Identification of a Tick Midgut Protein Involved in Babesia bovis Infection of Female Rhipicephalus microplus Ticks
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Ticks, and Pathogen
2.2. Proteomic Analysis of R. microplus Female Ticks Exposed to B. bovis
2.3. Synthesis of Double Stranded RNA
2.4. Injection of Ticks with dsRNA
2.5. Gene Expression and Silencing
2.6. Evaluation of Tick Fitness
2.7. Infection Rate of B. bovis in R. microplus Adult Females, Egg Mass, and Larvae
2.8. Statistical Analyses
3. Results
3.1. Proteomic Analysis
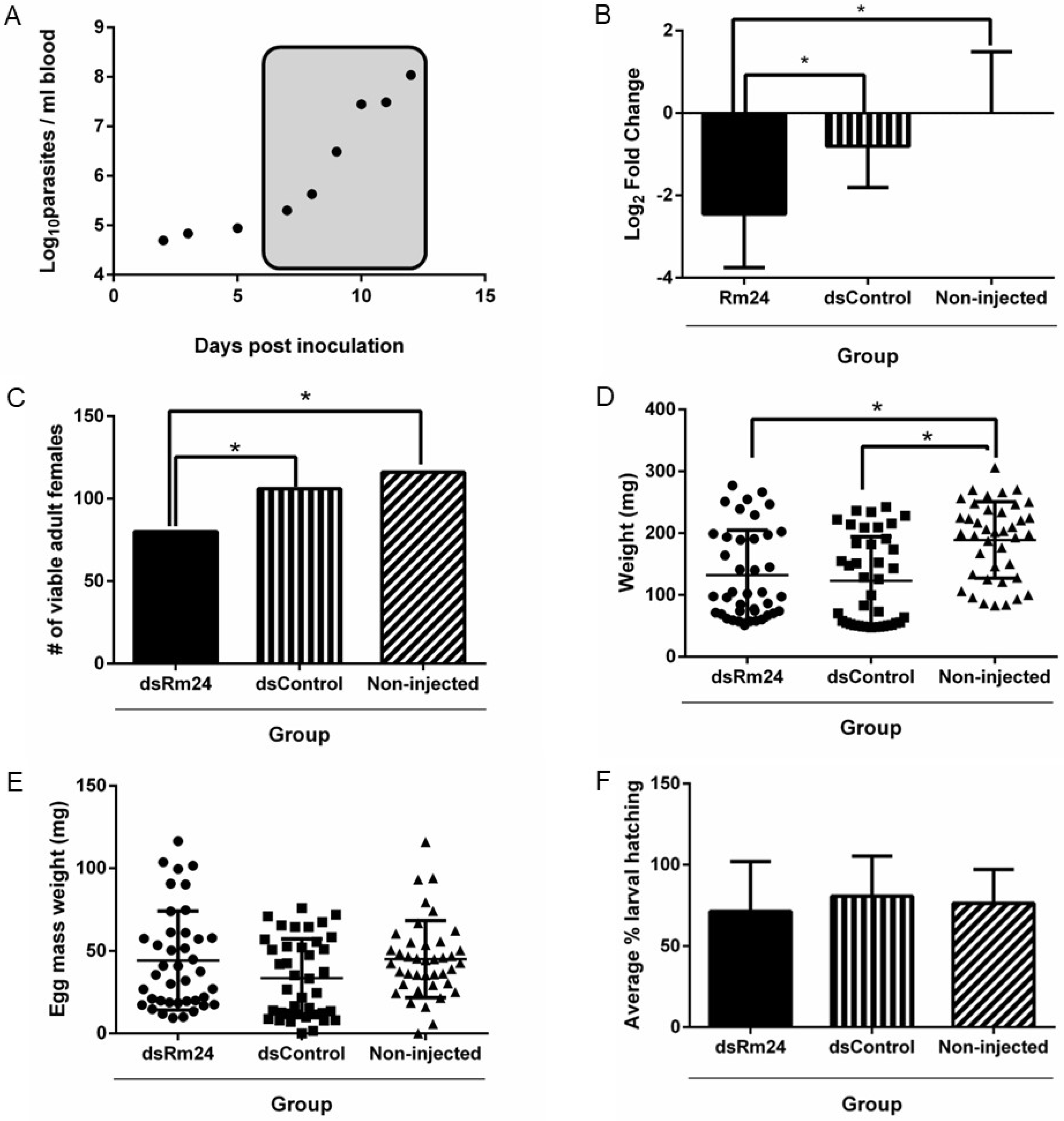
3.2. RNA Interference
3.3. Effect of Gene Silencing on Tick Fitness
3.4. Babesia Bovis Infection of Engorged R. microplus Female Ticks, Egg Mass, and Larvae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gou, H.; Guan, G.; Liu, A.; Ma, M.; Chen, Z.; Liu, Z.; Ren, Q.; Li, Y.; Yang, J.; Yin, H.; et al. Coevolutionary analyses of the relationships between piroplasmids and their hard tick hosts. Ecol. Evol. 2013, 3, 2985–2993. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; Mallón, A.R.; Bermúdez, S.; de la Fuente, J.; Domingos, A.; García, M.P.E.; Labruna, M.B.; Merino, O.; Mosqueda, J.; Nava, S.; et al. One Health Approach to Identify Research Needs on Rhipicephalus Microplus Ticks in the Americas. Pathogens 2022, 11, 1180. [Google Scholar] [CrossRef] [PubMed]
- Elsworth, B.; Duraisingh, M.T. A Framework for signaling throughout the life cycle of Babesia species. Mol. Microbiol. 2021, 115, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Homer, M.J.; Aguilar-Delfin, I.; Telford, S.R.; Krause, P.J.; Persing, D.H. Babesiosis. Clin. Microbiol. Rev. 2000, 13, 451–469. [Google Scholar] [CrossRef] [PubMed]
- Antunes, S.; Rosa, C.; Couto, J.; Ferrolho, J.; Domingos, A. Deciphering Babesia-Vector Interactions. Front. Cell. Infect. Microbiol. 2017, 7, 429. [Google Scholar] [CrossRef] [PubMed]
- Bock, R.E.; Jackson, L.A.; De Vos, A.J.; Jorgensen, W.K. Babesiosis of Cattle. In Ticks: Biology, Disease and Control; Cambridge University Press: Cambridge, UK, 2008; pp. 281–307. [Google Scholar]
- Dalgliesh, R.J.; Stewart, N.P. The use of tick transmission by Boophilus microplus to isolate pure strains of Babesia bovis, Babesia bigemina and Anaplasma marginale from cattle with mixed infections. Vet. Parasitol. 1983, 13, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Obaid, M.K.; Islam, N.; Alouffi, A.; Khan, A.Z.; da Silva Vaz, I.; Tanaka, T.; Ali, A. Acaricides resistance in ticks: Selection, diagnosis, mechanisms, and mitigation. Front. Cell. Infect. Microbiol. 2022, 12, 941831. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, J.; Rodríguez, M.; Montero, C.; Redondo, M.; García-García, J.C.; Méndez, L.; Serrano, E.; Valdés, M.; Enríquez, A.; Canales, M.; et al. Vaccination against ticks (Boophilus spp.): The Experience with the Bm86-based vaccine GavacTM. Genet. Anal. 1999, 15, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, N.N.; Matschoss, A.L.; Pepper, P.; Green, P.E.; Albrecht, M.S.; Hungerford, J.; Ansell, J. Evaluation of TickGARD(PLUS), a novel vaccine against Boophilus microplus, in lactating Holstein–Friesian cows. Vet. Parasitol. 