Mosquito-Borne Diseases Emergence/Resurgence and How to Effectively Control It Biologically
Abstract
1. Introduction
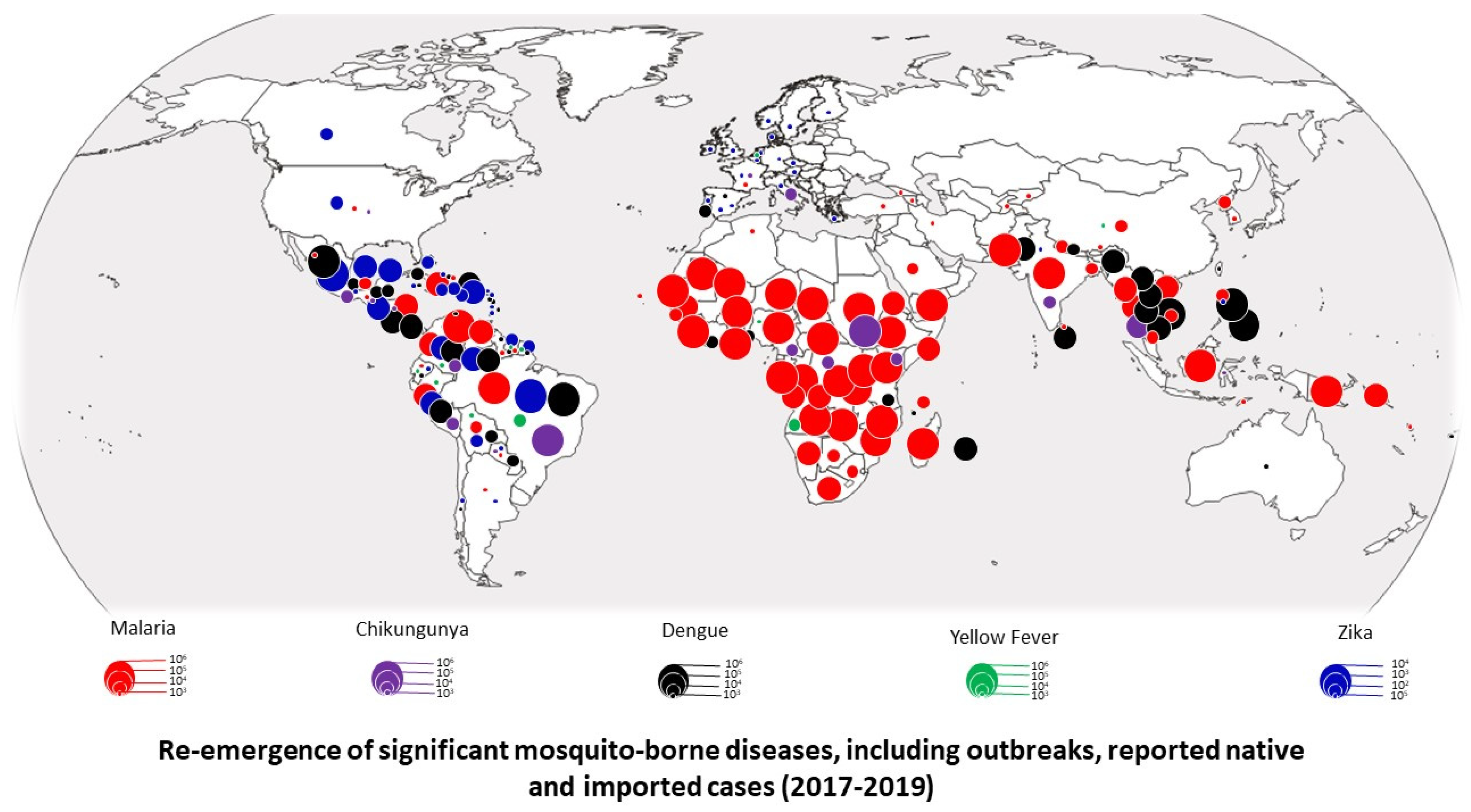
2. Resurgence of Diseases Transmitted by Mosquitoes
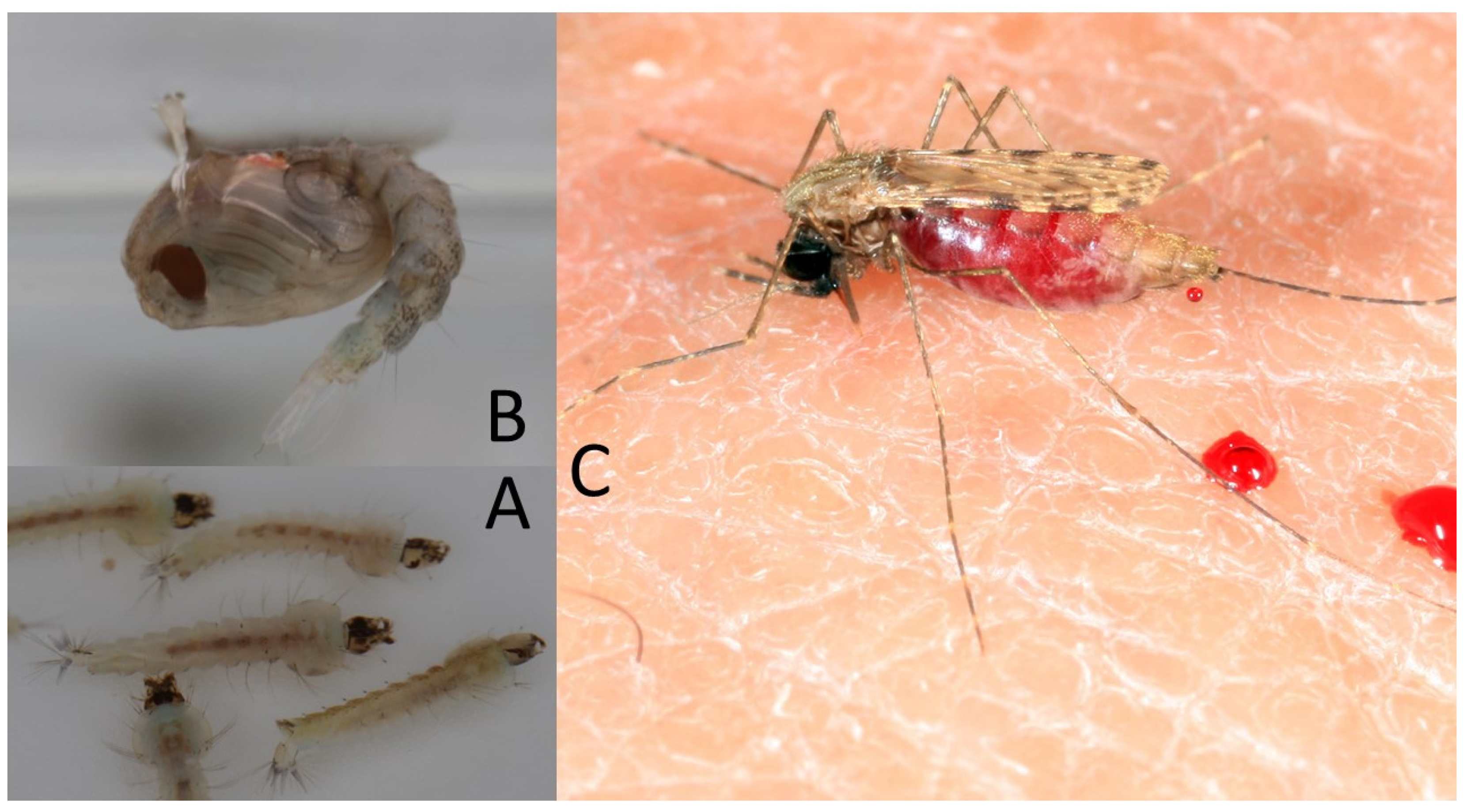
3. Actual Insecticide-Based Vector-Control Strategies
3.1. Indoor Residual Spraying (IRS)
3.2. Peridomestic Space Spraying
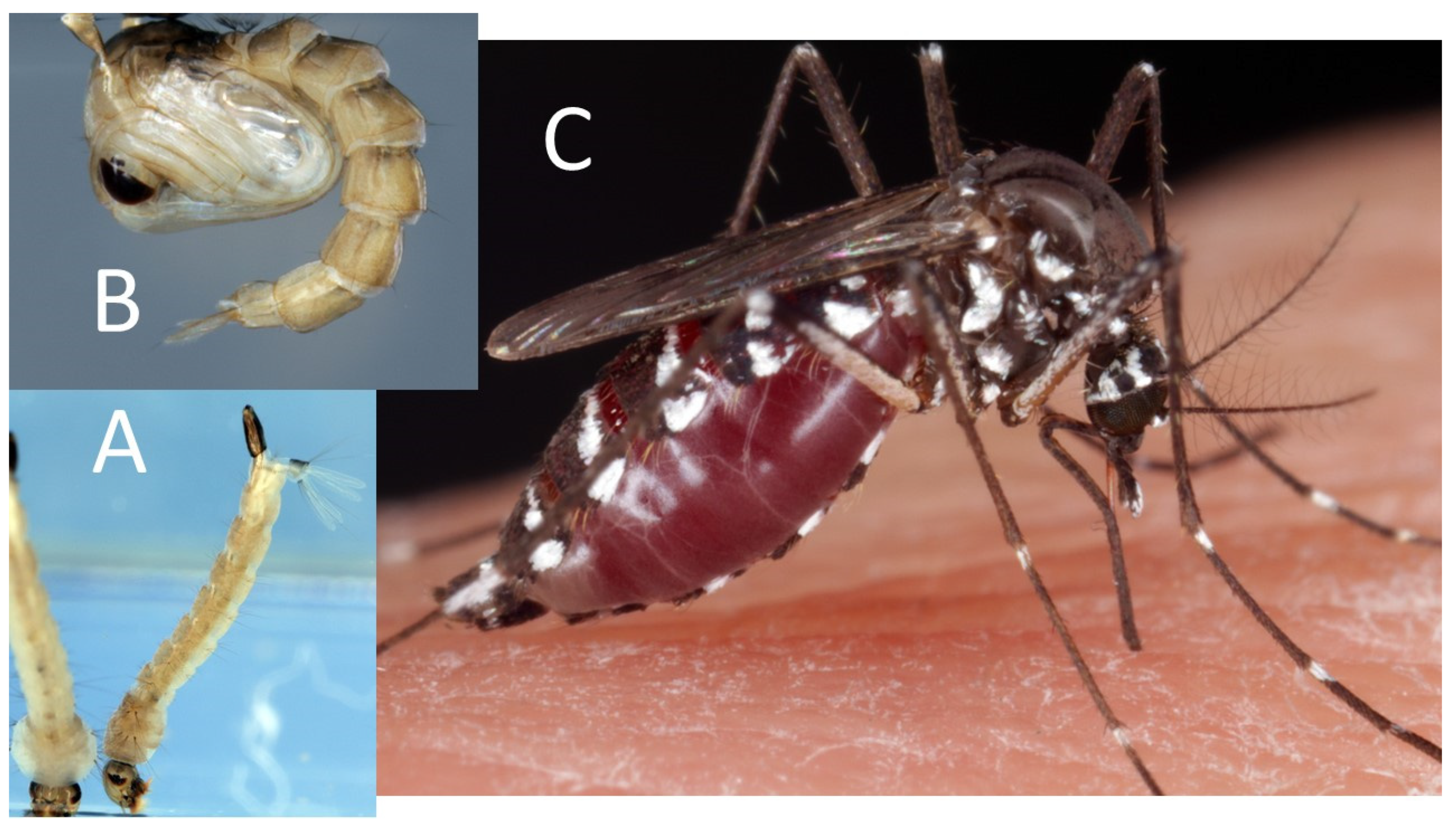
3.3. Long-Lasting Insecticide-Treated Nets (LLINs)
3.4. Mosquito Repellents
4. Biological Control
4.1. Genetic Modification
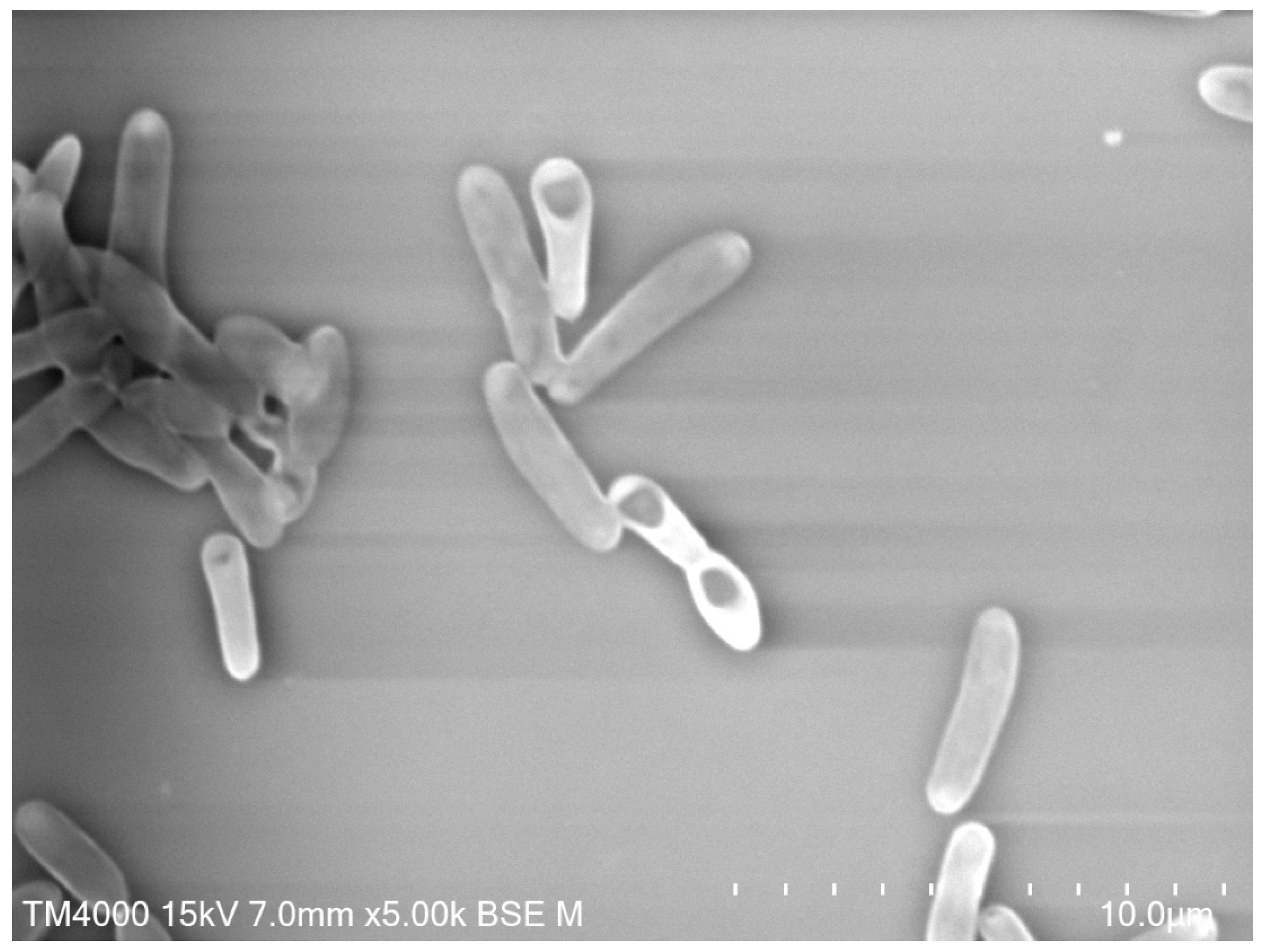
4.2. Fungi
4.3. Control of Aquatic Stages Using Elephant Mosquito and Fish Predators
4.4. Protozoan Control
5. Bacterial Agents Tested or Used in Control Strategies
5.1. Bacillus spp.
5.2. Insect Growth Regulators (IGRs)
5.3. Wolbachia spp.
5.4. Asaia
5.5. Spinosyns
5.6. Bacterial-Based Feeding Deterrents and Repellents
6. Biological Insecticide Resistance
6.1. Resistance to Bti
6.2. Resistance to Bs
6.3. Resistance Management
7. Current Challenges for a Prosperous Future
7.1. New Insecticide, IGR, and Repellent Compounds
7.2. Attractive Toxic Sugar Baits (ATSB)
7.3. Parasitic Nematodes
7.4. Acoustic Larvicides and Traps
7.5. Advanced Genetic Studies
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benelli, G. Research in mosquito control: Current challenges for a brighter future. Parasitol. Res. 2015, 114, 2801–2805. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, A.M. Globalization, land use and the invasion of West Nile virus NIH Public Access. Science 2011, 334, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, D.; Zuniga, R.; Morrison, D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 2005, 52, 273–288. [Google Scholar] [CrossRef]
- Mehlhorn, H. Encyclopedia of Parasitology; Institut für Zoomorphologie, Zellbiologie und Parasitologie: Düsseldorf, Germany, 2016; Volume 1, ISBN 978-3-662-43977-7. [Google Scholar]
- Mehlhorn, H.; Al-Rasheid, K.A.S.; Al-Quraishy, S.; Abdel-Ghaffar, F. Research and increase of expertise in arachno-entomology are urgently needed. Parasitol. Res. 2012, 110, 259–265. [Google Scholar] [CrossRef]
- Hubálek, Z.; Halouzka, J. West Nile fever—A reemerging mosquito-borne viral disease in Europe. Emerg. Infect. Dis. 1999, 5, 643–650. [Google Scholar] [CrossRef]
