Effects of a Simulated Acute Oil Spillage on Bacterial Communities from Arctic and Antarctic Marine Sediments
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Sites and Sediment Collection
2.2. Microcosm Set-Up
2.3. Analysis of Residual Hydrocarbons in Arctic and Antarctic Sediments
2.4. Microbial Community Characterization
2.4.1. Flow Cytometry
2.4.2. DNA Extraction and Fingerprinting Analyses
2.5. Statistical Analyses
3. Results
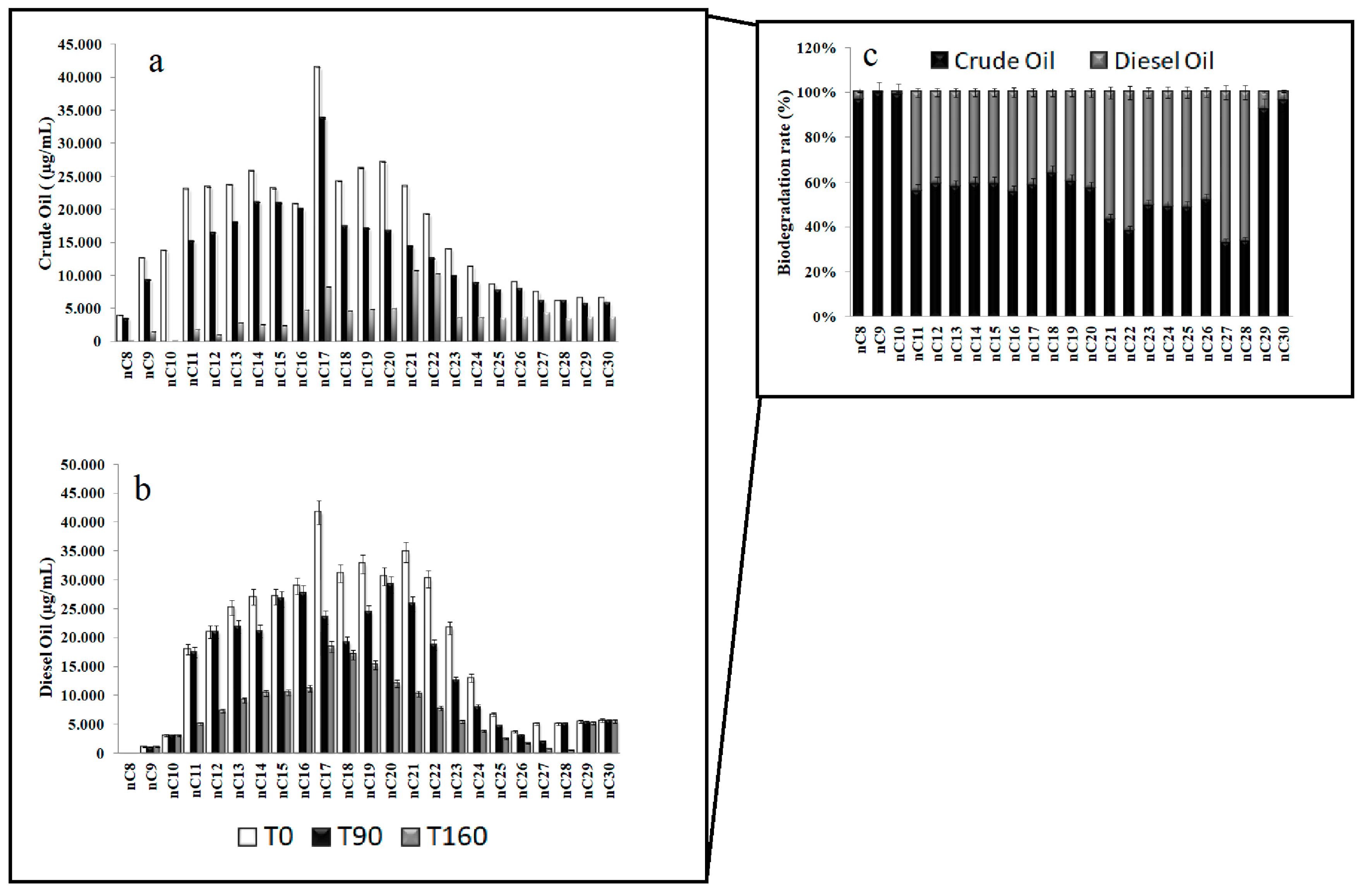
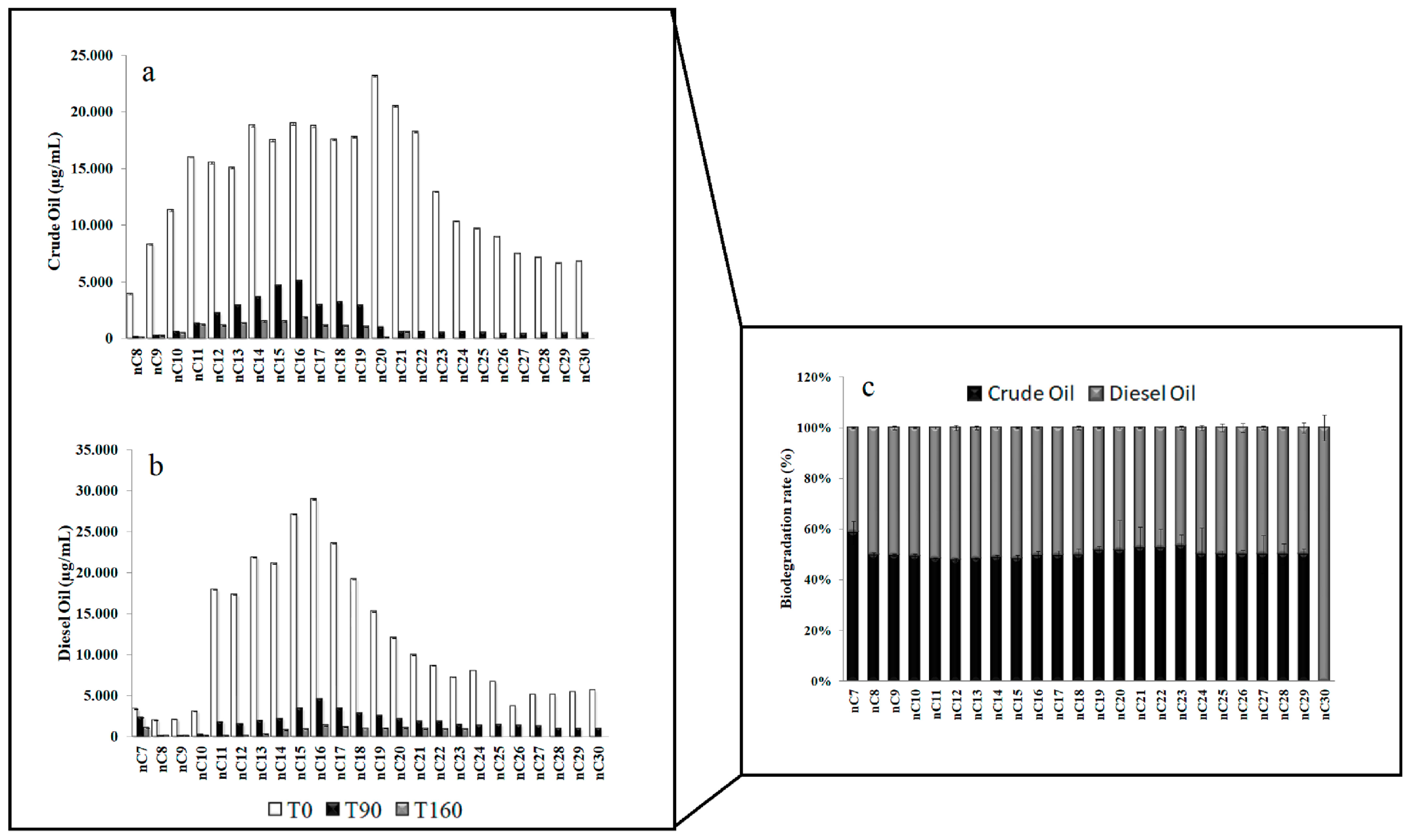
3.1. Residual Hydrocarbons in Arctic and Antarctic Microcosms
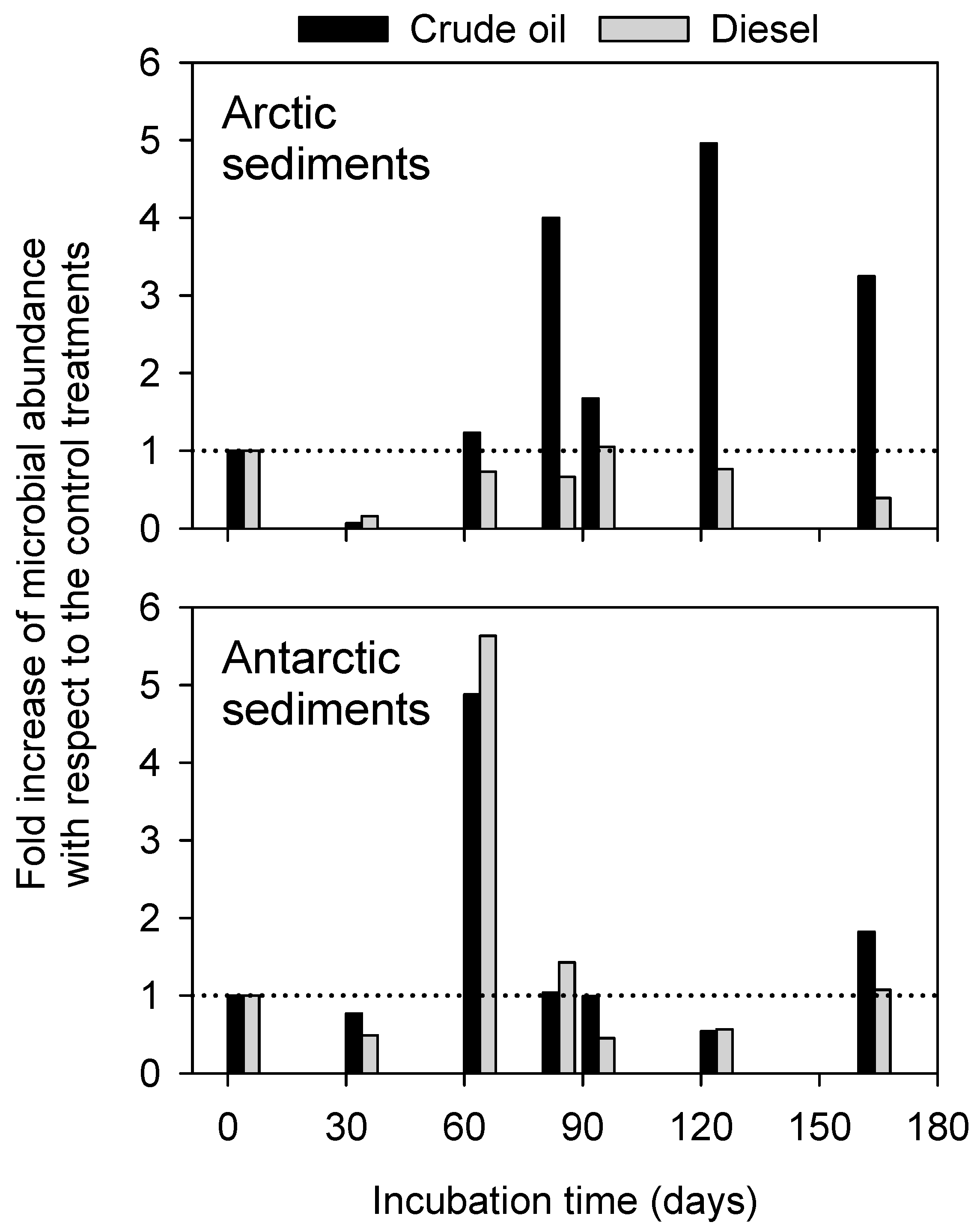
3.2. Patterns of Microbial Cell Abundance in Arctic and Antarctic Contaminated Sediments
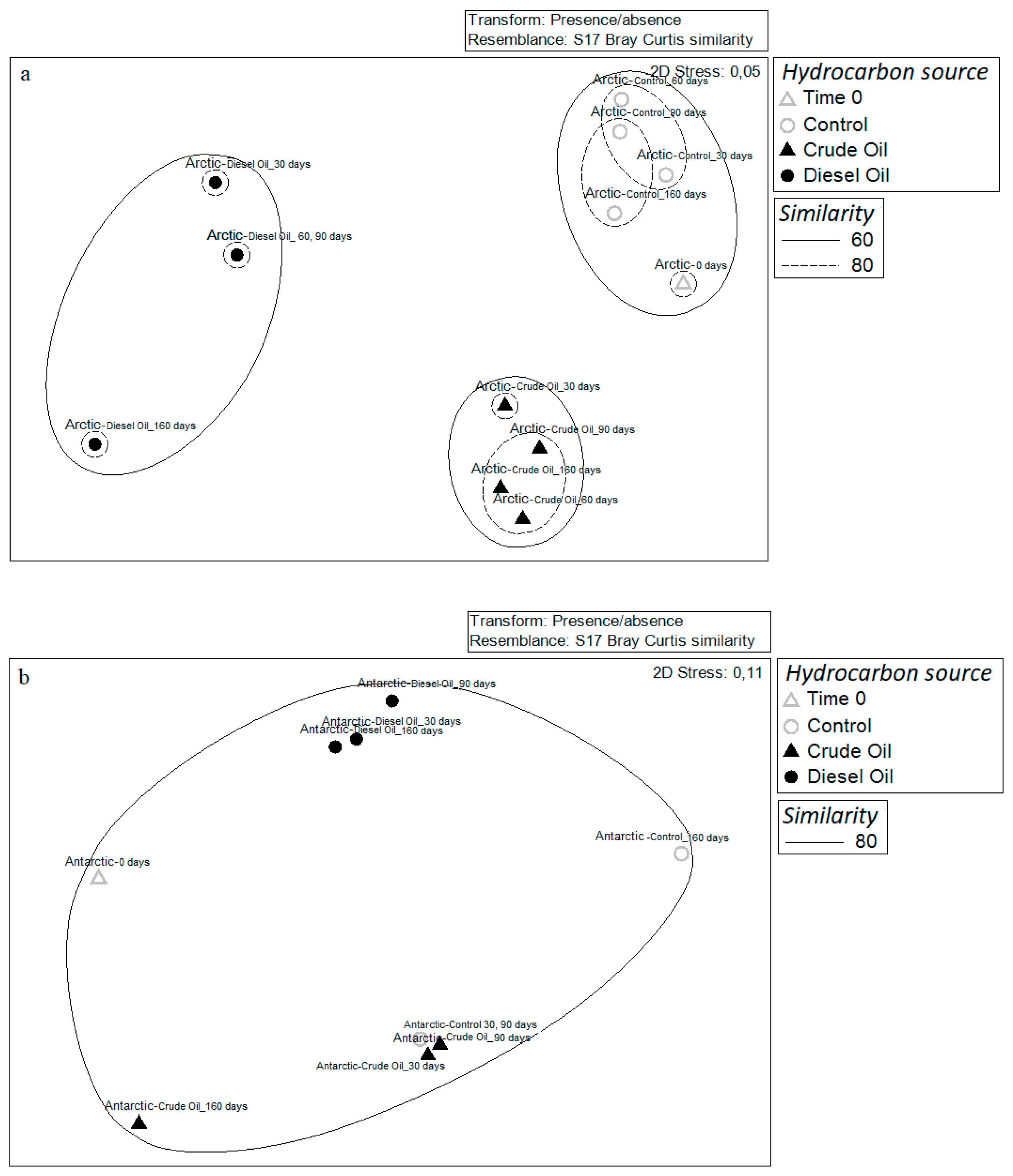
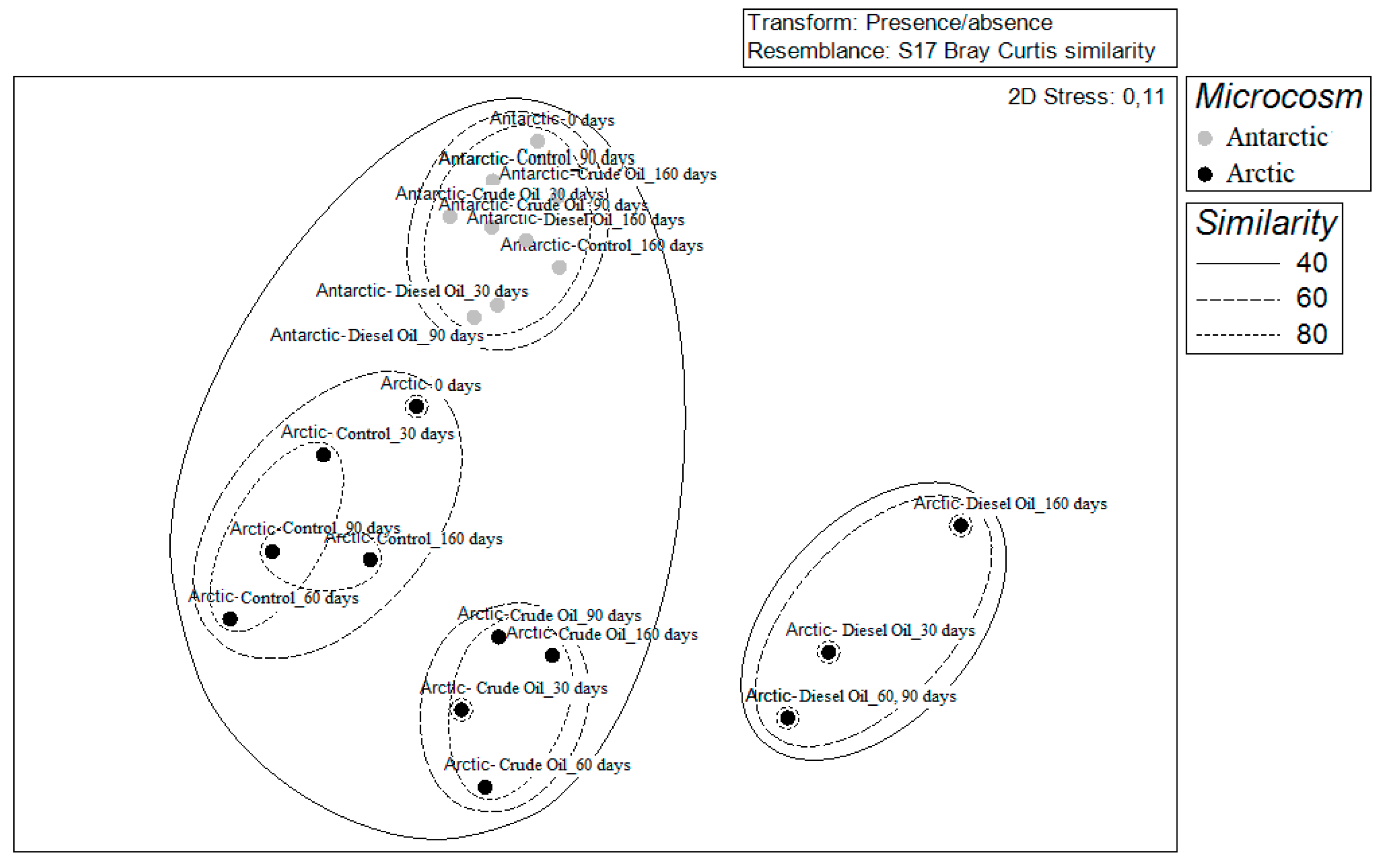
3.3. Community Diversity Profiles in Contaminated Sediments over the Incubation Time
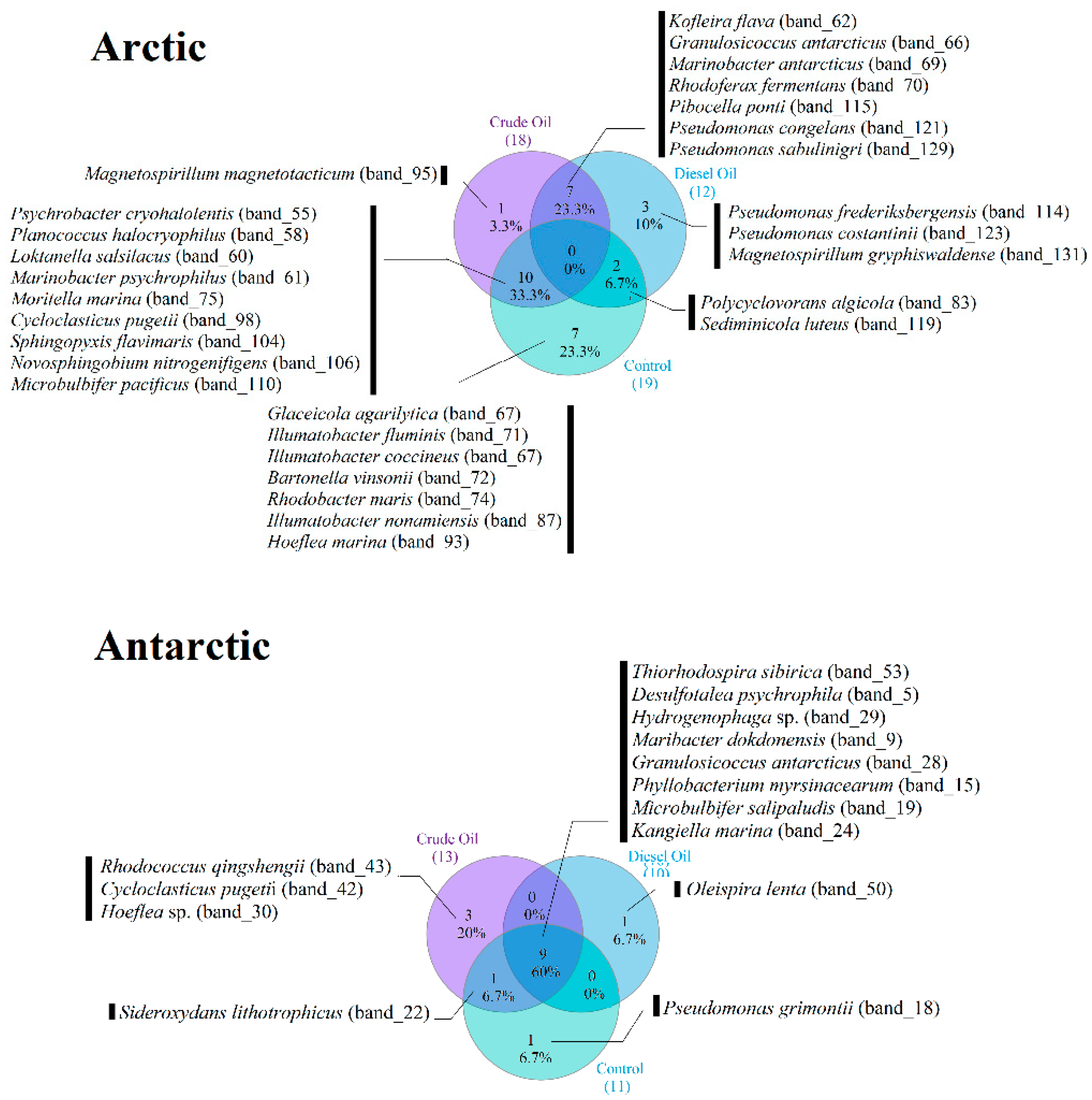
3.4. Occurrence of Dominant Bacterial Species across the Experimental Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cripps, G.C.; Shears, J. The fate in the marine environment of a minor diesel fuel spill from an Antarctic research station. Environ. Monit. Assess. 1997, 46, 221–232. [Google Scholar] [CrossRef]
- Malavenda, R.; Rizzo, C.; Michaud, L.; Gerce, B.; Bruni, V.; Syldatk, C.; Hausmann, R.; Lo Giudice, A. Biosurfactant production by Arctic and Antarctic bacteria growing on hydrocarbons. Polar Biol. 2015, 38, 1565–1574. [Google Scholar] [CrossRef]
- Gerdes, B.; Brinkmeyer, R.; Dieckmann, G.; Helmke, E. Influence of crude oil on changes of bacterial community in Arctic sea-ice. FEMS Microbiol. Ecol. 2005, 53, 129–139. [Google Scholar] [CrossRef]
- Delille, D.; Pelletier, E. Natural attenuation of diesel-oil contamination in a subantarctic soil (Crozet Island). Polar Biol. 2002, 25, 682–687. [Google Scholar]
- Powell, S.M.; Snapeb, I.; Bowmana, J.P.; Thompsona, B.; Starkb, J.; McCammon, S.A.; Riddleb, M.J. A comparison of the short term effects of diesel fuel and lubricant oils on Antarctic benthic microbial communities. J. Exp. Mar. Biol. Ecol. 2005, 322, 53–65. [Google Scholar] [CrossRef]
- Deppe, U.; Richnow, H.H.; Michaelis, W.; Antranikian, G. Degradation of crude oil by an Arctic microbial consortium. Extremophiles 2005, 9, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Conte, A.; Papale, M.; Amalfitano, S.; Mikkonen, A.; Rizzo, C.; De Domenico, E.; Michaud, L.; Lo Giudice, A. Bacterial community structure along the subtidal sandy sediment belt of a high Arctic fjord (Kongsfjorden, Svalbard Islands). Sci. Total Environ. 2018, 619–620, 203–211. [Google Scholar] [CrossRef]
