Nitrofurantoin–Aminoglycoside Synergy Against Common Uropathogens Evaluated by Disc Diffusion: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Analysis
2.2. Ethical Issues
3. Results
Relationship Between Inhibition Zone Diameter and Synergy
4. Discussion
4.1. Overview of Study Results
4.2. Comparison with Previous Synergy Studies
4.3. Clinical Implications
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMI | Amikacin |
| AMR | Antimicrobial resistance |
| DNA | Deoxyribonucleic acid |
| ESBL | Extended-spectrum beta-lactamases |
| EU | European Union |
| EUCAST | The European Committee on Antimicrobial Susceptibility Testing |
| GEN | Gentamicin |
| IQR | Interquartile range |
| McF | McFarland standard |
| MDR | Multidrug-resistant |
| mRNA | messenger RNA |
| MIC | Minimal inhibitory concentration |
| NIT | Nitrofurantoin |
| OHAMR | One Health Antimicrobial Resistance |
| RNA | Ribonucleic acid |
| TOB | Tobramycin |
| UPEC | Uropathogenic Escherichia coli |
| UTI | Urinary tract infection |
| WHO | World Health Organization |
Appendix A
| Species | ID | Inhibition Zone Diameter [mm] | Synergy (1 = Occurrence, 0 = Lack) | |||||
|---|---|---|---|---|---|---|---|---|
| NIT | AMI | GEN | TOB | NIT × AMI | NIT × GEN | NIT × TOB | ||
| Enterococcus faecalis | 01 | 19 | 20 | 18 | 19 | 1 | 0 | 1 |
| 02 | 20 | 18 | 16 | 18 | 1 | 0 | 1 | |
| 03 | 6 | 17 | 16 | 16 | 0 | 0 | 0 | |
| 04 | 12 | 17 | 7 | 10 | 1 | 0 | 0 | |
| 05 | 15 | 16 | 6 | 7 | 1 | 0 | 0 | |
| 06 | 20 | 9 | 10 | 10 | 0 | 0 | 0 | |
| 07 | 21 | 6 | 10 | 10 | 0 | 0 | 0 | |
| 08 | 11 | 15 | 6 | 7 | 1 | 0 | 0 | |
| 09 | 9 | 20 | 16 | 17 | 0 | 0 | 0 | |
| 10 | 20 | 20 | 20 | 18 | 0 | 0 | 0 | |
| 11 | 20 | 6 | 6 | 6 | 0 | 0 | 0 | |
| 12 | 21 | 6 | 6 | 6 | 0 | 0 | 0 | |
| 13 | 22 | 6 | 6 | 6 | 0 | 0 | 0 | |
| 14 | 11 | 17 | 16 | 14 | 1 | 0 | 0 | |
| 15 | 14 | 19 | 15 | 16 | 0 | 0 | 0 | |
| 16 | 21 | 6 | 10 | 10 | 1 | 1 | 1 | |
| 17 | 7 | 16 | 6 | 7 | 0 | 0 | 0 | |
| 18 | 21 | 18 | 9 | 9 | 1 | 1 | 1 | |
| 19 | 11 | 17 | 16 | 16 | 0 | 0 | 0 | |
| 20 | 22 | 9 | 13 | 13 | 1 | 1 | 1 | |
| 21 | 23 | 6 | 6 | 6 | 0 | 0 | 0 | |
| 22 | 19 | 6 | 6 | 6 | 1 | 0 | 0 | |
| 23 | 22 | 6 | 6 | 6 | 0 | 0 | 0 | |
| 24 | 20 | 6 | 10 | 9 | 1 | 1 | 1 | |
| 25 | 25 | 6 | 6 | 6 | 1 | 0 | 0 | |
| 26 | 20 | 6 | 6 | 6 | 1 | 0 | 0 | |
| 27 | 14 | 6 | 6 | 6 | 0 | 0 | 0 | |
| 28 | 20 | 6 | 6 | 6 | 0 | 0 | 0 | |
| 29 | 10 | 14 | 14 | 8 | 1 | 0 | 1 | |
| 30 | 11 | 16 | 16 | 15 | 0 | 0 | 0 | |
| Escherichia coli | 31 | 20 | 6 | 6 | 6 | 0 | 0 | 0 |
| 32 | 6 | 18 | 6 | 6 | 0 | 0 | 0 | |
| 33 | 19 | 17 | 15 | 16 | 1 | 0 | 0 | |
| 34 | 8 | 15 | 15 | 9 | 0 | 0 | 0 | |
| 35 | 6 | 16 | 6 | 7 | 0 | 0 | 0 | |
| 36 | 6 | 21 | 18 | 18 | 1 | 1 | 0 | |
| 37 | 25 | 10 | 12 | 12 | 0 | 0 | 0 | |
| 38 | 6 | 16 | 6 | 7 | 0 | 0 | 0 | |
| 39 | 10 | 17 | 6 | 6 | 1 | 0 | 1 | |
| 40 | 20 | 20 | 9 | 9 | 1 | 1 | 1 | |
| 41 | 23 | 6 | 10 | 9 | 0 | 0 | 0 | |
