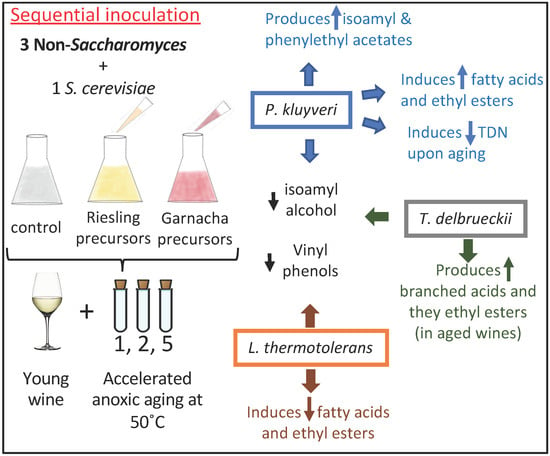
Modulating Fermentative, Varietal and Aging Aromas of Wine Using non-Saccharomyces Yeasts in a Sequential Inoculation Approach
Abstract
:1. Introduction
2. Material and Methods
2.1. Reagents and Standards
2.2. Glycosidic Precursors Extraction
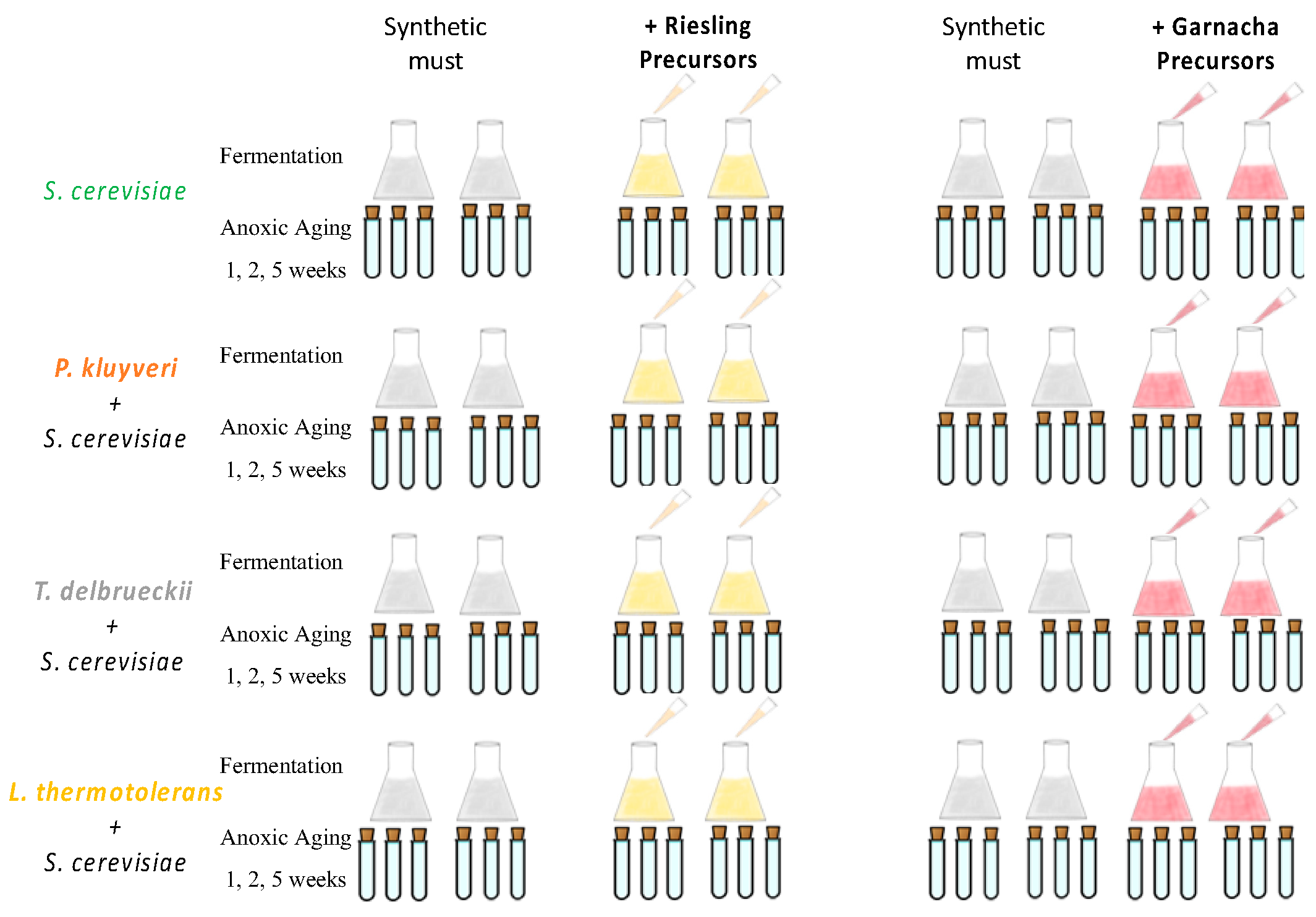
2.3. Synthetic Must
2.4. Fermentation
2.5. Analytical Methods
2.5.1. Analysis of Major Volatile Compounds
2.5.2. Analysis of Minor and Trace Volatile Compounds
3. Results
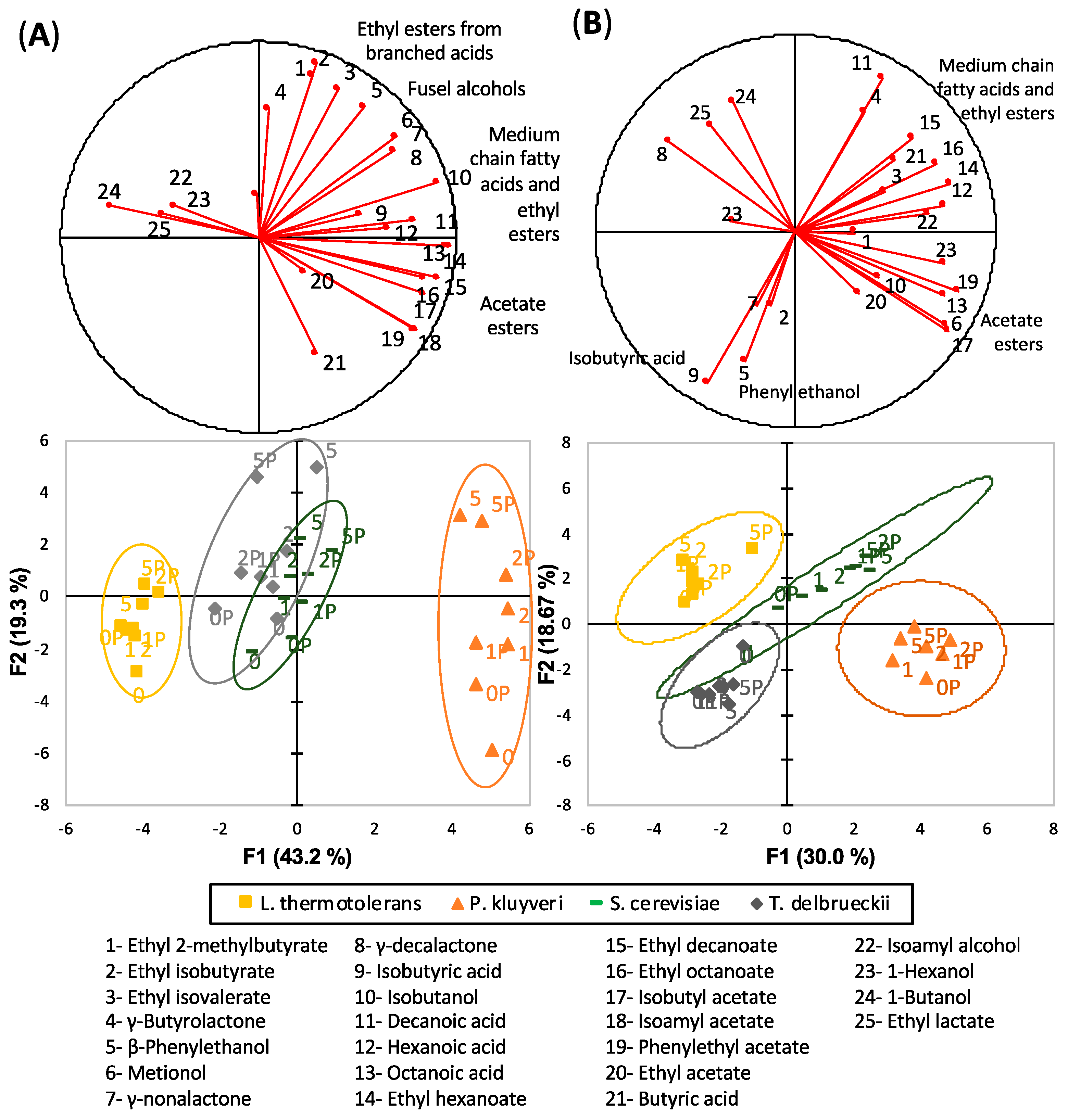
3.1. General Overview
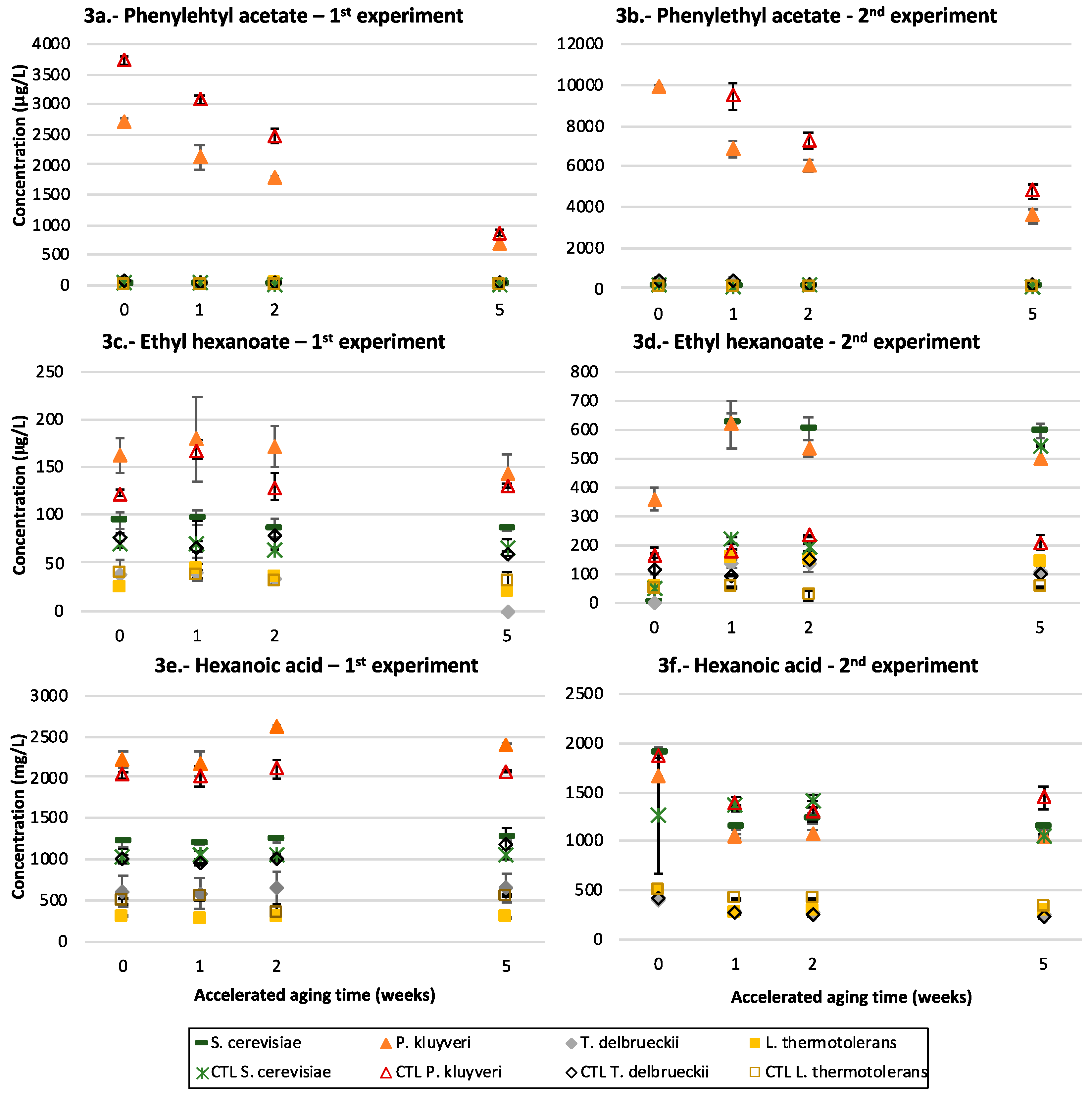
3.2. Fermentative Aroma Compounds
3.3. Acetates of Higher Alcohols
3.4. Medium Chain Fatty Acids and Their Ethyl Esters
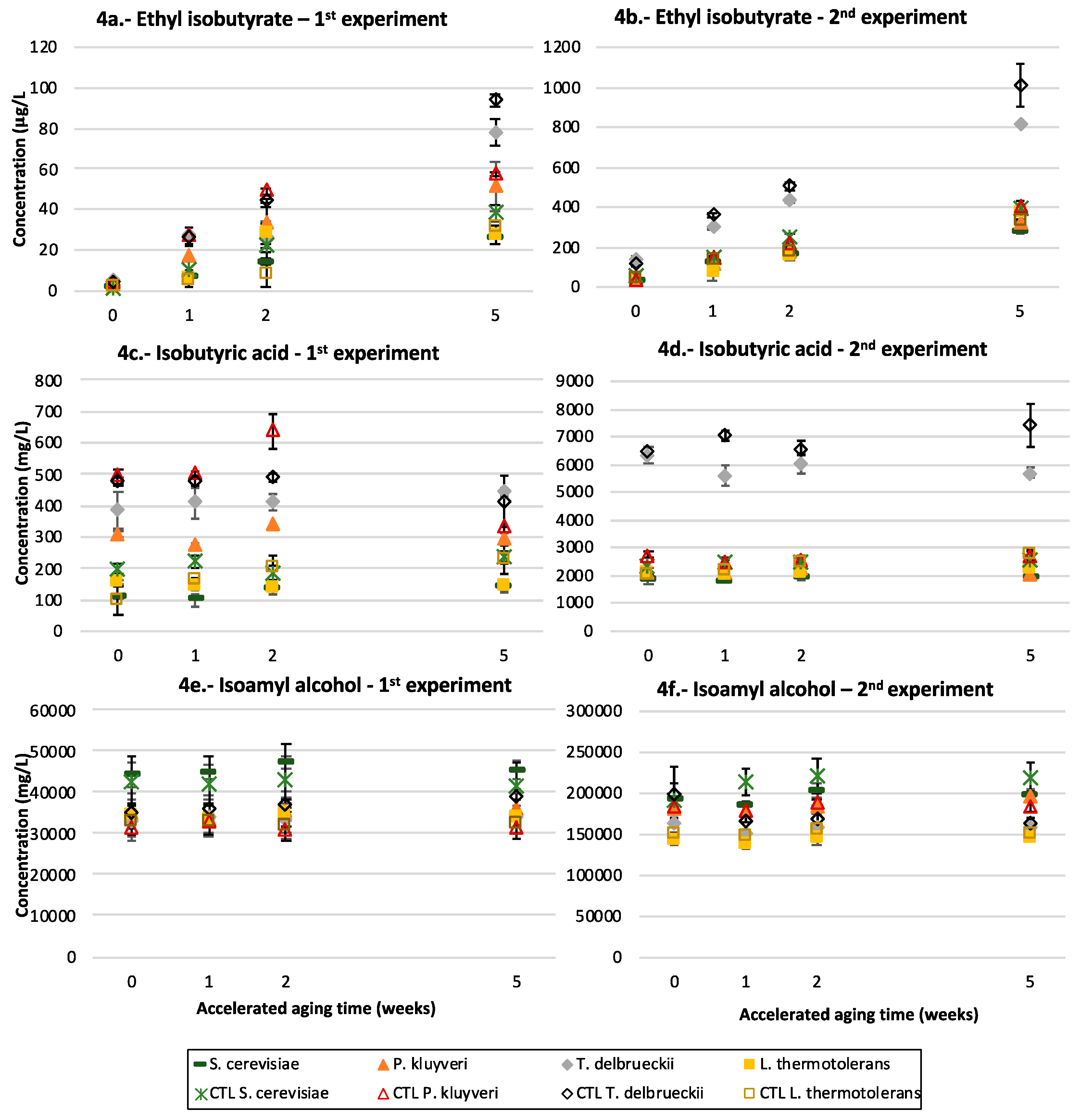
3.5. Ethyl Esters of Branched Acids
3.6. Isoamyl Alcohol
3.7. Varietal Aroma Compounds
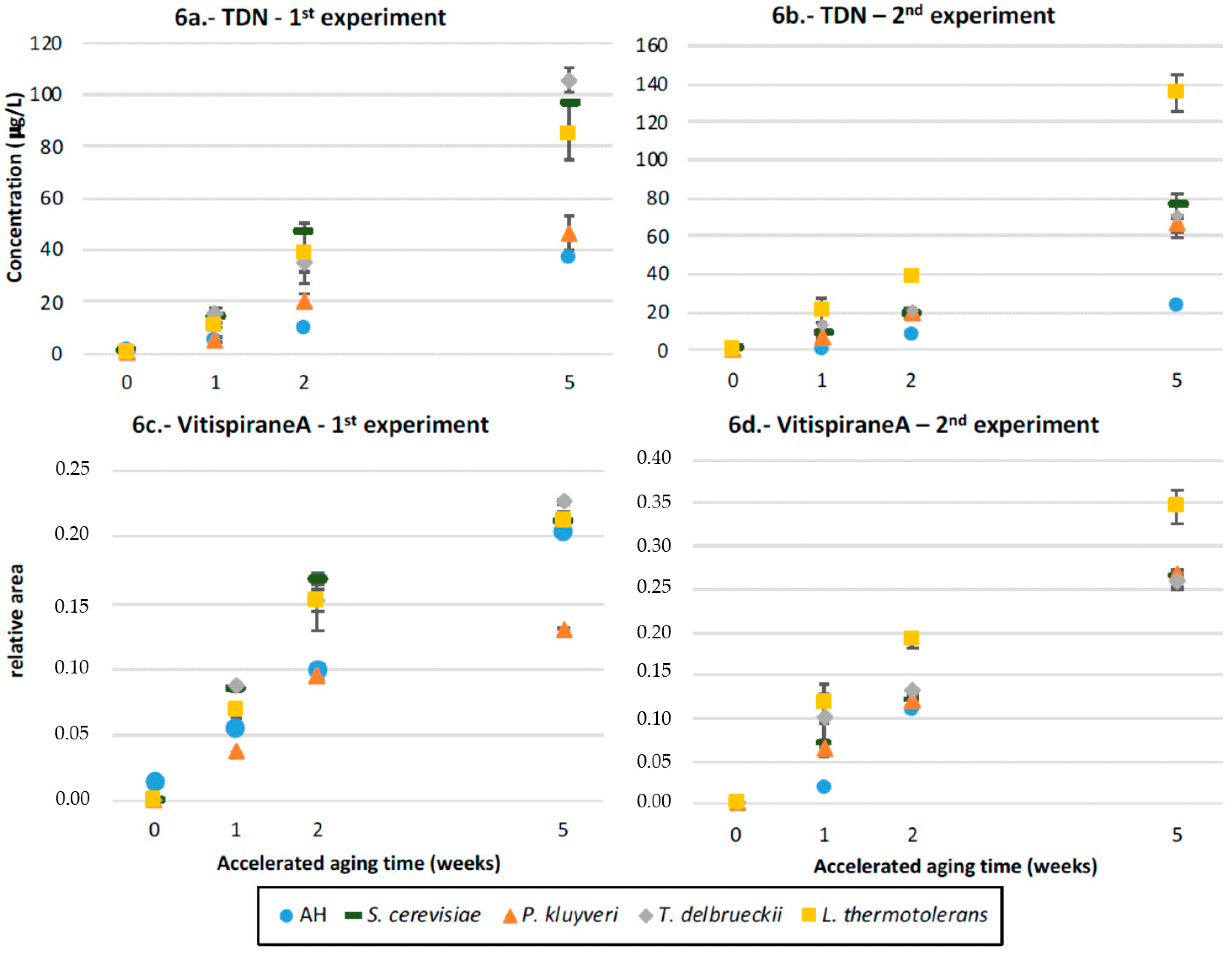
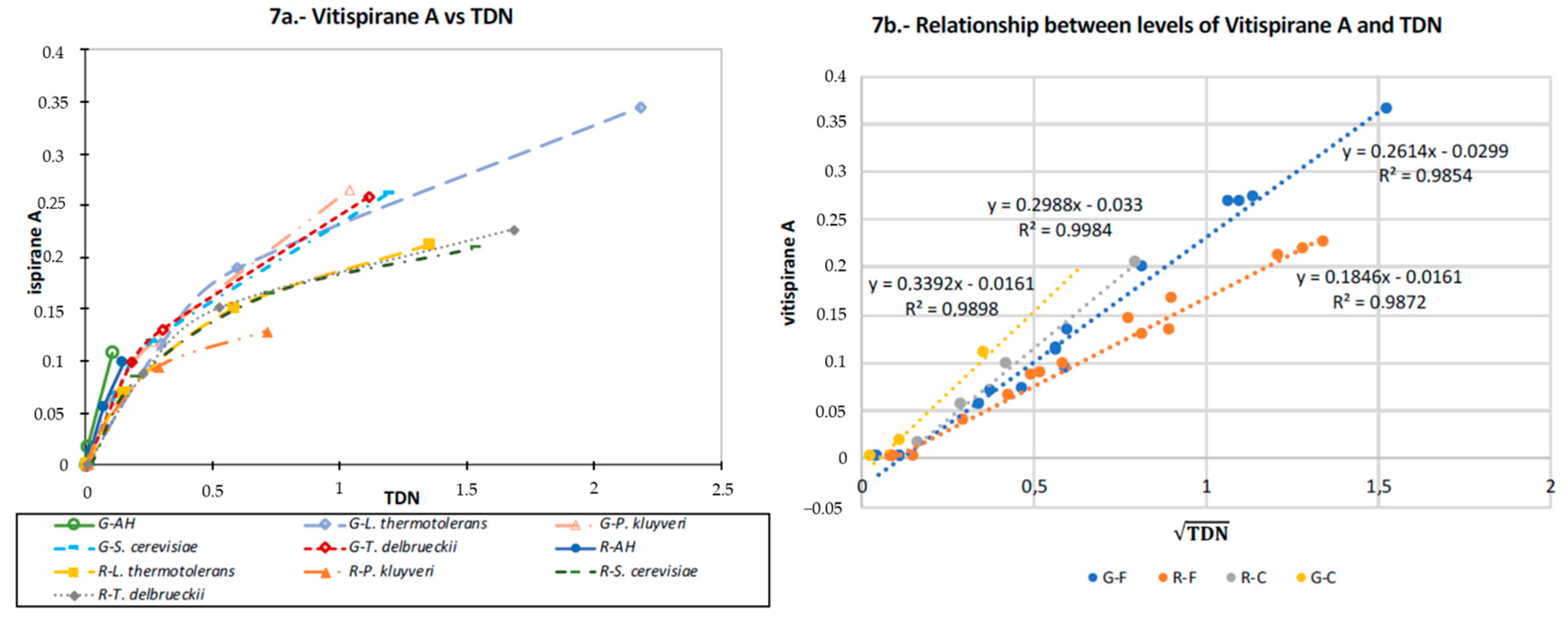
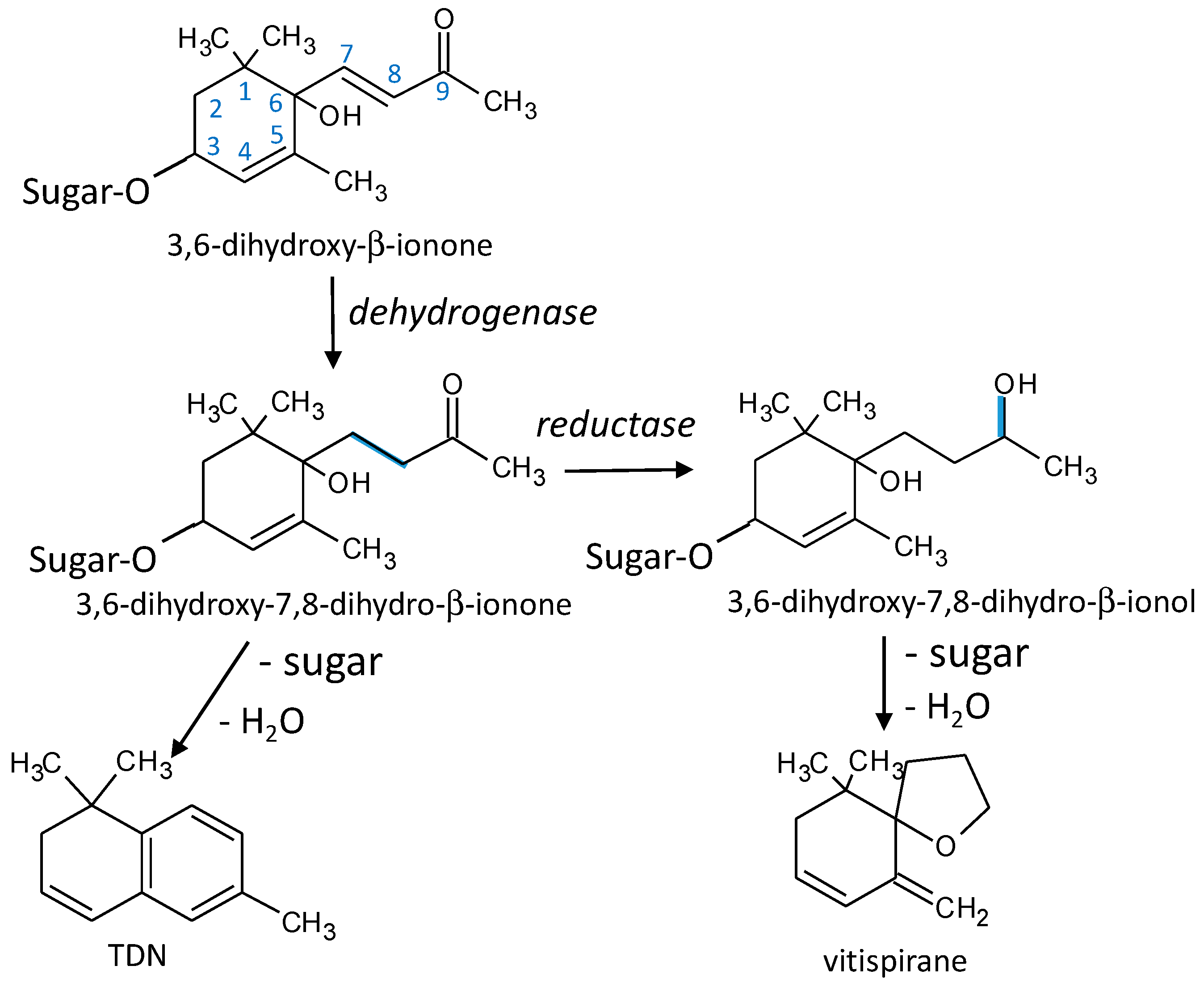
3.8. TDN and Vitispirane
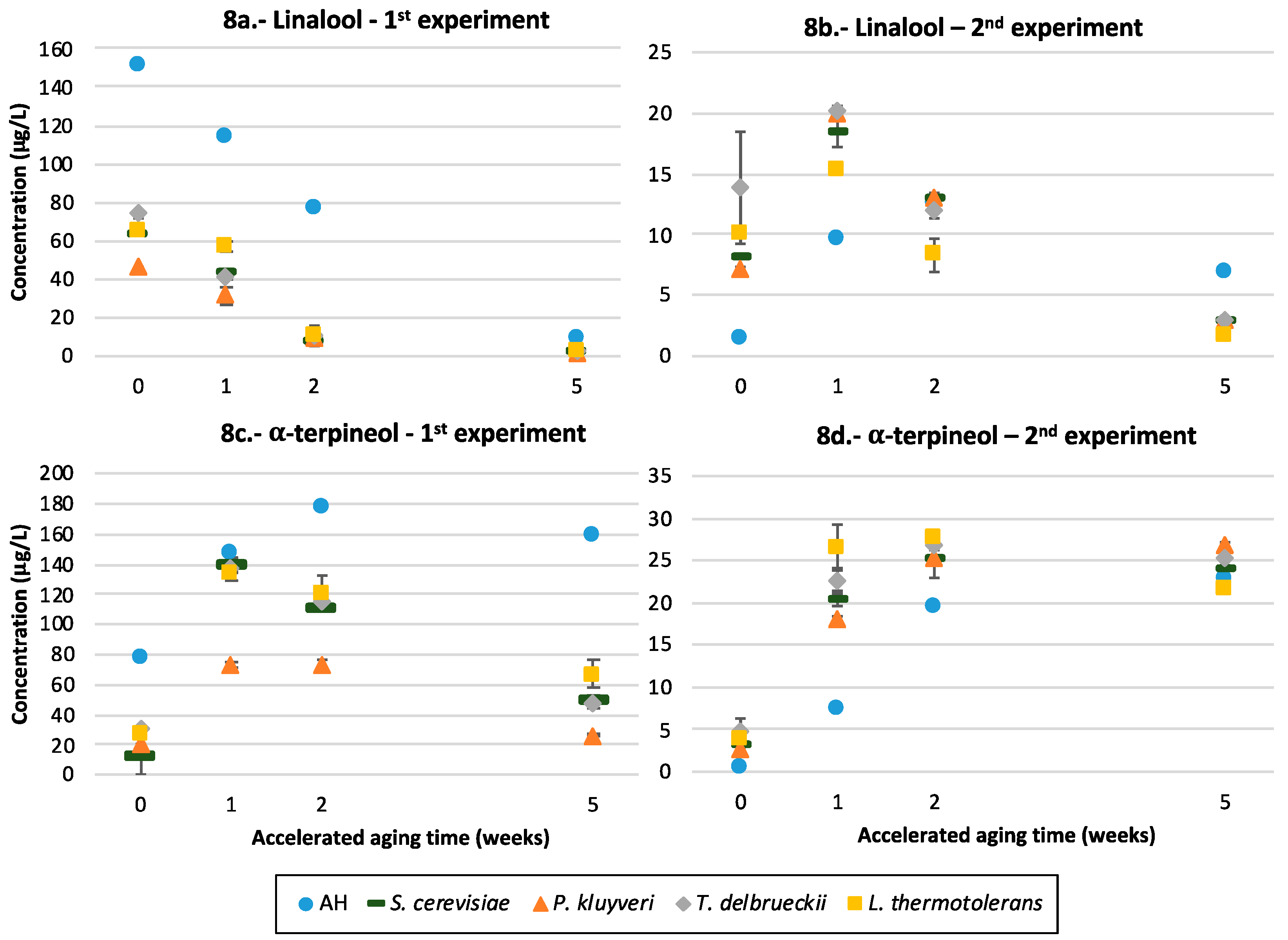
3.9. Terpenols
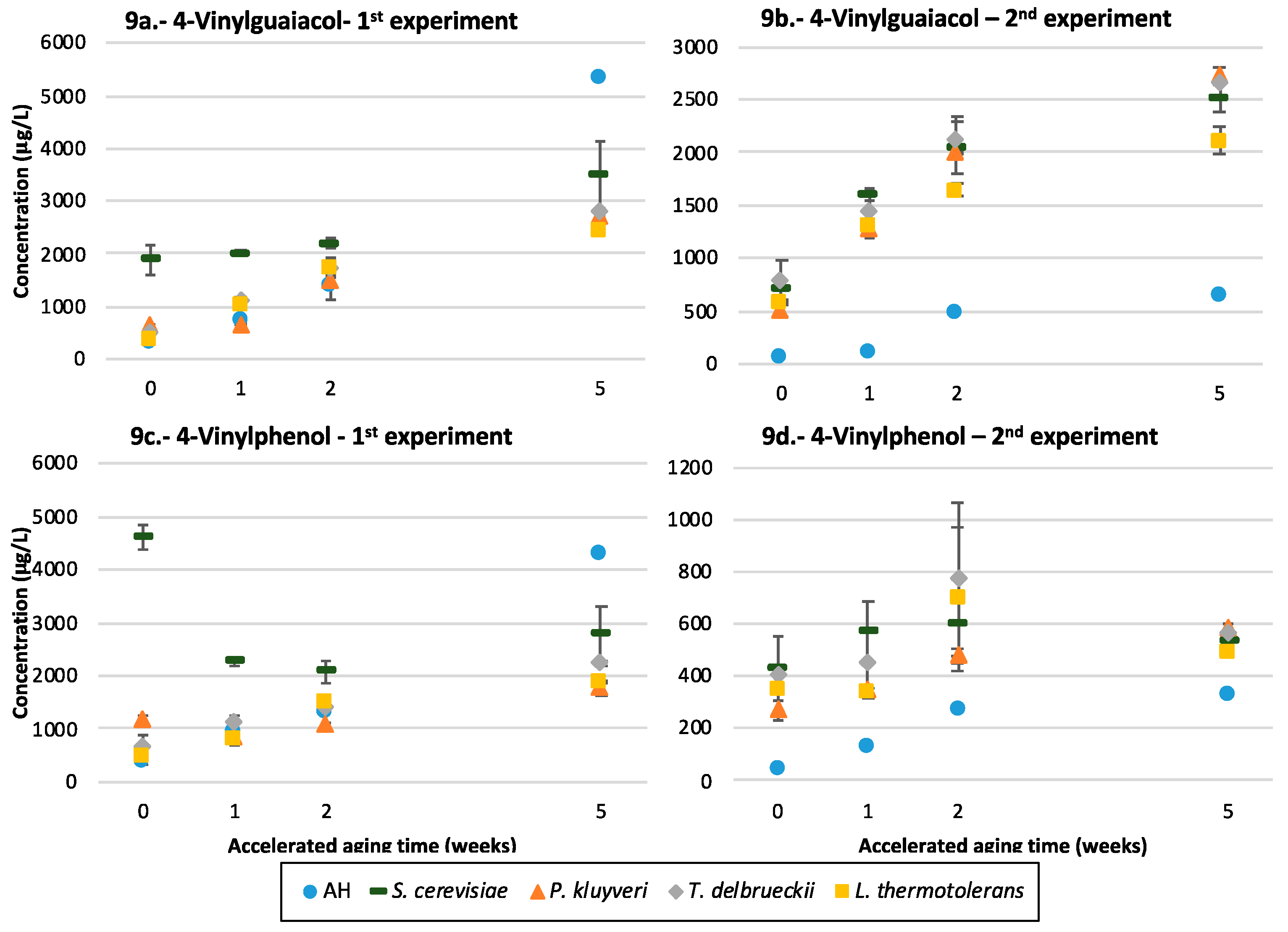
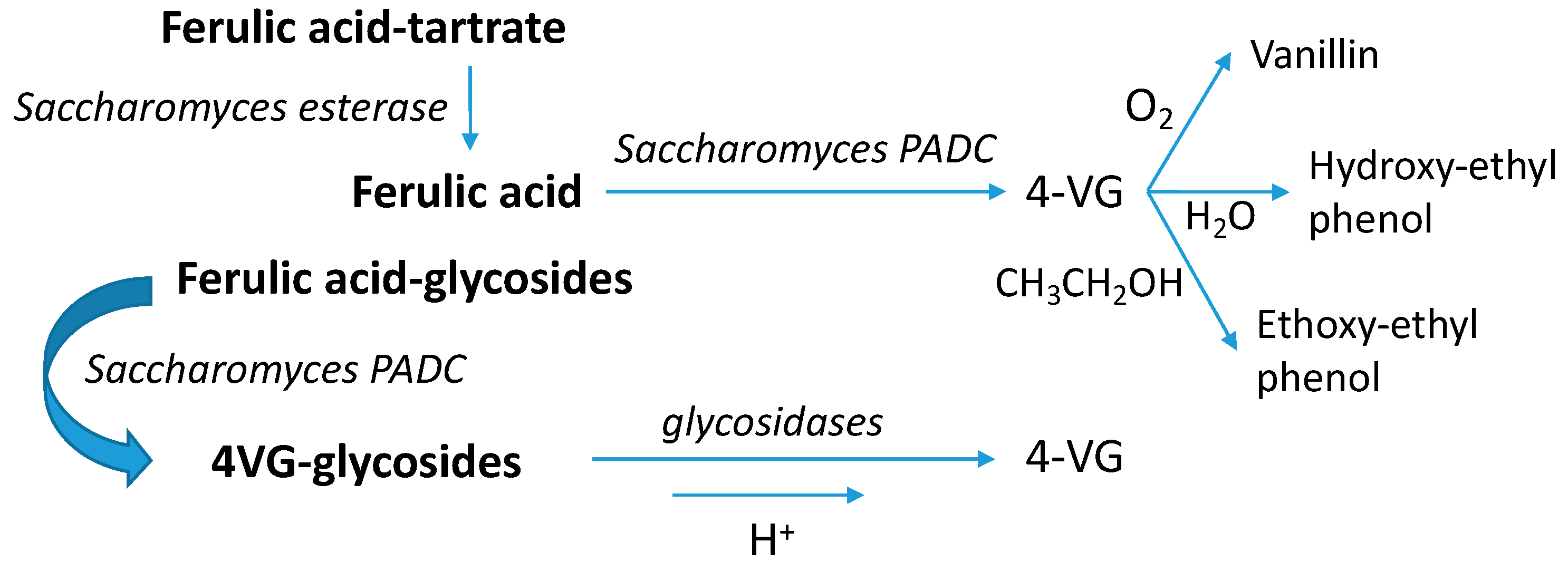
3.10. Volatile Phenols
4. Discussion
4.1. Differential Fermentative Profiles
4.2. P. kluyveri
4.3. T. delbrueckii
4.4. L. thermotolerans
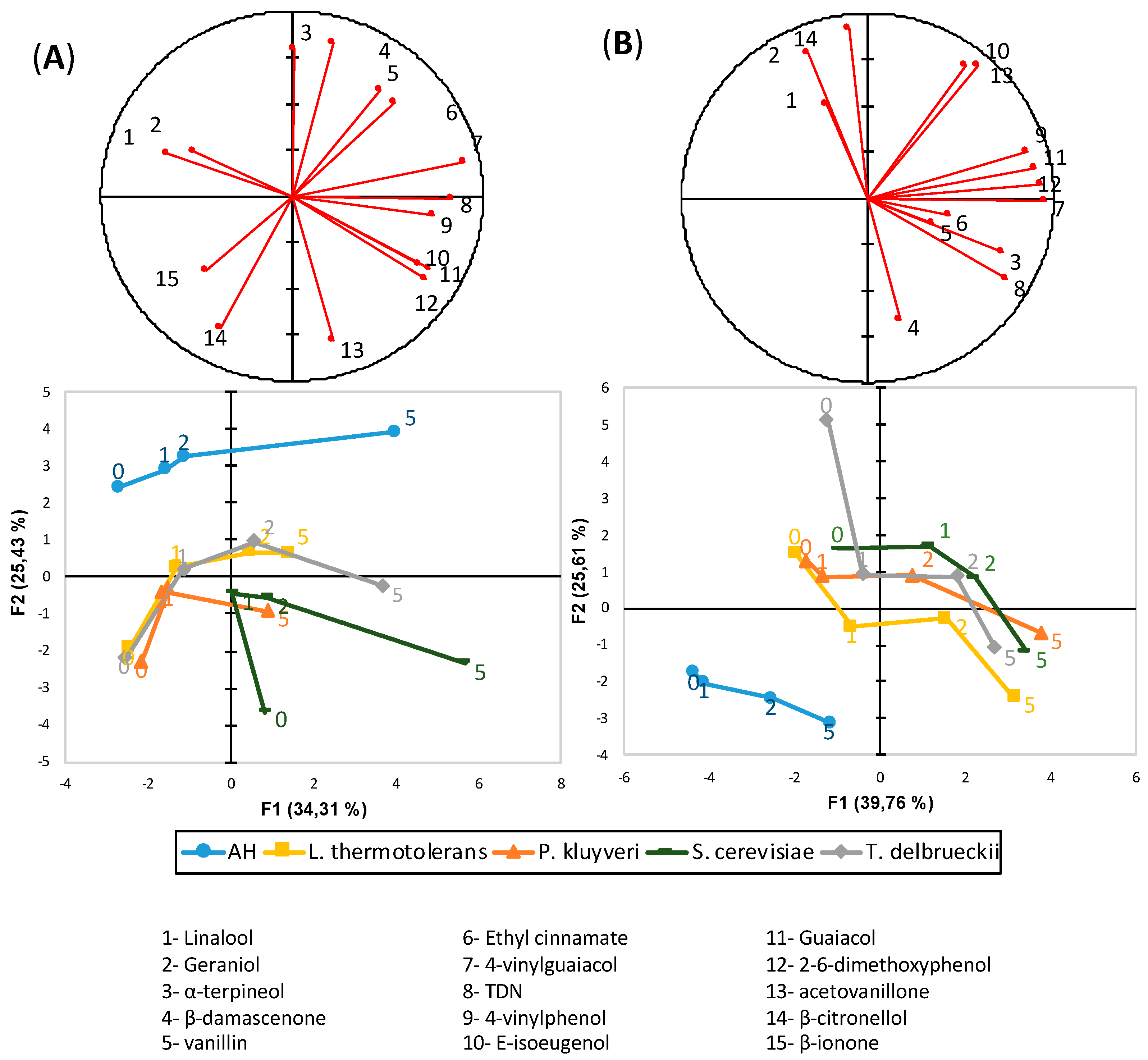
4.5. Effects on Varietal Aroma Profile
4.5.1. TDN
4.5.2. Terpenols
4.5.3. Vinylphenols
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ciani, M.; Ferraro, L. Enhanced glycerol content in wines made with immobilized Candida stellata cells. Appl. Environ. Microbiol. 1996, 62, 128–132. [Google Scholar] [PubMed]
- Ciani, M.; Ferraro, L. Combined use of immobilized Candida stellata cells and Saccharomyces cerevisiae to improve the quality of wines. J. Appl. Microbiol. 1998, 85, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, L.; Fatichenti, F.; Ciani, M. Pilot scale vinification process using immobilized Candida stellata cells and Saccharomyces cerevisiae. Process Biochem. 2000, 35, 1125–1129. [Google Scholar] [CrossRef]
- Rojas, V.; Gil, J.; Piñaga, F.; Manzanares, P. Studies on acetate ester production by non-Saccharomyces wine yeasts. Int. J. Food Microbiol. 2001, 70, 283–289. [Google Scholar] [CrossRef]