- Lo Giudice, A.; Bruni, V.; De Domenico, M.; Michaud, L. Cold-Adapted hydrocarbon-Degrading microorganisms. In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K.N., Ed.; Springer: Berlin, Germany, 2010; pp. 1897–1922. [Google Scholar]
- Crisafi, F.; Giuliano, L.; Yakimov, M.M.; Azzaro, M.; Denaro, R. Isolation and degradation potential of a cold-adapted oil/PAHdegrading marine bacterial consortium from Kongsfjorden (Arctic region). Rend. Lincei Sci. Fis. E Nat. 2016, 27, 261–270. [Google Scholar] [CrossRef]
- Yakimov, M.M.; Giuliano, L.; Gentile, G.; Crisafi, E.; Chernikova, T.N.; Abraham, W.R.; Lunsdorf, H.; Timmis, K.N.; Golyshin, P.N. Oleispira antarctica gen. nov., sp. nov., a novel hydrocarbonoclastic marine bacterium isolated from Antarctic coastal sea water. Int. J. Syst. Evol. Microbiol. 2003, 53, 779–785. [Google Scholar] [CrossRef]
- Gentile, G.; Bonasera, V.; D’Amico, C.; Giuliano, L.; Yakimov, M.M. Shewanella sp. GA-22, a psychrophilic hydrocarbonoclastic Antarctic bacterium producing polyunsaturated fatty acids. J. Appl. Microbiol. 2003, 95, 1124–1133. [Google Scholar] [CrossRef]
- Michaud, L.; Lo Giudice, A.; Saitta, M.; De Domenico, M.; Bruni, V. The biodegradation efficiency on diesel oil by two psychrotrophic Antarctic marine bacteria during a two-month-long experiment. Mar. Pollut. Bull. 2004, 49, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Yakimov, M.M.; Gentile, G.; Bruni, V.; Cappello, S.; D’Auria, G.; Golyshin, P.N.; Giuliano, L. Crude oil-induced structural shift of coastal bacterial communities of Rod Bay (Terra Nova Bay, Ross Sea) and characterization of cultured cold-adapted hydro-carbonoclastic bacteria. FEMS Microbiol. Ecol. 2004, 49, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Pini, F.; Grossi, C.; Nereo, S.; Michaud, L.; Lo Giudice, A.; Bruni, V.; Baldi, F.; Fani, R. Molecular and physiological characterisation of psychotrophic hydrocarbon-degrading bacteria isolated from Terra Nova Bay (Antarctica). Eur. J. Soil Biol. 2007, 43, 368–379. [Google Scholar] [CrossRef]
- Röling, W.F.M.; Milner, M.G.; Jones, D.M.; Lee, K.; Daniel, F.; Swannell, R.P.J.; Head, I.M. Robust hydrocarbon degradation during nutrient-enhanced oil spill bioremediation. Appl. Environ. Microbiol. 2002, 68, 5537–5548. [Google Scholar] [CrossRef]
- Das, N.; Chandran, P. Microbial degradation of petroleum hydrocarbon contaminants: An overview. Biotechnol. Res. Int. 2011, 1–13. [Google Scholar] [CrossRef]
