Gastric Candidiasis in Five Horses: A Case Series
Abstract
1. Introduction
2. Case Description
2.1. Case 1
2.2. Case 2
2.3. Case 3
2.4. Case 4
2.5. Case 5
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCS | Body Condition Score |
| KWPN | Koninklijk Warmbloed Paardenstamboek Nederland |
| EGUS | Equine Gastric Ulcer Syndrome |
| PCR | Polymerase Chain Reaction |
| NSAIDs | Non-Steroidal Anti-inflammatory Drugs |
| GGT | Gamma-Glutamyl Transferase |
| CPK | Creatin Phosphokinase |
| IBD | Inflammatory Bowel Disease |
References
- Sellon, D.C.; Long, M.T. Equine Infectious Diseases, 2nd ed.; Saunders/Elsevier: St. Louis, MO, USA, 2014; pp. 408–411. [Google Scholar]
- Cordeiro, R.D.A.; Bittencourt, P.V.; Brilhante, R.S.N.; Teixeira, C.E.C.; Castelo-Branco, D.D.S.C.M.; Silva, S.T.D.C.; De Alencar, L.P.; Souza, E.R.Y.; Bandeira, T.d.J.P.G.; Monteiro, A.J.; et al. Species of Candida as a component of the nasal microbiota of healthy horses. Med. Mycol. 2013, 51, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Linder, N.; Levit, O.; Klinger, G.; Kogan, I.; Levy, I.; Shalit, I.; Ashkenazi, S.; Sirota, L. Risk factors associated with candidaemia in the neonatal intensive care unit: A case–control study. J. Hosp. Infect. 2004, 57, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Pirrone, A.; Castagnetti, C.; Mariella, J.; Bonoli, C.; Stancampiano, L.; Tampieri, M.P.; Galuppi, R. Yeast Flora in Oropharyngeal and Rectal Mucous Membranes of Healthy and Critically Ill Neonatal Foals. J. Equine Vet. Sci. 2012, 32, 93–98. [Google Scholar] [CrossRef]
- Jawhara, S. How Gut Bacterial Dysbiosis Can Promote Candida albicans Overgrowth during Colonic Inflammation. Microorganisms 2022, 10, 1014. [Google Scholar] [CrossRef]
- Poulain, D. Candida albicans, plasticity and pathogenesis. Crit. Rev. Microbiol. 2013, 41, 208–217. [Google Scholar] [CrossRef]
- Kumamoto, C.A. Inflammation and gastrointestinal Candida colonization. Curr. Opin. Microbiol. 2011, 14, 386–391. [Google Scholar] [CrossRef]
- Bradford, K.; Meinkoth, J.; McKeirnen, K.; Love, B. Candida peritonitis in dogs: Report of 5 cases. Vet. Clin. Pathol. 2013, 4, 227–233. [Google Scholar] [CrossRef]