- Rojas, V.; Gil, J.V.; Piñaga, F.; Manzanares, P. Acetate ester formation in wine by mixed cultures in laboratory fermentations. Int. J. Food Microbiol. 2003, 86, 181–188. [Google Scholar] [CrossRef]
- Anfang, N.; Brajkovich, M.; Goddard, M.R. Co-fermentation with Pichia kluyveri increases varietal thiol concentrations in Sauvignon Blanc. Aust. J. Grape Wine Res. 2009, 15, 1–8. [Google Scholar] [CrossRef]
- Benito, S.; Hofmann, T.; Laier, M.; Lochbuhler, B.; Schuttler, A.; Ebert, K.; Fritsch, S.; Rocker, J.; Rauhut, D. Effect on quality and composition of Riesling wines fermented by sequential inoculation with non-Saccharomyces and Saccharomyces cerevisiae. Eur. Food Res. Technol. 2015, 241, 707–717. [Google Scholar] [CrossRef]
- Herraiz, T.; Reglero, G.; Herraiz, M.; Martin-Alvarez, P.J.; Cabezudo, M. The incluence of the yeast and type of culture on the volatile composition of wine fermented without sulfur dioxide. Am. J. Enol. Vitic. 1990, 41, 313–318. [Google Scholar]
- Bely, M.; Stoeckle, P.; Masneuf-Pomarède, I.; Dubourdieu, D. Impact of mixed Torulaspora delbrueckii-Saccharomyces cerevisiae culture on high-sugar fermentation. Int. J. Food Microbiol. 2008, 122, 312–320. [Google Scholar] [CrossRef]
- Izquierdo Cañas, P.M.; Palacios García, A.T.; García Romero, E. Enhancement of flavour properties in wines using sequential inoculations of non-Saccharomyces (Hansenula and Torulaspora) and Saccharomyces yeast starter. VITIS J. Grapevine Res. 2011, 50, 177–182. [Google Scholar]
- Azzolini, M.; Fedrizzi, B.; Tosi, E.; Finato, F.; Vagnoli, P.; Scrinzi, C.; Zapparoli, G. Effects of Torulaspora delbrueckii and Saccharomyces cerevisiae mixed cultures on fermentation and aroma of Amarone wine. Eur. Food Res. Technol. 2012, 235, 303–313. [Google Scholar] [CrossRef]
- Loira, I.; Vejarano, R.; Bañuelos, M.A.; Morata, A.; Tesfaye, W.; Uthurry, C.; Villa, A.; Cintora, I.; Suárez-Lepe, J.A. Influence of sequential fermentation with Torulaspora delbrueckii and Saccharomyces cerevisiae on wine quality. LWT Food Sci. Technol. 2014, 59, 915–922. [Google Scholar] [CrossRef]
