Abstract
The rapid global emergence of multidrug-resistant (MDR) Klebsiella pneumoniae threatens public health, as treatment options remain limited and resistance to last-line antibiotics is rising. Natural phenolic compounds emerge as promising adjuvants to restore antibiotic activity. This study pooled data from 216 in vitro assays evaluating interactions between phenolic compounds and conventional antibiotics against MDR K. pneumoniae. Fractional inhibitory concentration index (FICI) values were analyzed at the individual-test level, and structure–activity relationships were explored using a binary chemotype flagging approach. Overall, synergy was highly context-dependent, varying by both antibiotic class and phenolic chemotype. Polymyxin B combined with resveratrol demonstrated the most consistent and robust synergy (median FICI = 0.25, synergy rate = 96.2%), with no antagonism observed. For carbapenems, meropenem showed strong synergy when paired with flavonoids containing catechol or gallol motifs (e.g., quercetin, kaempferol), whereas curcumin exhibited inconsistent or antagonistic effects. Variability analysis revealed that combinations with low dispersion, such as polymyxin B + resveratrol, offer greater translational potential than high-variability pairs. These findings highlight the structural determinants of synergy and support further preclinical evaluation of select phenolic compounds as adjuvants to conventional antibiotics in the fight against MDR K. pneumoniae.
1. Introduction
The global rise of multidrug-resistant (MDR) Klebsiella pneumoniae represents a significant public health threat, particularly in clinical settings where treatment options are increasingly limited [1,2]. This Gram-negative opportunistic pathogen has developed various resistance mechanisms, including the production of extended-spectrum β-lactamases, carbapenemases, and alterations in membrane permeability, rendering it unresponsive to most available antibiotics [3]. Infections caused by MDR K. pneumoniae are associated with high morbidity, mortality, and substantial healthcare burdens [4,5].
Current treatment options are usually limited to last-line agents such as polymyxins (e.g., colistin), tigecycline, and aminoglycosides, frequently used in combination to enhance efficacy and reduce resistance emergence [6,7]. Novel β-lactam/β-lactamase inhibitor combinations, such as ceftazidime–avibactam and meropenem–vaborbactam, have expanded therapeutic possibilities, particularly against carbapenemase-producing strains [8]. However, resistance to these newer agents is already emerging [9,10,11,12].
Thus, the development of new antimicrobials has failed to keep pace with the rapid emergence of resistance [13], underscoring an urgent need for innovative approaches to combat MDR pathogens [14,15]. One promising avenue is the optimization of combination regimens and the use of adjuvant compounds capable of restoring or enhancing the activity of existing antibiotics [14,16]. Among these strategies, natural compounds—particularly phenolic secondary metabolites derived from plants—have attracted increasing attention due to their diverse biological activities and a relatively low potential for driving resistance [17,18,19,20]. Unlike many secondary metabolites that exert singular or indirect antimicrobial effects, phenolics possess multiple functional groups—particularly hydroxylated aromatic rings—that enable direct interaction with bacterial targets [20,21,22,23]. These structures confer membrane-disruptive capacity, efflux pump inhibition, metal chelation, enzyme modulation, and redox activity, all of which are directly relevant to reversing antimicrobial resistance and potentiating antibiotic activity [20,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39]. Phenolic compounds additionally interfere with quorum sensing (QS), the bacterial communication system that regulates biofilm formation and virulence factor expression [40,41]. Consequently, they disrupt signal production, receptor binding, and downstream gene activation, resulting in weaker biofilms, reduced motility, and diminished toxin secretion [42,43,44,45,46,47,48,49,50,51]. Beyond bacterial targets, phenolic compounds exhibit host-protective properties, including the downregulation of pro-inflammatory cytokines, the enhancement of antioxidant defenses, and the preservation of endothelial barrier integrity [52,53,54,55,56,57,58,59].
Here in, we aim to provide an in-depth analysis of the synergistic interactions between phenolic compounds and antibiotics against MDR K. pneumoniae strains. This study delivers the first pooled, assay-level analysis of phenolic–antibiotic combinations, integrating reproducibility metrics and a chemotype-based structure–activity framework. By synthesizing data on minimum inhibitory concentrations (MICs), fractional inhibitory concentration indices (FICIs), and underlying molecular mechanisms, we have effectively assessed the potential of these combinations against critical priority pathogens. This approach allows clear differentiation between consistent synergistic effects and non-reproducible interactions, thereby enhancing the predictive and translational relevance of phenolic adjuvants.
2. Materials and Methods
A systematic search was conducted in PubMed, Web of Science, and Google Scholar using the following keywords and Boolean operators: (“phenolic” OR “polyphenol” OR “flavonoid”) AND (“antibiotic” OR “antimicrobial” OR “antibacterial”) AND (“synerg*” OR “combination” OR “FICI” OR “checkerboard” OR “fractional inhibitory concentration”) AND (“Klebsiella pneumoniae” OR “MDR Klebsiella”). The search covered all articles published up to June 2024. Only articles published in English were considered eligible for inclusion. We performed title/abstract-level screening and removed duplicate records. All remaining articles were assessed at the full-text level.
Studies were included if they reported standardized checkerboard or micro-broth dilution assays evaluating interactions between purified natural phenolic compounds and conventional antibiotics against multidrug-resistant (MDR) K. pneumoniae and reported quantitative data (MIC, FICI).
Studies were excluded if they met any of the following criteria:
- Use of non-standardized crude extracts or essential oils.
- Lack of reported FICI values or MIC data for both single agents and their combinations.
- Use of K. pneumoniae isolates susceptible to polymyxins, carbapenems, quinolones, or cephalosporins.
- Duplicate data or overlapping isolate collections.
Following screening and duplicate removal, 13 studies met the inclusion criteria, yielding a total of 216 isolate-level records for analysis [60,61,62,63,64,65,66,67,68,69,70,71,72]. Our analysis was conducted at the assay level, treating each isolate-specific test as an independent experimental unit and pooling in vitro outcomes rather than aggregating study-level summary effect sizes.
For each isolate, the following variables were recorded:
- Antibiotic name and mechanistic class;
- Natural phenolic compound;
- MIC values for each agent alone and in combination;
- FICI values.
All statistical analyses were performed in R version 4.3.2. FICI values were treated as continuous variables. When studies reported FICI values as ‘<0.5’, these were assigned a value of 0.5 using a conservative truncation approach, consistent with established methodology to avoid arbitrary imputation and prevent artificial inflation of synergy estimates [73,74].
Global synergy was evaluated using a one-sample Wilcoxon signed-rank test. Between-class differences were assessed using the Kruskal–Wallis test, followed by Dunn–Bonferroni post hoc comparisons.
To assess reproducibility, variability in FICI values was calculated for each antibiotic–compound combination using the range, interquartile range (IQR), and standard deviation (SD). Based on these metrics, combinations were classified as follows:
- Robust: median FICI ≤ 0.5 and range ≤ 0.5;
- Inconsistent: median FICI ≤ 1.0 and range > 2.0;
- Moderate: not meeting the above thresholds;
- Inconclusive: fewer than three replicate observations (n < 3).
These thresholds were empirically defined to capture both the strength and consistency of synergistic effects, following established interpretive FICI guidelines and emphasizing inter-strain variability as a determinant of translational potential.
To explore structural determinants of synergy, we curated one canonical structure per natural compound (single parent, neutral, dominant tautomer) and assigned a priori binary chemotype flags (flavonoid, stilbene, phenolic acid, simple phenol, curcuminoid/Michael acceptor, glycoside, alkaloid) and a catechol/gallol substructure flag. FICI values were analyzed at the individual-assay level; for distributional contrasts we used log2(FICI). Synergy and antagonism were defined as FICI ≤ 0.5 and FICI ≥ 4.0, respectively. For categorical outcomes (synergy rates by flag), Fisher’s exact test was applied and effect sizes were reported as odds ratios (OR) and risk differences (RD). Continuous contrasts of log2(FICI) between flagged vs. non-flagged groups were evaluated with the Mann–Whitney test, and median shifts were summarized using the Hodges–Lehmann estimator; approximate fold-changes in FICI were derived from the median difference in log2(FICI). All analyses were performed in R (v4.3.2).
3. Results
3.1. Synergistic Activity of Natural Phenolic Compounds with Conventional Antibiotics
A PRISMA 2020 flow diagram was constructed to illustrate the study selection process. The initial database search identified a total of 296 records. After the removal of duplicates, 232 unique records were screened based on title and abstract. Of these, 40 full-text articles were assessed for eligibility according to predefined inclusion and exclusion criteria, resulting in a final set of 13 studies included in the pooled analysis (Figure 1). Our dataset comprises a total of 270 in vitro synergy tests evaluating the interaction between 18 unique combinations of conventional antibiotics and natural phenolic compounds [60].
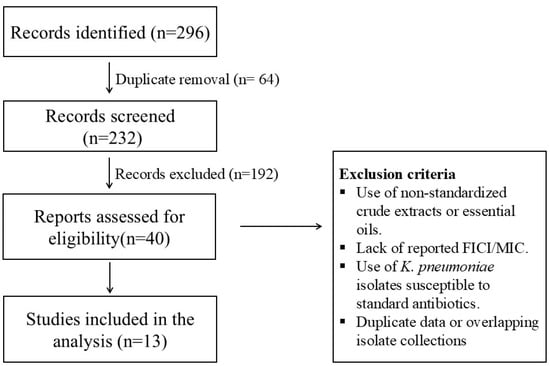
Figure 1.
