Early-Life Development of the Intestinal Microbiome in Preterm and Term Infants Hospitalized in the Neonatal Intensive Care Unit
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sample Collection and Analysis Method
2.2.1. Sample Collection
2.2.2. Fecal Microbiome Analysis Method: 16S rRNA Sequencing and Processing
2.3. Definitions
2.4. Statistical Analyses
3. Results
3.1. Study Cohort, Demographic, and Clinical Information
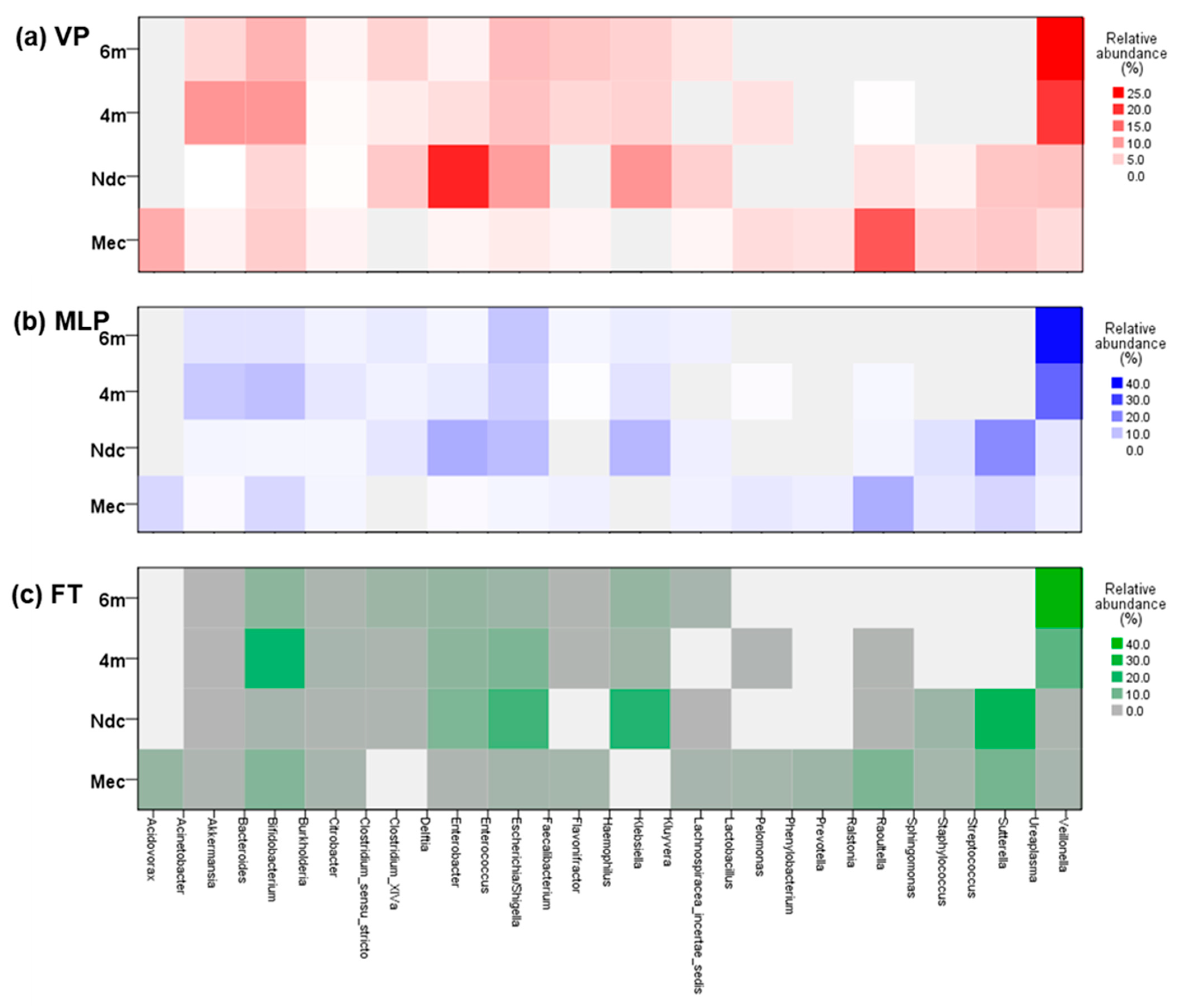
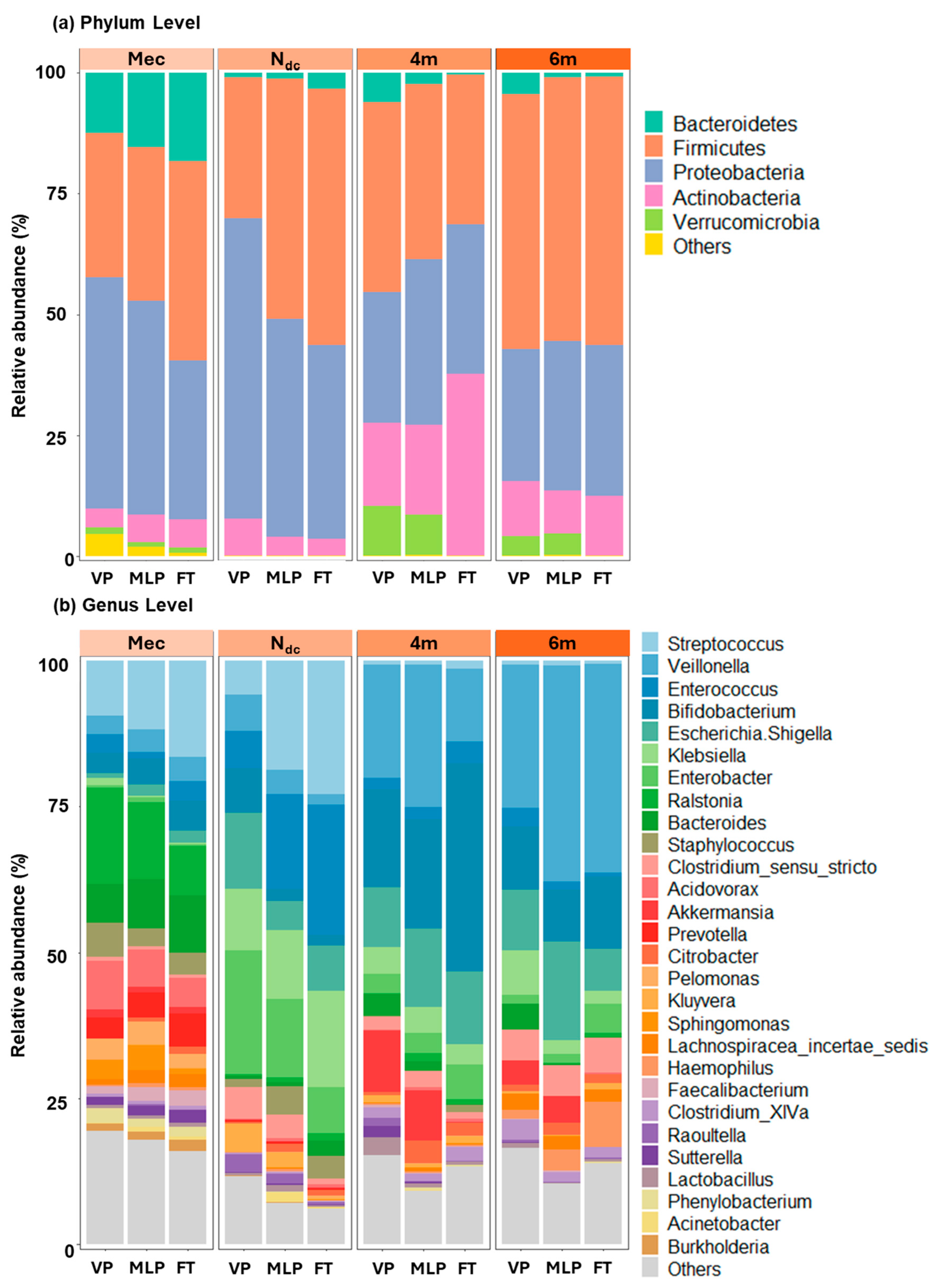
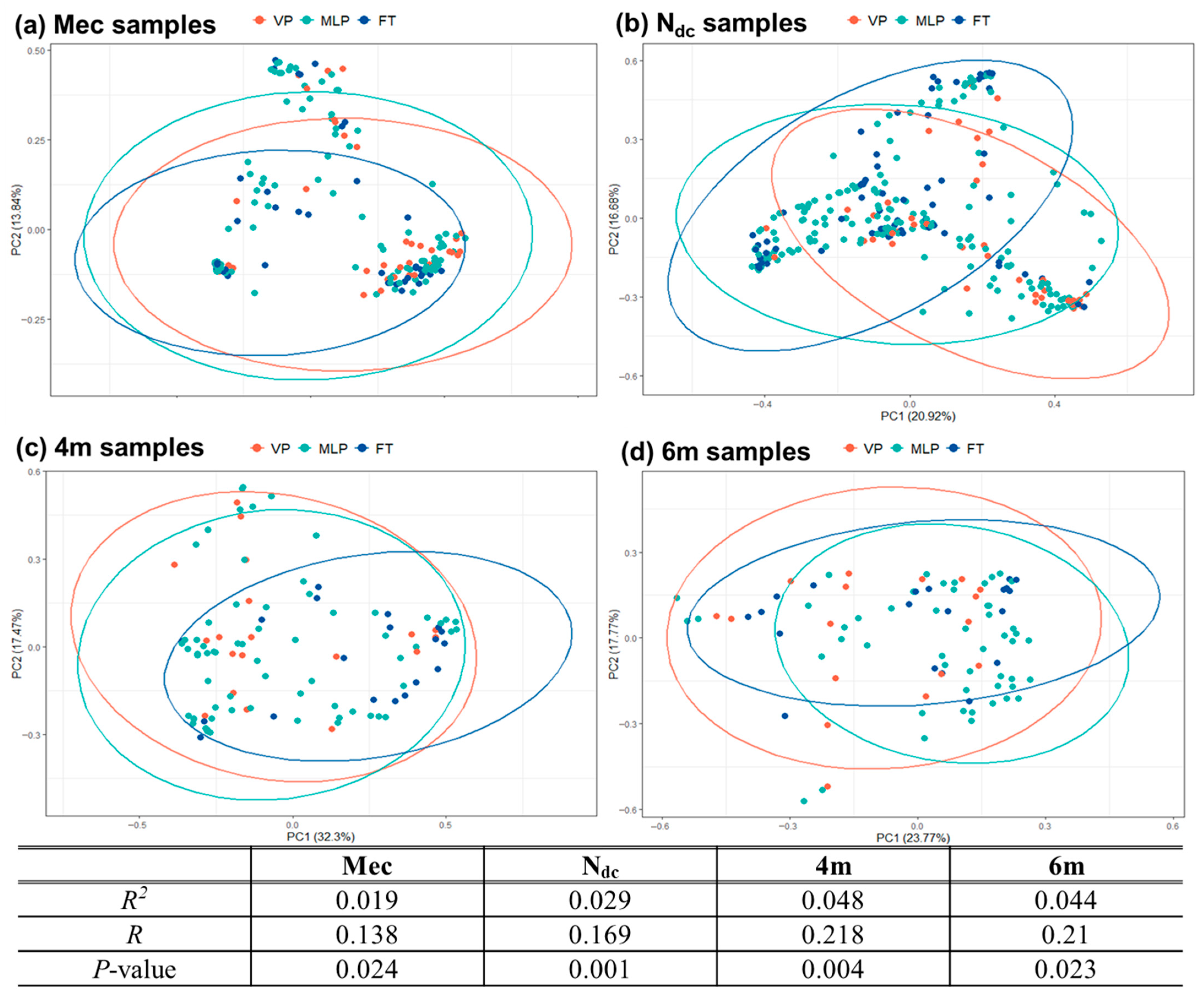
3.2. Structure of the Gut Microbiota
3.2.1. Meconium Stage (Mec Samples)
3.2.2. Changes During NICU Admission (Ndc Samples)
3.2.3. Changes After NICU Discharge (4m and 6m Samples)
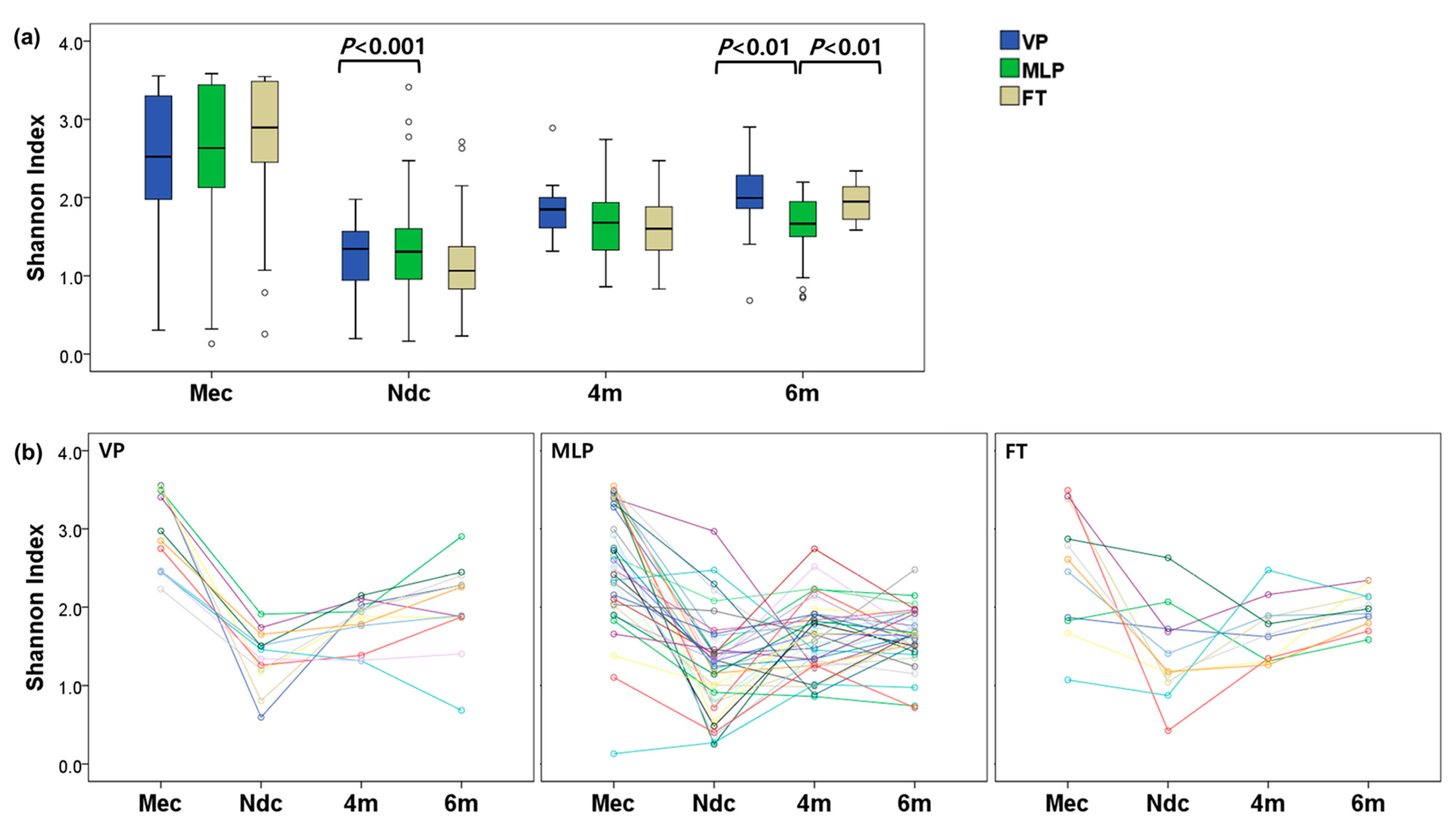
3.3. Microbial Diversities
4. Discussion
4.1. Dynamic Changes in NICU Were Exaggerated in VP Group
4.2. Development of Gut Microbiome in VP Infants During Early Home Period
4.3. Adaptation After Initiating the Weaning Diet
4.4. Decreased Diversity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Hui, Y.; Smith, B.; Mortensen, M.S.; Krych, L.; Sørensen, S.J.; Greisen, G.; Krogfelt, K.A.; Nielsen, D.S. The effect of early probiotic exposure on the preterm infant gut microbiome development. Gut Microbes 2021, 13, 1951113. [Google Scholar] [CrossRef]
- el Manouni el Hassani, S.; Niemarkt, H.J.; Berkhout, D.J.; Peeters, C.F.; Hulzebos, C.V.; van Kaam, A.H.; Kramer, B.W.; van Lingen, R.A.; Jenken, F.; de Boode, W.P. Profound pathogen-specific alterations in intestinal microbiota composition precede late-onset sepsis in preterm infants: A longitudinal, multicenter, case-control study. Clin. Infect. Dis. 2021, 73, e224–e232. [Google Scholar] [CrossRef]
- Lee, J.K.-F.; Hern Tan, L.T.; Ramadas, A.; Ab Mutalib, N.-S.; Lee, L.-H. Exploring the role of gut bacteria in health and disease in preterm neonates. Int. J. Environ. Res. Public Health 2020, 17, 6963. [Google Scholar] [CrossRef]
- Stewart, C.J.; Embleton, N.D.; Marrs, E.C.; Smith, D.P.; Fofanova, T.; Nelson, A.; Skeath, T.; Perry, J.D.; Petrosino, J.F.; Berrington, J.E. Longitudinal development of the gut microbiome and metabolome in preterm neonates with late onset sepsis and healthy controls. Microbiome 2017, 5, 75. [Google Scholar] [CrossRef]
