The Influence of Microbiota on Wild Birds’ Parental Coprophagy Behavior: Current Advances and Future Research Directions
Abstract
1. Introduction
2. Bird’s Gut Microbiota
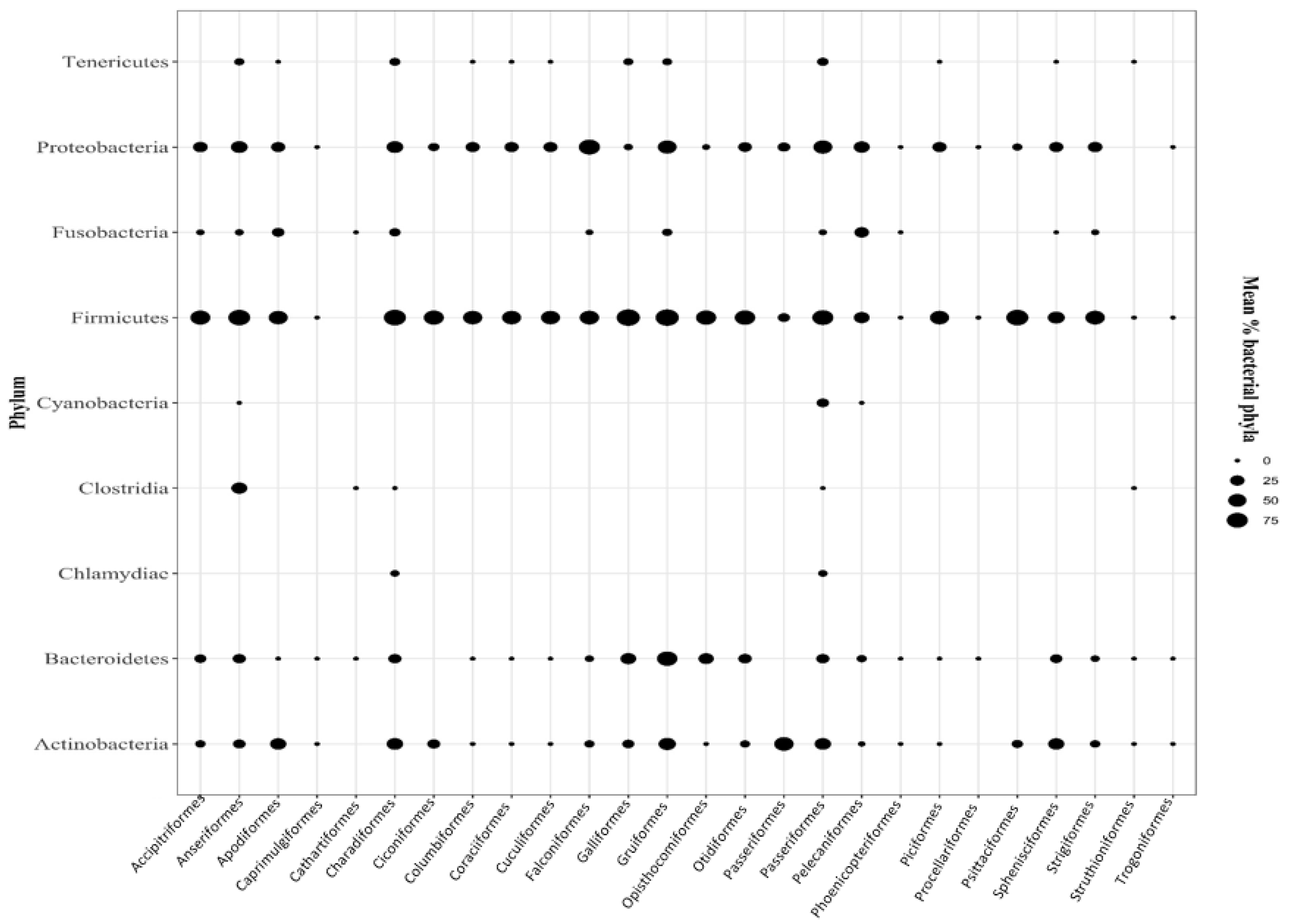
2.1. Microbial Phyla in Bird
2.1.1. Firmicutes
2.1.2. Proteobacteria
2.1.3. Actinobacteria
2.1.4. Bacteroidetes
3. Factors Influencing Microbiota Composition
3.1. Diet
3.2. Environmental Factors
3.3. Phylogeny and Genetics of Hosts
4. Nestling’s Fecal Consumption and How It Links to Gut Microbiota
4.1. Parental Coprophagy in Birds
4.2. Coprophagy and the Transfer of Microorganisms
4.3. Parental Coprophagy and Nutritional Implications
5. Research Gaps and Controversies
6. Implications and Future Directions
- (1)
- Longitudinal studies: Conducting longitudinal studies that track individual bird parents across multiple reproductive seasons would yield valuable insights into the long-term effects of fecal consumption on the microbiota. This could assist in determining whether the observed alterations in microbiota are temporary or permanent.
