Abstract
This study aimed to evaluate the effects of dietary supplementation with sodium butyrate (SB) in different forms on the growth performance, antioxidant capacity, immune response, and intestinal health of largemouth bass (Micropterus salmoides). Five diets were formulated: a basal diet (SB0), diets with 1000 (ESB1), 1500 (ESB2), and 2000 mg/kg encapsulated SB (ESB3), and a diet with 2000 mg/kg raw powder sodium butyrate (RSB, non-encapsulated). After 49 days of feeding trials, the ESB2 group exhibited significantly higher weight gain and specific growth rates and a lower feed coefficient than those of the SB0 group (p < 0.05). Compared with the SB0 group, proximal intestinal villus length and width were significantly increased in the ESB1, ESB2, and ESB3 groups (p < 0.05). The expressions of tight junction genes zo-1, claudin-1, and claudin-4 were up-regulated in these SB-supplemented groups and most pronounced in the ESB2 group (p < 0.05). Compared with the SB0 group, antioxidant enzyme activities (catalase and superoxide dismutase) and their gene expressions increased in the ESB1, ESB2, and RSB groups (p < 0.05). Immune-related genes il-10 and tgf-β1 were up-regulated in the ESB1 and ESB2 groups, while their il-8, il-1β, and tnf-α were down-regulated (p < 0.05). The ESB2 group had higher intestinal abundance of Firmicutes and Lactobacillus. In conclusion, dietary supplementation with 1500 mg/kg encapsulated SB (ESB2) improved growth, antioxidant capacity, immunity, and gut health in largemouth bass.
1. Introduction
With the rapid development of aquaculture and marine fishing industries, the demand for fishmeal as a high-quality protein source has continued to rise, and its insufficient supply has caused prices to soar [1]. Plant protein materials, due to their wide availability and low cost, are commonly used as alternatives to fishmeal in aquafeeds. However, anti-nutritional factors and amino acid imbalances in plant protein materials can affect the growth and health of aquatic animals to varying degrees [2,3]. When fishmeal is largely replaced by plant protein, functional feed additives (such as organic acids, nucleotides, and probiotics) are typically added to mitigate the negative effects of the alternative protein sources, thereby enhancing fish growth performance, boosting immunity and disease resistance, and improving gut health [4,5].
Sodium butyrate (SB), the sodium salt of the short-chain volatile fatty acid butyric acid, is widely used in poultry and aquaculture feed due to its high stability, rapid absorption, and environmental friendliness [6,7]. However, different forms of SB show significant differences in bioavailability and efficacy [8]. Raw SB powder, with its strong odor and short shelf life, is rapidly absorbed in the stomach, making it difficult to exert sustained effects in the intestine [9]. Encapsulated SB (ESB), encapsulated with lipids or polymers, forms protective and controlled-release layers. It is released gradually in the stomach and intestinal regions [10] and is targeted to the cecum and colon [11], enhancing intestinal digestion and absorption and reducing inflammation [12,13].
SB is an important energy source for intestinal epithelial cells, promoting cell growth and metabolism [14], and can reduce intestinal pH to suppress harmful bacteria and maintain gut homeostasis [15]. Studies have demonstrated that dietary SB can enhance the growth performance and digestive enzyme activities of juvenile thin-lipped mullet (Liza ramada) [16]. Moreover, dietary SB has been proven to enhance intestinal absorption and immunity in various fish species, including Nile tilapia (Oreochromis niloticus) [17], grass carp (Ctenopharyngodon idella) [18], turbot (Scophthalmus maximusa) [19], and rice field eel (Monopterus albus) [20]. Interestingly, Jesus et al. [21] observed that the addition of encapsulated SB at 0.5% in either buffer or oil improved the yield and biomass gain compared with the unprotected forms during sexual reversion of Nile tilapia. Additionally, Zhou et al. [22] found that different SB forms improved C. idella health by up-regulating myd88 and tlr22 gene expressions, enriching beneficial gut bacteria, and reducing lipid accumulation, despite showing no significant effects on growth. These findings highlight the need for further investigation into the optimal dietary supplementation levels of different forms of SB (raw powder or encapsulated), as well as their impacts on gut antioxidant status, microbiota composition, and immune function.
The largemouth bass (Micropterus salmoides), a carnivorous fish, preys on small fish and insects. It is highly regarded in the market for its firm flesh, delicious taste, few bones, and high nutritional value [23]. With its rapid growth and adaptability to intensive farming, it has a robust market demand. Largemouth bass has a high demand for dietary protein and is more sensitive to adverse intestinal effects caused by plant-based feed, making it a key species for enhancing feed efficiency and health management and an ideal model for studying carnivorous fish intestinal physiology [24]. Research showed that dietary supplementation with SB in feed could alleviate the negative influence of high-fat diets on fish growth, intestinal microbiota homeostasis, and liver health in largemouth bass [23,24]. However, the appropriate inclusion level of encapsulated SB in largemouth bass feed, along with comparisons to raw powder SB at equivalent doses, remains an area for further investigation. This study investigates how different doses of encapsulated SB and raw powder SB affect the growth, serum biochemistry, gut structure, antioxidant capacity, immunity, and gut microbiota of largemouth bass, aiming to guide the optimal use of encapsulated SB in feed.
2. Materials and Methods
2.1. Diet Formulation
Largemouth bass (M. salmoides) with an initial weight of 29.75 ± 0.25 g were used in this study. Sodium butyrate (SB) as the feed additive was obtained from Hunan Perfly Biotech Co., Ltd. (Hunan, China). Five experimental diets were formulated: a control diet (SB0, basal diet containing 25% fish meal by weight with no SB) and four treatment diets supplemented with 1000 mg/kg (ESB1), 1500 mg/kg (ESB2), and 2000 mg/kg (ESB3) of encapsulated SB, or 2000 mg/kg of raw powder SB (RSB). The addition concentration of SB in the feed referenced effective levels reported in published studies on grass carp (C. idella) [22], yellow drum (Nibea albiflora) [13], and Nile tilapia [21], with a gradient design covering 1000–2000 mg/kg to identify optimal concentrations for largemouth bass. The main ingredients and nutritional compositions of the five diets are listed in Table 1.

Table 1.
Experimental feed formulation and chemical composition (% dry matter).
The feed ingredients were finely ground through a 60-mesh sieve, uniformly mixed according to the formulated ratios, and extruded into strip-shaped pellets using an aquafeed extruder (Jiangsu Zhengchang Grain and Feed Machinery Co., Ltd., SZLH200, Liyang, China) at a temperature of 50 °C. The extruded feed was dried to a moisture content of less than 10% and then formed into approximately 3 mm diameter particles using a feed pellet mill (Jiangsu Zhengchang Grain and Feed Machinery Co., Ltd., 1208 Pellet Mill, Liyang, China) for low-temperature storage until use.
2.2. Experimental Animals and Management
After two weeks of acclimation, 600 largemouth bass with an initial weight of 29.75 ± 0.25 g were evenly distributed into five groups (n = 120 fish per group). Each group was allocated three net cages (2 m × 2 m × 1.6 m; total 15 cages) with 40 fish per cage. The cages were suspended in an aerated pond. The 49-day feeding trial involved daily feeding at 3% of body weight, distributed twice daily (07:00 and 17:00) until apparent satiation (1–1.5 h per feeding). Weekly, four fish per group were randomly weighed to adjust feeding rations. Water quality parameters were maintained within the following ranges: dissolved oxygen at 5–7 mg/L, water temperature at 16–32 °C, pH at 7.0–8.5, and pond water transparency at 30–40 cm.
2.3. Sample Collection and Ethics Statement
At the end of the 49-day rearing period, the largemouth bass were subjected to a 24 h fasting period. Subsequently, 20 fish were randomly sampled from each net cage (60 fish per group) for experimentation. The fish were anesthetized with an MS-222 solution (50 mg/L, Changsha Shanghe Biotechnology Co., Ltd., Changsha, China) for 5 min. All experiments complied with the Guidelines for the Care and Use of Laboratory Animals in China and adhered to local animal welfare regulations and institutional guidelines.