- Azzolini, M.; Tosi, E.; Lorenzini, M.; Finato, F.; Zapparoli, G. Contribution to the aroma of white wines by controlled Torulaspora delbrueckii cultures in association with Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2015, 31, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Belda, I.; Navascués, E.; Marquina, D.; Santos, A.; Calderon, F.; Benito, S. Dynamic analysis of physiological properties of Torulaspora delbrueckii in wine fermentations and its incidence on wine quality. Appl. Microbiol. Biotechnol. 2015, 99, 1911–1922. [Google Scholar] [CrossRef] [PubMed]
- de-la-Fuente-Blanco, A.; Sáenz-Navajas, M.-P.; Ferreira, V. On the effects of higher alcohols on red wine aroma. Food Chem. 2016, 210, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.Y.; Gong, H.S.; Jiang, X.M.; Zhao, Y.P. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae on alcoholic fermentation behaviour and wine aroma of cherry wines. Food Microbiol. 2014, 44, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Renault, P.; Coulon, J.; de Revel, G.; Barbe, J.C.; Bely, M. Increase of fruity aroma during mixed T. delbrueckii/S. cerevisiae wine fermentation is linked to specific esters enhancement. Int. J. Food Microbiol. 2015, 207, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Velázquez, R.; Zamora, E.; álvarez, M.L.; Hernández, L.M.; Ramírez, M. Effects of new Torulaspora delbrueckii killer yeasts on the must fermentation kinetics and aroma compounds of white table wine. Front. Microbiol. 2015, 6, 1222. [Google Scholar] [CrossRef] [PubMed]
- Beckner Whitener, M.E.; Stanstrup, J.; Panzeri, V.; Carlin, S.; Divol, B.; Du Toit, M.; Vrhovsek, U. Untangling the wine metabolome by combining untargeted SPME–GCxGC-TOF-MS and sensory analysis to profile Sauvignon blanc co-fermented with seven different yeasts. Metabolomics 2016, 12, 1–25. [Google Scholar] [CrossRef]
- Mora, J.; Barbas, J.I.; Ramis, B.; Mulet, A. Yeast microflora associated with some Majorcan musts and wines. Am. J. Enol. Vitic. 1988, 39, 344–346. [Google Scholar]
- Mora, J.; Barbas, J.I.; Mulet, A. Growth of yeast species during the fermentation of musts inculated with Kluyveromyces thermotolerans and Saccharomyces cerevisiae. Am. J. Enol. Vitic. 1990, 41, 156–159. [Google Scholar]
