Periodontal Health in Individuals Living with HIV: An Exploratory and Descriptive Molecular Approach of Microbial Interspecific and Intraspecific Diversity in Brazilian Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Sample Collection
2.2. Clinical Record
2.3. DNA Extraction
2.4. DNA Quantification
2.5. Primer Selection
2.6. Polymerase Chain Reaction (PCR)
2.7. DNA Sequencing
2.8. Sequence Analysis
3. Results
3.1. General Epidemiological Data
3.2. Clinical Measurements
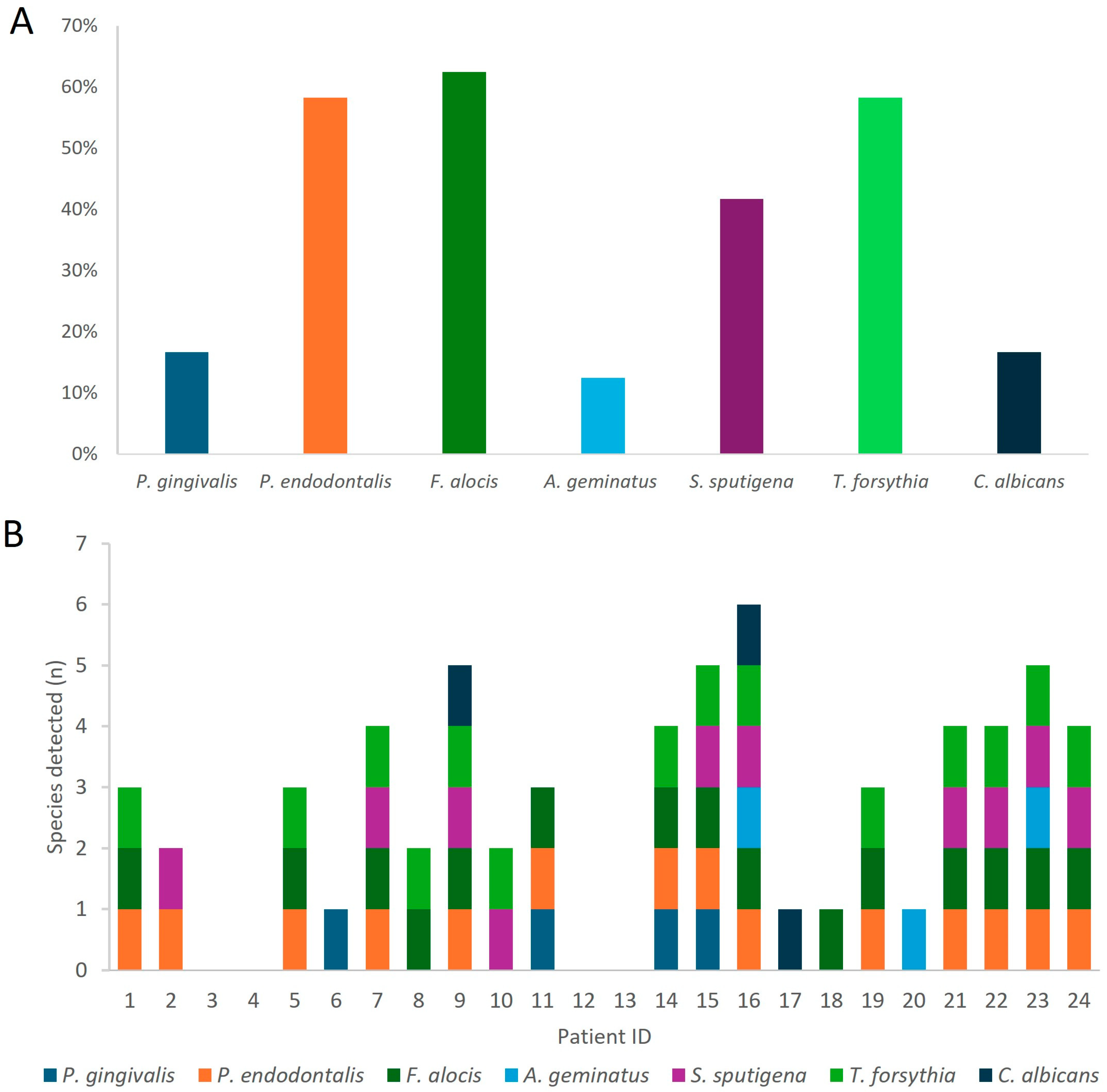
3.3. Molecular Identification of Targeted Oral Pathogens
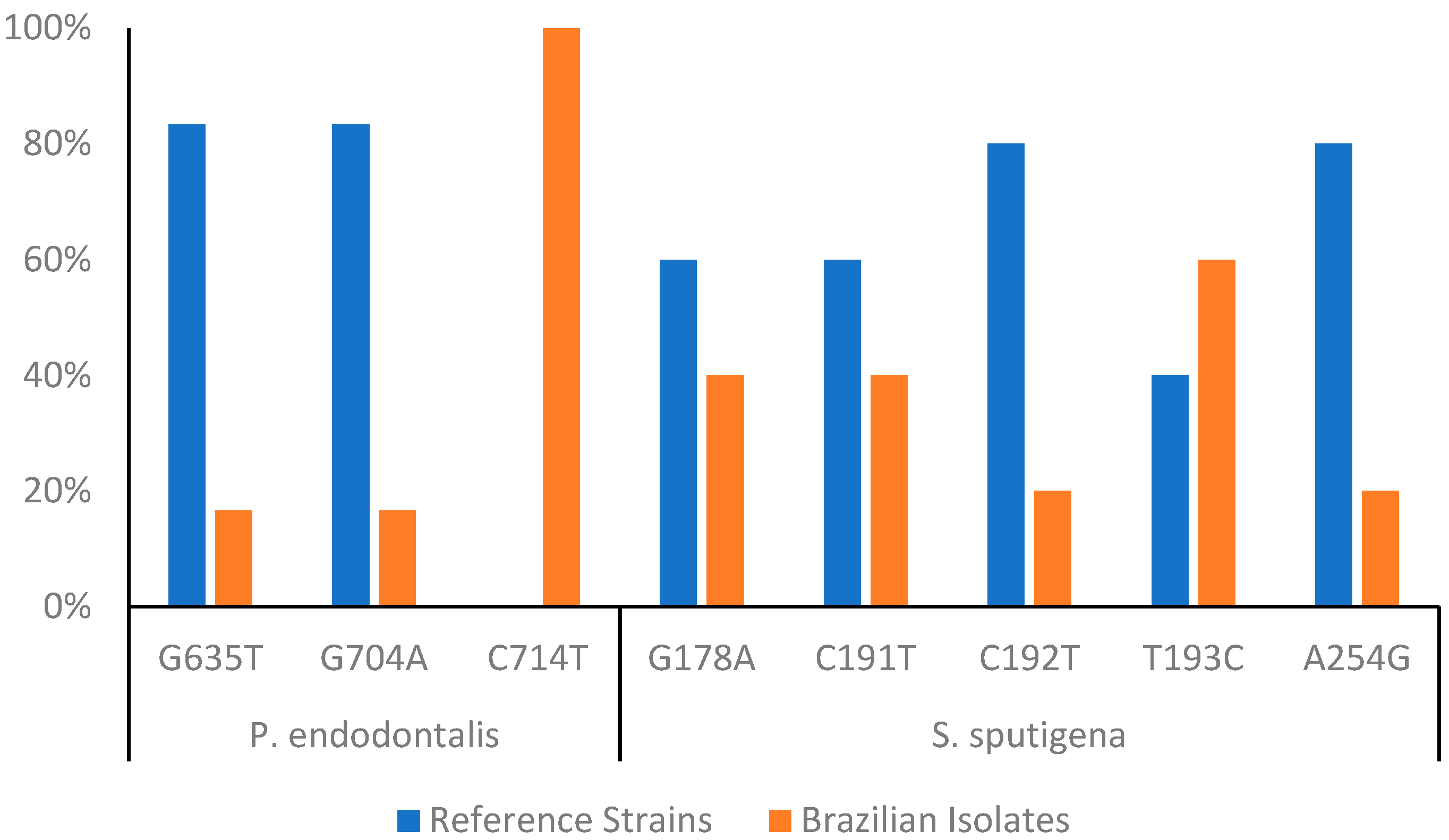
3.4. Intraspecific Diversity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nittayananta, W.; Talungchit, S.; Jaruratanasirikul, S.; Silpapojakul, K.; Chayakul, P.; Nilmanat, A.; Pruphetkaew, N. Effects of long-term use of HAART on oral health status of HIV-infected subjects. J. Oral Pathol. Med. 2010, 39, 397–406. [Google Scholar] [CrossRef]
- Glick, M.; Muzyca, B.C.; Lurie, D.; Salkin, L.M. Oral manifestations associated with HIV-related disease as markers for immune suppression and AIDS. Oral Surg. Oral Med. Oral Pathol. 1994, 77, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Chatzopoulos, G.; Doufexi, A.; Kalogirou, F. Association of susceptible genotypes to periodontal disease with the clinical outcome and tooth survival after non-surgical periodontal therapy: A systematic review and meta-analysis. Med. Oral 2016, 21, e14–e29. [Google Scholar] [CrossRef]
- Liu, B.; Faller, L.L.; Klitgord, N.; Mazumdar, V.; Ghodsi, M.; Sommer, D.D.; Gibbons, T.R.; Treangen, T.J.; Chang, Y.-C.; Li, S.; et al. Deep Sequencing of the Oral Microbiome Reveals Signatures of Periodontal Disease. PLoS ONE 2012, 7, e37919. [Google Scholar] [CrossRef] [PubMed]
- Ghensi, P.; Manghi, P.; Zolfo, M.; Armanini, F.; Pasolli, E.; Bolzan, M.; Bertelle, A.; Dell’Acqua, F.; Dellasega, E.; Waldner, R.; et al. Strong oral plaque microbiome signatures for dental implant diseases identified by strain-resolution metagenomics. NPJ Biofilms Microbiomes 2020, 6, 47. [Google Scholar] [CrossRef]
- Coker, M.O.; Cairo, C.; Garzino-Demo, A. HIV-Associated Interactions Between Oral Microbiota and Mucosal Immune Cells: Knowledge Gaps and Future Directions. Front. Immunol. 2021, 12, 676669. [Google Scholar] [CrossRef] [PubMed]