Surface moisture was gently removed from the 60 anesthetized fish per group using sterile gauze before body weight measurement. Blood was collected from the caudal vein of 30 randomly selected fish per group. The blood samples were left to stand at 4 °C for 24 h and then centrifuged at 3000× g for 15 min to collect the serum, which was stored at −80 °C for subsequent serum biochemical analysis. The intestines of these 30 fish were quickly dissected on ice, and tissue samples from the proximal (3 cm posterior to the stomach), middle, and distal (3 cm anterior to the anus) segments of the intestine were collected. Tissue samples from six fish were fixed in 4% paraformaldehyde at 4 °C for histological section preparation, while samples from the remaining 24 fish per experimental group (8 fish from each of the 3 tanks) were snap-frozen in liquid nitrogen for 12 h and then stored at −80 °C for total RNA extraction and antioxidant enzyme activity assays.
Finally, from the remaining 30 anesthetized fish in each group, 18 fish per experimental group (6 fish from each of the 3 tanks) were selected for gut content collection. The entire intestinal contents of these fish per experimental group (4 fish from each of the 3 tanks) were collected aseptically and stored in sterile vials. The samples were snap-frozen in liquid nitrogen for 12 h and then stored at −80 °C for subsequent gut microbiota sequencing. The remaining 12 fish per experimental group (4 fish from each of the 3 tanks) were stored at −20 °C for whole-body composition analysis (moisture, crude fat, crude protein, and crude ash).
2.4. Survival and Growth Performance
The survival and growth parameters measured in this study included initial body weight (IBW), final body weight (FBW), weight gain rate (WGR), specific growth rate (SGR), Feed coefficient (FC), feed intake (FI), viscera somatic index (VSI), and hepatosomatic index (HSI).
Weight gain rate, WGR (%) = 100 × [(final body weight − initial body weight)/initial body weight].
Specific growth rate, SGR (%/d) = (ln final body weight − ln initial body weight)/days × 100.
Feed intake, FI (g) = total amount of feed consumption (g)/[(initial body weight + final body weight)/2]/days.
Feed coefficient, FC = total amount of feed consumption (g)/(final body weight + death body weight (g) − initial body weight (g)).
Viscerosomatic index, VSI (%) = 100 × visceral weight (g)/body weight (g).
Hepatosomatic index, HSI (%) = 100 × liver weight (g)/body weight (g).
2.5. Proximate Composition Analysis
The crude protein content was determined using the Kjeldahl method (N × 6.25). Crude fat content was measured by Soxhlet extraction. Moisture content was determined by drying samples to constant weight in an oven at 105 °C. Ash content was analyzed by incinerating samples to constant weight in a muffle furnace at 550 °C. All procedures followed the methods described by Su et al. [25].
2.6. Histological Observation
Proximal, middle, and distal intestinal samples from each group (n = 3) were dehydrated through a graded ethanol series and embedded in paraffin. Transverse sections approximately 1 cm in length were cut into 5 μm thick slices and stained with hematoxylin and eosin (H&E) following the method of Su et al. [25]. H&E staining was performed and scanned by Wuhan Sevicebio Technology Co., Ltd. (Wuhan, China). Sections were scanned using a NanoZoomer S60 (Hamamatsu Photonics, Hamamatsu, Japan), and measurements of villus length, villus width, and muscle layer thickness were taken using Image-Pro Plus 6.0 software.
2.7. Serum Biochemical Assays
Blood samples collected from the caudal vein were left to clot at 4 °C, then centrifuged at 3000× g for 10 min to obtain serum, which was stored at −80 °C. Serum samples (n = 3) were analyzed for alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin (ALB), glucose (GLU), and total protein (TP) using a fully automatic biochemical analyzer (Mindray, BS280, Shenzhen, China) [26].
2.8. Intestinal Antioxidant Enzyme Activity Assays
Antioxidant enzyme activities in the proximal, middle, and distal intestines of largemouth bass were measured using commercial kits from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Superoxide dismutase (SOD) and catalase (CAT) activities and the total protein content (TP) were determined following the manufacturer’s protocols [25,26].
2.9. Real-Time Quantitative PCR Analysis
Total RNA was extracted from the proximal, middle, and distal intestine of each group of largemouth bass using the RNAex Pro Reagent (Hunan Akrie Biotechnology Co., Ltd., Changsha, China) and quantified using a NanoDrop 2000 (Thermo Scientific, Waltham, MA, USA). cDNA was synthesized from total RNA using the gDNA Clean Reaction Mix Ver.2 and 5X Evo M-MLV RT Reaction Mix II (Yeasen Biotechnology (Shanghai) Co., Ltd., Shanghai, China) and stored at −20 °C. Primers for real-time quantitative PCR (RT-qPCR) were designed using Primer Premier 5.0 and synthesized by Sangon Biotech (Shanghai) Co., Ltd. (Shanghai, China) (Table 2). RT-qPCR was performed using the CFX 96TM Real-Time System (Bio-Rad, Hercules, CA, USA). The total reaction volume was 20 μL, including 10 μL of SYBR Green, 0.4 μL of upstream and downstream specific primers (10 mM each), 1 μL of template cDNA, and 8.2 μL of sterile deionized water. The reaction protocol included an initial denaturation at 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s, annealing at 60 °C for 30 s, and a final melting curve analysis from 65 °C to 95 °C with a 5 s hold every 0.5 °C. β-actin was used as the reference gene, and relative gene expression was measured using the 2−△△Ct method [26,27].

Table 2.
RT-qPCR primer sequences for largemouth bass.
2.10. Gut Microbiota Analysis
Total intestinal genomic DNA was extracted using the E.Z.N.A.® Soil DNA Kit (Omega, Irving, TX, USA). The V3+V4 region of 16S rRNA was amplified with primers 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). Sequencing was performed on the Illumina MiSeq platform (Shanghai Majorbio Biopharm Technology Co., Ltd., Shanghai, China). Raw data were merged using FLASH (v1.2.7) and filtered for quality with QIIME2 (v2020.2), followed by chimera removal with UCHIME (v8.1) to obtain high-quality tags. Effective sequences were analyzed using USEARCH 10.0 for OTU clustering at 97% similarity. Taxonomic annotation of OTUs was performed with the RDP Classifier 2.2 against the Silva 16S rRNA gene database (v132, http://www.arb-silva.de accessed on 11 April 2025). Microbial community composition was profiled at various taxonomic levels (phylum, class, order, family, genus, species), and diversity indices (Shannon, Simpson, Chao, Ace) were calculated. β-diversity was assessed via principal coordinate analysis (PCoA, Vegan v2.4.3), and differentially abundant biomarkers were identified using linear discriminant analysis effect size (LEfSe) [28].
2.11. Data Analysis
Data are expressed as the mean ± standard error (mean ± S.E.) (n = 3). Statistical analyses were performed using IBM SPSS Statistics 26.0, and graphs were generated with GraphPad Prism 9.0. Prior to analysis, data that deviated from the population mean were identified and excluded through a one-sample t-test (α = 0.05). One-way ANOVA followed by Duncan’s multiple range test was used for intergroup comparisons. Paired-samples t-tests were used for intragroup comparisons of gut locations. Significance was set at p < 0.05. Significant differences among different gut locations within the same SB group are indicated by different capital letters, while significant differences among different SB groups within the same gut location are indicated by different lowercase letters.
3. Results
3.1. Growth Performance and Body Composition Analysis
As shown in Table 3, the WGR (303.53 ± 17.92%) and SGR (2.41 ± 0.13%/d) of the ESB2 group were significantly higher than those of the SB0 group (261.95 ± 1.39% and 2.10 ± 0.01%/d, respectively) (p < 0.05). Meanwhile, the FC (0.93 ± 0.09) of the ESB2 group was significantly lower than that of the SB0 group (1.20 ± 0.04) (p < 0.05). However, no significant differences were observed in these parameters among the remaining four groups (p > 0.05).

Table 3.
Growth performance analysis of five groups of largemouth bass.
From Table 4, it is evident that there were no significant differences in moisture, crude protein, crude fat, and crude ash content among the different groups (p > 0.05).

Table 4.
Effects of dietary SB levels on the final whole-body composition of largemouth bass.
3.2. Intestinal Histomorphology
The H&E staining results of the intestinal tissues (proximal, mid, and distal segments) in largemouth bass are presented in Figure 1. All groups exhibited intact intestinal architecture, orderly arranged villi, and smooth mucosal surfaces across the proximal, mid, and distal regions. In the ESB1, ESB2, and ESB3 groups, both the villus length and width in the proximal intestine were significantly increased compared to the control (p < 0.05). Notably, the muscularis thickness of the proximal intestine was significantly elevated in the ESB2 group (p < 0.05). Moreover, the villus width and muscularis thickness in the mid and distal intestines displayed a dose-dependent enhancement with increasing SB concentrations across the ESB groups. In the RSB group, the villus length in the distal intestine and villus width in the proximal intestine were significantly higher than those in the SB0 group (p < 0.05) (Table 5).