- Nadăş, G.C.; Taulescu, M.A.; Ciobanu, L.; Fiţ, N.I.; Flore, C.; Răpuntean, S.; Bouari, C.M.; Catoi, C. The Interplay Between NSAIDs and Candida albicans on the Gastrointestinal Tract of Guinea Pigs. Mycopathologia 2013, 175, 221–230. [Google Scholar] [CrossRef]
- Randolph, N.K.; Her, J.; McAloney, C.A.; Wellman, M. Gastrointestinal colonization by Diutina (Candida) rugosa in a 6-year-old Siberian Husky. Vet. Clin. Pathol. 2024, 53, 255–260. [Google Scholar] [CrossRef]
- Doyle, A.; López, A.; Pack, L.; Muckle, A. Candida osteomyelitis in a gelding. Can. Vet. J. 2013, 54, 176. [Google Scholar] [PubMed]
- Montes, A.J.; Montes, L.F.; Vaughan, J.; Wilborn, W.H.; Bado, G.; Comero, A.; Blaquier, P.C. Vulvo vaginal candidiasis in thoroughbred mares following progestogen administration intravaginal treatment with clotrimazole. J. Equine Vet. Sci. 2001, 21, 68–70. [Google Scholar] [CrossRef]
- Cafarchia, C.; Figueredo, L.A.; Otranto, D. Fungal diseases of horses. Vet. Microbiol. 2013, 167, 215–234. [Google Scholar] [CrossRef] [PubMed]
- Stefanetti, V.; Marenzoni, M.L.; Lepri, E.; Coletti, M.; Proietti, P.C.; Agnetti, F.; Crotti, S.; Pitzurra, L.; Del Sero, A.; Passamonti, F. A case of Candida guilliermondii abortion in an Arab mare. Med. Mycol. Case Rep. 2014, 4, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Gross, T.; Matyhew, I. Gastroesophageal ulceration and candidiasis in foals. J. Am. Vet. Med. Assoc. 1983, 15, 1370–1373. [Google Scholar] [CrossRef] [PubMed]
- Reilly, L.K.; Palmer, J.E. Systemic candidiasis in four foals. J. Am. Vet. Med. Assoc. 1994, 205, 464–466. [Google Scholar] [CrossRef] [PubMed]
- Henneke, D.R.; Potter, G.D.; Kreider, J.L.; Yeates, B.F. Relationship between condition score, physical measurements and body fat percentage in mares. Equine Vet. J. 1983, 15, 371–372. [Google Scholar] [CrossRef]
- Sykes, B.W.; Hewetson, M.; Hepburn, R.J.; Luthersson, N.; Tamzali, Y. European College of Equine Internal Medicine Consensus Statement—Equine Gastric Ulcer Syndrome in Adult Horses. J. Vet. Intern. Med. 2015, 29, 1288–1299. [Google Scholar] [CrossRef]