- Inaba, H.; Amano, A. Roles of Oral Bacteria in Cardiovascular Diseases—From Molecular Mechanisms to Clinical Cases: Implication of Periodontal Diseases in Development of Systemic Diseases. J. Pharmacol. Sci. 2010, 113, 103–109. [Google Scholar] [CrossRef]
- Ryder, M.I.; Nittayananta, W.; Coogan, M.; Greenspan, D.; Greenspan, J.S. Periodontal disease in HIV/AIDS. Periodontol. 2000 2012, 60, 78–97. [Google Scholar] [CrossRef]
- Tribble, G.D.; Kerr, J.E.; Wang, B.-Y. Genetic Diversity in the Oral Pathogen Porphyromonas Gingivalis: Molecular Mechanisms and Biological Consequences. Future Microbiol. 2013, 8, 607–620. [Google Scholar] [CrossRef]
- Westfelt, E.; Rylander, H.; Dahlén, G.; Lindhe, J. The effect of supragingival plaque control on the progression of advanced periodontal disease. J. Clin. Periodontol. 1998, 25, 536–541. [Google Scholar] [CrossRef]
- Ainamo, J.; Bay, I. Problems and proposals for recording gingivitis and plaque. Int. Dent. J 1975, 4, 229–235. [Google Scholar]
- Lombardo Bedran, T.B.; Marcantonio, R.A.C.; Spin Neto, R.; Alves Mayer, M.P.; Grenier, D.; Spolidorio, L.C.; Spolidorio, D.P. Porphyromonas endodontalis in chronic periodontitis: A clinical and microbiological cross-sectional study. J. Oral Microbiol. 2012, 4, 10123. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F.; Rôças, I.N. Detection of Filifactor alocis in endodontic infections associated with different forms of periradicular diseases. Oral Microbiol. Immunol. 2003, 18, 263–265. [Google Scholar] [CrossRef]
- Carlier, J.-P.; Marchandin, H.; Jumas-Bilak, E.; Lorin, V.; Henry, C.; Carrière, C.; Jean-Pierre, H. Anaeroglobus geminatus gen. nov., sp. nov., a novel member of the family Veillonellaceae. Int. J. Syst. Evol. Microbiol. 2002, 52, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Medikeri, R.S. Quantification of Selenomonas sputigena in Chronic Periodontitis in Smokers Using 16S rDNA Based PCR Analysis. J. Clin. Diagn. Res. 2015, 9, ZC13-7. [Google Scholar] [CrossRef]