- Kapsopoulou, K.; Kapaklis, A.; Spyropoulos, H. Growth and fermentation characteristics of a strain of the wine yeast Kluyveromyces thermotolerans isolated in greece. World J. Microbiol. Biotechnol. 2005, 21, 1599–1602. [Google Scholar] [CrossRef]
- Kapsopoulou, K.; Mourtzini, A.; Anthoulas, M.; Nerantzis, E. Biological acidification during grape must fermentation using mixed cultures of Kluyveromyces thermotolerans and Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2007, 23, 735–739. [Google Scholar] [CrossRef]
- Gobbi, M.; Comitini, F.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Lachancea thermotolerans and Saccharomyces cerevisiae in simultaneous and sequential co-fermentation: A strategy to enhance acidity and improve the overall quality of wine. Food Microbiol. 2013, 33, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Benito, A.; Calderon, F.; Palomero, F.; Benito, S. Combine use of selected Schizosaccharomyces pombe and Lachancea thermotolerans yeast strains as an alternative to the traditional malolactic fermentation in red wine production. Molecules 2015, 20, 9510–9523. [Google Scholar] [CrossRef] [PubMed]
- Balikci, E.K.; Tanguler, H.; Jolly, N.P.; Erten, H. Influence of Lachancea thermotolerans on cv. Emir wine fermentation. Yeast 2016, 33, 313–321. [Google Scholar] [CrossRef]
- Benito, A.; Calderon, F.; Palomero, F.; Benito, S. Quality and composition of Airen wines fermented by sequential inoculation of Lachancea thermotolerans and Saccharomyces cerevisiae. Food Technol. Biotechnol. 2016, 54, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Porter, T.J.; Divol, B.; Setati, M.E. Investigating the biochemical and fermentation attributes of Lachancea species and strains: Deciphering the potential contribution to wine chemical composition. Int. J. Food Microbiol. 2019, 290, 273–287. [Google Scholar] [CrossRef]
- Shekhawat, K.; Porter, T.J.; Bauer, F.F.; Setati, M.E. Employing oxygen pulses to modulate Lachancea thermotolerans-Saccharomyces cerevisiae Chardonnay fermentations. Ann. Microbiol. 2018, 68, 93–102. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Clímaco, M.C.; Faia, A.M. The role of non-Saccharomyces species in releasing glycosidic bound fraction of grape aroma components—A preliminary study. J. Appl. Microbiol. 2001, 91, 67–71. [Google Scholar] [CrossRef]