2000, 88, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Merino, O.; Almazán, C.; Canales, M.; Villar, M.; Moreno-Cid, J.A.; Galindo, R.C.; de la Fuente, J. Targeting the Tick Protective Antigen Subolesin Reduces Vector Infestations and Pathogen Infection by Anaplasma Marginale and Babesia Bigemina. Vaccine 2011, 29, 8575–8579. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.G.; Bastos, R.G.; Laughery, J.M.; Alzan, H.F.; Rathinasamy, V.A.; Cooke, B.M.; Suarez, C.E. Vaccination of cattle with the Babesia bovis sexual-stage protein HAP2 abrogates parasite transmission by Rhipicephalus microplus ticks. npj Vaccines 2023, 8, 140. [Google Scholar] [CrossRef] [PubMed]
- Suarez, C.E.; Noh, S. Emerging perspectives in the research of bovine babesiosis and anaplasmosis. Vet. Parasitol. 2011, 180, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold protein structure database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Blum, M.; Andreeva, A.; Florentino, L.C.; Chuguransky, S.R.; Grego, T.; Hobbs, E.; Pinto, B.L.; Orr, A.; Paysan-Lafosse, T.; Ponamareva, I.; et al. InterPro: The Protein sequence classification resource in 2025. Nucleic Acids Res. 2025, 53, D444–D456. [Google Scholar] [CrossRef] [PubMed]
- Jia, N.; Wang, J.; Shi, W.; Du, L.; Sun, Y.; Zhan, W.; Jiang, J.-F.; Wang, Q.; Zhang, B.; Ji, P.; et al. Large-scale comparative analyses of tick genomes elucidate their genetic diversity and vector capacities. Cell 2020, 182, 1328–1340.e13. [Google Scholar] [CrossRef] [PubMed]
- Horn, T.; Boutros, M. E-RNAi: A Web application for the multi-species design of RNAi Reagents—2010 Update. Nucleic Acids Res. 2010, 38, W332–W339. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.C.; Taus, N.S.; Reif, K.E.; Bohaliga, G.A.R.; Kappmeyer, L.S.; Ueti, M.W. Analysis of stage-specific protein expression during Babesia bovis development within female Rhipicephalus microplus. J. Proteome Res. 2017, 16, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Ueti, M.W.; Johnson, W.C.; Kappmeyer, L.S.; Herndon, D.R.; Mousel, M.R.; Reif, K.E.; Taus, N.S.; Ifeonu, O.O.; Silva, J.C.; Suarez, C.E.; et al. Comparative analysis of gene expression between Babesia bovis blood stages and kinetes allowed by improved genome annotation. Int. J. Parasitol. 2021, 51, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Waldman, J.; Ibanez-Carrasco, F.; Tirloni, L.; Waltero, C.; Calixo, C.; Braz, G.R.; Mulenga, A.; da Silva Vaz Junior, I.; Logullo, C. Stable internal reference genes for quantitative RT-PCR analyses in Rhipicephalus microplus during embryogenesis. Ticks Tick Borne Dis. 2023, 14, 102251. [Google Scholar] [CrossRef] [PubMed]
