Taxonomical, Physiological, and Biochemical Characteristics of Dunaliella salina DSTA20 from Hypersaline Environments of Taean Salt Pond, Republic of Korea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Isolation
2.2. Morphological Identification
2.3. Molecular Identification
2.4. Determination of Optimal Culture Conditions
2.5. Determination of Total Lipid, Fatty Acid Composition, Biodiesel Properties
2.6. Microalgal Carotenoid Extraction and Analysis
2.7. Extraction and Analysis of Microalgal Monosaccharides
2.8. Statistical Analysis
3. Results
3.1. Morphological Characteristics
3.2. Molecular Identification and Sequence Analysis
3.3. Verification of the Optimal Cultivation Conditions of the Isolated Strain
3.4. Proximate Composition and FAME Analysis, Along with the Evaluation of Biodiesel Properties
3.5. Analysis of Microalgal Carotenoid Profile
3.6. Analysis of the Monosaccharide Profile
4. Discussion
4.1. Morphological and Molecular Identification
4.2. Ecological and Growth Characteristics, Including Adaptability
4.3. Lipid Contents
4.4. Fatty Acid Composition and Biodiesel Properties
4.5. Carotenoid Composition
4.6. Monosaccharide Composition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Boutarfa, S.; Senoussi, M.M.; Gonzalez-Silvera, D.; López-Jiménez, J.A.; Aboal, M. The green microalga Coelastrella thermophila var. globulina (Scenedesmaceae, Chlorophyta) isolated from an Algerian hot spring as a potential source of fatty acids. Life 2022, 12, 560. [Google Scholar] [CrossRef]
- van Leeuwe, M.A.; Tedesco, L.; Arrigo, K.R.; Assmy, P.; Campbell, K.; Meiners, K.M.; Rintala, J.M.; Selz, V.; Thomas, D.N.; Stefels, J. Microalgal community structure and primary production in Arctic and Antarctic sea ice: A synthesis. Elementa 2018, 6, 267. [Google Scholar] [CrossRef]
- Oren, A. The ecology of Dunaliella in high-salt environments. J. Biol. Res. 2014, 21, 1–8. [Google Scholar] [CrossRef]
- Martínez, G.M.; Pire, C.; Martínez-Espinosa, R.M. Hypersaline environments as natural sources of microbes with potential applications in biotechnology: The case of solar evaporation systems to produce salt in Alicante County (Spain). Curr. Res. Microb. Sci. 2022, 3, 100136. [Google Scholar] [CrossRef]
- Saccò, M.; White, N.E.; Harrod, C.; Salazar, G.; Aguilar, P.; Cubillos, C.F.; Meredith, K.; Baxter, B.K.; Oren, A.; Anufriieva, E.; et al. Salt to conserve: A review on the ecology and preservation of hypersaline ecosystems. Biol. Rev. Camb. Philos. Soc. 2021, 96, 2828–2850. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication; National University of Ireland Galway: Galway, Ireland, 2024; Available online: http://www.algaebase.org (accessed on 19 July 2024).
- Goswami, R.K.; Agrawal, K.; Verma, P. Microalgae Dunaliella as biofuel feedstock and β-carotene production: An influential step towards environmental sustainability. Energy Convers. Manag. X 2022, 13, 100154. [Google Scholar] [CrossRef]
- Ben-Amotz, A.; Avron, M. Glycerol and ß-carotene metabolism in the halotolerant alga Dunaliella: A model system for biosolar energy conversion. Trends Biochem. Sci. 1981, 6, 297–299. [Google Scholar] [CrossRef]
- Ben-Amotz, A.; Avron, M. On the factors which determine massive ß-Carotene accumulation in the halotolerant alga Dunaliella bardawil. Plant Physiol. 1983, 72, 593–597. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Siva, C.J. The taxonomy of the genus Dunaliella (Chlorophyta, Dunaliellales) with emphasis on the marine and halophilic species. J. Appl. Phycol. 2007, 19, 567–590. [Google Scholar] [CrossRef]
- Blossom, H.E.; Markussen, B.; Daugbjerg, N.; Krock, B.; Norlin, A.; Hansen, P.J. The cost of toxicity in microalgae: Direct evidence from the dinoflagellate Alexandrium. Front. Microbiol. 2019, 10, 1065. [Google Scholar] [CrossRef]
- Cifuentes, A.S.; González, M.; Conejeros, M.; Dellarossa, V.; Parra, O. Growth and carotenogenesis in eight strains of Dunaliella salina Teodoresco from Chile. J. Appl. Phycol. 1992, 4, 111–118. [Google Scholar] [CrossRef]
- Kang, N.S.; Cho, K.; An, S.M.; Kim, E.S.; Ki, H.; Lee, C.H.; Choi, G.; Hong, J.W. Taxonomic and biochemical characterization of microalga Graesiella emersonii GEGS21 for its potential to become feedstock for biofuels and bioproducts. Energies 2022, 15, 8725. [Google Scholar] [CrossRef]
- Xu, Y.; Ibrahim, I.M.; Wosu, C.I.; Ben-Amotz, A.; Harvey, P.J. Potential of new isolates of Dunaliella salina for natural β-carotene production. Biology 2018, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.D.; Vasconcelos, V. Legal aspects of microalgae in the European food sector. Foods 2024, 13, 124. [Google Scholar] [CrossRef]
- Mendes, M.C.; Navalho, S.; Ferreira, A.; Paulino, C.; Figueiredo, D.; Silva, D.; Gao, F.; Gama, F.; Bombo, G.; Jacinto, R.; et al. Algae as food in Europe: An overview of species diversity and their application. Foods 2022, 11, 1871. [Google Scholar] [CrossRef]
- Yarkent, Ç.; Gürlek, C.; Oncel, S.S. Potential of microalgal compounds in trending natural cosmetics: A review. Sustain. Chem. Pharm. 2020, 17, 100304. [Google Scholar] [CrossRef]
- Global Market Insights. Dunaliella salina Market Size, Industry Analysis Report, Regional Outlook; Global Market Insights: Selbyville, DE, USA, 2023; Available online: https://www.gminsights.com/industry-analysis/dunaliella-salina-market (accessed on 7 October 2024).
- Parvin, M.; Zannat, M.N.; Habib, M.A.B. Two important techniques for isolation of microalgae. Asian Fish. Sci. 2007, 20, 117–124. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Kang, N.S.; Jeong, H.J.; Moestrup, Ø.; Shin, W.; Nam, S.W.; Park, J.Y.; De Salas, M.F.; Kim, K.W.; Noh, J.H. Description of a new planktonic mixotrophic dinoflagellate Paragymnodinium shiwhaense n. gen., n. sp. from the coastal waters off western Korea: Morphology, pigments, and ribosomal DNA gene sequence. J. Eukaryot. Microbiol. 2010, 57, 121–144. [Google Scholar] [CrossRef]
- Edler, D.; Klein, J.; Antonelli, A.; Silvestro, D. raxmlGUI 2.0: A graphical interface and toolkit for phylogenetic analyses using RAxML. Methods Ecol. Evol. 2021, 12, 373–377. [Google Scholar] [CrossRef]
- Medlin, L.; Elwood, H.J.; Stickel, S.; Sogin, M.L. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene 1988, 71, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Litaker, R.W.; Vandersea, M.W.; Kibler, S.R.; Reece, K.S.; Stokes, N.A.; Steidinger, K.A.; Millie, D.F.; Bendis, B.J.; Pigg, R.J.; Tester, P.A. Identification of Pfiesteria piscicida (Dinophyceae) and Pfiesteria-like organisms using internal transcribed spacer-specific PCR assays. J. Phycol. 2003, 39, 754–761. [Google Scholar] [CrossRef]
- Weekers, P.H.H.; Gast, R.J.; Fuerst, P.A.; Byers, T.J. Sequence variations in small-subunit ribosomal RNAs of Hartmannella vermiformis and their phylogenetic implications. Mol. Biol. Evol. 1994, 11, 684–690. [Google Scholar] [CrossRef]
- Scholin, C.A.; Herzog, M.; Sogin, M.; Anderson, D.M. Identification of group and strain-specific genetic markers for globally distributed Alexandrium (Dinophyceae). II. Sequence analysis of a fragment of the LSU rRNA gene. J. Phycol. 1994, 30, 999–1011. [Google Scholar] [CrossRef]
- Hadi, S.I.I.A.; Santana, H.; Brunale, P.P.M.; Gomes, T.G.; Oliveira, M.D.; Matthiensen, A.; Oliveira, M.E.C.; Silva, F.C.P.; Brasil, B.S.A.F. DNA barcoding green microalgae isolated from neotropical inland waters. PLoS ONE 2016, 11, e0149284. [Google Scholar] [CrossRef]
- Heo, J.; Cho, D.H.; Ramanan, R.; Oh, H.M.; Kim, H.S. PhotoBiobox: A tablet-sized, low-cost, high throughput photobioreactor for microalgal screening and culture optimization for growth, lipid content and CO2 sequestration. Biochem. Eng. J. 2015, 103, 193–197. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S.A. A simple method for total lipid extraction and purification. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Nayak, M.; Rath, S.S.; Thirunavoukkarasu, M.; Panda, P.K.; Mishra, B.K.; Mohanty, R.C. Maximizing biomass productivity and CO2 biofixation of microalga, Scenedesmus sp. by using sodium hydroxide. J. Microbiol. Biotechnol. 2013, 23, 1260–1268. [Google Scholar] [CrossRef]
- Breuer, G.; Evers, W.A.C.; de Vree, J.H.; Kleinegris, D.M.M.; Martens, D.E.; Wijffels, R.H.; Lamers, P.P. Analysis of fatty acid content and composition in microalgae. J. Vis. Exp. 2013, 80, 50628. [Google Scholar] [CrossRef]
- Islam, M.A.; Magnusson, M.; Brown, R.J.; Ayoko, G.A.; Nabi, M.N.; Heimann, K. Microalgal species selection for biodiesel production based on fuel properties derived from fatty acid profiles. Energies 2013, 6, 5676–5702. [Google Scholar] [CrossRef]
- Yang, H.W.; Song, J.Y.; Cho, S.M.; Kwon, H.C.; Pan, C.H.; Park, Y.I. Genomic survey of salt acclimation-related genes in the halophilic cyanobacterium Euhalothece sp. Z-M001. Sci. Rep. 2020, 10, 676. [Google Scholar] [CrossRef] [PubMed]
- Teodoresco, E.C. Organisation et développement du Dunaliella, nouveau genre de Volvocacée-Polyblépharidée. Bot. Zentralbl. Beih. 1905, 18, 215–223. [Google Scholar]
- Highfield, A.; Ward, A.; Pipe, R.; Schroeder, D.C. Molecular and phylogenetic analysis reveals new diversity of Dunaliella salina from hypersaline environments. J. Mar. Biol. Assoc. UK 2021, 101, 27–37. [Google Scholar] [CrossRef]
- Polle, J.E.W.; Roth, R.; Ben-Amotz, A.; Goodenough, U. Ultrastructure of the green alga Dunaliella salina strain CCAP19/18 (Chlorophyta) as investigated by quick-freeze deep-etch electron microscopy. Algal Res. 2020, 49, 101953. [Google Scholar] [CrossRef]
- EN 14214; Automotive Fuels-Fatty Acid Methyl Esters (FAME) for Diesel Engines-Requirements and Test Methods. European Committee for Standardization: Brussels, Belgium, 2008.
