Rhizosphere-Associated Bacteria of Saltgrass [Distichlis spicata (L.) Greene] Show Enhanced Ability to Tolerate Saline Environments and Stimulate Plant Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Area
2.2. Soil Analysis
2.3. Bacterial Isolation
2.4. Qualitative In Vitro Tests to Select Bacteria with Plant Growth-Promoting Traits
2.5. Quantitative Tests Under Salt Stress
2.6. Greenhouse Assay and Molecular Identification
2.7. Statistical Analysis
3. Results and Discussion
3.1. Chemical Properties of the Soil
3.2. Bacterial Isolations
3.3. Evaluation of Plant Growth-Promoting Traits
3.4. Siderophore Production and Phosphate Solubilization
3.5. Exopolysaccharide (EPS) Production
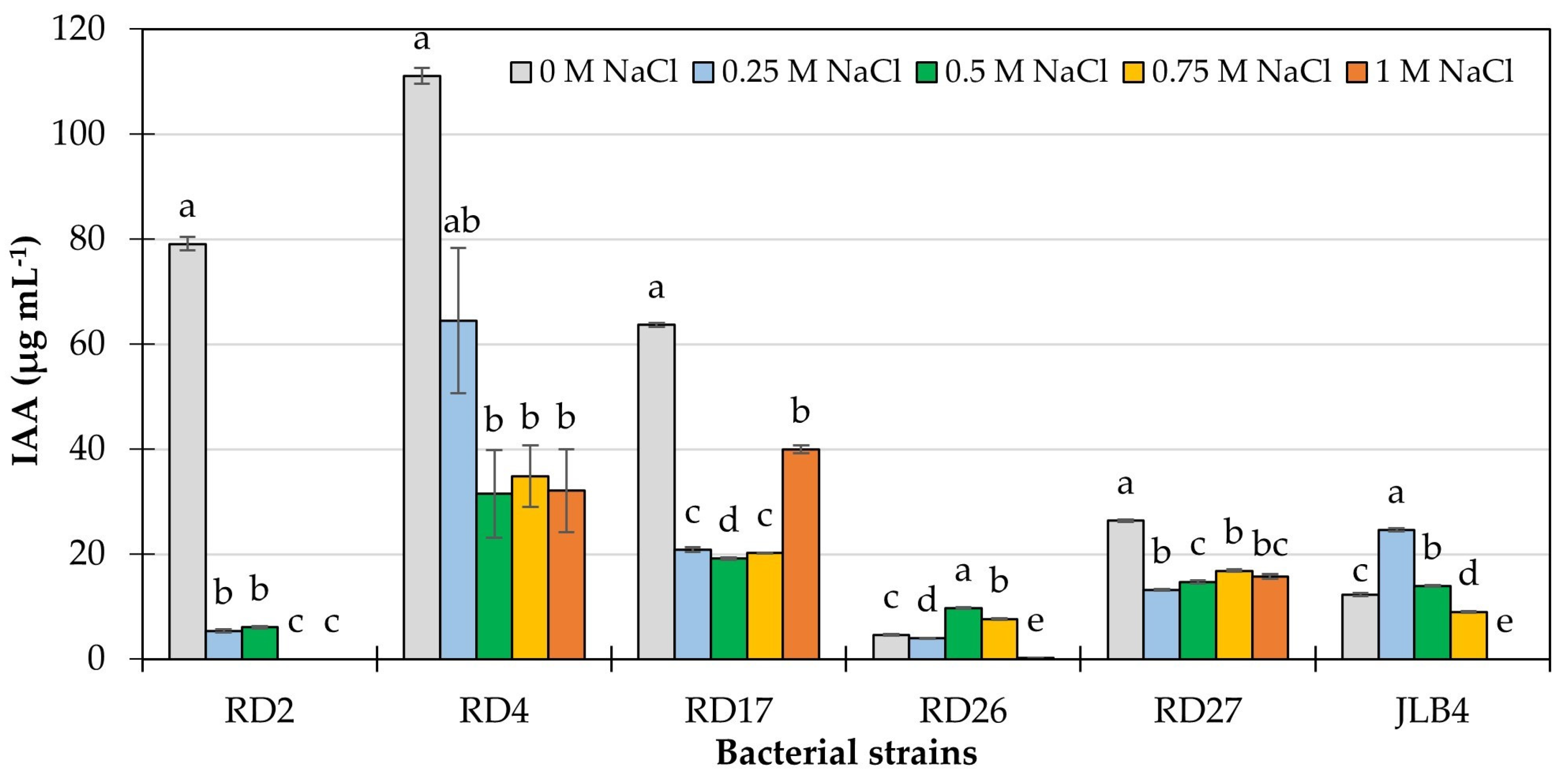
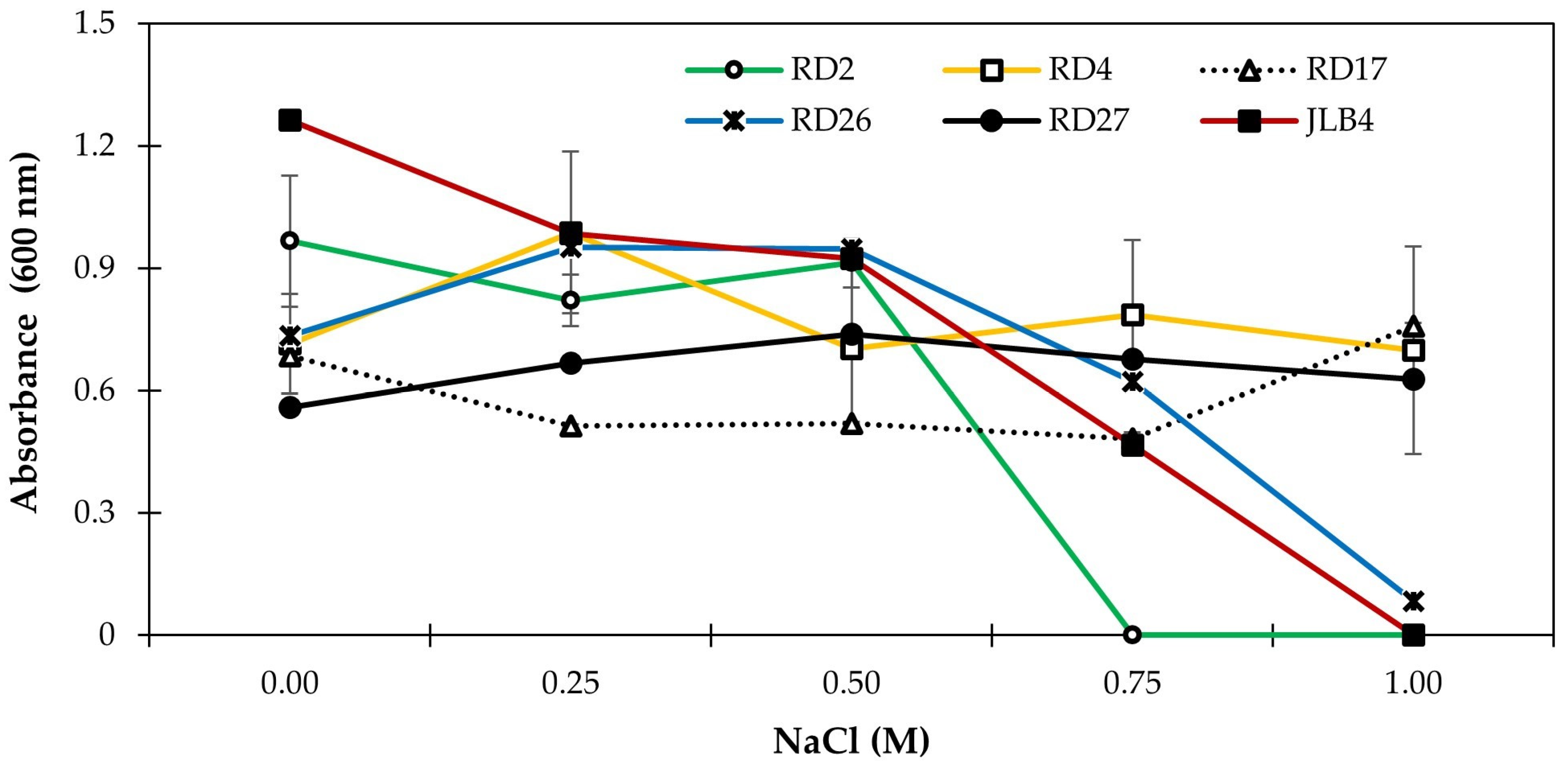
3.6. IAA Production and Bacterial Growth
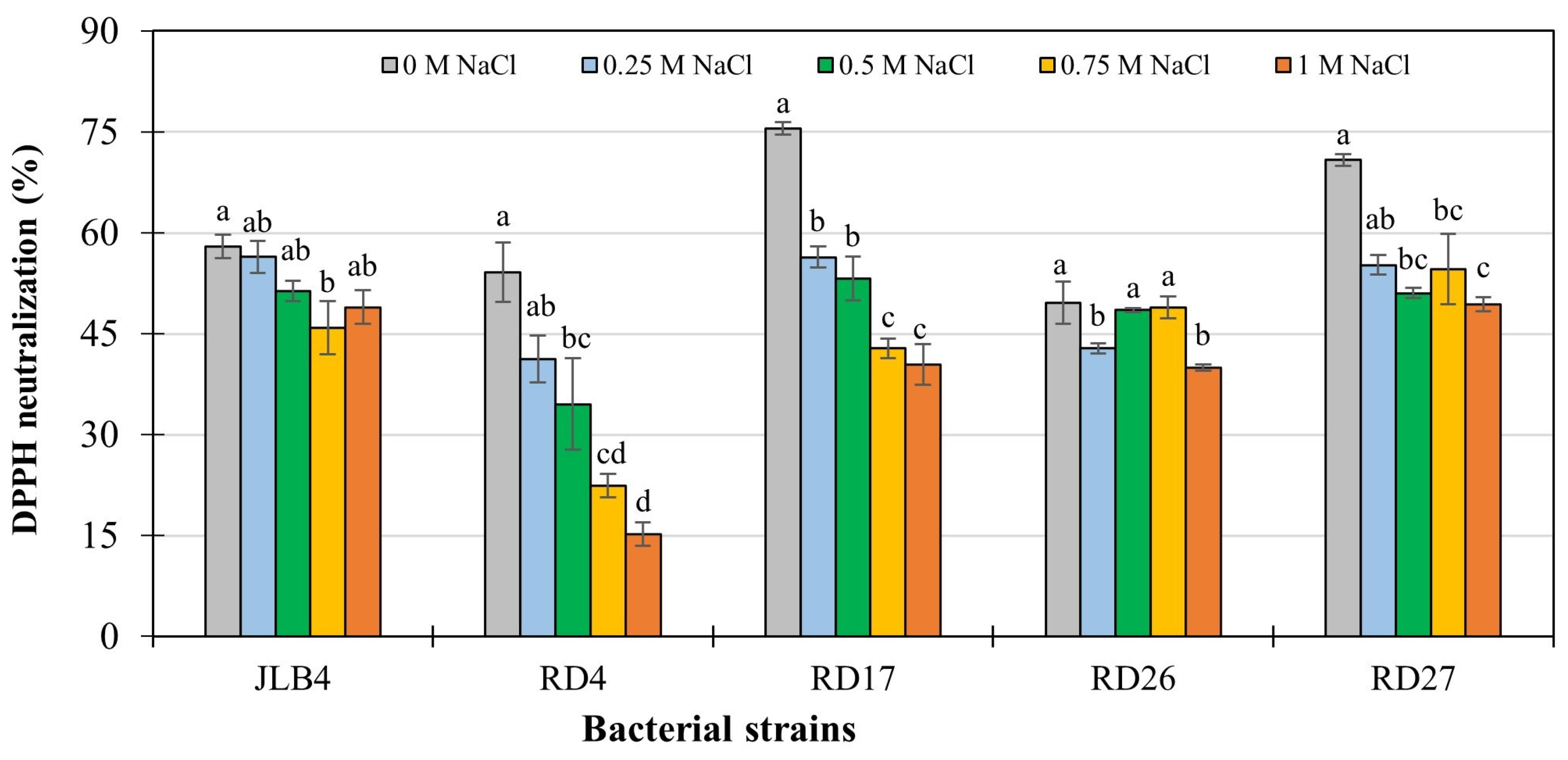
3.7. Antioxidant Activity
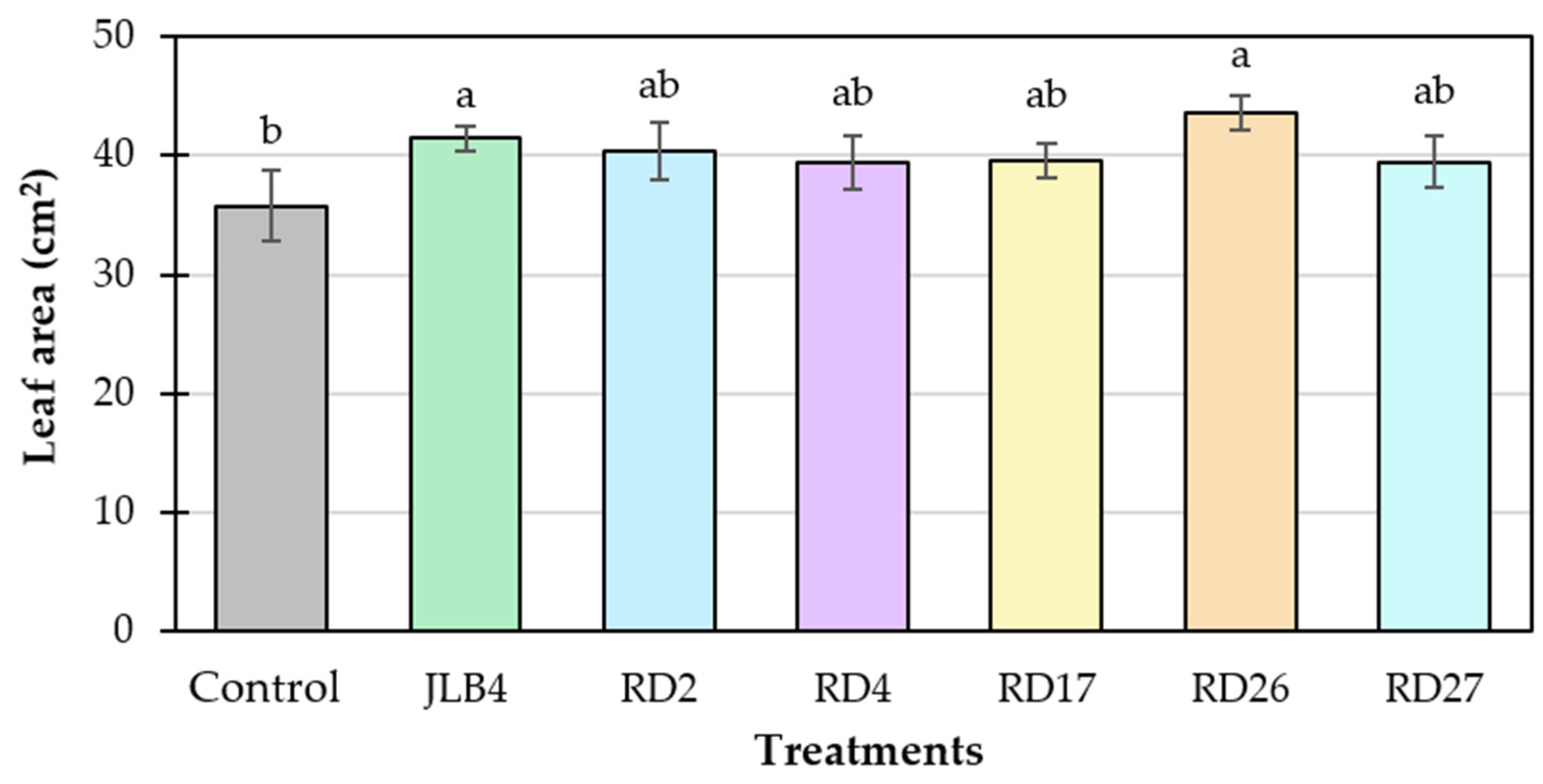
3.8. Plant Growth Promotion Induced by Bacteria in Tomato Seedlings Under Greenhouse Conditions
3.9. Molecular Identification of Effective Strains
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orozco-Mosqueda, M.D.C.; Flores, A.; Rojas-Sánchez, B.; Urtis-Flores, C.A.; Morales-Cedeño, L.R.; Valencia-Marin, M.F.; Chávez-Avila, S.; Rojas-solis, D.; Santoyo, G. Plant growth-promoting bacteria as bioinoculants: Attributes and challenges for sustainable crop improvement. Agronomy 2021, 11, 1167. [Google Scholar] [CrossRef]