PRISMA 2020 flow diagram illustrating the study selection process for in vitro synergy assays between phenolic compounds and antibiotics against MDR Klebsiella pneumoniae.
Table 1 below presents a comparative summary of the in vitro interactions between selected antibiotics and natural phenolic compounds against MDR Klebsiella pneumoniae strains. A FICI ≤ 0.5 indicates synergistic interaction, while a FICI between 0.5 and 1.0 is generally interpreted as additive. A FICI > 4.0 indicates an antagonistic effect [75].

Table 1.
Comparative in vitro synergistic effects of natural phenolic compounds and antibiotics against multidrug-resistant Klebsiella pneumoniae.
Among the antibiotic–natural compound combinations tested, substantial variability in synergistic activity was observed, as reflected by the range and median FICI values. Several combinations demonstrated strong synergistic potential, suggesting their promise in restoring antibiotic efficacy against MDR K. pneumoniae strains.
The combination of polymyxin B and resveratrol showed the most consistent synergy, with a median FICI of 0.199 and minimal variability (range: 0.002–0.502), based on 24 independent tests. Similarly, combinations of colistin and kaempferol or eugenol exhibited a very low median FICI of 0.26 or 0.27, respectively, indicating strong synergism. However, this finding is based on a limited number of replicates (n = 6). Notably, meropenem in association with baicalein yielded the lowest FICI recorded in the dataset (0.07). However, this result was derived from a single assay and is reported solely as a hypothesis-generating signal aligned with the broader structure–activity pattern of flavonoid synergy against carbapenems. Only combinations supported by sufficient replicates and low inter-assay variability were prioritized for translational interpretation.
Conversely, combinations such as cefotaxime + matrine (median FICI = 1.050) and ciprofloxacin + myricetin (median FICI = 1.34) failed to demonstrate synergy, indicating that not all phenolic compounds contribute beneficially to antibiotic activity. Furthermore, combinations involving cinnamaldehyde with either cefotaxime or ciprofloxacin displayed high variability.
Interestingly, within the same antibiotic class, the efficacy of natural compounds varied considerably. For example, meropenem combined with quercetin demonstrated moderate synergy, while its pairing with curcumin yielded a higher median FICI.
3.2. Comparative In Vitro Synergistic Effects of Natural Phenolic Compounds with Key Antibiotics Against MDR K. pneumoniae
The combinations of natural phenolic compounds and antibiotics tested against MDR K. pneumoniae, including only those with optimal median FICI values and enough replicates to allow for robust statistical analysis, are included in Table 2.

Table 2.
Synergistic activity of natural phenolic compounds combined with antibiotics against multidrug-resistant Klebsiella pneumoniae.
Pairwise comparisons within antibiotic groups confirmed that some phenolics consistently outperform others. To better visualize pairwise differences in synergy among the natural compounds tested in combination with individual antibiotics, we generated boxplots of FICI distributions for each antibiotic group (Figure 2, Figure 3 and Figure 4). The interaction profiles of cefotaxime with the tested natural compounds revealed statistically significant differences in FICI values (Kruskal–Wallis χ2 = 31.77, df = 2, p < 0.001). Post hoc Dunn–Bonferroni analysis grouped baicalein (median FICI = 0.82) and matrine (median FICI = 1.07) in the same statistical subset (CLD = A), indicating predominantly additive to indifferent effects. In contrast, cinnamaldehyde exhibited a markedly lower median FICI (0.455) and belonged to a distinct subset (CLD = B), suggesting a synergistic interaction with cefotaxime against the tested isolates (Figure 2).
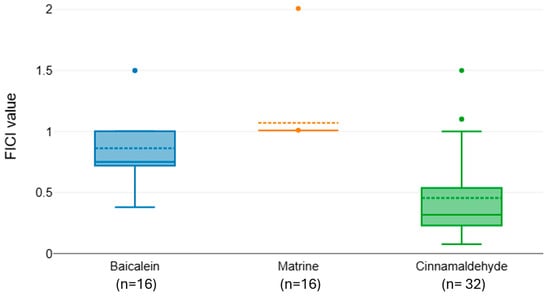
Figure 2.
Distribution of FICI values for cefotaxime combined with baicalein, matrine, and cinnamaldehyde.
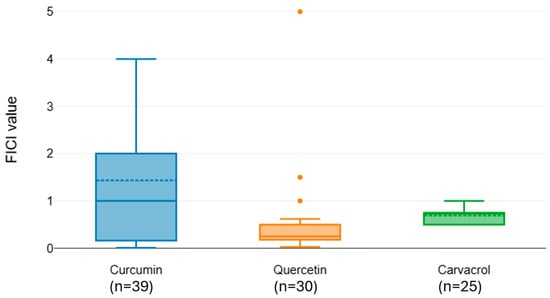
Figure 3.
Distribution of FICI values for meropenem combined with natural phenols.
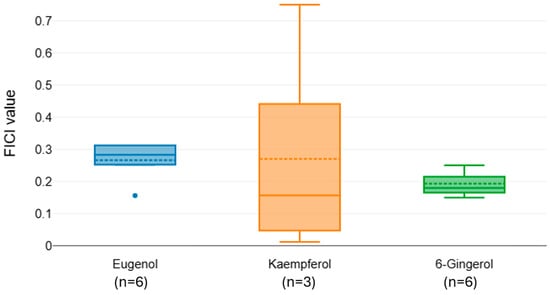
Figure 4.
Distribution of FICI values for colistin combined with natural phenols.
Meropenem exhibited significant variation in interaction outcomes with different natural compounds (Kruskal–Wallis χ2 = 16.83, df = 2, p < 0.001). Curcumin (median FICI = 1.436) and carvacrol (median FICI = 0.695) were classified within the same statistical group (CLD = A), indicating no consistent synergy. Quercetin, however, showed a substantially lower median FICI (0.5) and was placed in a separate group (CLD = B), consistent with a synergistic effect when combined with meropenem (Figure 3).
The FICI distributions for colistin in combination with eugenol, kaempferol, and 6-Gingerol indicate overall strong synergistic interactions, with median values below 0.5 for all compounds (Figure 4). Post hoc comparisons revealed no significant differences between pairings. Despite all compounds showing synergistic potential, 6-Gingerol yielded the lowest and most consistent FICI values, highlighting it as the most promising adjunctive agent to colistin among those tested. However, the small sample sizes (n = 3–6) limit the strength of our conclusions.
3.3. Synergistic Potency vs. FICI Variability
Although the synergistic potential of natural phenolic compounds combined with conventional antibiotics against MDR K. pneumoniae has been established in several instances, the clinical relevance of such findings requires not only high potency, but also consistent, predictable outcomes. The FICI is a robust quantitative metric for synergy assessment; however, relying solely on its median value can obscure variability across isolates.
To address this, we performed a dispersion-based analysis of FICI values for the most studied antibiotic–compound pairs (Table 3).

Table 3.
FICI summary analysis.
Analysis of dispersion metrics revealed notable differences in variability between combinations. Cefotaxime + matrine displayed exceptionally low variability, indicating highly consistent additive effects across replicates. Similarly, meropenem + carvacrol and colistin + eugenol exhibited narrow ranges (0.5 and 0.156, respectively) and low SD values (0.173 and 0.062), suggestive of stable interaction profiles. In contrast, meropenem + curcumin and meropenem + quercetin exhibited substantial dispersion, with wide ranges (3.991 and 4.97, respectively) and large SD values (1.266 and 0.900), reflecting variable interaction outcomes from strong synergy to pronounced antagonism.
Cefotaxime + baicalein and cefotaxime + cinnamaldehyde demonstrated moderate dispersion (IQR 0.28 and 0.314, respectively), with FICI values spanning both synergistic and indifferent interaction ranges. In contrast, the stability of cefotaxime + matrine suggests a reproducible interaction type with minimal fluctuation.
The observed variability in FICI values has important implications for prioritizing combinations for further development. Combinations with low dispersion are more likely to demonstrate reproducible efficacy in subsequent in vivo models, making them strong candidates for preclinical testing. High-dispersion combinations, while potentially capable of producing strong synergy, may be less reliable due to inconsistency, which could stem from strain-specific responses, compound instability, or experimental variability. Thus, dispersion-based analysis complements traditional mean FICI interpretation by identifying both robust and unstable interaction profiles, guiding rational selection of candidates for translational research.
The robustness classification of antibiotic–phenolic compound combinations based on synergy metrics is illustrated in Figure 5.
