Inhibition of Clostridium perfringens Spore Germination by the Synergistic Effects of the Natural Products Chitosan and Nisin
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Spore Preparation and Purification
2.3. Preparation of Antimicrobial Solutions
2.4. C. perfringens Spore Germination in the Presence of Antimicrobial Agents
2.5. C. perfringens Spore Outgrowth in the Presence of Antimicrobials
2.6. C. perfringens Vegetative Growth in the Presence of Antimicrobials
2.7. Growth of C. perfringens Spores in Chicken Meat in the Presence of Antimicrobials
2.8. Statistical Analyses
3. Results
3.1. Effect of pH on C. perfringens Spore Germination Inhibition by Chitosan and Nisin
3.2. C. perfringens Spore Germination Inhibition by a Chitosan–Nisin Combination Treatment at pH 6.0
3.3. Inhibition of C. perfringens SM101 Spore Germination by Nisin and Chitosan Combination Treatments at Different Concentrations
3.4. Spore Germination Inhibition of Clinically Important Strains Other Than SM101 by a Combination of Chitosan and Nisin
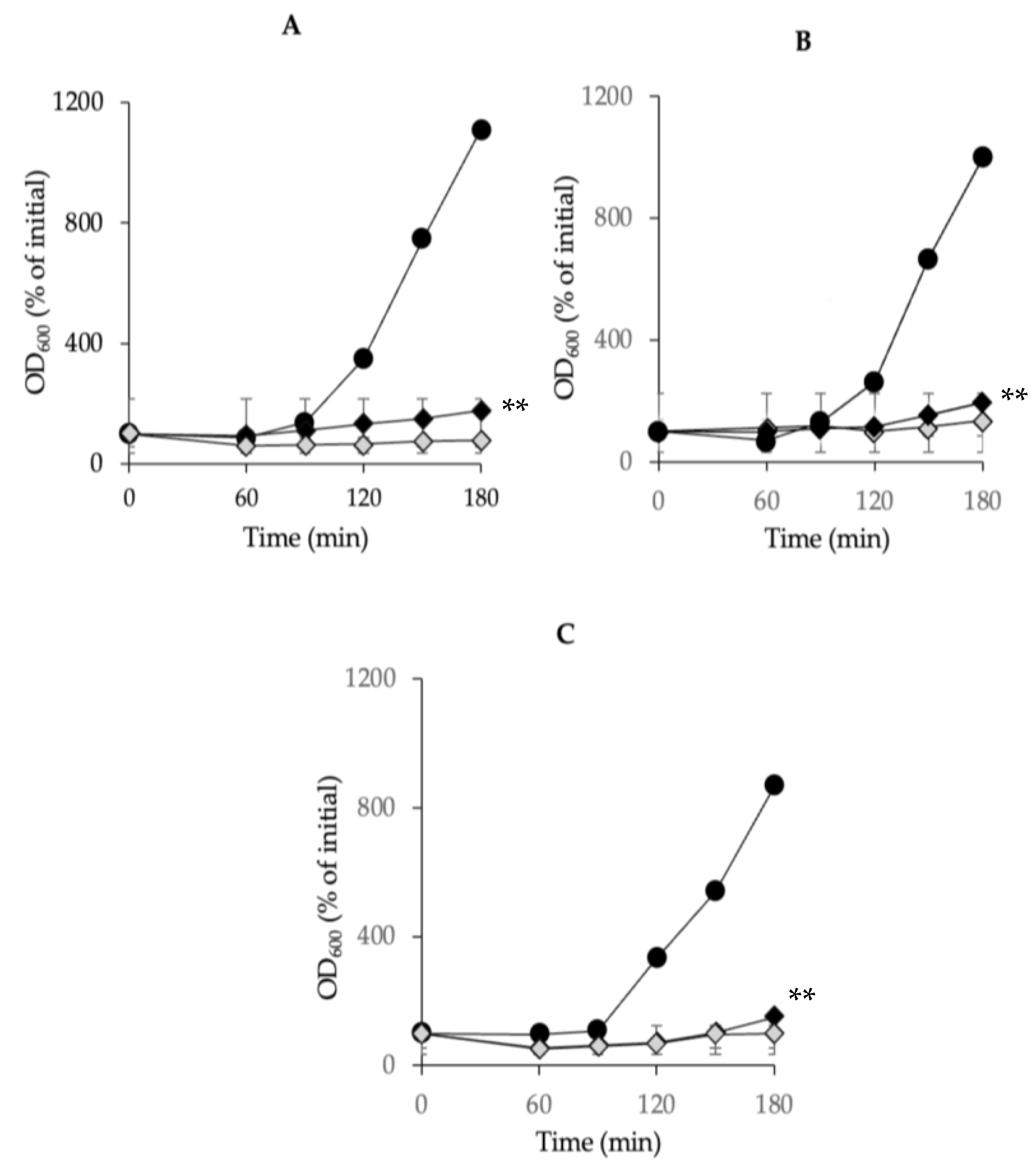
3.5. Inhibitory Effects of Combined Chitosan and Nisin on C. perfringens Spore Outgrowth
3.6. Effects of a Chitosan and Nisin Combination on the Growth of C. perfringens Vegetative Cells in Laboratory Media
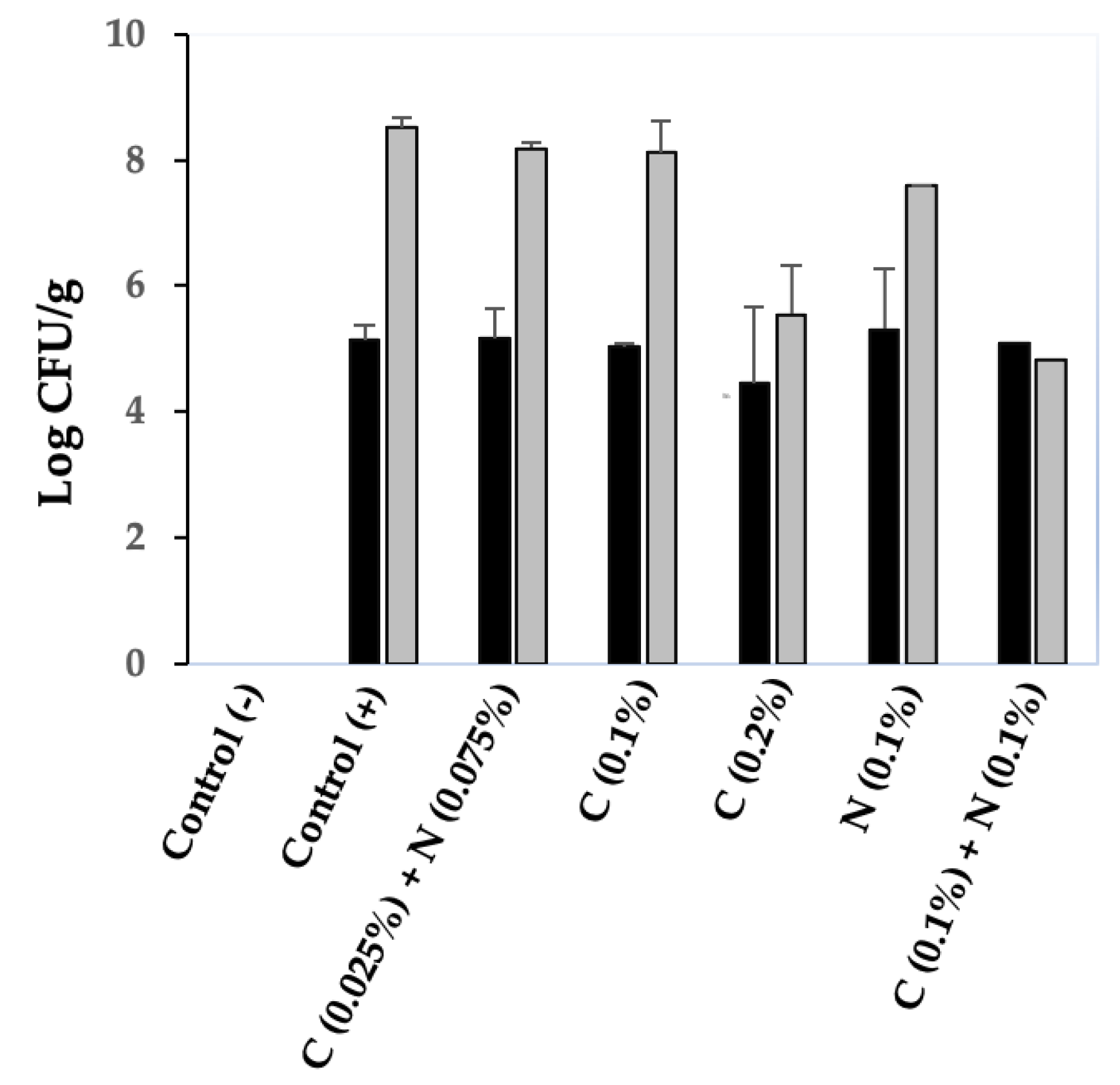
3.7. Together, Chitosan and Nisin Inhibit C. perfringens Spore Germination in Chicken Meat
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grass, J.E.; Gould, L.H.; Mahon, B.E. Epidemiology of Foodborne Disease Outbreaks Caused by Clostridium perfringens, United States, 1998–2010. Foodborne Pathog. Dis. 2013, 10, 131–136. [Google Scholar] [CrossRef]
- Kiu, R.; Hall, L.J. An Update on the Human and Animal Enteric Pathogen Clostridium perfringens. Emerg. Microbes Infect. 2018, 7, 141. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh Gohari, I.; Navarro, M.A.; Li, J.; Shrestha, A.; Uzal, F.; McClane, B.A. Pathogenicity and Virulence of Clostridium perfringens. Virulence 2021, 12, 723–753. [Google Scholar] [CrossRef] [PubMed]