- Jha, B.; Gontia, I.; Hartmann, A. The roots of the halophyte Salicornia brachiata are a source of new halotolerant diazotrophic bacteria with plant growth-promoting potential. Plant Soil 2012, 356, 265–277. [Google Scholar] [CrossRef]
- Yaish, M.W.; Antony, I.; Glick, B.R. Isolation and characterization of endophytic plant growth-promoting bacteria from date palm tree (Phoenix dactylifera L.) and their potential role in salinity tolerance. Antonie Van Leeuwenhoek 2015, 107, 1519–1532. [Google Scholar] [CrossRef]
- Kadmiri, I.M.; Chaouqui, L.; Azaroual, S.E.; Sijilmassi, B.; Yaakoubi, K.; Wahby, I. Phosphate-solubilizing and auxin-producing rhizobacteria promote plant growth under saline conditions. Arab. J. Sci. Eng. 2018, 43, 3403–3415. [Google Scholar] [CrossRef]
- Pan, L.; Cai, B. Phosphate-solubilizing bacteria: Advances in their physiology, molecular mechanisms and microbial community effects. Microorganisms 2023, 11, 2904. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Mukherjee, A.; Rastogi, R.P.; Verma, J.P. Salt-tolerant plant growth-promoting Bacillus pumilus strain JPVS11 to enhance plant growth attributes of rice and improve soil health under salinity stress. Microbiol. Res. 2021, 242, 126616. [Google Scholar] [CrossRef]