- Siqueira, J.F.; Rôças, I.N. Polymerase chain reaction-based analysis of microorganisms associated with failed endodontic treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 97, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F.; Rôças, I.N. PCR-based identification of Treponema maltophilum, T. amylovorum, T. medium, and T. lecithinolyticum in primary root canal infections. Arch. Oral Biol. 2003, 48, 495–502. [Google Scholar] [CrossRef]
- Rôças, I.N.; Neves, M.A.S.; Provenzano, J.C.; Siqueira, J.F. Susceptibility of As-yet-uncultivated and Difficult-to-culture Bacteria to Chemomechanical Procedures. J. Endod. 2014, 40, 33–37. [Google Scholar] [CrossRef]
- Ashimoto, A.; Chen, C.; Bakker, I.; Slots, J. Polymerase chain reaction detection of 8 putative periodontal pathogens in subgingival plaque of gingivitis and advanced periodontitis lesions. Oral Microbiol. Immunol. 1996, 11, 266–273. [Google Scholar] [CrossRef]
- Siqueira, J.F.; Rôças, I.N.; Favieri, A.; Santos, K.R.N. Detection of Treponema denticola in endodontic infections by 16S rRNA gene-directed polymerase chain reaction. Oral Microbiol. Immunol. 2000, 15, 335–337. [Google Scholar] [CrossRef]
- Kan, V.L. Polymerase Chain Reaction for the Diagnosis of Candidemia. J. Infect. Dis. 1993, 168, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Dumani, A.; Yoldas, O.; Yilmaz, S.; Koksal, F.; Kayar, B.; Akcimen, B.; Seydaoglu, G. Polymerase chain reaction of enterococcus faecalis and candida albicans in apical periodontitis from Turkish patients. J. Clin. Exp. Dent. 2012, 4, e34–e39. [Google Scholar] [CrossRef] [PubMed]
- Araújo, L.L.; Lourenço, T.G.B.; Colombo, A.P.V. Periodontal Disease Severity Is Associated to Pathogenic Consortia Comprising Putative and Candidate Periodontal Pathogens. J. Appl. Oral Sci. 2023, 31, e20220359. [Google Scholar] [CrossRef]
- Gonçalves, L.S.; Soares Ferreira, S.M.; Souza, C.O.; Souto, R.; Colombo, A.P. Clinical and Microbiological Profiles of Human Immunodeficiency Virus (HIV)–Seropositive Brazilians Undergoing Highly Active Antiretroviral Therapy and HIV-Seronegative Brazilians With Chronic Periodontitis. J. Periodontol. 2007, 78, 87–96. [Google Scholar] [CrossRef]
- Colombo, A.P.V.; Teles, R.P.; Torres, M.C.; Souto, R.; Rosalém, W., Jr.; Mendes, M.C.S.; Uzeda, M. Subgingival Microbiota of Brazilian Subjects With Untreated Chronic Periodontitis. J. Periodontol. 2002, 73, 360–369. [Google Scholar] [CrossRef]