- Kang, H.M.; Kim, S.; Hwang-Bo, S.; Yoo, I.H.; Seo, Y.-M.; Oh, M.Y.; Im, S.-A.; Youn, Y.-A. Compositional Differences of Meconium Microbiomes of Preterm and Term Infants, and Infants That Developed Necrotizing Enterocolitis or Feeding Intolerance. Pathogens 2022, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Beghetti, I.; Barone, M.; Turroni, S.; Biagi, E.; Sansavini, A.; Brigidi, P.; Corvaglia, L.; Aceti, A. Early-life gut microbiota and neurodevelopment in preterm infants: Any role for Bifidobacterium? Eur. J. Pediatr. 2022, 181, 1773–1777. [Google Scholar] [CrossRef] [PubMed]
- Krajmalnik-Brown, R.; Ilhan, Z.E.; Kang, D.W.; DiBaise, J.K. Effects of gut microbes on nutrient absorption and energy regulation. Nutr. Clin. Pract. 2012, 27, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Henderickx, J.G.; Zwittink, R.D.; Van Lingen, R.A.; Knol, J.; Belzer, C. The preterm gut microbiota: An inconspicuous challenge in nutritional neonatal care. Front. Cell. Infect. Microbiol. 2019, 9, 85. [Google Scholar] [CrossRef]
- Perez-Muñoz, M.E.; Arrieta, M.-C.; Ramer-Tait, A.E.; Walter, J. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: Implications for research on the pioneer infant microbiome. Microbiome 2017, 5, 48. [Google Scholar] [CrossRef]
- Stinson, L.F.; Boyce, M.C.; Payne, M.S.; Keelan, J.A. The not-so-sterile womb: Evidence that the human fetus is exposed to bacteria prior to birth. Front. Microbiol. 2019, 10, 1124. [Google Scholar] [CrossRef]
- He, Q.; Kwok, L.-Y.; Xi, X.; Zhong, Z.; Ma, T.; Xu, H.; Meng, H.; Zhao, F.; Zhang, H. The meconium microbiota shares more features with the amniotic fluid microbiota than the maternal fecal and vaginal microbiota. Gut Microbes 2020, 12, 1794266. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.; Dai, D.L.; Boutin, R.C.; Sbihi, H.; Sears, M.R.; Moraes, T.J.; Becker, A.B.; Azad, M.B.; Mandhane, P.J.; Subbarao, P. A rich meconium metabolome in human infants is associated with early-life gut microbiota composition and reduced allergic sensitization. Cell Rep. Med. 2021, 2, 100260. [Google Scholar] [CrossRef]
- Robertson, R.C.; Manges, A.R.; Finlay, B.B.; Prendergast, A.J. The human microbiome and child growth–first 1000 days and beyond. Trends Microbiol. 2019, 27, 131–147. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The human intestinal microbiome in health and disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar]