- Sultana, S.; Alam, S.; Karim, M.M. Screening of siderophore-producing salt-tolerant rhizobacteria suitable for supporting plant growth in saline soils with iron limitation. J. Agric. Food Res. 2021, 4, 100150. [Google Scholar] [CrossRef]
- Sarvepalli, M.; Velidandi, A.; Korrapati, N. Optimization of siderophore production in three marine bacterial isolates along with their heavy-metal chelation and seed germination potential determination. Microorganisms 2023, 11, 2873. [Google Scholar] [CrossRef]
- Palacio-Rodríguez, R.; Coria-Arellano, J.L.; López-Bucio, J.; Sánchez-Salas, J.; Muro-Pérez, G.; Castañeda-Gaytán, G.; Sáenz-Mata, J. Halophilic rhizobacteria from Distichlis spicata promote growth and improve salt tolerance in heterologous plant hosts. Symbiosis 2017, 73, 179–189. [Google Scholar] [CrossRef]
- Fatima, T.; Arora, N.K. Pseudomonas entomophila PE3 and its exopolysaccharides as biostimulants for enhancing growth, yield and tolerance responses of sunflower under saline conditions. Microbiol. Res. 2021, 244, 126671. [Google Scholar] [CrossRef] [PubMed]
- Kusale, S.P.; Attar, Y.C.; Sayyed, R.Z.; Malek, R.A.; Ilyas, N.; Suriani, N.L.; Khan, N.; El Enshasy, H.A. Production of plant beneficial and antioxidants metabolites by Klebsiella variicola under salinity stress. Molecules 2021, 26, 1894. [Google Scholar] [CrossRef]
- Ali, B.; Wang, X.; Saleem, M.H.; Azeem, M.A.; Afridi, M.S.; Nadeem, M.; Ghazal, M.; Batool, T.; Qayyum, A.; Alatawi, A.; et al. Bacillus mycoides PM35 reinforces photosynthetic efficiency, antioxidant defense, expression of stress-responsive genes, and ameliorates the effects of salinity stress in maize. Life 2022, 12, 219. [Google Scholar] [CrossRef]
- Fatima, T.; Mishra, I.; Verma, R.; Arora, N.K. Mechanisms of halotolerant plant growth promoting Alcaligenes sp. involved in salt tolerance and enhancement of the growth of rice under salinity stress. 3 Biotech 2020, 10, 361. [Google Scholar] [CrossRef]
- Rojas-Tapias, D.; Moreno-Galván, A.; Pardo-Díaz, S.; Obando, M.; Rivera, D.; Bonilla, R. Effect of inoculation with plant growth-promoting bacteria (PGPB) on amelioration of saline stress in maize (Zea mays). Appl. Soil Ecol. 2012, 61, 264–272. [Google Scholar] [CrossRef]
- Chávez-García, E. Distichlis spicata: Una Planta Para la sal del Suelo. Available online: https://repositorionacionalcti.mx/recurso/oai:cicy.repositorioinstitucional.mx:1003/2904 (accessed on 15 June 2025).