- Juneja, V.K.; Novak, J.S.; Labbe, R.J. Clostridium perfringens. In Pathogens and Toxins in Foods; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; pp. 53–70. ISBN 978-1-68367-131-2. [Google Scholar]
- Juneja, V.K.; Huang, L.; Thippareddi, H.H. Predictive Model for Growth of Clostridium perfringens in Cooked Cured Pork. Int. J. Food Microbiol. 2006, 110, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Márquez-González, M.; Cabrera-Díaz, E.; Hardin, M.D.; Harris, K.B.; Lucia, L.M.; Castillo, A. Survival and Germination of Clostridium perfringens Spores during Heating and Cooling of Ground Pork. J. Food Prot. 2012, 75, 682–689. [Google Scholar] [CrossRef]
- Valenzuela-Martinez, C.; Pena-Ramos, A.; Juneja, V.K.; Korasapati, N.R.; Burson, D.E.; Thippareddi, H. Inhibition of Clostridium perfringens Spore Germination and Outgrowth by Buffered Vinegar and Lemon Juice Concentrate during Chilling of Ground Turkey Roast Containing Minimal Ingredients. J. Food Prot. 2010, 73, 470–476. [Google Scholar] [CrossRef]
- Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocins: Developing Innate Immunity for Food. Nat. Rev. Microbiol. 2005, 3, 777–788. [Google Scholar] [CrossRef]
- Banerjee, M.; Sarkar, P.K. Antibiotic Resistance and Susceptibility to Some Food Preservative Measures of Spoilage and Pathogenic Micro-Organisms from Spices. Food Microbiol. 2004, 21, 335–342. [Google Scholar] [CrossRef]
- Eastoe, J.E.; Long, J.E.; Eastoe, J.E.; Long, J.E. The Effect of Nisin on the Growth of Cells and Spores of Clostridium welchii in Gelatine. J. Appl. Bacteriol. 1959, 22, 1–7. [Google Scholar] [CrossRef]
- Araújo, M.K.; Gumiela, A.M.; Bordin, K.; Luciano, F.B.; de Macedo, R.E.F. Combination of Garlic Essential Oil, Allyl Isothiocyanate, and Nisin Z as Bio-Preservatives in Fresh Sausage. Meat Sci. 2018, 143, 177–183. [Google Scholar] [CrossRef]
- Delves-Broughton, J. Nisin as a Food Preservative. Food Aust. 2005, 57, 525–527. [Google Scholar]
