Growth Response of Non-Conventional Yeasts on Sugar-Rich Media: Part 2: Citric Acid Production and Circular-Oriented Valorization of Glucose-Enriched Olive Mill Wastewaters Using Novel Yarrowia lipolytica Strains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Microorganisms (Maintenance and Inoculum Preparation)
2.2. Batch Shake Flask and Bioreactor Fermentations
2.3. Analytical Methods
2.4. Data Analysis
2.5. Nomenclature
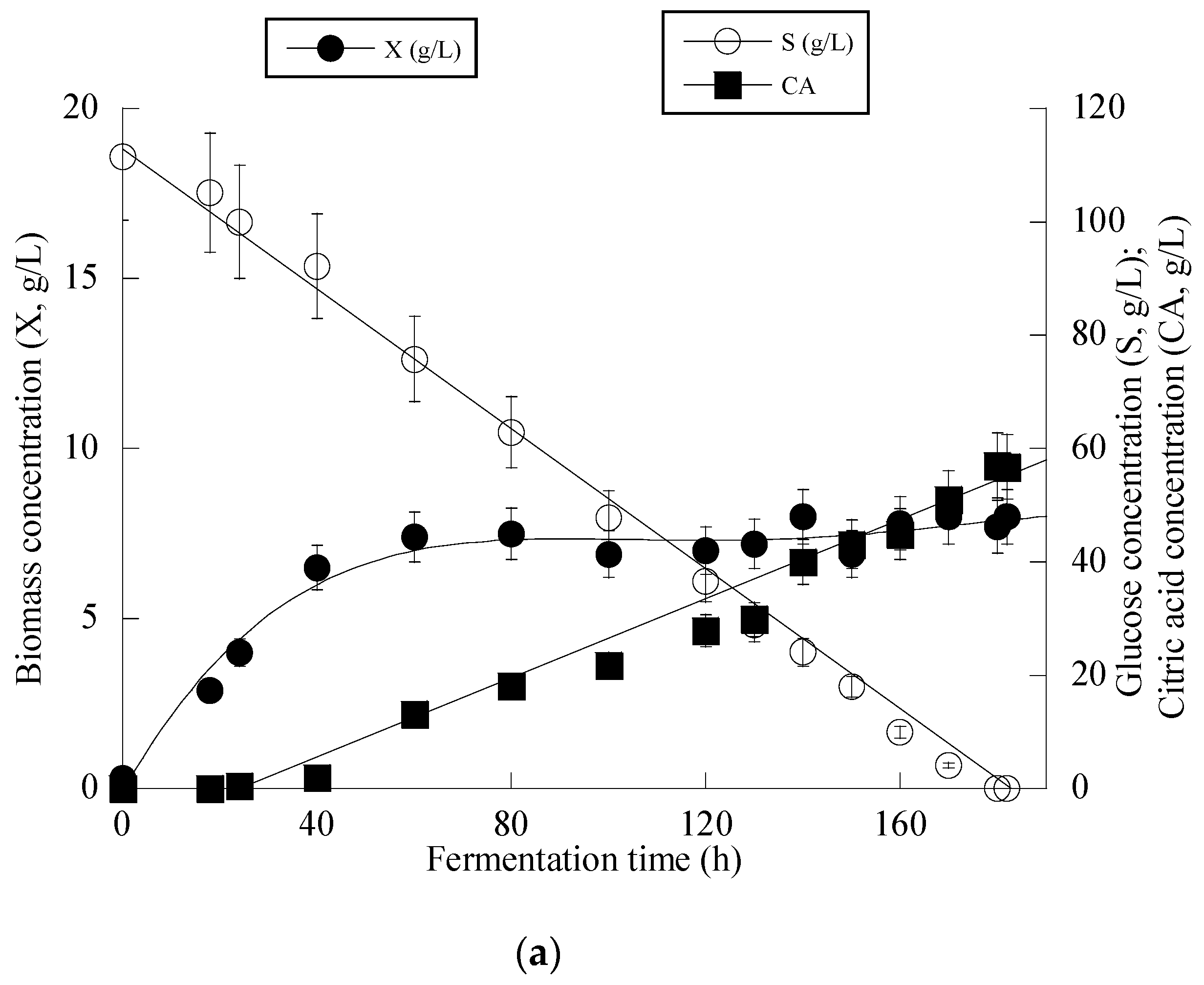
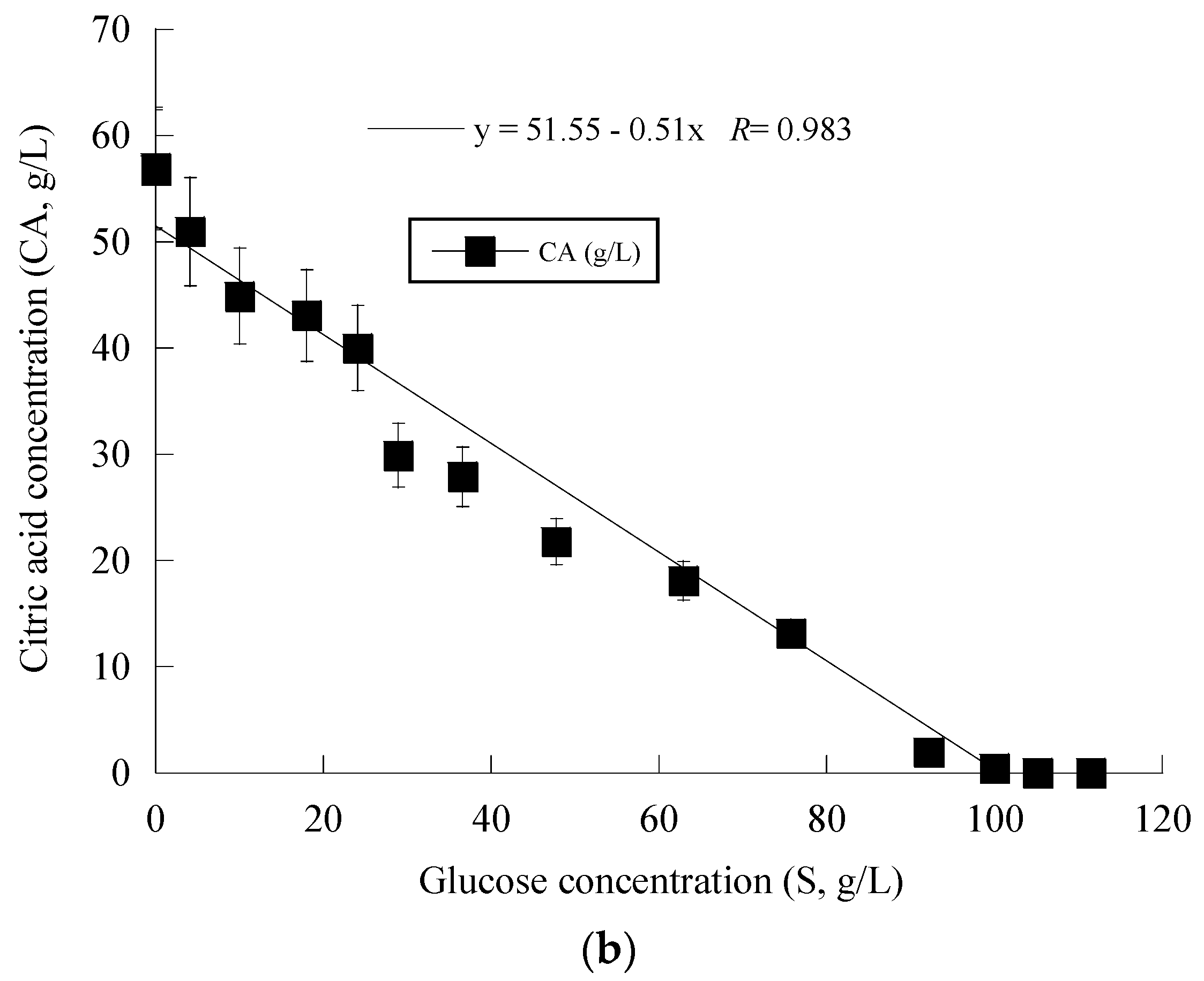
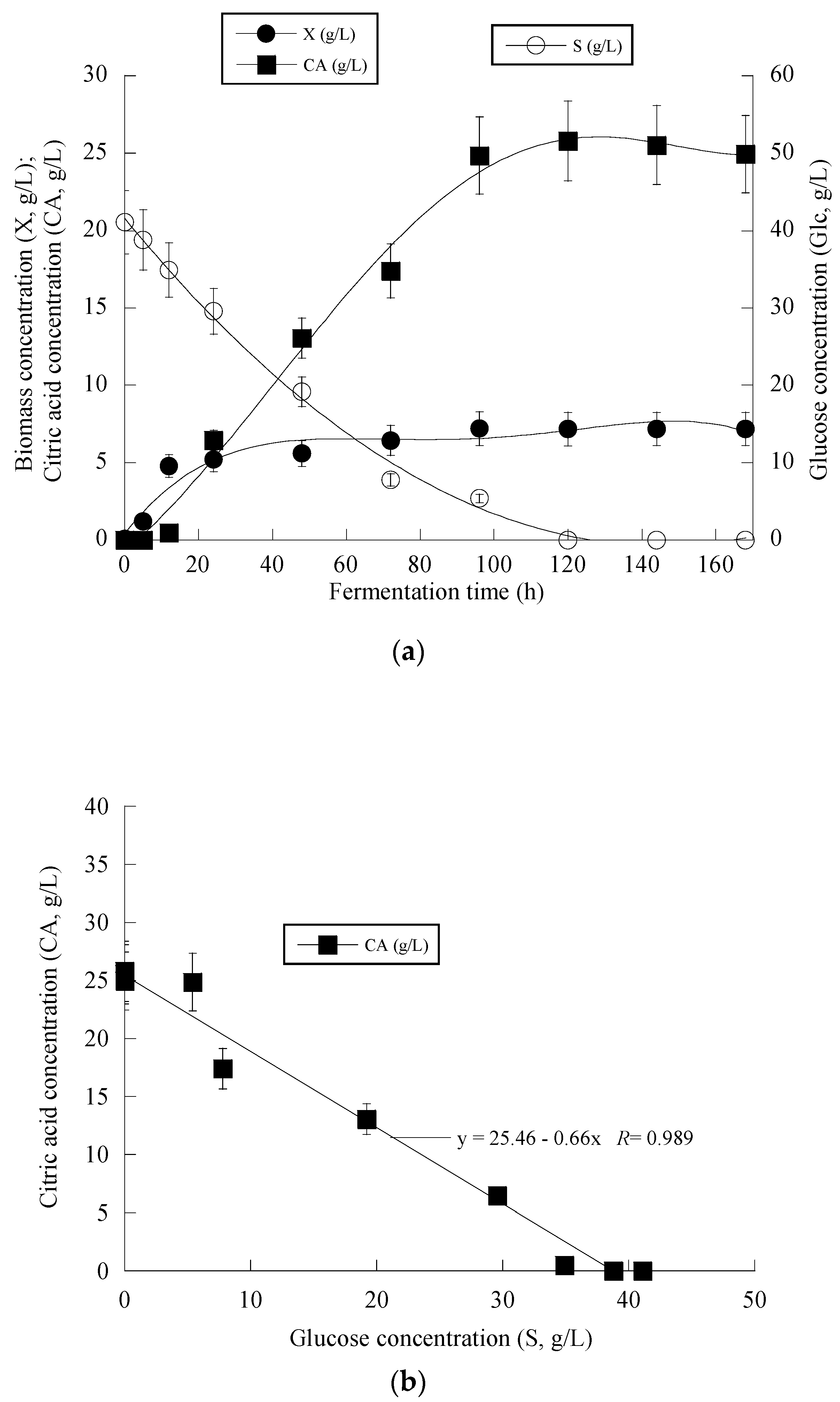
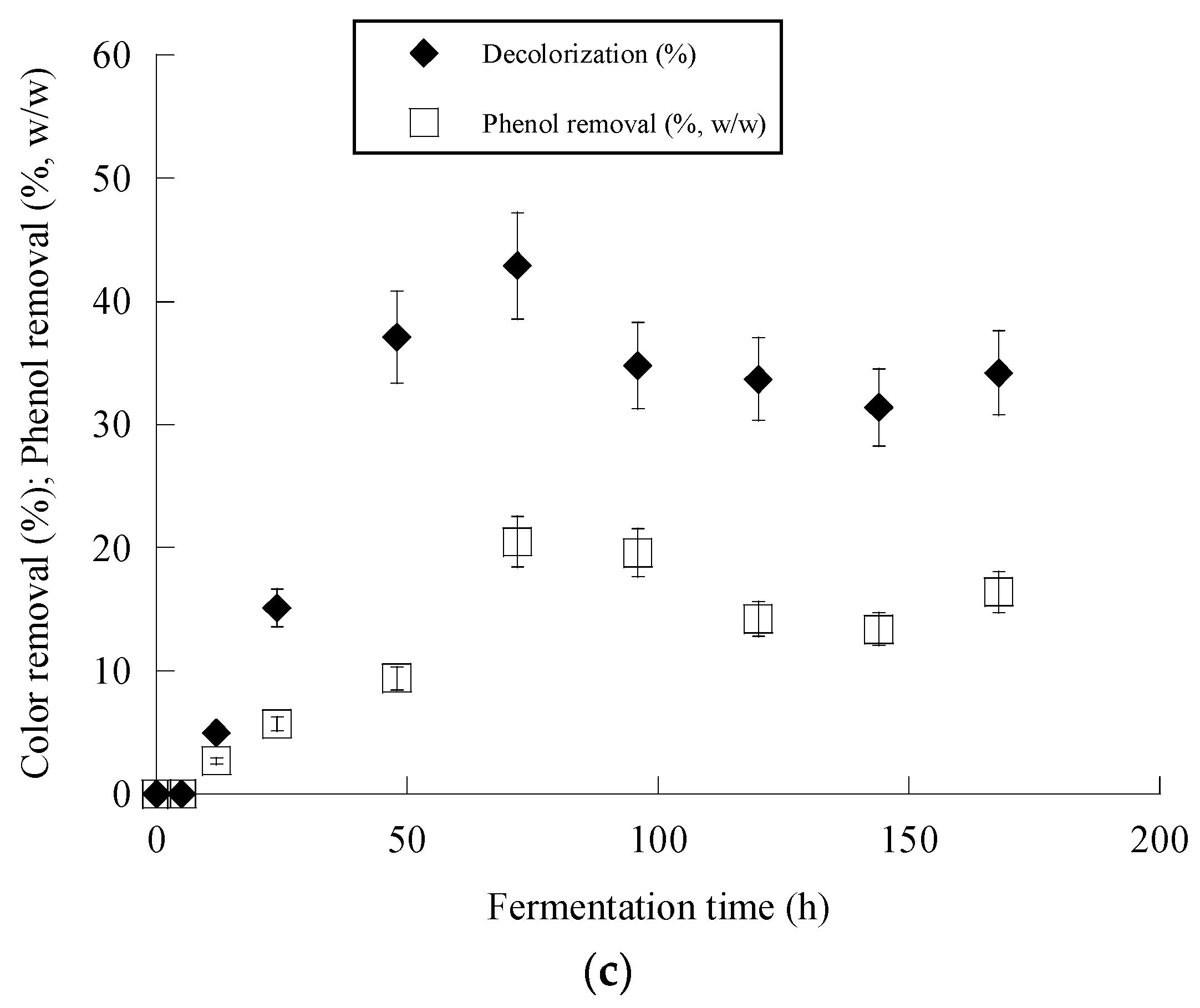
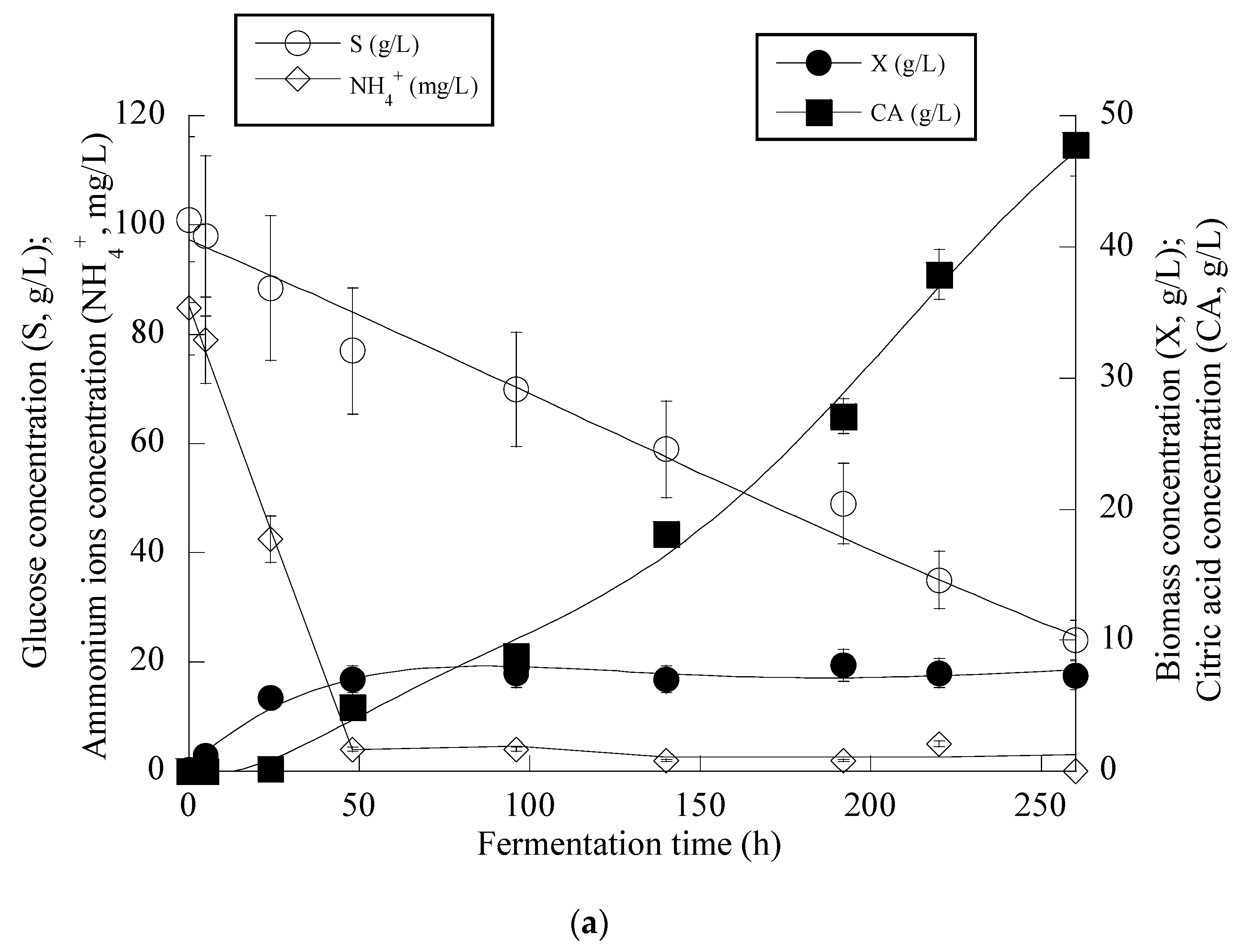
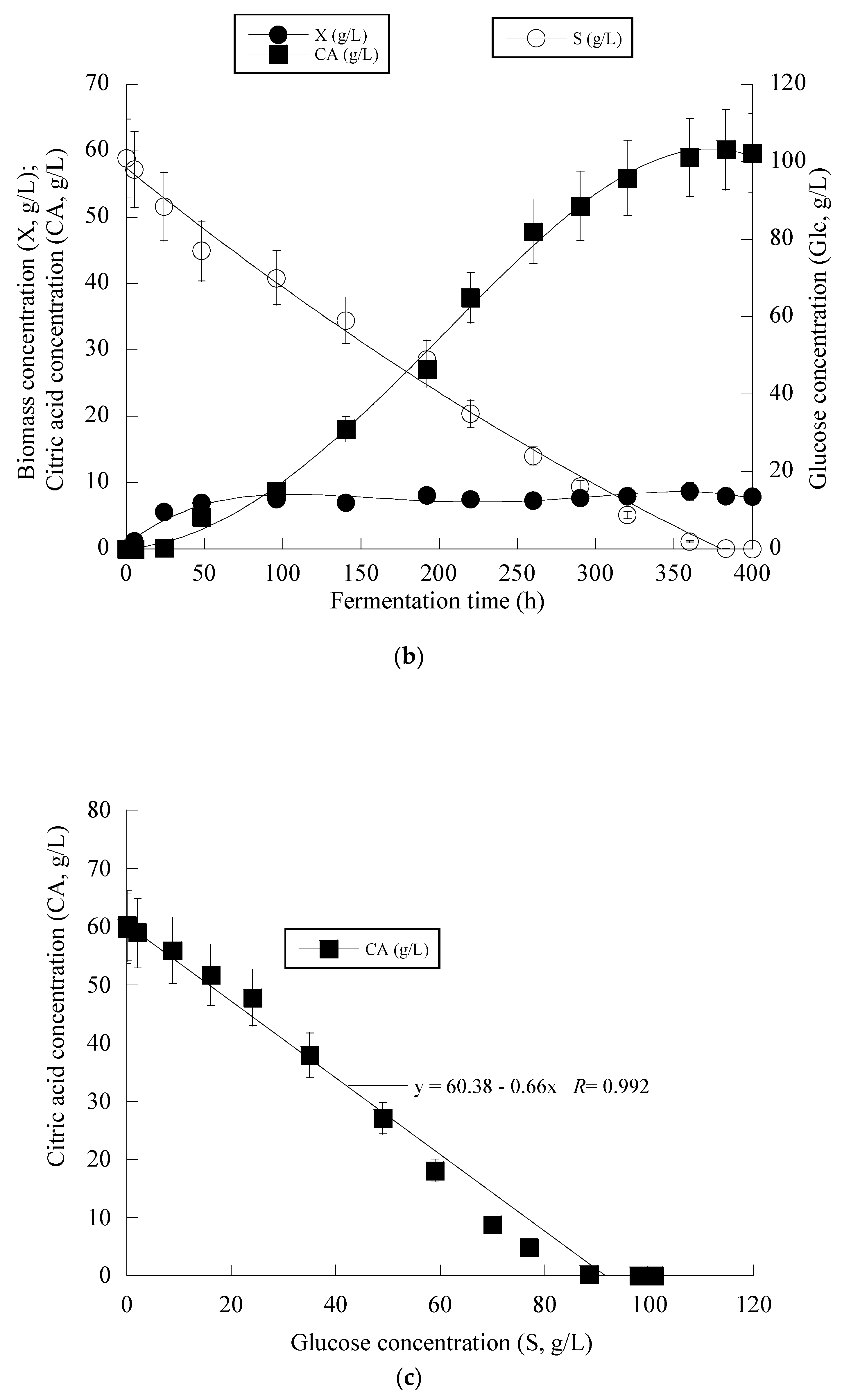
3. Results
4. Discussion
5. Conclusions/Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- United Nations. The 2030 Agenda for Sustainable Development; United Nations: San Francisco, CA, USA, 2023. [Google Scholar]
- Council of European Unior. Fit for 55. Available online: https://www.consilium.europa.eu/en/policies/green-deal/fit-for-55-the-eu-plan-for-a-green-transition/ (accessed on 27 August 2023).
- Tsouko, E.; Papadaki, A.; Papapostolou, H.; Ladakis, D.; Natsia, A.; Koutinas, A.; Kampioti, A.; Eriotou, E.; Kopsahelis, N. Valorization of Zante Currant Side-streams for the Production of Phenolic-rich Extract and Bacterial Cellulose: A Novel Biorefinery Concept. J. Chem. Technol. Biotechnol. 2020, 95, 427–438. [Google Scholar] [CrossRef]
- Pilafidis, S.; Diamantopoulou, P.; Gkatzionis, K.; Sarris, D. Valorization of Agro-Industrial Wastes and Residues through the Production of Bioactive Compounds by Macrofungi in Liquid State Cultures: Growing Circular Economy. Appl. Sci. 2022, 12, 11426. [Google Scholar] [CrossRef]
- Cavallo, E.; Nobile, M.; Cerrutti, P.; Foresti, M.L. Exploring the Production of Citric Acid with Yarrowia lipolytica Using Corn Wet Milling Products as Alternative Low-Cost Fermentation Media. Biochem. Eng. J. 2020, 155, 107463. [Google Scholar] [CrossRef]
- Data Bridgge, Market Research Global Citric Acid Market—Industry Trends and Forecast to 2029. Available online: https://www.databridgemarketresearch.com/reports/global-citric-acid-market (accessed on 25 June 2023).
- Vandenberghe, L.P.S.; Rodrigues, C.; Carvalho, J.C.; Medeiros, A.B.P.; Soccol, C.R. Production and Application of Citric Acid. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 557–575. ISBN 978-0-444-63662-1. [Google Scholar]
- Ajala, A.S.; Adeoye, A.O.; Olaniyan, S.A.; Fasonyin, O.T. A Study on Effect of Fermentation Conditions on Citric Acid Production from Cassava Peels. Sci. Afr. 2020, 8, e00396. [Google Scholar] [CrossRef]
- Morgunov, I.; Kamzolova, S.; Lunina, J. Citric Acid Production by Yarrowia lipolytica Yeast on Different Renewable Raw Materials. Fermentation 2018, 4, 36. [Google Scholar] [CrossRef]
- Soong, Y.V.; Liu, N.; Yoon, S.; Lawton, C.; Xie, D. Cellular and Metabolic Engineering of Oleaginous Yeast Yarrowia lipolytica for Bioconversion of Hydrophobic Substrates into High-value Products. Eng. Life Sci. 2019, 19, 423–443. [Google Scholar] [CrossRef]