- (2)
- Comparative studies: Comparing the gut microbiota of bird species that consume varying amounts of parental feces could cast light on the variation in microbiota dynamics and potential adaptations across species. Investigating closely and distantly associated species with differing levels of parental care could shed light on the evolutionary importance of this behavior.
- (3)
- Health implications: Investigating the ecological variables that govern the incidence and regularity of parental feces consumption among wild bird populations may shed light on the adaptive importance of this behavior. Taking into account factors such as the availability of food resources, breeding density, and prevailing environmental conditions could help achieve an in-depth understanding of the ecological determinants of this practice and its subsequent impact on individual fitness.
- (4)
- Experimental manipulations: Conducting controlled experiments in which the availability or composition of fecal matter is altered could assist in elucidating the causal relationship between fecal consumption and alterations in the microbiota. Providing bird parents with feces from various sources or altering the nutrient content of fecal matter, for instance, could aid in the identification of specific factors that influence microbiota composition.
- (5)
- Concerning evolutionary implications: Understanding the evolutionary significance of parental coprophagy in birds is essential. This behavior is hypothesized to have developed as an adaptive mechanism; however, its role in the broader context of avian evolutionary history is not fully understood. Future research endeavors should focus on the evolutionary consequences of coprophagy, particularly its influence on reproductive outcomes, overall fitness, and species survival. Comparative analyses across different avian taxa could reveal the selective pressures that have contributed to the emergence and maintenance of this behavior over time.
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Petrosino, J.F.; Highlander, S.; Luna, R.A.; Gibbs, R.A.; Versalovic, J. Metagenomic Pyrosequencing and Microbial Identification. Clin. Chem. 2009, 55, 856–866. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The Human Microbiome Project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- Parfrey, L.W.; Moreau, C.S.; Russell, J.A. Introduction: The Host-Associated Microbiome: Pattern, Process and Function. Mol. Ecol. 2018, 27, 1749–1765. [Google Scholar] [CrossRef]
- Hird, S.M. Evolutionary Biology Needs Wild Microbiomes. Front. Microbiol. 2017, 8, 725. [Google Scholar] [CrossRef]
- Ezenwa, V.O.; Gerardo, N.M.; Inouye, D.W.; Medina, M.; Xavier, J.B. Animal Behavior and the Microbiome. Science 2012, 338, 198–199. [Google Scholar] [CrossRef]
- Ballou, A.L.; Ali, R.A.; Mendoza, M.A.; Ellis, J.C.; Hassan, H.M.; Croom, W.J.; Koci, M.D. Development of the Chick Microbiome: How Early Exposure Influences Future Microbial Diversity. Front. Vet. Sci. 2016, 3, 2. [Google Scholar] [CrossRef]
- Videvall, E.; Song, S.J.; Bensch, H.M.; Strandh, M.; Engelbrecht, A.; Serfontein, N.; Hellgren, O.; Olivier, A.; Cloete, S.; Knight, R.; et al. Major Shifts in Gut Microbiota during Development and Its Relationship to Growth in Ostriches. Mol. Ecol. 2019, 28, 2653–2667. [Google Scholar] [CrossRef]
- Kohl, K.D. Diversity and Function of the Avian Gut Microbiota. J. Comp. Physiol. B 2012, 182, 591–602. [Google Scholar] [CrossRef]
- Grond, K.; Sandercock, B.K.; Jumpponen, A.; Zeglin, L.H. The Avian Gut Microbiota: Community, Physiology and Function in Wild Birds. J. Avian Biol. 2018, 49, e01788. [Google Scholar] [CrossRef]
- Park, W. Gut Microbiomes and Their Metabolites Shape Human and Animal Health. J. Microbiol. 2018, 56, 151–153. [Google Scholar] [CrossRef]
- Costello, E.K.; Stagaman, K.; Dethlefsen, L.; Bohannan, B.J.M.; Relman, D.A. The Application of Ecological Theory Toward an Understanding of the Human Microbiome. Science 2012, 336, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Coyte, K.Z.; Rao, C.; Rakoff-Nahoum, S.; Foster, K.R. Ecological Rules for the Assembly of Microbiome Communities. PLoS Biol. 2021, 19, e3001116. [Google Scholar] [CrossRef] [PubMed]
