Metabolic Responses, Cell Recoverability, and Protein Signatures of Three Extremophiles: Sustained Life During Long-Term Subzero Incubations
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures and Incubations
2.1.1. Cell Cultures
2.1.2. Harvest
2.1.3. Resuspensions
2.1.4. Incubations
2.2. Cell Abundance and Recoverability
2.3. Metabolic Activity
2.3.1. [3H]-Leucine Incorporation
2.3.2. Growth Rates
2.4. Stable Isotope Probing (SIP) and Proteomics
2.4.1. Sample Preparation
2.4.2. Mass Spectrometry
2.4.3. MS Data Analysis
3. Results
3.1. Temperature-Dependent Response in Cell Abundance, Recoverability and Activity
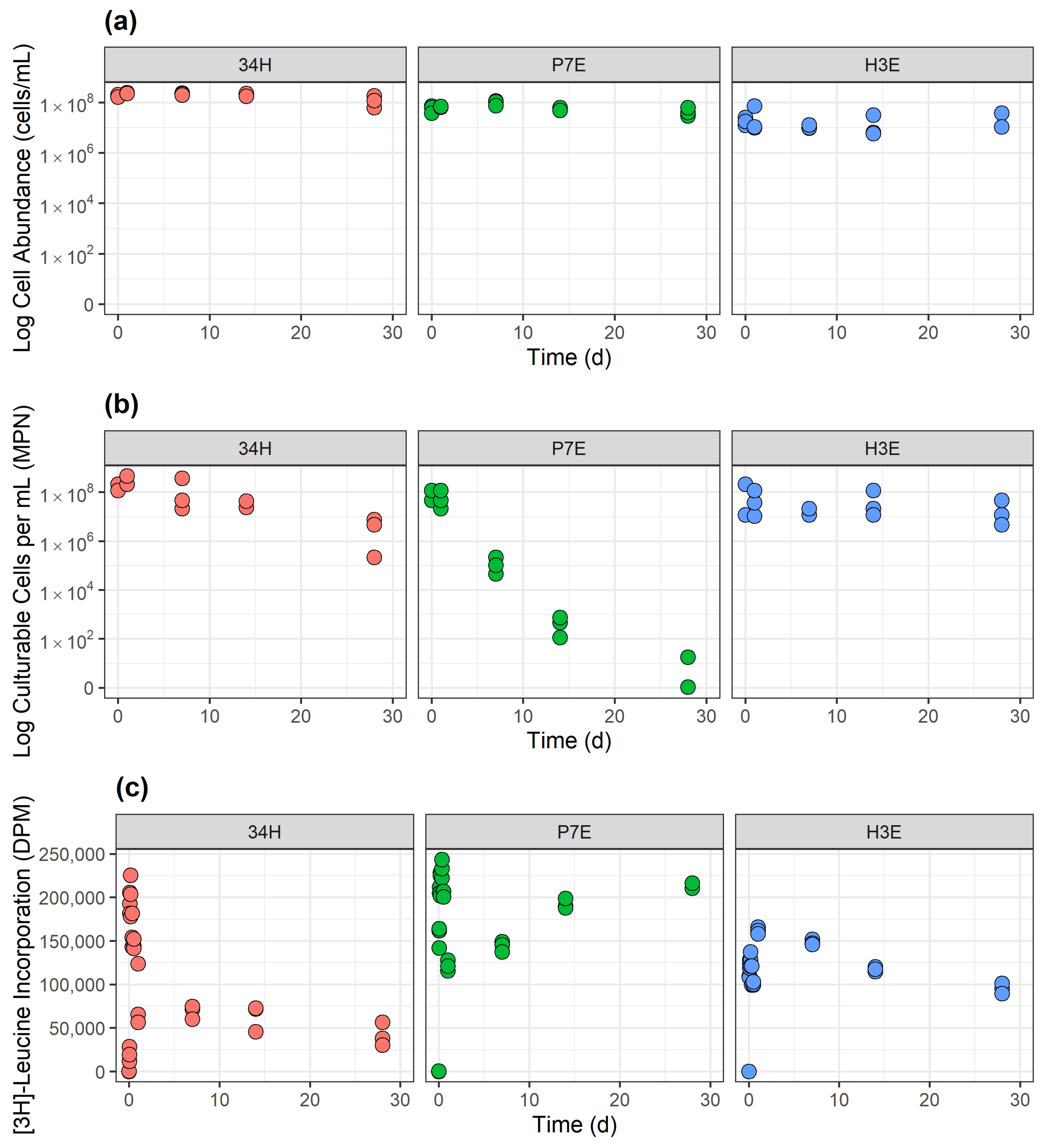
3.1.1. Control Temperature
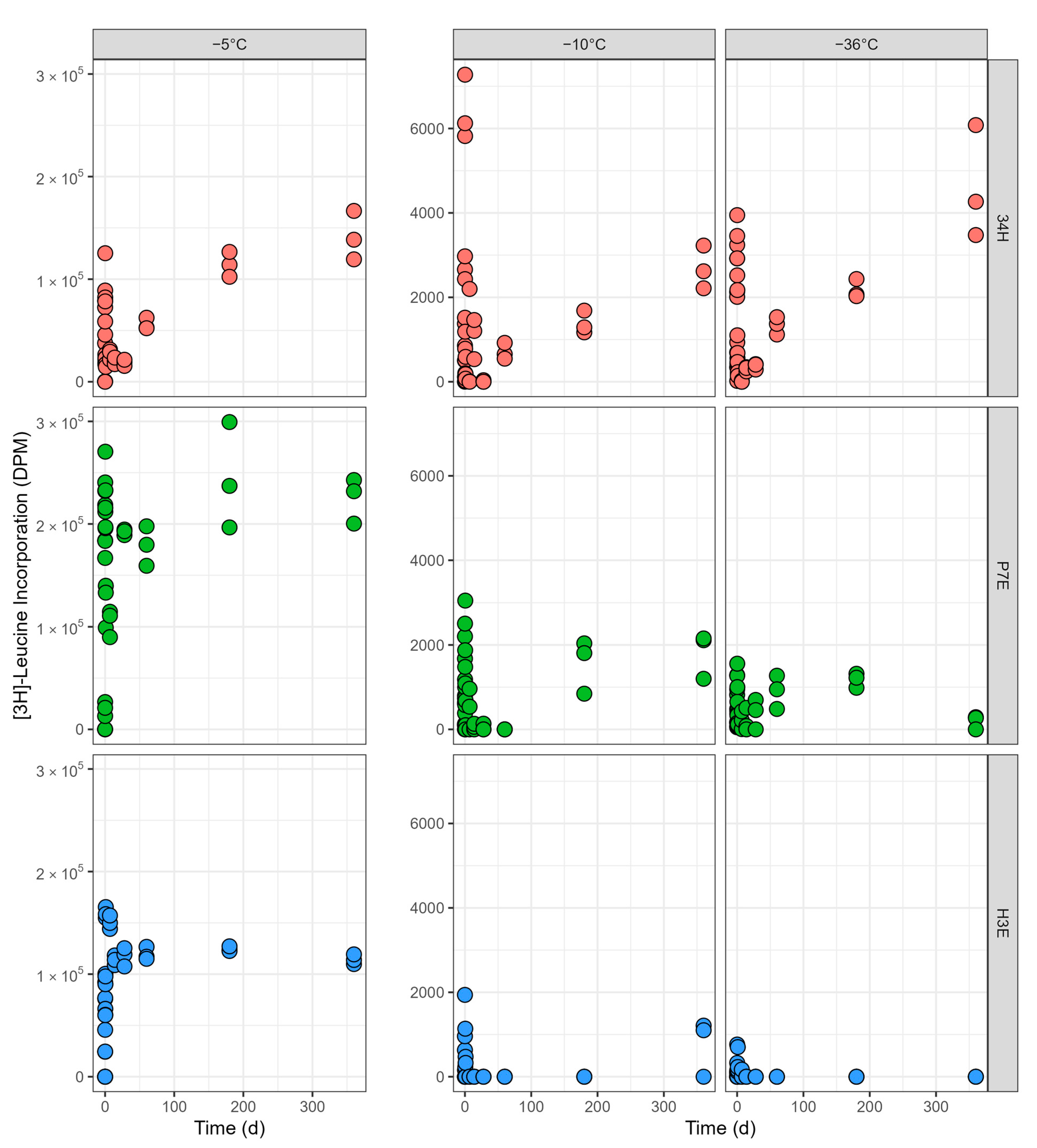
3.1.2. Incubations at −5 °C
3.1.3. Incubations at −10 °C
3.1.4. Incubations at −36 °C
3.2. Stable Isotope 13C6-Leucine Incorporation at −5 °C in Cp34H
4. Discussion
4.1. Strain-Specific Biosignatures
4.2. The Frozen Environment
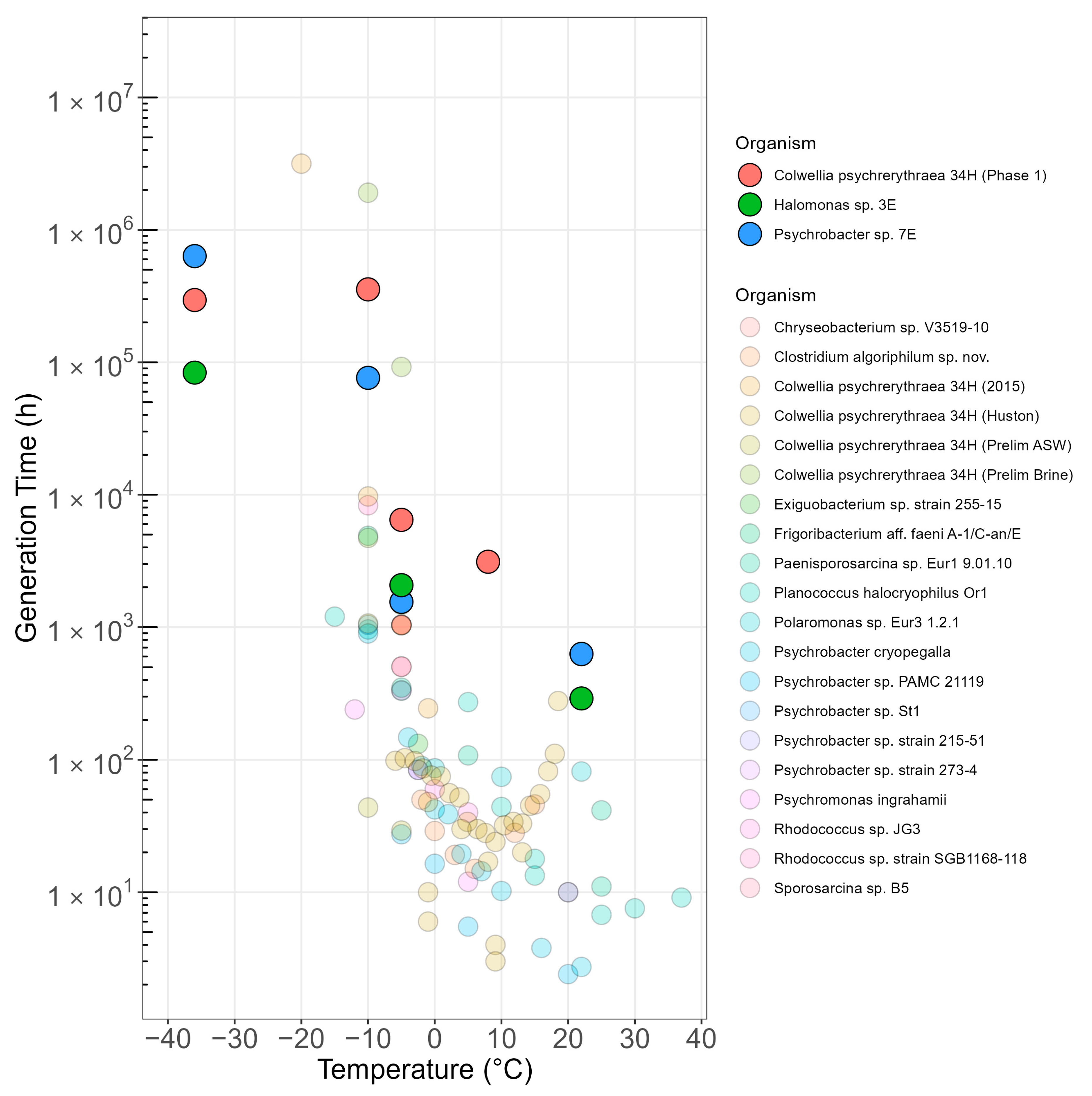
4.3. Metabolic Activity and Generation Times
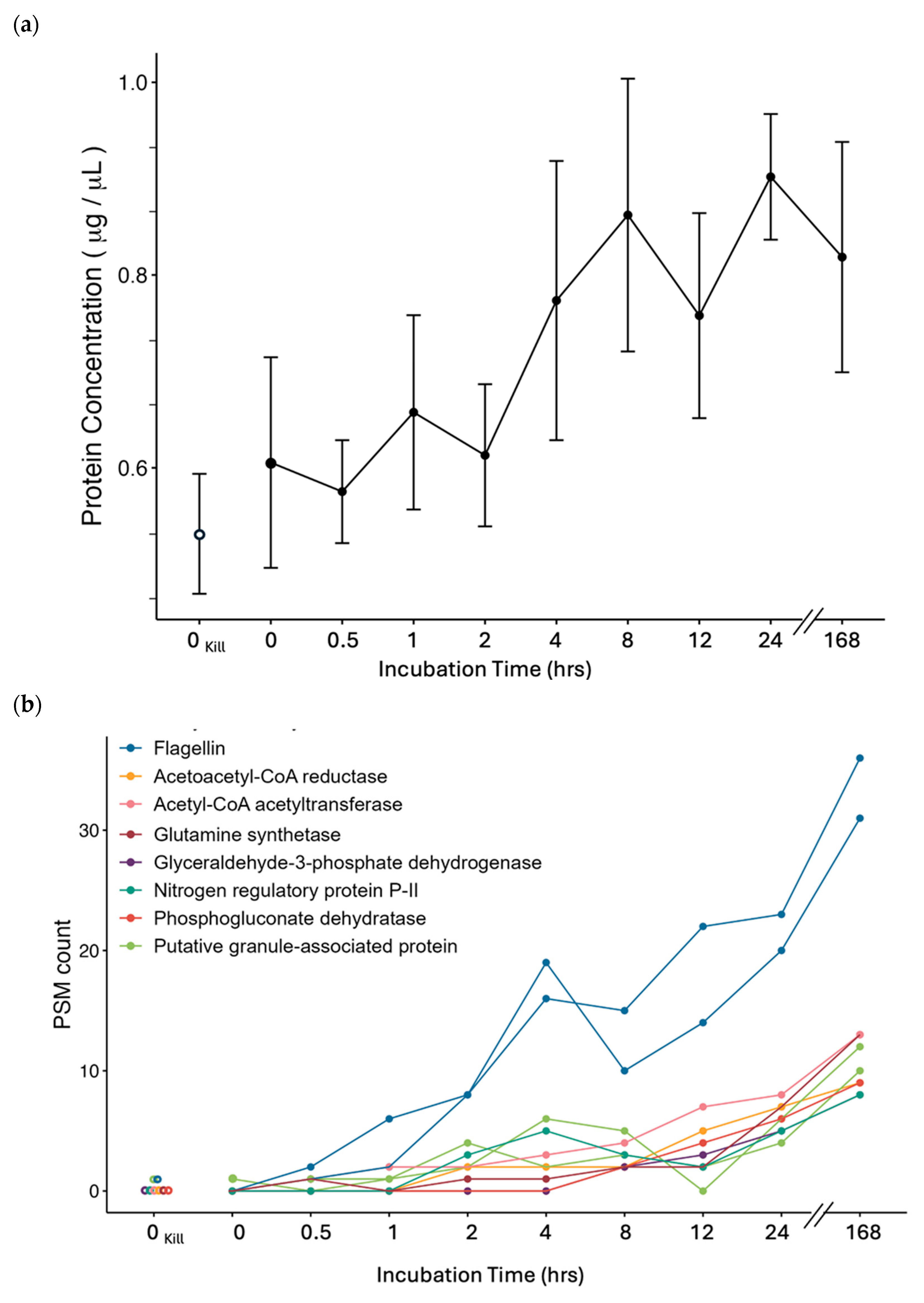
4.4. Proteomics
4.4.1. Motility
4.4.2. Storage
4.4.3. Future of Protein SIP in Life Detection
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Iess, L.; Stevenson, D.J.; Parisi, M.; Hemingway, D.; Jacobson, R.A.; Lunine, J.I.; Nimmo, F.; Armstrong, J.W.; Asmar, S.W.; Ducci, M.; et al. The Gravity Field and Interior Structure of Enceladus. Science 2014, 344, 78–80. [Google Scholar] [CrossRef] [PubMed]
- McKay, C.P.; Anbar, A.D.; Porco, C.; Tsou, P. Follow the Plume: The Habitability of Enceladus. Astrobiology 2014, 14, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.D.; Schubert, G.; Jacobson, R.A.; Lau, E.L.; Moore, W.B.; Sjogren, W.L. Europa’s Differentiated Internal Structure: Inferences from Four Galileo Encounters. Science 1998, 281, 2019–2022. [Google Scholar] [CrossRef] [PubMed]
- Vance, S.D.; Journaux, B.; Hesse, M.; Steinbrügge, G. The Salty Secrets of Icy Ocean Worlds. J. Geophys. Res. Planets 2021, 126, e2020JE006736. [Google Scholar] [CrossRef]
- Nimmo, F.; Hamilton, D.P.; McKinnon, W.B.; Schenk, P.M.; Binzel, R.P.; Bierson, C.J.; Beyer, R.A.; Moore, J.M.; Stern, S.A.; Weaver, H.A.; et al. Reorientation of Sputnik Planitia Implies a Subsurface Ocean on Pluto. Nature 2016, 540, 94–96. [Google Scholar] [CrossRef]
- Orosei, R.; Lauro, S.E.; Pettinelli, E.; Cicchetti, A.; Coradini, M.; Cosciotti, B.; Di Paolo, F.; Flamini, E.; Mattei, E.; Pajola, M.; et al. Radar Evidence of Subglacial Liquid Water on Mars. Science 2018, 361, 490–493. [Google Scholar] [CrossRef]
- Pappalardo, R.T.; Buratti, B.J.; Korth, H.; Senske, D.A.; Blaney, D.L.; Blankenship, D.D.; Burch, J.L.; Christensen, P.R.; Kempf, S.; Kivelson, M.G.; et al. Science Overview of the Europa Clipper Mission. Space Sci. Rev. 2024, 220, 40. [Google Scholar] [CrossRef]