| 42 | 20 | 18 | 9 | 14 | 1 | 1 | 1 | |
| 43 | 21 | 17 | 18 | 18 | 0 | 0 | 0 | |
| 44 | 6 | 18 | 6 | 7 | 1 | 0 | 0 | |
| 45 | 17 | 20 | 19 | 18 | 1 | 1 | 1 | |
| 46 | 9 | 19 | 7 | 10 | 1 | 0 | 0 | |
| 47 | 20 | 6 | 9 | 10 | 0 | 1 | 1 | |
| 48 | 6 | 16 | 17 | 11 | 0 | 0 | 0 | |
| 49 | 6 | 17 | 17 | 17 | 0 | 0 | 0 | |
| 50 | 15 | 18 | 17 | 17 | 1 | 0 | 0 | |
| 51 | 16 | 19 | 17 | 17 | 1 | 0 | 0 | |
| 52 | 20 | 18 | 6 | 6 | 1 | 1 | 1 | |
| 53 | 20 | 10 | 12 | 13 | 1 | 1 | 1 | |
| 54 | 10 | 20 | 10 | 12 | 1 | 0 | 1 | |
| 55 | 20 | 6 | 10 | 8 | 0 | 0 | 0 | |
| 56 | 23 | 6 | 6 | 6 | 1 | 0 | 0 | |
| 57 | 15 | 17 | 17 | 10 | 1 | 1 | 0 | |
| 58 | 19 | 6 | 6 | 6 | 0 | 0 | 0 | |
| 59 | 11 | 17 | 6 | 8 | 1 | 0 | 0 | |
| 60 | 20 | 6 | 6 | 6 | 0 | 0 | 0 | |
| Klebsiella pneumoniae | 61 | 23 | 6 | 6 | 6 | 0 | 0 | 0 |
| 62 | 6 | 17 | 17 | 10 | 1 | 0 | 0 | |
| 63 | 9 | 17 | 6 | 9 | 1 | 0 | 0 | |
| 64 | 13 | 17 | 7 | 10 | 1 | 0 | 1 | |
| 65 | 7 | 20 | 20 | 20 | 1 | 0 | 0 | |
| 66 | 10 | 17 | 15 | 15 | 1 | 1 | 0 | |
| 67 | 20 | 7 | 6 | 6 | 1 | 0 | 1 | |
| 68 | 18 | 11 | 6 | 7 | 0 | 0 | 0 | |
| 69 | 6 | 18 | 18 | 11 | 1 | 0 | 0 | |
| 70 | 15 | 6 | 6 | 6 | 0 | 0 | 0 | |
| 71 | 20 | 18 | 7 | 10 | 1 | 1 | 1 | |
| 72 | 7 | 17 | 17 | 18 | 0 | 0 | 0 | |
| 73 | 21 | 9 | 11 | 11 | 1 | 1 | 1 | |
| 74 | 21 | 7 | 6 | 6 | 1 | 0 | 0 | |
| 75 | 6 | 18 | 17 | 17 | 0 | 0 | 0 | |
| 76 | 6 | 17 | 18 | 10 | 0 | 0 | 0 | |
| 77 | 6 | 19 | 16 | 18 | 0 | 0 | 0 | |
| 78 | 6 | 18 | 6 | 12 | 1 | 0 | 0 | |
| 79 | 6 | 18 | 17 | 17 | 0 | 0 | 0 | |
| 80 | 12 | 20 | 20 | 20 | 0 | 0 | 0 | |
| 81 | 6 | 18 | 18 | 17 | 0 | 0 | 0 | |
| 82 | 14 | 18 | 18 | 17 | 1 | 0 | 0 | |
| 83 | 10 | 19 | 20 | 20 | 0 | 0 | 0 | |
| 84 | 11 | 20 | 19 | 20 | 0 | 0 | 0 | |
| 85 | 8 | 19 | 17 | 17 | 0 | 0 | 0 | |
| 86 | 10 | 18 | 17 | 11 | 1 | 0 | 0 | |
| 87 | 8 | 17 | 17 | 16 | 0 | 0 | 0 | |
| 88 | 11 | 16 | 6 | 7 | 1 | 0 | 0 | |
| 89 | 10 | 16 | 17 | 9 | 1 | 0 | 1 | |
| 90 | 10 | 20 | 19 | 19 | 0 | 0 | 0 | |
References
- Mancuso, G.; Midiri, A.; Gerace, E.; Marra, M.; Zummo, S.; Biondo, C. Urinary tract infections: The current scenario and future prospects. Pathogens 2023, 12, 623. [Google Scholar] [CrossRef]
- Broughton, E.; Bektas, M.; Colosia, A.; Kuper, K.; Fernandez, M.M.; Al-Taie, A.; Kotb, R. A systematic literature review of the epidemiology of complicated urinary tract infection. Infect. Dis. Ther. 2025, 14, 1157–1181. [Google Scholar] [CrossRef]
- Liu, J.; Xu, K.; Hu, J.; Wang, L.; Liu, Z. Recurrent uncomplicated lower urinary tract infections in women. Curr. Urol. 2025, 19, 90–94. [Google Scholar] [CrossRef]
- Sher, E.K.; Džidić-Krivić, A.; Sesar, A.; Farhat, E.K.; Čeliković, A.; Beća-Zećo, M.; Pinjic, E.; Sher, F. Current state and novel outlook on prevention and treatment of rising antibiotic resistance in urinary tract infections. Pharmacol. Ther. 2024, 261, 108688. [Google Scholar] [CrossRef]