- Sarris, D.; Stoforos, N.G.; Mallouchos, A.; Kookos, I.K.; Koutinas, A.A.; Aggelis, G.; Papanikolaou, S. Production of Added-Value Metabolites by Yarrowia lipolytica Growing in Olive Mill Wastewater-Based Media under Aseptic and Non-Aseptic Conditions. Eng. Life Sci. 2017, 17, 695–709. [Google Scholar] [CrossRef]
- Cavallo, E.; Charreau, H.; Cerrutti, P.; Foresti, M.L. Yarrowia lipolytica: A Model Yeast for Citric Acid Production. FEMS Yeast Res. 2017, 17, fox084. [Google Scholar] [CrossRef] [PubMed]
- Rakicka, M.; Wolniak, J.; Lazar, Z.; Rymowicz, W. Production of High Titer of Citric Acid from Inulin. BMC Biotechnol. 2019, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Sarris, D.; Rapti, A.; Papafotis, N.; Koutinas, A.A.; Papanikolaou, S. Production of Added-Value Chemical Compounds through Bioconversions of Olive-Mill Wastewaters Blended with Crude Glycerol by a Yarrowia lipolytica Strain. Molecules 2019, 24, 222. [Google Scholar] [CrossRef] [PubMed]
- Hamimed, S.; Landoulsi, A.; Chatti, A. The Bright Side of Olive Mill Wastewater: Valuables Bioproducts after Bioremediation. Int. J. Environ. Sci. Technol. 2021, 18, 4053–4074. [Google Scholar] [CrossRef]
- Nikolaou, A.; Kourkoutas, Y. Exploitation of Olive Oil Mill Wastewaters and Molasses for Ethanol Production Using Immobilized Cells of Saccharomyces cerevisiae. Environ. Sci. Pollut. Res. 2018, 25, 7401–7408. [Google Scholar] [CrossRef] [PubMed]
- Sarris, D.; Tsouko, E.; Kothri, M.; Anagnostou, M.; Karageorgiou, E.; Papanikolaou, S. Upgrading Major Waste Streams Derived from the Biodiesel Industry and Olive Mills via Microbial Bioprocessing with Non-Conventional Yarrowia lipolytica Strains. Fermentation 2023, 9, 251. [Google Scholar] [CrossRef]
- Diamantopoulou, P.; Gardeli, C.; Papanikolaou, S. Impact of Olive Mill Wastewaters on the Physiological Behavior of a Wild-Type New Ganoderma resinaceum Isolate. Environ. Sci. Pollut. Res. 2021, 28, 20570–20585. [Google Scholar] [CrossRef]
- Foti, P.; Romeo, F.V.; Russo, N.; Pino, A.; Vaccalluzzo, A.; Caggia, C.; Randazzo, C.L. Olive Mill Wastewater as Renewable Raw Materials to Generate High Added-Value Ingredients for Agro-Food Industries. Appl. Sci. 2021, 11, 7511. [Google Scholar] [CrossRef]
- Crognale, S.; Federici, F.; Petruccioli, M. β-Glucan Production by Botryosphaeria rhodina on Undiluted Olive-Mill Wastewaters. Biotechnol. Lett. 2003, 25, 2013–2015. [Google Scholar] [CrossRef] [PubMed]
- Crognale, S.; D’Annibale, A.; Federici, F.; Fenice, M.; Quaratino, D.; Petruccioli, M. Olive Oil Mill Wastewater Valorisation by Fungi. J. Chem. Technol. Biotechnol. 2006, 81, 1547–1555. [Google Scholar] [CrossRef]
- Sarris, D.; Galiotou-Panayotou, M.; Koutinas, A.A.; Komaitis, M.; Papanikolaou, S. Citric Acid, Biomass and Cellular Lipid Production by Yarrowia lipolytica Strains Cultivated on Olive Mill Wastewater-based Media. J. Chem. Technol. Biotechnol. 2011, 86, 1439–1448. [Google Scholar] [CrossRef]
- Diamantis, I.; Melanouri, E.-M.; Dedousi, M.; Panagopoulou, I.; Papanikolaou, S.; Stoforos, N.G.; Diamantopoulou, P. Sustainable and Eco-Friendly Conversions of Olive Mill Wastewater-Based Media by Pleurotus pulmonarius Cultures. Fermentation 2022, 8, 129. [Google Scholar] [CrossRef]