- Juneja, V.; Thippareddi, H.; Bari, L.; Inatsu, Y.; Kawamoto, S.; Friedman, M. Chitosan Protects Cooked Ground Beef and Turkey Against Clostridium perfringens Spores During Chilling. J. Food Sci. 2006, 71, M236–M240. [Google Scholar] [CrossRef]
- Raafat, D.; Sahl, H.-G. Chitosan and Its Antimicrobial Potential—A Critical Literature Survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef]
- Xing, K.; Zhu, X.; Peng, X.; Qin, S. Chitosan Antimicrobial and Eliciting Properties for Pest Control in Agriculture: A Review. Agron. Sustain. Dev. 2015, 35, 569–588. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial Properties of Chitosan and Mode of Action: A State of the Art Review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Juneja, V.K. Review of Antimicrobial and Antioxidative Activities of Chitosans in Food. J. Food Prot. 2010, 73, 1737–1761. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Zivanovic, S.; D’Souza, D.H. Effect of Chitosan on the Infectivity of Murine Norovirus, Feline Calicivirus, and Bacteriophage MS2. J. Food Prot. 2009, 72, 2623–2628. [Google Scholar] [CrossRef]
- Udompijitkul, P.; Paredes-Sabja, D.; Sarker, M.R. Inhibitory Effects of Nisin against Clostridium perfringens Food Poisoning and Nonfood-Borne Isolates. J. Food Sci. 2012, 77, M51–M56. [Google Scholar] [CrossRef]
- Paredes-Sabja, D.; Torres, J.A.; Setlow, P.; Sarker, M.R. Clostridium perfringens Spore Germination: Characterization of Germinants and Their Receptors. J. Bacteriol. 2008, 190, 1190–1201. [Google Scholar] [CrossRef]
- Banawas, S.; Paredes-Sabja, D.; Korza, G.; Li, Y.; Hao, B.; Setlow, P.; Sarker, M.R. The Clostridium perfringens Germinant Receptor Protein GerKC Is Located in the Spore Inner Membrane and Is Crucial for Spore Germination. J. Bacteriol. 2013, 195, 5084–5091. [Google Scholar] [CrossRef]