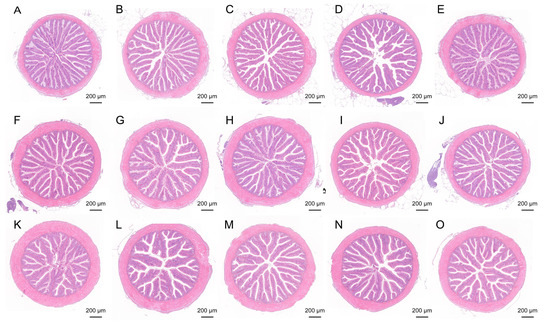
Figure 1.
Histological HE staining results of intestinal tissue in five groups of largemouth bass. (A–E) HE staining of the proximal intestine; (F–J) HE staining of the mid intestine; (K–O) HE staining of the distal intestine.

Table 5.
Effects of dietary SB levels on intestinal morphological parameters in largemouth bass.
3.3. Tight Junction Gene Expression
As shown in Figure 2, the expression of tight junction-related genes (zo-1, claudin-1, and claudin-4) in the proximal, middle, and distal intestine was significantly up-regulated in the ESB1, ESB2, ESB3, and RSB groups compared to the SB0 group (p < 0.05). Among these, the highest gene expression levels were observed in the ESB2 group, indicating that the intestinal tight junction function was strongest when the diet contained 1500 mg/kg of encapsulated SB.
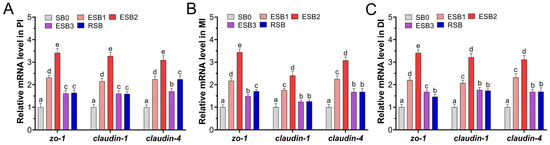
Figure 2.
Effects of dietary SB levels on intestinal tight junction gene expression in largemouth bass. (A) Expression of tight junction genes in the proximal intestine (PI); (B) expression of tight junction genes in the mid intestine (MI); (C) expression of tight junction genes in the distal intestine (DI). Different lowercase letters indicate significant differences between groups (p < 0.05), while the same lowercase letters indicate no significant differences (p > 0.05).
3.4. Serum Biochemical Parameters
In the serum biochemistry parameters of largemouth bass, the levels of GLU, ALB, and AST showed no significant differences among the five groups (p > 0.05). The TP content was lowest in the ESB3 group (29.89 ± 2.86 g/L) and was significantly lower than in the SB0 (35.62 ± 4.20 g/L) and ESB2 (35.43 ± 3.26 g/L) groups (p < 0.05). The ALT level in the SB0 group (3.65 ± 1.51 U/L) was significantly lower than in the RSB group (5.49 ± 1.39 U/L) (p < 0.05), but no significant differences were found between the SB0 group and the ESB1, ESB2, or ESB3 groups (p > 0.05) (Table 6).

Table 6.
Effects of dietary SB on serum biochemical indicators in largemouth bass.
3.5. Intestinal Antioxidant Capacity
As shown in Figure 3, the activity trends of the antioxidant enzymes SOD and CAT in the intestines of largemouth bass were similar across the five groups, with a stepwise decrease in enzyme activity from the proximal to middle and distal intestines. Compared to the SB0 group, the SOD activity in the proximal, middle, and distal intestine was significantly increased in the ESB1, ESB2, ESB3, and RSB groups (p < 0.05) (Figure 3A–C). In the proximal intestine, the CAT activity in the ESB1, ESB2, ESB3, and RSB groups was significantly higher than in the SB0 group (p < 0.05) (Figure 3A). In the middle intestine, only the CAT activity in the ESB3 group was significantly lower than in the SB0 and the other three groups (p < 0.05) (Figure 3B). In the distal intestine, the CAT activity in the ESB2 and RSB groups was significantly higher than in the SB0 group (p < 0.05), while the CAT activity in the ESB3 group was significantly lower than in the SB0 group (p < 0.05) (Figure 3C). Overall, the ESB2 group exhibited the highest enzyme activity for both CAT and SOD across the proximal, middle, and distal intestines, indicating that dietary supplementation with 1500 mg/kg encapsulated SB enhanced antioxidant capacity the most.
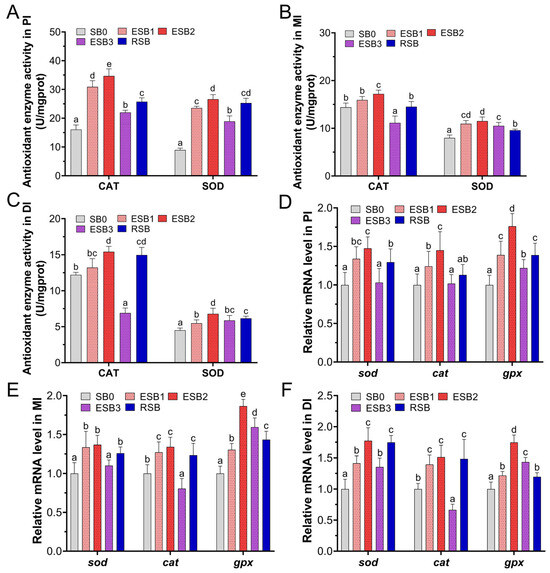
Figure 3.
Effects of dietary SB levels on intestinal antioxidant enzyme activities and antioxidant gene expression in largemouth bass. (A) CAT and SOD enzyme activities in the proximal intestine (PI); (B) CAT and SOD enzyme activities in the mid intestine (MI); (C) CAT and SOD enzyme activities in the distal intestine (DI); (D) antioxidant gene expression in the proximal intestine (PI); (E) antioxidant gene expression in the mid intestine (MI); (F) antioxidant gene expression in the distal intestine (DI). Different lowercase letters indicate significant differences between groups (p < 0.05), while the same lowercase letters indicate no significant differences (p > 0.05).
At the gene expression level, in the proximal and middle intestines, the expression of the sod gene was significantly up-regulated in the ESB1, ESB2, and RSB groups compared to the SB0 group (p < 0.05) (Figure 3D,E). In the distal intestine, sod gene expression was significantly higher in the four SB-supplemented groups than in the SB0 group (p < 0.05) (Figure 3F). In the proximal intestine, cat gene expression was significantly higher in the ESB1 and ESB2 groups than in the SB0 group (p < 0.05) (Figure 3D). In the middle and distal intestine, cat gene expression was significantly higher in the ESB1, ESB2, and RSB groups than in the SB0 group (p < 0.05), while the ESB3 group showed significantly lower cat gene expression than the SB0 group (p < 0.05) (Figure 3E,F). The expression of the gpx gene was significantly higher in the four SB-supplemented groups than in the SB0 group across the proximal, middle, and distal intestine (p < 0.05) (Figure 3D–F).
3.6. Intestinal Immunity
As shown in Figure 4, the expression of the immune-related genes tgf-β1 and il-10 in the proximal intestine of largemouth bass was significantly higher in the ESB1, ESB2, and RSB groups than in the SB0 group (p < 0.05) (Figure 4A). In the middle intestine, the expression of these two genes was significantly higher in all four groups compared to the SB0 group (p < 0.05) (Figure 4B). In the distal intestine, the expression of tgf-β1 and il-10 genes was significantly higher in the ESB1 and ESB2 groups than in the SB0 group (p < 0.05) (Figure 4C). The expression of the il-1β gene in the RSB group was significantly higher than in the SB0 group in the middle and distal intestine (p < 0.05) (Figure 4B,C), but no significant difference was found in the proximal intestine (p > 0.05) (Figure 4A). The expression of il-8 and tnf-α genes was significantly higher in the ESB1, ESB2, ESB3, and RSB groups than in the SB0 group across all intestinal segments (Figure 4A–C) (p < 0.05).
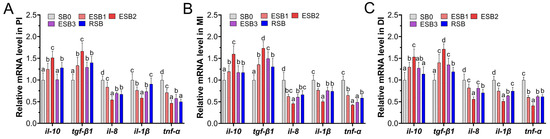
Figure 4.
Effects of dietary SB levels on intestinal immune gene expression in largemouth bass. (A) Immune gene expression in the proximal intestine (PI); (B) immune gene expression in the mid intestine (MI); (C) immune gene expression in the distal intestine (DI). Different lowercase letters indicate significant differences between groups (p < 0.05), while the same lowercase letters indicate no significant differences (p > 0.05).