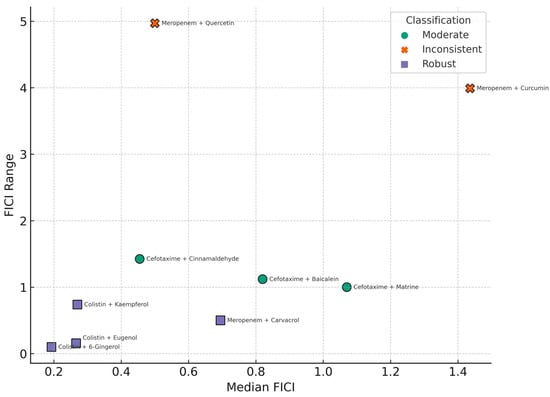
Figure 5.
Scatterplot showing the relationship between synergistic potency (median FICI) and variability (FICI range) across 11 antibiotic–phenolic compound combinations tested against multidrug-resistant K. pneumoniae. Each point represents one combination and is color-coded by robustness classification: robust (median FICI ≤ 0.5 and range ≤ 0.5), inconsistent (median FICI ≤ 1.0 and range > 2.0), moderate (other qualifying combinations), and inconclusive (n < 3). Vertical and horizontal dashed lines indicate the 0.5 thresholds used for defining synergy and consistency, respectively.
This analysis highlights that FICI dispersion is a critical component in evaluating the translational potential of antibiotic-phenolic combinations. Highly variable combinations, even with low median FICI values, may not consistently yield synergistic effects across diverse clinical strains. Therefore, such inconsistencies must be considered when prioritizing candidates for preclinical development or clinical trials.
3.4. SAR Analysis
We next asked whether simple, mechanism-motivated chemotype flags could predict functional outcomes. We curated one canonical structure per compound (single parent, neutral, dominant tautomer) and assigned binary flags a priori (flavonoid, stilbene, phenolic acid, simple phenol, curcuminoid/Michael acceptor, glycoside, alkaloid; plus a catechol/gallol substructure flag), as seen in Table 4.

Table 4.
Binary structural classification of natural compounds by chemical class and functional motifs.
FICI values were analyzed at the individual-test level. For distributional contrasts we used log2(FICI). Table 5 summarizes the distribution of synergy and antagonism across structural classes of natural products, as well as continuous contrasts of log2(FICI) values. These analyses identify structural motifs associated with enhanced or reduced combinatorial activity.

Table 5.
Summary of synergy (FICI ≤ 0.5) and antagonism (FICI ≥ 4.0) by structural class.
The analysis demonstrated distinct structure–activity patterns among the tested natural products. Flavonoids showed a significantly higher synergy rate (64.9%) compared to non-flavonoids (23.8%), with an odds ratio of 5.94 and a 2.7-fold reduction in median FICI, indicating a robust and reproducible potentiating effect. This trend was even more pronounced for compounds containing catechol or gallol substructures, which exhibited the highest synergy rate among all groups (80.9%) and a fourfold reduction in FICI. Conversely, curcuminoids and other Michael acceptor motifs showed lower synergy rates (30.8%) relative to compounds lacking this feature (44.9%), with a slight increase in median FICI, indicating a trend toward neutral or antagonistic interactions.
Simple phenols demonstrated reduced synergy (28%) and a statistically significant negative risk difference (−28.8%), suggesting that small, monofunctional phenolic scaffolds lack sufficient structural complexity to potentiate antibiotics effectively in this context.
Phenolic acids displayed a nominal synergy rate of 100%; however, this was based on a limited sample size (wide confidence interval) and should be interpreted cautiously. Alkaloids showed no synergistic activity (0%), with a strongly significant negative association, indicating that this chemotype is unsuitable for antibiotic potentiation against K. pneumoniae.
4. Discussion
The primary objective of this study was to systematically evaluate the potential of natural phenolic compounds to enhance the activity of conventional antibiotics against MDR Klebsiella pneumoniae, a critical priority pathogen. By pooling and analyzing 216 in vitro assays, we aimed to identify combinations with consistent synergistic interactions, explore variability in synergy across different antibiotic–compound pairs, and provide a SAR perspective to guide the rational selection of adjunctive agents. This approach addresses an urgent clinical need to restore the efficacy of existing antibiotics amid rapidly increasing resistance and a lag in the slow pace of new antibiotics development [76,77].
Phenolic compounds were selected for investigation due to their unique structural and mechanistic attributes that distinguish them from other natural product classes. Their multifaceted mechanisms support their potential role as antibiotic adjuvants. Furthermore, extensive evidence supports the translational potential of these natural compounds. These characteristics make phenolic compounds uniquely suited for systematic evaluation in combination therapies, justifying their prioritization over other natural compound classes.
Our findings indicate that synergy between phenolic compounds and antibiotics is not uniform, but rather highly dependent on the mechanistic group of the antibiotic and the chemical class of the natural compound. To determine which structural elements drive this potentiation, we conducted a chemotype-based structure–activity analysis to identify phenolic motifs most strongly associated with synergistic outcomes across antibiotic classes.
Within this heterogeneous class, flavonoids were classified as a distinct chemotype due to their conserved C6-C3-C6 scaffold and associated mechanistic features—including membrane interaction, efflux pump inhibition, metal ion chelation, and virulence modulation—which collectively differentiate them from other phenolic subclasses and justify their independent evaluation in SAR analysis [78]. Overall, flavonoid compounds were strongly enriched for synergy compared to non-flavonoid chemotypes. This aligns with previous studies reporting potentiation of β-lactams by flavonoids through outer membrane destabilization and interference with resistance enzymes such as carbapenemases [79,80,81,82].
The markedly higher synergy observed for meropenem in combination with flavonoids was driven specifically by the presence of catechol and gallate motifs, rather than the flavonoid scaffold alone. These motifs were therefore classified as distinct chemotypes because they confer unique mechanistic advantages beyond the core flavonoid structure. Catechol-containing phenolics possess adjacent hydroxyl groups (–OH in ortho position) that enable iron chelation and reduction (Fe3+ to Fe2+), driving ROS generation and disrupting metal homeostasis. In MDR K. pneumoniae, catechol-containing flavonoids (such as luteolin and myricetin) were shown to potentiate colistin’s efficacy, whereas structurally related flavonoids lacking adjacent hydroxyls showed no synergy [78]. Catechol-mediated iron reduction dysregulates the PmrA/PmrB signaling pathway, increases outer membrane negative charge, and enhances antibiotic binding [83]. These combined effects mechanistically explain the consistent association between catechol motifs and synergistic antibiotic interactions. Additionally, the gallate group (3,4,5-trihydroxybenzoyl moiety) further enhances phenolic antimicrobial potency by increasing lipophilicity and membrane affinity. Galloylated catechins such as epigallocatechin gallate more effectively insert into bacterial membranes, disrupting lipid organization, increasing permeability, and facilitating antibiotic uptake [84]. Gallate substitution also enhances hydrogen bonding interactions with cell envelope components, leading to membrane leakage and interference with cell wall synthesis [85]. Consequently, galloylated phenolics show superior synergy profiles compared to their ungalloylated analogs, supporting inclusion of this motif as a key predictor of antibiotic potentiation.
Overall, flavonoid compounds were strongly enriched for synergy compared to non-flavonoid chemotypes, with catechol/gallol-bearing scaffolds emerging as the most consistent predictors of enhanced activity across combinations.
In contrast, curcuminoid/Michael acceptor and simple phenol classes tended to display lower synergy rates and higher FICI values, indicating less favorable interactions.
Curcuminoids constitute a structurally distinct class of phenolics defined by a linear diarylheptanoid backbone containing conjugated α,β-unsaturated carbonyl groups (Michael acceptors). This electrophilic scaffold enables interaction with nucleophilic targets, modulation of redox pathways, and insertion into bacterial membranes, all of which can influence antimicrobial responses [86]. However, these same electrophilic properties may activate bacterial stress defense mechanisms or neutralize antibiotic-generated ROS, leading to inconsistent outcomes when combined with antibiotics [28,87,88]. In our analysis, curcumin exhibited variable or antagonistic interactions, reflected by higher FICI values compared to catechol- or gallate-bearing compounds. Thus, while curcuminoids possess intrinsic antimicrobial potential, their promiscuous reactivity and redox-modulating effects make them less reliable as antibiotic adjuvants, justifying their classification as a separate chemotype with limited translational potential compared to synergy-promoting motifs.
Simple phenols such as carvacrol were largely neutral, supporting the idea that small hydrophobic compounds exert a more limited adjuvant effect in this context [89].
In our analysis, stilbenes (represented herein by resveratrol) were categorized as a distinct chemotype based on their C6-C2-C6 scaffold, which promotes membrane intercalation, QS disruption, and potentiation of polymyxin activity through unique physicochemical interactions that are not exhibited by other phenolic subclasses. This chemotype was found to possess a very high and consistent rate of synergy with polymyxin B. This is noteworthy, given the increasing clinical reliance on polymyxins as last-line therapies, and suggests that certain stilbene structures may complement their membrane-targeting activity [28,90,91,92]. The consistency of this effect across multiple strains highlights its potential for translation to in vivo models. However, for other antibiotic groups, such as cephalosporins and fluoroquinolones, the evidence base remains limited due to smaller sample sizes.