- ASTM D6751-02; Standard Specification for Biodiesel Fuel (B100) Blend Stock for Distillate Fuels. American Society for Testing and Materials: West Conshohocken, PA, USA, 2002.
- Jo, S.W.; Kang, N.S.; Lee, J.A.; Kim, E.S.; Kim, K.M.; Yoon, M.; Hong, J.W.; Yoon, H.-S. Characterization of MABIK microalgae with biotechnological potentials. J. Mar. Sci. Eng. 2020, 12, 40–49. [Google Scholar]
- Brown, M.R. The amino-acid and sugar composition of 16 species of microalgae used in mariculture. J. Exp. Mar. Biol. Ecol. 1991, 145, 79–99. [Google Scholar] [CrossRef]
- Kang, N.S.; Lee, J.A.; Jang, H.S.; Kim, K.M.; Kim, E.S.; Yoon, M.; Hong, J.W. First record of a marine microalgal species, Chlorella gloriosa (Trebouxiophyceae) isolated from the Dokdo Islands, Korea. J. Environ. Biol. 2019, 37, 526–534. [Google Scholar] [CrossRef]
- Shanmugavelan, P.; Kim, S.Y.; Kim, J.B.; Kim, H.W.; Cho, S.M.; Kim, S.N.; Kim, S.Y.; Cho, Y.S.; Kim, H.R. Evaluation of sugar content and composition in commonly consumed Korean vegetables, fruits, cereals, seed plants, and leaves by HPLC-ELSD. Carbohydr. Res. 2013, 380, 112–117. [Google Scholar] [CrossRef]
- Santhosh, S.; Dhandapani, R.; Hemalatha, N. Bioactive compounds from microalgae and its different applications: A review. Adv. Appl. Sci. Res. 2016, 7, 153–158. [Google Scholar]
- da Silva, M.R.O.B.; Moura, Y.A.S.; Converti, A.; Porto, A.L.F.; de Araújo Viana Marques, D.; Bezerra, R.P. Assessment of the potential of Dunaliella microalgae for different biotechnological applications: A systematic review. Algal Res. 2021, 58, 102396. [Google Scholar] [CrossRef]
- Massyuk, N.P. Mass culture of the carotene-bearing alga Dunaliella salina Teod. Ukr. Bot. Zh. 1966, 23, 12–19. [Google Scholar]
- Christensen, T. Two new families and some new names and combinations in the Algae. Blumea Biodivers. Evol. Biogeogr. Plants 1967, 15, 91–94. [Google Scholar]
- Assunção, P.; Jaén-Molina, R.; Caujapé-Castells, J.; Wolf, M.; Buchheim, M.A.; de la Jara, A.; Freijanes, K.; Carmona, L.; Mendoza, H. Phylogenetic analysis of ITS2 sequences suggests the taxonomic re-structuring of Dunaliella viridis (Chlorophyceae, Dunaliellales). Phycol. Res. 2013, 61, 81–88. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Huisman, J.M. The ecology of Dunaliella salina (Chlorophyceae, Volvocales): Effect of environmental conditions on aplanospore formation. Bot. Mar. 1993, 36, 233–244. [Google Scholar] [CrossRef]
- Leonardi, P.I.; Caceres, E.J. Light and electron microscope observations of the life cycle of Dunaliella salina (Polyblepharidaceae, Chlorophyceae). Nova Hedwigia 1997, 64, 621–633. [Google Scholar] [CrossRef]
- Hamburger, C. Zur Kenntnis der Dunaliella salina und einer Amöbe aus Salinenwasser von Cagliari. Arch. Protistenk. 1905, 6, 111–130. [Google Scholar]
- Liebetanz, R. Hydrobiologische Studien an Kujawischen Brackwässern. Bull. Int. Acad. Pol. Sci. Lett. Sér. B 1925, 1, 1–116. [Google Scholar]
- Loeblich, A.R. Aplanospores of Dunaliella salina (Chlorophyta). J. Protozool. 1969, 16, 22–23. [Google Scholar]
- Loeblich, A.R. Studies on the Brine Flagellate Dunaliella salina. PhD Thesis, University of California, San Diego, CA, USA, 1972. [Google Scholar]
- Margulis, L.; Barghoorn, E.S.; Ashendorf, D.; Banerjee, S.; Chase, D.; Francis, S.; Giovannoni, S.; Stolz, J. The microbial community in the layered sediments at Laguna Figueroa, Baja California, Mexico: Does it have Precambrian analogues? Precambrian Res. 1980, 11, 93–123. [Google Scholar] [CrossRef]
- Samanamud, M. Crecimiento e historia de vida de Dunaliella salina de las salinas de los Chimus, Ancash y de Chilca, Lima, Peru. In Anais do IV Congresso Latino-Americano, II Reuniao Iberio-Americana, VII Reunião Brasileira, de Ficologia; de Paula, E.J., Corediro-Marino, M., Santos, D.P., Plastino, E.M., Fujii, M.T., Yokoya, N.S., Eds.; Sociedade Ficologica de America Latina e Caribe and Sociatade Brasiliera de Ficologia: Caxambu, Brazil, 1998; Volume II, pp. 309–324. [Google Scholar]
- Achour, H.Y.; Doumandji, A.; Bouras, N.; Sabaou, N.; Assunção, P. Isolation, molecular identification and the carotenogenesis process of the microalgae Dunaliella salina strain DunaDZ1 isolated from an Algerian Salt Lake. Turk. J. Fish. Aquat. Sci. 2019, 19, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Borovkov, A.B.; Gudvilovich, I.N.; Avsiyan, A.L. Scale-up of Dunaliella salina cultivation: From strain selection to open ponds. J. Appl. Phycol. 2020, 32, 1545–1558. [Google Scholar] [CrossRef]
- Gómez-Pinchetti, J.L.; Ramazanov, Z.; Fontes, A.; García-Reina, G. Photosynthetic characteristics of Dunaliella salina (Chlorophyceae, Dunaliellales) in relation to β-carotene content. J. Appl. Phycol. 1992, 4, 11–15. [Google Scholar] [CrossRef]
- Paniagua-Michel, J.; Capa-Robles, W.; Olmos-Soto, J.; Gutierrez-Millan, L.E. The carotenogenesis pathway via the isoprenoid-β-carotene interference approach in a new strain of Dunaliella salina isolated from Baja California, Mexico. Mar. Drugs 2009, 7, 45–56. [Google Scholar] [CrossRef]
- Polle, J.E.W.; Struwe, L.; Jin, E. Identification and characterization of a new strain of the unicellular green alga Dunaliella salina (Teod.) from Korea. J. Microbiol. Biotechnol. 2008, 18, 821–827. [Google Scholar]
- Preetha, K.; John, L.; Subin, C.S.; Vijayan, K.K. Phenotypic and genetic characterization of Dunaliella (Chlorophyta) from Indian salinas and their diversity. Aquat. Biosyst. 2012, 8, 27. [Google Scholar] [CrossRef]
- Tavallaie, S.; Emtyazjoo, M.; Rostami, K.; Kosari, H.; Assadi, M.M. Comparative studies of β-carotene and protein production from Dunaliella salina isolated from Lake Hoze-Soltan, Iran. J. Aquat. Food Prod. Technol. 2015, 24, 79–90. [Google Scholar] [CrossRef]
- Reshma, R.; Chitra Devi, K.; Dinesh Kumar, S.; Santhanam, P.; Perumal, P.; Krishnaveni, N.; Begum, A.; Pragnya, M.; Arthikha, R.; Dhanalakshmi, B.; et al. Enhancement of pigments production in the green microalga Dunaliella salina (PSBDU05) under optimized culture condition. Bioresour. Technol. Rep. 2021, 14, 100672. [Google Scholar] [CrossRef]
- Mallick, N.; Mandal, S.; Singh, A.K.; Bishai, M.; Dash, A. Green microalga Chlorella vulgaris as a potential feedstock for biodiesel. J. Chem. Technol. Biotechnol. 2012, 87, 137–145. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- BenMoussa-Dahmen, I.; Chtourou, H.; Rezgui, F.; Sayadi, S.; Dhouib, A. Salinity stress increases lipid, secondary metabolites and enzyme activity in Amphora subtropica and Dunaliella sp. for biodiesel production. Bioresour. Technol. 2016, 218, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Converti, A.; Casazza, A.A.; Ortiz, E.Y.; Perego, P.; Del Borghi, M. Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem. Eng. Process. 2009, 48, 1146–1151. [Google Scholar] [CrossRef]