- Simoni, A.; Schwartz, L.; Junquera, G.Y.; Ching, C.B.; Spencer, J.D. Current and emerging strategies to curb antibiotic-resistant urinary tract infections. Nat. Rev. Urol. 2024, 21, 707–722. [Google Scholar] [CrossRef]
- Huttner, A.; Verhaegh, E.M.; Harbarth, S.; Muller, A.E.; Theuretzbacher, U.; Mouton, J.W. Nitrofurantoin revisited: A systematic review and meta-analysis of controlled trials. J. Antimicrob. Chemother. 2015, 70, 2456–2464. [Google Scholar] [CrossRef]
- McOsker, C.C.; Fitzpatrick, P.M. Nitrofurantoin: Mechanism of action and implications for resistance development in common uropathogens. J. Antimicrob. Chemother. 1994, 33 (Suppl. A), 23–30. [Google Scholar] [CrossRef]
- Khamari, B.; Bulagonda, E.P. Unlocking nitrofurantoin: Understanding molecular mechanisms of action and resistance in Enterobacterales. Med. Princ. Pract. 2024, 34, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Chen, N.; Zhang, M.; Lin, L.; Lin, B.; Fang, Y.; Hua, Z.; Liang, C. Bibliometric analysis of global research on the clinical applications of aminoglycoside antibiotics: Improving efficacy and decreasing risk. Front. Microbiol. 2025, 16, 1532231. [Google Scholar] [CrossRef] [PubMed]
- Krause, K.M.; Serio, A.W.; Kane, T.R.; Connolly, L.E. Aminoglycosides: An overview. Cold Spring Harb. Perspect. Med. 2016, 6, a027029. [Google Scholar] [CrossRef] [PubMed]
- Forge, A.; Schacht, J. Aminoglycoside antibiotics. Audiol. Neurotol. 2000, 5, 3–22. [Google Scholar] [CrossRef]
- Le, J.; McKee, B.; Srisupha-Olarn, W.; Burgess, D.S. In vitro activity of carbapenems alone and in combination with amikacin against KPC-producing Klebsiella pneumoniae. J. Clin. Med. Res. 2011, 3, 106–110. [Google Scholar] [CrossRef][Green Version]
- Thy, M.; Timsit, J.-F.; de Montmollin, E. Aminoglycosides for the treatment of severe infection due to resistant Gram-negative pathogens. Antibiotics 2023, 12, 860. [Google Scholar] [CrossRef]
- Tyers, M.; Wright, G.D. Drug combinations: A strategy to extend the life of antibiotics in the 21st century. Nat. Rev. Microbiol. 2019, 17, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Fatsis-Kavalopoulos, N.; Roemhild, R.; Tang, P.C.; Kreuger, J.; Andersson, D.I. CombiANT: Antibiotic interaction testing made easy. PLoS Biol. 2020, 18, e3000856. [Google Scholar] [CrossRef]
- Tian, P.; Li, Q.-Q.; Guo, M.-J.; Zhu, Y.-Z.; Zhu, R.-Q.; Guo, Y.-Q.; Yang, Y.; Liu, Y.-Y.; Yu, L.; Li, Y.-S.; et al. Zidovudine in synergistic combination with nitrofurantoin or omadacycline: In vitro and in murine urinary tract or lung infection evaluation against multidrug-resistant Klebsiella Pneumoniae. Antimicrob. Agents Chemother. 2024, 68, e00344-24. [Google Scholar] [CrossRef]
- Zhong, Z.X.; Cui, Z.H.; Li, X.J.; Tang, T.; Zheng, Z.J.; Ni, W.N.; Fang, L.X.; Zhou, Y.F.; Yu, Y.; Liu, Y.H.; et al. Nitrofurantoin combined with amikacin: A promising alternative strategy for combating MDR uropathogenic Escherichia coli. Front. Cell. Infect. Microbiol. 2020, 10, 608547. [Google Scholar] [CrossRef]