- Sarris, D.; Matsakas, L.; Aggelis, G.; Koutinas, A.A.; Papanikolaou, S. Aerated vs Non-Aerated Conversions of Molasses and Olive Mill Wastewaters Blends into Bioethanol by Saccharomyces cerevisiae under Non-Aseptic Conditions. Ind. Crops Prod. 2014, 56, 83–93. [Google Scholar] [CrossRef]
- Tzirita, M.; Kremmyda, M.; Sarris, D.; Koutinas, A.A.; Papanikolaou, S. Effect of Salt Addition upon the Production of Metabolic Compounds by Yarrowia lipolytica Cultivated on Biodiesel-Derived Glycerol Diluted with Olive-Mill Wastewaters. Energies 2019, 12, 3649. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Galiotou-Panayotou, M.; Fakas, S.; Komaitis, M.; Aggelis, G. Citric Acid Production by Yarrowia lipolytica Cultivated on Olive-Mill Wastewater-Based Media. Bioresour. Technol. 2008, 99, 2419–2428. [Google Scholar] [CrossRef]
- Valdés, G.; Mendonça, R.T.; Aggelis, G. Lignocellulosic Biomass as a Substrate for Oleaginous Microorganisms: A Review. Appl. Sci. 2020, 10, 7698. [Google Scholar] [CrossRef]
- Tryfinopoulou, P.; Tsakalidou, E.; Nychas, G.-J. Characterization of Pseudomonas Spp. Associated with Spoilage of Gilt-Head Sea Bream Stored under Various Conditions. Appl. Environ. Microbiol. 2002, 68, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Paramithiotis, S.; Müller, M.R.A.; Ehrmann, M.A.; Tsakalidou, E.; Seiler, H.; Vogel, R.; Kalantzopoulos, G. Polyphasic Identification of Wild Yeast Strains Isolated from Greek Sourdoughs. Syst. Appl. Microbiol. 2000, 23, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Filippousi, R.; Tsouko, E.; Mordini, K.; Ladakis, D.; Koutinas, A.A.; Aggelis, G.; Papanikolaou, S. Sustainable Arabitol Production by a Newly Isolated Debaryomyces prosopidis Strain Cultivated on Biodiesel-Derived Glycerol. Carbon Resour. Convers. 2022, 5, 92–99. [Google Scholar] [CrossRef]
- Sarantou, S.; Stoforos, N.G.; Kalantzi, O.; Papanikolaou, S. Biotechnological Valorization of Biodiesel-derived Glycerol: Trials with the Non-conventional Yeasts Yarrowia lipolytica and Rhodosporidium sp. Carbon Resour. Convers. 2021, 4, 61–75. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Muniglia, L.; Chevalot, I.; Aggelis, G.; Marc, I. Yarrowia lipolytica as a Potential Producer of Citric Acid from Raw Glycerol. J. Appl. Microbiol. 2002, 92, 737–744. [Google Scholar] [CrossRef]
- Tsouko, E.; Papadaki, A.; Papanikolaou, S.; Danezis, G.P.; Georgiou, C.A.; Freire, D.M.G.; Koutinas, A. Enzymatic Production of Isopropyl and 2-Ethylhexyl Esters Using γ-Linolenic Acid Rich Fungal Oil Produced from Spent Sulphite Liquor. Biochem. Eng. J. 2021, 169, 107956. [Google Scholar] [CrossRef]
- Bellou, S.; Triantaphyllidou, I.-E.; Aggeli, D.; Elazzazy, A.M.; Baeshen, M.N.; Aggelis, G. Microbial Oils as Food Additives: Recent Approaches for Improving Microbial Oil Production and Its Polyunsaturated Fatty Acid Content. Curr. Opin. Biotechnol. 2016, 37, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Koukoumaki, D.I.; Tsouko, E.; Papanikolaou, S.; Ioannou, Z.; Diamantopoulou, P.; Sarris, D. Recent Advances in the Production of Single Cell Protein from Renewable Resources and Applications. Carbon Resour. Convers. 2023, in press. [CrossRef]