- Morales-Sánchez, D.; Martinez-Rodriguez, O.A.; Kyndt, J.; Martinez, A. Heterotrophic growth of microalgae: Metabolic aspects. World J. Microbiol. Biotechnol. 2015, 31, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Wu, J.H.Y. Omega-3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef]
- Randrianarison, G.; Ashraf, M.A. Microalgae: A potential plant for energy production. Geol. Ecol. Landscapes 2017, 1, 104–120. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Rimm, E.B. Fish intake, contaminants, and human health. JAMA 2006, 296, 1885–1900. [Google Scholar] [CrossRef]
- Venegas-Calerón, M.; Sayanova, O.; Napier, J.A. An alternative to fish oils: Metabolic engineering of oil-seed crops to produce omega-3 long-chain polyunsaturated fatty acids. Prog. Lipid Res. 2010, 49, 108–119. [Google Scholar] [CrossRef]
- Saifullah, A.Z.A.; Karim, M.A.; Ahmad-Yazid, A. Microalgae: An alternative source of renewable energy. Am. J. Eng. Res. 2014, 3, 330–338. [Google Scholar]
- Hawrot-Paw, M.; Ratomski, P.; Koniuszy, A.; Golimowski, W.; Teleszko, M.; Grygier, A. Fatty acid profile of microalgal oils as a criterion for selection of the best feedstock for biodiesel production. Energies 2021, 14, 7334. [Google Scholar] [CrossRef]
- Remize, M.; Brunel, Y.; Silva, J.L.; Berthon, J.Y.; Filaire, E. Microalgae n-3 PUFAs production and use in food and feed industries. Mar. Drugs 2021, 19, 113. [Google Scholar] [CrossRef]
- Deshmukh, S.; Bala, K.; Kumar, R. Selection of microalgae species based on their lipid content, fatty acid profile and apparent fuel properties for biodiesel production. Environ. Sci. Pollut. Res. 2019, 26, 24462–24473. [Google Scholar] [CrossRef] [PubMed]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Ceniceros, E.; Natarajan, M. Review of biodiesel composition, properties, and specifications. Renew. Sustain. Energy Rev. 2012, 16, 143–169. [Google Scholar] [CrossRef]
- Misra, R.D.; Murthy, M.S. Blending of additives with biodiesels to improve the cold flow properties, combustion and emission performance in a compression ignition engine—A review. Renew. Sustain. Energy Rev. 2011, 15, 2413–2422. [Google Scholar] [CrossRef]
- Palani, Y.; Devarajan, C.; Manickam, D.; Thanikodi, S. Performance and emission characteristics of biodiesel-blend in diesel engine: A review. Environ. Eng. Res. 2022, 27, 200338. [Google Scholar] [CrossRef]
- Ahmed, R.A.; He, M.; Aftab, R.A.; Zheng, S.; Nagi, M.; Bakri, R.; Wang, C. Bioenergy application of Dunaliella salina SA 134 grown at various salinity levels for lipid production. Sci. Rep. 2017, 7, 8118. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Borowitzka, L.J.; Kessly, D. Effects of salinity increase on carotenoid accumulation in the green alga Dunaliella salina. J. Appl. Phycol. 1990, 2, 111–119. [Google Scholar] [CrossRef]
- Ren, Y.; Sun, H.; Deng, J.; Huang, J.; Chen, F. Carotenoid production from microalgae: Biosynthesis, salinity responses and novel biotechnologies. Mar. Drugs 2021, 19, 713. [Google Scholar] [CrossRef]
- Wu, M.; Zhu, R.; Lu, J.; Lei, A.; Zhu, H.; Hu, Z.; Wang, J. Effects of different abiotic stresses on carotenoid and fatty acid metabolism in the green microalga Dunaliella salina Y6. Ann. Microbiol. 2020, 70, 48. [Google Scholar] [CrossRef]
- Chekanov, K. Diversity and distribution of carotenogenic algae in Europe: A review. Mar. Drugs 2023, 21, 108. [Google Scholar] [CrossRef]
- Chen, B.H.; Chuang, J.R.; Lin, J.H.; Chiu, C.P. Quantification of pro-vitamin A compounds in Chinese vegetables by high-performance liquid chromatography. J. Food Prot. 1993, 56, 51–54. [Google Scholar] [CrossRef]
- Leach, G.; Oliveira, G.; Morais, R. Spray-drying of Dunaliella salina to produce a β-carotene-rich powder. J. Ind. Microbiol. Biotechnol. 1998, 20, 82–85. [Google Scholar] [CrossRef]
- Vega, P.J.; Balaban, M.O.; Sims, C.A.; O’Keefe, S.F.; Cornell, J.A. Supercritical carbon dioxide extraction efficiency for carotenes from carrots by RSM. J. Food Sci. 1996, 61, 757–759. [Google Scholar] [CrossRef]
- Favati, F.; King, J.W.; Friedrich, J.P.; Esking, K. Supercritical CO2 extraction of carotene and lutein from leaf protein concentrates. J. Food Sci. 1988, 53, 1532–1536. [Google Scholar] [CrossRef]
- Keat, O.C.; May, C.Y.; Hock, A.O.S. Recovery of Carotenoids. U.S. Patent 5,019,668, 28 May 1991. [Google Scholar]
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef]
- Ben-Amotz, A. Production of β-carotene and vitamins by the halotolerant alga Dunaliella. In Pharmaceutical and Bioactive Natural Products; Springer: Boston, MA, USA, 1993; pp. 411–417. [Google Scholar]
- Banskota, A.H.; Sperker, S.; Stefanova, R.; McGinn, P.J.; O’Leary, S.J.B. Antioxidant properties and lipid composition of selected microalgae. J. Appl. Phycol. 2019, 31, 309–318. [Google Scholar] [CrossRef]
- Jambo, S.A.; Abdulla, R.; Mohd Azhar, S.H.; Marbawi, H.; Gansau, J.A.; Ravindra, P. A review on third generation bioethanol feedstock. Renew. Sustain. Energy Rev. 2016, 65, 756–769. [Google Scholar] [CrossRef]
- Debnath, C.; Bandyopadhyay, T.K.; Bhunia, B.; Mishra, U.; Narayanasamy, S.; Muthuraj, M. Microalgae: Sustainable resource of carbohydrates in third-generation biofuel production. Renew. Sustain. Energy Rev. 2021, 150, 111464. [Google Scholar] [CrossRef]
- Jain, S.; Kumar, S. A comprehensive review of bioethanol production from diverse feedstocks: Current advancements and economic perspectives. Energy 2024, 296, 131130. [Google Scholar] [CrossRef]
- Ho, S.H.; Huang, S.W.; Chen, C.Y.; Hasunuma, T.; Kondo, A.; Chang, J.S. Bioethanol production using carbohydrate-rich microalgae biomass as feedstock. Bioresour. Technol. 2013, 135, 191–198. [Google Scholar] [CrossRef]
- Tse, T.J.; Wiens, D.J.; Reaney, M.J.T. Production of bioethanol—A review of factors affecting ethanol yield. Fermentation 2021, 7, 268. [Google Scholar] [CrossRef]
- Huang, B.; Qu, G.; He, Y.; Zhang, J.; Fan, J.; Tang, T. Study on high-CO2 tolerant Dunaliella salina and its mechanism via transcriptomic analysis. Front. Bioeng. Biotechnol. 2022, 10, 1086357. [Google Scholar] [CrossRef] [PubMed]
- Fiume, M.M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety assessment of monosaccharides, disaccharides, and related ingredients as used in cosmetics. Int. J. Toxicol. 2019, 38, 5S–38S. [Google Scholar] [CrossRef] [PubMed]
- Okolie, O.; Kumar, A.; Edwards, C.; Lawton, L.A.; Oke, A.; McDonald, S.; Thakur, V.K.; Njuguna, J. Bio-based sustainable polymers and materials: From processing to biodegradation. J. Compos. Sci. 2023, 7, 213. [Google Scholar] [CrossRef]
- Araujo, R.; Peteiro, C. Algae as Food and Food Supplements in Europe; EUR 30779 EN, Publications Office of the European Union: Luxembourg, 2021; ISBN 978-92-76-40548-1. [Google Scholar]
- U.S. Food and Drug Administration. GRAS Notices: Dunaliella. Available online: https://www.hfpappexternal.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&sort=GRN_No&order=DESC&startrow=1&type=basic&search=dunaliella (accessed on 7 October 2024).