- Strauss, M.L.A.; Jolly, N.P.; Lambrechts, M.G.; Van Rensburg, P. Screening for the production of extracellular hydrolytic enzymes by non-Saccharomyces wine yeasts. J. Appl. Microbiol. 2001, 91, 182–190. [Google Scholar] [CrossRef]
- Escribano, R.; González-Arenzana, L.; Garijo, P.; Berlanas, C.; López-Alfaro, I.; López, R.; Gutiérrez, A.R.; Santamaría, P. Screening of enzymatic activities within different enological non-Saccharomyces yeasts. J. Food Sci. Technol. 2017, 54, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Whitener, M.E.B.; Stanstrup, J.; Carlin, S.; Divol, B.; Du Toit, M.; Vrhovsek, U. Effect of non-Saccharomyces yeasts on the volatile chemical profile of Shiraz wine. Aust. J. Grape Wine Res. 2017, 23, 179–192. [Google Scholar] [CrossRef]
- Bely, M.; Sablayrolles, J.-M.; Barre, P. Automatic detection of assimilable nitrogen deficiencies during alcoholic fermentation in oenological conditions. J. Ferment. Bioeng. 1990, 70, 246–252. [Google Scholar] [CrossRef]
- Ortega, C.; Lopez, R.; Cacho, J.; Ferreira, V. Fast analysis of important wine volatile compounds Development and validation of a new method based on gas chromatographic—Flame ionisation detection analysis of dichloromethane microextracts. J. Chromatogr. A 2001, 923, 205–214. [Google Scholar] [CrossRef]
- Lopez, R.; Aznar, M.; Cacho, J.; Ferreira, V. Determination of minor and trace volatile compounds in wine by solid-phase extraction and gas chromatography with mass spectrometric detection. J. Chromatogr. A 2002, 966, 167–177. [Google Scholar] [CrossRef]
- de-la-Fuente-Blanco, A.; Sáenz-Navajas, M.-P.; Ferreira, V. Levels of higher alcohols inducing aroma changes and modulating experts’ preferences in wine model solutions: Levels of higher alcohols inducing aroma changes. Aust. J. Grape Wine Res. 2017, 23, 162–169. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Boekhout, T.; Gojkovic, Z.; Katz, M. Evaluation of non-Saccharomyces yeasts in the fermentation of wine, beer and cider for the development of new beverages. J. Inst. Brew. 2018, 124, 389–402. [Google Scholar] [CrossRef]