- Koutrotsios, G.; Kalogeropoulos, N.; Kaliora, A.C.; Zervakis, G.I. Toward an Increased Functionality in Oyster (Pleurotus) Mushrooms Produced on Grape Marc or Olive Mill Wastes Serving as Sources of Bioactive Compounds. J. Agric. Food Chem. 2018, 66, 5971–5983. [Google Scholar] [CrossRef] [PubMed]
- Khdair, A.; Abu-Rumman, G. Sustainable Environmental Management and Valorization Options for Olive Mill Byproducts in the Middle East and North Africa (MENA) Region. Processes 2020, 8, 671. [Google Scholar] [CrossRef]
- Lopes, M.; Araujo, C.; Aguedo, M.; Gomes, N.; Goncalves, C.; Teixeira, J.A.; Belo, I. The Use of Olive Mill Wastewater by Wild Type Yarrowia lipolytica Strains: Medium Supplementation and Surfactant Presence Effect. J. Chem. Technol. Biotechnol. 2009, 84, 533–537. [Google Scholar] [CrossRef]
- Vastaroucha, E.-S.; Maina, S.; Michou, S.; Kalantzi, O.; Pateraki, C.; Koutinas, A.A.; Papanikolaou, S. Bioconversions of Biodiesel-Derived Glycerol into Sugar Alcohols by Newly Isolated Wild-Type Yarrowia lipolytica Strains. Reactions 2021, 2, 499–513. [Google Scholar] [CrossRef]
- Fickers, P.; Cheng, H.; Sze Ki Lin, C. Sugar Alcohols and Organic Acids Synthesis in Yarrowia lipolytica: Where Are We? Microorganisms 2020, 8, 574. [Google Scholar] [CrossRef] [PubMed]
- Carsanba, E.; Papanikolaou, S.; Fickers, P.; Erten, H. Lipids by Yarrowia lipolytica Strains Cultivated on Glucose in Batch Cultures. Microorganisms 2020, 8, 1054. [Google Scholar] [CrossRef]
- Carsanba, E.; Papanikolaou, S.; Fickers, P.; Agirman, B.; Erten, H. Citric Acid Production by Yarrowia lipolytica. In Non-conventional Yeasts: From Basic Research to Application; Sibirny, A., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 91–117. ISBN 978-3-030-21110-3. [Google Scholar]
- Liu, J.; Li, J.; Shin, H.; Liu, L.; Du, G.; Chen, J. Protein and Metabolic Engineering for the Production of Organic Acids. Bioresour. Technol. 2017, 239, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.; Lopes, M.; Mota, M.; Belo, I. Oxygen Transfer Rate and PH as Major Operating Parameters of Citric Acid Production from Glycerol by Yarrowia lipolytica W29 and CBS 2073. Chem. Pap. 2016, 70, 869–876. [Google Scholar] [CrossRef]
- Levinson, W.E.; Kurtzman, C.P.; Kuo, T.M. Characterization of Yarrowia lipolytica and Related Species for Citric Acid Production from Glycerol. Enzyme Microb. Technol. 2007, 41, 292–295. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Morgunov, I.G. Metabolic Peculiarities of the Citric Acid Overproduction from Glucose in Yeasts Yarrowia lipolytica. Bioresour. Technol. 2017, 243, 433–440. [Google Scholar] [CrossRef]
- Zhang, S.; Jagtap, S.S.; Deewan, A.; Rao, C.V. pH Selectively Regulates Citric Acid and Lipid Production in Yarrowia lipolytica W29 during Nitrogen-Limited Growth on Glucose. J. Biotechnol. 2019, 290, 10–15. [Google Scholar] [CrossRef]
- Moeller, L.; Grünberg, M.; Zehnsdorf, A.; Strehlitz, B.; Bley, T. Biosensor Online Control of Citric Acid Production from Glucose by Yarrowia lipolytica Using Semicontinuous Fermentation. Eng. Life Sci. 2010, 10, 311–320. [Google Scholar] [CrossRef]