- Szwarcwald, C.L.; Malta, D.C.; Barros, M.B.A.; de Souza Júnior, P.R.B.; Romero, D.; de Almeida, W.D.S.; Damacena, G.N.; Werneck, A.O.; da Silva, D.R.P.; Lima, M.G.; et al. Associations of Sociodemographic Factors and Health Behaviors with the Emotional Well-Being of Adolescents during the COVID-19 Pandemic in Brazil. Int. J. Environ. Res. Public Health 2021, 18, 6160. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, L.F.H.; Fermiano, D.; Feres, M.; Figueiredo, L.C.; Teles, F.R.P.; Mayer, M.P.A.; Faveri, M. Levels of Selenomonas Species in Generalized Aggressive Periodontitis. J. Periodontal Res. 2012, 47, 711–718. [Google Scholar] [CrossRef]
- de Oliveira, M.C.; Gomes-Filho, I.S.; Stöcker, A.; Neto, L.O.B.; do Nascimento Santos, A.; da Cruz, S.S.; Passos-Soares, J.S.; Falcão, M.M.L.; Meireles, J.R.C.; Seymour, G.J.; et al. Microbiological Findings of the Maternal Periodontitis Associated to Low Birthweight. Einstein 2020, 18, eAO4209. [Google Scholar] [CrossRef]
- Gonçalves, C.; Soares, G.M.S.; Faveri, M.; Pérez-Chaparro, P.J.; Lobão, E.; Figueiredo, L.C.; Baccelli, G.T.; Feres, M. Association of Three Putative Periodontal Pathogens with Chronic Periodontitis in Brazilian Subjects. J. Appl. Oral Sci. 2016, 24, 181–185. [Google Scholar] [CrossRef]
- Shaikh, H.F.M.; Oswal, P.U.; Kugaji, M.S.; Katti, S.S.; Bhat, K.G.; Kandaswamy, E.; Joshi, V.M. Association of F. Alocis and D. Pneumosintes with Periodontitis Disease Severity and Red Complex Bacteria. Dent J (Basel) 2024, 12, 105. [Google Scholar] [CrossRef]
- Serra e Silva Filho, W.; Casarin, R.C.; Nicolela Junior, E.L.; Passos, H.M.; Sallum, A.W.; Gonçalves, R.B. Microbial Diversity Similarities in Periodontal Pockets and Atheromatous Plaques of Cardiovascular Disease Patients. PLoS ONE 2014, 9, e109761. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, F.G.; Silva, D.S.; Hebling, J.; Spolidorio, L.C.; Spolidorio, D.M.P. Presence of Mutans Streptococci and Candida Spp. in Dental Plaque/Dentine of Carious Teeth and Early Childhood Caries. Arch Oral Biol 2006, 51, 1024–1028. [Google Scholar] [CrossRef]