- Fulde, M.; Hornef, M.W. Maturation of the enteric mucosal innate immune system during the postnatal period. Immunol. Rev. 2014, 260, 21–34. [Google Scholar] [CrossRef]
- Thänert, R.; Schwartz, D.J.; Keen, E.C.; Hall-Moore, C.; Wang, B.; Shaikh, N.; Ning, J.; Rouggly-Nickless, L.C.; Thänert, A.; Ferreiro, A. Clinical sequelae of gut microbiome development and disruption in hospitalized preterm infants. Cell Host Microbe 2024, 32, 1822–1837. [Google Scholar] [CrossRef]
- Bargheet, A.; Klingenberg, C.; Esaiassen, E.; Hjerde, E.; Cavanagh, J.P.; Bengtsson-Palme, J.; Pettersen, V.K. Development of early life gut resistome and mobilome across gestational ages and microbiota-modifying treatments. EBioMedicine 2023, 92, 104613. [Google Scholar] [CrossRef] [PubMed]
- Korpela, K.; Blakstad, E.W.; Moltu, S.J.; Strømmen, K.; Nakstad, B.; Rønnestad, A.E.; Brække, K.; Iversen, P.O.; Drevon, C.A.; de Vos, W. Intestinal microbiota development and gestational age in preterm neonates. Sci. Rep. 2018, 8, 2453. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Lopez, M.; Dinsmoor, A.M.; Ho, T.T.; Donovan, S.M. A systematic review of the factors influencing microbial colonization of the preterm infant gut. Gut Microbes 2021, 13, 1884514. [Google Scholar] [CrossRef]
- Kim, S.Y.; Youn, Y.-A. Gut Dysbiosis in the First-Passed Meconium Microbiomes of Korean Preterm Infants Compared to Full-Term Neonates. Microorganisms 2024, 12, 1271. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.; Kim, S.Y.; Kang, H.M.; Im, S.-A.; Youn, Y.-A. Dysbiosis of the initial stool microbiota increases the risk of developing necrotizing enterocolitis or feeding intolerance in newborns. Sci. Rep. 2024, 14, 24416. [Google Scholar] [CrossRef]
- Klopp, J.; Ferretti, P.; Meyer, C.U.; Hilbert, K.; Haiß, A.; Marißen, J.; Henneke, P.; Hudalla, H.; Pirr, S.; Viemann, D. Meconium microbiome of very preterm infants across Germany. MSphere 2022, 7, e00808–e00821. [Google Scholar] [CrossRef]
- Shi, Y.-C.; Guo, H.; Chen, J.; Sun, G.; Ren, R.-R.; Guo, M.-Z.; Peng, L.-H.; Yang, Y.-S. Initial meconium microbiome in Chinese neonates delivered naturally or by cesarean section. Sci. Rep. 2018, 8, 3255. [Google Scholar] [CrossRef]