Glycosylated phenolics such as forsythin and forsythoside B were classified as a distinct chemotype due to the presence of sugar moieties that markedly alter physicochemical behavior, reducing membrane permeability, increasing hydrophilicity, and requiring metabolic activation. These can diminish antimicrobial potentiation compared with their aglycone counterparts [93]. In our dataset, both glycosides yielded an FICI of approximately 1 (no interaction), suggesting that glycosylation may impair their ability to synergize with antibiotics. However, as only single determinations were available for each, these findings should be considered preliminary and hypothesis-generating rather than definitive.
The novelty of this work was the incorporation of FICI variability into the assessment of clinical relevance. While median FICI values provide a measure of central tendency, they can mask high dispersion and strain-specific differences. Our analysis revealed that combinations such as meropenem + quercetin, while often synergistic, displayed considerable variability across isolates, whereas polymyxin B + resveratrol demonstrated both low median FICI and minimal dispersion, making it a stronger candidate for predictable clinical performance. This emphasizes the need to consider reproducibility and stability of effects alongside potency when prioritizing combinations for further development.
The broader implications of our findings are twofold. First, they provide a rational framework for prioritizing natural compounds as antibiotic adjuvants, emphasizing those with structural features predictive of consistent synergy [94]. This is particularly relevant for carbapenem-resistant K. pneumoniae, where treatment options are severely limited [95]. Second, the study highlights the value of integrating quantitative synergy metrics with SAR analysis to generate mechanistically coherent hypotheses for future drug development. Moving forward, research should focus on confirming these in vitro findings in animal infection models, elucidating the molecular mechanisms underlying synergy, and exploring pharmacokinetic and pharmacodynamic considerations [96,97]. Additionally, expanding the dataset to include a broader range of phenolic compounds and antibiotic classes will improve statistical power and generalizability.
In summary, this work demonstrates that the potentiation of antibiotics by natural phenolic compounds is structurally determined and highly context-dependent. Flavonoids, particularly those with catechol/gallol motifs, emerge as the most promising partners for restoring carbapenem activity, while Michael acceptor chemotypes warrant caution due to their inconsistent effects. These results lay the groundwork for developing targeted combination therapies that leverage natural products to address the pressing challenge of MDR K. pneumoniae and other Gram-negative pathogens.
To translate these promising in vitro interactions into clinical applications, however, it is essential to address the pharmacological limitations of phenolic compounds. Despite their biological potency, many phenolics exhibit poor solubility, limited oral bioavailability, extensive metabolism into inactive forms, and unpredictable pharmacokinetic/pharmacodynamic profiles [98,99,100,101,102,103,104,105]. Overcoming these challenges will require advanced formulation strategies such as nanocarriers, liposomal formulations, and inclusion complexes, which can be employed to improve tissue penetration, ensure targeted delivery, and maintain sustained therapeutic drug levels [106,107,108,109,110]. Additional pharmaceutical solutions—such as co-crystallization, amorphous solid dispersions, nanocapsules, and matrix-based systems—further address bioavailability challenges [111,112,113,114]. The appeal of phenolic compounds is also supported by favorable safety profiles derived from toxicological data, traditional medicinal use, and their marketing as dietary supplements. Nonetheless, issues persist around standardization, dose uniformity, long-term safety, and potential effects on the gut microbiota [115,116,117,118]. Importantly, the ability of low-dose phenolics to restore antibiotic susceptibility and induce synergy supports their cautious advancement in translational research [21]. Still, applying in vitro synergy findings to clinical contexts remains challenging due to pathogen heterogeneity, complex resistance mechanisms, and host-dependent pharmacokinetics [119].
This study has several limitations that should be considered when interpreting the findings. First, the pooled data were derived from in vitro studies conducted under heterogeneous experimental conditions, including variation in media composition, inoculum size, antibiotic concentration ranges, and methodological approaches to FICI determination. Such variability may influence synergy estimates and limit direct comparability across studies, although the stratification by chemotype and antibiotic class was designed to partially mitigate this effect [120].
A potential limitation of our chemotype-based analysis is that individual phenolic compounds often possess multiple structural motifs (e.g., flavonoids simultaneously containing catechol or gallate groups), which may lead to overlapping classification. This structural overlap could contribute to interaction effects that inflate the observed odds ratios for synergy when motifs co-occur, making it difficult to attribute potentiation to a single chemotype. To mitigate this, we stratified compounds using a binary flagging approach and interpreted results in terms of mechanistic enrichment rather than exclusive causality. However, the synergistic effects observed for catechol- and gallate-bearing flavonoids likely reflect combined contributions of both the core flavonoid scaffold and the redox-active functional groups. Therefore, chemotype-specific odds ratios should be interpreted as indicators of association rather than isolated causative determinants, and future multivariate or machine learning models will be required to deconvolute these overlapping effects.
The lack of in vivo validation or pharmacodynamic modeling limits the translational relevance of the observed interactions, particularly given the known pharmacokinetic constraints of phenolic compounds mentioned above.
Finally, the potential for publication bias cannot be excluded. Studies reporting synergistic outcomes are more likely to be published, while neutral or antagonistic findings may be underrepresented in the literature. Although our analysis included combinations across a range of outcomes, including antagonism and no interaction, the reported rates of synergy may overestimate the true clinical potential [121].
Despite these limitations, the study provides a robust framework for identifying structural predictors of antibiotic potentiation and prioritizing phenolic compounds for further investigation in translational models.
5. Conclusions
This pooled analysis of 216 in vitro assays shows that synergy between phenolic compounds and antibiotics against multidrug-resistant Klebsiella pneumoniae is structure- and context-dependent. The most reproducible effect was observed for polymyxin B + resveratrol, which showed a low median FICI and minimal dispersion. For carbapenems, meropenem + catechol/gallol-bearing flavonoids (e.g., quercetin, kaempferol) outperformed non-flavonoids, whereas curcumin displayed inconsistent or antagonistic interactions. Because synergy magnitude alone can be misleading, low dispersion should guide prioritization for in vivo testing. Future work should validate top low-dispersion pairs in animal models and assess PK/PD and safety to enable clinical translation.