- Hand, K.P.; Phillips, C.B.; Murray, A.; Garvin, J.B.; Maize, E.H.; Gibbs, R.G.; Reeves, G.; Martin, A.M.S.; Tan-Wang, G.H.; Krajewski, J.; et al. Science Goals and Mission Architecture of the Europa Lander Mission Concept. Planet. Sci. J. 2022, 3, 22. [Google Scholar] [CrossRef]
- Farley, K.A.; Williford, K.H.; Stack, K.M.; Bhartia, R.; Chen, A.; de la Torre, M.; Hand, K.; Goreva, Y.; Herd, C.D.K.; Hueso, R.; et al. Mars 2020 Mission Overview. Space Sci. Rev. 2020, 216, 142. [Google Scholar] [CrossRef]
- Junge, K.; Cameron, K.; Nunn, B. Chapter 12—Diversity of Psychrophilic Bacteria in Sea and Glacier Ice Environments—Insights Through Genomics, Metagenomics, and Proteomics Approaches. In Microbial Diversity in the Genomic Era; Das, S., Dash, H.R., Eds.; Academic Press: San Diego, CA, USA, 2019; pp. 197–216. ISBN 978-0-12-814849-5. [Google Scholar]
- Margesin, R.; Collins, T. Microbial Ecology of the Cryosphere (Glacial and Permafrost Habitats): Current Knowledge. Appl. Microbiol. Biotechnol. 2019, 103, 2537–2549. [Google Scholar] [CrossRef]
- Bakermans, C. Limits for Microbial Life at Subzero Temperatures. In Psychrophiles: From Biodiversity to Biotechnology; Margesin, R., Schinner, F., Marx, J.-C., Gerday, C., Eds.; Springer: Berlin, Germany, 2008; pp. 17–28. ISBN 978-3-540-74335-4. [Google Scholar]
- Bakermans, C.; Tollaksen, S.L.; Giometti, C.S.; Wilkerson, C.; Tiedje, J.M.; Thomashow, M.F. Proteomic Analysis of Psychrobactercryohalolentis K5 During Growth at Subzero Temperatures. Extremophiles 2007, 11, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Bakermans, C.; Skidmore, M.L. Microbial Metabolism in Ice and Brine at −5°C. Environ. Microbiol. 2011, 13, 2269–2278. [Google Scholar] [CrossRef] [PubMed]
- Christner, B.C. Detection, Recovery, Isolation and Characterization of Bacteria in Glacial Ice and Lake Vostok Accretion Ice. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 2002. [Google Scholar]
- Mykytczuk, N.C.S.; Foote, S.J.; Omelon, C.R.; Southam, G.; Greer, C.W.; Whyte, L.G. Bacterial Growth at −15 °C.; Molecular Insights from the Permafrost Bacterium Planococcus halocryophilus Or1. ISME J. 2013, 7, 1211–1226. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, R.C.; Radtke, K.J.; Mykytczuk, N.C.S.; Greer, C.W.; Whyte, L.G. Life at the Wedge: The Activity and Diversity of Arctic Ice Wedge Microbial Communities. Astrobiology 2012, 12, 347–360. [Google Scholar] [CrossRef]
- Amato, P.; Christner, B.C. Energy Metabolism Response to Low-Temperature and Frozen Conditions in Psychrobacter cryohalolentis. Appl. Environ. Microbiol. 2009, 75, 711–718. [Google Scholar] [CrossRef]