From the above analysis, the expression levels of the anti-inflammatory genes tgf-β1 and il-10 in the ESB2 group were higher than in the other groups across the proximal, middle, and distal intestines (p < 0.05). In contrast, the expression levels of the pro-inflammatory genes il-1β, il-8, and tnf-α in the ESB2 group were lower than in the other groups. Thus, dietary supplementation with 1500 mg/kg encapsulated SB enhances the anti-inflammatory ability of largemouth bass and reduce the incidence of inflammation in the intestine.
3.7. Intestinal Microbiota and Immune Gene Correlation
As shown in Table 7, the microbial diversity indices (Sobs, Chao, Ace, and Shannon) were significantly higher in the SB0 group than in the RSB group (p < 0.05), while no significant differences were found between the three encapsulated SB groups (ESB1, ESB2, ESB3) and the SB0 or RSB groups (p > 0.05). The Simpson index was significantly lower in the SB0 group than in the RSB group (p < 0.05), with no significant differences among the ESB groups (p > 0.05).

Table 7.
Effects of dietary SB levels on the alpha diversity of intestinal microbiota in largemouth bass.
At the phylum level, the dominant phyla in the gut microbiota of largemouth bass were Firmicutes, Proteobacteria, Actinobacteria, and Cyanobacteria. The abundance of Firmicutes was significantly higher in the ESB2 group than in the SB0 group (p < 0.05), while the abundance of Proteobacteria and Actinobacteria showed a decreasing trend but without significant differences (p > 0.05) (Figure 5A). At the genus level, the dominant genera across all groups were Achromobacter, Mycoplasma, Weissella, and Lactobacillus. The abundances of Weissella and Lactobacillus were significantly higher in the ESB2 group than in the SB0 group (p < 0.05) (Figure 5B). Principal coordinate analysis (PCoA) revealed distinct differences in gut microbiota composition between the SB-supplemented groups and the SB0 group at the phylum level (Figure 5C).
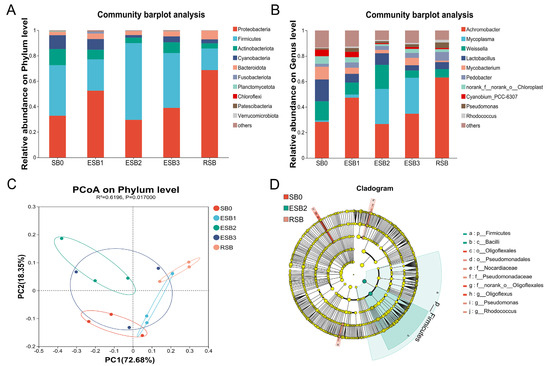
Figure 5.
Changes in the intestinal microbiota composition in different SB groups. (A) Intestinal microbiota composition analysis at the phylum level; (B) intestinal microbiota composition analysis at the genus level; (C) principal coordinate analysis (PCoA); (D) LEfse analysis of intestinal microbiota from phylum to genus.
LEfse analysis (Figure 5D) indicated that the OTU abundance of beneficial bacteria, including Firmicutes and Bacilli, was significantly higher in the ESB2 group than in the other groups (p < 0.05). In contrast, the OTU abundance of Pseudomonadales, Nocardiaceae, Pseudomonadaceae, Pseudomonas, and Rhodococcus was significantly higher in the RSB group (p < 0.05). The OTU abundance of Oligoflexales and Oligoflexus was significantly higher in the SB0 group than in the other groups (p < 0.05).
As shown in Figure 6, the expression level of the anti-inflammatory gene il-10 was significantly negatively correlated with Bdellovibrionota (p < 0.05). The expression levels of pro-inflammatory genes tnf-α, il-1β, and il-8 were significantly positively correlated with Planctomycetota, Verrucomicrobiota, Cyanobacteria, Actinobacteriota, and Gemmatimonadota (p < 0.05).
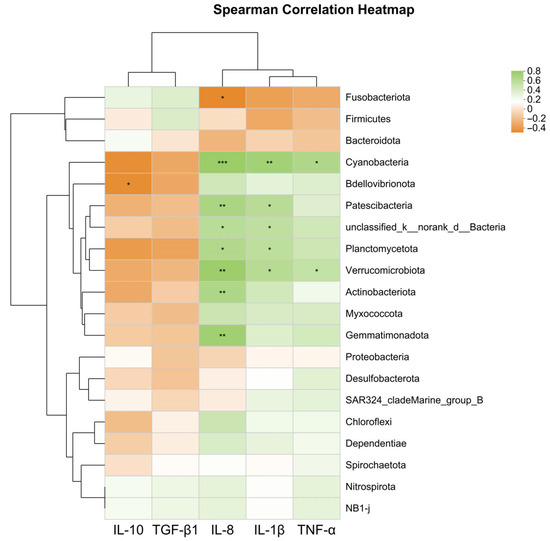
Figure 6.
Heatmap correlation analysis of intestinal microbiota at the phylum level and immune gene expression levels affected by SB. * Indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001.
4. Discussion
4.1. Effects of SB Supplementation on Growth Performance of Largemouth Bass
As a novel green feed additive, SB has been widely applied in aquatic diets to enhance growth performance. In herbivorous species, dietary supplementation with 300 mg/kg SB improved weight gain rate (WGR) and feed coefficient (FC) in common carp (Cyprinus carpio) [29]. Similarly, 1000–2000 mg/kg SB significantly elevated the specific growth rate (SGR) of grass carp (C. idellus) [30]. For omnivorous fish, 300 mg/kg SB increased WGR, SGR, and FC in Nile tilapia (O. niloticus) [31,32], while 2000–4000 mg/kg SB enhanced growth performance in crucian carp (Carassius auratus) [33]. In carnivorous species, 3000 mg/kg palm oil-encapsulated SB improved WGR, SGR, and FC in gilthead sea bream (Sparus aurata) [34]. However, no significant growth benefits were observed in Atlantic salmon (Salmo salar) or rainbow trout (Oncorhynchus mykiss) fed 5000–20,000 mg/kg SB [35,36].
Notably, Abdel-Mohsen et al. [37] reported that 0.2% SB promoted growth in European sea bass (Dicentrarchus labrax) juveniles, whereas 0.3% SB showed no significant effects. A recent study by Hou et al. [38] demonstrated that 2000 mg/kg SB significantly enhanced growth in largemouth bass. In the present study, dietary supplementation with 1500 mg/kg encapsulated SB (ESB2) significantly increased WGR and SGR while reducing FC in largemouth bass (p < 0.05). In contrast, neither lower (1000 mg/kg) nor higher (2000 mg/kg) encapsulated SB doses nor 2000 mg/kg raw powder SB improved these parameters. These discrepancies may stem from dose-dependent efficacy, formulation differences (encapsulated vs. raw powder), and species-specific responses influenced by feeding habits and metabolic pathways.
4.2. Effects of SB Addition on Intestinal Morphology and Tight Junction Capacity in Largemouth Bass
Intestinal morphological parameters, such as villus length, crypt depth, and muscle layer thickness, are key biomarkers for evaluating the digestive and absorptive efficiency of fish [39]. In this study, the addition of encapsulated SB (1000–2000 mg/kg) to feed significantly increased the villus length and width in the proximal, mid, and distal regions of the intestine of largemouth bass, with a positive correlation observed as the concentration of SB increased. These results are consistent with findings in Nile tilapia (O. niloticus) fry [40] and grass carp (C. idellus) [22]. In this study, largemouth bass fed with feed containing 2000 mg/kg raw powder SB showed a significant increase in villus length only in the distal intestine and a significant increase in villus width only in the proximal intestine compared to the control group. This suggests that the effect of 2000 mg/kg raw powder SB on improving intestinal villus structure in largemouth bass is inferior to that of encapsulated SB, indicating that the sustained-release technology of encapsulated SB facilitates its gradual release throughout the intestine, thereby enhancing digestive and absorptive capacity through improved villus structure.