- Abdulkareem, H.; Hasan, M.; Abdulhamza, F. Efficacy of combination of nitrofurantoin with gentamicin, and ciprofloxacin against resistant E. coli isolated from patients with urinary tract infections: In vitro study. Mintage J. Pharm. Med. Sci. 2014, 3, 5–9. Available online: https://www.mjpms.in/articles/efficacy-of-combination-of-nitrofurantoin-with-gentamicin-and-ciprofloxacin-against-resistant-e-coliisolated-from-patien.pdf (accessed on 20 July 2020).
- World Health Organization (WHO). Global Research Agenda for Antimicrobial Resistance in Human Health. Available online: https://cdn.who.int/media/docs/default-source/antimicrobial-resistance/amr-spc-npm/who-global-research-agenda-for-amr-in-human-health---policy-brief.pdf (accessed on 14 July 2025).
- Health Emergency Preparedness and Response Authority (HERA). Health Union: Identifying Top 3 Priority Health Threats. 2025. Available online: https://health.ec.europa.eu/publications/hera-factsheet-health-union-identifying-top-3-priority-health-threats_en (accessed on 14 July 2025).
- European Partnership on One Health Antimicrobial Resistance (OHAMR). Roadmap of Actions 2025–2032. 2024. Available online: https://www.jpiamr.eu/app/uploads/2024/03/OHAMR-Roadmap-of-Actions_v2024-03-08.pdf (accessed on 14 July 2025).
- Dellit, T.H.; Owens, R.C.; McGowan, J.E.; Gerding, D.N., Jr.; Weinstein, R.A.; Burke, J.P.; Huskins, W.C.; Paterson, D.L.; Fishman, N.O.; Carpenter, C.F.; et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin. Infect. Dis. 2007, 44, 159–177. [Google Scholar] [CrossRef] [PubMed]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Antimicrobial Susceptibility Testing EUCAST Disk Diffusion Method (Version 13.0). 2025. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/2025_manuals/Manual_v_13.0_EUCAST_Disk_Test_2025.pdf (accessed on 20 July 2020).
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Reading Guide—EUCAST Disk Diffusion Method for Antimicrobial Susceptibility Testing (Version 11.0). 2025. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/2025_manuals/Reading_guide_v_11.0_EUCAST_Disk_Test_2025.pdf (accessed on 20 July 2020).
- Laishram, S.; Pragasam, A.K.; Bakthavatchalam, Y.D.; Veeraraghavan, B. An update on technical, interpretative and clinical relevance of antimicrobial synergy testing methodologies. Indian J. Med. Microbiol. 2017, 35, 445–468. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Good Clinical Laboratory Practice (GCLP). 2009. Available online: https://iris.who.int/bitstream/handle/10665/44092/9789241597852_eng.pdf (accessed on 20 July 2020).
- World Medical Association. WMA Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. 2013. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki/ (accessed on 20 July 2020).