- Bell, T.H.; Yergeau, E.; Maynard, C.; Juck, D.; Whyte, L.G.; Greer, C.W. Predictable bacterial composition and hydrocarbon degradation in Arctic soils following diesel and nutrient disturbance. ISME J. 2013, 7, 1200–1210. [Google Scholar] [CrossRef]
- Garneau, M.; Michel, C.; Meisterhans, G.; Fortin, N.; King, T.; Greer, C.; Lee, K. Hydrocarbon biodegradation by Arctic sea-ice and sub-ice microbial communities during microcosm experiments, Northwest Passage (Nunavut, Canada). FEMS Microbiol. Ecol. 2016, 92. [Google Scholar] [CrossRef]
- Amalfitano, S.; Fazi, S. Recovery and quantification of bacterial cells associated with streambed sediments. J. Microbiol. Methods 2008, 75, 237–243. [Google Scholar] [CrossRef]
- Amalfitano, S.; Fazi, S.; Ejarque, E.; Freixa, A.; Romaní, A.M.; Butturini, A. Deconvolution model to resolve cytometric microbial community patterns in flowing waters. Cytom. Part A 2018, 93, 194–200. [Google Scholar] [CrossRef]
- Luna, G.M.; Dell’Anno, A.; Danovaro, R. DNA extraction procedure: A critical issue for bacterial diversity assessment in marine sediments. Environ. Microbiol. 2006, 8, 308–320. [Google Scholar] [CrossRef]
- Lukow, T.; Dunfield, P.F.; Liesack, W. Use of the T-RFLP technique to assess spatial and temporal changes in the bacterial community structure within an agricultural soil planted with transgenic and non-transgenic potato plants. FEMS Microbiol. Ecol. 2000, 32, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Danilowicz, B.S.; Clear, A.K.; Costello, F.J.; Wilson, B.; Meijer, W.G.T. Align, a web-based tool for comparison of multiple terminal restriction fragment length polymorphism profiles. FEMS Microbiol. Ecol. 2005, 54, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Baldi, F.; Marchetto, D.; Pini, F.; Fani, R.; Michaud, L.; Lo Giudice, A.; Berto, D.; Giani, M. Biochemical and microbial features of shallow marine sediments along the Terra Nova Bay (Ross Sea, Antarctica). Cont. Shelf Res. 2010, 15, 1614–1625. [Google Scholar] [CrossRef]
- Engebretson, J.J.; Moyer, C.L. Fidelity of select restriction endonucleases in determining microbial diversity by terminal-restriction fragment length polymorphism. Appl. Environ. Microbiol. 2003, 69, 4823–4829. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Danovaro, R.; Luna, G.M.; Dell’Anno, A.; Pietrangeli, B. Comparison of two fingerprinting techniques, terminal restriction fragment length polymorphism and automated ribosomal intergenic spacer analysis, for determination of bacterial diversity in aquatic environments. Appl. Environ. Microbiol. 2006, 72, 5982–5989. [Google Scholar] [CrossRef] [PubMed]