- Vila, M.; Hulme, P.E. Impact of Biological Invasions on Ecosystem Services; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; ISBN 978-3-319-45121-3. [Google Scholar]
- Shukla, D.; Wijayapala, S.; Vankar, P.S. Effective mosquito repellent from plant based formulation. Int. J. Mosq. Res. 2018, 5, 19–24. [Google Scholar]
- Moyes, C.L.; Vontas, J.; Martins, A.J.; Ng, L.C.; Koou, S.Y.; Dusfour, I.; Raghavendra, K.; Pinto, J.; Corbel, V.; David, J.-P.; et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl. Trop. Dis. 2017, 11, e0005625. [Google Scholar] [CrossRef]
- Toledo Marrelli, M.; Barretto Bruno WILKE, A.; Toledo Marrelli, M. Genetic Control of Mosquitoes: Population suppression strategies. Rev. Inst. Med. Trop. São Paulo 2012, 54, 287–292. [Google Scholar]
- Benelli, G.; Beier, J.C. Current vector control challenges in the fight against malaria. Acta Trop. 2017, 174, 91–96. [Google Scholar] [CrossRef]
- Achee, N.L.; Grieco, J.P.; Vatandoost, H.; Seixas, G.; Pinto, J.; Ching-Ng, L.; Martins, A.J.; Juntarajumnong, W.; Corbel, V.; Gouagna, C.; et al. Alternative strategies for mosquito-borne arbovirus control. PLoS Negl. Trop. Dis. 2019, 13, e0006822. [Google Scholar]
- Omori, N. A review of the role of mosquitoes in the transmission of Malayan and bancroftian filariasis in Japan. Bull. World Health Organ. 1962, 27, 585–594. [Google Scholar] [PubMed]
- Barretto, A.; Wilke, B.; Toledo Marrelli, M. Paratransgenesis: A promising new strategy for mosquito vector control. Parasites Vectors 2015, 8, 342. [Google Scholar]
- World Malaria Report. 2019. Available online: https://www.who.int/news-room/feature-stories/detail/world-malaria-report-2019 (accessed on 26 March 2020).
- Rahmah, Z.; Sasmito, S.D.; Siswanto, B.; Sardjono, T.W.; Fitri, L.E. Malaria. Malays. J. Med. Sci. 2015, 22, 25–32. [Google Scholar] [PubMed]
- Feged-Rivadeneira, A.; Ángel, A.; González-Casabianca, F.; Rivera, C. Malaria intensity in Colombia by regions and populations. PLoS ONE 2018, 13, e0203673. [Google Scholar] [CrossRef]