- Repac Antić, D.; Kovač, B.; Kolenc, M.; Brčić Karačonji, I.; Gobin, I.; Petković Didović, M. Combinatory effect of nitroxoline and gentamicin in the control of uropathogenic Enterococci infections. Antibiotics 2024, 13, 829. [Google Scholar] [CrossRef]
- Mahdizade Ari, M.; Dashtbin, S.; Ghasemi, F.; Shahroodian, S.; Kiani, P.; Bafandeh, E.; Darbandi, T.; Ghanavati, R.; Darbandi, A. Nitrofurantoin: Properties and potential in treatment of urinary tract infection: A narrative review. Front. Cell. Infect. Microbiol. 2023, 13, 1148603. [Google Scholar] [CrossRef] [PubMed]
- Umemura, T.; Kato, H.; Hagihara, M.; Hirai, J.; Yamagishi, Y.; Mikamo, H. Efficacy of combination therapies for the treatment of multi-drug resistant Gram-negative bacterial infections based on meta-analyses. Antibiotics 2022, 11, 524. [Google Scholar] [CrossRef] [PubMed]
- Bielec, F.; Wenecka, M.; Brauncajs, M.; Pastuszak-Lewandoska, D. Analysis of cumulative antibiogram reports in search for optimal empirical urinary tract infection treatment at the Central Teaching Hospital of the Medical University of Lodz, Poland: Results of a 3-year surveillance. J. Clin. Med. 2023, 12, 6270. [Google Scholar] [CrossRef] [PubMed]