- Gerçe, B.; Schwartz, T.; Syldatk, C.; Hausmann, R. Differences between bacterial communities associated with the surface or tissue of Mediterranean sponge species. Microb. Ecol. 2011, 61, 769–782. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biolology 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Mason, O.U.; Hazen, T.C.; Borglin, S.; Chain, P.S.G.; Dubinsky, E.A.; Fortney, J.L.; Han, J.; Holman, H.Y.; Hultman, J.; Lamendella, R.; et al. Metagenomics, metatranscriptomics and single cell sequencing reveal bacterial response to the Gulf oil spill. ISME J. 2012, 6, 1715–1727. [Google Scholar] [CrossRef]
- Yergeau, E.; Maynard, C.; Sanschagrin, S.; Champagne, J.; Juck, D.; Lee, K.; Greer, C.W. Microbial community composition, functions, and activities in the Gulf of Mexico 1 year after the Deepwater Horizon accident. Appl. Environ. Microbiol. 2015, 81, 5855–5866. [Google Scholar] [CrossRef]
- Bacosa, H.P.; Erdner, D.L.; Rosenheim, B.E.; Shetty, P.; Seitz, K.W.; Baker, B.J.; Liu, Z. Hydrocarbon degradation and response of seafloor sediment bacterial community in the northern Gulf of Mexico to light Louisiana sweet crude oil. ISME J. 2018, 12, 2532–2543. [Google Scholar] [CrossRef]
- Acosta-González, A.; Martirani-von Abercron, S.M.; Rosselló-Móra, R.; Wittich, R.M.; Marqués, S. The effect of oil spills on the bacterial diversity and catabolic function in coastal sediments: A case study on the Prestige oil spill. Environ. Sci. Pollut. Res. 2015, 22, 15200–15214. [Google Scholar] [CrossRef] [PubMed]
- Duran, R.; Cravo-Laureau, C. Role of environmental factors and microorganisms in determining the fate of polycyclic aromatic hydrocarbons in the marine environment. FEMS Microbiol. Rev. 2016, 40, 814–830. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Nigro, L.M.; Gutierrez, T.; D’Ambrosio, L.; Joye, S.B.; Highsmith, R.; Teske, A. Pulsed blooms and persistent oil-degrading bacterial populations in the water column during and after the Deepwater Horizon blowout. Deep Sea Res. Part II Top. Stud. Oceanogr. 2016, 129, 282–291. [Google Scholar] [CrossRef]
- Saul, D.J.; Aislabie, J.M.; Brown, C.E.; Harris, L.; Foght, J.M. Hydrocarbon contamination changes the bacterial diversity of soil from around Scott Base, Antarctica. FEMS Microbiol. Ecol. 2005, 53, 141–155. [Google Scholar] [CrossRef]
- Horz, H.P.; Tchawa Yimga, M.; Liesack, W. Detection of methanotroph diversity on roots of submerged rice plants by molecular retrieval of pmoA, mmoX, mxaF, and 16S rRNA and ribosomal DNA, including terminal restriction fragment length polymorphism profiling. Appl. Environ. Microbiol. 2001, 67, 4177–4185. [Google Scholar] [CrossRef]