- Asghar, N.; Hameed, M.; Ahmad, M.S.A.; Ahmad, F. Soil-root relationship in a leaf succulent halophyte Suaeda vera from differently salt-affected habitats. Water Air Soil Poll. 2023, 234, 431. [Google Scholar] [CrossRef]
- SEMARNAT. Norma Oficial Mexicana NOM-021-RECNAT-2000, Que Establece las Especificaciones de Fertilidad, Sanidad y Clasificación de Suelos. Estudios, Muestreo y Análisis. Available online: https://platiica.economia.gob.mx/normalizacion/nom-021-semarnat-2000/ (accessed on 15 June 2025).
- Amin, D.; Ray, S.; Wagh, V. Mother Culture, Broth, and Peat Test. In Practical Handbook on Agricultural Microbiology; Amaresan, N., Patel, P., Amin, D., Eds.; Springer Protocols Handbooks: New York, NY, USA, 2022; pp. 395–403. [Google Scholar]
- Daza-Martínez, Y.M.; Almaraz-Suárez, J.J.; Rodríguez-Mendoza, M.N.; Angulo-Castro, A.; Silva-Rojas, H.V. Aislamiento de rizobacterias asociadas a tomate (Solanum lycopersicum L.) y su potencial para promover crecimiento vegetal. ITEA-Inf. Tec. Econ. 2022, 118, 345–360. [Google Scholar] [CrossRef]
- Louden, B.C.; Haarmann, D.; Lynne, A.M. Use of blue agar CAS assay for siderophore detection. J. Microbiol. Biol. Educ. 2011, 12, 51–53. [Google Scholar] [CrossRef]
- Pikovskaya, R.I. Mobilization of phosphorus in soil connection with the vital activity of some microbial species. Microbiology 1948, 17, 362–370. [Google Scholar]
- Gordon, S.A.; Weber, R.P. Colorimetric estimation of indoleacetic acid. Plant Physiol. 1951, 26, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.A.; Tette, P.A.S.; Mendonça, R.C.S.; Soares, A.D.S.; Carvalho, M.M.D. Detection of exopolysaccharide production and biofilm-related genes in Staphylococcus spp. isolated from a poultry processing plant. Food Sci. Technol. 2014, 34, 710–716. [Google Scholar] [CrossRef]
- Xing, J.; Wang, G.; Zhang, Q.; Liu, X.; Gu, Z.; Zhang, H.; Chen, Y.Q.; Chen, W. Determining antioxidant activities of lactobacilli cell-free supernatants by cellular antioxidant assay: A comparison with traditional methods. PLoS ONE 2015, 10, e0119058. [Google Scholar] [CrossRef]
- Steiner, A.A. The universal nutrient solution. In Proceedings of the Sixth International Congress on Soilless Culture, ISOSC, Lunteren, The Netherlands, 29 April–5 May 1984; pp. 633–650. [Google Scholar]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 15 June 2025).
- Osman, K.T. (Ed.) Saline and sodic soils. In Management of Soil Problems; Springer Nature: Cham, Switzerland, 2018; pp. 255–298. [Google Scholar]
- Zhang, X.; Zhang, D.; Sun, W.; Wang, T. The adaptive mechanism of plants to iron deficiency via iron uptake, transport, and homeostasis. Int. J. Mol. Sci. 2019, 20, 2424. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, S.; Płociniczak, T.; Piotrowska-Seget, Z.; Złoch, M.; Ruppel, S.; Hrynkiewicz, K. Metabolic potential and community structure of endophytic and rhizosphere bacteria associated with the roots of the halophyte Aster tripolium L. Microbiol. Res. 2016, 182, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wu, J.; Shang, X.; Xue, L.; Ji, G.; Chang, S.; Niu, J.; Emaneghemi, B. Screening of siderophore-producing bacteria and their effects on promoting the growth of plants. Curr. Microbiol. 2022, 79, 150. [Google Scholar] [CrossRef]
- Northover, G.H.; Mao, Y.; Ahmed, H.; Blasco, S.; Vilar, R.; Garcia-España, E.; Weiss, D.J. Effect of salinity on the zinc (II) binding efficiency of siderophore functional groups and implications for salinity tolerance mechanisms in barley. Sci. Rep. 2021, 11, 16704. [Google Scholar] [CrossRef]
- Idris, A.; Labuschagne, N.; Korsten, L. Efficacy of rhizobacteria for growth promotion in sorghum under greenhouse conditions and selected modes of action studies. J. Agric. Sci. 2009, 147, 17–30. [Google Scholar] [CrossRef]
- Naseem, H.; Ahsan, M.; Shahid, M.A.; Khan, N. Exopolysaccharides producing rhizobacteria and their role in plant growth and drought tolerance. J. Basic Microbiol. 2018, 58, 1009–1022. [Google Scholar] [CrossRef]