- Bore, E.K.; Apostel, C.; Halicki, S.; Kuzyakov, Y.; Dippold, M.A. Microbial Metabolism in Soil at Subzero Temperatures: Adaptation Mechanisms Revealed by Position-Specific 13C Labeling. Front. Microbiol. 2017, 8, 946. [Google Scholar] [CrossRef]
- Schwendner, P.; Nguyen, A.N.; Schuerger, A.C. Use of NanoSIMS to Identify the Lower Limits of Metabolic Activity and Growth by Serratia liquefaciens Exposed to Sub-Zero Temperatures. Life 2021, 11, 459. [Google Scholar] [CrossRef]
- Tuorto, S.J.; Darias, P.; McGuinness, L.R.; Panikov, N.; Zhang, T.; Häggblom, M.M.; Kerkhof, L.J. Bacterial Genome Replication at Subzero Temperatures in Permafrost. ISME J. 2014, 8, 139–149. [Google Scholar] [CrossRef]
- Folgar-Cameán, Y.; Barták, M. Evaluation of Photosynthetic Processes in Antarctic Mosses and Lichens Exposed to Controlled Rate Cooling: Species-Specific Responses. Czech Polar Rep. 2019, 9, 114–124. [Google Scholar] [CrossRef]
- Nunn, B.L.; Slattery, K.V.; Cameron, K.A.; Timmins-Schiffman, E.; Junge, K. Proteomics of Colwellia psychrerythraea at Subzero Temperatures—A Life with Limited Movement, Flexible Membranes and Vital DNA Repair. Environ. Microbiol. 2015, 17, 2319–2335. [Google Scholar] [CrossRef]
- Hecht, M.H.; Kounaves, S.P.; Quinn, R.C.; West, S.J.; Young, S.M.M.; Ming, D.W.; Catling, D.C.; Clark, B.C.; Boynton, W.V.; Hoffman, J.; et al. Detection of Perchlorate and the Soluble Chemistry of Martian Soil at the Phoenix Lander Site. Science 2009, 325, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Gentilhomme, A.S.; Dhakar, K.; Timmins-Schiffman, E.; Chaw, M.; Firth, E.; Junge, K.; Nunn, B.L. Surviving the Stress: MS-Based Proteomics Unravels the Molecular Strategies in a Psychrophile Confronting Perchlorate-Rich Subzero Conditions. Astrobiology 2024. accepted. [Google Scholar]
- Lipps, J.H.; Rieboldt, S. Habitats and Taphonomy of Europa. Icarus 2005, 177, 515–527. [Google Scholar] [CrossRef]
- Amato, P.; Doyle, S.M.; Battista, J.R.; Christner, B.C. Implications of Subzero Metabolic Activity on Long-Term Microbial Survival in Terrestrial and Extraterrestrial Permafrost. Astrobiology 2010, 10, 789–798. [Google Scholar] [CrossRef]
- Neveu, M.; Hays, L.E.; Voytek, M.A.; New, M.H.; Schulte, M.D. The Ladder of Life Detection. Astrobiology 2018, 18, 1375–1402. [Google Scholar] [CrossRef]
- Christner, B.C.; Mosley-Thompson, E.; Thompson, L.G.; Zagorodnov, V.; Sandman, K.; Reeve, J.N. Recovery and Identification of Viable Bacteria Immured in Glacial Ice. Icarus 2000, 144, 479–485. [Google Scholar] [CrossRef]
- Domagal-Goldman, S.D.; Wright, K.E.; Adamala, K.; Arina de la Rubia, L.; Bond, J.; Dartnell, L.R.; Goldman, A.D.; Lynch, K.; Naud, M.-E.; Paulino-Lima, I.G.; et al. The Astrobiology Primer v2.0. Astrobiology 2016, 16, 561–653. [Google Scholar] [CrossRef]