- McClure, J.J.; Addison, J.D.; Miller, R.I. Immunodeficiency manifested by oral candidiasis and bacterial septicemia in foals. J. Am. Vet. Med. Assoc. 1985, 186, 1195–1197. [Google Scholar] [CrossRef] [PubMed]
- Preis, I.S.; Silva, R.O.S.; Pires, P.S.; Lobato, F.C.F.; Palhares, M.S.; Maranhão, R.P.A.; Ecco, R. Enteritis associated with Clostridium difficile and opportunistic candidiasis in a foal. Braz. J. Vet. Path. 2012, 5, 10–12. Available online: https://bjvp.org.br/wp-content/uploads/2015/07/DOWNLOAD-FULL-ARTICLE-2-20881_2012_3_30_8_11.pdf (accessed on 15 May 2024).
- Cohen, J.M.; Ross, M.W.; Busschers, E. Diagnosis and management of Candida utilis infectious arthritis in a Standardbred filly. Equine Vet. Educ. 2008, 20, 348–352. [Google Scholar] [CrossRef]
- Daniell, H.W. Acid suppressing therapy as a risk factor for Candida esophagitis. Dis. Esophagus 2016, 29, 479–483. [Google Scholar] [CrossRef]
- Larner, A.J.; Lendrum, R. Oesophageal candidiasis after omeprazole therapy. Gut 1992, 33, 860–861. [Google Scholar] [CrossRef]
- Mottaghi, B.; Emami, M.H.; Riahi, P.; Fahim, A.; Rahimi, H. Candida colonization of the esophagus and gastric mucosa; a comparison of patients taking proton pump inhibitors and those taking histamine receptor antagonist drugs. Gastroenterol. Hepatol. Bed Bench 2021, 14, 349–355. [Google Scholar] [PubMed] [PubMed Central]
- Shah, M.D.N.; Cavanagh, Y.; Shulik, O.; Patel, P.; DeBari, V.A.; Baddoura, W. Proton pump inhibitors and corticosteroids as synergistic risk factors for Candida esophagitis. J. Adv. Med. Res. 2015, 10, 1–6. [Google Scholar] [CrossRef]
- Brzozowski, T.; Zwolinska-Wcislo, M.; Konturek, P.C.; Kwiecien, S.; Drozdowicz, D.; Konturek, S.J.; Stachura, J.; Budak, A.; Bogdal, J.; Pawlik, W.W.; et al. Influence of gastric colonization with Candida albicans on ulcer healing in rats: Effect of ranitidine, aspirin and probiotic therapy. Scand. J. Gastroenterol. 2005, 40, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Perkins, G.A.; Bakker, H.C.D.; Burton, A.J.; Erb, H.N.; McDonough, S.P.; McDonough, P.L.; Parker, J.; Rosenthal, R.L.; Wiedmann, M.; Dowd, S.E.; et al. Equine Stomachs Harbor an Abundant and Diverse Mucosal Microbiota. Appl. Environ. Microbiol. 2012, 78, 2522–2532. [Google Scholar] [CrossRef]