- Sipos, R.; Székely, A.J.; Palatinszky, M.; Révész, S.; Márialigeti, K.; Nikolausz, M. Effect of primer mismatch, annealing temperature and PCR cycle number on 16S rRNA gene-targeting bacterial community analysis. FEMS Microbiol. Ecol. 2007, 60, 341–350. [Google Scholar] [CrossRef]
- Tiquia, S.M.; Michel, F.C. Bacterial diversity in livestock manure composts as characterized by terminal restriction fragment length polymorphisms (T-RFLP) of PCR-amplified 16S rRNA and gene sequences. In Microbiology of Composting and Other Biodegradation Processes; Insam, H., Ed.; Springer: Heidelberg, Germany, 2002. [Google Scholar]
- Sanchez, J.I.; Rossetti, L.; Martinez, B.; Rodriguez, A.; Giraffa, G. Application of reverse transcriptase PCR-based T-RFLP to perform semi-quantitative analysis of metabolically active bacteria in dairy fermentations. J. Microbiol. Methods 2006, 65, 268–277. [Google Scholar] [CrossRef]
- Moeseneder, M.M.; Arrieta, J.M.; Muyzer, G.; Winter, C.; Herndl, G.J. Optimization of terminal-restriction fragment length polymorphism analysis for complex marine bacterioplankton communities and comparison with denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 1999, 65, 3518–3525. [Google Scholar]
- Nikolausz, M.; Sipos, R.; Revesz, S.; Szekely, A.; Marialigeti, K. Observation of bias associated with re-amplification of DNA isolated from denaturing gradient gels. FEMS Microbiol. Lett. 2005, 244, 385–390. [Google Scholar] [CrossRef]
- Nunan, N.; Daniell, T.J.; Singh, B.K.; Papert, A.; McNicol, J.W.; Prosser, J.I. Links between plant and rhizoplane bacterial communities in grassland soils, characterized using molecular techniques. Appl. Environ. Microbiol. 2005, 71, 6784–6792. [Google Scholar] [CrossRef]
- Eriksson, M.; Ka, J.O.; Mohn, W.W. Effects of low temperature and freeze-thaw cycles on hydrocarbon biodegradation in arctic tundra soil. Appl. Environ. Microbiol. 2001, 67, 5107–5112. [Google Scholar] [CrossRef] [PubMed]
- Whyte, L.G.; Smits, T.H.M.; Labbè, D.; Witholt, B.; Greer, C.W.; Van Beilen, J.B. Gene cloning and characterization of multiple alkane hydroxilase systems in Rhodococcus strains Q15 and NRRL B-16531. Appl. Environ. Microbiol. 2002, 68, 5933–5942. [Google Scholar] [CrossRef] [PubMed]