- Shultana, R.; Tan Kee, Z.A.; Yusop, M.R.; Mohd, S.H.; Ayanda, A.F. Effect of salt-tolerant bacterial inoculations on rice seedlings differing in salt-tolerance under saline soil conditions. Agronomy 2020, 10, 1030. [Google Scholar]
- Shultana, R.; Kee, Z.A.T.; Yusop, M.R.; Saud, H.M. Characterization of salt-tolerant plant growth-promoting rhizobacteria and the effect on growth and yield of saline-affected rice. PLoS ONE 2020, 15, e0238537. [Google Scholar] [CrossRef]
- Redha, A. Removal of heavy metals from aqueous media by biosorption. Arab. J. Basic Appl. Sci. 2020, 27, 183–193. [Google Scholar] [CrossRef]
- Meng, J.; Huang, C.; Huang, X.; Liu, D.; Han, B.; Chen, J. Osmoregulated periplasmic glucans transmit external signals through Rcs phosphorelay pathway in Yersinia enterocolitica. Front. Microbiol. 2020, 11, 122. [Google Scholar] [CrossRef]
- Rodriguez-Ayala, F.; Bartolini, M.; Grau, R. The stress-responsive alternative Sigma Factor SigB of Bacillus subtilis and its relatives: An old friend with new functions. Front. Microbiol. 2020, 11, 1761. [Google Scholar] [CrossRef] [PubMed]
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.E. Microbial extracellular polymeric substances: Ecological function and impact on soil aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.M.; Biswas, M.S.; Mosharaf, M.K.; Haque, M.A.; Islam, M.S.; Nahar, K.; Islam, M.M.; Shozib, H.B.; Islam, M.M.; Ferdous-E-Elahi. Halotolerant biofilm-producing rhizobacteria mitigate seawater-induced salt stress and promote growth of tomato. Sci. Rep. 2022, 12, 5599. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.K.; Sing, J.S.; Sing, D.P. Exopolysaccharide plant growth promoting rhizobacteria under salinity condition. Pedosphere 2011, 21, 214–222. [Google Scholar] [CrossRef]
- Shurigin, V.; Egamberdieva, D.; Li, L.; Davranov, K.; Panosyan, H.; Birkeland, N.K.; Wirth, S.; Bellingrath-Kimura, S.D. Endophytic bacteria associated with halophyte Seidlitzia rosmarinus Ehrenb. ex Boiss. from saline soil of Uzbekistan and their plant beneficial traits. J. Arid Land 2020, 12, 730–740. [Google Scholar] [CrossRef]
- Yasin, N.A.; Khan, W.U.; Ahmad, S.R.; Ali, A.; Ahmed, S.; Ahmad, A. Effect of Bacillus fortis 162 on growth, oxidative stress tolerance and phytoremediation potential of Catharanthus roseus under chromium stress. Int. J. Agric. Biol. 2018, 20, 1513–1522. [Google Scholar]
- Ali, B.; Wang, X.; Saleem, M.H.; Hafeez, A.; Afridi, M.S.; Khan, S.; Nisa, Z.U.; Ullah, I.; Júnior, A.T.A.; Alatawa, A.; et al. PGPR-mediated salt tolerance in maize by modulating plant physiology, antioxidant defense, compatible solutes accumulation and bio-surfactant producing genes. Plants 2022, 11, 345. [Google Scholar] [CrossRef]
- Khan, M.A.; Sahile, A.A.; Jan, R.; Asaf, S.; Hamayun, M.; Imran, M.; Adhikari, A.; Kang, S.M.; Kim, K.M.; Lee, I.J. Halotolerant bacteria mitigate the effects of salinity stress on soybean growth by regulating secondary metabolites and molecular responses. BMC Plant Biol. 2021, 21, 176. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, C.; Feng, Q.; Liou, R.-M.; Lin, Y.-F.; Qiao, J.; Lu, Y.; Chang, Y. The mechanisms of sodium chloride stress mitigation by salt-tolerant plant growth promoting rhizobacteria in wheat. Agronomy 2022, 12, 543. [Google Scholar] [CrossRef]
- Mokashe, N.; Chaudhari, B.; Patil, U. Operative utility of salt-stable proteases of halophilic and halotolerant bacteria in the biotechnology sector. Int. J. Biol. Macromol. 2018, 117, 493–522. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, N.; Mazhar, R.; Yasmin, H.; Khan, W.; Iqbal, S.; Enshasy, H.E.; Dailin, D.J. Rhizobacteria isolated from saline soil induce systemic tolerance in wheat (Triticum aestivum L.) against salinity stress. Agronomy 2020, 10, 989. [Google Scholar] [CrossRef]
- Mahmoud, O.M.B.; Hidri, R.; Talbi-Zribi, O.; Taamalli, W.; Abdelly, C.; Djébali, N. Auxin and proline producing rhizobacteria mitigate salt-induced growth inhibition of barley plants by enhancing water and nutrient status. S. Afr. J. Bot. 2020, 128, 209–217. [Google Scholar] [CrossRef]