- Ewert Sarmiento, M. Microbial Challenges and Solutions to Inhabiting the Dynamic Architecture of Saline Ice Formations. Ph.D. Thesis, University of Washington, Seattle, WA, USA, 2013. [Google Scholar]
- Ewert, M.; Deming, J.W. Bacterial Responses to Fluctuations and Extremes in Temperature and Brine Salinity at the Surface of Arctic Winter Sea Ice. FEMS Microbiol. Ecol. 2014, 89, 476–489. [Google Scholar] [CrossRef]
- Bowman, J.P.; Gosink, J.J.; McCammon, S.A.; Lewis, T.E.; Nichols, D.S.; Nichols, P.D.; Skerratt, J.H.; Staley, J.T.; McMeekin, T.A. Colwellia demingiae sp. nov., Colwellia hornerae sp. nov., Colwellia rossensis sp. nov. and Colwellia psychrotropica sp. nov.: Psychrophilic Antarctic Species with the Ability to Synthesize Docosahexaenoic Acid (22:6ω3). Int. J. Syst. Evol. Microbiol. 1998, 48, 1171–1180. [Google Scholar] [CrossRef]
- Huston, A.L.; Methe, B.; Deming, J.W. Purification, Characterization, and Sequencing of an Extracellular Cold-Active Aminopeptidase Produced by Marine Psychrophile Colwellia psychrerythraea Strain 34H. Appl. Environ. Microbiol. 2004, 70, 3321–3328. [Google Scholar] [CrossRef]
- Huston, A.L. Bacterial Adaptation to the Cold: In Situ Activities of Extracellular Enzymes in the North Water Polynya and Characterization of a Cold-Active Aminopeptidase from Colwellia psychrerythraea Strain 34H. Ph.D. Thesis, University of Washington, Seattle, WA, USA, 2003. [Google Scholar]
- Wells, L.E.; Deming, J.W. Modelled and Measured Dynamics of Viruses in Arctic Winter Sea-Ice Brines. Environ. Microbiol. 2006, 8, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Mudge, M.C.; Nunn, B.L.; Firth, E.; Ewert, M.; Hales, K.; Fondrie, W.E.; Noble, W.S.; Toner, J.; Light, B.; Junge, K.A. Subzero, Saline Incubations of Colwellia psychrerythraea Reveal Strategies and Biomarkers for Sustained Life in Extreme Icy Environments. Environ. Microbiol. 2021, 23, 3840–3866. [Google Scholar] [CrossRef] [PubMed]
- Golden, K.M.; Eicken, H.; Heaton, A.L.; Miner, J.; Pringle, D.J.; Zhu, J. Thermal Evolution of Permeability and Microstructure in Sea Ice. Geophys. Res. Lett. 2007, 34, L16501. [Google Scholar] [CrossRef]
- Marion, G.M.; Farren, R.E.; Komrowski, A.J. Alternative Pathways for Seawater Freezing. Cold Reg. Sci. Technol. 1999, 29, 259–266. [Google Scholar] [CrossRef]
- Vancoppenolle, M.; Madec, G.; Thomas, M.; McDougall, T.J. Thermodynamics of Sea Ice Phase Composition Revisited. J. Geophys. Res. Oceans 2019, 124, 615–634. [Google Scholar] [CrossRef]
- Alcolombri, U.; Pioli, R.; Stocker, R.; Berry, D. Single-Cell Stable Isotope Probing in Microbial Ecology. ISME Commun. 2022, 2, 55. [Google Scholar] [CrossRef]