- Belibasakis, G.N. Grand Challenges in Oral Infections and Microbes. Front. Oral. Health 2020, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Džunková, M.; Martinez-Martinez, D.; Gardlík, R.; Behuliak, M.; Janšáková, K.; Jiménez, N.; Vázquez-Castellanos, J.F.; Martí, J.M.; D’Auria, G.; Bandara, H.M.H.N.; et al. Oxidative stress in the oral cavity is driven by individual-specific bacterial communities. NPJ Biofilms Microbiomes 2018, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Wolff, D.; Frese, C.; Schoilew, K.; Dalpke, A.; Wolff, B.; Boutin, S. Amplicon-based microbiome study highlights the loss of diversity and the establishment of a set of species in patients with dentin caries. PLoS ONE 2019, 14, e0219714. [Google Scholar] [CrossRef]
- Jarosz, L.M.; Deng, D.M.; Van Der Mei, H.C.; Crielaard, W.; Krom, B.P. Streptococcus mutans Competence-Stimulating Peptide Inhibits Candida albicans Hypha Formation. Eukaryot. Cell 2009, 8, 1658–1664. [Google Scholar] [CrossRef]
- Li, X.; Kolltveit, K.M.; Tronstad, L.; Olsen, I. Systemic Diseases Caused by Oral Infection. Clin. Microbiol. Rev. 2000, 13, 547–558. [Google Scholar] [CrossRef]
- Atanasova, K.R.; Yilmaz, Ö. Looking in the Porphyromonas gingivalis cabinet of curiosities: The microbium, the host and cancer association. Mol. Oral Microbiol. 2014, 29, 55–66. [Google Scholar] [CrossRef]
- Griffen, A.L.; Thompson, Z.A.; Beall, C.J.; Lilly, E.A.; Granada, C.; Treas, K.D.; DuBois, K.R.; Hashmi, S.B.; Mukherjee, C.; Gilliland, A.E.; et al. Significant effect of HIV/HAART on oral microbiota using multivariate analysis. Sci. Rep. 2019, 9, 19946. [Google Scholar] [CrossRef]
- Abranches, J.; Zeng, L.; Kajfasz, J.K.; Palmer, S.R.; Chakraborty, B.; Wen, Z.T.; Richards, V.P.; Brady, L.J.; Lemos, J.A. Biology of Oral Streptococci. Microbiol. Spectr. 2018, 6, 5. [Google Scholar] [CrossRef]
- Whitmore, S.E.; Lamont, R.J. Oral Bacteria and Cancer. PLoS Pathog 2014, 10, e1003933. [Google Scholar] [CrossRef] [PubMed]
- Téllez-Corral, M.A.; Herrera-Daza, E.; Cuervo-Jimenez, H.K.; Arango-Jimenez, N.; Morales-Vera, D.Z.; Velosa-Porras, J.; Latorre-Uriza, C.; Escobar-Arregoces, F.M.; Hidalgo-Martinez, P.; Cortés, M.E.; et al. Patients with obstructive sleep apnea can favor the predisposing factors of periodontitis by the presence of P. melaninogenica and C. albicans, increasing the severity of the periodontal disease. Front. Cell. Infect. Microbiol. 2022, 12, 934298. [Google Scholar] [CrossRef] [PubMed]