- Duncan, C.L.; Strong, D.H. Improved Medium for Sporulation of Clostridium perfringens. Appl. Microbiol. 1968, 16, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Romero, M.C.; Murphy, T.; Morris, M.; Cummins, E.; Kerry, J.P. Antimicrobial Activity of Chitosan, Organic Acids and Nano-Sized Solubilisates for Potential Use in Smart Antimicrobially-Active Packaging for Potential Food Applications. Food Control 2013, 34, 393–397. [Google Scholar] [CrossRef]
- Li, X.; Feng, X.; Yang, S.; Fu, G.; Wang, T.; Su, Z. Chitosan Kills Escherichia coli through Damage to Be of Cell Membrane Mechanism. Carbohydr. Polym. 2010, 79, 493–499. [Google Scholar] [CrossRef]
- Cortezzo, D.E.; Setlow, B.; Setlow, P. Analysis of the Action of Compounds That Inhibit the Germination of Spores of Bacillus Species. J. Appl. Microbiol. 2004, 96, 725–741. [Google Scholar] [CrossRef]
- Alnoman, M.; Udompijitkul, P.; Sarker, M.R. Chitosan Inhibits Enterotoxigenic Clostridium perfringens Type A in Growth Medium and Chicken Meat. Food Microbiol. 2017, 64, 15–22. [Google Scholar] [CrossRef]
- Ettayebi, K.; El Yamani, J.; Rossi-Hassani, B.-D. Synergistic Effects of Nisin and Thymol on Antimicrobial Activities in Listeria monocytogenes and Bacillus subtilis. FEMS Microbiol. Lett. 2000, 183, 191–195. [Google Scholar] [CrossRef]
- Nerandzic, M.M.; Donskey, C.J. Activate to Eradicate: Inhibition of Clostridium difficile Spore Outgrowth by the Synergistic Effects of Osmotic Activation and Nisin. PLoS ONE 2013, 8, e54740. [Google Scholar] [CrossRef]
- Wu, M.; Ma, Y.; Dou, X.; Aslam, M.Z.; Liu, Y.; Xia, X.; Yang, S.; Wang, X.; Qin, X.; Hirata, T.; et al. A review of potential antibacterial activities of nisin against Listeria monocytogenes: The combined use of nisin shows more advantages than single use. Food Res. Int. 2023, 164, 112363. [Google Scholar] [CrossRef]
- Nasaj, M.; Chehelgerdi, M.; Asghari, B.; Ahmadieh-Yazdi, A.; Asgari, M.; Kabiri-Samani, S.; Sharifi, E.; Arabestania, M. Factors influencing the antimicrobial mechanism of chitosan action and its derivatives: A review. Int. J. Biol. Macromol. 2024, 277, 134321. [Google Scholar] [CrossRef]