- Torres-Tiji, Y.; Fields, F.J.; Mayfield, S.P. Microalgae as a future food source. Biotechnol. Adv. 2020, 41, 107536. [Google Scholar] [CrossRef]
- Almutairi, A.W. Effects of nitrogen and phosphorus limitations on fatty acid methyl esters and fuel properties of Dunaliella salina. Environ. Sci. Pollut. Res. Int. 2020, 27, 32296–32303. [Google Scholar] [CrossRef]
- Mendoza Guzmán, H.; de la Jara Valido, A.; Freijanes Presmanes, K.; Carmona Duarte, L. Quick estimation of intraspecific variation of fatty acid composition in Dunaliella salina using flow cytometry and Nile Red. J. Appl. Phycol. 2012, 24, 1237–1243. [Google Scholar] [CrossRef]
- Talebi, A.F.; Tohidfar, M.; Bagheri, A.; Lyon, S.R.; Salehi-Ashtiani, K.; Tabatabaei, M. Manipulation of carbon flux into fatty acid biosynthesis pathway in Dunaliella salina using AccD and ME genes to enhance lipid content and to improve produced biodiesel quality. Biofuel Res. J. 2014, 1, 91–97. [Google Scholar] [CrossRef]
- Jo, S.W.; Do, J.M.; Kang, N.S.; Park, J.M.; Lee, J.H.; Kim, H.S.; Hong, J.W.; Yoon, H.S. Isolation, identification, and biochemical characteristics of a cold-tolerant Chlorella vulgaris KNUA007 isolated from King George Island, Antarctica. J. Mar. Sci. Eng. 2020, 8, 935. [Google Scholar] [CrossRef]
- Mohmmed, S. Screening of four green microalgae potentially used as feedstock for biodiesel and nutraceuticals. J. Appl. Phycol. 2022, 34, 1565–1581. [Google Scholar]
- Nascimento, I.A.; Marques, S.S.I.; Cabanelas, I.T.D.; Pereira, S.A.; Druzian, J.I.; De Souza, C.O.; De Vich, D.V.; De Carvalho, G.C.; De Nascimento, M.A. Screening microalgae strains for biodiesel production: Lipid productivity and estimation of fuel quality based on fatty acids profiles as selective criteria. Bioenergy Res. 2013, 6, 1–13. [Google Scholar] [CrossRef]
- Reitan, K.I.; Øie, G.; Jørgensen, H.; Wang, X. Chemical composition of selected marine microalgae, with emphasis on lipid and carbohydrate production for potential use as feed resources. J. Appl. Phycol. 2021, 33, 3831–3842. [Google Scholar] [CrossRef]
- Vazhappilly, R.; Chen, F. Eicosapentaenoic acid and docosahexaenoic acid production potential of microalgae and their heterotrophic growth. J. Amer. Oil Chem. Soc. 1998, 75, 393–397. [Google Scholar] [CrossRef]
- Afi, L.; Metzger, P.; Largeau, C.; Connan, J.; Berkaloff, C.; Rousseau, B. Bacterial degradation of green microalgae: Incubation of Chlorella emersonii and Chlorella vulgaris with Pseudomonas oleovorans and Flavobacterium aquatile. Org. Geochem. 1996, 25, 117–130. [Google Scholar] [CrossRef]
- Kabir, F.; Gulfraz, M.; Raja, G.K.; Inam-ul-Haq, M.; Awais, M.; Mustafa, M.S.; Khan, S.U.; Tlili, I.; Shadloo, M.S. Screening of native hyper-lipid producing microalgae strains for biomass and lipid production Screening of native hyper-lipid producing microalgae strains for biomass and lipid production. Renew. Energy 2020, 160, 1295–1307. [Google Scholar] [CrossRef]