- Jameson, E.; Taubert, M.; Coyotzi, S.; Chen, Y.; Eyice, Ö.; Schäfer, H.; Murrell, J.C.; Neufeld, J.D.; Dumont, M.G. DNA-, RNA-, and Protein-Based Stable-Isotope Probing for High-Throughput Biomarker Analysis of Active Microorganisms. In Metagenomics: Methods and Protocols; Streit, W.R., Daniel, R., Eds.; Springer: New York, NY, USA, 2017; pp. 57–74. ISBN 978-1-4939-6691-2. [Google Scholar]
- von Bergen, M.; Jehmlich, N.; Taubert, M.; Vogt, C.; Bastida, F.; Herbst, F.-A.; Schmidt, F.; Richnow, H.-H.; Seifert, J. Insights from Quantitative Metaproteomics and Protein-Stable Isotope Probing into Microbial Ecology. ISME J. 2013, 7, 1877–1885. [Google Scholar] [CrossRef]
- Jehmlich, N.; Vogt, C.; Lünsmann, V.; Richnow, H.H.; von Bergen, M. Protein-SIP in Environmental Studies. Curr. Opin. Biotechnol. 2016, 41, 26–33. [Google Scholar] [CrossRef]
- Czajka, J.J.; Abernathy, M.H.; Benites, V.T.; Baidoo, E.E.K.; Deming, J.W.; Tang, Y.J. Model Metabolic Strategy for Heterotrophic Bacteria in the Cold Ocean Based on Colwellia psychrerythraea 34H. Proc. Natl. Acad. Sci. USA 2018, 115, 12507–12512. [Google Scholar] [CrossRef]
- Marx, J.G.; Carpenter, S.D.; Deming, J.W. Production of Cryoprotectant Extracellular Polysaccharide Substances (EPS) by the Marine Psychrophilic Bacterium Colwellia psychrerythraea Strain 34H under Extreme conditions. Can. J. Microbiol. 2009, 55, 63–72. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Bacteriological Analytical Manual (BAM); U.S. Food and Drug Administration: Silver Spring, MD, USA, 2023. [Google Scholar]
- Junge, K.; Eicken, H.; Deming, J.W. Bacterial Activity at −2 to −20°C in Arctic Wintertime Sea Ice. Appl. Environ. Microbiol. 2004, 70, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Junge, K.; Eicken, H.; Swanson, B.D.; Deming, J.W. Bacterial Incorporation of Leucine into Protein Down to −20 °C with Evidence for Potential Activity in Sub-Eutectic Saline Ice Formations. Cryobiology 2006, 52, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Azam, F. Protein Content and Protein Synthesis Rates of Planktonic Marine Bacteria. Mar. Ecol. Prog. Ser. 1989, 51, 201–213. [Google Scholar] [CrossRef]
- Kirchman, D.L. Incorporation of Thymidine and Leucine in the Subarctic Pacific: Application to Estimating Bacterial Production. Mar. Ecol. Prog. Ser. 1992, 82, 301–309. [Google Scholar] [CrossRef]
- Price, P.B.; Sowers, T. Temperature Dependence of Metabolic Rates for Microbial Growth, Maintenance, and Survival. Proc. Natl. Acad. Sci. USA 2004, 101, 4631–4636. [Google Scholar] [CrossRef]
- Ludwig, K.R.; Schroll, M.M.; Hummon, A.B. Comparison of In-Solution, FASP, and S-Trap Based Digestion Methods for Bottom-Up Proteomic Studies. J. Proteome Res. 2018, 17, 2480–2490. [Google Scholar] [CrossRef]