Author Contributions
Conceptualization, S.N. and A.Z.; methodology, A.Z. and C.A.; formal analysis, S.N. and V.-P.O.; data curation, V.-P.O., O.T.O. and C.P.; writing—original draft preparation, V.-P.O. and C.P.; writing—review and editing, A.Z. and O.T.O.; visualization, C.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Xu, P.; Mao, Y.; Chen, Q.; Luo, X.; Lin, R.; Zheng, C. Clinical Characteristics and Independent Risk Factors for Multidrug-Resistant Klebsiella Pneumoniae Bloodstream Infections: A Retrospective Analysis from China. Infect. Drug Resist. 2025, 18, 3993–4006. [Google Scholar] [CrossRef]
- Santella, B.; Boccella, M.; Folliero, V.; Iervolino, D.; Pagliano, P.; Fortino, L.; Serio, B.; Vozzella, E.A.; Schiavo, L.; Galdiero, M.; et al. Antimicrobial Susceptibility Profiles of Klebsiella Pneumoniae Strains Collected from Clinical Samples in a Hospital in Southern Italy. Can. J. Infect. Dis. Med. Microbiol. = J. Can. Mal. Infect. Microbiol. Méd. 2024, 2024, 5548434. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kumar, S.; Zhang, L.; Wu, H.; Wu, H. Characteristics of Antibiotic Resistance Mechanisms and Genes of Klebsiella Pneumoniae. Open Med. 2023, 18, 20230707. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Xu, C.; Zhu, Z.; Zhang, C.; Qin, C.; Liu, J.; Kong, X.; Zhu, Z.; Xu, W.; Zhu, M. Multidrug-Resistant Klebsiella Pneumoniae Coinfection with Multiple Microbes: A Retrospective Study on Its Risk Factors and Clinical Outcomes. mSystems 2025, 10, e01757-24. [Google Scholar] [CrossRef] [PubMed]
- Willy, C.; Bröcker, F. Health Economic Significance of Antimicrobial Resistance. Bundesgesundheitsbl.-Gesundheitsforsch.-Gesundheitsschutz 2025, 68, 584–592. [Google Scholar] [CrossRef]
- Yi, H.; Yuan, G.; Li, S.; Xu, X.; Guan, Y.; Zhang, L.; Yan, Y. Drug Combinations to Prevent Antimicrobial Resistance: Various Correlations and Laws, and Their Verifications, Thus Proposing Some Principles and a Preliminary Scheme. Antibiotics 2022, 11, 1279. [Google Scholar] [CrossRef]
- Jacobs, D.M.; Safir, M.C.; Huang, D.; Minhaj, F.; Parker, A.; Rao, G.G. Triple Combination Antibiotic Therapy for Carbapenemase-Producing Klebsiella Pneumoniae: A Systematic Review. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 76. [Google Scholar] [CrossRef]
- Marino, A.; Maraolo, A.E.; Mazzitelli, M.; Oliva, A.; Geremia, N.; De Vito, A.; Gullotta, C.; Scaglione, V.; Vania, E.; Lo Menzo, S.; et al. Head-to-Head: Meropenem/Vaborbactam versus Ceftazidime/Avibactam in ICUs Patients with KPC-Producing K. Pneumoniae Infections–Results from a Retrospective Multicentre Study. Infection 2025. [Google Scholar] [CrossRef]
- Mott, M.P.; Moreira, N.K.; Collar, G.; Scalco, S.; Martins, J.B.; Vieira, P.; Pereira, D.; de Oliveira, G.S.; Martins, A.F.; Barth, A.; et al. First Report of Ceftazidime-Avibactam Resistance in Non-Mutated BlaKPC-3 K. Pneumoniae Recovered from Patients with No Prior Treatment, in Latin America. Diagn. Microbiol. Infect. Dis. 2025, 113, 116965. [Google Scholar] [CrossRef]
- Tang, B.; Meng, T.; Tian, L.; Zhong, M.; Dai, Y.; Tian, R.; Pan, T.; Sun, J.; Tan, R.; Wang, X.; et al. Within-Host Resistance Evolution of ST15 Klebsiella Pneumoniae in an ICU Immunosuppressed Patient under Antibiotic Pressure of Polymyxins, Ceftazidime-Avibactam, and Meropenem. Int. J. Antimicrob. Agents 2025, 66, 107554. [Google Scholar] [CrossRef]
- Gaibani, P.; Lombardo, D.; Bussini, L.; Bovo, F.; Munari, B.; Giannella, M.; Bartoletti, M.; Viale, P.; Lazzarotto, T.; Ambretti, S. Epidemiology of Meropenem/Vaborbactam Resistance in KPC-Producing Klebsiella Pneumoniae Causing Bloodstream Infections in Northern Italy, 2018. Antibiotics 2021, 10, 536. [Google Scholar] [CrossRef]
- Halder, G.; Chaudhuri, B.N.; Veeraraghavan, B.; Denny, P.; Dutta, P.; Chakraborty, M.; Khan, U.R.; Ganguly, S.S.; Mandal, S.; Upadhyaya, Y.P.; et al. Antimicrobial Resistance and Phylogenetic Lineages of KPC-2-Producing Blood-Borne Klebsiella Pneumoniae Subsp. Pneumoniae from Kolkata, India during 2015–2024: Emergence of Klebsiella Pneumoniae Subsp. Pneumoniae with BlaKPC-2, BlaNDM, and BlaOXA-48-like Triple Carbapenemases. Microbiol. Spectr. 2025, 13, e00126-25. [Google Scholar] [CrossRef]
- Brüssow, H. The Antibiotic Resistance Crisis and the Development of New Antibiotics. Microb. Biotechnol. 2024, 17, e14510. [Google Scholar] [CrossRef]
- Dhanda, G.; Acharya, Y.; Haldar, J. Antibiotic Adjuvants: A Versatile Approach to Combat Antibiotic Resistance. ACS Omega 2023, 8, 10757. [Google Scholar] [CrossRef] [PubMed]
- D’andrea, M.M.; Fraziano, M.; Thaller, M.C.; Rossolini, G.M. The Urgent Need for Novel Antimicrobial Agents and Strategies to Fight Antibiotic Resistance. Antibiotics 2019, 8, 254. [Google Scholar] [CrossRef] [PubMed]
- Worthington, R.J.; Melander, C. Combination Approaches to Combat Multi-Drug Resistant Bacteria. Trends Biotechnol. 2013, 31, 177. [Google Scholar] [CrossRef] [PubMed]
- Duda-Madej, A.; Viscardi, S.; Niezgódka, P.; Szewczyk, W.; Wińska, K. The Impact of Plant-Derived Polyphenols on Combating Efflux-Mediated Antibiotic Resistance. Int. J. Mol. Sci. 2025, 26, 4030. [Google Scholar] [CrossRef]
- Mandal, S.M.; Dias, R.O.; Franco, O.L. Phenolic Compounds in Antimicrobial Therapy. J. Med. Food 2017, 20, 1031–1038. [Google Scholar] [CrossRef]
- Saifi, S.; Ashraf, A.; Hasan, G.M.; Shamsi, A.; Hassan, M.I. Insights into the Preventive Actions of Natural Compounds against Klebsiella Pneumoniae Infections and Drug Resistance. Fitoterapia 2024, 173, 105811. [Google Scholar] [CrossRef]
- Luna-Pineda, V.M.; Rodríguez-Martínez, G.; Salazar-García, M.; Romo-Castillo, M. Plant-Origin Components: New Players to Combat Antibiotic Resistance in Klebsiella Pneumoniae. Int. J. Mol. Sci. 2024, 25, 2134. [Google Scholar] [CrossRef]
- Santos, C.A.; Lima, E.M.F.; de Franco, B.D.G.M.; Pinto, U.M. Exploring Phenolic Compounds as Quorum Sensing Inhibitors in Foodborne Bacteria. Front. Microbiol. 2021, 12, 735931. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.M.F.; Winans, S.C.; Pinto, U.M. Quorum Sensing Interference by Phenolic Compounds—A Matter of Bacterial Misunderstanding. Heliyon 2023, 9, e17657. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, K.; Rajabi, E.; Varpaei, H.A.; Iranzadasl, M.; Khodaparast, S.; Salehi, M. Thymol and Carvacrol against Klebsiella: Anti-Bacterial, Anti-Biofilm, and Synergistic Activities—A Systematic Review. Front. Pharmacol. 2024, 15, 1487083. [Google Scholar] [CrossRef] [PubMed]
- Karasu, E.; Nilsson, B.; Köhl, J.; Lambris, J.D.; Huber-Lang, M. Targeting Complement Pathways in Polytrauma- and Sepsis-Induced Multiple-Organ Dysfunction. Front. Immunol. 2019, 10, 543, Erratum in Front. Immunol. 2019, 10, 994. [Google Scholar] [CrossRef]
- Zhou, Z.; Duan, Y.; Li, Y.; Zhang, P.; Li, Q.; Yu, L.; Han, C.; Huo, J.; Chen, W.; Xiao, Y. CYP98A Monooxygenases: A Key Enzyme Family in Plant Phenolic Compound Biosynthesis. Hortic. Res. 2025, 12, uhaf074. [Google Scholar] [CrossRef]
- Guo, P.; Li, Z.; Cai, T.; Guo, D.; Yang, B.; Zhang, C.; Shan, Z.; Wang, X.; Peng, X.; Liu, G.; et al. Inhibitory Effect and Mechanism of Oregano Essential Oil on Listeria Monocytogenes Cells, Toxins and Biofilms. Microb. Pathog. 2024, 194, 106801. [Google Scholar] [CrossRef]
- Shih, Y.H.; Tsai, P.J.; Chen, Y.L.; Pranata, R.; Chen, R.J. Assessment of the Antibacterial Mechanism of Pterostilbene against Bacillus Cereus through Apoptosis-like Cell Death and Evaluation of Its Beneficial Effects on the Gut Microbiota. J. Agric. Food Chem. 2021, 69, 12219–12229. [Google Scholar] [CrossRef]