- Moeller, L.; Grünberg, M.; Zehnsdorf, A.; Aurich, A.; Bley, T.; Strehlitz, B. Repeated Fed-Batch Fermentation Using Biosensor Online Control for Citric Acid Production by Yarrowia lipolytica. J. Biotechnol. 2011, 153, 133–137. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Diamantopoulou, P.; Blanchard, F.; Lambrinea, E.; Chevalot, I.; Stoforos, N.G.; Rondags, E. Physiological Characterization of a Novel Wild-Type Yarrowia lipolytica Strain Grown on Glycerol: Effects of Cultivation Conditions and Mode on Polyols and Citric Acid Production. Appl. Sci. 2020, 10, 7373. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Morgunov, I.G.; Aurich, A.; Perevoznikova, O.A.; Shishkanova, N.V.; Stottmeister, U.; Finogenova, T.V. Lipase Secretion and Citric Acid Production in Yarrowia lipolytica Yeast Grown on Animal and Vegetable Fat. Food Technol. Biotechnol. 2005, 43, 113–122. [Google Scholar]
- Förster, A.; Aurich, A.; Mauersberger, S.; Barth, G. Citric Acid Production from Sucrose Using a Recombinant Strain of the Yeast Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2007, 75, 1409–1417. [Google Scholar] [CrossRef]
- Rywinska, A.; Rymowicz, W.; Marcinkiewicz, M. Valorization of Raw Glycerol for Citric Acid Production by Yarrowia lipolytica Yeast. Electron. J. Biotechnol. 2010, 13, 9–10. [Google Scholar] [CrossRef]
- Madzak, C. Yarrowia lipolytica Strains and Their Biotechnological Applications: How Natural Biodiversity and Metabolic Engineering Could Contribute to Cell Factories Improvement. J. Fungi 2021, 7, 548. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, S.; Kampisopoulou, E.; Blanchard, F.; Rondags, E.; Gardeli, C.; Koutinas, A.A.; Chevalot, I.; Aggelis, G. Production of Secondary Metabolites through Glycerol Fermentation under Carbon-Excess Conditions by the Yeasts Yarrowia lipolytica and Rhodosporidium toruloides. Eur. J. Lipid Sci. Technol. 2017, 119, 1600507. [Google Scholar] [CrossRef]
- Giacomobono, R.; Albergo, R.; Valerio, V.; Caporusso, A.; De Bari, I. Modelling of the Citric Acid Production from Crude Glycerol by Wild-Type Yarrowia lipolytica DSM 8218 Using Response Surface Methodology (RSM). Life 2022, 12, 621. [Google Scholar] [CrossRef]
- Liu, X.; Lv, J.; Zhang, T.; Deng, Y. Citric Acid Production from Hydrolysate of Pretreated Straw Cellulose by Yarrowia lipolytica SWJ-1b Using Batch and Fed-Batch Cultivation. Prep. Biochem. Biotechnol. 2015, 45, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lv, J.; Xu, J.; Xia, J.; He, A.; Zhang, T.; Li, X.; Xu, J. Effects of Osmotic Pressure and pH on Citric Acid and Erythritol Production from Waste Cooking Oil by Yarrowia lipolytica. Eng. Life Sci. 2018, 18, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Imandi, S.B.; Bandaru, V.V.R.; Somalanka, S.R.; Bandaru, S.R.; Garapati, H.R. Application of Statistical Experimental Designs for the Optimization of Medium Constituents for the Production of Citric Acid from Pineapple Waste. Bioresour. Technol. 2008, 99, 4445–4450. [Google Scholar] [CrossRef]