- Verster, A.J.; Borenstein, E. Competitive Lottery-Based Assembly of Selected Clades in the Human Gut Microbiome. Microbiome 2018, 6, 186. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.F. Alien Invasions and Gut “Island Biogeography”. Cell 2014, 159, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Wenny, D.G.; DeVault, T.L.; Johnson, M.D.; Kelly, D.; Sekercioglu, C.H.; Tomback, D.F.; Whelan, C.J. The Need to Quantify Ecosystem Services Provided by Birds. Auk 2011, 128, 1–14. [Google Scholar] [CrossRef]
- Sekercioglu, C. Increasing Awareness of Avian Ecological Function. Trends Ecol. Evol. 2006, 21, 464–471. [Google Scholar] [CrossRef]
- Whelan, C.J.; Wenny, D.G.; Marquis, R.J. Ecosystem Services Provided by Birds. Ann. N. Y. Acad. Sci. 2008, 1134, 25–60. [Google Scholar] [CrossRef]
- Barnes, E.M. The Avian Intestinal Flora with Particular Reference to the Possible Ecological Significance of the Cecal Anaerobic Bacteria. Am. J. Clin. Nutr. 1972, 25, 1475–1479. [Google Scholar] [CrossRef]
- McKnite, A.M.; Perez-Munoz, M.E.; Lu, L.; Williams, E.G.; Brewer, S.; Andreux, P.A.; Bastiaansen, J.W.M.; Wang, X.; Kachman, S.D.; Auwerx, J.; et al. Murine Gut Microbiota Is Defined by Host Genetics and Modulates Variation of Metabolic Traits. PLoS ONE 2012, 7, e39191. [Google Scholar] [CrossRef]
- Benson, A.K.; Kelly, S.A.; Legge, R.; Ma, F.; Low, S.J.; Kim, J.; Zhang, M.; Oh, P.L.; Nehrenberg, D.; Hua, K.; et al. Individuality in Gut Microbiota Composition Is a Complex Polygenic Trait Shaped by Multiple Environmental and Host Genetic Factors. Proc. Natl. Acad. Sci. USA 2010, 107, 18933–18938. [Google Scholar] [CrossRef]
- Campbell, J.H.; Foster, C.M.; Vishnivetskaya, T.; Campbell, A.G.; Yang, Z.K.; Wymore, A.; Palumbo, A.V.; Chesler, E.J.; Podar, M. Host Genetic and Environmental Effects on Mouse Intestinal Microbiota. ISME J. 2012, 6, 2033–2044. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, F.; Nguyen, T.L.A.; Brinkman, B.; Yunta, R.G.; Cauwe, B.; Vandenabeele, P.; Liston, A.; Raes, J. Inflammation-Associated Enterotypes, Host Genotype, Cage and Inter-Individual Effects Drive Gut Microbiota Variation in Common Laboratory Mice. Genome Biol. 2013, 14, R4. [Google Scholar] [CrossRef] [PubMed]
- Sullam, K.E.; Essinger, S.D.; Lozupone, C.A.; O’Connor, M.P.; Rosen, G.L.; Knight, R.; Kilham, S.S.; Russell, J.A. Environmental and Ecological Factors That Shape the Gut Bacterial Communities of Fish: A Meta-Analysis. Mol. Ecol. 2012, 21, 3363–3378. [Google Scholar] [CrossRef] [PubMed]
- Engel, P.; Moran, N.A. The Gut Microbiota of Insects–Diversity in Structure and Function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef] [PubMed]
- Vickery, J.A.; Ewing, S.R.; Smith, K.W.; Pain, D.J.; Bairlein, F.; Škorpilová, J.; Gregory, R.D. The Decline of Afro-Palaearctic Migrants and an Assessment of Potential Causes. Ibis 2014, 156, 1–22. [Google Scholar] [CrossRef]
- Pappas, S.; Benson, T.J.; Bednarz, J.C. Effects of Brown-Headed Cowbird Parasitism on Provisioning Rates of Swainson’s Warblers. Wilson J. Ornithol. 2010, 122, 75–81. [Google Scholar] [CrossRef]
- Lima, S.L. Predation Risk and Unpredictable Feeding Conditions: Determinants of Body Mass in Birds. Ecology 1986, 67, 377–385. [Google Scholar] [CrossRef]
- Soave, O.; Brand, C.D. Coprophagy in Animals: A Review. Cornell Vet. 1991, 81, 357–364. [Google Scholar]
- Hirakawa, H. Coprohagy in Leporids and Other Mammalian Herbivores. Mamm. Rev. 2001, 31, 61–80. [Google Scholar] [CrossRef]
- Sakamaki, T. Coprophagy in Wild Bonobos (Pan paniscus) at Wamba in the Democratic Republic of the Congo: A Possibly Adaptive Strategy? Primates 2010, 51, 87–90. [Google Scholar] [CrossRef]
- Barnes, R.H. Nutritional implications of coprophagy. Nutr. Rev. 2009, 20, 289–291. [Google Scholar] [CrossRef] [PubMed]