- Oktyabrsky, O.N.; Bezmaternykh, K.V.; Smirnova, G.V.; Tyulenev, A.V. Effect of Resveratrol and Quercetin on the Susceptibility of Escherichia Coli to Antibiotics. World J. Microbiol. Biotechnol. 2020, 36, 167. [Google Scholar] [CrossRef]
- Surendran Nair, M.; Ma, F.; Lau, P.; Upadhyaya, I.; Venkitanarayanan, K. Inactivation of Escherichia Coli O157:H7 in Apple Cider by Resveratrol and Naringenin. Food Microbiol. 2020, 86, 103327. [Google Scholar] [CrossRef]
- Goswami, S.; Ghosh, M.; Roy, S.; Basak, S.; Bhattacharjee, S. Quercetin Combined with Ciprofloxacin and Gentamicin Inhibits Biofilm Formation and Virulence in Staphylococcus Aureus. Microb. Pathog. 2025, 200, 107297. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, Y.; Li, B.; Zhang, Z.; Qin, G.; Chen, T.; Tian, S. Molecular Mechanisms Underlying Multi-Level Defense Responses of Horticultural Crops to Fungal Pathogens. Hortic. Res. 2022, 9, uhac066. [Google Scholar] [CrossRef] [PubMed]
- Surjadinata, B.B.; Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. UVA, UVB and UVC Light Enhances the Biosynthesis of Phenolic Antioxidants in Fresh-Cut Carrot through a Synergistic Effect with Wounding. Molecules 2017, 22, 668. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An Overview of Plant Phenolic Compounds and Their Importance in Human Nutrition and Management of Type 2 Diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef]
- Safe, S.; Jayaraman, A.; Chapkin, R.S.; Howard, M.; Mohankumar, K.; Shrestha, R. Flavonoids: Structure–Function and Mechanisms of Action and Opportunities for Drug Development. Toxicol. Res. 2021, 37, 147. [Google Scholar] [CrossRef]
- Botten, D.; Fugallo, G.; Fraternali, F.; Molteni, C. Structural Properties of Green Tea Catechins. J. Phys. Chem. B 2015, 119, 12860–12867. [Google Scholar] [CrossRef]
- Varga, K.; Paszternák, A.; Kovács, V.; Guczogi, A.; Sikur, N.; Patakfalvi, D.; Bagaméry, F.; Szökő, É.; Tábi, T. Differential Cytoprotective Effect of Resveratrol and Its Derivatives: Focus on Antioxidant and Autophagy-Inducing Effects. Int. J. Mol. Sci. 2024, 25, 11274. [Google Scholar] [CrossRef]
- Kashi, M.; Farahani, A.; Ahmadi, A.; Shariati, A.; Akbari, M. Antibacterial and Antibiofilm Efficacy of Eugenol, Carvacrol, and Cinnamaldehyde against Colistin-Resistant Klebsiella Pneumoniae. Mol. Biol. Rep. 2025, 52, 480. [Google Scholar] [CrossRef]
- Rochin-Medina, J.J.; Mendoza-Lopez, I.A.; Castro-Del Campo, N.; Bastidas-Bastidas, P.J.; Ramirez, K. Activity of Plant Essential Oils against Clinically and Environmentally Isolated Salmonella Enterica Serotypes: In Vitro Assays and Molecular Docking. Lett. Appl. Microbiol. 2023, 76, ovad045. [Google Scholar] [CrossRef]
- Moreno-Gámez, S.; Hochberg, M.E.; van Doorn, G.S. Quorum Sensing as a Mechanism to Harness the Wisdom of the Crowds. Nat. Commun. 2023, 14, 3415. [Google Scholar] [CrossRef]
- Haque, S.; Yadav, D.K.; Bisht, S.C.; Yadav, N.; Singh, V.; Dubey, K.K.; Jawed, A.; Wahid, M.; Dar, S.A. Quorum Sensing Pathways in Gram-Positive and -Negative Bacteria: Potential of Their Interruption in Abating Drug Resistance. J. Chemother. 2019, 31, 161–187. [Google Scholar] [CrossRef]
- Datta, S.; Singh, V.; Nag, S.; Roy, D.N. Carvacrol, a Monoterpenoid, Binds Quorum Sensing Proteins (LasI and LasR) and Swarming Motility Protein BswR of Pseudomonas Aeruginosa, Resulting in Loss of Pathogenicity: An in Silico Approach. Can. J. Microbiol. 2025, 71, 1–15. [Google Scholar] [CrossRef]
- Gao, X.; Gao, X.; Hua, Z.; Alhomrani, M.; Shi, C.; Lin, L.; Zhu, Y. Exploring Inhibition of Listeria Monocytogenes Biofilm by Carvacrol Based on Action to Quorum Sensing. Food Biosci. 2025, 63, 105790. [Google Scholar] [CrossRef]
- Salim, S.A.; Mohan, M.S.; Ranganathan, S.; Parasuraman, P.; Lee, J.K.; Ramatchandirane, M.; Suchiang, K.; Busi, S. Derrisisoflavone-B Interferes with AHL-Mediated Quorum Sensing of Pseudomonas Aeruginosa and Decreased Pathogenicity in Caenorhabditis Elegans Infection Model. Microb. Pathog. 2025, 206, 107738. [Google Scholar] [CrossRef] [PubMed]
- Frikha, F.; Jardak, M.; Aifa, S.; Mnif, S. A Novel Perspective on Eugenol as a Natural Anti-Quorum Sensing Molecule against Serratia sp. Microb. Pathog. 2024, 189, 106576. [Google Scholar] [CrossRef]
- Allahyari, H.; Shamsini, L.; Zamani, H. Dual Encapsulation of Curcumin and Ciprofloxacin in Chitosan Nanoparticles Attenuates Pseudomonas Aeruginosa Virulence, Elastinolytic Potential and Quorum Sensing Genes. Microb. Pathog. 2025, 202, 107438. [Google Scholar] [CrossRef]
- Deng, J.; Yuan, Y.; Wu, Y.; Wen, F.; Yang, X.; Gou, S.; Chu, Y.; Zhao, K. Isovanillin Decreases the Virulence Regulated by the Quorum Sensing System of Pseudomonas Aeruginosa. Microb. Pathog. 2024, 196, 107010. [Google Scholar] [CrossRef]
- Pandey, P.; Rao, L.; Shekhar, B.R.; Das, D.K.; Vavilala, S.L. Molecular Insights into Flavone-Mediated Quorum Sensing Interference: A Novel Strategy against Serratia Marcescens Biofilm-Induced Antibiotic Resistance. Chem. Biol. Interact. 2024, 396, 111027. [Google Scholar] [CrossRef]
- Dey, P.; De, R.; Parai, D.; Hossain, S.T.; Mukherjee, S.K. Enhanced Antimicrobial Activity of Naringin-Ciprofloxacin Combination against Pseudomonas Aeruginosa PAO1: Unveiling Quorum-Sensing Mediated Molecular Mechanisms in Biofilm Formation and Virulence. Microbe 2024, 5, 100171. [Google Scholar] [CrossRef]
- Wijesundara, N.M.; Lee, S.F.; Rupasinghe, H.P.V. Carvacrol Inhibits Streptococcus Pyogenes Biofilms by Suppressing the Expression of Genes Associated with Quorum-Sensing and Reducing Cell Surface Hydrophobicity. Microb. Pathog. 2022, 169, 105684. [Google Scholar] [CrossRef]
- Leitão, M.M.; Gonçalves, A.S.C.; Sousa, S.F.; Borges, F.; Simões, M.; Borges, A. Two Cinnamic Acid Derivatives as Inhibitors of Pseudomonas Aeruginosa Las and Pqs Quorum-Sensing Systems: Impact on Biofilm Formation and Virulence Factors. Biomed. Pharmacother. 2025, 187, 118090. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; He, B.; Li, Z.P.; Zhong, Q.; Liu, Y.C.; Zhang, H.Y.; Li, Y.; Yan, H.L.; Hu, Y.L.; Zheng, Z.J.; et al. Rutin Synergizes with Colistin to Eradicate Salmonellosis in Mice by Enhancing the Efficacy and Reducing the Toxicity. J. Agric. Food Chem. 2025, 73, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Elshimy, R.; El-Shiekh, R.A.; Okba, M.M.; Ashour, R.M.S.; Ibrahim, M.A.; Hassanen, E.I.; Aboul-Ella, H.; Ali, M.E. Unveiling the Antimicrobial, Antivirulence, and Wound-Healing Accelerating Potentials of Resveratrol against Carbapenem-Resistant Pseudomonas Aeruginosa (CRPA)-Septic Wound in a Murine Model. Inflammopharmacology 2024, 33, 401. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Zhu, X.; Gao, X.J.; Yang, H.; Li, H.; Du, Y.; Gao, J.; Chen, Z.; Dong, H.; Wang, B.; et al. Kaempferol Mitigates Sepsis-Induced Acute Lung Injury by Modulating the SphK1/S1P/S1PR1/MLC2 Signaling Pathway to Restore the Integrity of the Pulmonary Endothelial Cell Barrier. Chem. Biol. Interact. 2024, 398, 111085. [Google Scholar] [CrossRef]
- Li, Z.; Yu, Y.; Bu, Y.; Liu, C.; Liu, E.; Jin, J.; Chen, G.; Li, C.; Wang, H.; Li, H.; et al. Targeting Macrophagic RasGRP1 with Catechin Hydrate Ameliorates Sepsis-Induced Multiorgan Dysfunction. Phytomedicine 2024, 130, 155733. [Google Scholar] [CrossRef]
- Wei, H.; Xia, D.; Li, L.; Liang, L.; Ning, L.; Gan, C.; Wu, Y. Baicalin Modulates Glycolysis via the PKC/Raf/MEK/ERK and PI3K/AKT Signaling Pathways to Attenuate IFN-I-Induced Neutrophil NETosis. Mediat. Inflamm. 2025, 2025, 8822728. [Google Scholar] [CrossRef]
- Nayak, S.P.R.R.; Boopathi, S.; Priya, P.S.; Pasupuleti, M.; Pachaiappan, R.; Almutairi, B.O.; Arokiyaraj, S.; Arockiaraj, J. Luteolin, a Promising Quorum Quencher Mitigates Virulence Factors Production in Pseudomonas Aeruginosa-In Vitro and In Vivoapproach. Microb. Pathog. 2023, 180, 106123. [Google Scholar] [CrossRef]