- Amato, R.; Pearson, R.D.; Almagro-Garcia, J.; Amaratunga, C.; Lim, P.; Suon, S.; Sreng, S.; Drury, E.; Stalker, J.; Miotto, O.; et al. Origins of the current outbreak of multidrug-resistant malaria in southeast Asia: A retrospective genetic study. Lancet Infect. Dis. 2018, 18, 337–345. [Google Scholar] [CrossRef]
- Lok, P.; Dijk, S. Malaria outbreak in Burundi reaches epidemic levels with 5.7 million infected this year. BMJ 2019, 366. [Google Scholar] [CrossRef]
- Erickson, S.M.; Thomsen, E.K.; Keven, J.B.; Vincent, N.; Koimbu, G.; Siba, P.M.; Christensen, B.M.; Reimer, L.J. Mosquito-parasite interactions can shape filariasis transmission dynamics and impact elimination programs. PLoS Negl. Trop. Dis. 2013, 7, e2433. [Google Scholar] [CrossRef]
- Gleave, K.; Cook, D.; Taylor, M.J.; Reimer, L.J. Filarial infection influences mosquito behaviour and fecundity. Sci. Rep. 2016, 6, 36319. [Google Scholar] [CrossRef]
- Ughasi, J.; Bekard, H.E.; Coulibaly, M.; Adabie-Gomez, D.; Gyapong, J.; Appawu, M.; Wilson, M.D.; Boakye, D.A. Mansonia africana and Mansonia uniformis are vectors in the transmission of Wuchereria bancrofti lymphatic filariasis in Ghana. Parasit. Vectors 2012, 5, 89. [Google Scholar] [CrossRef]
- Joseph, H.; Moloney, J.; Maiava, F.; McClintock, S.; Lammie, P.; Melrose, W. First evidence of spatial clustering of lymphatic filariasis in an Aedes polynesiensis endemic area. Acta Trop. 2011, 120, S39–S47. [Google Scholar] [CrossRef] [PubMed]
- Southgate, B.A.; Bryan, J.H. Factors affecting transmission of Wuchereria bancrofti by anopheline mosquitoes. 4. Facilitation, limitation, proportionality and their epidemiological significance. Trans. R Soc. Trop. Med. Hyg. 1992, 86, 523–530. [Google Scholar] [CrossRef]
- Wada, Y. Vector mosquitoes of filariasis in Japan. Trop. Med. Health 2011, 39, 39–45. [Google Scholar] [PubMed]
- Lymphatic Filariasis. Available online: https://www.who.int/news-room/fact-sheets/detail/lymphatic-filariasis (accessed on 26 March 2020).
- Ramos-Castañeda, J.; Barreto Dos Santos, F.; Martínez-Vega, R.; Lio, J.; Galvão De Araujo, M.; Joint, G.; Sarti, E. Dengue in Latin America: Systematic Review of Molecular Epidemiological Trends. PLoS Negl. Trop. Dis. 2017, 11, e0005224. [Google Scholar] [CrossRef] [PubMed]
- Dengue Worldwide Overview. Available online: https://www.ecdc.europa.eu/en/dengue-monthly (accessed on 26 March 2020).