The physical barrier function of fish intestines maintains the stability of intestinal epithelial cells and their interconnections, ensuring normal function and integrity [41]. Tight junction proteins, such as Claudins, Occludin, and ZOs, play a critical role in limiting macromolecular passage and regulating cellular barrier permeability [42,43]. Studies have shown that adding 2000 mg/kg SB to a high-soybean-meal diet (37.9%) for turbot (S. maximus) significantly up-regulated the expression of zo-1, claudin-4, and occludin genes, which were previously down-regulated [19]. In this study, the addition of 1000–2000 mg/kg encapsulated SB and 2000 mg/kg raw powder SB to a basal diet (containing 25% fishmeal) significantly up-regulated the gene expression of zo-1, claudin-1, and claudin-4 in the intestines of largemouth bass. In Nile tilapia (O. niloticus), adding 2–4 g/kg SB to the 60 % fava bean feed showed that the structural integrity of the intestinal barrier was associated with improved muscle texture, because genes associated with intestinal tight junctions (zo-1 and claudin-14) and the anti-apoptotic gene bcl2 showed significant positive correlations with muscle texture parameters (hardness, chewiness, gumminess, springiness, adhesiveness, resilience, and cohesiveness) [44]. In summary, both encapsulated SB and raw powder SB can improve intestinal morphology, promote tight junctions in intestinal epithelial cells, and enhance the physical barrier function of the intestine in largemouth bass (Figure 7).
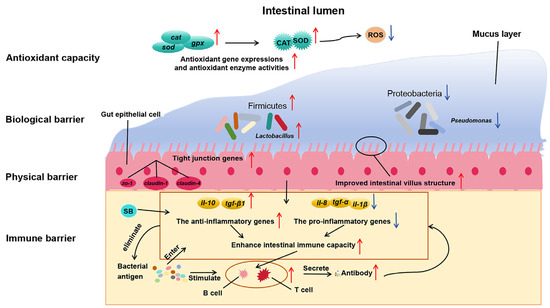
Figure 7.
Schematic of SB action on the intestine of largemouth bass. The red upward arrow indicates an increase in the enzyme activities, gene expressions or abundance of gut microbiota, while the blue downward arrow indicates a decrease in the enzyme activities, gene expressions or abundance of gut microbiota.
4.3. Effects of Sodium Butyrate Supplementation on Serum Biochemical Parameters of Largemouth Bass
Serum biochemical parameters serve as critical indicators of physiological status and overall health in fish, providing essential diagnostic tools for disease detection [45,46]. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) are sensitive biomarkers for evaluating hepatopancreatic function and cellular integrity [47]. In the present study, AST levels showed no significant differences among groups (p > 0.05), indicating neither encapsulated nor non-encapsulated SB induced substantial hepatocyte or myocardial injury. ALT was significantly elevated in the RSB group versus SB0 (p < 0.05), while encapsulated SB groups (ESB1-ESB3) remained comparable to controls, potentially attributable to rapid gastric release of non-encapsulated SB. Contrastingly, studies in crucian carp (C. auratus) reported decreased AST and ALT following 1000–2000 mg/kg SB supplementation [48], which may be explained by differences in fish species and feed formulations. Total protein (TP), a core indicator of immune function and nutritional metabolism [49], significantly decreased in the ESB3 group relative to SB0 and ESB2 (p < 0.05), suggesting that a high concentration of encapsulated SB (2000 mg/kg) may inhibit hepatic protein synthesis in largemouth bass.
4.4. Effects of SB Addition on Intestinal Antioxidant Capacity in Largemouth Bass
Antioxidant capacity is a core indicator for evaluating the health status and environmental adaptability of aquatic animals [50]. Superoxide dismutase (SOD) and catalase (CAT), as key antioxidant enzymes in fish, play an important role in combating oxidative stress damage [51]. In this study, RT-qPCR analysis showed that both encapsulated SB (1000–2000 mg/kg) and raw powder SB (2000 mg/kg) significantly up-regulated the mRNA expression of sod, cat, and gpx genes in the intestine of largemouth bass, which is consistent with previous findings in largemouth bass [52]. Results of antioxidant enzyme activity demonstrated that the addition of encapsulated SB and raw powder SB to feed significantly enhanced SOD activity in the proximal, mid, and distal regions of the intestine of largemouth bass. These results align with previous studies on the antioxidant effects of SB in other fish species. For example, adding 500 mg/kg SB to the feed of rice field eel (M. albus) and grass carp (C. idellus) improved SOD and GSH-Px activities in the hepatopancreas and intestine [20,22]. Similarly, adding 0.05–0.2% raw powder SB to the feed of largemouth bass significantly increased the activity levels of T-SOD, CAT, and GPx [23,24].
Notably, in this study, the CAT activity in the group fed with 2000 mg/kg encapsulated SB was significantly lower than that in the group fed with 2000 mg/kg raw powder SB and in groups fed with low to medium concentrations (1000–1500 mg/kg) of encapsulated SB. Additionally, the SOD and CAT activities in the intestine of largemouth bass fed with encapsulated SB and raw powder SB showed a gradual decline in the proximal, mid, and distal regions of the intestine. This suggests that low concentrations of SB can enhance antioxidant capacity in fish, but its gradual absorption in the intestine leads to a weakening of its antioxidant effects. However, high concentrations (2000 mg/kg) of encapsulated SB may cause oxidative stress imbalance, disrupt intracellular redox homeostasis, and lead to excessive reactive oxygen species (ROS) production, exceeding the clearance capacity of antioxidant enzymes, thereby reducing CAT activity [53]. This study found that adding 1500 mg/kg encapsulated SB provided the strongest antioxidant capacity in fish, while adding 2000 mg/kg encapsulated SB may promote intestinal stress responses (Figure 7). This indicates that higher concentrations of SB in feed are not necessarily better. Furthermore, the addition of raw powder SB should be appropriately higher than that of encapsulated SB to achieve equivalent effects in the intestine.
4.5. Effects of SB Addition on Intestinal Immune Capacity in Largemouth Bass
Cytokines play a crucial role in fish immune health. Pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-8 are involved in immune responses, while anti-inflammatory cytokines such as TGF-β1 and IL-10 help modulate inflammation [54]. In this study, the addition of 1000 and 1500 mg/kg encapsulated SB to feed significantly up-regulated the expression levels of anti-inflammatory cytokine genes il-10 and tgf-β1 in the proximal, mid, and distal regions of the intestine of largemouth bass, while down-regulating the expression levels of pro-inflammatory cytokine genes il-8, il-1β, and tnf-α. Similar results were observed in common carp (C. carpio), where supplementing 300 mg/kg SB alleviated soybean oil-induced enteritis by suppressing il-1β and tnf-α gene expressions and promoting tgf-β gene expression [19]. In yellow drum (N. albiflora, Richardson), adding 0.15% SB to a high-soybean-meal diet reduced the gene expressions of tnf-α, il-1β, and il-6 while significantly increasing tgf-β1 and il-10 gene expressions [13]. In largemouth bass fed with high-soybean-meal (28.6%) or high-fat (17.80%) diets, adding 0.2% raw powder SB significantly reduced tnf-α and il-1β gene expressions, effectively mitigating intestinal inflammation caused by these diets [54,55].
However, this study found that adding high concentrations (2000 mg/kg) of encapsulated SB or raw powder SB to feed reduced the gene expression levels of il-8, il-1β, and tnf-α in the intestine and increased tgf-β1 gene expression in the distal intestine but showed no significant changes in il-10 gene expression. In European sea bass (D. labrax), feeding a soybean diet containing 0.2% SB significantly increased tnf-α gene expression while showing no significant changes in il-1β, il-8, or il-10 gene expressions [56], likely due to interactions between anti-nutritional factors in soybean meal and SB. Ge et al. [57] found that adding 2000 and 20,000 mg/kg raw powder SB to feed increased tnf-α and il-1β gene expressions in the liver of largemouth bass, with il-1β gene expression increasing with SB concentrations while reducing tnf-α gene expression in the intestine. This suggests that high concentrations of SB may affect liver immune function. In summary, adding 1500 mg/kg encapsulated SB to feed promotes the gene expression of anti-inflammatory factors and reduces pro-inflammatory factors in the proximal, mid, and distal regions of the intestine, thereby enhancing the mucosal immune system and serving as an immune barrier in fish (Figure 7).