- Boucher, L.; Leduc, L.; Leclère, M.; Costa, M.C. Current Understanding of Equine Gut Dysbiosis and Microbiota Manipulation Techniques: Comparison with Current Knowledge in Other Species. Animals 2024, 14, 758. [Google Scholar] [CrossRef]
- Pinto, N.C.; Bosch, J.N.; Ng-Wong, Y.K.; Menowsky, M.; Shine, R.; Moya, M.; Galindo, J.; Serna, S.; Ii, M.M.; Malcolm, J. A Case Report of Candida-Induced Emphysematous Gastritis. Cureus 2023, 15, 47870. [Google Scholar] [CrossRef]
- Gottlieb, K.; Iber, F.L.; Livak, A.; Leya, J.; Mobarhan, S. Oral Candida Colonizes the Stomach and Gastrostomy Feeding Tubes. J. Parenter. Enter. Nutr. 1994, 18, 264–267. [Google Scholar] [CrossRef]
- Kreulen, I.A.M.; De Jonge, W.J.; Van Den Wijngaard, R.M.; Van Thiel, I.A.M. Candida spp. in Human Intestinal Health and Disease: More than a Gut Feeling. Mycopathologia 2023, 188, 845–862. [Google Scholar] [CrossRef]
- Veses, V.; Gow, N.A.R. Pseudohypha budding patterns of Candida albicans. Med. Mycol. 2009, 47, 268–275. [Google Scholar] [CrossRef]
- Sasaki, K. Candida-associated gastric ulcer relapsing in a different position with a different appearance. World J. Gastroenterol. 2012, 18, 4450. [Google Scholar] [CrossRef]
- Akpan, A.; Morgan, R. Oral candidiasis. Postgrad. Med. J. 2002, 78, 455–459. [Google Scholar] [CrossRef]
- Vitale, V. Inflammatory bowel diseases in horses: What do we know? Equine Vet. Educ. 2022, 34, 493–500. [Google Scholar] [CrossRef]
- Patnaik, S.; Durairajan, S.S.K.; Singh, A.K.; Krishnamoorthi, S.; Iyaswamy, A.; Mandavi, S.P.; Jeewon, R.; Williams, L.L. Role of Candida Species in Pathogenesis, Immune Regulation, and Prognostic Tools for Managing Ulcerative Colitis and Crohn’s Disease. World J. Gastroenterol. 2024, 30, 5212–5220. [Google Scholar] [CrossRef]
- Di Febo, G.; Miglioli, M.; Calò, G.; Biasco, G.; Luzza, F.; Gizzi, G.; Cipollini, F.; Rossi, A.; Barbara, L. Candida albicans infection of gastric ulcer frequency and correlation with medical treatment: Results of a multicenter study. Dig. Dis. Sci. 1985, 30, 178–181. [Google Scholar] [CrossRef]