- Yong, G.J.; Porsche, C.E.; Sitarik, A.R.; Fujimura, K.E.; McCauley, K.; Nguyen, D.T.; Levin, A.M.; Woodcroft, K.J.; Ownby, D.R.; Rundle, A.G. Precocious infant fecal microbiome promotes enterocyte barrier dysfuction, altered neuroendocrine signaling and associates with increased childhood obesity risk. Gut Microbes 2024, 16, 2290661. [Google Scholar] [CrossRef]
- Yoon, B.H.; Romero, R.; Kim, C.J.; Jun, J.K.; Gomez, R.; Choi, J.-H.; Syu, H.C. Amniotic fluid interleukin-6: A sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am. J. Obstet. Gynecol. 1995, 172, 960–970. [Google Scholar] [CrossRef]
- Fenton, T.R.; Kim, J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013, 13, 59. [Google Scholar] [CrossRef]
- Jobe, A.H.; Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2001, 163, 1723–1729. [Google Scholar] [CrossRef]
- Bell, M.J.; Ternberg, J.L.; Feigin, R.D.; Keating, J.P.; Marshall, R.; Barton, L.; Brotherton, T. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 1978, 187, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Carlos, M.A.; Babyn, P.S.; Marcon, M.A.; Moore, A.M. Changes in gastric emptying in early postnatal life. J. Pediatr. 1997, 130, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Garner, A. An international classification of retinopathy of prematurity. Arch. Ophthalmol. 1984, 102, 1130–1134. [Google Scholar]
- International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch. Ophthalmol. 2005, 123, 991–999. [Google Scholar] [CrossRef]
- Hartz, L.E.; Bradshaw, W.; Brandon, D.H. Potential NICU environmental influences on the neonate’s microbiome: A systematic review. Adv. Neonatal Care 2015, 15, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Groer, M.W.; Luciano, A.A.; Dishaw, L.J.; Ashmeade, T.L.; Miller, E.; Gilbert, J.A. Development of the preterm infant gut microbiome: A research priority. Microbiome 2014, 2, 38. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Lee, T.y.; Kim, H.-Y.; Jeong, S.J.; Han, J.H.; Shin, J.E.; Lee, J.-H.; Kang, C.-M. Natal factors influencing newborn’s oral microbiome diversity. Sci. Rep. 2024, 14, 28161. [Google Scholar] [CrossRef] [PubMed]
- Underwood, M.A.; Sohn, K. The microbiota of the extremely preterm infant. Clin. Perinatol. 2017, 44, 407. [Google Scholar] [CrossRef]
- Xiang, Q.; Yan, X.; Shi, W.; Li, H.; Zhou, K. Early gut microbiota intervention in premature infants: Application perspectives. J. Adv. Res. 2023, 51, 59–72. [Google Scholar] [CrossRef]