- Ebino, K.Y. Studies on Coprophagy in Experimental Animals. Exp. Anim. 1993, 42, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hörnicke, H.; Björnhag, G. Coprophagy and Related Strategies for Digesta Utilization. In Digestive Physiology and Metabolism in Ruminants, Proceedings of the 5th International Symposium on Ruminant Physiology, Clermont-Ferrand, France, 3–7 September 1979; Springer: Dordrecht, The Netherlands, 1980; pp. 707–730. [Google Scholar]
- Troyer, K. Transfer of Fermentative Microbes Between Generations in a Herbivorous Lizard. Science 1982, 216, 540–542. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Tsuchida, S.; Ueda, A.; Yamada, T.; Murata, K.; Nakamura, H.; Ushida, K. Role of Coprophagy in the Cecal Microbiome Development of an Herbivorous Bird Japanese Rock Ptarmigan. JVMS 2019, 81, 1389–1399. [Google Scholar] [CrossRef] [PubMed]
- Scupham, A.J.; Patton, T.G.; Bent, E.; Bayles, D.O. Comparison of the Cecal Microbiota of Domestic and Wild Turkeys. Microb. Ecol. 2008, 56, 322–331. [Google Scholar] [CrossRef]
- Wilkinson, N.; Hughes, R.J.; Aspden, W.J.; Chapman, J.; Moore, R.J.; Stanley, D. The Gastrointestinal Tract Microbiota of the Japanese Quail, Coturnix Japonica. Appl. Microbiol. Biotechnol. 2016, 100, 4201–4209. [Google Scholar] [CrossRef]
- McWhorter, T.J.; Caviedes-Vidal, E.; Karasov, W.H. The Integration of Digestion and Osmoregulation in the Avian Gut. Biol. Rev. Camb. Philos. Soc. 2009, 84, 533–565. [Google Scholar] [CrossRef]
- Blair, R.H.; Tucker, B.W. Nest Sanitation. Br. Birds 1941, 34, 215. [Google Scholar]
- Guigueno, M.F.; Sealy, S.G. Nest Sanitation in Passerine Birds: Implications for Egg Rejection in Hosts of Brood Parasites. J. Ornithol. 2012, 153, 35–52. [Google Scholar] [CrossRef]
- Leggett, K. Coprophagy and Unusual Thermoregulatory Behaviour in Desert-Dwelling Elephants of North-Western Namibia. Pachyderm 2004, 36, 113–115. [Google Scholar] [CrossRef]
- Waite, D.W.; Taylor, M.W. Characterizing the Avian Gut Microbiota: Membership, Driving Influences, and Potential Function. Front. Microbiol. 2014, 5, 223. [Google Scholar] [CrossRef] [PubMed]
- Waite, D.W.; Taylor, M.W. Exploring the Avian Gut Microbiota: Current Trends and Future Directions. Front. Microbiol. 2015, 6, 673. [Google Scholar] [CrossRef] [PubMed]
- Knutie, S.A. Food Supplementation Affects Gut Microbiota and Immunological Resistance to Parasites in a Wild Bird Species. J. Appl. Ecol. 2020, 57, 536–547. [Google Scholar] [CrossRef]
- Berlow, M.; Phillips, J.N.; Derryberry, E.P. Effects of Urbanization and Landscape on Gut Microbiomes in White-Crowned Sparrows. Microb. Ecol. 2021, 81, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Kropáčková, L.; Těšický, M.; Albrecht, T.; Kubovčiak, J.; Čížková, D.; Tomášek, O.; Martin, J.F.; Bobek, L.; Králová, T.; Procházka, P.; et al. Codiversification of Gastrointestinal Microbiota and Phylogeny in Passerines Is Not Explained by Ecological Divergence. Mol. Ecol. 2017, 26, 5292–5304. [Google Scholar] [CrossRef]
- Michel, A.J.; Ward, L.M.; Goffredi, S.K.; Dawson, K.S.; Baldassarre, D.T.; Brenner, A.; Gotanda, K.M.; McCormack, J.E.; Mullin, S.W.; O’Neill, A.; et al. The Gut of the Finch: Uniqueness of the Gut Microbiome of the Galápagos Vampire Finch 06 Biological Sciences 0602 Ecology 05 Environmental Sciences 0502 Environmental Science and Management. Microbiome 2018, 6, 167. [Google Scholar] [CrossRef]
- Knutie, S.A.; Chaves, J.A.; Gotanda, K.M. Human Activity Can Influence the Gut Microbiota of Darwin’s Finches in the Galapagos Islands. Mol. Ecol. 2019, 28, 2441–2450. [Google Scholar] [CrossRef]
- San Juan, P.A.; Hendershot, J.N.; Daily, G.C.; Fukami, T. Land-Use Change Has Host-Specific Influences on Avian Gut Microbiomes. ISME J. 2020, 14, 318–321. [Google Scholar] [CrossRef]