- Howell, J.M.; Ueti, M.W.; Palmer, G.H.; Scoles, G.A.; Knowles, D.P. Transovarial transmission efficiency of Babesia bovis tick stages acquired by Rhipicephalus (Boophilus) microplus during acute infection. J. Clin. Microbiol. 2007, 45, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Dalgliesh, R.J.; Stewart, N.P. Some effects of time, temperature and feeding on infection rates with Babesia bovis and Babesia bigemina in Boophilus microplus larvae. Int. J. Parasitol. 1982, 12, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Rachinsky, A.; Guerrero, F.D.; Scoles, G.A. Proteomic profiling of Rhipicephalus (Boophilus) microplus midgut responses to infection with Babesia bovis. Vet. Parasitol. 2008, 152, 294–313. [Google Scholar] [CrossRef] [PubMed]
- Antunes, S.; Couto, J.; Ferrolho, J.; Sanches, G.S.; Merino Charrez, J.O.; De la Cruz Hernández, N.; Mazuz, M.; Villar, M.; Shkap, V.; de la Fuente, J.; et al. Transcriptome and proteome response of Rhipicephalus annulatus tick vector to Babesia bigemina infection. Front. Physiol. 2019, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Heekin, A.M.; Guerrero, F.D.; Bendele, K.G.; Saldivar, L.; Scoles, G.A.; Dowd, S.E.; Gondro, C.; Nene, V.; Djikeng, A.; Brayton, K.A. Gut Transcriptome of engorged adult female cattle ticks, Rhipicephalus (Boophilus) microplus, feeding upon a Babesia bovis-infected bovine host. Parasitol. Res. 2013, 112, 3075–3090. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.E.; Johnson, W.C.; Ueti, M.W. Differential paired stage-specific expression of Babesia bovis cysteine-rich GCC2/GCC3 domain family proteins (BboGDP) during development within Rhipicephalus microplus. Parasites Vectors 2023, 16, 16. [Google Scholar] [CrossRef] [PubMed]
- Earls, K.N.; Poh, K.; Ueti, M.; Oyen, K. Infection with Babesia bovis alters metabolic rates of Rhipicephalus microplus ticks across life stages. Parasit Vectors 2025, 18, 81. [Google Scholar] [CrossRef] [PubMed]
- Martins da Silva, R.; de Oliveira Daumas Filho, C.R.; Calixto, C.; Nascimento da Silva, J.; Lopes, C.; da Silva Vaz, I., Jr.; Logullo, C. PEPCK and glucose metabolism homeostasis in arthropods. Insect Biochem. Mol. Biol. 2023, 160, 103986. [Google Scholar] [CrossRef] [PubMed]
- Cabezas-Cruz, A.; Espinosa, P.J.; Obregón, D.A.; Alberdi, P.; de la Fuente, J. Ixodes scapularis tick cells control Anaplasma phagocytophilum infection by increasing the synthesis of phosphoenolpyruvate from tyrosine. Front. Cell. Infect. Microbiol. 2017, 7, 375. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Khan, S.; Ali, I.; Karim, S.; da Silva Vaz, I., Jr.; Termignoni, C. Probing the functional role of tick metalloproteases. Physiol. Entomol. 2015, 40, 177–188. [Google Scholar] [CrossRef]
- Sojka, D.; Hajdušek, O.; Dvořák, J.; Sajid, M.; Franta, Z.; Schneider, E.L.; Craik, C.S.; Vancová, M.; Burešová, V.; Bogyo, M.; et al. IrAE—An Asparaginyl endopeptidase (Legumain) in the gut of the hard tick Ixodes ricinus. Int. J. Parasitol. 