- Baranowski, J.R.; Claud, E.C. Necrotizing enterocolitis and the preterm infant microbiome. In Probiotics and Child Gastrointestinal Health: Advances in Microbiology, Infectious Diseases and Public Health Volume 10; Springer International Publishing: Cham, Switzerland, 2019; pp. 25–36. [Google Scholar]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Maharshak, N.; Packey, C.D.; Ellermann, M.; Manick, S.; Siddle, J.P.; Huh, E.Y.; Plevy, S.; Sartor, R.B.; Carroll, I.M. Altered enteric microbiota ecology in interleukin 10-deficient mice during development and progression of intestinal inflammation. Gut Microbes 2013, 4, 316–324. [Google Scholar] [CrossRef]
- Berrington, J.E.; Stewart, C.J.; Cummings, S.P.; Embleton, N.D. The neonatal bowel microbiome in health and infection. Curr. Opin. Infect. Dis. 2014, 27, 236–243. [Google Scholar] [CrossRef]
- Pammi, M.; Cope, J.; Tarr, P.I.; Warner, B.B.; Morrow, A.L.; Mai, V.; Gregory, K.E.; Kroll, J.S.; McMurtry, V.; Ferris, M.J. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: A systematic review and meta-analysis. Microbiome 2017, 5, 31. [Google Scholar] [CrossRef]
- Iqbal, F.; Barche, A.; Shenoy, P.A.; Lewis, L.E.S.; Purkayastha, J.; Vandana, K. Gram-Negative Colonization and Bacterial Translocation Drive Neonatal Sepsis in the Indian Setting. J. Epidemiol. Glob. Health 2024, 14, 1525–1535. [Google Scholar] [CrossRef]
- Jia, Q.; Yu, X.; Chang, Y.; You, Y.; Chen, Z.; Wang, Y.; Liu, B.; Chen, L.; Ma, D.; Xing, Y. Dynamic changes of the gut microbiota in preterm infants with different gestational age. Front. Microbiol. 2022, 13, 923273. [Google Scholar] [CrossRef]
- Ryan, M.P.; Adley, C.C. Ralstonia spp.: Emerging global opportunistic pathogens. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 291–304. [Google Scholar] [CrossRef]
- Padiyar, S.; Nandakumar, V.; Kollikonda, S.; Karnati, S.; Sangwan, N.; Aly, H. Maternal and infant microbiome and birth anthropometry. Iscience 2024, 27, 110312. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, J.; Li, X.; Zhao, H.; Ai, Q.; Zhang, L.; Tong, Y.; Meng, L.; Yang, H. No microorganism was detected in amniotic fluid of healthy pregnancies from the second trimester to the delivery. Microbiome 2025, 13, 20. [Google Scholar] [CrossRef]