- Eng, J.K.; Jahan, T.A.; Hoopmann, M.R. Comet: An Open-Source MS/MS Sequence Database Search Tool. Proteomics 2013, 13, 22–24. [Google Scholar] [CrossRef]
- Eng, J.K.; Hoopmann, M.R.; Jahan, T.A.; Egertson, J.D.; Noble, W.S.; MacCoss, M.J. A Deeper Look into Comet—Implementation and Features. J. Am. Soc. Mass Spectrom. 2015, 26, 1865–1874. [Google Scholar] [CrossRef]
- Nesvizhskii, A.I.; Keller, A.; Kolker, E.; Aebersold, R. A Statistical Model for Identifying Proteins by Tandem Mass Spectrometry. Anal. Chem. 2003, 75, 4646–4658. [Google Scholar] [CrossRef]
- Riffle, M.; Hoopmann, M.R.; Jaschob, D.; Zhong, G.; Moritz, R.L.; MacCoss, M.J.; Davis, T.N.; Isoherranen, N.; Zelter, A. Discovery and Visualization of Uncharacterized Drug-Protein Adducts Using Mass Spectrometry. Anal. Chem. 2022, 94, 3501–3509. [Google Scholar] [CrossRef]
- Showalter, G.M.; Deming, J.W. Low-Temperature Chemotaxis, Halotaxis and Chemohalotaxis by the Psychrophilic Marine Bacterium Colwellia psychrerythraea 34H. Environ. Microbiol. Rep. 2018, 10, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Showalter, G.M.; Deming, J.W. Extracellular Enzyme Activity of Model Cold-Adapted Bacteria and Arctic Sea-Ice Microbial Communities under Subzero Hypersaline Conditions. Aquat. Microb. Ecol. 2021, 87, 99–111. [Google Scholar] [CrossRef]
- Kaye, J.Z.; Márquez, M.C.; Ventosa, A.; Baross, J.A. Halomonas neptunia sp. nov., Halomonas sulfidaeris sp. nov., Halomonas axialensis sp. nov. and Halomonas hydrothermalis sp. nov.: Halophilic Bacteria Isolated from Deep-Sea Hydrothermal-Vent Environments. Int. J. Syst. Evol. Microbiol. 2004, 54, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Mnif, S.; Chamkha, M.; Sayadi, S. Isolation and Characterization of Halomonas sp. Strain C2SS100, a Hydrocarbon-degrading Bacterium Under Hypersaline Conditions. J. Appl. Microbiol. 2009, 107, 785–794. [Google Scholar] [CrossRef]
- Niederberger, T.D.; Perreault, N.N.; Tille, S.; Lollar, B.S.; Lacrampe-Couloume, G.; Andersen, D.; Greer, C.W.; Pollard, W.; Whyte, L.G. Microbial Characterization of a Subzero, Hypersaline Methane Seep in the Canadian High Arctic. ISME J. 2010, 4, 1326–1339. [Google Scholar] [CrossRef]
- Petrich, C.; Eicken, H. Overview of Sea Ice Growth and Properties. In Sea Ice; John Wiley & Sons: Chichester, UK, 2017; pp. 1–41. ISBN 978-1-118-77837-1. [Google Scholar]
- Gault, S.; Fonseca, F.; Cockell, C.S. Preservation of Bacillus Subtilis’ Cellular Liquid State at Deep Sub-Zero Temperatures in Perchlorate Brines. Commun. Biol. 2024, 7, 588. [Google Scholar] [CrossRef]
- Clarke, A.; Morris, G.J.; Fonseca, F.; Murray, B.J.; Acton, E.; Price, H.C. A Low Temperature Limit for Life on Earth. PLoS ONE 2013, 8, e66207. [Google Scholar] [CrossRef]
