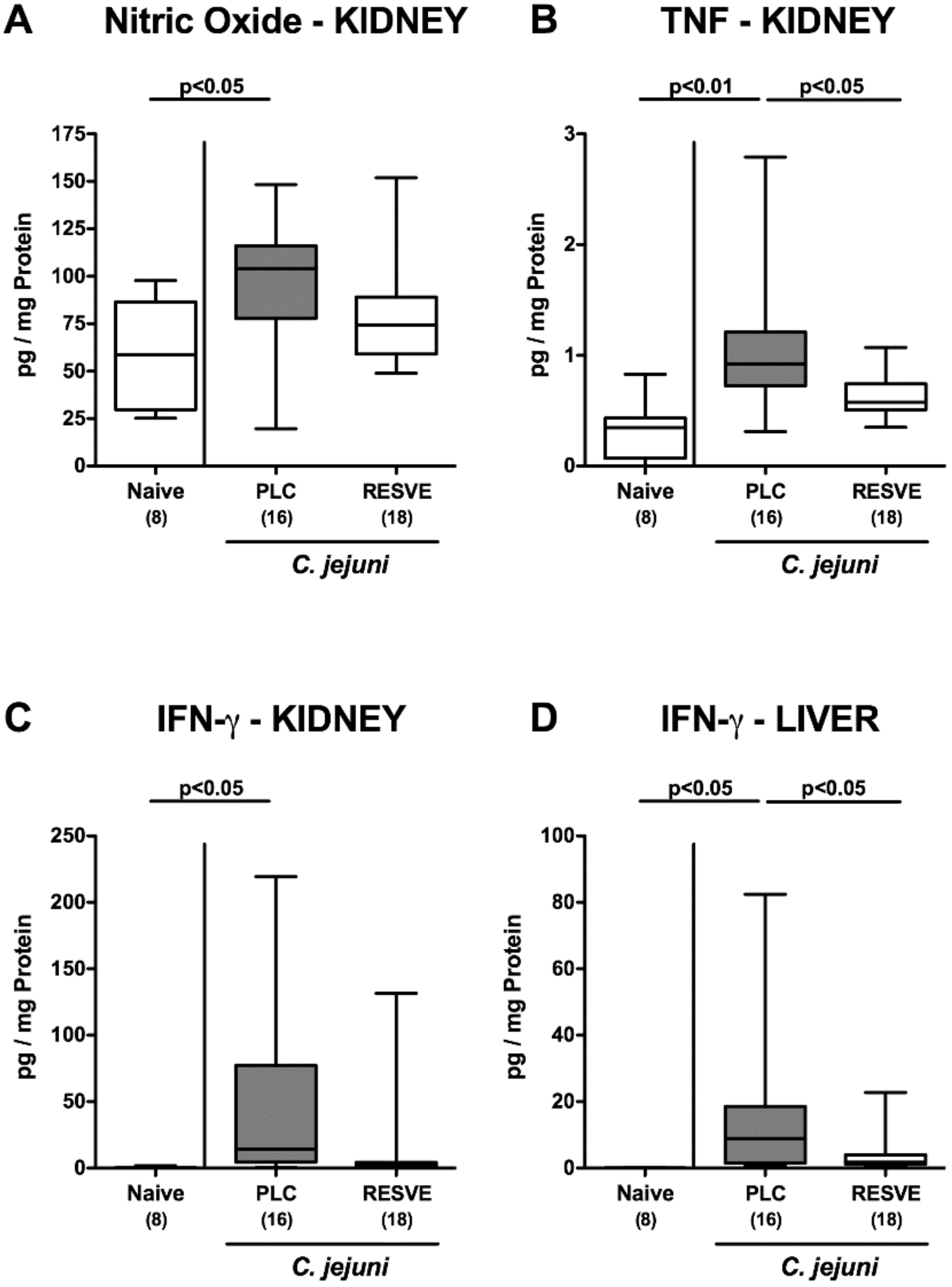
3.5. Inflammatory Immune Responses in the Large Intestines of Resveratrol-Treated Mice Suffering from C. jejuni Induced Enterocolitis
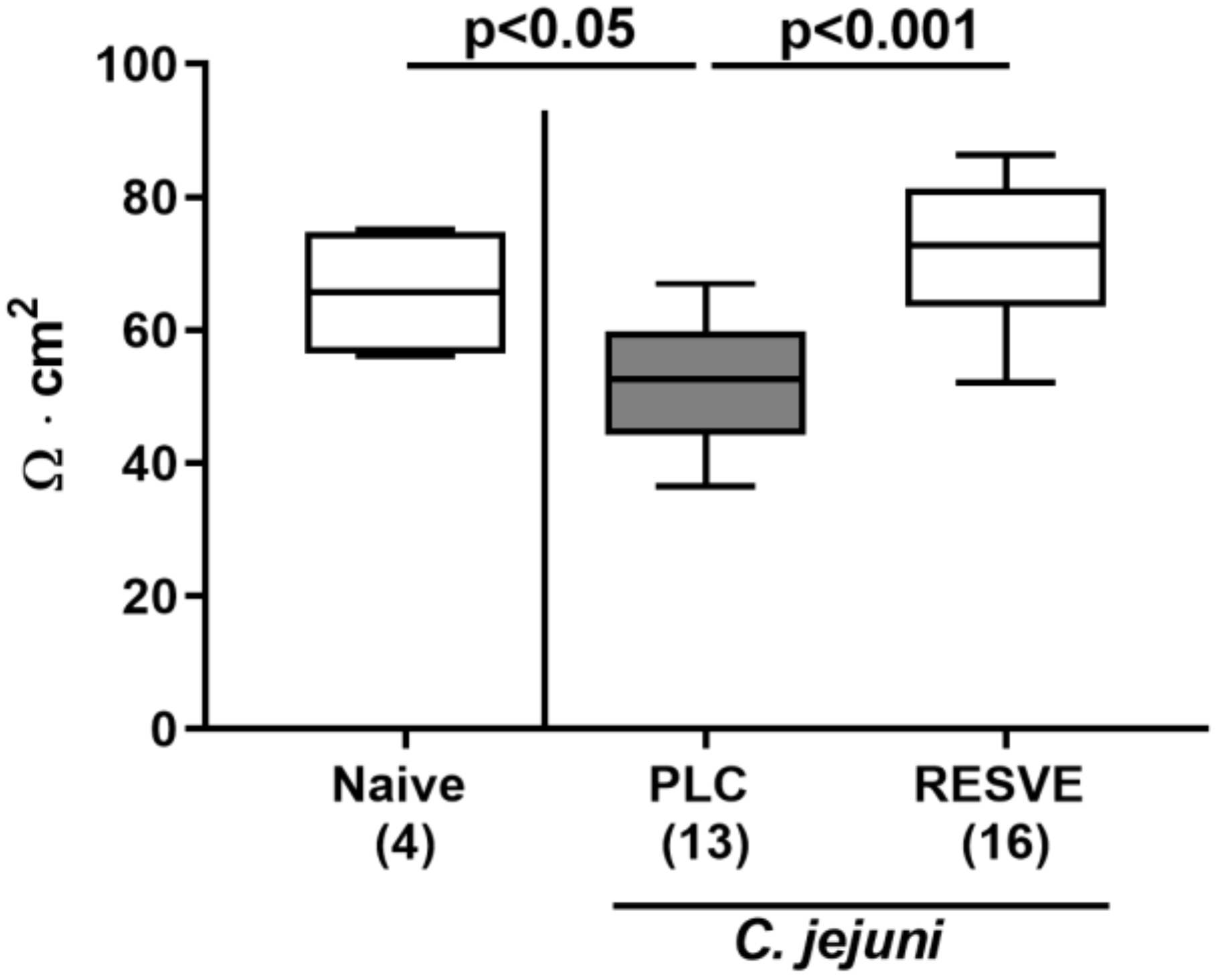
3.6. Colonic Epithelial Barrier Function of Resveratrol-Treated Mice Suffering from C. jejuni Induced Enterocolitis
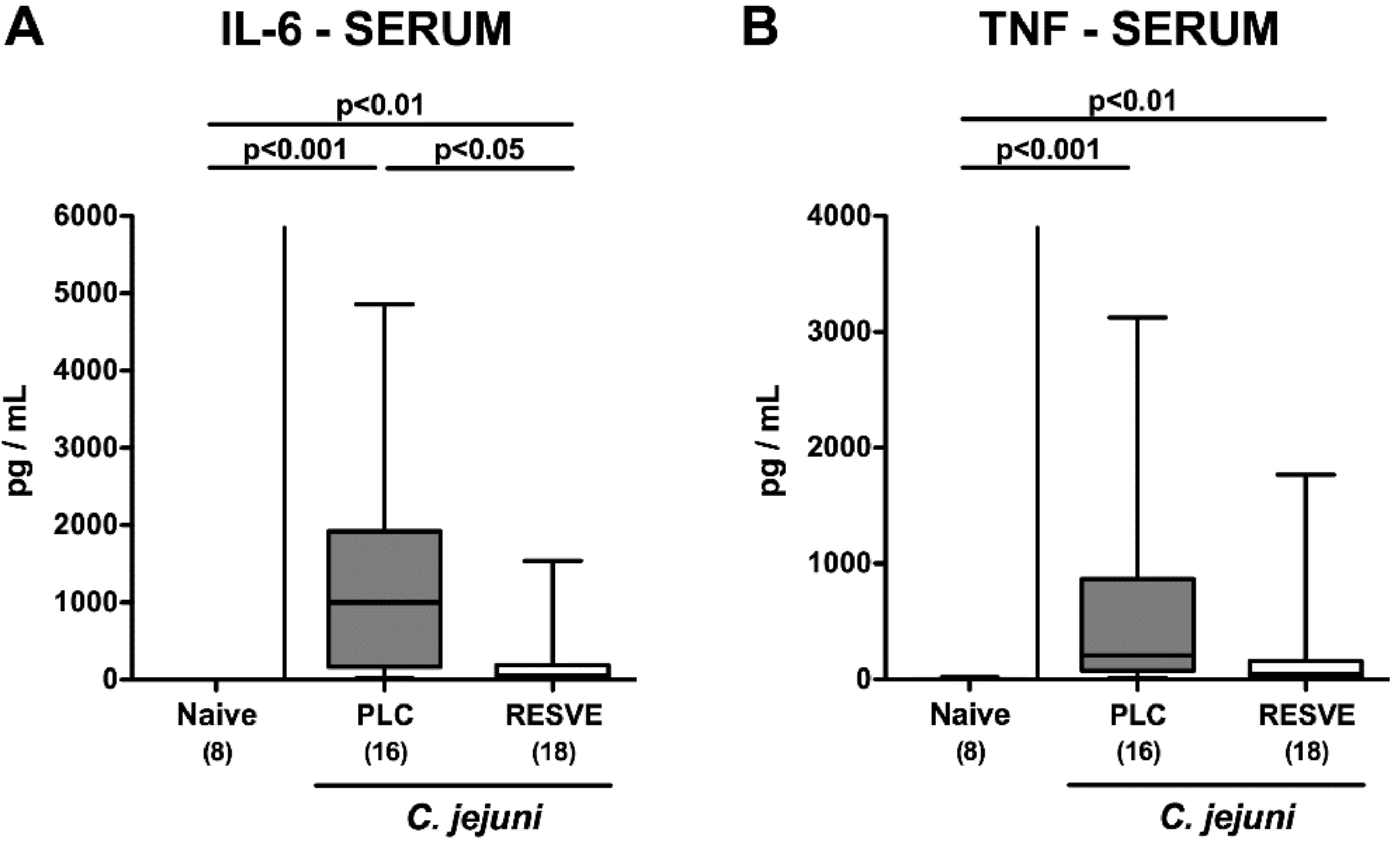
3.7. Extra-Intestinal Including Systemic Pro-Inflammatory Responses in Resveratrol-Treated Mice Suffering from C. jejuni Induced Enterocolitis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CBA | cytometric bead array |
| CFU | colony forming units |
| CLSI | Clinical and Laboratory Standards Institute |
| HPF | high-power fields |
| IFN | interferon |
| IL | interleukin |
| iNOS | inducible nitric oxide synthase |
| LOS | lipooligosaccharide |
| LPS | lipopolysaccharide |
| MIC | minimal inhibitory concentration |
| NF | nuclear factor |
| PBS | phosphate-buffered saline |
| p.i. | post-infection |
| Rt | transmural electrical resistance |
| TLR | Toll-like receptor |
| TNF-α | tumor necrosis factor-α |
| Th-1 cells | T helper-1 cells |
References
- WHO. Campylobacter. Available online: https://www.who.int/news-room/fact-sheets/detail/campylobacter (accessed on 4 June 2020).