- Damiani, M.C.; Popovich, C.A.; Constenla, D.; Leonardi, P.I. Lipid analysis in Haematococcus pluvialis to assess its potential use as a biodiesel feedstock. Bioresour. Technol. 2010, 101, 3801–3807. [Google Scholar] [CrossRef]
- Vello, V.; Phang, S.M.; Poong, S.W.; Lim, Y.K.; Ng, F.L.; Shanmugam, J.; Gopal, M. New report of Halamphora subtropica (Bacillariophyta) from the Strait of Malacca and its growth and biochemical characterisation under nutrient deprivation. Reg. Stud. Mar. Sci. 2023, 62, 102947. [Google Scholar] [CrossRef]
- Kim, K.M.; Kang, N.S.; Jang, H.S.; Park, J.S.; Jeon, B.H.; Hong, J.W. Characterization of Heterochlorella luteoviridis (Trebouxiaceae, Trebouxiophyceae) isolated from the Port of Jeongja in Ulsan, Korea. J. Mar. Biosci. Biotechnol. 2017, 9, 22–29. [Google Scholar] [CrossRef]
- Daroch, M.; Shao, C.; Liu, Y.; Geng, S.; Cheng, J.J. Induction of lipids and resultant FAME profiles of microalgae from coastal waters of Pearl River Delta. Bioresour. Technol. 2013, 146, 192–199. [Google Scholar] [CrossRef]
- Lin, Y.; Ge, J.; Zhang, Y.; Ling, H.; Yan, X.; Ping, W. Monoraphidium sp. HDMA-20 is a new potential source of α-linolenic acid and eicosatetraenoic acid. Lipids Health Dis. 2019, 18, 56. [Google Scholar] [CrossRef]
- Kezlya, E.; Maltsev, Y.; Genkal, S.; Krivova, Z.; Kulikovskiy, M. Phylogeny and fatty acid profiles of new Pinnularia (Bacillariophyta) species from soils of Vietnam. Cells 2022, 11, 2446. [Google Scholar] [CrossRef]
- Kumar, N.; Banerjee, C.; Jagadevan, S. Identification, characterization, and lipid profiling of microalgae Scenedesmus sp. NC1, isolated from coal mine effluent with potential for biofuel production. Biotechnol. Rep. 2021, 30, e00621. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Makkar, H.P.S. Jatropha curcas: A potential source for tomorrow’s oil and biodiesel. Lipid Technol. 2008, 20, 104–107. [Google Scholar] [CrossRef]
- Goembira, F.; Saka, S. Advanced supercritical methyl acetate method for biodiesel production from Pongamia pinnata oil. Renew. Energy 2015, 83, 1245–1249. [Google Scholar] [CrossRef]
- Saravanan, N.; Nagarajan, G.; Puhan, S. Experimental investigation on a DI diesel engine fuelled with Madhuca indica ester and diesel blend. Biomass Bioenergy 2010, 34, 838–843. [Google Scholar] [CrossRef]
- Crabbe, E.; Nolasco-Hipolito, C.; Kobayashi, G.; Sonomoto, K.; Ishizaki, A. Biodiesel production from crude palm oil and evaluation of butanol extraction and fuel properties. Process Biochem. 2001, 37, 65–71. [Google Scholar] [CrossRef]
- An, S.M.; Cho, K.; Kim, E.S.; Ki, H.; Choi, G.; Kang, N.S. Description and characterization of the Odontella aurita OAOSH22, a marine diatom rich in eicosapentaenoic acid and fucoxanthin, isolated from Osan Harbor, Korea. Mar. Drugs 2023, 21, 563. [Google Scholar] [CrossRef]