- Godoy-Vitorino, F.; Goldfarb, K.C.; Karaoz, U.; Leal, S.; Garcia-Amado, M.A.; Hugenholtz, P.; Tringe, S.G.; Brodie, E.L.; Dominguez-Bello, M.G. Comparative Analyses of Foregut and Hindgut Bacterial Communities in Hoatzins and Cows. ISME J. 2012, 6, 531–541. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The Role of Short-Chain Fatty Acids in the Interplay between Diet, Gut Microbiota, and Host Energy Metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Benskin, C.M.H.; Wilson, K.; Jones, K.; Hartley, I.R. Bacterial Pathogens in Wild Birds: A Review of the Frequency and Effects of Infection. Biol. Rev. 2009, 84, 349–373. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, A.; Yang, Y.; Wang, F.; Liu, Y.; Zhang, Y.; Sharshov, K.; Gui, L. Composition, Diversity and Function of Gastrointestinal Microbiota in Wild Red-Billed Choughs (Pyrrhocorax pyrrhocorax). Int. J. Microbiol. 2019, 22, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Wallménius, K.; Barboutis, C.; Fransson, T.; Jaenson, T.G.; Lindgren, P.E.; Nyström, F.; Olsen, B.; Salaneck, E.; Nilsson, K. Spotted Fever Rickettsia Species in Hyalomma and Ixodes Ticks Infesting Migratory Birds in the European Mediterranean Area. Parasit. Vectors 2014, 7, 318. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.; Grond, K.; Verheijen, B.; Elk, M.; Buehler, D.M.; Santo Domingo, J.W. Intestinal Microbiota and Species Diversity of Campylobacter and Helicobacter Spp. in Migrating Shorebirds in Delaware Bay. Appl. Environ. Microbiol. 2014, 80, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.I.; Shriver, W.G.; Waldenströ, J.; Griekspoor, P.; Rn Olsen, B. Prevalence of Campylobacter in Wild Birds of the Mid-Atlantic Region, USA. J. Wildl. Dis. 2011, 47, 750–754. [Google Scholar] [CrossRef]
- Diakou, A.; Norte, A.C.; Lopes de Carvalho, I.; Núncio, S.; Nováková, M.; Kautman, M.; Alivizatos, H.; Kazantzidis, S.; Sychra, O.; Literák, I. Ticks and Tick-Borne Pathogens in Wild Birds in Greece. Parasitol. Res. 2016, 115, 2011–2016. [Google Scholar] [CrossRef]
- Ley, R.E.; Hamady, M.; Lozupone, C.; Turnbaugh, P.J.; Ramey, R.R.; Bircher, J.S.; Schlegel, M.L.; Tucker, T.A.; Schrenzel, M.D.; Knight, R.; et al. Evolution of Mammals and Their Gut Microbes. Science 2008, 320, 1647–1651. [Google Scholar] [CrossRef]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Klenk, H.-P.; Clément, C.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, Physiology, and Natural Products of Actinobacteria. Microbiol. Mol. Biol. 2016, 80, 1–43. [Google Scholar] [CrossRef]
- Janssen, P.H. Identifying the Dominant Soil Bacterial Taxa in Libraries of 16S RRNA and 16S RRNA Genes. Appl. Environ. Microbiol. 2006, 72, 1719–1728. [Google Scholar] [CrossRef]
- Kailasapathy, K.; Chin, J. Survival and Therapeutic Potential of Probiotic Organisms with Reference to Lactobacillus Acidophilus and Bifidobacterium spp. Immunol. Cell Biol. 2000, 78, 80–88. [Google Scholar] [CrossRef]
- Hird, S.M.; Sánchez, C.; Carstens, B.C.; Brumfield, R.T. Comparative Gut Microbiota of 59 Neotropical Bird Species. Front. Microbiol. 2015, 6, 1403. [Google Scholar] [CrossRef] [PubMed]
- Colston, T.J.; Jackson, C.R. Microbiome Evolution along Divergent Branches of the Vertebrate Tree of Life: What Is Known and Unknown. Mol. Ecol. 2016, 25, 3776–3800. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F.; Hehemann, J.H.; Rebuffet, E.; Czjzek, M.; Michel, G. Environmental and Gut Bacteroidetes: The Food Connection. Front. Microbiol. 2011, 2, 93. [Google Scholar] [CrossRef] [PubMed]
- Matsui, H.; Kato, Y.; Chikaraishi, T.; Moritani, M.; Ban-Tokuda, T.; Wakita, M. Microbial Diversity in Ostrich Ceca as Revealed by 16S Ribosomal RNA Gene Clone Library and Detection of Novel Fibrobacter Species. Anaerobe 2010, 16, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.C.; Tun, H.M.; Kim, J.E.; Leung, F.C.; Cheng, K.M. Characterization of Cecal Microbiota of the Emu (Dromaius novaehollandiae). Vet. Microbiol. 2013, 166, 304–310. [Google Scholar] [CrossRef]
- Kohl, K.D.; Amaya, J.; Passement, C.A.; Dearing, M.D.; Mccue, M.D. Unique and Shared Responses of the Gut Microbiota to Prolonged Fasting: A Comparative Study across Five Classes of Vertebrate Hosts. FEMS Microbiol. Ecol. 2014, 90, 883–894. [Google Scholar] [CrossRef]
- Bauer, S.; Hoye, B.J. Migratory Animals Couple Biodiversity and Ecosystem Functioning Worldwide. Science 2014, 344, 1242552. [Google Scholar] [CrossRef]