- Magdouli, S.; Guedri, T.; Tarek, R.; Brar, S.K.; Blais, J.F. Valorization of Raw Glycerol and Crustacean Waste into Value Added Products by Yarrowia lipolytica. Bioresour. Technol. 2017, 243, 57–68. [Google Scholar] [CrossRef]
- Yalcin, S.K.; Bozdemir, M.T.; Ozbas, Z.Y. Utilization of Whey and Grape Must for Citric Acid Production by Two Yarrowia lipolytica Strains. Food Biotechnol. 2009, 23, 266–283. [Google Scholar] [CrossRef]
- Arslan, N.P.; Aydogan, M.N.; Taskin, M. Citric Acid Production from Partly Deproteinized Whey under Non-Sterile Culture Conditions Using Immobilized Cells of Lactose-Positive and Cold-Adapted Yarrowia lipolytica B9. J. Biotechnol. 2016, 231, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Rymowicz, W.; Fatykhova, A.R.; Kamzolova, S.V.; Rywińska, A.; Morgunov, I. Citric acid production from glycerol-containing waste of biodiesel industry by Yarrowia lipolytica in batch, repeated batch, and cell recycle regimes. Appl Microbiol Biotechnol 2010, 87, 971–979. [Google Scholar] [CrossRef]
- Rywińska, A.; Rymowicz, W. High-yield production of citric acid by Yarrowia lipolytica on glycerol in repeated-batch bioreactors. J. Ind. Microbiol. Biotechnol. 2010, 37, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Tsouko, E.; Papanikolaou, S.; Koutinas, A.A. Production of Fuels from Microbial Oil Using Oleaginous Microorganisms. In Handbook of Biofuels Production; Elsevier: Amsterdam, The Netherlands, 2016; pp. 201–236. ISBN 978-0-08-100455-5. [Google Scholar]
- Bhutada, G.; Kavšček, M.; Ledesma-Amaro, R.; Thomas, S.; Rechberger, G.N.; Nicaud, J.-M.; Natter, K. Sugar versus Fat: Elimination of Glycogen Storage Improves Lipid Accumulation in Yarrowia lipolytica. FEMS Yeast Res. 2017, 17, fox020. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Zhang, X.; Gong, Z.; Wang, Y.; Yu, X.; Yang, X.; Zhao, Z.K. Compositional Profiles of Rhodosporidium toruloides Cells under Nutrient Limitation. Appl. Microbiol. Biotechnol. 2017, 101, 3801–3809. [Google Scholar] [CrossRef] [PubMed]
- Dourou, M.; Mizerakis, P.; Papanikolaou, S.; Aggelis, G. Storage Lipid and Polysaccharide Metabolism in Yarrowia lipolytica and Umbelopsis isabellina. Appl. Microbiol. Biotechnol. 2017, 101, 7213–7226. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, R.; Dufreche, S.; Zappi, M.; Bajpai, R. Microbial Lipids from Renewable Resources: Production and Characterization. J. Ind. Microbiol. Biotechnol. 2010, 37, 1271–1287. [Google Scholar] [CrossRef]
- Diamantopoulou, P.; Papanikolaou, S.; Kapoti, M.; Komaitis, M.; Aggelis, G.; Philippoussis, A. Mushroom Polysaccharides and Lipids Synthesized in Liquid Agitated and Static Cultures. Part I: Screening Various Mushroom Species. Appl. Biochem. Biotechnol. 2012, 167, 536–551. [Google Scholar] [CrossRef] [PubMed]
- Diamantopoulou, P.; Papanikolaou, S.; Aggelis, G.; Philippoussis, A. Adaptation of Volvariella volvacea Metabolism in High Carbon to Nitrogen Ratio Media. Food Chem. 2016, 196, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Tchakouteu, S.S.; Chatzifragkou, A.; Kalantzi, O.; Koutinas, A.A.; Aggelis, G.; Papanikolaou, S. Oleaginous Yeast Cryptococcus curvatus Exhibits Interplay between Biosynthesis of Intracellular Sugars and Lipids. Eur. J. Lipid Sci. Technol. 2015, 117, 657–672. [Google Scholar] [CrossRef]