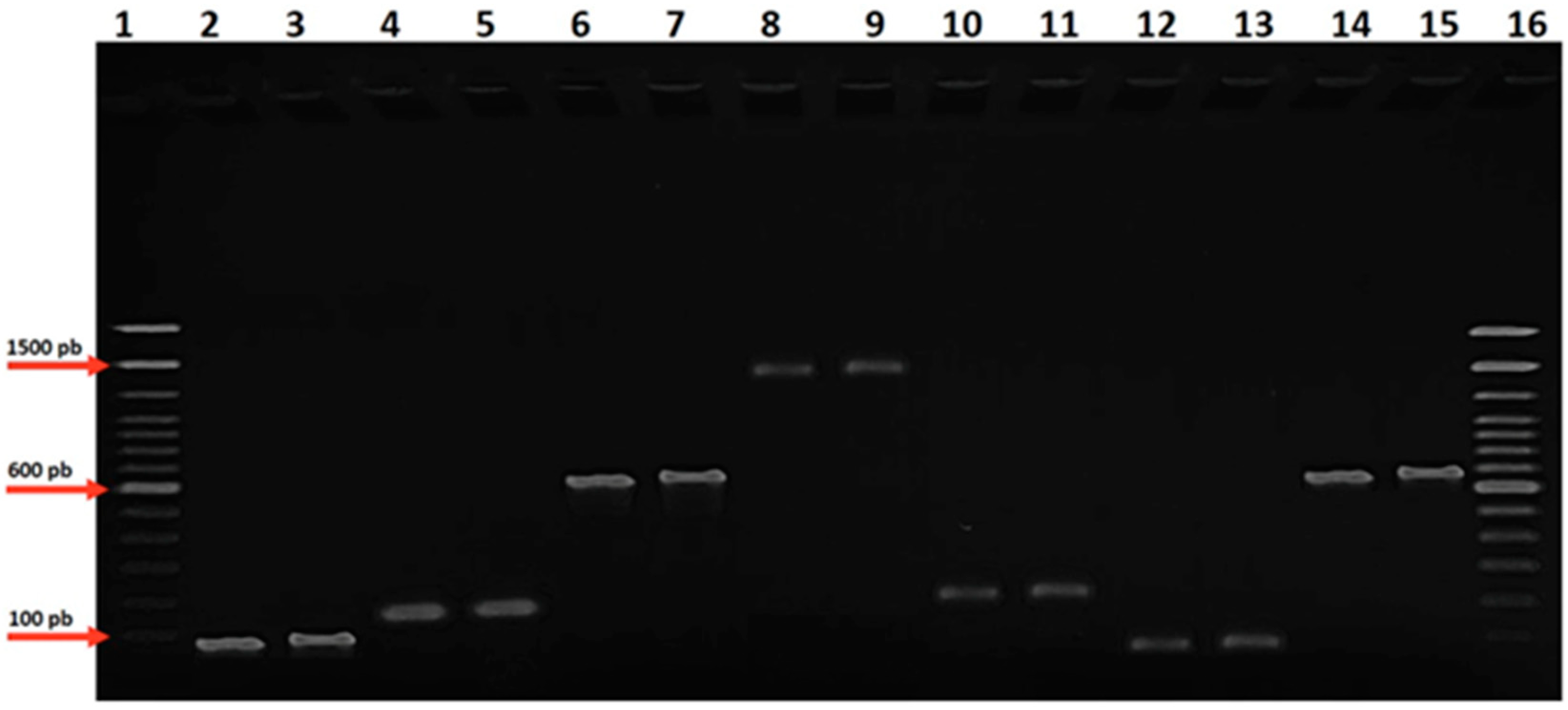
| Targets | Primer Pairs (5′–3′) | Amplicon Length (bp) | Reference |
|---|---|---|---|
| Porphyromonas gingivalis | ACC TTA CCC GGG ATT GAA ATG | 83 | [12] |
| CAA CCA TGC AGC ACCT AC ATA GAA | |||
| Porphyromonas endodontalis | GCT GCA GCT CAA CTG TAG TCT TG | 110 | [12] |
| TCA GTG TCA GAC GGA GCC TAG TAC | |||
| Filifactor alocis | CAG GTG GTT TAA CAA GTT AGT GG | 594 | [13] |
| CTA AGT TGT CCT TAG CTG TCT CG | |||
| Anaeroglobus geminatus | GTGCTGCAGAGAGTTTGATCCTGGCTCAG | 1408 | [14] |
| CACGGATCCTACGGGTACCTTGTTACGACTT | |||
| Selenomonas sputigena | AGAGTTTGATCCTGGCTCAG | 700 | [15] |
| CTCAATATTCTCAA GCTCGGTT | |||
| Enterococcus faecalis | GTT TAT GCC GCA TGG CAT AAG AG | 310 | [16] |
| CCG TCA GGG GAC GTT CAG | |||
| Treponema lecithinolyticum | CTT GCT CCT TTC TGA GAG TGG CGG | 950 | [17] |
| ACG CAT CCG TAT CTC TAC GAA CTT | |||
| Treponema médium | AGA GTT TGA TCC TGG CTC AG | 156 | [17] |
| CCT TAT GAA GCA CTG AGT GTA TTC | |||
| Fretibacterium fastidiuosum | AGA GTT TGA TCC TGG CTC AG | 969 | [18] |
| GAA AGT ACG TCG TCG CCC TTT CAG | |||
| Treponema denticola | TAA TAC CGA ATG TGC TCA TTT ACA T | 316 | [19,20] |
| TCA AAG AAG CAT TCC CTC TTC TTC TTA | |||
| Tannerella forsythia | AGC GAT GGT AGC AAT ACC TGT C | 88 | [12] |
| TTC GCC GGG TTA TCC CTC | |||
| Candida albicans | GCC GGT GAC GAC GCT CCA AGA GCT G | 158 | [21,22] |
| CCG TGT TCA ATT GGG TAT CTC AAG GTC |
| Summarized Data from Studied Patients (n = 24) | |
|---|---|
| Clinical and epidemiological | Mean ± SD |
| Age | 42.5 ± 7.9 |
| Time elapsed since the diagnosis (years) | 8.2 ± 6.2 |
| Leukocytes | 4783 ± 2096 |
| Linfocytes | 1758 ± 696 |
| Neutrophils | 2319 ± 1158 |
| T-CD4 cells count | 661.4 ± 354.1 |
| T-CD8 cells count | 792.9 ± 397.1 |
| CD4/CD8 ratio | 0.865 ± 0.286 |
| Co-morbities | Frequency (%) |
| Diabetes | 17% |
| Hipertension | 22% |
| Herpes | 28% |
| Zoster | 28% |
| Patient | % VSB | % BOP | PPD (mm) | CAL (mm) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Median | Range | Mean | SD | Median | Range | |||