- Backert, S.; Tegtmeyer, N.; Cróinín, T.Ó.; Boehm, M.; Heimesaat, M.M. Chapter 1—Human campylobacteriosis. In Campylobacter; Klein, G., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 1–25. [Google Scholar] [CrossRef]
- Skirrow, M. Campylobacter enteritis: A “new” disease. Br. Med. J. 1977, 2, 9–11. [Google Scholar] [CrossRef]
- Ellis-Iversen, J.; Ridley, A.; Morris, V.; Sowa, A.; Harris, J.; Atterbury, R.; Sparks, N.; Allen, V. Persistent environmental reservoirs on farms as risk factors for Campylobacter in commercial poultry. Epidemiol. Infect. 2012, 140, 916–924. [Google Scholar] [CrossRef]
- Butkevych, E.; de Sá, F.D.L.; Nattramilarasu, P.K.; Bücker, R. Contribution of Epithelial Apoptosis and Subepithelial Immune Responses in Campylobacter jejuni-Induced Barrier Disruption. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Young, K.T.; Davis, L.M.; Dirita, V.J. Campylobacter jejuni: Molecular biology and pathogenesis. Nat. Rev. Microbiol. 2007, 5, 665–679. [Google Scholar] [CrossRef]
- Kist, M.; Bereswill, S. Campylobacter jejuni . Contrib. Microbiol. 2001, 8, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Kaakoush, N.O.; Castano-Rodriguez, N.; Mitchell, H.M.; Man, S.M. Global Epidemiology of Campylobacter Infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef] [PubMed]
- Haag, L.M.; Fischer, A.; Otto, B.; Plickert, R.; Kuhl, A.A.; Gobel, U.B.; Bereswill, S.; Heimesaat, M.M. Campylobacter jejuni induces acute enterocolitis in gnotobiotic IL-10-/- mice via Toll-like-receptor-2 and -4 signaling. PLoS ONE 2012, 7, e40761. [Google Scholar] [CrossRef] [PubMed]
- Taveira da Silva, A.M.; Kaulbach, H.C.; Chuidian, F.S.; Lambert, D.R.; Suffredini, A.F.; Danner, R.L. Brief report: Shock and multiple-organ dysfunction after self-administration of Salmonella endotoxin. New Engl. J. Med. 1993, 328, 1457–1460. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.; Bereswill, S.; Heimesaat, M.M. Novel Clinical Campylobacter jejuni Infection Models Based on Sensitization of Mice to Lipooligosaccharide, a Major Bacterial Factor Triggering Innate Immune Responses in Human Campylobacteriosis. Microorganisms 2020, 8, 482. [Google Scholar] [CrossRef]
- Mousavi, S.; Schmidt, A.M.; Escher, U.; Kittler, S.; Kehrenberg, C.; Thunhorst, E.; Bereswill, S.; Heimesaat, M.M. Carvacrol ameliorates acute campylobacteriosis in a clinical murine infection model. Gut. Pathog. 2020, 12, 2. [Google Scholar] [CrossRef]
- Mousavi, S.; Escher, U.; Thunhorst, E.; Kittler, S.; Kehrenberg, C.; Bereswill, S.; Heimesaat, M.M. Vitamin C alleviates acute enterocolitis in Campylobacter jejuni infected mice. Sci. Rep. 2020, 10, 2921. [Google Scholar] [CrossRef] [PubMed]
- Lobo de Sá, F.D.; Butkevych, E.; Nattramilarasu, P.K.; Fromm, A.; Mousavi, S.; Moos, V.; Golz, J.C.; Stingl, K.; Kittler, S.; Seinige, D. Curcumin mitigates immune-induced epithelial barrier dysfunction by Campylobacter jejuni. Int. J. Mol. Sci. 2019, 20, 4830. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.; Lobo de Sa, F.D.; Schulzke, J.D.; Bucker, R.; Bereswill, S.; Heimesaat, M.M. Vitamin D in Acute Campylobacteriosis-Results From an Intervention Study Applying a Clinical Campylobacter jejuni Induced Enterocolitis Model. Front. Immunol. 2019, 10, 2094. [Google Scholar] [CrossRef] [PubMed]
- Heimesaat, M.M.; Mousavi, S.; Klove, S.; Genger, C.; Weschka, D.; Giladi, E.; Bereswill, S.; Gozes, I. Immune-modulatory Properties of the Octapeptide NAP in Campylobacter jejuni Infected Mice Suffering from Acute Enterocolitis. Microorganisms 2020, 8, 802. [Google Scholar] [CrossRef] [PubMed]