- Diamantopoulou, P.; Filippousi, R.; Antoniou, D.; Varfi, E.; Xenopoulos, E.; Sarris, D.; Papanikolaou, S. Production of Added-Value Microbial Metabolites during Growth of Yeast Strains on Media Composed of Biodiesel-Derived Crude Glycerol and Glycerol/Xylose Blends. FEMS Microbiol. Lett. 2020, 367, fnaa063. [Google Scholar] [CrossRef] [PubMed]
- Fountoulakis, M.S.; Dokianakis, S.N.; Kornaros, M.E.; Aggelis, G.G.; Lyberatos, G. Removal of Phenolics in Olive Mill Wastewaters Using the White-Rot Fungus Pleurotus ostreatus. Water Res. 2002, 36, 4735–4744. [Google Scholar] [CrossRef]


| Strain | Point | Time (h) | Scons (g/L) | X (g/L) | L (g/L) | IPs (g/L) | YIPs/X (g/g) | YL/X (g/g) |
|---|---|---|---|---|---|---|---|---|
| ACA-YC 5033 | a | 24 | 6.1 ± 1.7 | 4.8 ± 0.9 | 0.7 ± 0.2 | 1.6 ± 0.7 | 0.34 | 0.15 |
| b | 195 | 50.4 ± 1.1 | 7.1 ± 1.4 | 0.6 ± 0.2 | 2.2 ± 0.5 | 0.31 | 0.08 | |
| LMBF Y-46 | a | 120 | 42.9 ± 2.4 | 9.5 ± 1.8 | 2.5 ± 0.4 | 3.3 ± 0.4 | 0.35 | 0.26 |
| b | 170 | 47.1 ± 2.1 | 11.4 ± 1.9 | 1.8 ± 0.4 | 3.9 ± 0.9 | 0.34 | 0.16 | |
| LMBF Y-47 | a | 152 | 44.1 ± 2.1 | 11.4 ± 1.8 | 3.4 ± 1.0 | 2.9 ± 0.8 | 0.25 | 0.30 |
| b | 172 | 48.2 ± 2.3 | 12.4 ± 2.0 | 3.5 ± 0.9 | 2.7 ± 0.8 | 0.22 | 0.28 |
| Yeast Strain | C16:0 | C18:0 | C18:1 | C18:2 |
|---|---|---|---|---|
| Y. lipolytica ACA-YC 5033 | 16.1 ± 1.9 | 4.5 ± 0.6 | 53.1 ± 3.6 | 12.7 ± 2.4 |
| Y. lipolytica LMBF Y-46 | 11.1 ± 1.9 | 5.0 ± 1.6 | 41.2 ± 2.9 | 22.4 ± 1.9 |
| Y. lipolytica LMBF Y-47 | 25.9 ± 2.9 | 6.4 ± 1.3 | 46.9 ± 2.5 | 15.1 ± 2.9 |
| Phen0 (g/L) | Time (h) | X (g/L) | L (g/L) | IPs (g/L) | CA (g/L) | YX/S (g/g) | YL/X (g/g) | YIPs/X (g/g) | YCA/S (g/g) | Productivity (g/L/h) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a | 0 | 120 | 7.5 ± 0.2 | 0.7 ± 0.1 | 1.2 ± 0.2 | 11.8 ± 0.1 | 0.21 | 0.09 | 0.16 | 0.33 | |
| b | 0 | 168 | 7.9 ± 0.4 | 0.3 ± 0.1 | 1.2 ± 0.1 | 18.9 ± 0.9 | 0.20 | 0.04 | 0.15 | 0.49 | 0.113 |
| a | ≈1.0 | 96 | 7.2 ± 0.4 | 0.7 ± 0.1 | 1.2 ± 0.2 | 24.9 ± 0.8 | 0.20 | 0.10 | 0.16 | 0.70 | |
| b | ≈1.0 | 120 | 7.2 ± 0.6 | 0.2 ± 0.1 | 1.3 ± 0.1 | 25.8 ± 0.9 | 0.18 | 0.03 | 0.18 | 0.63 | 0.215 |
| a | ≈2.0 | 120 | 7.3 ± 0.5 | 0.4 ± 0.1 | 1.3 ± 0.1 | 12.3 ± 0.5 | 0.22 | 0.05 | 0.18 | 0.37 | |
| b | ≈2.0 | 192 | 6.2 ± 0.6 | 0.4 ± 0.1 | 1.0 ± 0.2 | 20.8 ± 1.1 | 0.16 | 0.06 | 0.16 | 0.54 | 0.108 |
| Phen0 (g/L) | Time (h) | X (g/L) | L (g/L) | IPs (g/L) | YX/S (g/g) | YL/X (g/g) | YIPs/X (g/g) |
|---|---|---|---|---|---|---|---|
| 0 | 144 | 15.7 ± 0.5 | 0.3 ± 0.1 | 2.5 ± 0.8 | 0.38 | 0.02 | 0.16 |
| ≈1.0 | 120 | 25.3 ± 0.8 | 0.5 ± 0.1 | 3.3 ± 0.1 | 0.60 | 0.02 | 0.13 |
| ≈2.0 | 96 | 22.9 ± 0.9 | 0.4 ± 0.1 | 3.9 ± 0.2 | 0.60 | 0.02 | 0.17 |
| Fatty Acid Methyl Esters (% w/w) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Phen0 (g/L) | Time (h) | C16:0 | C16:1 | C18:0 | C18:1 | C18:2 | RSU | |
| C/N ≈ 114 | 0 | 48 | 14.8 ± 0.3 | 9.9 ± 0.2 | 7.8 ± 0.2 | 56.8 ± 0.8 | 10.7 ± 0.2 | 0.3 |