- San Juan, F.; Cacho, J.; Ferreira, V.; Escudero, A. Aroma chemical composition of red wines from different price categories and its relationship to quality. J. Agric. Food Chem. 2012, 60, 5045–5056. [Google Scholar] [CrossRef]
- Simpson, R.F. 1,1,6-trimethyl-1,2-dihydronaphthalene: An important contributor to the bottle aged bouquet of wine. Chem. Ind. 1978, 1, 37. [Google Scholar]
- Winterhalter, P. 1,1,6-Trimethyl-1,2-dihydronaphthalene (TDN) formation in wine. I. Studies on the hydrolysis of 2,6,10,10-tetramethyl-1-oxaspiro[4.5]dec-6-ene-2,8-diol rationalizing the origin of TDN and related C13 norisoprenoids in Riesling wine. J. Agric. Food Chem. 1991, 39, 1825–1829. [Google Scholar] [CrossRef]
- Sacks, G.L.; Gates, M.J.; Ferry, F.X.; Lavin, E.H.; Kurtz, A.J.; Acree, T.E. Sensory threshold of 1,1,6-Trimethyl-1,2-dihydronaphthalene (TDN) and concentrations in young Riesling and non-Riesling wines. J. Agric. Food Chem. 2012, 60, 2998–3004. [Google Scholar] [CrossRef] [PubMed]
- Winterhalter, P.; Gok, R. TDN and beta-Damascenone: Two important carotenoid metabolites in wine. In Carotenoid Cleavage Products; Winterhalter, P., Ebeler, S.E., Eds.; ACS Symposium Series; ACS Publications: Washington, DC, USA, 2013; Volume 1134, pp. 125–137. ISBN 978-0-8412-2778-1. [Google Scholar]
- Loscos, N.; Hernández-Orte, P.; Cacho, J.; Ferreira, V. Evolution of the aroma composition of wines supplemented with grape flavour precursors from different varietals during accelerated wine ageing. Food Chem. 2010, 120, 205–216. [Google Scholar] [CrossRef]
- Roscher, R.; Winterhalter, P. Application of multylayer coil countercurrent chromatography for the study of Vitis-vinifera CV Riesling leaf glycosides. J. Agric. Food Chem. 1993, 41, 1452–1457. [Google Scholar] [CrossRef]
- Skouroumounis, G.K.; Winterhalter, P. Glycosidically bound norisoprenoids from vitis-vinifera CV Riesling leaves. J. Agric. Food Chem. 1994, 42, 1068–1072. [Google Scholar] [CrossRef]
- Stingl, C.; Knapp, H.; Winterhalter, P. 3,4-Dihydroxy-7,8-dihydro-beta-ionone 3-O-beta-d-glucopyranoside and other glycosidic constituents from apple leaves. Nat. Prod. Lett. 2002, 16, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.A.; Capone, D.L.; Sefton, M.A.; Elsey, G.M. Riesling acetal is precursor to 1,1,6-trimethyl-1,2-dihydronapthalene (TDN) in wine. Aust. J. Grape Wine Res. 2009, 15, 93–96. [Google Scholar] [CrossRef]