- Gu, M.; Pang, Z. Luteolin Inhibits Inflammation and M1 Macrophage Polarization in the Treatment of Pseudomonas Aeruginosa-Induced Acute Pneumonia through Suppressing EGFR/PI3K/AKT/NF-ΚB and EGFR/ERK/AP-1 Signaling Pathways. Phytomedicine 2025, 141, 156663. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, T.; Liu, J.; Gu, L. Vitexin Attenuates Lipopolysaccharide-Induced Acute Lung Injury by Controlling the Nrf2 Pathway. PLoS ONE 2018, 13, e0196405. [Google Scholar] [CrossRef]
- Cai, W.; Fu, Y.; Zhang, W.; Chen, X.; Zhao, J.; Song, W.; Li, Y.; Huang, Y.; Wu, Z.; Sun, R.; et al. Synergistic Effects of Baicalein with Cefotaxime against Klebsiella Pneumoniae through Inhibiting CTX-M-1 Gene Expression. BMC Microbiol. 2016, 16, 181. [Google Scholar] [CrossRef]
- Dhara, L.; Tripathi, A. Cinnamaldehyde: A Compound with Antimicrobial and Synergistic Activity against ESBL-Producing Quinolone-Resistant Pathogenic Enterobacteriaceae. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 65–73. [Google Scholar] [CrossRef]
- Köse, E.O. In Vitro Activity of Carvacrol in Combination with Meropenem against Carbapenem-Resistant Klebsiella Pneumoniae. Folia Microbiol. 2022, 67, 143–156. [Google Scholar] [CrossRef]
- Gülen, D.; Şafak, B.; Erdal, B.; Günaydın, B. Curcumin-Meropenem Synergy in Carbapenem Resistant Klebsiella Pneumoniae Curcumin-Meropenem Synergy. Iran. J. Microbiol. 2021, 13, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yu, J.; Shen, X.; Cao, X.; Zhan, Q.; Guo, Y.; Yu, F. Resveratrol Enhances the Antimicrobial Effect of Polymyxin B on Klebsiella Pneumoniae and Escherichia Coli Isolates with Polymyxin B Resistance. BMC Microbiol. 2020, 20, 306. [Google Scholar] [CrossRef] [PubMed]
- Buchmann, D.; Schultze, N.; Borchardt, J.; Böttcher, I.; Schaufler, K.; Guenther, S. Synergistic Antimicrobial Activities of Epigallocatechin Gallate, Myricetin, Daidzein, Gallic Acid, Epicatechin, 3-hydroxy-6-methoxyflavone and Genistein Combined with Antibiotics against ESKAPE Pathogens. J. Appl. Microbiol. 2022, 132, 949–963. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, M.; Behera, D.U.; Sahoo, R.K.; Sahoo, S.; Dey, S.; Subudhi, E. Synergistic Action of 6-Gingerol as an Adjuvant to Colistin for Susceptibility Enhancement in Multidrug-Resistant Klebsiella Pneumoniae Isolates. RSC Adv. 2024, 14, 7779–7785. [Google Scholar] [CrossRef]
- Kong, J.; Wang, Y.; Yao, Z.; Lin, Y.; Zhang, Y.; Han, Y.; Zhou, T.; Ye, J.; Cao, J. Eugenol Works Synergistically with Colistin against Colistin-Resistant Pseudomonas Aeruginosa and Klebsiella Pneumoniae Isolates by Enhancing Membrane Permeability. Microbiol. Spectr. 2023, 11, e03666-22. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, M.; Guo, W.; Yao, Z.; Du, X.; Chen, L.; Sun, Y.; Shi, S.; Cao, J.; Zhou, T. The Antibacterial Activity of Kaempferol Combined with Colistin against Colistin-Resistant Gram-Negative Bacteria. Microbiol. Spectr. 2022, 10, e02265-22. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, A.; Chen, Q.; Hu, Z. Synergistic Antibacterial Activity of EGCGcombined with Imipenem against Planktonic Carbapenem-Resistant Klebsiella Pneumoniae and the Evaluation of Independent Antibiofilm Activity of EGCG. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Tan, S.; Gao, J.; Li, Q.; Guo, T.; Dong, X.; Bai, X.; Yang, J.; Hao, S.; He, F. Synergistic Effect of Chlorogenic Acid and Levofloxacin against Klebsiella Pneumonia Infection in Vitro and in Vivo. Sci. Rep. 2020, 10, 20013, Erratum in Sci. Rep. 2025, 15, 7824. [Google Scholar] [CrossRef]
- Aydemir, Ö.; Ormanoğlu, G.; Ayhancı, T.; Zengin, M.; Köroğlu, M. Investigation of in Vitro Efficacy of Quercetin-Meropenem Combination in Carbapenemase-Producing Klebsiella Pneumoniae Isolates. J. Infect. Dev. Ctries. 2023, 17, 1325–1329. [Google Scholar] [CrossRef]
- Qin, X.; Wu, Y.; Zhao, Y.; Qin, S.; Ji, Q.; Jia, J.; Huo, M.; Zhao, X.; Ma, Q.; Wang, X.; et al. Revealing Active Constituents within Traditional Chinese Medicine Used for Treating Bacterial Pneumonia, with Emphasis on the Mechanism of Baicalein against Multi-Drug Resistant Klebsiella Pneumoniae. J. Ethnopharmacol. 2024, 321, 117488. [Google Scholar] [CrossRef]
- Odds, F.C. Synergy, Antagonism, and What the Chequerboard Puts between Them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef] [PubMed]
- Doern, C.D. When Does 2 plus 2 Equal 5? A Review of Antimicrobial Synergy Testing. J. Clin. Microbiol. 2014, 52, 4124–4128. [Google Scholar] [CrossRef] [PubMed]
- Donkor, M.N.; Donkor, A.M.; Mosobil, R. Combination Therapy: Synergism among Three Plant Extracts against Selected Pathogens. BMC Res. Notes 2023, 16, 83. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.E.; Theuretzbacher, U.; Mouton, J.W. Use of Old Antibiotics Now and in the Future from a Pharmacokinetic/Pharmacodynamic Perspective. Clin. Microbiol. Infect. 2015, 21, 881–885. [Google Scholar] [CrossRef]
- Ferraz, M.P. Antimicrobial Resistance: The Impact from and on Society According to One Health Approach. Societies 2024, 14, 187. [Google Scholar] [CrossRef]
- Wang, S.; Yao, J.; Zhou, B.; Yang, J.; Chaudry, M.T.; Wang, M.; Xiao, F.; Li, Y.; Yin, W. Bacteriostatic Effect of Quercetin as an Antibiotic Alternative in Vivo and Its Antibacterial Mechanism in Vitro. J. Food Prot. 2018, 81, 68–78. [Google Scholar] [CrossRef]
- Odabaş Köse, E.; Koyuncu Özyurt, Ö.; Bilmen, S.; Er, H.; Kilit, C.; Aydemir, E. Quercetin: Synergistic Interaction with Antibiotics against Colistin-Resistant Acinetobacter Baumannii. Antibiotics 2023, 12, 739. [Google Scholar] [CrossRef]
- Pal, A.; Tripathi, A. Quercetin Inhibits Carbapenemase and Efflux Pump Activities among Carbapenem-Resistant Gram-Negative Bacteria. J. Pathol. Microbiol. Immunol. 2020, 128, 251–259. [Google Scholar] [CrossRef]
- Pal, A.; Tripathi, A. Demonstration of Bactericidal and Synergistic Activity of Quercetin with Meropenem among Pathogenic Carbapenem Resistant Escherichia Coli and Klebsiella Pneumoniae. Microb. Pathog. 2020, 143, 104120. [Google Scholar] [CrossRef]
- Güran, M.; Çakıral, K.; Teralı, K.; Kandemir, T.; Sanlıtürk, G.; Öcal, M.M.; Nagiyev, T.; Köksal, F. Meropenem in Combination with Baicalein Exhibits Synergism against Extensively Drug Resistant and Pan-Drug-Resistant Acinetobacter Baumannii Clinical Isolates in Vitro. Pathog. Dis. 2023, 81, ftad007. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, M.; Shang, Z.; Xu, J.; Chen, X.; Zhu, Z.; Wang, W.; Wei, X.; Zhou, X.; Bai, Y.; et al. Research Progress on the Antibacterial Activity of Natural Flavonoids. Antibiotics 2025, 14, 334. [Google Scholar] [CrossRef]
- Tarahovsky, Y.S.; Kim, Y.A.; Yagolnik, E.A.; Muzafarov, E.N. Flavonoid–Membrane Interactions: Involvement of Flavonoid–Metal Complexes in Raft Signaling. Biochim. Biophys. Acta-Biomembr. 2014, 1838, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Abiko, Y.; Washio, J.; Luo, Y.; Zhang, L.; Takahashi, N. Green Tea-Derived Epigallocatechin Gallate Inhibits Acid Production and Promotes the Aggregation of Streptococcus Mutans and Non-Mutans Streptococci. Caries Res. 2021, 55, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Morão, L.G.; Polaquini, C.R.; Kopacz, M.; Torrezan, G.S.; Ayusso, G.M.; Dilarri, G.; Cavalca, L.B.; Zielińska, A.; Scheffers, D.J.; Regasini, L.O.; et al. A Simplified Curcumin Targets the Membrane of Bacillus Subtilis. Microbiologyopen 2019, 8, e00683. [Google Scholar] [CrossRef] [PubMed]
- Jha, N.S.; Mishra, S.; Jha, S.K.; Surolia, A. Antioxidant Activity and Electrochemical Elucidation of the Enigmatic Redox Behavior of Curcumin and Its Structurally Modified Analogues. Electrochim. Acta 2015, 151, 574–583. [Google Scholar] [CrossRef]