- Ruiz-Rodríguez, M.; Martín-Vivaldi, M.; Martínez-Bueno, M.; Soler, J. Correction: Ruiz-Rodríguez et al. Gut Microbiota of Great Spotted Cuckoo Nestlings Is a Mixture of Those of Their Foster Magpie Siblings and of Cuckoo Adults. Genes 2018, 9, 381. Genes 2018, 9, 530. [Google Scholar] [CrossRef]
- Youngblut, N.D.; Reischer, G.H.; Walters, W.; Schuster, N.; Walzer, C.; Stalder, G.; Ley, R.E.; Farnleitner, A.H. Host Diet and Evolutionary History Explain Different Aspects of Gut Microbiome Diversity among Vertebrate Clades. Nat. Commun. 2019, 10, 2200. [Google Scholar] [CrossRef]
- Grond, K.; Lanctot, R.B.; Jumpponen, A.; Sandercock, B.K. Recruitment and Establishment of the Gut Microbiome in Arctic Shorebirds. FEMS Microbiol. Ecol. 2017, 93, 12. [Google Scholar] [CrossRef]
- Pan, D.; Yu, Z. Intestinal Microbiome of Poultry and Its Interaction with Host and Diet. Gut Microbes 2014, 5, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Davidson, G.L.; Wiley, N.; Cooke, A.C.; Johnson, C.N.; Fouhy, F.; Reichert, M.S.; de la Hera, I.; Crane, J.M.S.; Kulahci, I.G.; Ross, R.P.; et al. Diet Induces Parallel Changes to the Gut Microbiota and Problem Solving Performance in a Wild Bird. Sci. Rep. 2020, 10, 20783. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Meng, D.; Gong, M.; Li, H.; Wen, W.; Wang, Y.; Zhou, J. Effects of Sex and Diet on Gut Microbiota of Farmland-Dependent Wintering Birds. Front. Microbiol. 2020, 11, 587873. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, Y.; Gong, M.; Zheng, C.; Zhang, C.; Li, H.; Wen, W.; Wang, Y.; Liu, G. Diet-Induced Microbiome Shifts of Sympatric Overwintering Birds. Appl. Microbiol. Biotechnol. 2021, 105, 5993–6005. [Google Scholar] [CrossRef] [PubMed]
- Roggenbuck, M.; Bærholm Schnell, I.; Blom, N.; Bælum, J.; Bertelsen, M.F.; Pontén, T.S.; Sørensen, S.J.; Gilbert, M.T.P.; Graves, G.R.; Hansen, L.H. The Microbiome of New World Vultures. Nat. Commun. 2014, 5, 5498. [Google Scholar] [CrossRef]
- Cho, H.; Lee, W.Y. Interspecific Comparison of the Fecal Microbiota Structure in Three Arctic Migratory Bird Species. Ecol. Evol. 2020, 10, 5582–5594. [Google Scholar] [CrossRef]
- Teyssier, A.; Matthysen, E.; Hudin, N.S.; de Neve, L.; White, J.; Lens, L. Diet Contributes to Urban-Induced Alterations in Gut Microbiota: Experimental Evidence from a Wild Passerine. Proc. Biol. Sci. 2020, 287, 1920. [Google Scholar] [CrossRef]
- Gubert, C.; Kong, G.; Renoir, T.; Hannan, A.J. Exercise, Diet and Stress as Modulators of Gut Microbiota: Implications for Neurodegenerative Diseases. Neurobiol. Dis. 2020, 134, 104621. [Google Scholar] [CrossRef]
- Angelstam, P.; Roberge, J.-M.; Lohmus, A.; Bergmanis, M. Habitat Modelling as a Tool for Landscape- Scale Conservation- A Review of Parameters for Focal Forest Birds. Ecol. Bull. 2004, 51, 427–453. [Google Scholar] [CrossRef]
- Hird, S.M.; Carstens, B.C.; Cardiff, S.W.; Dittmann, D.L.; Brumfield, R.T. Sampling Locality Is More Detectable than Taxonomy or Ecology in the Gut Microbiota of the Brood-Parasitic Brown-Headed Cowbird (Molothrus ater). PeerJ 2014, 2014. [Google Scholar] [CrossRef]
- Grond, K.; Ryu, H.; Baker, A.J.; Santo Domingo, J.W.; Buehler, D.M. Gastro-Intestinal Microbiota of Two Migratory Shorebird Species during Spring Migration Staging in Delaware Bay, USA. J. Ornithol. 2014, 155, 969–977. [Google Scholar] [CrossRef]
- Yang, Y.; Deng, Y.; Cao, L. Characterising the Interspecific Variations and Convergence of Gut Microbiota in Anseriformes Herbivores at Wintering Areas. Sci. Rep. 2016, 6, 32655. [Google Scholar] [CrossRef] [PubMed]
- Herder, E.A.; Spence, A.R.; Tingley, M.W.; Hird, S.M. Elevation Correlates With Significant Changes in Relative Abundance in Hummingbird Fecal Microbiota, but Composition Changes Little. Front. Ecol. Evol. 2021, 8, 597756. [Google Scholar] [CrossRef]