2007, 37, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, A.; Valdés, J.J.; Kotsyfakis, M. The Role of Cystatins in tick physiology and blood reeding. Ticks Tick Borne Dis. 2012, 3, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ueda, M.; Umemiya, R.; Battsetseg, B.; Boldbaatar, D.; Xuan, X.; Fujisaki, K. A secreted cystatin from the tick Haemaphysalis longicornis and its distinct expression patterns in relation to innate immunity. Insect Biochem. Mol. Biol. 2006, 36, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Ceraul, S.M.; Chung, A.; Sears, K.T.; Popov, V.L.; Beier-Sexton, M.; Rahman, M.S.; Azad, A.F. A Kunitz Protease inhibitor from Dermacentor variabilis, a vector for spotted fever group Rickettsiae, limits Rickettsia montanensis invasion. Infect. Immun. 2011, 79, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Agwunobi, D.O.; Wang, T.; Zhang, M.; Wang, T.; Jia, Q.; Zhang, M.; Shi, X.; Yu, Z.; Liu, J. Functional implication of heat shock protein 70/90 and tubulin in cold stress of Dermacentor silvarum. Parasit Vectors 2021, 14, 542. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, E.P.; Talactac, M.R.; Fujisaki, K.; Tanaka, T. The case for oxidative stress molecule involvement in the tick-pathogen interactions -an omics approach. Dev. Comp. Immunol. 2019, 100, 103409. [Google Scholar] [CrossRef] [PubMed]
- Sabadin, G.A.; Salomon, T.B.; Leite, M.S.; Benfato, M.S.; Oliveira, P.L.; da Silva Vaz, I. An insight into the functional role of antioxidant and detoxification enzymes in adult Rhipicephalus microplus female ticks. Parasitol. Int. 2021, 81, 102274. [Google Scholar] [CrossRef] [PubMed]
- Busby, A.T.; Ayllón, N.; Kocan, K.M.; Blouin, E.F.; De La Fuente, G.; Galindo, R.C.; Villar, M.; De La Fuente, J. Expression of heat shock proteins and subolesin affects stress responses, Anaplasma phagocytophilum infection and questing behaviour in the tick, Ixodes scapularis. Med. Vet. Entomol. 2012, 26, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.B.; McVicar, M.; Beniwal, S.; Sharma, A.; Tillett, R.; Petereit, J.; Nuss, A.; Gulia-Nuss, M. A Multi-Omics Approach for Understanding Blood Digestion Dynamics in Ixodes Scapularis and Identification of Anti-Tick Vaccine Targets. Ticks Tick Borne Dis. 2024, 15, 102379. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Sonenshine, D.E.; Valenzuela, J.G. Exploring the Mialome of Ticks: An Annotated Catalogue of Midgut Transcripts from the Hard Tick, Dermacentor Variabilis (Acari: Ixodidae). BMC Genom. 2008, 9, 552. [Google Scholar] [CrossRef] [PubMed]
- Dean, T.; Xu, R.; Joiner, W.; Sehgal, A.; Hoshi, T. Drosophila QVR/SSS Modulates the Activation and C-Type Inactivation Kinetics of Shaker K(+) Channels. J. Neurosci. 2011, 31, 11387–11395. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Robinson, J.E.; Joiner, W.J. SLEEPLESS Is a Bifunctional Regulator of Excitability and Cholinergic Synaptic Transmission. Curr. Biol. 2014, 24, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Aung, K.M.; Boldbaatar, D.; Liao, M.; Umemiya-Shirafuji, R.; Nakao, S.; Matsuoka, T.; Tanaka, T.; Fujisaki, K. Identification and Characterization of Class B Scavenger Receptor CD36 from the Hard Tick, Haemaphysalis Longicornis. Parasitol. Res. 2011, 108, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Healy, J.A. Phosphoglucomutase Polymorphism in the Tick Ixodes Ricinus. Parasitology 1979, 78, 7–17. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Kitsou, C.; Sonenshine, D.E.; Pedra, J.H.F.; Fikrig, E.; Kassis, J.A.; Pal, U. Epigenetic regulation of tick biology and vectorial capacity. Trends Genet. 2021, 37, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Sojka, D.; Franta, Z.; Horn, M.; Caffrey, C.R.; Mareš, M.; Kopáček, P. New insights into the machinery of blood digestion by ticks. Trends Parasitol. 2013, 29, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Budachetri, K.; Crispell, G.; Karim, S. Amblyomma Maculatum SECIS Binding Protein 2 and Putative Selenoprotein P Are Indispensable for Pathogen Replication and Tick Fecundity. Insect Biochem. Mol. Biol. 2017, 88, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Embers, M.; Mather, T.N.; Karim, S. Is Selenoprotein K Required for Borrelia Burgdorferi Infection within the Tick Vector Ixodes Scapularis? Parasites Vectors 2019, 12, 289. [Google Scholar] [CrossRef] [PubMed]
- Burtenshaw, S.M.; Su, P.P.; Zhang, J.R.; Tobe, S.S.; Dayton, L.; Bendena, W.G. A Putative Farnesoic Acid O-Methyltransferase (FAMeT) Orthologue in Drosophila Melanogaster (CG10527): Relationship to Juvenile Hormone Biosynthesis? Peptides 2008, 29, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Buarque, D.S.; Braz, G.R.C.; Martins, R.M.; Tanaka-Azevedo, A.M.; Gomes, C.M.; Oliveira, F.A.A.; Schenkman, S.; Tanaka, A.S. Differential expression profiles in the midgut of Triatoma infestans infected with Trypanosoma cruzi. PLoS ONE 2013, 8, e61203. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, S.; Schuijt, T.J.; Abraham, N.M.; Rajeevan, N.; Coumou, J.; Graham, M.; Robson, A.; Wu, M.-J.; Daffre, S.; Hovius, J.W.; et al. Modulation of the tick gut milieu by a secreted tick protein favors Borrelia burgdorferi colonization. Nat. Commun. 2017, 8, 184. [Google Scholar] [CrossRef] [PubMed]
- Bastos, R.G.; Ueti, M.W.; Guerrero, F.D.; Knowles, D.P.; Scoles, G.A. Silencing of a putative immunophilin gene in the cattle tick Rhipicephalus (Boophilus) microplus increases the infection rate of Babesia bovis in larval progeny. Parasit Vectors 2009, 2, 57. [Google Scholar] [CrossRef] [PubMed]
- Willadsen, P.; Riding, G.A.; McKenna, R.V.; Kemp, D.H.; Tellam, R.L.; Nielsen, J.N.; Lahnstein, J.; Cobon, G.S.; Gough, J.M. Immunologic Control of a Parasitic Arthropod. Identification of a Protective Antigen from Boophilus microplus. J. Immunol. 1989, 143, 1346–1351. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Garg, R.; Yadav, C.L.; Vatsya, S.; Kumar, R.R.; Sugumar, P.; Chandran, D.; Mangamoorib, L.N.; Bedarkar, S.N. Immune Responses against Recombinant Tick Antigen, Bm95, for the Control of Rhipicephalus (Boophilus) Microplus Ticks in Cattle. Vet. Parasitol. 2009, 165, 119–124. [Google Scholar] [CrossRef] [PubMed]


| Accession | Protein Description | Sequest HT Score | Coverage % | Sum PEP Score | # Peptide | # PSMs | Up/Down Regulation | p Value |
|---|---|---|---|---|---|---|---|---|
| A0A6M2D867 | Conserved secreted | 419.11 | 62 | 68.00 | 9 | 132 | 2.1-fold increase | 0.022 |
| A0A6G5AC63 | Selenium binding | 424.94 | 61 | 149.80 | 21 | 138 | 2.3-fold increase | 0.008 |