- Williams, P.J.; Strauss, C.R.; Wilson, B. New linalool derivatives in Muscat of Alexandria grapes and wines. Phytochemistry 1980, 19, 1137–1139. [Google Scholar] [CrossRef]
- Chatonnet, P.; Dubourdieu, D.; Boidron, J.; Lavigne, V. Synthesis of volatile phenols by Saccharomyces cerevisiae in wines. J. Sci. Food Agric. 1993, 62, 191–202. [Google Scholar] [CrossRef]
- Shinohara, T.; Kubodera, S.; Yanagida, F. Distribution of phenolic yeasts and production of phenolic off-flavors in wine fermentation. J. Biosci. Bioeng. 2000, 90, 90–97. [Google Scholar] [CrossRef]
- Smit, A.; Otero, R.R.C.; Lambrechts, M.G.; Pretorius, I.S.; Van Rensburg, P. Enhancing volatile phenol concentrations in wine by expressing various phenolic acid decarboxylase genes in Saccharomyces cerevisiae. J. Agric. Food Chem. 2003, 51, 4909–4915. [Google Scholar] [CrossRef] [PubMed]
- Dugelay, I.; Gunata, Z.; Sapis, J.C.; Baumes, R.; Bayonove, C. Role of cinnamoyl esterase-activities from enzyme preparations on the formation of volatile phenols during winemaking. J. Agric. Food Chem. 1993, 41, 2092–2096. [Google Scholar] [CrossRef]
- Baderschneider, B.; Winterhalter, P. Isolation and characterization of novel benzoates, cinnamates, flavonoids, and lignans from Riesling wine and screening for antioxidant activity. J. Agric. Food Chem. 2001, 49, 2788–2798. [Google Scholar] [CrossRef] [PubMed]
- Tomou, E.M.; Skaltsa, H. Phytochemical investigation of the fern Asplenium ceterach (Aspleniaceae). Nat. Prod. Commun. 2018, 13, 849–850. [Google Scholar] [CrossRef]
- Vanbeneden, N.; Saison, D.; Delvaux, F.; Delvaux, F.R. Decrease of 4-vinylguaiacol during beer aging and formation of apocynol and vanillin in beer. J. Agric. Food Chem. 2008, 56, 11983–11988. [Google Scholar] [CrossRef] [PubMed]
- Soleas, G.J.; Dam, J.; Carey, M.; Goldberg, D.M. Toward the fingerprinting of wines: Cultivar-related patterns of polyphenolic constituents in Ontario wines. J. Agric. Food Chem. 1997, 45, 3871–3880. [Google Scholar] [CrossRef]
| (A) Riesling | Volatile Acidity | pH | Total Acidity | Residual Sugars |
| CTL S. cerevisiae | 0.51 ± 0.07 | 3.32 ± 0.06 | 5.06 ± 0.11 | 0.30 ± 0.1 |