- Heimesaat, M.M.; Mousavi, S.; Klove, S.; Genger, C.; Weschka, D.; Tamas, A.; Reglodi, D.; Bereswill, S. Pituitary Adenylate Cyclase-Activating Polypeptide Alleviates Intestinal, Extra-Intestinal and Systemic Inflammatory Responses during Acute Campylobacter jejuni-induced Enterocolitis in Mice. Pathogens 2020, 9, 805. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A double-edged sword in health benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, L. Influence of resveratrol on the immune response. Nutrients 2019, 11, 946. [Google Scholar] [CrossRef] [PubMed]
- CLSI. VET01-A5: Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, Approved Standard, 5th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; Volume 34. [Google Scholar]
- Bereswill, S.; Fischer, A.; Plickert, R.; Haag, L.M.; Otto, B.; Kuhl, A.A.; Dasti, J.I.; Zautner, A.E.; Munoz, M.; Loddenkemper, C.; et al. Novel murine infection models provide deep insights into the “menage a trois” of Campylobacter jejuni, microbiota and host innate immunity. PLoS ONE 2011, 6, e20953. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Bereswill, S.; Fischer, A.; Fuchs, D.; Struck, D.; Niebergall, J.; Jahn, H.K.; Dunay, I.R.; Moter, A.; Gescher, D.M.; et al. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J. Immunol. 2006, 177, 8785–8795. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Alutis, M.; Grundmann, U.; Fischer, A.; Tegtmeyer, N.; Bohm, M.; Kuhl, A.A.; Gobel, U.B.; Backert, S.; Bereswill, S. The role of serine protease HtrA in acute ulcerative enterocolitis and extra-intestinal immune responses during Campylobacter jejuni infection of gnotobiotic IL-10 deficient mice. Front. Cell Infect. Microbiol. 2014, 4, 77. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Nogai, A.; Bereswill, S.; Plickert, R.; Fischer, A.; Loddenkemper, C.; Steinhoff, U.; Tchaptchet, S.; Thiel, E.; Freudenberg, M.A.; et al. MyD88/TLR9 mediated immunopathology and gut microbiota dynamics in a novel murine model of intestinal graft-versus-host disease. Gut 2010, 59, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Heimesaat, M.M.; Giladi, E.; Kuhl, A.A.; Bereswill, S.; Gozes, I. The octapetide NAP alleviates intestinal and extra-intestinal anti-inflammatory sequelae of acute experimental colitis. Peptides 2018, 101, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Paulo, L.; Ferreira, S.; Gallardo, E.; Queiroz, J.A.; Domingues, F. Antimicrobial activity and effects of resveratrol on human pathogenic bacteria. World J. Microbiol. Biotechnol. 2010, 26, 1533–1538. [Google Scholar] [CrossRef]
- Hwang, D.; Lim, Y. Resveratrol antibacterial activity against Escherichia coli is mediated by Z-ring formation inhibition via suppression of FtsZ expression. Sci. Rep. 2015, 5, 10029. [Google Scholar] [CrossRef]
- Lee, W.; Lee, D.G. Resveratrol induces membrane and DNA disruption via pro-oxidant activity against Salmonella typhimurium. Biochem. Biophys. Res. Commun. 2017, 489, 228–234. [Google Scholar] [CrossRef]
- Selma, M.V.; Larrosa, M.; Beltrán, D.; Lucas, R.; Morales, J.C.; Tomás-Barberán, F.; Espín, J.C. Resveratrol and some glucosyl, glucosylacyl, and glucuronide derivatives reduce Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes Scott A adhesion to colonic epithelial cell lines. J. Agric. Food Chem. 2012, 60, 7367–7374. [Google Scholar] [CrossRef]