- Metpally, R.P.R.; Reddy, B.V.B. Comparative Proteome Analysis of Psychrophilic versus Mesophilic Bacterial Species: Insights into the Molecular Basis of Cold Adaptation of Proteins. BMC Genom. 2009, 10, 11. [Google Scholar] [CrossRef]
- Methé, B.A.; Nelson, K.E.; Deming, J.W.; Momen, B.; Melamud, E.; Zhang, X.; Moult, J.; Madupu, R.; Nelson, W.C.; Dodson, R.J.; et al. The Psychrophilic Lifestyle as Revealed by the Genome Sequence of Colwellia psychrerythraea 34H Through Genomic and Proteomic Analyses. Proc. Natl. Acad. Sci. USA 2005, 102, 10913–10918. [Google Scholar] [CrossRef]
- Shah, M.P.; Dey, S. Extremophiles: A Paradox of Nature with Biotechnological Implications; De Gruyter: Berlin, Germany, 2022; ISBN 978-3-11-078848-8. [Google Scholar]
- Myka, K.K.; Allcock, D.J.; Eloe-Fadrosh, E.A.; Tryfona, T.; Haag, A.F.; Lauro, F.M.; Bartlett, D.H.; Ferguson, G.P. Adaptations of Cold- and Pressure-Loving Bacteria to the Deep-Sea Environment: Cell Envelope and Flagella. In Microbial Ecology of Extreme Environments; Chénard, C., Lauro, F.M., Eds.; Springer International Publishing: Champaign, IL, USA, 2017; pp. 51–80. ISBN 978-3-319-51686-8. [Google Scholar]
- Obruca, S.; Sedlacek, P.; Slaninova, E.; Fritz, I.; Daffert, C.; Meixner, K.; Sedrlova, Z.; Koller, M. Novel Unexpected Functions of PHA Granules. Appl. Microbiol. Biotechnol. 2020, 104, 4795–4810. [Google Scholar] [CrossRef]
- Martin, D.; Bartlett, D.H.; Roberts, M.F. Solute Accumulation in the Deep-Sea Bacterium Photobacterium profundum. Extremophiles 2002, 6, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Klumpp, S.; Zhang, Z.; Hwa, T. Growth Rate-Dependent Global Effects on Gene Expression in Bacteria. Cell 2009, 139, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; García-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE Database Resources in 2022: A Hub for Mass Spectrometry-Based Proteomics Evidences. Nucleic Acids Res. 2022, 50, D543–D552. [Google Scholar] [CrossRef] [PubMed]
- Breezee, J.; Cady, N.; Staley, J.T. Subfreezing Growth of the Sea Ice Bacterium “Psychromonas ingrahamii”. Microb. Ecol. 2004, 47, 300–304. [Google Scholar] [CrossRef]
- Goordial, J.; Raymond-Bouchard, I.; Zolotarov, Y.; de Bethencourt, L.; Ronholm, J.; Shapiro, N.; Woyke, T.; Stromvik, M.; Greer, C.W.; Bakermans, C.; et al. Cold Adaptive Traits Revealed by Comparative Genomic Analysis of the Eurypsychrophile rhodococcus sp. JG3 Isolated from High Elevation McMurdo Dry Valley Permafrost, Antarctica. FEMS Microbiol. Ecol. 2016, 92, fiv154. [Google Scholar] [CrossRef]
- Koh, H.Y.; Park, H.; Lee, J.H.; Han, S.J.; Sohn, Y.C.; Lee, S.G. Proteomic and Transcriptomic Investigations on Cold-Responsive Properties of the Psychrophilic Antarctic Bacterium Psychrobacter sp. PAMC 21119 at Subzero Temperatures. Environ. Microbiol. 2017, 19, 628–644. [Google Scholar] [CrossRef]