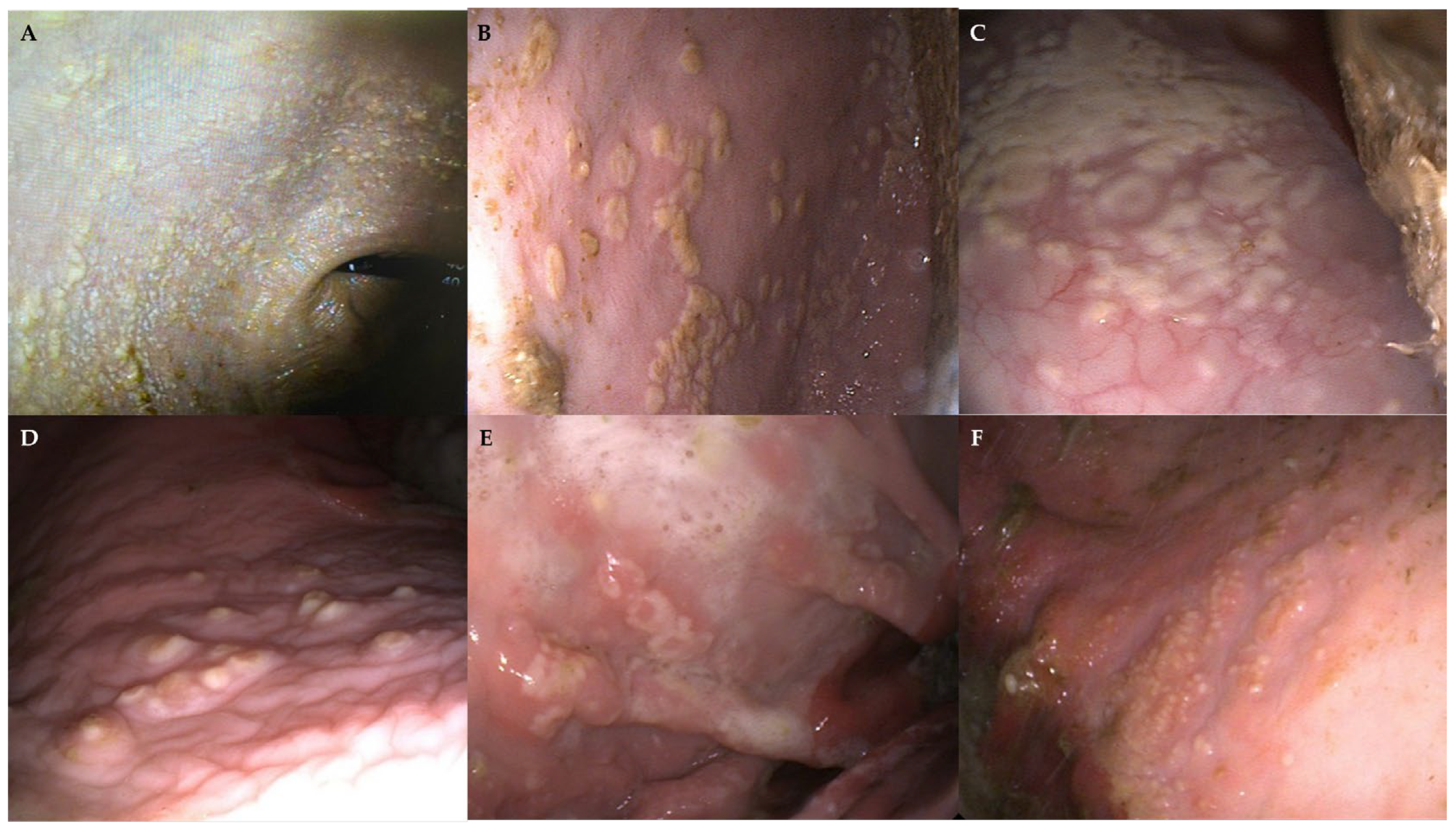
| Macroscopic Findings | |||
|---|---|---|---|
| Squamous Mucosa | Glandular Mucosa | Duodenum | |
| Case 1 | White, cotton-like material Ulcers grade 2/4 | Hyperemic areas in the antrum, pylorus, and duodenum | Hyperemic areas and duodenal reflux |
| Case 2 | Friable white pseudo-membranous multifocal to coalescing plaques | Normal | Hyperemic areas |
| Case 3 | Two dispersed white, pearl-shaped, well-demarcated pseudomembranous patches | Normal | |
| Case 4 | White ring-shaped and multifocal pseudo-membranous friable plaques, most of them being closer to the greater curvature. An underlying hyperaemic area, extending beyond the plaques, was visible. | Linear raised fibrinosuppurative ulcer that originated from the pyloric region and extended towards the antrum | Presence of two parasites (Anoplocephala spp.) |
| Case 5 | White multifocal miliary vesicles surrounded by a reddish halo. Several ulcers grade 2/4 at the level of the greater and lesser curvature close to the margo plicatum. | Two severe fibrinohemorragic and depressed ulcers adjacent to the pylorus, one of them with fibrinosuppurative centre | Normal |
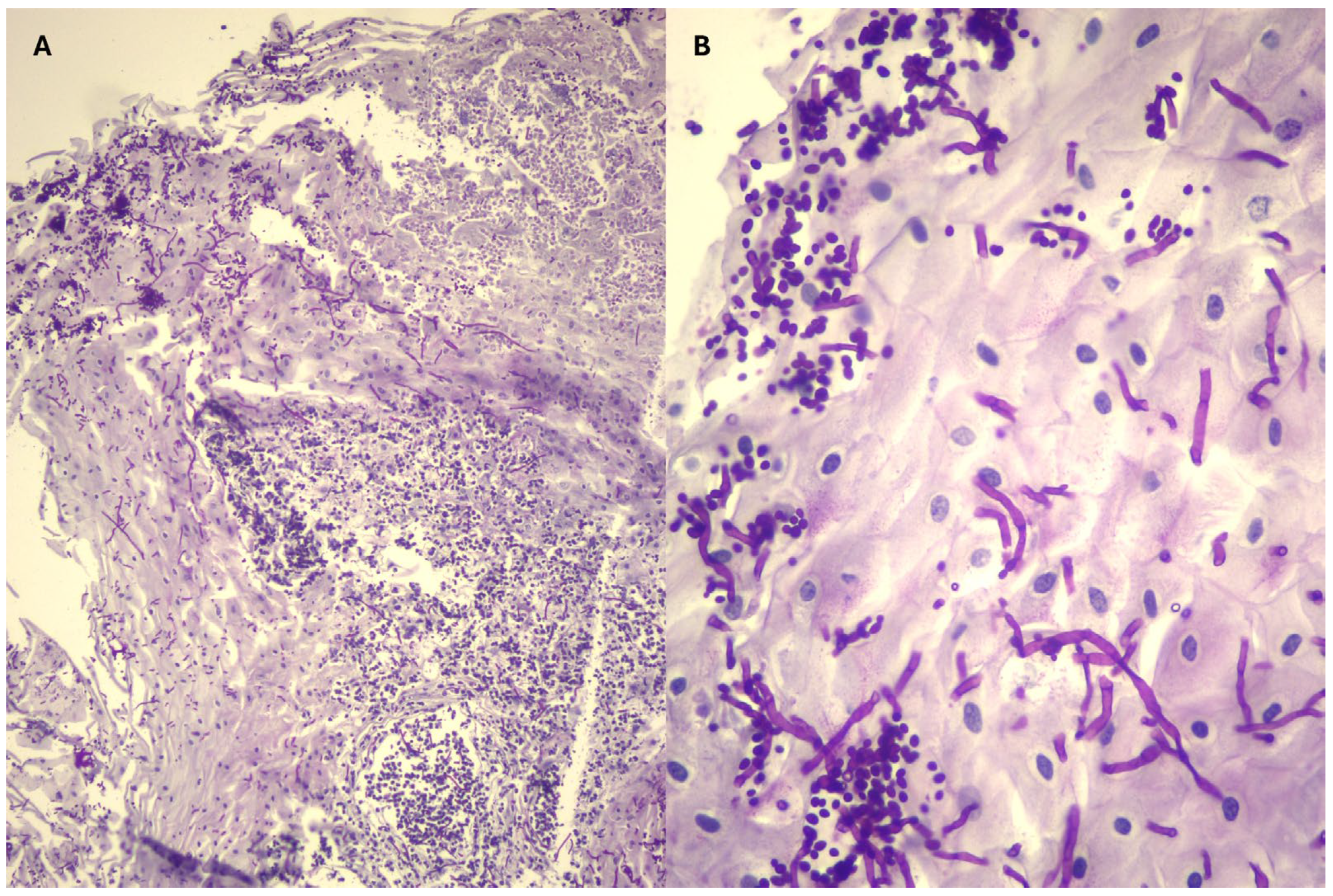
| Cases | Gastric Biopsy | Duodenal Biopsies | Culture |
|---|---|---|---|
| 1 | Squamous epithelium without hyperkeratosis | Mild lymphoplasmacytic infiltrate | Candida spp. |
| 2 | Squamous epithelium without hyperkeratosis with formation of micro-pustules and dilated necrotic-suppurative material. PAS staining showed heavy infiltration of the surface layers by hyphae. | Lymphoplasmacytic infiltrate (mild in the lamina propria) with marked villous atrophy, erosions, with cryptid lesions. Bacterial proliferation on the surface, with no evidence of fungal elements. | Candida spp. |
| 3 | Normal squamous epithelium and necrotic-purulent material with mycotic elements (hyphae) and bacteria. Rare hyphae identified by PAS staining. | Mild to moderate lymphoplasmacytic infiltrate with bacterial proliferation laying on top. No fungal, parasitic or fungal or elements in the PAS staining. | No growth |