- Katzelnick, L.C.; Coloma, J.; Harris, E. Dengue: Knowledge gaps, unmet needs, and research priorities. Lancet Infect. Dis. 2017, 17, e88–e100. [Google Scholar] [CrossRef]
- Villamil-Gómez, W.E.; Rodríguez-Morales, A.J.; Uribe-García, A.M.; González-Arismendy, E.; Castellanos, J.E.; Calvo, E.P.; Álvarez-Mon, M.; Musso, D. Zika, dengue, and chikungunya co-infection in a pregnant woman from Colombia. Int. J. Infect. Dis. 2016, 51, 135–138. [Google Scholar] [CrossRef]
- Giron, S.; Franke, F.; Decoppet, A.; Cadiou, B.; Travaglini, T.; Thirion, L.; Durand, G.; Jeannin, C.; L’Ambert, G.; Grard, G.; et al. Vector-borne transmission of Zika virus in Europe, southern France, August 2019. Euro Surveill. 2019, 24. Available online: https://doi.org/10.2807/1560-7917.ES.2019.24.45.1900655 (accessed on 20 April 2020). [CrossRef]
- Ruchusatsawat, K.; Wongjaroen, P.; Posanacharoen, A.; Rodriguez-Barraquer, I.; Sangkitporn, S.; Cummings, D.A.; Salje, H. Long-term circulation of Zika virus in Thailand: An observational study. Lancet Infect. Dis. 2019, 19, 439–446. [Google Scholar] [CrossRef]
- Brady, O.J.; Hay, S.I. The first local cases of Zika virus in Europe. Lancet 2019, 394, 1991–1992. [Google Scholar] [CrossRef]
- Ledermann, J.P.; Guillaumot, L.; Yug, L.; Saweyog, S.C.; Tided, M.; Machieng, P.; Pretrick, M.; Marfel, M.; Griggs, A.; Bel, M.; et al. Aedes hensilli as a potential vector of Chikungunya and Zika viruses. PLoS Negl. Trop. Dis. 2014, 8, e3188. [Google Scholar] [CrossRef]
- Diallo, D.; Sall, A.A.; Diagne, C.T.; Faye, O.; Faye, O.; Ba, Y.; Hanley, K.A.; Buenemann, M.; Weaver, S.C.; Diallo, M. Zika virus emergence in mosquitoes in southeastern Senegal, 2011. PLoS ONE 2014, 9, e109442. [Google Scholar] [CrossRef]
- Grard, G.; Caron, M.; Mombo, I.M.; Nkoghe, D.; Mboui Ondo, S.; Jiolle, D.; Fontenille, D.; Paupy, C.; Leroy, E.M. Zika virus in Gabon (Central Africa)—2007: A new threat from Aedes albopictus? PLoS Negl. Trop. Dis. 2014, 8, e2681. [Google Scholar] [CrossRef] [PubMed]
- Song, B.H.; Yun, S.I.; Woolley, M.; Lee, Y.M. Zika virus: History, epidemiology, transmission, and clinical presentation. J. Neuroimmunol. 2017, 308, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Nilles, E.J.; Cao-Lormeau, V.-M. Rapid spread of emerging Zika virus in the Pacific area. Clin. Microbiol. Infect. 2014, 20, O595–O596. [Google Scholar] [CrossRef] [PubMed]
- Edington, F.; Varjão, D.; Melo, P. Incidence of articular pain and arthritis after chikungunya fever in the Americas: A systematic review of the literature and meta-analysis. Jt. Bone Spine 2018, 85, 669–678. [Google Scholar] [CrossRef]
- Spoto, S.; Riva, E.; Fogolari, M.; Cella, E.; Costantino, S.; Angeletti, S.; Ciccozzi, M. Diffuse maculopapular rash: A family cluster during the last Chikungunya virus epidemic in Italy. Clin. Case Rep. 2018, 6, 2322–2325. [Google Scholar] [CrossRef]
- Rahman, M.M.; Jakaria, S.K.; Sayed, B.; Moniruzzaman, M.; Humayon Kabir, A.K.M.; Mallik, M.U.; Hasan, M.R.; Siddique, A.B.; Hossain, M.A.; Uddin, N.; et al. Clinical and Laboratory Characteristics of an Acute Chikungunya Outbreak in Bangladesh in 2017. Am. J. Trop. Med. Hyg. 2018, 100, 405–410. [Google Scholar] [CrossRef]
- Da Silva Junior, G.B.; Pinto, J.R.; Mota, R.M.S.; da Pires Neto, R.J.; Daher, E.D.F. Risk factors for death among patients with Chikungunya virus infection during the outbreak in northeast Brazil, 2016–2017. Trans. R. Soc. Trop. Med. Hyg. 2018, 113, 221–226. [Google Scholar] [CrossRef]
- De Azevedo Fernandes, N.C.C.; Cunha, M.S.; Guerra, J.M.; Réssio, R.A.; Cirqueira, C.D.S.; Iglezias, S.D.; de Carvalho, J.; Araujo, E.L.L.; Catão-Dias, J.L.; Díaz-Delgado, J. Outbreak of Yellow Fever among Nonhuman Primates, Espirito Santo, Brazil, 2017. Emerg. Infect. Dis. 2017, 23, 2038–2041. [Google Scholar] [CrossRef]
- Possas, C.; Lourenço-de-Oliveira, R.; Tauil, P.L.; de Pinheiro, F.P.; Pissinatti, A.; da Cunha, R.V.; Freire, M.; Martins, R.M.; Homma, A. Yellow fever outbreak in Brazil: The puzzle of rapid viral spread and challenges for immunisation. Mem. Inst. Oswaldo Cruz 2018, 113, e180278. [Google Scholar] [CrossRef]
- Simon, L.V.; Hashmi, M.F.; Torp, K.D. Yellow Fever; StatPearls: Tampa/St. Petersburg, FL, USA, 2018. [Google Scholar]
- Nwachukwu, W.E.; Yusuff, H.; Nwangwu, U.; Okon, A.; Ogunniyi, A.; Imuetinyan-Clement, J.; Besong, M.; Ayo-Ajayi, P.; Nikau, J.; Baba, A.; et al. The response to re-emergence of yellow fever in Nigeria, 2017. Int. J. Infect. Dis. 2020, 92, 189–196. [Google Scholar] [CrossRef]
- WHO. Yellow Fever—Nigeria; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Silva, N.I.O.; Sacchetto, L.; De Rezende, I.M.; Trindade, G.D.S.; Labeaud, A.D.; De Thoisy, B.; Drumond, B.P. Recent sylvatic yellow fever virus transmission in Brazil: The news from an old disease. Virol. J. 2020, 17, 9. [Google Scholar] [CrossRef]
- Auguste, A.J.; Lemey, P.; Bergren, N.A.; Giambalvo, D.; Moncada, M.; Morón, D.; Hernandez, R.; Navarro, J.-C.; Weaver, S.C. Enzootic transmission of yellow fever virus, Venezuela. Emerg. Infect. Dis. 2015, 21, 99–102. [Google Scholar] [CrossRef]
- Selemane, I. Epidemiological monitoring of the last outbreak of yellow fever in Brazil—An outlook from Portugal. Travel Med. Infect. Dis. 2019, 28, 46–51. [Google Scholar] [CrossRef]
- Solomon, T.; Hombach, J.; Jacobson, J.; Hoke, C.; Marfin, A.; Campbell, G.; Ginsburg, A.; Fischer, M.; Tsai, T.; Hills, S.; et al. Estimated Global Incidence of Japanese Encephalitis: A Systematic Review. Bull. World Health Organ. 2011, 89, 766–774. [Google Scholar]
- Fang, Y.; Zhang, Y.; Zhou, Z.B.; Xia, S.; Shi, W.Q.; Xue, J.B.; Li, Y.Y.; Wu, J.T. New strains of Japanese encephalitis virus circulating in Shanghai, China after a ten-year hiatus in local mosquito surveillance. Parasites Vectors 2019, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Griesemer, S.B.; Kramer, L.D.; Van Slyke, G.A.; Pata, J.D.; Gohara, D.W.; Cameron, C.E.; Ciota, A.T. Mutagen resistance and mutation restriction of St. Louis encephalitis virus. J. Gen. Virol. 2017, 98, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.; Coffey, L.L.; Burkett-Cadena, N.; Day, J.F. Reemergence of St. Louis encephalitis virus in the Americas. Emerg. Infect. Dis. 2018, 24, 2150–2157. [Google Scholar] [CrossRef]
- Castillo-Olivares, J.; Wood, J. West Nile virus infection of horses. Vet. Res. 2004, 35, 467–483. [Google Scholar] [CrossRef]
- Barrett, A.D.T. West Nile in Europe: An increasing public health problem. J. Travel Med. 2018, 25, tay096. [Google Scholar] [CrossRef] [PubMed]