- Teyssier, A.; Rouffaer, L.O.; Saleh Hudin, N.; Strubbe, D.; Matthysen, E.; Lens, L.; White, J. Inside the Guts of the City: Urban-Induced Alterations of the Gut Microbiota in a Wild Passerine. Sci. Total Environ. 2018, 612, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.N.; Berlow, M.; Derryberry, E.P. The Effects of Landscape Urbanization on the Gut Microbiome: An Exploration into the Gut of Urban and Rural White-Crowned Sparrows. Front. Ecol. Evol. 2018, 6, 148. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Y.; Cao, L.; Yin, H.; Xu, M.; Wang, Z.; Liu, Y.; Wang, X.; Deng, Y. Habitat Environments Impacted the Gut Microbiome of Long-Distance Migratory Swan Geese but Central Species Conserved. Sci. Rep. 2018, 8, 13314. [Google Scholar] [CrossRef]
- Baldo, L.; Pretus, J.L.; Riera, J.L.; Musilova, Z.; Bitja Nyom, A.R.; Salzburger, W. Convergence of Gut Microbiotas in the Adaptive Radiations of African Cichlid Fishes. ISME J. 2017, 11, 1975–1987. [Google Scholar] [CrossRef]
- Koskella, B.; Hall, L.J.; Metcalf, C.J.E. The Microbiome beyond the Horizon of Ecological and Evolutionary Theory. Nat. Ecol. Evol. 2017, 1, 1606–1615. [Google Scholar] [CrossRef]
- Song, S.J.; Sanders, J.G.; Delsuc, F.; Metcalf, J.; Amato, K.; Taylor, M.W.; Mazel, F.; Lutz, H.L.; Winker, K.; Graves, G.R.; et al. Comparative Analyses of Vertebrate Gut Microbiomes Reveal Convergence between Birds and Bats. mBio 2020, 11, e02901-19. [Google Scholar] [CrossRef]
- Grond, K.; Santo Domingo, J.W.; Lanctot, R.B.; Jumpponen, A.; Bentzen, R.L.; Boldenow, M.L.; Brown, S.C.; Casler, B.; Cunningham, J.A.; Doll, A.C.; et al. Composition and Drivers of Gut Microbial Communities in Arctic-Breeding Shorebirds. Front. Microbiol. 2019, 10, 2258. [Google Scholar] [CrossRef]
- Bodawatta, K.H.; Sam, K.; Jønsson, K.A.; Poulsen, M. Comparative Analyses of the Digestive Tract Microbiota of New Guinean Passerine Birds. Front. Microbiol. 2018, 9, 1830. [Google Scholar] [CrossRef] [PubMed]
- Trevelline, B.K.; Sosa, J.; Hartup, B.K.; Kohl, K.D. A Bird’s-Eye View of Phylosymbiosis: Weak Signatures of Phylosymbiosis among All 15 Species of Cranes. Proc. Biol. Sci. 2020, 287, 20192988. [Google Scholar] [CrossRef] [PubMed]
- Groussin, M.; Mazel, F.; Sanders, J.G.; Smillie, C.S.; Lavergne, S.; Thuiller, W.; Alm, E.J. Unraveling the Processes Shaping Mammalian Gut Microbiomes over Evolutionary Time. Nat. Commun. 2017, 8, 14319. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.X.; Li, H.R.; Han, W.; Luo, W. Comparison of Gut Microbiota between Gentoo and Adélie Penguins Breeding Sympatrically on Antarctic Ardley Island as Revealed by Fecal DNA Sequencing. Diversity 2021, 13, 500. [Google Scholar] [CrossRef]
- Royle, N.J.; Smiseth, P.T.; Kölliker, M. The Evolution of Parental Care: Summary, Conclusions, and Implications. In The Evolution of Parental Care; Oxford University Press: Oxford, UK, 2012; pp. 326–345. [Google Scholar]
- Ibáñez-Álamo, J.D.; Rubio, E.; Soler, J.J. Evolution of Nestling Faeces Removal in Avian Phylogeny. Anim. Behav. 2017, 124, 1–5. [Google Scholar] [CrossRef]
- Ibáñez-Álamo, J.D.; Sanllorente, O.; Arco, L.; Soler, M. Does Nest Predation Risk Induce Parent Birds to Eat Nestlings’ Fecal Sacs? An Experimental Study. Ann. Zool. Fennici. 2013, 50, 71–78. [Google Scholar] [CrossRef]
- Ibáñez-Álamo, J.D.; Ruiz-Rodríguez, M.; Soler, J.J. The Mucous Covering of Fecal Sacs Prevents Birds from Infection with Enteric Bacteria. J. Avian Biol. 2014, 45, 354–358. [Google Scholar] [CrossRef]
- Quan, R.C.; Li, H.; Wang, B.; Goodale, E. The Relationship between Defecation and Feeding in Nestling Birds: Observational and Experimental Evidence. Front. Zool. 2015, 12, 21. [Google Scholar] [CrossRef]
- Smith, J.; Petrovic, P.; Rose, M.; De Souz, C.; Muller, L.; Nowak, B.; Martinez, J. Impacts of coprophagic foraging behaviour on the avian gut microbiome. Biol. Rev. 2024, 99, 582–597. [Google Scholar] [CrossRef]
- Glück, E. Why Do Parent Birds Swallow the Feces of Their Nestlings? Experientia 1988, 44, 537–539. [Google Scholar] [CrossRef]
- Hurd, P.L.; Weatherhead, P.J.; McRae, S.B. Parental Consumption of Nestling Feces: Good Food or Sound Economics? Behav. Ecol. 1991, 2, 69–76. [Google Scholar] [CrossRef]