- Ramadhas, A.S.; Muraleedharan, C.; Jayaraj, S. Performance and emission evaluation of a diesel engine fueled with methyl esters of rubber seed oil. Renew. Energy 2005, 30, 1789–1800. [Google Scholar] [CrossRef]
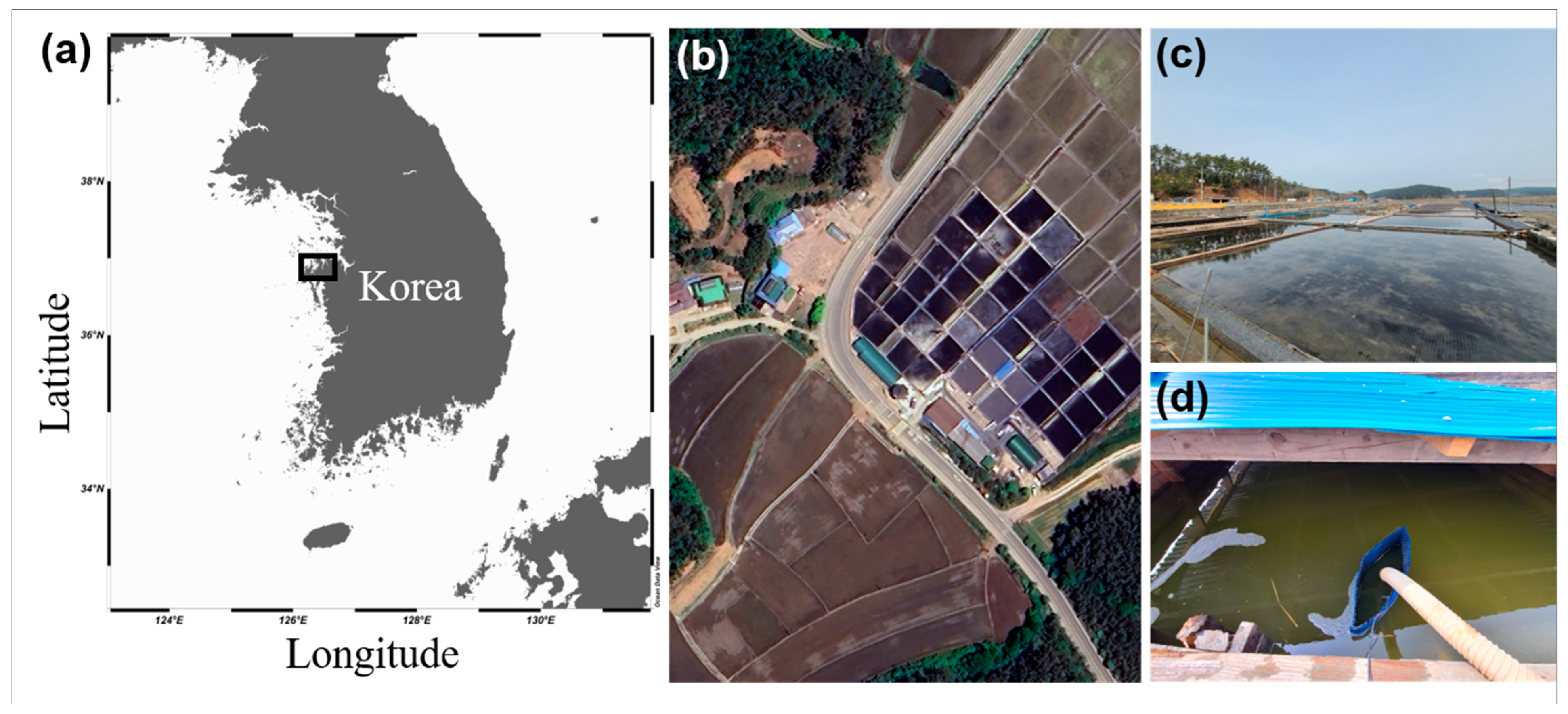

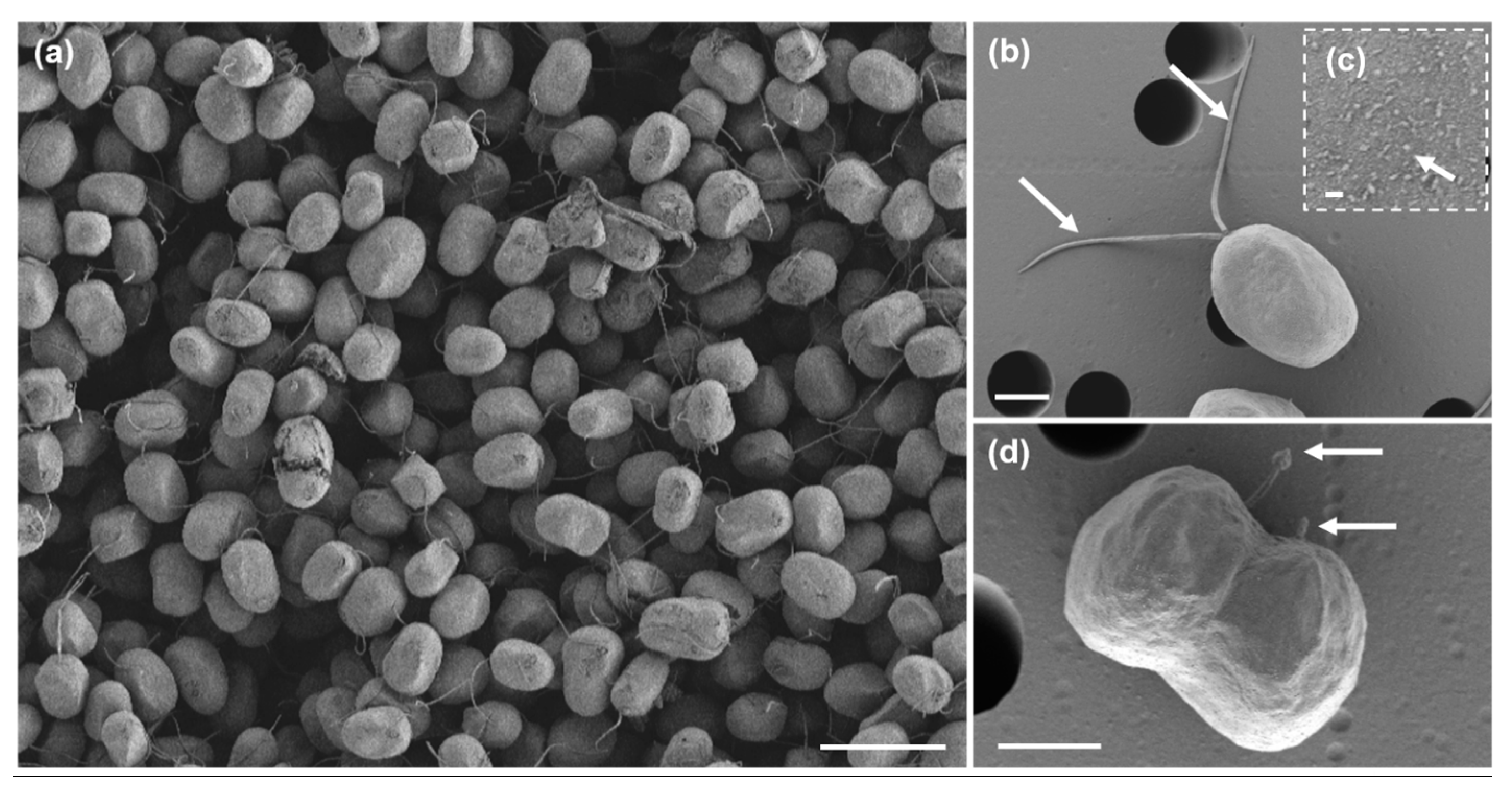

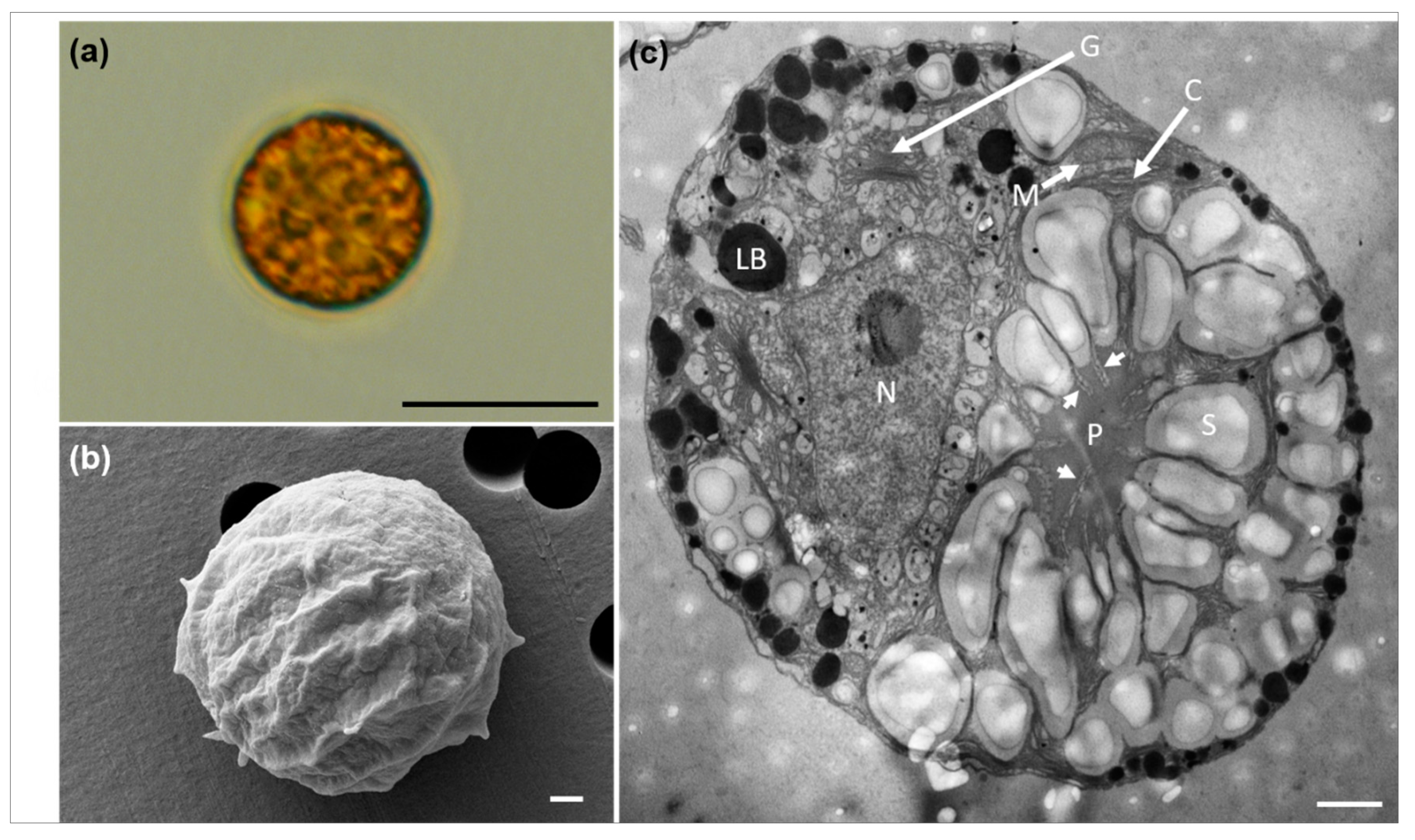
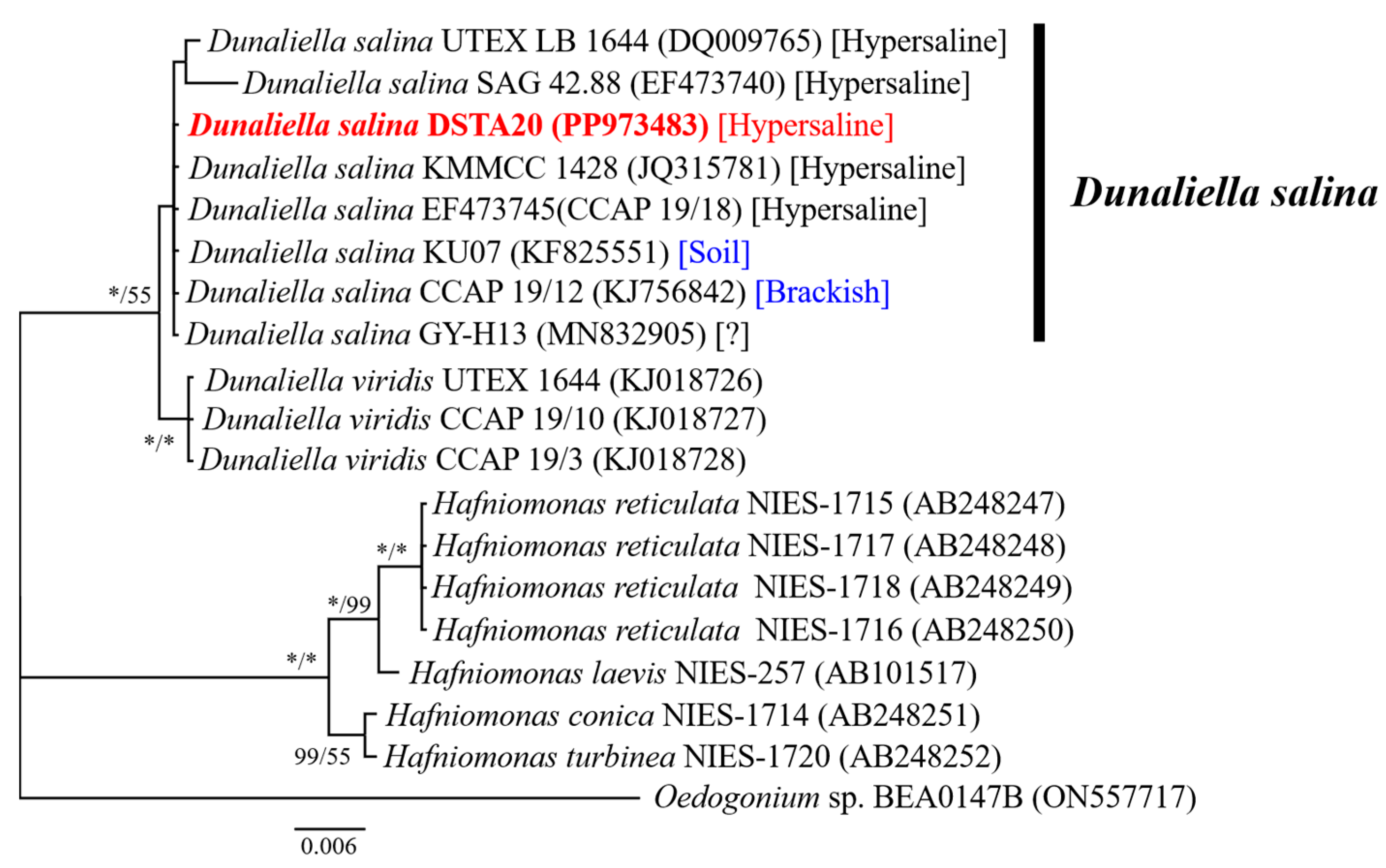
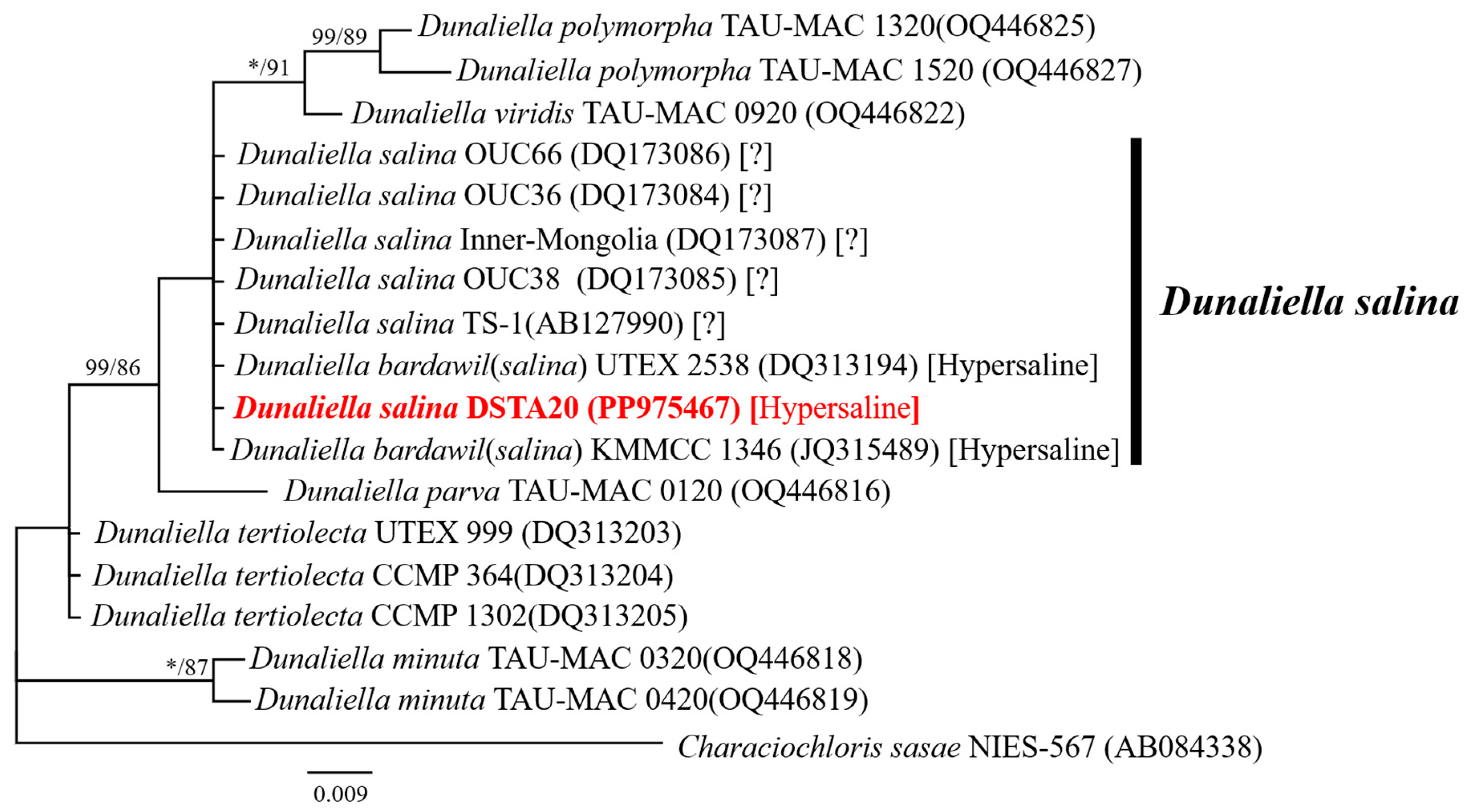
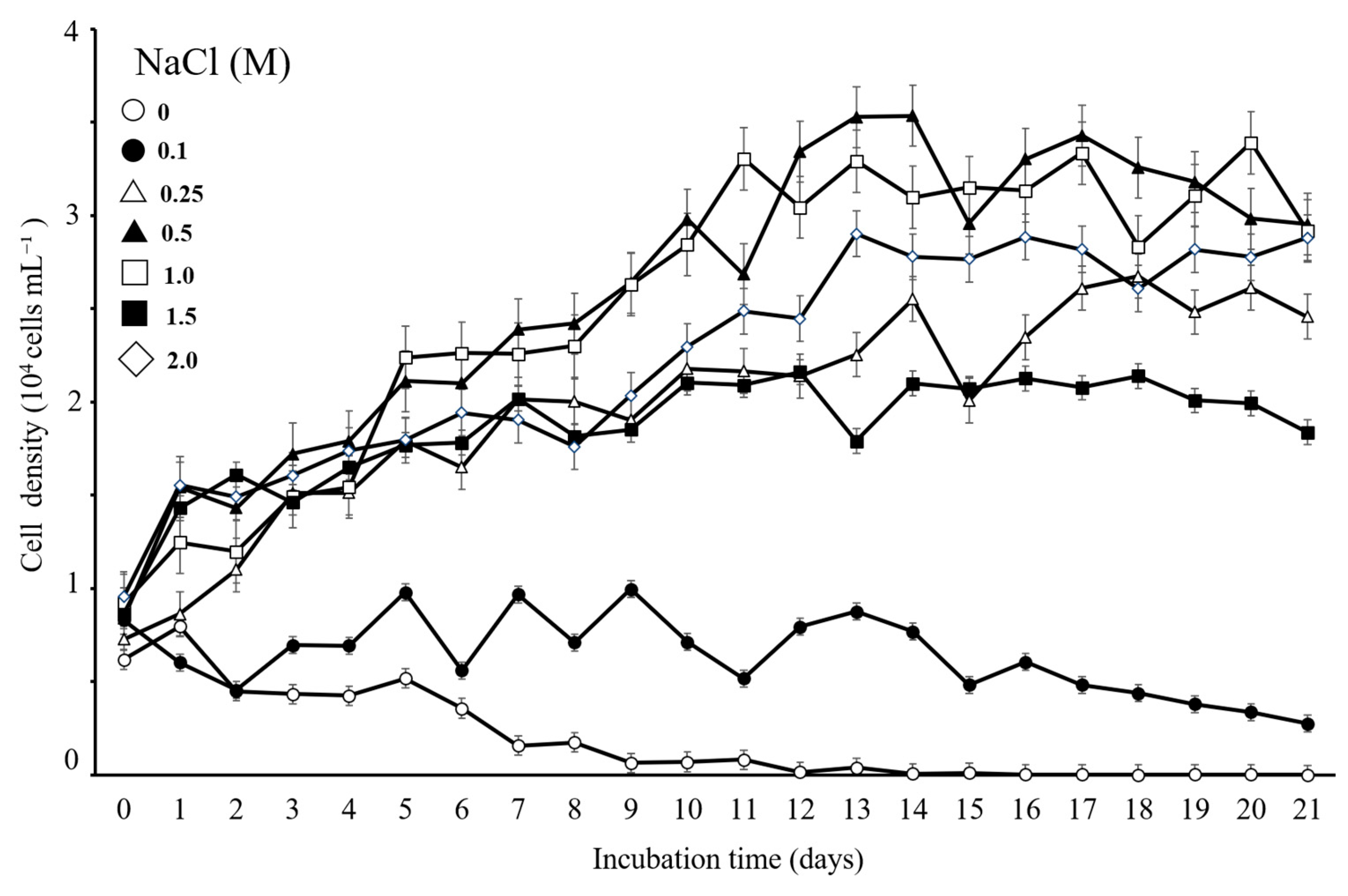
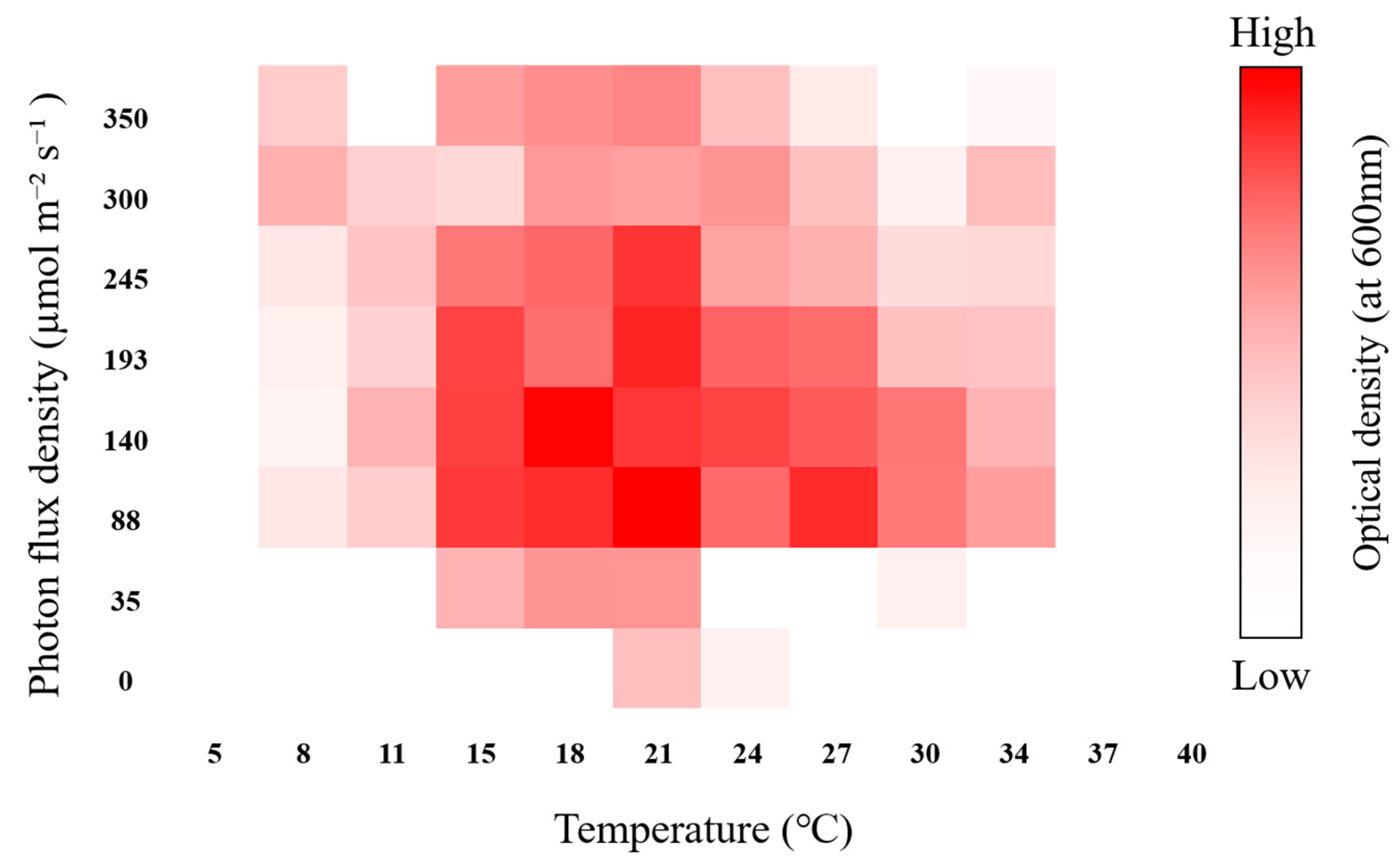


| Species | Strain | LC | Date | T (°C) | S (PSU) | Marker Gene | Amplicon Length (bp) | GBAN |
|---|---|---|---|---|---|---|---|---|
| D. salina | DSTA20 | Mandae Solhyanggi-gil Salt pond | July 2020 | 27.2 | >100 | SSU | 1460 | PP973483 |
| ITS | 602 | PP974559 | ||||||
| LSU | 746 | PP973494 | ||||||
| rbcL | 345 | PP975467 |
| Primer Name | Primer Region | Sequence (5′-3′) | References |
|---|---|---|---|
| EukA | Forward, SSU | AACCTGGTTGATCCTGCCAG | [24] |
| G18R | Reverse, SSU | GCATCACAGACCTGTTATTG | [25] |
| 570F | Forward, SSU | GTAATTCCAGCTCCAATAGC | [26] |
| EukB | Reverse, SSU | TGATCCTTCTGCAGGTTCACCTAC | [24] |
| ITSF2 | Forward, ITS | ACCCGCTGAATTTAAGCATA | [25] |
| ITSFR2 | Reverse, ITS | ACGAACGATTTGCACGTCAG | [25] |
| D1R | Forward, LSU | ACCCGCTGAATTTAAGCATA | [27] |
| LSUB | Reverse, LSU | ACGAACGATTTGCACGTCAG | [25] |
| rbcL-192 | Forward, rbcL | GGTACTTGGACAACWGTWTGGAC | [28] |
| rbcL-657 | Reverse, rbcL | GAAACGGTCTCKCCARCGCAT | [28] |
| Traits | Dunaliella salina Strains | |||
|---|---|---|---|---|
| Cell shape | Spherical or oval | Elongated ellipsoidal or cylindrical | Spherical or oval | Oval |
| Cell length (μm) | 9.3–14 (11.3) | 16–24, up to 28 for aged zoospores | 5–29 (10.9–16.9) | 10.2–15.4 (14.1) |
| Cell width (μm) | 7.8–10 (8.1) | ND | 3.8–20.3 (7.9–13.2) | 9.8–15 (11.1) |
| Flagella | Two flagella, approximately equal to or longer than cell length in some cells | Two flagella, longer than the total body length | Two flagella, approximately equal to cell length | Two flagella |
| Chloroplast | Cup-shaped | Bell-shaped | Cup-shaped | Cup-shaped |
| Pyrenoid | Present, surrounded by the starch grains | Present, surrounded by the starch grains | Present, surrounded by the starch grains | Present, surrounded by the starch grains |
| Eyespots | Present | Present | Present | Present |
| Aplanospores | Aplanospores present, spherical, 12–19 (14.6) μm in diameter, with a thick, rugose wall | ND | Aplanospores present, spherical, 12–20 μm in diameter, with a thick, rugose wall | ND |
| Reference | This study | [35] | [10] | [36,37] |
| Marker Gene | Collection Location | Strain Habitat (Isolation Source) | Strain Name | GenBank Accession No. | Dunaliella salina DSTA20 * |
|---|---|---|---|---|---|
| SSU | Australia | Hypersaline | CCAP 19/18 | EF473745 | 0 (0) |
| Israel | Hypersaline | SAG 42.88 | EF473740 | 0 (0) | |
| Israel | Brackish | CCAP 19/12 | KJ756842 | 0 (0) | |
| Republic of Korea | Hypersaline | KMMCC 1428 | JQ315781 | 0 (0) | |