- Bakermans, C.; Tsapin, A.I.; Souza-Egipsy, V.; Gilichinsky, D.A.; Nealson, K.H. Reproduction and Metabolism at −10°C of Bacteria Isolated from Siberian Permafrost. Environ. Microbiol. 2003, 5, 321–326. [Google Scholar] [CrossRef]
- Raymond-Bouchard, I.; Goordial, J.; Zolotarov, Y.; Ronholm, J.; Stromvik, M.; Bakermans, C.; Whyte, L.G. Conserved Genomic and Amino Acid Traits of Cold Adaptation in Subzero-Growing Arctic Permafrost Bacteria. FEMS Microbiol. Ecol. 2018, 94, fiy023. [Google Scholar] [CrossRef]
- Ponder, M.A.; Gilmour, S.J.; Bergholz, P.W.; Mindock, C.A.; Hollingsworth, R.; Thomashow, M.F.; Tiedje, J.M. Characterization of Potential Stress Responses in Ancient Siberian Permafrost Psychroactive Bacteria. FEMS Microbiol. Ecol. 2005, 53, 103–115. [Google Scholar] [CrossRef]
- Shcherbakova, V.A.; Chuvilskaya, N.A.; Rivkina, E.M.; Pecheritsyna, S.A.; Laurinavichius, K.S.; Suzina, N.E.; Osipov, G.A.; Lysenko, A.M.; Gilichinsky, D.A.; Akimenko, V.K. Novel Psychrophilic Anaerobic Spore-Forming Bacterium from the Overcooled Water Brine in Permafrost: Description Clostridium Algoriphilum sp. nov. Extremophiles 2005, 9, 239–246. [Google Scholar] [CrossRef]
- Bakermans, C.; Nealson, K.H. Relationship of Critical Temperature to Macromolecular Synthesis and Growth Yield in Psychrobacter cryopegella. J. Bacteriol. 2004, 186, 2340–2345. [Google Scholar] [CrossRef]
- WebPlotDigitizer V4.5. Available online: https://automeris.io/ (accessed on 10 October 2022).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ewert, M.; Nunn, B.L.; Firth, E.; Junge, K. Metabolic Responses, Cell Recoverability, and Protein Signatures of Three Extremophiles: Sustained Life During Long-Term Subzero Incubations. Microorganisms 2025, 13, 251. https://doi.org/10.3390/microorganisms13020251
Ewert M, Nunn BL, Firth E, Junge K. Metabolic Responses, Cell Recoverability, and Protein Signatures of Three Extremophiles: Sustained Life During Long-Term Subzero Incubations. Microorganisms. 2025; 13(2):251. https://doi.org/10.3390/microorganisms13020251
Chicago/Turabian StyleEwert, Marcela, Brook L. Nunn, Erin Firth, and Karen Junge. 2025. "Metabolic Responses, Cell Recoverability, and Protein Signatures of Three Extremophiles: Sustained Life During Long-Term Subzero Incubations" Microorganisms 13, no. 2: 251. https://doi.org/10.3390/microorganisms13020251
APA StyleEwert, M., Nunn, B. L., Firth, E., & Junge, K. (2025). Metabolic Responses, Cell Recoverability, and Protein Signatures of Three Extremophiles: Sustained Life During Long-Term Subzero Incubations. Microorganisms, 13(2), 251. https://doi.org/10.3390/microorganisms13020251