- Molina, R.; Rivera, D.; Mora, V.; López, G.; Rosas, S.; Spaepen, S.; Vanderleyden, J.; Cassán, F. Regulation of IAA biosynthesis in Azospirillum brasilense under environmental stress conditions. Curr. Microbiol. 2018, 75, 1408–1418. [Google Scholar] [CrossRef]
- Abeed, A.H.; Mahdy, R.E.; Alshehri, D.; Hammami, I.; Eissa, M.A.; Abdel Latef, A.A.H.; Mahmoud, G.A.E. Induction of resilience strategies against biochemical deteriorations prompted by severe cadmium stress in sunflower plant when Trichoderma and bacterial inoculation were used as biofertilizers. Front. Plant Sci. 2022, 13, 1004173. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Li, Y.; Zhang, L.; Mu, J.; Jiang, Y.; Fu, H.; Zhang, Y.; Cui, H.; Yu, X.; Ye, Z. Biosynthetic pathways and functions of indole-3-acetic acid in microorganisms. Microorganisms 2023, 11, 2077. [Google Scholar] [CrossRef]
- Bhutani, N.; Maheshwari, R.; Negi, M.; Suneja, P. Optimization of IAA production by endophytic Bacillus spp. from Vigna radiata for their potential use as plant growth promoters. Isr. J. Plant Sci. 2018, 65, 83–96. [Google Scholar] [CrossRef]
- Ona, O.; Van Impe, J.; Prinsen, E.; Vanderleyden, J. Growth and indole-3-acetic acid biosynthesis of Azospirillum brasilense Sp245 is environmentally controlled. FEMS Microbiol. Lett. 2005, 246, 125–132. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH radical scavenging assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Alonazi, M.A.; Alwathnani, H.A.; AL-Barakah, F.N.I.; Alotaibi, F. Native plant growth-promoting rhizobacteria containing acc deaminase promote plant growth and alleviate salinity and heat stress in maize (Zea mays L.) plants in Saudi Arabia. Plants 2025, 14, 1107. [Google Scholar] [CrossRef]
- Fanai, A.; Bohia, B.; Lalremruati, F.; Lalhriatpuii, N.; Lalrokimi; Lalmuanpuii, R.; Singh, P.K.; Zothanpuia. Plant growth promoting bacteria (PGPB)-induced plant adaptations to stresses: An updated review. PeerJ 2024, 12, e17882. [Google Scholar] [CrossRef]
- De Mello-Prado, R. Introduction to plant nutrition. In Mineral Nutrition of Tropical Plants; Mello-Prado, R., Ed.; Springer Nature: Cham, Switzerland, 2021; pp. 1–38. [Google Scholar]
- Weraduwage, S.M.; Chen, J.; Anozie, F.C.; Morales, A.; Weise, S.E.; Sharkey, T.D. The relationship between leaf area growth and biomass accumulation in Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 167. [Google Scholar] [CrossRef] [PubMed]
- Diel, M.I.; Lúcio, A.D.C.; Tartaglia, F.D.L.; Tischler, A.L.; Lambrecht, D.M.; Zemolin, J.A.; Marques, L.E. New insights on the influence of the quality of tomato seedlings on production of fruits cultivated in substracts. Ciênc. Rural. 2022, 52, e20210428. [Google Scholar] [CrossRef]
- Zheng, Y.; Zou, J.; Lin, S.; Jin, C.; Shi, M.; Yang, B.; Yang, Y.; Jin, D.; Li, R.; Li, Y.; et al. Effects of different light intensity on the growth of tomato seedlings in a plant factory. PLoS ONE 2023, 18, e0294876. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, M.; Thompson, R.B.; Valdez, L.C.; Fernández, M.D. Use of stem diameter variations to detect plant water stress in tomato. Irrig. Sci. 2006, 24, 241–255. [Google Scholar] [CrossRef]
- Thomas, B. Sources Sinks. In Encyclopedia of Applied Plant Science; Elsevier: Oxford, UK, 2017; pp. 119–127. [Google Scholar]
- Fageria, N.K.; Moreira, A. The role of mineral nutrition on root growth of crop plants. Adv. Agron. 2011, 110, 251–331. [Google Scholar]

| Ca | K | Mg | Na | CEC | P | Cu | Fe | Mn | Zn | O. M. | Nt |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (cmol(+) kg−1) | (mg kg−1) | (%) | |||||||||
| 17.39 | 20.22 | 1.15 | 45.93 | 86.95 | 84.96 | 0.88 | 6.34 | 4.04 | 0.30 | 2.41 | 0.12 |
| HCO3- | CO32− | Cl- | SO42− | B | Na+ | P | K+ | Ca2+ | Mg2+ |
|---|---|---|---|---|---|---|---|---|---|
| (mg L−1) | |||||||||
| 2334.01 | 249.00 | 3515.62 | 2122.13 | 39.77 | 963.80 | 31.60 | 154.56 | 11.07 | 2.10 |
| Strain | NaCl (M) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 0.25 | 0.50 | 0.75 | 1 | 2 | 3 | |
| RD1 | + | + | + | + | + | − | − |
| RD2 | + | + | + | + | + | − | − |