- Yakimov, M.M.; Timmis, K.N.; Golyshin, P.N. Obligate oil-degrading marine bacteria. Curr. Opin. Biotechnol. 2007, 18, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Kube, M.; Chernikova, T.N.; Al-Ramahi, Y.; Beloqui, A.; Lopez-Cortez, N.; Guazzaroni, M.-A.; Heipieper, H.J.; Klages, S.; Kotsyurbenko, O.R.; Langer, I.; et al. Genome sequence and functional genomic analysis of the oil-degrading bacterium Oleispira antarctica. Nat. Commun. 2013, 23, 2156. [Google Scholar] [CrossRef]
- Coulon, F.; McKew, B.A.; Osborn, A.M.; McGenity, T.J.; Timmis, K.N. Effects of temperature and biostimulation on oil-degrading microbial communities in temperate estuarine waters. Environ. Microbiol. 2007, 9, 177–186. [Google Scholar] [CrossRef]
- Gentile, G.; Bonsignore, M.; Santisi, S.; Catalfamo, M.; Giuliano, L.; Genovese, L.; Yakimov, M.M.; Denaro, R.; Genovese, M.; Cappello, S. Biodegradation potentiality of psychrophilic bacterial strain Oleispira antarctica RB-8T. Mar. Pollut. Bull. 2016, 105, 125–130. [Google Scholar] [CrossRef]
- Whyte, L.G.; Hawari, J.; Zhou, E.; Bourbonniére, L.; Inniss, W.E.; Greer, C.W. Biodegradation of variable chain-length alkanes at low temperatures by a psychrotrophic Rhodococcus sp. Appl. Environ. Microbiol. 1998, 64, 2578–2584. [Google Scholar]
- Grossman, M.J.; Prince, R.C.; Garrett, R.M.; Garrett, K.K.; Bare, R.E.; O’Neil, K.R.; Sowlay, M.R.; Hinton, S.M.; Lee, K.; Sergy, G.; et al. Microbial diversity in oiled and un-oiled shoreline sediments in the Norwegian Arctic. In Microbial Biosystems: New Frontiers Proceedings of the 8th International Symposium on Microbial Ecology; Bell, C.R., Brylinsky, M., Johnson-Green, P., Eds.; Atlantic Canada Society for Microbial Ecology: Halifax, Canada, 2000; pp. 775–787. [Google Scholar]
- Terrisse, F.; Cravo-Laureau, C.; Noël, C.; Cagnon, C.; Dumbrell, A.J.; McGenity, T.J.; Duran, R. Variation of oxygenation conditions on a hydrocarbonoclastic microbial community reveals Alcanivorax and Cycloclasticus ecotypes. Front. Microbiol. 2017, 8, 1549. [Google Scholar] [CrossRef]
- Brito, E.M.S.; Guyoneaud, R.; Goñi-Urriza, M.; Ranchou-Peyruse, A.; Verbaere, A.; Crapez, M.A.C.; Wasserman, J.C.A.; Duran, R. Characterization of hydrocarbonoclastic bacterial communities from mangrove sediments in Guanabara Bay, Brazil. Res. Microbiol. 2006, 157, 752–762. [Google Scholar] [CrossRef]
- LaRoe, S.L.; Wang, B.; Han, J.I. Isolation and characterization of a novel polycyclic aromatic hydrocarbon-degrading bacterium, Sphingopyxis sp strain M2R2, capable of passive spreading motility through soil. Environ. Eng. Sci. 2010, 27, 452–456. [Google Scholar] [CrossRef]