| 1 | 18.8 | 26.8 | 2.58 | 0.74 | 3.0 | 1.0–5.0 | 2.66 | 0.73 | 3.0 | 1.0–5.0 |
| 2 | 38.2 | 12.0 | 1.87 | 0.65 | 2.0 | 1.0–3.0 | 1.87 | 0.68 | 2.0 | 0.0–3.0 |
| 3 | - | - | - | - | - | - | - | - | - | - |
| 4 | 42.7 | 2.8 | 2.10 | 0.32 | 2.0 | 1.0–3.0 | 2.22 | 0.50 | 2.0 | 1.0–4.0 |
| 5 | 90.5 | 19.3 | 2.61 | 0.74 | 3.0 | 1.0–6.0 | 2.68 | 0.71 | 3.0 | 1.0–6.0 |
| 6 | - | - | - | - | - | - | - | - | - | - |
| 7 | 48.2 | 28.6 | 2.40 | 0.70 | 2.0 | 1.0–6.0 | 1.29 | 1.94 | 0.0 | 0.0–6.0 |
| 8 | - | - | 2.50 | 0.72 | 2.0 | 1.0–5.0 | 0.0 | 0.00 | 0.0 | 0.0–0.0 |
| 9 | 37.5 | 42.1 | 2.39 | 0.75 | 2.0 | 1.0–4.0 | 0.20 | 0.73 | 0.0 | 0.0–3.0 |
| 10 | 65.3 | 26.0 | 3.59 | 1.73 | 3.0 | 2.0–9.0 | 4.47 | 2.00 | 4.0 | 2.0–10 |
| 11 | 69.4 | 49.5 | 2.89 | 0.73 | 3.0 | 2.0–6.0 | 3.01 | 2.22 | 4.0 | 0.0–7.0 |
| 12 | 84.4 | 77.7 | 3.40 | 1.30 | 3.0 | 2.0–8.0 | 3.88 | 2.43 | 5.0 | 0.0–9.0 |
| 13 | 15.1 | 49.2 | 1.91 | 1.99 | 2.0 | 1.0–3.0 | 0.59 | 0.85 | 0.0 | 0.0–4.0 |
| 14 | 6.8 | 3.4 | 1.69 | 0.70 | 2.0 | 1.0–4.0 | 0.94 | 1.12 | 0.0 | 0.0–4.0 |
| 15 | 52.2 | 37.3 | 2.00 | 0.84 | 2.0 | 1.0–6.0 | 2.14 | 1.32 | 2.0 | 0.0–7.0 |
| 16 | - | - | - | - | - | - | - | - | - | - |
| 17 | 67.5 | 23.3 | 2.48 | 0.71 | 2.0 | 1.0–5.0 | 0.75 | 1.35 | 0.0 | 0.0–5.0 |
| 18 | 38.0 | 14.7 | 2.65 | 1.25 | 2.0 | 1.0–7.0 | 3.05 | 1.42 | 3.0 | 1.0–8.0 |
| 19 | 36.6 | 13.9 | 2.23 | 0.69 | 2.0 | 1.0–4.0 | 2.78 | 0.82 | 3.0 | 1.0–5.0 |
| 20 | 25.8 | 40.2 | 1.79 | 0.46 | 2.0 | 1.0–3.0 | 2.05 | 0.87 | 2.0 | 1.0–8.0 |
| 21 | - | - | 1.29 | 0.80 | 1.0 | 0.0–5.0 | 1.68 | 1.47 | 1.0 | 0.0–8.0 |
| 22 | 46.4 | 12.7 | 3.26 | 1.52 | 3.0 | 1.0–9.0 | 3.38 | 1.53 | 3.0 | 1.0–10.0 |
| 23 | - | - | - | - | - | - | - | - | - | - |
| 24 | 70.8 | 37.5 | 2.71 | 0.92 | 3.0 | 1.0–5.0 | 2.91 | 1.11 | 3.0 | 1.0–7.0 |
| Species | HIV (+) Studied Individuals | HIV (−) Individuals from Other Brazilian Studies | Refs. |
|---|---|---|---|
| Frequency | Mean Frequency (Range) | ||
| P. gingivalis | 16.7% | 29.4% (7.5–58.4%) | [23,24,25,26,27,28] |
| P. endodontalis | 58.3% | 63.5% (50.0–77.0%) | [29] |
| F. alocis | 62.5% | 43.0% (20.0–67.0%) | [23,29,30,31] |
| A. geminatus | 12.5% | 33.0% (33.0%) | [27,31] |