| A0A6M2CI31 | Farnesoic acid o-methyltransferase | 83.32 | 87 | 49.43 | 8 | 29 | 11.5-fold increase | 0.049 |
| A0A6G5A0W4 | Metallopeptidase | 55.28 | 25 | 29.37 | 6 | 15 | 3.3-fold increase | 0.048 |
| A0A6G4ZX34 | Myosin class ii heavy chain | 171.62 | 27 | 69.24 | 14 | 44 | 1.5-fold decrease | 0.041 |
| A0A034WTW0 | Kunitz domain-containing protein | 308.11 | 24 | 61.48 | 11 | 97 | 1.3-fold decrease | 0.034 |
| A0A6G5A749 | cystatin | 75.3 | 51 | 19.71 | 5 | 25 | 3.8-fold decrease | 0.027 |
| A0A6M2CKK4 | Calmodulin | 71.47 | 26 | 24.96 | 2 | 17 | 3.3-fold decrease | 0.035 |
| A0A6M2CYW0 | Proline and glutamine-rich splicing factor | 65.99 | 10 | 29.64 | 5 | 18 | 3.0-fold decrease | 0.031 |
| A0A6M2CKP6 | Phosphoenolpyruvate carboxykinase (GTP) | 264.53 | 40 | 94.60 | 15 | 70 | 1.9-fold decrease | 0.006 |
| A0A6M2CGM2 | Legumain-like protease | 58.15 | 21 | 26.82 | 4 | 16 | 4.9-fold decrease | 0.022 |
| A0A6G5A5X8 | Protein quiver | 39.38 | 12 | 14.64 | 2 | 11 | 9.0-fold decrease | 0.049 |
| A0A6M2CJA6 | Plasma membrane glycoprotein | 39.21 | 12 | 15.41 | 4 | 11 | 3.3-fold decrease | 0.032 |
| A0A6M2CM57 | Phosphoglucomutase phosphomannomutase | 24.19 | 11 | 14.49 | 4 | 7 | 3.0-fold decrease | 0.023 |
| A0A6G4ZZC0 | metalloprotease m41 | 23.56 | 8 | 18.94 | 3 | 7 | 2.9-fold decrease | 0.046 |
| A0A6G5ADT3 | transcriptional regulator | 14.42 | 13 | 9.49 | 2 | 5 | 5.8-fold decrease | 0.022 |
| Q2XW15 | Glutathione peroxidase | 13.94 | 29 | 14.391 | 3 | 6 | 10.2-fold decrease | 0.043 |
| A0A6M2D080 | Molecular chaperone | 10.26 | 9 | 5.591 | 2 | 3 | 9.8-fold decrease | 0.047 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izaguirre, S.; Capelli-Peixoto, J.; Vimonish, R.; Poh, K.C.; Davis, S.; Peltier, K.; Brayton, K.A.; Taus, N.; Chung, C.; Ueti, M.W. Identification of a Tick Midgut Protein Involved in Babesia bovis Infection of Female Rhipicephalus microplus Ticks. Microorganisms 2025, 13, 1713. https://doi.org/10.3390/microorganisms13081713
Izaguirre S, Capelli-Peixoto J, Vimonish R, Poh KC, Davis S, Peltier K, Brayton KA, Taus N, Chung C, Ueti MW. Identification of a Tick Midgut Protein Involved in Babesia bovis Infection of Female Rhipicephalus microplus Ticks. Microorganisms. 2025; 13(8):1713. https://doi.org/10.3390/microorganisms13081713
Chicago/Turabian StyleIzaguirre, Sadie, Janaina Capelli-Peixoto, Rubikah Vimonish, Karen C. Poh, Sara Davis, Kierra Peltier, Kelly A. Brayton, Naomi Taus, Chungwon Chung, and Massaro W. Ueti. 2025. "Identification of a Tick Midgut Protein Involved in Babesia bovis Infection of Female Rhipicephalus microplus Ticks" Microorganisms 13, no. 8: 1713. https://doi.org/10.3390/microorganisms13081713
APA StyleIzaguirre, S., Capelli-Peixoto, J., Vimonish, R., Poh, K. C., Davis, S., Peltier, K., Brayton, K. A., Taus, N., Chung, C., & Ueti, M. W. (2025). Identification of a Tick Midgut Protein Involved in Babesia bovis Infection of Female Rhipicephalus microplus Ticks. Microorganisms, 13(8), 1713. https://doi.org/10.3390/microorganisms13081713