| ND | ND | GY-H13 | MN832905 | 0 (0) | |
| Mexico | Hypersaline | UTEX LB 1644 | DQ009765 | 2 (0.1) | |
| Thailand | soil | KU07 | KF825551 | 7 (0.5) | |
| rbcL | China | ND | OUC66 | DQ173086 | 0 (0) |
| China | ND | OUC36 | DQ173084 | 0 (0) | |
| Inner Mongolia | ND | Inner Mongolia | DQ173087 | 0 (0) | |
| China | ND | OUC38 | DQ173085 | 0 (0) | |
| ND | ND | TS-1 | AB127990 | 0 (0) | |
| USA | ND | UTEX2538 | DQ313194 | 0 (0) | |
| Republic of Korea | ND | KMMCC1346 | JQ315489 | 0 (0) |
| Component | Content (%) | Note |
|---|---|---|
| Myristic acid (C14:0) | 0.49 | |
| Palmitic acid (C16:0) | 21.06 | SFA (major) |
| Palmitoleic acid (C16:1 ω-7) | 1.63 | |
| Hexadecadienoic acid (C16:2 ω-6) | 1.08 | |
| Hexadecatrienoic acid (C16:3 ω-3) | 2.75 | |
| Hexadecatetraenoic acid (C16:4 ω-3) | 13.23 | ω-3 PUFA (major) |
| Stearic acid (C18:0) | 0.53 | |
| Oleic acid (C18:1 ω-9) | 2.77 | |
| Linoleic acid (C18:2 ω-6) | 6.81 | ω-6 PUFA (major) |
| γ-linolenic Acid (C18:3 ω-6) | 3.84 | |
| α-linolenic acid (C18:3 ω-3) | 31.55 | ω-3 PUFA (major) |
| Total saturated fatty acids | 22.08 | |
| Total monounsaturated fatty acids | 4.40 | |
| Total polyunsaturated fatty acids | 59.26 |
| Source | SV (mg KOH/g) | IV (g I2/100 g) | DU | MUFA (%) | PUFA (%) | LCSF | CFPP (°C) | CN | OS (h) |
|---|---|---|---|---|---|---|---|---|---|
| Jatropha | 190.98 | 105.42 | 122.1 | 37.3 | 42.4 | 4.54 | −2.21 | 51.16 | 5.37 |
| Karanja | 184.05 | 94.22 | 105.2 | 65.6 | 19.8 | 2.64 | −8.18 | 54.76 | 8.55 |
| Mahua | 191.58 | 67.72 | 78.62 | 39.1 | 19.76 | 11.65 | 20.12 | 59.55 | 8.56 |
| Palm | 194.82 | 48.05 | 55.7 | 37.04 | 9.33 | 6.91 | 5.22 | 63.5 | 15.23 |
| Rapeseed | 188.61 | 115.07 | 125.46 | 64.4 | 30.53 | 0.77 | −14.05 | 49.35 | 6.45 |
| Dunaliella salina DSTA20 | 171.39 | 169.05 | 122.92 | 4.4 | 59.26 | 2.37 | −9.03 | 35.04 | 8.7 |
| Dunaliella salina LIMS-PS-1511 | 121.29 | 95.41 | 78.3 | 3.7 | 37.3 | 2.73 | −7.9 | 69.83 | 5.75 |
| Asterarcys quadricellulare AQYS21 | 205.11 | 171.65 | 134.04 | 13.3 | 60.37 | 2.99 | −7.08 | 34.29 | 4.54 |
| Chlamydomonas hedleyi MM0020 | 95.6 | 62.09 | 55.0 | 2.6 | 26.2 | 2.43 | −8.84 | 89.42 | 7.09 |
| Chlorella salina MM0063 | 132.19 | 100.26 | 85.0 | 2.6 | 41.2 | 2.75 | −7.84 | 65.03 | 5.45 |
| Coelastrum microporum IBL-C119 | 181.83 | 82.61 | 84.64 | 45.24 | 19.7 | 4.02 | −3.84 | 57.73 | 8.58 |
| Graesiella emersonii GEGS21 | 204.86 | 131.06 | 121.3 | 22.5 | 49.4 | 3.05 | −6.89 | 43.45 | 4.98 |
| Haematococcus lacustris | 162.7 | 98.86 | 95.81 | 20.13 | 37.84 | 3.82 | −4.46 | 57.6 | 5.75 |
| Microglena monadina NFW3 | 188.54 | 166.15 | 138.24 | 3.28 | 67.48 | 2.71 | −7.97 | 37.86 | 4.34 |
| Mychonastes homosphaera UTEX 2341 | 142.74 | 162.79 | 98.9 | 23.9 | 37.5 | 1.45 | −11.92 | 47.91 | 23.28 |
| Jaagichlorella luteoviridis MM0014 | 157.6 | 109.69 | 110.7 | 7.1 | 51.8 | 2.77 | −7.77 | 56.25 | 4.87 |
| Tetradesmus obliquus MM0026 | 138.92 | 98.63 | 85.4 | 18.4 | 33.5 | 2.45 | −8.78 | 63.4 | 6.11 |