- Mitchell, C.M.; Mazzoni, C.; Hogstrom, L.; Bryant, A.; Bergerat, A.; Cher, A.; Pochan, S.; Herman, P.; Carrigan, M.; Sharp, K. Delivery mode affects stability of early infant gut microbiota. Cell Rep. Med. 2020, 1, 100156. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Huang, L.; Wang, Y.; Huang, C.; Luo, Y.; Qin, X.; Zeng, J. Effect of different delivery modes on intestinal microbiota and immune function of neonates. Sci. Rep. 2024, 14, 17452. [Google Scholar] [CrossRef] [PubMed]
- Toubon, G.; Patin, C.; Delannoy, J.; Rozé, J.-C.; Barbut, F.; Ancel, P.-Y.; Charles, M.-A.; Butel, M.-J.; Lepage, P.; Aires, J. Very preterm gut microbiota development from the first week of life to 3.5 years of age: A prospective longitudinal multicenter study. Microbiol. Spectr. 2025, 13, e01636-24. [Google Scholar] [CrossRef] [PubMed]
- Binda, C.; Lopetuso, L.R.; Rizzatti, G.; Gibiino, G.; Cennamo, V.; Gasbarrini, A. Actinobacteria: A relevant minority for the maintenance of gut homeostasis. Dig. Liver Dis. 2018, 50, 421–428. [Google Scholar] [CrossRef]
- Anteneh, Y.S.; Franco, C.M. Symbiosis and pathogenicity of Actinobacteria. In Biology and Biotechnology of Actinobacteria; Springer International Publishing: Cham, Switzerland, 2017; pp. 233–268. [Google Scholar]
- Hughes, H.K.; Rose, D.; Ashwood, P. The gut microbiota and dysbiosis in autism spectrum disorders. Curr. Neurol. Neurosci. Rep. 2018, 18, 81. [Google Scholar] [CrossRef]
- Moore, R.E.; Townsend, S.D. Temporal development of the infant gut microbiome. Open Biol. 2019, 9, 190128. [Google Scholar] [CrossRef] [PubMed]
- Aatsinki, A.-K.; Keskitalo, A.; Laitinen, V.; Munukka, E.; Uusitupa, H.-M.; Lahti, L.; Kortesluoma, S.; Mustonen, P.; Rodrigues, A.J.; Coimbra, B. Maternal prenatal psychological distress and hair cortisol levels associate with infant fecal microbiota composition at 2.5 months of age. Psychoneuroendocrinology 2020, 119, 104754. [Google Scholar] [CrossRef] [PubMed]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef]
- Dahlgren, A.F.; Pan, A.; Lam, V.; Gouthro, K.C.; Simpson, P.M.; Salzman, N.H.; Nghiem-Rao, T.H. Longitudinal changes in the gut microbiome of infants on total parenteral nutrition. Pediatr. Res. 2019, 86, 107–114. [Google Scholar] [CrossRef]