| 4 | 1st gastroscopy: Hyperkeratotic epithelium with hyphae or pseusohyphae, which appeared branched but not really segmented. There were often budding yeasts with a narrow base (suggesting Candida). Neutrophilic inflammation and bacterial colonisation (mostly bacilli and cocci). Pyloric lesion: Ulcerated surface with abundant fibrinopurulent material. The PAS staining did not reveal any mycosis. Giemsa staining did not reveal any significant bacterial flora. 2nd gastroscopy: Thick squamous epithelium with regular well differentiated cells. Some superficial bacteria. No inflammatory infiltrate. No fungal elements on PAS staining. | Second gastroscopy: Normal duodenal epithelium | Candida spp. |
| 5 | Hyperkeratosis infiltrated by a purulent inflammatory reaction and mycotic proliferation (hyphae and pseudohyphae). | Normal duodenal epithelium | Candida glabrata |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neira-Egea, P.; Alamar Malvoisin, C.; de la Cuesta-Torrado, M.; Bautista-Erler, C.; Vitale, V.; Jolly, S.; Cesarini, C. Gastric Candidiasis in Five Horses: A Case Series. Microorganisms 2025, 13, 1746. https://doi.org/10.3390/microorganisms13081746
Neira-Egea P, Alamar Malvoisin C, de la Cuesta-Torrado M, Bautista-Erler C, Vitale V, Jolly S, Cesarini C. Gastric Candidiasis in Five Horses: A Case Series. Microorganisms. 2025; 13(8):1746. https://doi.org/10.3390/microorganisms13081746
Chicago/Turabian StyleNeira-Egea, Patricia, Clara Alamar Malvoisin, María de la Cuesta-Torrado, Claudia Bautista-Erler, Valentina Vitale, Sandra Jolly, and Carla Cesarini. 2025. "Gastric Candidiasis in Five Horses: A Case Series" Microorganisms 13, no. 8: 1746. https://doi.org/10.3390/microorganisms13081746
APA StyleNeira-Egea, P., Alamar Malvoisin, C., de la Cuesta-Torrado, M., Bautista-Erler, C., Vitale, V., Jolly, S., & Cesarini, C. (2025). Gastric Candidiasis in Five Horses: A Case Series. Microorganisms, 13(8), 1746. https://doi.org/10.3390/microorganisms13081746