| 72 | 18.6 ± 0.5 | 7.2 ± 0.3 | 7.8 ± 0.2 | 54.9 ± 0.7 | 11.5 ± 0.4 | 0.4 | ||
| 144 | 14.5 ± 0.6 | 10.0 ± 0.4 | 4.7 ± 0.2 | 60.2 ± 0.4 | 10.5 ± 0.1 | 0.2 | ||
| ≈1.0 | 48 | 14.8 ± 0.2 | 8.0 ± 0.2 | 4.9 ± 0.1 | 57.9 ± 0.3 | 14.4 ± 0.4 | 0.2 | |
| 72 | 17.3 ± 0.5 | 10.0 ± 0.4 | 4.2 ± 0.1 | 54.1 ± 0.5 | 14.3 ± 0.4 | 0.3 | ||
| 120 | 15.7 ± 0.5 | 8.1 ± 0.3 | 5.1 ± 0.4 | 57.4 ± 0.8 | 13.7 ± 0.1 | 0.3 | ||
| 168 | 21.2 ± 0.3 | 6.4 ± 0.2 | 7.5 ± 0.3 | 52.8 ± 0.4 | 12.1 ± 0.4 | 0.4 | ||
| ≈2.0 | 48 | 11.5 ± 0.2 | 3.0 ± 0.1 | 4.3 ± 0.2 | 62.9 ± 0.5 | 18.3 ± 0.4 | 0.2 | |
| 96 | 12.3 ± 0.1 | 4.3 ± 0.1 | 5.1 ± 0.2 | 63.7 ± 0.8 | 14.6 ± 0.3 | 0.2 | ||
| 144 | 12.1 ± 0.3 | 3.4 ± 0.3 | 3.9 ± 0.1 | 66.2 ± 0.5 | 14.4 ± 0.5 | 0.2 | ||
| 192 | 14.7 ± 0.2 | 4.6 ± 0.1 | 5.1 ± 0.3 | 60.3 ± 0.3 | 15.3 ± 0.4 | 0.2 | ||
| C/N ≈ 16 | 0 | 24 | 16.6 ± 0.4 | 8.6 ± 0.2 | 5.0 ± 0.2 | 52.6 ± 0.2 | 17.2 ± 0.4 | 0.3 |
| 144 | 15.9 ± 0.2 | 8.9 ± 0.4 | 5.0 ± 0.2 | 52.9 ± 0.2 | 17.3 ± 0.2 | 0.3 | ||
| ≈1.0 | 24 | 21.5 ± 0.2 | 2.0 ± 0.1 | 9.2 ± 0.2 | 53.7 ± 0.5 | 13.6 ± 0.5 | 0.4 | |
| 48 | 18.0 ± 0.4 | 8.0 ± 0.2 | 8.2 ± 0.3 | 50.6 ± 0.7 | 15.2 ± 0.2 | 0.4 | ||
| 144 | 19.8 ± 0.4 | 9.8 ± 0.2 | 10.0 ± 0.3 | 48.9 ± 0.5 | 11.5 ± 0.4 | 0.4 | ||
| ≈2.0 | 24 | 10.0 ± 0.4 | 3.5 ± 0.1 | 2.6 ± 0.1 | 70.2 ± 0.7 | 11.6 ± 0.4 | 0.1 | |
| 48 | 8.2 ± 0.1 | 4.2 ± 0.1 | 2.3 ± 0.1 | 67.8 ± 0.8 | 17.4 ± 0.3 | 0.1 | ||
| 96 | 17.0 ± 0.3 | 2.3 ± 0.1 | 4.9 ± 0.1 | 61.4 ± 0.5 | 14.3 ± 0.4 | 0.3 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarris, D.; Tsouko, E.; Photiades, A.; Tchakouteu, S.S.; Diamantopoulou, P.; Papanikolaou, S. Growth Response of Non-Conventional Yeasts on Sugar-Rich Media: Part 2: Citric Acid Production and Circular-Oriented Valorization of Glucose-Enriched Olive Mill Wastewaters Using Novel Yarrowia lipolytica Strains. Microorganisms 2023, 11, 2243. https://doi.org/10.3390/microorganisms11092243
Sarris D, Tsouko E, Photiades A, Tchakouteu SS, Diamantopoulou P, Papanikolaou S. Growth Response of Non-Conventional Yeasts on Sugar-Rich Media: Part 2: Citric Acid Production and Circular-Oriented Valorization of Glucose-Enriched Olive Mill Wastewaters Using Novel Yarrowia lipolytica Strains. Microorganisms. 2023; 11(9):2243. https://doi.org/10.3390/microorganisms11092243
Chicago/Turabian StyleSarris, Dimitris, Erminta Tsouko, Angelos Photiades, Sidoine Sadjeu Tchakouteu, Panagiota Diamantopoulou, and Seraphim Papanikolaou. 2023. "Growth Response of Non-Conventional Yeasts on Sugar-Rich Media: Part 2: Citric Acid Production and Circular-Oriented Valorization of Glucose-Enriched Olive Mill Wastewaters Using Novel Yarrowia lipolytica Strains" Microorganisms 11, no. 9: 2243. https://doi.org/10.3390/microorganisms11092243
APA StyleSarris, D., Tsouko, E., Photiades, A., Tchakouteu, S. S., Diamantopoulou, P., & Papanikolaou, S. (2023). Growth Response of Non-Conventional Yeasts on Sugar-Rich Media: Part 2: Citric Acid Production and Circular-Oriented Valorization of Glucose-Enriched Olive Mill Wastewaters Using Novel Yarrowia lipolytica Strains. Microorganisms, 11(9), 2243. https://doi.org/10.3390/microorganisms11092243