- López-Ruiz, N.; del Montaño-Remacha, M.C.; Durán-Pla, E.; Pérez-Ruiz, M.; Navarro-Marí, J.M.; Salamanca-Rivera, C.; Miranda, B.; Oyonarte-Gómez, S.; Ruiz-Fernández, J. West nile virus outbreak in humans and epidemiological surveillance, West Andalusia, Spain, 2016. Eurosurveillance 2018, 23, 17-00261. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, G.; Li, J.; Kelly, P.; Li, M.; Wang, J.; Huang, K.; Qiu, H.; You, J.; Zhang, R.; et al. Molecular Detection of Rickettsia felis and Rickettsia bellii in Mosquitoes. Vector Borne Zoonotic Dis. 2019, 19, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.P.; Tian, J.H.; Lin, X.D.; Ni, X.B.; Chen, X.P.; Liao, Y.; Yang, S.Y.; Dumler, J.S.; Holmes, E.C.; Zhang, Y.Z. Extensive genetic diversity of Rickettsiales bacteria in multiple mosquito species. Sci. Rep. 2016, 6, 38770. [Google Scholar] [CrossRef] [PubMed]
- Krajacich, B.J.; Huestis, D.L.; Dao, A.; Yaro, A.S.; Diallo, M.; Krishna, A.; Xu, J.; Lehmann, T. Investigation of the seasonal microbiome of Anopheles coluzzii mosquitoes in Mali. PLoS ONE 2018, 13, e0194899. [Google Scholar] [CrossRef] [PubMed]
- Socolovschi, C.; Pagés, F.; Raoult, D. Rickettsia felis in aedes albopictus mosquitoes, libreville, gabon. Emerg. Infect. Dis. 2012, 18, 1688–1689. [Google Scholar] [CrossRef] [PubMed]
- Dieme, C.; Bechah, Y.; Socolovschi, C.; Audoly, G.; Berenger, J.M.; Faye, O.; Raoult, D.; Parola, P. Transmission potential of rickettsia felis infection by Anopheles gambiae mosquitoes. Proc. Natl. Acad. Sci. USA 2015, 112, 8088–8093. [Google Scholar] [CrossRef]
- Eliasson, H.; Broman, T.; Forsman, M.; Bäck, E. Tularemia: Current Epidemiology and Disease Management. Infect. Dis. Clin. North Am. 2006, 20, 289–311. [Google Scholar] [CrossRef]
- San Martín, J.L.; Brathwaite, O.; Zambrano, B.; Solórzano, J.O.; Bouckenooghe, A.; Dayan, G.H.; Guzmán, M.G. The epidemiology of dengue in the americas over the last three decades: A worrisome reality. Am. J. Trop. Med. Hyg. 2010, 82, 128–135. [Google Scholar] [CrossRef]
- Bouwman, H.; van den Berg, H.; Kylin, H. DDT and Malaria Prevention: Addressing the Paradox. Environ. Health Perspect. 2011, 119, 744–747. [Google Scholar] [CrossRef]
- Cailly, P.; Tran, A.; Balenghien, T.; L’Ambert, G.; Toty, C.; Ezanno, P. A climate-driven abundance model to assess mosquito control strategies. Ecol. Model. 2012, 227, 7–17. [Google Scholar] [CrossRef]
- Hemingway, J.; Beaty, B.J.; Rowland, M.; Scott, T.W.; Sharp, B.L. The Innovative Vector Control Consortium: Improved control of mosquito-borne diseases. Trends Parasitol. 2006, 22, 308–312. [Google Scholar] [CrossRef]
- Oxborough, R.M.; Kitau, J.; Jones, R.; Mosha, F.W.; Rowland, M.W. Experimental hut and bioassay evaluation of the residual activity of a polymer-enhanced suspension concentrate (SC-PE) formulation of deltamethrin for IRS use in the control of Anopheles arabiensis. Parasites Vectors 2014, 7, 454. [Google Scholar] [CrossRef] [PubMed]
- Dzul-Manzanilla, F.; Ibarra-López, J.; Marín, W.B.; Martini-Jaimes, A.; Leyva, J.T.; Correa-Morales, F.; Huerta, H.; Manrique-Saide, P.; Vazquez-Prokopec, G.M.; Day, J. Indoor resting behavior of Aedes aegypti (Diptera: Culicidae) in Acapulco, Mexico. J. Med. Entomol. 2018, 54, 501–504. [Google Scholar]
- Hladish, T.J.; Pearson, C.A.B.; Rojas, D.P.; Gomez-Dantes, H.; Halloran, M.E.; Vazquez-Prokopec, G.M.; Longini, I.M. Forecasting the effectiveness of indoor residual spraying for reducing dengue burden. PLoS Negl. Trop. Dis. 2018, 12, e0006570. [Google Scholar] [CrossRef] [PubMed]
- Giglioli, G. An Investigation of the House-Frequenting Habits of Mosquitoes of the British Guiana Coastland in Relation to the Use of DDT 1. Am. J. Trop. Med. Hyg. 1948, 1, 43–70. [Google Scholar] [CrossRef]
- Nathan, M.B.; Giglioli, M.E. Eradication of Aedes aegypti on Cayman Brac and Little Cayman, West Indies, with Abate (Temephos) in 1970–1971. Bull. Pan Am. Health Organ. 1982, 16, 28–39. [Google Scholar]
- Oxborough, R.M.; Kitau, J.; Jones, R.; Feston, E.; Matowo, J.; Mosha, F.W.; Rowland, M.W. Long-lasting control of Anopheles arabiensis by a single spray application of micro-encapsulated pirimiphos-methyl (Actellic® 300 CS). Malar. J. 2014, 13, 37. [Google Scholar] [CrossRef]
- WHO. Pesticides and Their Application: For the Control of Vectors and Pests of Public Health Importance, 6th ed.; WHO: Geneva, Switzerland, 2006; Available online:https://apps.who.int/iris/handle/10665/69223 (accessed on 20 April 2020).
- Vazquez-Prokopec, G.M.; Medina-Barreiro, A.; Che-Mendoza, A.; Dzul-Manzanilla, F.; Correa-Morales, F.; Guillermo-May, G.; Bibiano-Marín, W.; Uc-Puc, V.; Geded-Moreno, E.; Vadillo-Sánchez, J.; et al. Deltamethrin resistance in Aedes aegypti results in treatment failure in Merida, Mexico. PLoS Negl. Trop. Dis. 2017, 11, e0005656. [Google Scholar] [CrossRef]
- Uragayala, S.; Kamaraju, R.; Tiwari, S.N.; Sreedharan, S.; Ghosh, S.K.; Valecha, N. Village-scale (Phase III) evaluation of the efficacy and residual activity of SumiShield ® 50 WG (Clothianidin 50%, w/w) for indoor spraying for the control of pyrethroid-resistant Anopheles culicifacies Giles in Karnataka state, India. Trop. Med. Int. Heal. 2018, 23, 605–615. [Google Scholar] [CrossRef]
- Zaim, M.; Guillet, P. Alternative insecticides: An urgent need. Trends Parasitol. 2002, 18, 161–163. [Google Scholar] [CrossRef]
- Esu, E.; Lenhart, A.; Smith, L.; Horstick, O. Effectiveness of peridomestic space spraying with insecticide on dengue transmission; Systematic review. Trop. Med. Int. Heal. 2010, 15, 619–631. [Google Scholar] [CrossRef]
- Peterson, R.K.D.; Macedo, P.A.; Davis, R.S. A human-health risk assessment for West Nile virus and insecticides used in mosquito management. Environ. Health Perspect. 2006, 114, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Bonds, J.A.S. Ultra-low-volume space sprays in mosquito control: A critical review. Med. Vet. Entomol. 2012, 26, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Boyce, W.M.; Lawler, S.P.; Schultz, J.M.; McCauley, S.J.; Kimsey, L.S.; Niemela, M.K.; Nielsen, C.F.; Reisen, W.K. Nontarget effects of the mosquito adulticide pyrethrin applied aerially during a West Nile virus outbreak in an urban California environment. J. Am. Mosq. Control Assoc. 2007, 23, 335–339. [Google Scholar] [CrossRef]
- Nathan, M.; Reiter, P.; WHO. Guidelines for Assessing the Efficacy of Insecticidal Space Sprays for Control of the Dengue Vector: Aedes aegypti; WHO: Geneva, Switzerland, 2001; Available online: https://apps.who.int/iris/handle/10665/67047 (accessed on 20 April 2020).