| RD3 | + | + | + | + | + | − | − |
| RD4 | + | + | + | + | + | + | − |
| RD5 | + | + | + | + | − | − | − |
| RD6 | + | + | + | + | + | − | − |
| RD7 | + | + | + | + | + | + | − |
| RD8 | + | + | + | + | + | + | − |
| RD9 | + | + | + | + | + | + | − |
| RD10 | + | + | + | + | + | + | − |
| RD11 | + | + | + | + | + | + | − |
| RD12 | + | + | + | + | + | − | − |
| RD13 | + | + | + | + | + | + | − |
| RD14 | + | + | + | + | + | + | − |
| RD15 | + | + | + | + | + | + | − |
| RD16 | + | + | + | + | + | + | − |
| RD17 | + | + | + | + | + | + | − |
| RD18 | + | + | + | + | + | + | − |
| RD19 | + | + | + | + | + | + | − |
| RD20 | + | + | + | + | + | + | − |
| RD21 | + | + | + | + | + | + | − |
| RD22 | + | + | + | + | + | + | − |
| RD23 | + | + | + | + | + | + | − |
| RD24 | + | + | + | + | + | − | − |
| RD25 | + | + | + | + | + | − | − |
| RD26 | + | + | + | + | − | − | − |
| RD27 | + | + | + | + | + | + | − |
| RD28 | + | + | + | + | + | + | − |
| RD29 | + | + | + | + | + | + | − |
| Strain | Growth-Promoting Traits | ||||
|---|---|---|---|---|---|
| Siderophores | PS | IAA | EPS | ||
| Sucrose | Glucose | ||||
| RD1 | − | − | − | − | − |
| RD2 | + | − | + | − | − |
| RD4 | + | − | + | − | + |
| RD7 | − | − | − | − | − |
| RD8 | − | − | − | − | − |
| RD9 | − | − | − | − | − |
| RD10 | − | − | − | + | + |
| RD11 | − | − | − | + | − |
| RD13 | − | − | − | − | − |
| RD14 | − | − | − | − | + |
| RD15 | − | − | − | − | − |
| RD16 | − | − | − | − | − |
| RD17 | + | − | + | − | − |
| RD18 | − | − | − | − | − |
| RD19 | − | − | − | − | − |
| RD20 | − | − | − | − | − |
| RD21 | − | − | − | − | − |
| RD22 | − | − | − | − | − |
| RD23 | − | − | − | − | − |
| RD24 | − | − | − | − | − |
| RD25 | − | − | − | − | − |
| RD26 | + | − | + | − | − |
| RD27 | + | − | + | − | − |
| RD28 | − | − | − | + | − |
| RD29 | − | − | − | + | + |
| Bacterial Strains | p Value Correlation | Correlation Coefficient |
|---|---|---|
| JLB4 | 0.140 p | NS |
| RD2 | <0.001 s | 1 *** |
| RD4 | 0.350 s | NS |
| RD17 | 0.110 p | NS |
| RD26 | 0.170 p | NS |
| RD27 | 0.100 p | NS |
| Treatments | Stem Diameter (mm) | Shoot Dry Biomass (mg) | Root Dry Biomass (mg) |
|---|---|---|---|
| Control | 2.24 ± 0.07 c | 186.96 ± 18.08 b | 64.04 ± 6.60 b |
| JLB4 | 2.39 ± 0.02 ab | 227.47 ± 6.02 ab | 75.26 ± 5.62 ab |
| RD2 | 2.31 ± 0.08 bc | 221.86 ± 28.39 ab | 69.47 ± 8.77 ab |
| RD4 | 2.45 ± 0.05 a | 229.70 ± 23.13 a | 71.46 ± 10.18 ab |
| RD17 | 2.36 ± 0.03 abc | 224.53 ± 9.86 ab | 72.16 ± 4.63 ab |
| RD26 | 2.38 ± 0.06 ab | 243.15 ± 8.12 a | 81.44 ± 5.47 a |
| RD27 | 2.38 ± 0.04 ab | 233.70 ± 17.76 a | 64.92 ± 4.78 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mena-García, Á.; Alarcón, A.; Gómez-Merino, F.C.; Peralta-Sánchez, M.G.; Trejo-Téllez, L.I. Rhizosphere-Associated Bacteria of Saltgrass [Distichlis spicata (L.) Greene] Show Enhanced Ability to Tolerate Saline Environments and Stimulate Plant Growth. Microorganisms 2025, 13, 2046. https://doi.org/10.3390/microorganisms13092046
Mena-García Á, Alarcón A, Gómez-Merino FC, Peralta-Sánchez MG, Trejo-Téllez LI. Rhizosphere-Associated Bacteria of Saltgrass [Distichlis spicata (L.) Greene] Show Enhanced Ability to Tolerate Saline Environments and Stimulate Plant Growth. Microorganisms. 2025; 13(9):2046. https://doi.org/10.3390/microorganisms13092046
Chicago/Turabian StyleMena-García, Ángel, Alejandro Alarcón, Fernando C. Gómez-Merino, María G. Peralta-Sánchez, and Libia I. Trejo-Téllez. 2025. "Rhizosphere-Associated Bacteria of Saltgrass [Distichlis spicata (L.) Greene] Show Enhanced Ability to Tolerate Saline Environments and Stimulate Plant Growth" Microorganisms 13, no. 9: 2046. https://doi.org/10.3390/microorganisms13092046
APA StyleMena-García, Á., Alarcón, A., Gómez-Merino, F. C., Peralta-Sánchez, M. G., & Trejo-Téllez, L. I. (2025). Rhizosphere-Associated Bacteria of Saltgrass [Distichlis spicata (L.) Greene] Show Enhanced Ability to Tolerate Saline Environments and Stimulate Plant Growth. Microorganisms, 13(9), 2046. https://doi.org/10.3390/microorganisms13092046