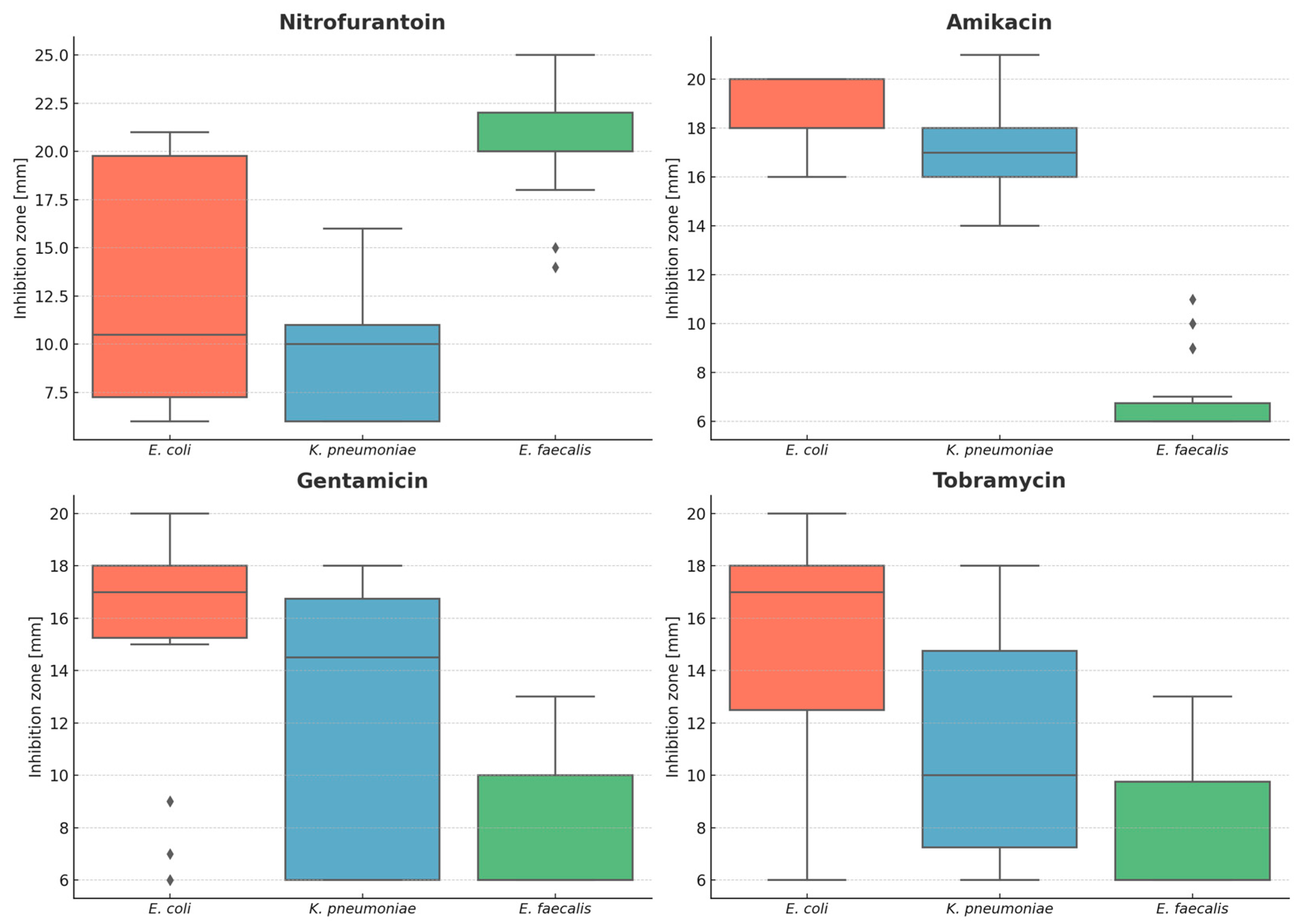

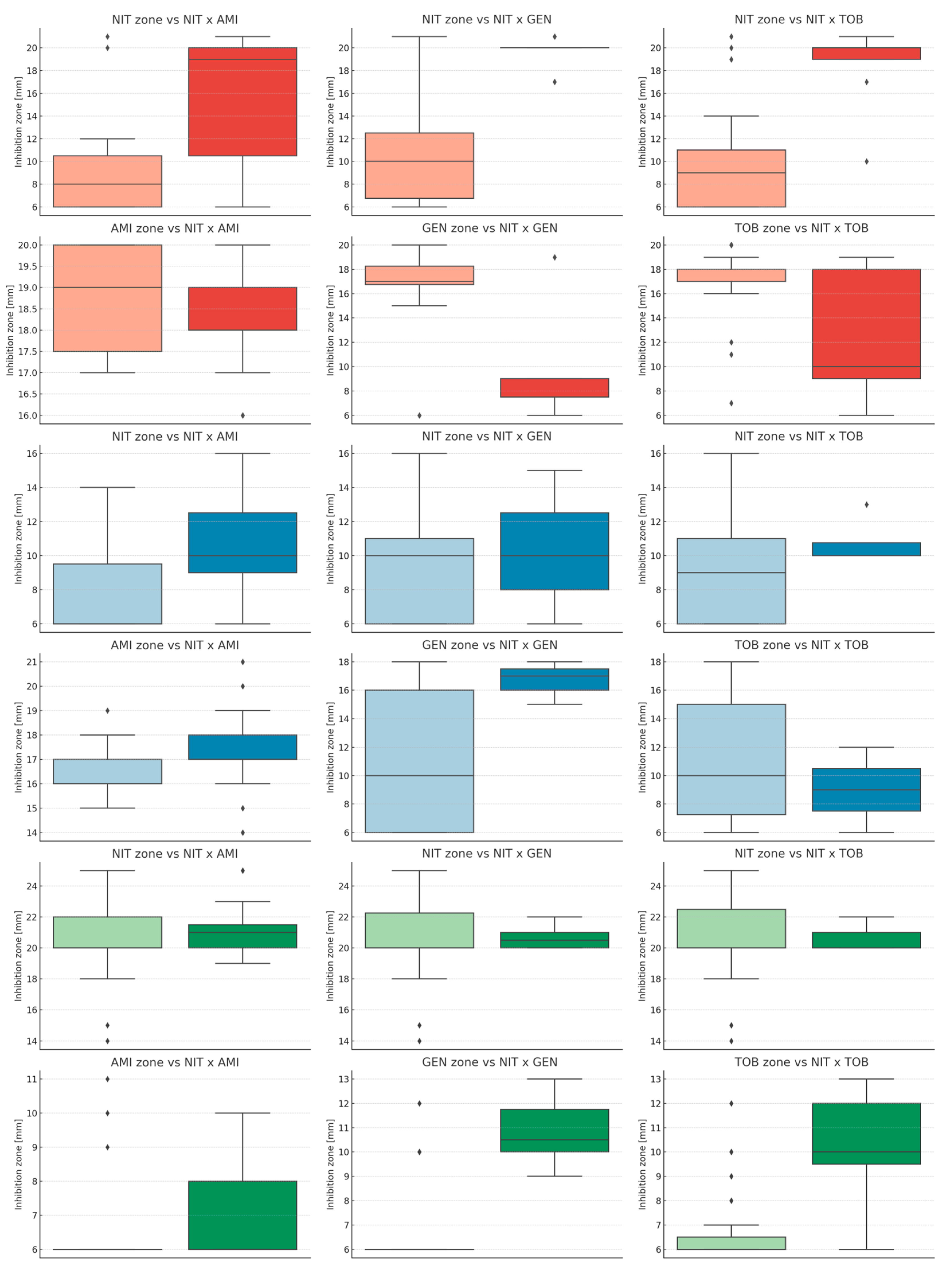
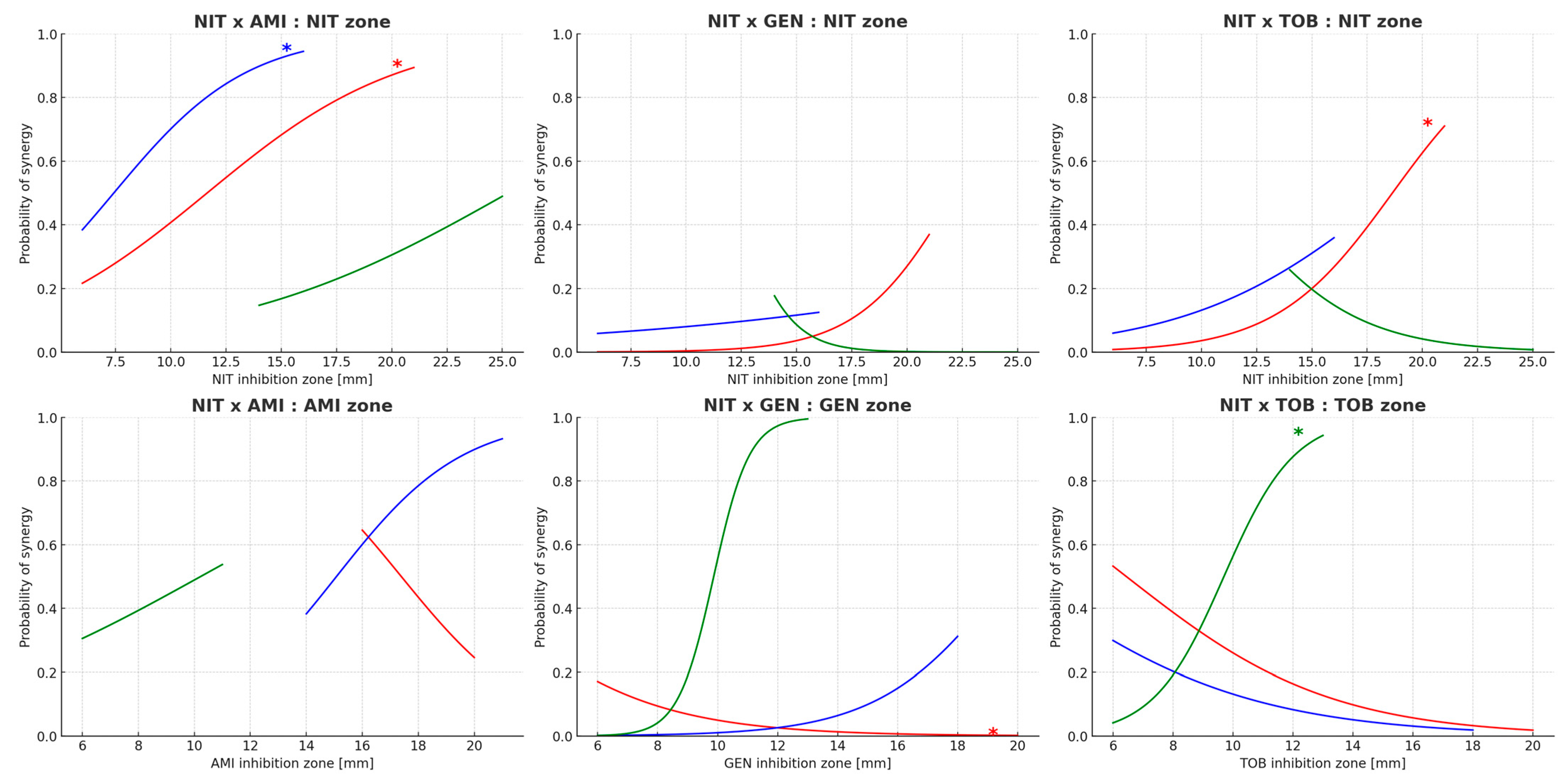
| Antimicrobial | Species | Mean [mm] | Median [mm] | Minimum [mm] | Maximum [mm] | Range [mm] |
|---|---|---|---|---|---|---|
| Nitrofurantoin | E. faecalis | 20.6 | 20.0 | 14 | 25 | 11 |
| E. coli | 12.7 | 10.5 | 6 | 21 | 15 | |
| K. pneumoniae | 9.6 | 10.0 | 6 | 16 | 10 | |
| Amikacin | E. faecalis | 6.8 | 6.0 | 6 | 11 | 5 |
| E. coli | 18.4 | 18.0 | 16 | 20 | 4 | |
| K. pneumoniae | 17.1 | 17.0 | 14 | 21 | 7 | |
| Gentamicin | E. faecalis | 7.7 | 6.0 | 6 | 13 | 7 |
| E. coli | 15.3 | 17.0 | 6 | 20 | 14 | |
| K. pneumoniae | 11.8 | 14.5 | 6 | 18 | 12 | |
| Tobramycin | E. faecalis | 7.7 | 6.0 | 6 | 13 | 7 |
| E. coli | 15.5 | 17.0 | 6 | 20 | 14 | |
| K. pneumoniae | 10.8 | 10.0 | 6 | 18 | 12 |
| Antimicrobial | H Statistics | p-Value |
|---|---|---|