- Teng, H.-J.; Chen, T.-J.; Tsai, S.-F.; Lin, C.-P.; Chiou, H.-Y.; Lin, M.-C.; Yang, S.-Y.; Lee, Y.-W.; Kang, C.-C.; Hsu, H.-C.; et al. Emergency vector control in a DENV-2 outbreak in 2002 in Pingtung City, Pingtung County, Taiwan. Jpn. J. Infect. Dis. 2007, 60, 271–279. [Google Scholar] [PubMed]
- Guillet, P.; Alnwick, D.; Cham, M.K.; Neira, M.; Zaim, M.; Heymann, D.; Mukelabai, K. Long-lasting treated mosquito nets: A breakthrough in malaria prevention. Bull. World Health Organ. 2001, 79, 998. [Google Scholar] [PubMed]
- Yang, G.G.; Kim, D.; Pham, A.; Paul, C.J. A meta-regression analysis of the effectiveness of mosquito nets for malaria control: The value of long-lasting insecticide nets. Int. J. Environ. Res. Public Health 2018, 15, 1–12. [Google Scholar] [CrossRef]
- Rowland, M.; Webster, J.; Saleh, P.; Chandramohan, D.; Freeman, T.; Pearcy, B.; Durrani, N.; Rab, A.; Mohammed, N. Prevention of malaria in Afghanistan through social marketing of insecticide-treated nets: Evaluation of coverage and effectiveness by cross-sectional surveys and passive surveillance. Trop. Med. Int. Heal. 2002, 7, 813–822. [Google Scholar] [CrossRef]
- Arroz, J.A.H.; Candrinho, B.; Mendis, C.; Varela, P.; Pinto, J.; Do, M.; Martins, R.O. Effectiveness of a new long-lasting insecticidal nets delivery model in two rural districts of Mozambique: A before-after study. Malar J. 2018, 17, 66. [Google Scholar] [CrossRef]
- Girond, F.; Madec, Y.; Kesteman, T.; Randrianarivelojosia, M.; Randremanana, R.; Randriamampionona, L.; Randrianasolo, L.; Ratsitorahina, M.; Herbreteau, V.; Hedje, J.; et al. Evaluating effectiveness of mass and continuous long-lasting insecticidal net distributions over time in Madagascar: A sentinel surveillance based epidemiological study. EClinicalMedicine 2018, 1, 62–69. [Google Scholar] [CrossRef]
- Hounkonnou, C.; Djènontin, A.; Egbinola, S.; Houngbegnon, P.; Bouraima, A.; Soares, C.; Fievet, N.; Accrombessi, M.; Yovo, E.; Briand, V.; et al. Impact of the use and efficacy of long lasting insecticidal net on malaria infection during the first trimester of pregnancy—A pre-conceptional cohort study in southern Benin. BMC Public Health 2018, 18, 683. [Google Scholar] [CrossRef]
- Trape, J.F.; Tall, A.; Diagne, N.; Ndiath, O.; Ly, A.B.; Faye, J.; Dieye-Ba, F.; Roucher, C.; Bouganali, C.; Badiane, A.; et al. Malaria morbidity and pyrethroid resistance after the introduction of insecticide-treated bednets and artemisinin-based combination therapies: A longitudinal study. Lancet Infect. Dis. 2011, 11, 925–932. [Google Scholar] [CrossRef]
- Sougoufara, S.; Thiaw, O.; Cailleau, A.; Diagne, N.; Harry, M.; Bouganali, C.; Sembène, P.M.; Doucoure, S.; Sokhna, C. The Impact of Periodic Distribution Campaigns of Long-Lasting Insecticidal-Treated Bed Nets on Malaria Vector Dynamics and Human Exposure in Dielmo, Senegal. Am. J. Trop. Med. Hyg. 2018, 98, 1343–1352. [Google Scholar] [CrossRef]
- Soleimani-Ahmadi, M.; Vatandoost, H.; Shaeghi, M.; Raeisi, A.; Abedi, F.; Eshraghian, M.R.; Madani, A.; Safari, R.; Oshaghi, M.A.; Abtahi, M.; et al. Field evaluation of permethrin long-lasting insecticide treated nets (Olyset®) for malaria control in an endemic area, southeast of Iran. Acta Trop. 2012, 123, 146–153. [Google Scholar] [CrossRef]
- Kajla, M.K.; Barrett-Wilt, G.A.; Paskewitz, S.M. Bacteria: A novel source for potent mosquito feeding-deterrents. Sci. Adv. 2019, 5, eaau6141. [Google Scholar] [CrossRef] [PubMed]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent activity of essential oils: A review. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef]
- Trongtokit, Y.; Rongsriyam, Y.; Komalamisra, N.; Apiwathnasorn, C. Comparative repellency of 38 essential oils against mosquito bites. Phyther. Res. 2005, 19, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Daisy, B.H.; Strobel, G.A.; Castillo, U.; Ezra, D.; Sears, J.; Weaver, D.K.; Runyon, J.B. Naphthalene, an insect repellent, is produced by Muscodor vitigenus, a novel endophytic fungus. Microbiology 2002, 148, 3737–3741. [Google Scholar] [CrossRef]
- Naseem, S.; Munir, T.; Faheem Malik, M. Mosquito management: A review. J. Entomol. Zool. Stud. 2016, 4, 73–79. [Google Scholar]
- Balaji, A.P.B.; Ashu, A.; Manigandan, S.; Sastry, T.P.; Mukherjee, A.; Chandrasekaran, N. Polymeric nanoencapsulation of insect repellent: Evaluation of its bioefficacy on Culex quinquefasciatus mosquito population and effective impregnation onto cotton fabrics for insect repellent clothing. J. King Saud Univ. Sci. 2017, 29, 517–527. [Google Scholar] [CrossRef]
- Soni, N.; Prakash, S. Green nanoparticles for mosquito control. Sci. World J. 2014, 2014, 496362. [Google Scholar] [CrossRef]
- Knipling, E.F. Possibilities of Insect Control or Eradication Through the Use of Sexually Sterile Males1. J. Econ. Entomol. 1955, 48, 459–462. [Google Scholar] [CrossRef]
- Phuc, H.K.; Andreasen, M.H.; Burton, R.S.; Vass, C.; Epton, M.J.; Pape, G.; Fu, G.; Condon, K.C.; Scaife, S.; Donnelly, C.A.; et al. Late-acting dominant lethal genetic systems and mosquito control. BMC Biol. 2007, 5, 11. [Google Scholar] [CrossRef]
- Reiter, P. Oviposition, Dispersal, and Survival in Aedes aegypti: Implications for the Efficacy of Control Strategies. Vector Borne Zoonotic Dis. 2007, 7, 261–273. [Google Scholar] [CrossRef]
- Onyekwere, J.; Nnamonu, E.; Bede, E.; Okoye, C.; Okoro, J.; Nnamonu, E.; Bede, E.; Okoye, I.C.; Onyekwere, J.; Nnamonu, E.; et al. Application of genetically modified mosquitoes (Anopheles species) in the control of malaria transmission. Asian J. Biotechnol. Genet. Eng. 2018, 1, 1–16. [Google Scholar]
- Meghani, Z.; Boëte, C. Genetically engineered mosquitoes, Zika and other arboviruses, community engagement, costs, and patents: Ethical issues. PLoS Negl. Trop. Dis. 2018, 12, e0006501. [Google Scholar] [CrossRef]
- GMWATCH. New Documents Show Oxitec’s GM Mosquitoes Ineffective and Risky. Available online: https://www.gmwatch.org/en/news/latest-news/17828-new-documents-show-oxitec-s-gm-mosquitoes-ineffective-and-risky (accessed on 6 April 2019).