- Yergeau, E.; Sanschagrin, S.; Beaumier, D.; Greer, C.W. Metagenomic analysis of the bioremediation of diesel- contaminated Canadian high arctic soils. PLoS ONE 2012, 7, e30058. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.Y.; Lee, I.; Cho, Y.; Lee, Y.M.; Jung, Y.J.; Baek, K.; Nam, S.; Lee, H.K. Sediminicola arcticus sp. nov., a psychrophilic bacterium isolated from deep-sea sediment, and emended description of the genus Sediminicola. Int. J. Syst. Evol. Microbiol. 2015, 65, 1567–1571. [Google Scholar] [CrossRef] [PubMed]

| Site | Phylum or Class | DGGE Bands | Next relative by Genbank Alignment (Accession Number, Microorganism) | Hom (%) |
|---|---|---|---|---|
| MicroBy | Alphaproteobacteria | 30 | KC160704, Hoeflea sp. SS10.8 | 86 |
| 15 | NR_113874, Phyllobacterium myrsinacearum strain NBRC100019 | 94 | ||
| Betaproteobacteria | 29 | JQ799976, Hydrogenophaga sp. FS13-2 | 99 | |
| 22 | NR_074731, Sideroxydans lithotrophicus strain ES-1 | 82 | ||
| Gammaproteobacteria | 42 | NR_025955, Cycloclasticus pugetiiPS-1 | 93 | |
| 28 | NR_044255; Granulosicoccus antarcticusIMCC3135 | 99 | ||
| 24 | NR_109475, Kangiella marina strain KM1 | 90 | ||
| 18 | NR_043223, Marinimicrobium agarilyticum strain M18 | 93 | ||
| 19 | NR_025232, Microbulbifer salipaludis strain SM-1 | 97 | ||
| 50 | NR_108293, Oleispira lenta strain DFH11 | 99 | ||
| 51 | NR_025102, Pseudomonas grimontii CFML 97-514 | 99 | ||
| 53 | NR_028867, Thiorhodospira sibirica strain A12 | 95 | ||
| Deltaproteobacteria | 5 | NR_028729, Desulfotalea psychrophila LSv54 | 93 | |
| Bacteroidetes | 9 | NR_043294, Maribacter dokdonensis strain DSW-8 | 89 | |
| Actinobacteria | 43 | KT962173, Rhodococcus qingshengii strain CN-S1 | 100 | |
| 8 | KF306368, Salinibacterium amurskyense strain y182 | 95 | ||
| MicroSval | Alphaproteobacteria | 74 | NR_104902, Bartonella vinsonii subsp. Arupensis strain OK 94-513 | 93 |
| 93 | NR_043007, Hoeflea marina strain LMG 128 | 93 | ||
| 60 | NR_025539, Loktanella salsilacus strain R-8904 | 100 | ||
| 95 | NR_026381, Magnetospirillum magnetotacticum DSM 3856 | 89 | ||
| 131 | NR_121771, Magnetospirillum gryphiswaldense strain MSR-1 | 86 | ||
| 106 | NR_043857, Novosphingobium nitrogenifigens DSM 19370 Y88 | 95 | ||
| 87 | NR_042629, Rhodobacter maris strain JA276 | 88 | ||
| 104 | NR_025814, Sphingopyxis flavimaris strain SW-151 | 99 | ||
| Betaproteobacteria | 70 | NR_104835, Rhodoferax antarcticus strain ANT.BR | 92 | |
| 111 | NR_117864, Thiobacillus thioparus strain THI 111 | 95 | ||
| Deltaproteobacteria | 62 | NR_041981, Kofleria flava strain Pl vt1 | 92 | |
| Gammaproteobacteria | 98 | NR_025955, Cycloclasticus pugetiiPS-1 | 97 | |
| 67 | NR_043956, Glaciecola agarilytica strain NO2 | 94 | ||