- Klančnik, A.; Šikić Pogačar, M.; Trošt, K.; Tušek Žnidarič, M.; Mozetič Vodopivec, B.; Smole Možina, S. Anti-Campylobacter activity of resveratrol and an extract from waste Pinot noir grape skins and seeds, and resistance of Camp. jejuni planktonic and biofilm cells, mediated via the Cme ABC efflux pump. J. Appl. Microbiol. 2017, 122, 65–77. [Google Scholar] [CrossRef]
- Duarte, A.; Alves, A.C.; Ferreira, S.; Silva, F.; Domingues, F.C. Resveratrol inclusion complexes: Antibacterial and anti-biofilm activity against Campylobacter spp. and Arcobacter butzleri. Food Res. Int. 2015, 77, 244–250. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef]
- Bode, L.M.; Bunzel, D.; Huch, M.; Cho, G.S.; Ruhland, D.; Bunzel, M.; Bub, A.; Franz, C.M.; Kulling, S.E. In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am. J. Clin. Nutr. 2013, 97, 295–309. [Google Scholar] [CrossRef]
- Zhou, Z.X.; Mou, S.F.; Chen, X.Q.; Gong, L.L.; Ge, W.S. Anti-inflammatory activity of resveratrol prevents inflammation by inhibiting NF-κB in animal models of acute pharyngitis. Mol. Med. Rep. 2018, 17, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Schwager, J.; Richard, N.; Widmer, F.; Raederstorff, D. Resveratrol distinctively modulates the inflammatory profiles of immune and endothelial cells. BMC Complement. Altern. Med. 2017, 17, 309. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Xu, Y.X.; Janakiraman, N.; Chapman, R.A.; Gautam, S.C. Immunomodulatory activity of resveratrol: Suppression of lymphocyte proliferation, development of cell-mediated cytotoxicity, and cytokine production. Biochem. Pharmacol. 2001, 62, 1299–1308. [Google Scholar] [CrossRef]
- Švajger, U.; Jeras, M. Anti-inflammatory effects of resveratrol and its potential use in therapy of immune-mediated diseases. Int. Rev. Immunol. 2012, 31, 202–222. [Google Scholar] [CrossRef]
- Bereswill, S.; Munoz, M.; Fischer, A.; Plickert, R.; Haag, L.M.; Otto, B.; Kuhl, A.A.; Loddenkemper, C.; Gobel, U.B.; Heimesaat, M.M. Anti-inflammatory effects of resveratrol, curcumin and simvastatin in acute small intestinal inflammation. PLoS ONE 2010, 5, e15099. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L.; Luo, X.F.; Li, M.T.; Xu, D.; Zhou, S.; Chen, H.Z.; Gao, N.; Chen, Z.; Zhang, L.L.; Zeng, X.F. Resveratrol possesses protective effects in a pristane-induced lupus mouse model. PLoS ONE 2014, 9, e114792. [Google Scholar] [CrossRef]
- Voloshyna, I.; Hai, O.; Littlefield, M.J.; Carsons, S.; Reiss, A.B. Resveratrol mediates anti-atherogenic effects on cholesterol flux in human macrophages and endothelium via PPARγ and adenosine. Eur. J. Pharmacol. 2013, 698, 299–309. [Google Scholar] [CrossRef]
- Riba, A.; Deres, L.; Sumegi, B.; Toth, K.; Szabados, E.; Halmosi, R. Cardioprotective Effect of Resveratrol in a Postinfarction Heart Failure Model. Oxid. Med. Cell Longev. 2017, 2017, 6819281. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, X.H.; Yang, L.; Chen, X.Y.; Jiang, R.S.; Jin, S.H.; Geng, Z.Y. Resveratrol alleviates heat stress-induced impairment of intestinal morphology, microflora, and barrier integrity in broilers. Poult. Sci. 2017, 96, 4325–4332. [Google Scholar] [CrossRef]
- Den Hartogh, D.J.; Tsiani, E. Health Benefits of Resveratrol in Kidney Disease: Evidence from In Vitro and In Vivo Studies. Nutrients 2019, 11, 1624. [Google Scholar] [CrossRef] [PubMed]