4.6. Effects of SB Addition on the Intestinal Microbial Community Structure of Largemouth Bass
Intestinal microbiota, as part of the intestinal biological barrier, play a crucial role in maintaining host immune function and resisting pathogens [23]. LEfse analysis showed that adding 1500 mg/kg encapsulated SB significantly increased the abundance of Firmicutes in the intestine of largemouth bass, while the abundance of Cyanobacteria, Actinobacteria, and Proteobacteria showed a downward trend, with no significant effect on Bacteroidetes. Firmicutes can improve gut microbiota structure, regulate host metabolism of nutrients such as fats and carbohydrates, and enhance intestinal digestion and utilization of nutrients [44,58,59]. The decrease in Proteobacteria abundance may be due to the inhibitory effect of 1500 mg/kg encapsulated SB on pathogenic bacteria in the intestine. Chen et al. found that adding 0.2% raw SB to the diet significantly increased the abundance of Firmicutes and Bacteroidetes at the phylum level [23,24]. However, in this study, adding 2000 mg/kg raw powder SB reduced the abundance of Firmicutes and increased that of Proteobacteria. The outer membrane of Proteobacteria is mainly composed of lipopolysaccharides, and an increase in their abundance may induce intestinal inflammation in fish [60]. These differences in microbial changes may be due to variations in feed formulation (e.g., protein source), farming environment (e.g., water temperature, dissolved oxygen), or sample processing methods across different studies. Additionally, adding 2000 mg/kg raw powder SB significantly increased the abundance of Pseudomonas and Rhodococcus. Pseudomonas can produce virulence factors and metabolic products in the fish intestine, release inflammatory factors, directly damage the intestinal mucosal barrier, trigger inflammatory responses, and increase the risk of infection in fish [61]. Pseudomonas has a high degree of metabolic diversity and can utilize various carbon sources (e.g., butyrate derivatives). Rhodococcus is known for its lipid-degrading ability and may obtain energy by breaking down SB. The increase in the abundance of these two genera may be due to the addition of SB providing them with sufficient carbon sources and energy.
In summary, this study found that adding 1500 mg/kg encapsulated SB to feed significantly increased the abundance of Firmicutes in the intestine of largemouth bass, with a downward trend in the abundance of Proteobacteria and Actinobacteria. This indicates that SB addition promotes the colonization and growth of beneficial bacteria in the intestine of largemouth bass and inhibits the adhesion of harmful bacteria. By regulating the dynamic balance of intestinal microbiota, it enhances the biological barrier function of the intestine in largemouth bass (Figure 7).
4.7. Outlook
While this study examined the effects of three encapsulated SB concentrations on largemouth bass growth performance, antioxidant capacity, immunity, and intestinal health—including comparisons between encapsulated and non-encapsulated forms—subsequent research will optimize dosage through 5–7 concentration gradients. Future investigations will evaluate sensory attributes via muscle quality and volatile flavor compound analyses, integrated with carbohydrate, protein, and lipid metabolism studies, employing response surface methodology or nonlinear regression (e.g., quadratic polynomial fitting) to identify optimal inclusion levels under multifactorial interactions.
5. Conclusions
In this study, both encapsulated SB and raw powder SB up-regulated the expression of tight junction genes (zo-1, claudin-1, and claudin-4), thereby enhancing the intestinal physical barrier function and improving nutrient absorption efficiency. They also promoted the gene expression of anti-inflammatory factors (tgf-β1 and il-10) and inhibited the gene expression of pro-inflammatory factors (tnf-α, il-1β, and il-8), thereby strengthening the intestinal immune barrier. Moreover, adding 1000 and 1500 mg/kg encapsulated SB enhanced the expression of antioxidant-related genes (sod, cat, and gpx) and enzyme activities (SOD and CAT), alleviating oxidative stress damage and systematically improving the fish’s antioxidant capacity. In this study, adding 1500 mg/kg encapsulated SB improved nutrient absorption efficiency, thereby achieving efficient growth conversion, enhancing the growth performance of largemouth bass, and significantly increasing the abundance of beneficial bacteria (Firmicutes) while reducing the proportion of potential pathogenic bacteria (Proteobacteria), thus maintaining intestinal microbial balance and enhancing the intestinal biological barrier. In summary, adding 1500 mg/kg encapsulated SB (ESB2 group) was the most effective, significantly improving the growth performance, intestinal health, and immune function of largemouth bass. This study provides a theoretical basis for developing efficient and eco-friendly feed additives and reduces the farming cost of largemouth bass.
Author Contributions
M.H., conceptualization, methodology, validation, investigation, writing—original draft and visualization; Z.Z., conceptualization, methodology, validation, investigation, writing—original draft and visualization; W.Z., software, formal analysis, investigation, and visualization; H.L., software, formal analysis, investigation, and visualization; T.L., software, formal analysis, investigation, and visualization; L.L., conceptualization, supervision and project administration; J.S., conceptualization, writing—review and editing, supervision, project administration and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 31800027), the Open Funds of Hubei Key Laboratory of Regional Development and Environmental Response (No. 2019(A)002), and the Key Project of Science and Technology Research Program of Hubei Educational Commission (No. D20211004).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
We would like to acknowledge the support provided by Cuiwei Chang and Rui Yu from Hunan Perfly Biotech Co., Ltd., Hunan, China.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ALB | albumin |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| CAT | catalase |
| DM | dry matter |
| DMPT | Dimethyl-β-propiothetin |
| ESB | encapsulated sodium butyrate |
| FBW | final body weight |
| FC | feed coefficient |
| FI | feed intake |
| GLU | glucose |
| HSI | hepatosomatic index |
| IBW | initial body weight |
| RSB | raw powder sodium butyrate |
| RT-qPCR | real-time quantitative polymerase chain reaction |
| SB | sodium butyrate |
| SGR | specific growth rate |
| SOD | superoxide dismutase |
| SR | survival rate |
| TP | total protein |
| VSI | viscerosomatic index |
| WGR | weight gain rate |
References
- Jannathulla, R.; Rajaram, V.; Kalanjiam, R.; Ambasankar, K.; Muralidhar, M.; Dayal, J.S. Fishmeal availability in the scenarios of climate change: Inevitability of fishmeal replacement in aquafeeds and approaches for the utilization of plant protein sources. Aquac. Res. 2019, 50, 3493–3506. [Google Scholar] [CrossRef]
- Krogdahl, A.; Penn, M.; Thorsen, J.; Refstie, S.; Bakke, A.M. Important antinutrients in plant feedstuffs for aquaculture: An update on recent findings regarding responses in salmonids. Aquac. Res. 2010, 41, 333–344. [Google Scholar] [CrossRef]
- Urán, P.; Goncalves, A.; Taverne-Thiele, J.; Schrama, J.; Verreth, J.; Rombout, J. Soybean meal induces intestinal inflammation in common carp (Cyprinus carpio L.). Fish Shellfish Immun. 2008, 25, 751–760. [Google Scholar] [CrossRef]
- Hossain, M.; Pandey, A.; Satoh, S. Effects of organic acids on growth and phosphorus utilization in red sea bream Pagrus major. Fish. Sci. 2007, 73, 1309–1317. [Google Scholar] [CrossRef]