- Seixas, A.F.; Quendera, A.P.; Sousa, J.P.; Silva, A.F.Q.; Arraiano, C.M.; Andrade, J.M. Bacterial Response to Oxidative Stress and RNA Oxidation. Front. Genet. 2022, 12, 821535. [Google Scholar] [CrossRef]
- Gan, C.; Langa, E.; Wang, G.; Van Bambeke, F.; Ballestero, D.; Pino-Otín, M.R. Mechanisms of Action and Resistance Prevention of Synergistic Thymol and Carvacrol Combinations with Antibiotics in Staphylococcus Aureus and Acinetobacter Baumannii. Nat. Prod. Bioprospect. 2025, 15, 36. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Lin, Y.; Cao, J.; Xu, C.; Chen, L.; Wang, Y.; Sun, Y.; Zheng, X.; Liu, Y.; et al. Resveratrol Increases Sensitivity of Clinical Colistin-Resistant Pseudomonas Aeruginosa to Colistin In Vitro and In Vivo. Microbiol. Spectr. 2023, 11, e01992-22. [Google Scholar] [CrossRef]
- Prava Rout, B.; Behera, B.; Kumar Sahu, K.; Praharaj, I.; Otta, S. An Overview of Colistin Resistance: A Breach in Last Line Defense. Med. J. Armed Forces India 2023, 79, 516. [Google Scholar] [CrossRef]
- Andrade, F.F.; Silva, D.; Rodrigues, A.; Pina-Vaz, C. Colistin Update on Its Mechanism of Action and Resistance, Present and Future Challenges. Microorganisms 2020, 8, 1716. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.H.; Bijsman, M.N.C.P.; Van Gameren, Y.; Cnossen, E.P.J.; De Vries, J.H.M.; Katan, M.B. The Sugar Moiety Is a Major Determinant of the Absorption of Dietary Flavonoid Glycosides in Man. Free Radic. Res. 1999, 31, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Stan, D.; Enciu, A.M.; Mateescu, A.L.; Ion, A.C.; Brezeanu, A.C.; Stan, D.; Tanase, C. Natural Compounds With Antimicrobial and Antiviral Effect and Nanocarriers Used for Their Transportation. Front. Pharmacol. 2021, 12, 723233. [Google Scholar] [CrossRef] [PubMed]
- Abhinand, K.; Menon, A.M.; Thomas, S.S.; Anil, A.B.; Parvathi Mohanan, P.C.; Arun, K.B.; Edison, L.K.; Babu, P.; Kumar, G.B.; Nair, B.G.; et al. Klebsiella Pneumoniae: Host Interactions, Virulence Mechanisms, and Novel Therapeutic Strategies. Microb. Pathog. 2025, 207, 107856. [Google Scholar] [CrossRef]
- Swearengen, J.R. Choosing the Right Animal Model for Infectious Disease Research. Anim. Model. Exp. Med. 2018, 1, 100. [Google Scholar] [CrossRef]
- Bissantz, C.; Zampaloni, C.; David-Pierson, P.; Dieppois, G.; Guenther, A.; Trauner, A.; Winther, L.; Stubbings, W. Translational PK/PD for the Development of Novel Antibiotics—A Drug Developer’s Perspective. Antibiotics 2024, 13, 72. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of Phenolic Compounds: A Review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxid. Med. Cell. Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.; Ma, Y.; Bai, L.; Li, Q.; Zhou, X.; Xu, P.; Li, X.; Xue, M. A UPLC-MS/MS Method Reveals the Pharmacokinetics and Metabolism Characteristics of Kaempferol in Rats under Hypoxia. Drug Metab. Pharmacokinet. 2022, 43, 100440. [Google Scholar] [CrossRef]
- Li, H.; Li, H.; Jiang, S.; Xu, J.; Cui, Y.; Wang, H.; Dai, L.; Lin, Y.; Zhang, J. Study of the Metabolism of Myricetin in Rat Urine, Plasma and Feces by Ultra-High-Performance Liquid Chromatography. Biomed. Chromatogr. 2022, 36, e5281. [Google Scholar] [CrossRef]
- Springer, M.; Moco, S. Resveratrol and Its Human Metabolites—Effects on Metabolic Health and Obesity. Nutrients 2019, 11, 143. [Google Scholar] [CrossRef]
- Zhang, B.; Dong, Y.; Yu, N.; Sun, Y.; Xing, Y.; Yang, F.; Yu, X.; Sun, W.; Sun, J.; Li, X.; et al. Intestinal Metabolism of Baicalein after Oral Administration in Mice: Pharmacokinetics and Mechanisms. J. Funct. Foods 2019, 54, 53–63. [Google Scholar] [CrossRef]
- Rodríguez-Gascón, A.; Solinís, M.Á.; Isla, A. The Role of PK/PD Analysis in the Development and Evaluation of Antimicrobials. Pharmaceutics 2021, 13, 833. [Google Scholar] [CrossRef] [PubMed]
- Alikhani, M.S.; Nazari, M.; Hatamkhani, S. Enhancing Antibiotic Therapy through Comprehensive Pharmacokinetic/Pharmacodynamic Principles. Front. Cell. Infect. Microbiol. 2025, 15, 1521091. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yang, Z.; Liu, H.; Man, J.; Oladejo, A.O.; Ibrahim, S.; Wang, S.; Hao, B. Novel Drug Delivery Systems: An Important Direction for Drug Innovation Research and Development. Pharmaceutics 2024, 16, 674. [Google Scholar] [CrossRef]
- Kapare, H.; Kanadje, S.; Bhole, R. Quercetin Nano-Formulations as a Potential Approach for Skin Cancer. Pharm. Nanotechnol. 2025, 13, 827–838. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, W.; Zhu, Y.; Zhu, H.; Ho, C.-T. Pharmacokinetic Profiles and Improvement of Resveratrol and Derived Stilbenes. J. Food Bioact. 2025, 30, 6–18. [Google Scholar] [CrossRef]
- Alanazi, A.Z.; Alqinyah, M.; Alhamed, A.S.; Mohammed, H.; Raish, M.; Aljerian, K.; Alsabhan, J.F.; Alhazzani, K. Cardioprotective Effects of Liposomal Resveratrol in Diabetic Rats: Unveiling Antioxidant and Anti-Inflammatory Benefits. Redox Rep. 2024, 29, 2416835. [Google Scholar] [CrossRef]
- Radeva, L.; Yordanov, Y.; Spassova, I.; Kovacheva, D.; Tibi, I.P.E.; Zaharieva, M.M.; Kaleva, M.; Najdenski, H.; Petrov, P.D.; Tzankova, V.; et al. Incorporation of Resveratrol-Hydroxypropyl-β-Cyclodextrin Complexes into Hydrogel Formulation for Wound Treatment. Gels 2024, 10, 396. [Google Scholar] [CrossRef]
- Furniturewalla, A.; Barve, K. Approaches to Overcome Bioavailability Inconsistencies of Epigallocatechin Gallate, a Powerful Anti-Oxidant in Green Tea. Food Chem. Adv. 2022, 1, 100037. [Google Scholar] [CrossRef]
- Abdi Syahputra, R.; Dalimunthe, A.; Utari, Z.D.; Halim, P.; Sukarno, M.A.; Zainalabidin, S.; Salim, E.; Gunawan, M.; Nurkolis, F.; Park, M.N.; et al. Nanotechnology and Flavonoids: Current Research and Future Perspectives on Cardiovascular Health. J. Funct. Foods 2024, 120, 106355. [Google Scholar] [CrossRef]
- Gao, J.; Fan, Y.; Lu, C.; Zhao, X.; He, X. The Baicalein Amorphous Solid Dispersion to Enhance the Dissolution and Bioavailability and Effects on Growth Performance, Meat Quality, Antioxidant Capacity and Intestinal Flora in Taihang Chickens. Poult. Sci. 2024, 103, 103768. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zheng, C.; Tian, F.; Xiao, Z.; Sun, Z.; Lu, L.; Dai, W.; Zhang, Q.; Mei, X. Improving the Dissolution Rate and Bioavailability of Curcumin via Co-Crystallization. Pharmaceuticals 2024, 17, 489. [Google Scholar] [CrossRef] [PubMed]
- Eseberri, I.; Trepiana, J.; Léniz, A.; Gómez-García, I.; Carr-Ugarte, H.; González, M.; Portillo, M.P. Variability in the Beneficial Effects of Phenolic Compounds: A Review. Nutrients 2022, 14, 1925. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Zhang, Q. The Potential Toxic Side Effects of Flavonoids. Biocell 2021, 46, 357–366. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Xiong, H.H.; Lin, S.Y.; Chen, L.L.; Ouyang, K.H.; Wang, W.J. The Interaction between Flavonoids and Intestinal Microbes: A Review. Foods 2023, 12, 320. [Google Scholar] [CrossRef]
- Tängdén, T.; Lundberg, C.V.; Friberg, L.E.; Huttner, A. How Preclinical Infection Models Help Define Antibiotic Doses in the Clinic. Int. J. Antimicrob. Agents 2020, 56, 106008. [Google Scholar] [CrossRef]
- Gajic, I.; Kabic, J.; Kekic, D.; Jovicevic, M.; Milenkovic, M.; Mitic Culafic, D.; Trudic, A.; Ranin, L.; Opavski, N. Antimicrobial Susceptibility Testing: A Comprehensive Review of Currently Used Methods. Antibiotics 2022, 11, 427. [Google Scholar] [CrossRef]
- Thornton, A.; Lee, P. Publication Bias in Meta-Analysis: Its Causes and Consequences. J. Clin. Epidemiol. 2000, 53, 207–216. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).