- Vallès, Y.; Artacho, A.; Pascual-García, A.; Ferrús, M.L.; Gosalbes, M.J.; Abellán, J.J.; Francino, M.P. Microbial succession in the gut: Directional trends of taxonomic and functional change in a birth cohort of Spanish infants. PLoS Genet. 2014, 10, e1004406. [Google Scholar] [CrossRef]
- Zhou, P.; Manoil, D.; Belibasakis, G.N.; Kotsakis, G.A. Veillonellae: Beyond bridging species in oral biofilm ecology. Front. Oral Health 2021, 2, 774115. [Google Scholar] [CrossRef]
- Grier, A.; Qiu, X.; Bandyopadhyay, S.; Holden-Wiltse, J.; Kessler, H.A.; Gill, A.L.; Hamilton, B.; Huyck, H.; Misra, S.; Mariani, T.J. Impact of prematurity and nutrition on the developing gut microbiome and preterm infant growth. Microbiome 2017, 5, 158. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Xun, P.; Wang, X.; He, K.; Tang, Q.; Zhang, T.; Wang, Y.; Tang, W.; Lu, L.; Yan, W. Impact of postnatal antibiotics and parenteral nutrition on the gut microbiota in preterm infants during early life. J. Parenter. Enter. Nutr. 2020, 44, 639–654. [Google Scholar] [CrossRef]
- Stinson, L.F.; Keelan, J.A.; Payne, M.S. Comparison of meconium DNA extraction methods for use in microbiome studies. Front. Microbiol. 2018, 9, 270. [Google Scholar] [CrossRef]
- Li, F.; Hooi, S.L.; Choo, Y.M.; Teh, C.S.J.; Toh, K.Y.; Lim, L.W.Z.; Lee, Y.Q.; Chong, C.W.; Ahmad Kamar, A. Progression of gut microbiome in preterm infants during the first three months. Sci. Rep. 2025, 15, 12104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Tang, H.; Chen, Y.; Chen, Z.; Xu, Y.; Fu, X.; Sun, Y.; Zhao, Z. Impact of environmental characteristics on children’s gut microbiota–A pilot study in assessing the role of indoor microbiome and metabolites. Environ. Res. 2023, 234, 116114. [Google Scholar] [CrossRef] [PubMed]

| VP (<32 wk) | MLP (32≤, <37 wk) | FT (≥37 wk) | p | P * | ||||
|---|---|---|---|---|---|---|---|---|
| [N = 51] | [N = 195] | [N = 93] | VP vs. MLP | VP vs. FT | MLP vs. FT | |||
| Maternal characteristics | Assisted pregnancy | 13 (25.5%) | 66 (33.8%) | 11 (11.8%) | <0.001 | |||
| ACS | 49 (96.1%) | 152 (77.9%) | 1 (1.1%) | <0.001 | ||||
| PROM > 18 h | 13 (25.5%) | 30 (15.4%) | 4 (4.3%) | 0.001 | ||||
| HCAM | 10 (76.9%) | 3 (1.5%) | 0 (0.0%) | <0.001 | ||||
| maternal DM | 13 (25.5%) | 28 (14.4%) | 7 (7.5%) | 0.013 | ||||
| oligohydramnios | 12 (60.0%) | 6 (3.1%) | 2 (2.2%) | <0.001 | ||||
| Placenta abruption | 5 (9.8%) | 5 (2.6%) | 1 (1.1%) | 0.013 | ||||