| Nitrofurantoin | 48.47 | 2.99 × 10−11 |
| Amikacin | 66.20 | 4.21 × 10−15 |
| Gentamycin | 31.61 | 1.37 × 10−7 |
| Tobramycin | 40.77 | 1.40 × 10−9 |
| Species | Synergy Kind | Inhibition Zone | U Statistics | p-Value |
|---|---|---|---|---|
| E. coli | NIT × AMI | NIT | 51.5 | 0.011 |
| AMI | 129.0 | 0.489 | ||
| NIT × GEN | NIT | 13.5 | 0.002 | |
| GEN | 116.0 | 0.022 | ||
| NIT × TOB | NIT | 22.0 | 0.001 | |
| TOB | 137.5 | 0.052 | ||
| K. pneumoniae | NIT × AMI | NIT | 58.0 | 0.043 |
| AMI | 66.0 | 0.089 | ||
| NIT × GEN | NIT | 37.0 | 0.832 | |
| GEN | 16.0 | 0.089 | ||
| NIT × TOB | NIT | 39.5 | 0.454 | |
| TOB | 66.5 | 0.389 | ||
| E. faecalis | NIT × AMI | NIT | 90.5 | 0.550 |
| AMI | 78.5 | 0.158 | ||
| NIT × GEN | NIT | 71.0 | 0.979 | |
| GEN | 12.5 | <0.001 | ||
| NIT × TOB | NIT | 84.0 | 0.880 | |
| TOB | 23.0 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bielec, F.; Łysakowska, M.; Brauncajs, M.; Bekier, A.; Klimaszewski, S.; Pastuszak-Lewandoska, D. Nitrofurantoin–Aminoglycoside Synergy Against Common Uropathogens Evaluated by Disc Diffusion: A Pilot Study. Microorganisms 2025, 13, 2117. https://doi.org/10.3390/microorganisms13092117
Bielec F, Łysakowska M, Brauncajs M, Bekier A, Klimaszewski S, Pastuszak-Lewandoska D. Nitrofurantoin–Aminoglycoside Synergy Against Common Uropathogens Evaluated by Disc Diffusion: A Pilot Study. Microorganisms. 2025; 13(9):2117. https://doi.org/10.3390/microorganisms13092117
Chicago/Turabian StyleBielec, Filip, Monika Łysakowska, Małgorzata Brauncajs, Adrian Bekier, Stanisław Klimaszewski, and Dorota Pastuszak-Lewandoska. 2025. "Nitrofurantoin–Aminoglycoside Synergy Against Common Uropathogens Evaluated by Disc Diffusion: A Pilot Study" Microorganisms 13, no. 9: 2117. https://doi.org/10.3390/microorganisms13092117
APA StyleBielec, F., Łysakowska, M., Brauncajs, M., Bekier, A., Klimaszewski, S., & Pastuszak-Lewandoska, D. (2025). Nitrofurantoin–Aminoglycoside Synergy Against Common Uropathogens Evaluated by Disc Diffusion: A Pilot Study. Microorganisms, 13(9), 2117. https://doi.org/10.3390/microorganisms13092117