| EN14214 | - | ≤120 | - | - | - | - | ≤−20~5 | ≥51 | ≥6 |
| ASTM D6751-02 | - | - | - | - | - | - | - | ≥47 | ≥3 |
| Carotenoids | Salinity (M) | Retention Time (min) | Peak Area (Arbitrary Units) | Amount (mg g−1) |
|---|---|---|---|---|
| β-carotene | 0.1 | 17.038 | 110.3 | 0.98 |
| 0.25 | 17.048 | 205.1 | 2.47 | |
| 0.5 | 17.083 | 410.8 | 1.27 | |
| 1 | 17.082 | 408.1 | 1.26 | |
| 1.5 | 17.108 | 16 | 0.12 | |
| 2 | 17.146 | 159.8 | 0.54 | |
| Lutein | 0.1 | 7.576 | 51.4 | 0.73 |
| 0.25 | 7.58 | 118.5 | 1.39 | |
| 0.5 | 7.596 | 306.9 | 0.61 | |
| 1 | 7.595 | 661.6 | 1.06 | |
| 1.5 | 7.603 | 31.7 | 0.27 | |
| 2 | 7.631 | 185.9 | 0.46 | |
| Zeaxanthin | 0.1 | - | - | - |
| 0.25 | 8.435 | 14 | 0.69 | |
| 0.5 | 8.45 | 52 | 0.65 | |
| 1 | 8.447 | 30.8 | 0.39 | |
| 1.5 | - | - | - | |
| 2 | 8.486 | 30.8 | 0.39 |
| Species | Strain | Monosaccharides (mg g−1 DW) | References | |||
|---|---|---|---|---|---|---|
| Arabinose | Fructose | Galactose | Glucose | |||
| Dunaliella salina | DSTA20 | - | 13.2 | 15.7 | 195.5 | This study |
| D. salina | LIMS-PS-1511 | - | - | 26.9 | 107.4 | [40] |
| D. tertiolecta | CS-175 | 0.65 | - | 1.1 | 85.3 | [41] |
| Chlorella salina | MM0063 | 27.8 | 19.0 | 75.1 | 124.1 | [42] |
| Picochlorum atomus | CS-183 | 0.16 | - | 10.6 | 55.2 | [41] |
| Terrestrial plants | ||||||
| Sweet potato | - | - | 25.3 | - | 22.8 | [43] |
| Cabbage | - | - | 261.8 | - | 258.5 | [43] |
| Grape | - | - | 126.4 | - | 108.2 | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, C.R.; Cho, K.; An, S.M.; Do, J.-M.; Hong, J.W.; Kim, J.H.; Kim, S.Y.; Jeong, H.G.; Kang, N.S. Taxonomical, Physiological, and Biochemical Characteristics of Dunaliella salina DSTA20 from Hypersaline Environments of Taean Salt Pond, Republic of Korea. Microorganisms 2024, 12, 2467. https://doi.org/10.3390/microorganisms12122467
Jo CR, Cho K, An SM, Do J-M, Hong JW, Kim JH, Kim SY, Jeong HG, Kang NS. Taxonomical, Physiological, and Biochemical Characteristics of Dunaliella salina DSTA20 from Hypersaline Environments of Taean Salt Pond, Republic of Korea. Microorganisms. 2024; 12(12):2467. https://doi.org/10.3390/microorganisms12122467
Chicago/Turabian StyleJo, Chang Rak, Kichul Cho, Sung Min An, Jeong-Mi Do, Ji Won Hong, Ju Hyoung Kim, Sun Young Kim, Hyeon Gyeong Jeong, and Nam Seon Kang. 2024. "Taxonomical, Physiological, and Biochemical Characteristics of Dunaliella salina DSTA20 from Hypersaline Environments of Taean Salt Pond, Republic of Korea" Microorganisms 12, no. 12: 2467. https://doi.org/10.3390/microorganisms12122467
APA StyleJo, C. R., Cho, K., An, S. M., Do, J.-M., Hong, J. W., Kim, J. H., Kim, S. Y., Jeong, H. G., & Kang, N. S. (2024). Taxonomical, Physiological, and Biochemical Characteristics of Dunaliella salina DSTA20 from Hypersaline Environments of Taean Salt Pond, Republic of Korea. Microorganisms, 12(12), 2467. https://doi.org/10.3390/microorganisms12122467