- Yan, D.; Li, Y.; Liu, Y.; Li, N.; Zhang, X.; Yan, C. Antimicrobial Properties of Chitosan and Chitosan Derivatives in the Treatment of Enteric Infections. Molecules 2021, 26, 7136. [Google Scholar] [CrossRef]
- Zimeta, P.; Mombrúa, A.W.; Mombrúb, D.; Castroa, A.; Villanuevaa, J.P.; Pardoa, H.; Rufo, C. Physico-chemical and antilisterial properties of nisin-incorporated chitosan/carboxymethyl chitosan films. Carbohydr. Polym. 2019, 219, 334–343. [Google Scholar] [CrossRef]
- Chai, C.; Lee, K.S.; Oh, S.W. Synergistic inhibition of Clostridium difficile with nisin-lysozyme combination treatment. Anaerobe 2015, 34, 24–26. [Google Scholar] [CrossRef]
- Iulietto, M.F.; Sechi, P.; Borgogni, E.; Cenci-Goga, B.T. Meat Spoilage: A Critical Review of a Neglected Alteration Due to Ropy Slime Producing Bacteria. Ital. J. Anim. Sci. 2016, 14, 4011. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhabeeb, R.S.; Almatrafi, R.; Banawas, S.S.; Alnoman, M.; Sarker, M.R. Inhibition of Clostridium perfringens Spore Germination by the Synergistic Effects of the Natural Products Chitosan and Nisin. Microorganisms 2025, 13, 2116. https://doi.org/10.3390/microorganisms13092116
Alhabeeb RS, Almatrafi R, Banawas SS, Alnoman M, Sarker MR. Inhibition of Clostridium perfringens Spore Germination by the Synergistic Effects of the Natural Products Chitosan and Nisin. Microorganisms. 2025; 13(9):2116. https://doi.org/10.3390/microorganisms13092116
Chicago/Turabian StyleAlhabeeb, Rabiaa S., Roua Almatrafi, Saeed S. Banawas, Maryam Alnoman, and Mahfuzur R. Sarker. 2025. "Inhibition of Clostridium perfringens Spore Germination by the Synergistic Effects of the Natural Products Chitosan and Nisin" Microorganisms 13, no. 9: 2116. https://doi.org/10.3390/microorganisms13092116
APA StyleAlhabeeb, R. S., Almatrafi, R., Banawas, S. S., Alnoman, M., & Sarker, M. R. (2025). Inhibition of Clostridium perfringens Spore Germination by the Synergistic Effects of the Natural Products Chitosan and Nisin. Microorganisms, 13(9), 2116. https://doi.org/10.3390/microorganisms13092116