- Defoirdt, T.; Boon, N.; Sorgeloos, P.; Verstraete, W.; Bossier, P. Short-chain fatty acids and poly-β-hydroxyalkanoates: (New) Biocontrol agents for a sustainable animal production. Biotechnol. Adv. 2009, 27, 680–685. [Google Scholar] [CrossRef]
- Hassanin, A.; Tony, M.; Sawiress, F.; Abdl-Rahman, M.; Saleh, S. Influence of dietary supplementation of coated sodium butyrate and/or synbiotic on growth performances, caecal fermentation, intestinal morphometry and metabolic profile of growing rabbits. J. Agr. Sci. 2015, 7, 180. [Google Scholar] [CrossRef]
- Sikandar, A.; Zaneb, H.; Younus, M.; Masood, S.; Aslam, A.; Khattak, F.; Ashraf, S.; Yousaf, M.; Rehman, H. Effect of sodium butyrate on performance, immune status, microarchitecture of small intestinal mucosa and lymphoid organs in broiler chickens. Asian Australas. J. Anim. 2017, 30, 690–699. [Google Scholar] [CrossRef]
- Piva, A.; Pizzamiglio, V.; Morlacchini, M.; Tedeschi, M.; Piva, G. Lipid microencapsulation allows slow release of organic acids and natural identical flavors along the swine intestine. J. Anim. Sci. 2007, 85, 486–493. [Google Scholar] [CrossRef]
- Bedford, A.; Gong, J. Implications of butyrate and its derivatives for gut health and animal production. Anim. Nutr. 2018, 4, 151–159. [Google Scholar] [CrossRef]
- Roda, A.; Simoni, P.; Magliulo, M.; Nanni, P.; Baraldini, M.; Roda, G.; Roda, E. A new oral formulation for the release of sodium butyrate in the ileo-cecal region and colon. World J. Gastroenterol. 2007, 13, 1079. [Google Scholar] [CrossRef]
- Van Immerseel, F.; Fievez, V.; De Buck, J.; Pasmans, F.; Martel, A.; Haesebrouck, F.; Ducatelle, R. Microencapsulated short-chain fatty acids in feed modify colonization and invasion early after infection with Salmonella enteritidis in young chickens. Poult. Sci. 2004, 83, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Xiao, Z.; Dong, Y.; An, W.; Zhang, B. Dietary sodium butyrate improves intestinal development and function by modulating the microbial community in broilers. PLoS ONE 2018, 13, e0197762. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, L.; Xie, Q.; Tan, P. Effects of dietary sodium butyrate on growth, diet conversion, body chemical compositions and distal intestinal health in yellow drum (Nibea albiflora, Richardson). Aquac. Res. 2020, 51, 69–79. [Google Scholar] [CrossRef]
- Piekarska, J.; Miśta, D.; Houszka, M.; Króliczewska, B.; Zawadzki, W.; Gorczykowski, M. Trichinella spiralis: The influence of short chain fatty acids on the proliferation of lymphocytes, the goblet cell count and apoptosis in the mouse intestine. Exp. Parasitol. 2011, 128, 419–426. [Google Scholar] [CrossRef]
- Sun, W.; Sun, J.; Li, M.; Xu, Q.; Zhang, X.; Tang, Z.; Chen, J.; Zhen, J.; Sun, Z. The effects of dietary sodium butyrate supplementation on the growth performance, carcass traits and intestinal microbiota of growing-finishing pigs. J. Appl. Microbiol. 2020, 128, 1613–1623. [Google Scholar] [CrossRef] [PubMed]
- Eman, A.; Ibrahim, M.; Asem, A.; Ali, A.; Mustafa, S.; Mahmoud, S.; Guillermo, T.; Zulhisyam, A.; Mahmoud, A. Effects of sodium butyrate on the growth performance, digestive enzyme activity, intestinal health, and immune responses of Thinlip Grey Mullet (Liza ramada) juveniles. Aquac. Res. 2023, 30, 101530. [Google Scholar]
- Hany, M.; Basma, M.; Mustafa, S.; Nagi, M.; Ibrahim, M.; Mahmoud, A.; Mohsen, A. Effects of sodium butyrate nanoparticles on the hemato-immunological indices, hepatic antioxidant capacity, and gene expression responses in Oreochromis niloticus. Fish Shellfish Immun. 2021, 119, 516–523. [Google Scholar]
- Tian, L.; Zhou, X.Q.; Jiang, W.D.; Liu, Y.; Wu, P.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A.; et al. Sodium butyrate improved intestinal immune function associated with NF-κB and p38MAPK signalling pathways in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immun. 2017, 66, 548–563. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Dai, J.; Yang, P.; Xu, W.; Ai, Q.; Zhang, W.; Zhang, Y.; Zhang, Y.; Mai, K. Sodium butyrate supplementation in high-soybean meal diets for turbot (Scophthalmus maximus L.): Effects on inflammatory status, mucosal barriers and microbiota in the intestine. Fish Shellfish Immun. 2019, 88, 65–75. [Google Scholar] [CrossRef]
- Zhang, J.; Zhong, L.; Chi, S.; Chu, W.; Liu, Y.; Hu, Y. Sodium butyrate supplementation in high-soybean meal diets for juvenile rice field eel (Monopterus albus): Effects on growth, immune response and intestinal health. Aquaculture 2020, 520, 734952. [Google Scholar] [CrossRef]
- Jesus, G.; Pereiraa, S.; Owataria, M.; Syracusea, N.; Silvab, B.; Silvaa, A.; Pierria, B.; Lehmanna, N.; Figueiredoc, H.; Fracalossia, D.; et al. Protected forms of sodium butyrate improve the growth and health of Nile tilapia fingerlings during sexual reversion. Aquaculture 2019, 499, 119–127. [Google Scholar] [CrossRef]
- Zhou, J.; Guo, P.; Yu, H.; Ji, H.; Lai, Z.; Chen, Y. Growth performance, lipid metabolism, and health status of grass carp (Ctenopharyngodon idella) fed three different forms of sodium butyrate. Fish Physiol. Biochem. 2019, 45, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Gao, S.; Chang, K.; Zhao, X.; Niu, B. Dietary sodium butyrate supplementation improves fish growth, intestinal microbiota composition, and liver health in largemouth bass (Micropterus salmoides) fed high-fat diets. Aquaculture 2023, 564, 739040. [Google Scholar] [CrossRef]
- Chen, W.; Gao, S.; Sun, P.; Han, L.; Zhang, Z. Sodium butyrate ameliorates high-fat diet-induced growth retardation and gut injury in largemouth bass (Micropterus salmoides) by modulating gut mucosal barrier and microbiota. Anim. Feed Sci. Technol. 2025, 323, 116283. [Google Scholar] [CrossRef]
- Su, J.; Hou, H.; Wang, C.; Luo, Y. Effects of replacing soybean meal with cottonseed meal on growth, muscle amino acids, and hematology of juvenile common carp, Cyprinus carpio. Aquacult. Int. 2019, 27, 555–566. [Google Scholar] [CrossRef]
- Li, Y.; Yang, R.; He, M.; Su, J.; Liu, L. Chronic ammonia stress in Chinese perch (Siniperca chuatsi): Oxidative response, nitrogen metabolism, and multi-enzyme-mediated molecular detoxification defense mechanisms. Antioxidants 2025, 14, 768. [Google Scholar] [CrossRef]
- Yang, R.; Liu, X.; Liu, Y.; Tian, Q.; Wang, Z.; Zhu, D.; Qian, Z.; Yi, Y.; Hu, J.; Li, Y.; et al. Dissolved oxygen and ammonia affect ammonia production via GDH/AMPK signaling pathway and alter flesh quality in Chinese perch (Siniperca chuatsi). Fish Physiol. Biochem. 2024, 50, 1237–1249. [Google Scholar] [CrossRef]
- Zeng, Z.; Yang, Y.; Zhong, X.; Dai, F.; Chen, S.; Tong, X. Ameliorative effects of lactobacillus paracasei L14 on oxidative stress and gut microbiota in type 2 diabetes Mellitus rats. Antioxidants 2023, 12, 1515. [Google Scholar] [CrossRef]
- Liu, W.; Yang, Y.; Zhang, J.; Gatlin, D.M.; Ringø, E.; Zhou, Z. Effects of dietary microencapsulated sodium butyrate on growth, intestinal mucosal morphology, immune response and adhesive bacteria in juvenile common carp (Cyprinus carpio) pre-fed with or without oxidised oil. Brit. J. Nutr. 2014, 112, 15–29. [Google Scholar] [CrossRef]
- Liu, M.; Guo, W.; Wu, F.; Qu, Q.; Tan, Q.; Gong, W. Dietary supplementation of sodium butyrate may benefit growth performance and intestinal function in juvenile grass carp (Ctenopharyngodon idellus). Aquac. Res. 2017, 48, 4102–4111. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Sadek, K.M. Impact of dietary supplementation of sodium butyrate and/or protexin on the growth performance, some blood parameters, and immune response of Oreochromis niloticus. Int. J. Agric. Innov. Res. 2015, 3, 985–991. [Google Scholar]