| 66 | NR_044255, Granulosicoccus antarcticusIMCC3135 | 99 | ||
| 83 | NR_116560, Polycyclovorans algicola strain TG408 | 99 | ||
| 61 | NR_043513, Marinobacter psychrophilus strain BSi20041 | 86 | ||
| 69 | NR_108299, Marinobacter antarcticus strain ZS2-30 | 92 | ||
| 110 | NR_11592, Microbulbifer pacificus strain SPO729 | 95 | ||
| 75 | NR_040842, Moritella marina strain ATCC15381 | 92 | ||
| 121 | NR_028985, Pseudomonas congelans strain P 538/23 | 99 | ||
| 123 | NR_025164, Pseudomonas costantinii strain CFBP 5705 | 99 | ||
| 114 | NR_028906, Pseudomonas frederiksbergensis strain JAJ28 | 100 | ||
| 129 | NR_044415, Pseudomonas sabulinigri strain J64 | 99 | ||
| 55 | NR_043079, Psychrobacter cryohalolentis K5 | 100 | ||
| Bacteroidetes | 119 | NR_041301, Sediminicola luteus strain CNI-3 | 99 | |
| 115 | NR_025821, Pibocella ponti strain KMM 6031 | 93 | ||
| Actinobacteria | 72 | NR_112714, Ilumatobacter coccineus strain YM16-304 | 92 | |
| 71 | NR_041633, 1Ilumatobacter fluminis strain YM22-133 | 93 | ||
| 88 | NR_112713, Ilumatobacter nonamiensis strain YM16-303 | 95 | ||
| Firmicutes | 58 | NR_118149, Planococcus halocryophilus strain | 100 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzo, C.; Malavenda, R.; Gerçe, B.; Papale, M.; Syldatk, C.; Hausmann, R.; Bruni, V.; Michaud, L.; Lo Giudice, A.; Amalfitano, S. Effects of a Simulated Acute Oil Spillage on Bacterial Communities from Arctic and Antarctic Marine Sediments. Microorganisms 2019, 7, 632. https://doi.org/10.3390/microorganisms7120632
Rizzo C, Malavenda R, Gerçe B, Papale M, Syldatk C, Hausmann R, Bruni V, Michaud L, Lo Giudice A, Amalfitano S. Effects of a Simulated Acute Oil Spillage on Bacterial Communities from Arctic and Antarctic Marine Sediments. Microorganisms. 2019; 7(12):632. https://doi.org/10.3390/microorganisms7120632
Chicago/Turabian StyleRizzo, Carmen, Roberta Malavenda, Berna Gerçe, Maria Papale, Christoph Syldatk, Rudolf Hausmann, Vivia Bruni, Luigi Michaud, Angelina Lo Giudice, and Stefano Amalfitano. 2019. "Effects of a Simulated Acute Oil Spillage on Bacterial Communities from Arctic and Antarctic Marine Sediments" Microorganisms 7, no. 12: 632. https://doi.org/10.3390/microorganisms7120632
APA StyleRizzo, C., Malavenda, R., Gerçe, B., Papale, M., Syldatk, C., Hausmann, R., Bruni, V., Michaud, L., Lo Giudice, A., & Amalfitano, S. (2019). Effects of a Simulated Acute Oil Spillage on Bacterial Communities from Arctic and Antarctic Marine Sediments. Microorganisms, 7(12), 632. https://doi.org/10.3390/microorganisms7120632