- Aktaş, H.S.; Ozel, Y.; Ahmad, S.; Pençe, H.H.; Ayaz-Adakul, B.; Kudas, I.; Tetik, S.; Şekerler, T.; Canbey-Göret, C.; Kabasakal, L.; et al. Protective effects of resveratrol on hepatic ischemia reperfusion injury in streptozotocin-induced diabetic rats. Mol. Cell Biochem. 2019, 460, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Heebøll, S.; Thomsen, K.L.; Pedersen, S.B.; Vilstrup, H.; George, J.; Grønbæk, H. Effects of resveratrol in experimental and clinical non-alcoholic fatty liver disease. World J. Hepatol. 2014, 6, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Xiao, Z.; Zhang, W.; Chen, H.; Liu, H.; Pan, J.; Cai, X.; Liang, G.; Zhou, B.; Shan, X.; et al. A novel resveratrol-curcumin hybrid, a19, attenuates high fat diet-induced nonalcoholic fatty liver disease. Biomed. Pharmacother. 2019, 110, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Kløve, S.; Genger, C.; Mousavi, S.; Weschka, D.; Bereswill, S.; Heimesaat, M.M. Toll-Like Receptor-4 Dependent Intestinal and Systemic Sequelae Following Peroral Campylobacter coli Infection of IL10 Deficient Mice Harboring a Human Gut Microbiota. Pathogens 2020, 9, 386. [Google Scholar] [CrossRef]
- Youn, H.S.; Lee, J.Y.; Fitzgerald, K.A.; Young, H.A.; Akira, S.; Hwang, D.H. Specific inhibition of MyD88-independent signaling pathways of TLR3 and TLR4 by resveratrol: Molecular targets are TBK1 and RIP1 in TRIF complex. J. Immunol. 2005, 175, 3339–3346. [Google Scholar] [CrossRef]
- Chen, J.; Cao, X.; Cui, Y.; Zeng, G.; Chen, J.; Zhang, G. Resveratrol alleviates lysophosphatidylcholine-induced damage and inflammation in vascular endothelial cells. Mol. Med. Rep. 2018, 17, 4011–4018. [Google Scholar] [CrossRef]
- Tomé-Carneiro, J.; Gonzálvez, M.; Larrosa, M.; Yáñez-Gascón, M.J.; García-Almagro, F.J.; Ruiz-Ros, J.A.; Tomás-Barberán, F.A.; García-Conesa, M.T.; Espín, J.C. Grape resveratrol increases serum adiponectin and downregulates inflammatory genes in peripheral blood mononuclear cells: A triple-blind, placebo-controlled, one-year clinical trial in patients with stable coronary artery disease. Cardiovasc. Drugs Ther. 2013, 27, 37–48. [Google Scholar] [CrossRef]
- Patel, K.R.; Scott, E.; Brown, V.A.; Gescher, A.J.; Steward, W.P.; Brown, K. Clinical trials of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 161–169. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heimesaat, M.M.; Mousavi, S.; Escher, U.; Lobo de Sá, F.D.; Peh, E.; Schulzke, J.-D.; Kittler, S.; Bücker, R.; Bereswill, S. Resveratrol Alleviates Acute Campylobacter jejuni Induced Enterocolitis in a Preclinical Murine Intervention Study. Microorganisms 2020, 8, 1858. https://doi.org/10.3390/microorganisms8121858
Heimesaat MM, Mousavi S, Escher U, Lobo de Sá FD, Peh E, Schulzke J-D, Kittler S, Bücker R, Bereswill S. Resveratrol Alleviates Acute Campylobacter jejuni Induced Enterocolitis in a Preclinical Murine Intervention Study. Microorganisms. 2020; 8(12):1858. https://doi.org/10.3390/microorganisms8121858
Chicago/Turabian StyleHeimesaat, Markus M., Soraya Mousavi, Ulrike Escher, Fábia Daniela Lobo de Sá, Elisa Peh, Jörg-Dieter Schulzke, Sophie Kittler, Roland Bücker, and Stefan Bereswill. 2020. "Resveratrol Alleviates Acute Campylobacter jejuni Induced Enterocolitis in a Preclinical Murine Intervention Study" Microorganisms 8, no. 12: 1858. https://doi.org/10.3390/microorganisms8121858
APA StyleHeimesaat, M. M., Mousavi, S., Escher, U., Lobo de Sá, F. D., Peh, E., Schulzke, J.-D., Kittler, S., Bücker, R., & Bereswill, S. (2020). Resveratrol Alleviates Acute Campylobacter jejuni Induced Enterocolitis in a Preclinical Murine Intervention Study. Microorganisms, 8(12), 1858. https://doi.org/10.3390/microorganisms8121858