- Yamada, H.; Kraupa, C.; Lienhard, C.; Parker, A.G.; Maiga, H.; de Oliveira Carvalho, D.; Zheng, M.; Wallner, T.; Bouyer, J. Mosquito mass rearing: Who’s eating the eggs? Parasite 2019, 26, 75. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Blaustein, L. Effects of predator type and alternative prey on mosquito egg raft predation and destruction. Hydrobiologia 2019, 846, 215–221. [Google Scholar] [CrossRef]
- Lopez, S.B.G.; Guimarães-Ribeiro, V.; Rodriguez, J.V.G.; Dorand, F.A.P.S.; Salles, T.S.; Sá-Guimarães, T.E.; Alvarenga, E.S.L.; Melo, A.C.A.; Almeida, R.V.; Moreira, M.F. RNAi-based bioinsecticide for Aedes mosquito control. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Lee, J.Y.; Woo, R.M.; Choi, C.J.; Shin, T.Y.; Gwak, W.S.; Woo, S.D. Beauveria bassiana for the simultaneous control of Aedes albopictus and Culex pipiens mosquito adults shows high conidia persistence and productivity. AMB Express 2019, 9, 206. [Google Scholar] [CrossRef]
- Scholte, E.-J.; Knols, B.G.J.; Samson, R.A.; Takken, W. Entomopathogenic fungi for mosquito control: A review. J. Insect Sci. 2004, 4, 19. [Google Scholar] [CrossRef]
- Noskov, Y.A.; Polenogova, O.V.; Yaroslavtseva, O.N.; Belevich, O.E.; Yurchenko, Y.A.; Chertkova, E.A.; Kryukova, N.A.; Kryukov, V.Y.; Glupov, V.V. Combined effect of the entomopathogenic fungus Metarhizium robertsii and avermectins on the survival and immune response of Aedes aegypti larvae. PeerJ 2019, 7, e7931. [Google Scholar] [CrossRef] [PubMed]
- Lovett, B.; Bilgo, E.; Diabate, A.; St Leger, R. A review of progress toward field application of transgenic mosquitocidal entomopathogenic fungi. Pest Manag. Sci. 2019, 75, 2316–2324. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.E.I.; Paula, A.R.; Ribeiro, A.; Butt, T.M.; Silva, C.P.; Samuels, R.I. A new method of deploying entomopathogenic fungi to control adult Aedes aegypti mosquitoes. J. Appl. Entomol. 2018, 142, 59–66. [Google Scholar] [CrossRef]
- Louca, V.; Lucas, M.C.; Green, C.; Majambere, S.; Fillinger, U.; Lindsay, S.W. Role of fish as predators of mosquito larvae on the floodplain of the Gambia River. J. Med. Entomol. 2009, 46, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Aditya, G.; Pal, S.; Saha, N.; Saha, G. Efficacy of indigenous larvivorous fishes against Culex quinquefasciatus in the presence of alternative prey: Implications for biological control. J. Vector Borne Dis. 2012, 49, 217–225. [Google Scholar]
- Sareein, N.; Phalaraksh, C.; Rahong, P.; Techakijvej, C.; Seok, S.; Bae, Y.J. Relationships between predatory aquatic insects and mosquito larvae in residential areas in northern Thailand. J. Vector Ecol. 2019, 44, 223–232. [Google Scholar] [CrossRef]
- Früh, L.; Kampen, H.; Schaub, G.A.; Werner, D. Predation on the invasive mosquito Aedes japonicus (Diptera: Culicidae) by native copepod species in Germany. J. Vector Ecol. 2019, 44, 241–247. [Google Scholar] [CrossRef]
- Cuthbert, R.N.; Callaghan, A.; Sentis, A.; Dalal, A.; Dick, J.T.A. Additive multiple predator effects can reduce mosquito populations. Ecol. Entomol. 2019, 45, 243–250. [Google Scholar] [CrossRef]
- Digma, J.R.; Sumalde, A.C.; Salibay, C.C. Laboratory evaluation of predation of Toxorhynchites amboinensis (Diptera:Culicidae) on three mosquito vectors of arboviruses in the Philippines. Biol. Control 2019, 137, 104009. [Google Scholar] [CrossRef]
- Focks, D.A. Toxorhynchites as biocontrol agents. J. Am. Mosq. Control Assoc. 2007, 23, 118–127. [Google Scholar] [CrossRef]
- Schiller, A.; Allen, M.; Coffey, J.; Fike, A.; Carballo, F. Updated Methods for the Production of Toxorhynchites rutilus septentrionalis (Diptera, Culicidae) for Use as Biocontrol Agent Against Container Breeding Pest Mosquitoes in Harris County, Texas. J. Insect Sci. 2019, 19, 8. [Google Scholar] [CrossRef] [PubMed]
- Das, B.P. Chilodonella uncinate—A protozoa pathogenic to mosquito larvae. Curr. Sci. 2003, 85, 483–489. [Google Scholar]
- Dhanasekaran, D.; Thangaraj, R. Microbial secondary metabolites are an alternative approaches against insect vector to prevent zoonotic diseases. Asian Pac. J. Trop. Dis. 2014, 4, 253–261. [Google Scholar] [CrossRef]
- Lacey, L.A. Bacillus thuringiensis serovariety israelensis and Bacillus sphaericus for mosquito control. J. Am. Mosq. Control Assoc. 2007, 23, 93–109. [Google Scholar] [CrossRef]
- Derua, Y.A.; Kahindi, S.C.; Mosha, F.W.; Kweka, E.J.; Atieli, H.E.; Wang, X.; Zhou, G.; Lee, M.; Githeko, A.K.; Yan, G. Microbial larvicides for mosquito control: Impact of long lasting formulations of Bacillus thuringiensis var. israelensis and Bacillus sphaericus on non-target organisms in western Kenya highlands. Ecol. Evol. 2018, 8, 7563–7573. [Google Scholar] [CrossRef]
- Zhang, Q.; Hua, G.; Adang, M.J. Effects and mechanisms of Bacillus thuringiensis crystal toxins for mosquito larvae. Insect Sci. 2017, 24, 714–729. [Google Scholar] [CrossRef]
- Palma, L.; Muñoz, D.; Berry, C.; Murillo, J.; Caballero, P. Bacillus thuringiensis toxins: An overview of their biocidal activity. Toxins 2014, 6, 3296–3325. [Google Scholar] [CrossRef]
- Chee Dhang, C.; Han Lim, L.; Wasi Ahmad, N.; Benjamin, S.; Koon Weng, L.; Abdul Rahim, D.; Syafinaz Safian, E.; Sofian-Azirun, M. Field effectiveness of Bacillus thuringiensis israelensis (Bti) against Aedes (Stegomyia) aegypti (Linnaeus) in ornamental ceramic containers with common aquatic plants. Trop. Biomed. 2009, 26, 100–105. [Google Scholar]
- Saliha, B.; Wafa, H.; Laid, O.M. Effect of Bacillus thuringiensis var krustaki on the mortality and development of Culex pipiens (Diptera; Cullicidae). Int. J. Mosq. Res. 2017, 4, 20–23. [Google Scholar]
- Delécluse, A.; Rosso, M.L.; Ragni, A. Cloning and expression of a novel toxin gene from Bacillus thuringiensis subsp. jegathesan encoding a highly mosquitocidal protein. Appl. Environ. Microbiol. 1995, 61, 4230–4235. [Google Scholar] [CrossRef]
- López-Meza, J.; Federici, B.A.; Poehner, W.J.; Martinez-Castillo, A.; Ibarra, J.E. Highly mosquitocidal isolates of Bacillus thuringiensis subspecies kenyae and entomocidus from Mexico. Biochem. Syst. Ecol. 1995, 23, 461–468. [Google Scholar] [CrossRef]
- De Barjac, H.; Sebald, M.; Charles, J.F.; Cheong, W.H.; Lee, H.L. Clostridium bifermentans serovar malaysia, a new anaerobic bacterium pathogen to mosquito and blackfly larvae. C. R. Acad. Sci. Iii. 1990, 310, 383–387. [Google Scholar] [PubMed]