| maternal preeclampsia | 20 (39.2%) | 50 (25.6%) | 3 (3.2%) | <0.001 | ||||
| Caesarean section | 49 (96.1%) | 189 (96.9%) | 73 (78.5%) | <0.001 | ||||
| neonatal characteristics | Gestational age a, week | 28.0 ± 2.6 | 34.1 ± 1.2 | 37.9 ± 1.0 | <0.001 | <0.001 | <0.001 | <0.001 |
| Birth weight a, g | 1125.8 ± 453.5 | 2186.7 ± 429.7 | 3160.2 ± 412.5 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Male | 22 (43.1%) | 94 (48.2%) | 53 (57.0%) | 0.220 | ||||
| Requiring intubation at birth | 27 (52.9%) | 7 (3.9%) | 2 (2.2%) | <0.001 | ||||
| SGA | 14 (27.5%) | 18 (9.2%) | 7 (7.5%) | 0.001 | ||||
| RDS | 42 (82.4%) | 50 (25.6%) | 16 (17.2%) | <0.001 | ||||
| NEC or FI | 12 (23.5%) | 20 (10.3%) | 3 (3.2%) | 0.001 | ||||
| BPD | 20 (39.2%) | 1 (0.5%) | - | <0.001 | ||||
| Sepsis | 9 (17.6%) | 1 (0.5%) | 0 (0.0%) | <0.001 | ||||
| ROP requiring surgery | 11 (21.6%) | 0 (0.0%) | - | <0.001 | ||||
| TPN duration | 46.1 ± 51.3 | 4.3 ± 3.0 | 2.2 ± 1.9 | <0.001 | <0.001 | <0.001 | 1.000 | |
| LOS | 101.4 ± 48.0 | 22.5 ± 13.3 | 10.5 ± 4.0 | <0.001 | <0.001 | <0.001 | <0.001 | |
| AGA at discharge | 30 (58.8%) | 32 (16.5%) | 69 (74.2%) | <0.001 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, J.; Choi, C.W.; Kang, H.M.; Kim, S.Y.; Youn, Y.-A. Early-Life Development of the Intestinal Microbiome in Preterm and Term Infants Hospitalized in the Neonatal Intensive Care Unit. Microorganisms 2025, 13, 2158. https://doi.org/10.3390/microorganisms13092158
Shin J, Choi CW, Kang HM, Kim SY, Youn Y-A. Early-Life Development of the Intestinal Microbiome in Preterm and Term Infants Hospitalized in the Neonatal Intensive Care Unit. Microorganisms. 2025; 13(9):2158. https://doi.org/10.3390/microorganisms13092158
Chicago/Turabian StyleShin, Jeongmin, Chang Won Choi, Hyun Mi Kang, Sae Yun Kim, and Young-Ah Youn. 2025. "Early-Life Development of the Intestinal Microbiome in Preterm and Term Infants Hospitalized in the Neonatal Intensive Care Unit" Microorganisms 13, no. 9: 2158. https://doi.org/10.3390/microorganisms13092158
APA StyleShin, J., Choi, C. W., Kang, H. M., Kim, S. Y., & Youn, Y.-A. (2025). Early-Life Development of the Intestinal Microbiome in Preterm and Term Infants Hospitalized in the Neonatal Intensive Care Unit. Microorganisms, 13(9), 2158. https://doi.org/10.3390/microorganisms13092158


_Di_Marco.png)