- Abdel-Tawwab, M.; Shukry, M.; Farrag, F.; El-Shafai, N.; Dawood, M.; Abdel-Latif, H. Dietary sodium butyrate nanoparticles enhanced growth, digestive enzyme activities, intestinal histomorphometry, and transcription of growth-related genes in Nile tilapia juveniles. Aquaculture 2021, 536, 736467. [Google Scholar] [CrossRef]
- Fang, L.; Wang, Q.; Guo, X.; Pan, X.; Li, X. Effects of dietary sodium butyrate on growth performance, antioxidant capacity, intestinal histomorphology and immune response in juvenile Pengze crucian carp (Carassius auratus Pengze). Aquac. Res. 2021, 21, 100828. [Google Scholar] [CrossRef]
- Robles, R.; Lozabo, A.; Sevilla, A.; Sevilla, A.; Márquez, L.; Nuez–Ortín, W.; Moyano, F. Effect of partially protected butyrate used as feed additive on growth and intestinal metabolism in sea bream (Sparus aurata). Fish Physiol. Biochem. 2013, 39, 1567–1580. [Google Scholar] [CrossRef] [PubMed]
- Bjerkeng; Storebakken; Wathne. Cholesterol and short-chain fatty acids in diets for Atlantic salmon Salmo salar (L.): Effects on growth, organ indices, macronutrient digestibility, and fatty acid composition. Aquacult. Nutr. 1999, 5, 181–191. [Google Scholar] [CrossRef]
- Gao, Y.; Storebakken, T.; Shearer, K.; Penn, M.; Øverland, M. Supplementation of fishmeal and plant protein-based diets for rainbow trout with a mixture of sodium formate and butyrate. Aquaculture 2011, 31, 233–240. [Google Scholar] [CrossRef]
- Mohsen, H.; Wassef, E.; El-Bermawy, N.; Abdel-Meguid, N.; Saleh, N.; Barakat, K.; Shaltout, O. Advantageous effects of dietary butyrate on growth, immunity response, intestinal microbiota and histomorphology of European Seabass (Dicentrarchus labrax) fry. Egypt. J. Aquat. Biol. Fish. 2018, 22, 93–110. [Google Scholar] [CrossRef]
- Hou, D.; Li, M.; Li, P.; Chen, B.; Huang, W.; Guo, H.; Cao, J.; Zhao, H. Effects of sodium butyrate on growth performance, antioxidant status, inflammatory response and resistance to hypoxic stress in juvenile largemouth bass (Micropterus salmoides). Front. Immunol. 2023, 14, 1265963. [Google Scholar] [CrossRef]
- Dawood, M.; Metwally, A.; El-Sharawy, M.; Attaa, A.; Elbialyb, Z.; Abdel-Latifc, H.; Paray, B. The role of β-glucan in the growth, intestinal morphometry, and immune-related gene and heat shock protein expressions of Nile tilapia (Oreochromis niloticus) under different stocking densities. Aquaculture 2020, 523, 735205. [Google Scholar] [CrossRef]
- Jesus, G.; Pereira, S.; Owatari, M.; Addam, K.; Silvab, B.; Sterzelleki, F.; Sugai, J.; Carddoso, L.; Jatobá, A.; Mouriñoa, J.; et al. Use of protected forms of sodium butyrate benefit the development and intestinal health of Nile tilapia during the sexual reversion period. Aquaculture 2019, 504, 326–333. [Google Scholar] [CrossRef]
- Niklasson, L.; Sundh, H.; Fridell, F.; Taranger, G.L.; Sundell, K. Disturbance of the intestinal mucosal immune system of farmed Atlantic salmon (Salmo salar), in response to long-term hypoxic conditions. Fish Shellfish Immun. 2011, 31, 1072–1080. [Google Scholar] [CrossRef]
- Wen, L.; Jiang, W.; Liu, Y.; Wu, P.; Zhao, J.; Jiang, J.; Kuang, S.; Tang, L.; Tang, W.; Zhang, Y.; et al. Evaluation the effect of thiamin deficiency on intestinal immunity of young grass carp (Ctenopharyngodon idella). Fish Shellfish Immun. 2015, 46, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Oshima, T.; Miwa, H.; Joh, T. Changes in the expression of claudins in active ulcerative colitis. J. Gastroenterol. Hepatol. 2008, 23, S146–S150. [Google Scholar] [CrossRef]
- Qi, H.; Cheng, K.; Dong, L.; Guo, Z.; Luo, Y.; Tian, J.; Peng, D.; Wen, H.; Liu, M.; Yang, R.; et al. Effects of sodium butyrate on meat quality improvement and intestinal injury induced by high-level fava beans in Nile tilapia (Oreochromis niloticus). Aquaculture 2025, 607, 742692. [Google Scholar] [CrossRef]
- Kim, J.; Yu, Y.; Choi, J. Toxic effects on bioaccumulation, hematological parameters, oxidative stress, immune responses and neurotoxicity in fish exposed to microplastics: A review. J. Hazard. Mater. 2021, 413, 125423. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Liu, X.; Wu, T.; Zhao, J. Study on acute toxicity of ammonia nitrogen to juvenile tilapia “Neogyphus". Aquat. Sci. 2017, 6, 42–46. [Google Scholar]
- Habte-Tsion, H.; Liu, B.; Ge, X.; Xu, P.; Ren, M.; Zhou, Q.; Pan, L.; Chen, R. Effects of dietary protein level on growth performance, muscle composition, blood composition, and digestive enzyme activity of Wuchang Bream (Megalobrama amblycephala) fry. Isr. J. Aquacult. Bamid. 2013, 65, 1–9. [Google Scholar] [CrossRef]
- Mehrgan, M.; Shekarabi, S.; Azari, A.; Yilmaz, S.; Lückstädt, C.; Islami, H. Synergistic effects of sodium butyrate and sodium propionate on the growth performance, blood biochemistry, immunity, and immune-related gene expression of goldfish (Carassius auratus). Aquacult. Int. 2022, 30, 3179–3193. [Google Scholar] [CrossRef]
- Hoseini, S.; Tarkhani, R. Effect of short-term treatment with potassium permanganate on stress markers and blood biochemistry in goldfish Carassius auratus. Aquac. Res. 2013, 44, 869–875. [Google Scholar] [CrossRef]
- Lin, S.; Shi, C.; Mu, M.; Chen, Y.; Luo, L. Effect of high dietary starch levels on growth, hepatic glucose metabolism, oxidative status and immune response of juvenile largemouth bass, Micropterus salmoides. Fish Shellfish Immun. 2018, 78, 121–126. [Google Scholar] [CrossRef]
- Cadenas, E.; Davies, K. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 2000, 2, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Cheng, L.; Wang, M.; Shen, L.; Zhang, C.; Mu, J.; Hu, Y.; Yang, Y.; He, K.; Yan, H.; et al. Dietary sodium acetate and sodium butyrate improve high-carbohydrate diet utilization by regulating gut microbiota, liver lipid metabolism, oxidative stress, and inflammation in largemouth bass (Micropterus salmoides). J. Anim. Sci. Biotechnol. 2024, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Benson, J.; Shepherd, M. Dietary ligands of the aryl hydrocarbon receptor induce anti-inflammatory and immunoregulatory effects on murine dendritic cells. Toxicol. Sci. 2011, 124, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chang, K.; Chen, J.; Zhao, X.; Gao, S. Dietary sodium butyrate supplementation attenuates intestinal inflammatory response and improves gut microbiota composition in largemouth bass (Micropterus salmoides) fed with a high soybean meal diet. Fish Physiol. Biochem. 2021, 47, 1805–1819. [Google Scholar] [CrossRef]
- Rimoldi, S.; Finzi, G.; Ceccotti, C.; Girardello, R.; Grimaldi1, A.; Ascione, C.; Terova, G. Butyrate and taurine exert a mitigating effect on the inflamed distal intestine of European sea bass fed with a high percentage of soybean meal. Fish. Aquat. Sci. 2016, 19, 40. [Google Scholar] [CrossRef]
- Ge, Y.; Yao, S.; Shi, Y.; Cai, C.; Wang, C.; Wu, P.; Cao, X.; Ye, Y. Effects of low or high dosages of dietary sodium butyrate on the growth and health of the liver and intestine of largemouth bass, Micropterus salmoides. Aquacult. Nutr. 2022, 2022, 6173245. [Google Scholar] [CrossRef]
- Liu, J.; Bian, G.; Zhu, W.; Mao, S. High-grain feeding causes strong shifts in ruminal epithelial bacterial community and expression of Toll-like receptor genes in goats. Front. Microbiol. 2015, 6, 167. [Google Scholar] [CrossRef]
- Nurul, A.; Danish-Daniel, A.; Okomoda, V.; Asma, N. Microbiota composition of captive bluestreak cleaner wrasse Labroides dimidiatus (Valenciennes, 1839). Appl. Microbiol. Biotechnol. 2020, 104, 7391–7407. [Google Scholar] [CrossRef]
- Ni, J.; Yan, Q.; Yu, Y.; Zhang, T. Factors influencing the grass carp gut microbiome and its effect on metabolism. Fems Microbiol. Ecol. 2014, 87, 704–714. [Google Scholar] [CrossRef]
- Tripathy, S.; Kumar, N.; Mohanty, S.; Samanta, M.; Mandal, R.; Maiti, N. Characterisation of Pseudomonas aeruginosa isolated from freshwater culture systems. Microbiol. Res. 2007, 162, 391–396. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).