| PR S. cerevisiae | 0.48 ± 0.04 | 3.36 ± 0.05 | 5.03 ± 0.08 | 0.30 ± 0.2 |
| CTL P. kluyveri | 0.49 ± 0.02 | 3.52 ± 0.03 | 4.76 ± 0.04 | 0.5 ± 0.2 |
| PR P. kluyveri | 0.40 ± 0.0 | 3.56 ± 0.04 | 4.95 ± 0.08 | 0.20 ± 0.1 |
| CTL L. thermotolerans | 0.22 ± 0.0 | 3.46 ± 0.02 | 4.65 ± 0.08 | 0.20 ± 0.1 |
| PR L. thermotolerans | 0.26 ± 0.0 | 3.49 ± 0.01 | 4.58 ± 0.0 | 0.05 ± 0.05 |
| CTL T. delbrueckii | 0.35 ± 0.02 | 3.52 ± 0.01 | 4.91 ± 0.11 | 0.15 ± 0.05 |
| PR T. delbrueckii | 0.24 ± 0.02 | 3.58 ± 0.01 | 4.80 ± 0.23 | 0.20 ± 0.0 |
| (B) Garnacha | Volatile Acidity | pH | Total Acidity | Residual Sugars |
| CTL S. cerevisiae | 0.6 ± 0.03 | 3.43 ± 0.01 | 6.5 ± 0.05 | 0.75 ± 0.07 |
| PG S. cerevisiae | 0.6 ± 0.08 | 3.43 ± 0.01 | 6.46 ± 0.11 | 1.75 ± 0.78 |
| CTL P. kluyveri | 0.9 ± 0.03 | 3.5 ± 0.08 | 6.27 ± 0.27 | 1.5 ± 0.71 |
| PG P. kluyveri | 0.93 ± 0.03 | 3.46 ± 0.02 | 6.16 ± 0 | 1 ± 0.14 |
| CTL L. thermotolerans | 0.79 ± 0 | 3.26 ± 0.01 | 8.89 ± 0.11 | 5.85 ± 1.41 |
| PG L. thermotolerans | 0.71 ± 0.03 | 3.44 ± 0.16 | 7.98 ± 0.32 | 11 ± 0.85 |
| CTL T. delbrueckii | 0.75 ± 0.03 | 3.41 ± 0.08 | 6.92 ± 0 | 4.5 ± 0.14 |
| PG T. delbrueckii | 0.75 ± 0.03 | 3.46 ± 0.01 | 6.57 ± 0.05 | 8 ± 0.71 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, I.; Ferreira, V. Modulating Fermentative, Varietal and Aging Aromas of Wine Using non-Saccharomyces Yeasts in a Sequential Inoculation Approach. Microorganisms 2019, 7, 164. https://doi.org/10.3390/microorganisms7060164
Oliveira I, Ferreira V. Modulating Fermentative, Varietal and Aging Aromas of Wine Using non-Saccharomyces Yeasts in a Sequential Inoculation Approach. Microorganisms. 2019; 7(6):164. https://doi.org/10.3390/microorganisms7060164
Chicago/Turabian StyleOliveira, Inês, and Vicente Ferreira. 2019. "Modulating Fermentative, Varietal and Aging Aromas of Wine Using non-Saccharomyces Yeasts in a Sequential Inoculation Approach" Microorganisms 7, no. 6: 164. https://doi.org/10.3390/microorganisms7060164
APA StyleOliveira, I., & Ferreira, V. (2019). Modulating Fermentative, Varietal and Aging Aromas of Wine Using non-Saccharomyces Yeasts in a Sequential Inoculation Approach. Microorganisms, 7(6), 164. https://doi.org/10.3390/microorganisms7060164