| S. sputigena | 41.7% | 44.6% (15.4–67.0%) | [31] |
| T. forsythia | 58.3% | 46.2% (12.0–100%) | [24,26,28] |
| C. albicans | 16.7% | 27.5% (14.5–50.0%) | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponce, P.N.O.; Chaves, L.B.; Perce-da-Silva, D.d.S.; Carneiro-Alencar, A.L.; Rodolphi, C.M.; Soares, I.F.; Rodrigues-da-Silva, R.N.; Alves-da-Silva, A.C.; Marques, F.V.; Peres, R.V.; et al. Periodontal Health in Individuals Living with HIV: An Exploratory and Descriptive Molecular Approach of Microbial Interspecific and Intraspecific Diversity in Brazilian Patients. Microorganisms 2025, 13, 867. https://doi.org/10.3390/microorganisms13040867
Ponce PNO, Chaves LB, Perce-da-Silva DdS, Carneiro-Alencar AL, Rodolphi CM, Soares IF, Rodrigues-da-Silva RN, Alves-da-Silva AC, Marques FV, Peres RV, et al. Periodontal Health in Individuals Living with HIV: An Exploratory and Descriptive Molecular Approach of Microbial Interspecific and Intraspecific Diversity in Brazilian Patients. Microorganisms. 2025; 13(4):867. https://doi.org/10.3390/microorganisms13040867
Chicago/Turabian StylePonce, Patricia N. Olivares, Lana Bitencourt Chaves, Daiana de Souza Perce-da-Silva, Ana Luiza Carneiro-Alencar, Cinthia Magalhães Rodolphi, Isabela Ferreira Soares, Rodrigo Nunes Rodrigues-da-Silva, Ana Caroline Alves-da-Silva, Fabio Vidal Marques, Rafael Vidal Peres, and et al. 2025. "Periodontal Health in Individuals Living with HIV: An Exploratory and Descriptive Molecular Approach of Microbial Interspecific and Intraspecific Diversity in Brazilian Patients" Microorganisms 13, no. 4: 867. https://doi.org/10.3390/microorganisms13040867
APA StylePonce, P. N. O., Chaves, L. B., Perce-da-Silva, D. d. S., Carneiro-Alencar, A. L., Rodolphi, C. M., Soares, I. F., Rodrigues-da-Silva, R. N., Alves-da-Silva, A. C., Marques, F. V., Peres, R. V., Ferreira, D. d. C., de Souza, R. C., Gonçalves, C., Gonçalves, L. S., & Lima-Junior, J. d. C. (2025). Periodontal Health in Individuals Living with HIV: An Exploratory and Descriptive Molecular Approach of Microbial Interspecific and Intraspecific Diversity in Brazilian Patients. Microorganisms, 13(4), 867. https://doi.org/10.3390/microorganisms13040867