- Gao, L.-F.; Zhang, W.; Zhang, H.-Y.; Zhu, Z.-Q.; Zhang, X.-D.; Li, J.-C.; Fan, L.-Q.; Du, B. Fecal Consumption by Adults of Altricial Birds in Relation to the Temporal Change in Nestling Gut Microbiota. Curr. Zool. 2020, 66, 689–691. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zeng, J.; Shi, Y.; Song, S. Are Microbes and Metabolites Influencing the Parental Consumption of Nestlings’ Feces in Gray-Backed Shrikes? Curr. Zool. 2022, 68, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Barnes, E.M.; Impey, C.S.; Cooper, D.M. Manipulation of the Crop and Intestinal Flora of the Newly Hatched Chick. Am. J. Clin. Nutr. 1980, 33, 2426–2433. [Google Scholar] [CrossRef]
- Bornbusch, S.L.; Harris, R.L.; Grebe, N.M.; Roche, K.; Dimac-Stohl, K.; Drea, C.M. Antibiotics and Fecal Transfaunation Differentially Affect Microbiota Recovery, Associations, and Antibiotic Resistance in Lemur Guts. Anim. Microbiome 2021, 3, 65. [Google Scholar] [CrossRef]
- Niederwerder, M.C. Fecal Microbiota Transplantation as a Tool to Treat and Reduce Susceptibility to Disease in Animals. Vet. Immunol. Immunopathol. 2018, 206, 65–72. [Google Scholar] [CrossRef]
- Guo, W.; Ren, K.; Ning, R.; Li, C.; Zhang, H.; Li, D.; Xu, L.; Sun, F.; Dai, M. Fecal Microbiota Transplantation Provides New Insight into Wildlife Conservation. Glob. Ecol. Conserv. 2020, 24, e01234. [Google Scholar] [CrossRef]
- Waite, D.W.; Deines, P.; Taylor, M.W. Quantifying the Impact of Storage Procedures for Faecal Bacteriotherapy in the Critically Endangered New Zealand Parrot, the Kakapo (Strigops habroptilus). Zoo. Biol. 2013, 32, 620–625. [Google Scholar] [CrossRef]
- Bo, T.B.; Zhang, X.Y.; Kohl, K.D.; Wen, J.; Tian, S.J.; Wang, D.H. Coprophagy Prevention Alters Microbiome, Metabolism, Neurochemistry, and Cognitive Behavior in a Small Mammal. ISME J. 2020, 14, 2625–2645. [Google Scholar] [CrossRef]
- Videvall, E.; Bensch, H.M.; Engelbrecht, A.; Cloete, S.; Cornwallis, C.K. Coprophagy Rapidly Matures Juvenile Gut Microbiota in a Precocial Bird. Evol. Lett. 2023, 7, 240–251. [Google Scholar] [CrossRef]
- Dell’omo, G.; Alleva, E.; Carere, C. Parental Recycling of Nestling Faeces in the Common Swift. Anim. Behav. 1998, 56, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Morton, M.L.; Orejuela, J.E.; Budd, S.M. The Biology of Immature Mountain White-Crowned Sparrows (Zonotrichia leucophrys Oriantha) on the Breeding Ground. Condor 1972, 74, 423–430. [Google Scholar] [CrossRef]
- Morton, M.L. Fecal Sac Ingestion in the Mountain White-Crowned Sparrow. Condor 1979, 81, 72–77. [Google Scholar] [CrossRef]
- Negro, J.J.; Grande, J.M.; Tella, J.L.; Garrido, J.; Hornero, D.; Donázar, J.A.; Sanchez-Zapata, J.A.; BenÍtez, J.R.; Barcell, M. An Unusual Source of Essential Carotenoids. Nature 2002, 416, 807–808. [Google Scholar] [CrossRef] [PubMed]
- Aziz, Q.; Doré, J.; Emmanuel, A.; Guarner, F.; Quigley, E.M.M. Gut Microbiota and Gastrointestinal Health: Current Concepts and Future Directions. Neurogastroenterol. Motility 2013, 25, 4–15. [Google Scholar] [CrossRef]
- Lindsay, E.C.; Metcalfe, N.B.; Llewellyn, M.S. The Potential Role of the Gut Microbiota in Shaping Host Energetics and Metabolic Rate. J. Anim. Ecol. 2020, 89, 2415–2426. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gul, S.; Shi, Y.; Hu, J.; Song, S. The Influence of Microbiota on Wild Birds’ Parental Coprophagy Behavior: Current Advances and Future Research Directions. Microorganisms 2024, 12, 2468. https://doi.org/10.3390/microorganisms12122468
Gul S, Shi Y, Hu J, Song S. The Influence of Microbiota on Wild Birds’ Parental Coprophagy Behavior: Current Advances and Future Research Directions. Microorganisms. 2024; 12(12):2468. https://doi.org/10.3390/microorganisms12122468
Chicago/Turabian StyleGul, Saba, Yurou Shi, Jie Hu, and Sen Song. 2024. "The Influence of Microbiota on Wild Birds’ Parental Coprophagy Behavior: Current Advances and Future Research Directions" Microorganisms 12, no. 12: 2468. https://doi.org/10.3390/microorganisms12122468
APA StyleGul, S., Shi, Y., Hu, J., & Song, S. (2024). The Influence of Microbiota on Wild Birds’ Parental Coprophagy Behavior: Current Advances and Future Research Directions. Microorganisms, 12(12), 2468. https://doi.org/10.3390/microorganisms12122468