- Darriet, F.; Hougard, J.-M. An isolate of Bacillus circulans toxic to mosquito larvae. J. Am. Mosq. Control Assoc. 2002, 18, 65–67. [Google Scholar] [PubMed]
- Favret, M.E.; Yousten, A.A. Insecticidal activity of Bacillus laterosporus. J. Invertebr. Pathol. 1985, 45, 195–203. [Google Scholar] [CrossRef]
- Orlova, M.V.; Smirnova, T.A.; Ganushkina, L.A.; Yacubovich, V.Y.; Azizbekyan, R.R. Insecticidal activity of Bacillus laterosporus. Appl. Environ. Microbiol. 1998, 64, 2723–2725. [Google Scholar] [CrossRef]
- Pener, M.P. An Overview of Insect Growth Disruptors; Applied Aspects. Adv. Insect Phys. 2012, 43, 1–162. [Google Scholar]
- Yapabandara, A.M.G.M.; Curtis, C.F. Laboratory and field comparisons of pyriproxyfen, polystyrene beads and other larvicidal methods against malaria vectors in Sri Lanka. Acta Trop. 2002, 81, 211–223. [Google Scholar] [CrossRef]
- Ansari, M.A.; Razdan, R.K.; Sreehari, U. Laboratory and field evaluation of Hilmilin against mosquitoes. J. Am. Mosq. Control Assoc. 2005, 21, 432–436. [Google Scholar] [CrossRef]
- Raghavendra, K.; Barik, T.K.; Reddy, B.P.N.; Sharma, P.; Dash, A.P. Malaria vector control: From past to future. Parasitol. Res. 2011, 108, 757–779. [Google Scholar] [CrossRef]
- Paul, A.; Harrington, L.C.; Zhang, L.; Scott, J.G. Insecticide resistance in Culex pipiens from New York. J. Am. Mosq. Control Assoc. 2005, 21, 305–309. [Google Scholar] [CrossRef]
- De Silva, J.J.; Mendes, J. Susceptibility of Aedes aegypti (L) to the insect growth regulators diflubenzuron and methoprene in Uberlândia, State of Minas Gerais. Rev. Soc. Bras. Med. Trop. 2007, 40, 612–616. [Google Scholar] [CrossRef][Green Version]
- Dennehy, T.J.; Degain, B.A.; Harpold, V.S.; Zaborac, M.; Morin, S.; Fabrick, J.A.; Nichols, R.L.; Brown, J.K.; Byrne, F.J.; Li, X. Extraordinary resistance to insecticides reveals exotic Q biotype of Bemisia tabaci in the New World. J. Econ. Entomol. 2010, 103, 2174–2186. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Young CHOI, J.; Ram LEE, B.; Fang, Y.; Hoon KIM, J.; Hwan Park, D.; Gu Park, M.; Mi Woo, R.; Jin Kim, W.; Ho, Y.J.; et al. Insect growth regulatory and larvicidal activity of chalcones against Aedes albopictus. Entomol. Rep. 2018, 48, 55–59. [Google Scholar] [CrossRef]
- Niang, E.H.A.; Bassene, H.; Fenollar, F.; Mediannikov, O. Biological Control of Mosquito-Borne Diseases: The Potential of Wolbachia -Based Interventions in an IVM Framework. J. Trop. Med. 2018, 2018, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Jeffries, C.L.; Walker, T. Biological Control of Mosquito Vectors: Past, Present, and Future. Insects 2016, 7, 52. [Google Scholar] [CrossRef]
- Zug, R.; Hammerstein, P. Still a Host of Hosts for Wolbachia: Analysis of Recent Data Suggests That 40% of Terrestrial Arthropod Species Are Infected. PLoS ONE 2012, 7, 38544. [Google Scholar] [CrossRef]
- Skerman, V.B.D.; McGowan, V.F.; Sneath, P.H.A.; Peter, H.A. Approved Lists of Bacterial Names; Skerman, V.B.D., McGowan, V.F., Sneath, P.H.A., Eds.; American Society for Microbiology: Washington, DC, USA, 1989; ISBN 9781555810146. [Google Scholar]
- Ogunbiyi, T.S.; Eromon, P.; Oluniyi, P.; Ayoade, F.; Oloche, O.; Oguzie, J.U.; Folarin, O.; Happi, C.; Komolafe, I. First Report of Wolbachia from Field Populations of Culex Mosquitoes in South-Western Nigeria. Afr. Zool. 2019, 54, 181–185. [Google Scholar] [CrossRef]
- Balaji, S.; Jayachandran, S.; Prabagaran, S.R. Evidence for the natural occurrence of Wolbachia in Aedes aegypti mosquitoes. Fems Microbiol. Lett. 2019, 366. Available online: https://doi.org/10.1093/femsle/fnz055 (accessed on 20 April 2020). [CrossRef]
- Mohanty, I.; Rath, A.; Swain, S.P.; Pradhan, N.; Hazra, R.K. Wolbachia Population in Vectors and Non-vectors: A Sustainable Approach Towards Dengue Control. Curr. Microbiol. 2019, 76, 133–143. [Google Scholar] [CrossRef]
- Laven, H. Eradication of Culex pipiens fatigans through Cytoplasmic Incompatibility. Nature 1967, 216, 383–384. [Google Scholar] [CrossRef]
- Zhang, D.; Zheng, X.; Xi, Z.; Bourtzis, K.; Gilles, J.R.L. Combining the Sterile Insect Technique with the Incompatible Insect Technique: I-Impact of Wolbachia Infection on the Fitness of Triple- and Double-Infected Strains of Aedes albopictus. PLoS ONE 2015, 10, e0121126. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, L.; Plichart, C.; Sang, A.C.; Brelsfoard, C.L.; Bossin, H.C.; Dobson, S.L. Open Release of Male Mosquitoes Infected with a Wolbachia Biopesticide: Field Performance and Infection Containment. PLoS Negl. Trop. Dis. 2012, 6, e1797. [Google Scholar] [CrossRef]
- Joubert, D.A.; Walker, T.; Carrington, L.B.; De Bruyne, J.T.; Kien, D.H.T.; Hoang, N.L.T.; Chau, N.V.V.; Iturbe-Ormaetxe, I.; Simmons, C.P.; O’Neill, S.L. Establishment of a Wolbachia Superinfection in Aedes aegypti Mosquitoes as a Potential Approach for Future Resistance Management. PLoS Pathog. 2016, 12, e1005434. [Google Scholar] [CrossRef] [PubMed]
- Aliota, M.T.; Walker, E.C.; Yepes, A.U.; Velez, I.D.; Christensen, B.M.; Osorio, J.E. The wMel Strain of Wolbachia Reduces Transmission of Chikungunya Virus in Aedes aegypti. PLoS Negl. Trop. Dis. 2016, 10, e0004677. [Google Scholar] [CrossRef]
- Van den Hurk, A.F.; Hall-Mendelin, S.; Pyke, A.T.; Frentiu, F.D.; McElroy, K.; Day, A.; Higgs, S.; O’Neill, S.L. Impact of Wolbachia on infection with chikungunya and yellow fever viruses in the mosquito vector Aedes aegypti. PLoS Negl. Trop. Dis. 2012, 6, e1892. [Google Scholar] [CrossRef] [PubMed]
- Bian, G.; Joshi, D.; Dong, Y.; Lu, P.; Zhou, G.; Pan, X.; Xu, Y.; Dimopoulos, G.; Xi, Z. Wolbachia Invades Anopheles stephensi Populations and Induces Refractoriness to Plasmodium Infection. Science 2013, 340, 748–751. [Google Scholar] [CrossRef]
- Hughes, G.L.; Koga, R.; Xue, P.; Fukatsu, T.; Rasgon, J.L. Wolbachia Infections Are Virulent and Inhibit the Human Malaria Parasite Plasmodium Falciparum in Anopheles Gambiae. PLoS Pathog. 2011, 7, e1002043. [Google Scholar] [CrossRef]
- Ford, S.A.; Allen, S.L.; Ohm, J.R.; Sigle, L.T.; Sebastian, A.; Albert, I.; Chenoweth, S.F.; McGraw, E.A. Selection on Aedes aegypti alters Wolbachia-mediated dengue virus blocking and fitness. Nat. Microbiol. 2019, 4, 1832–1839. [Google Scholar] [CrossRef]
- Dutra, H.L.C.; Rocha, M.N.; Dias, F.B.S.; Mansur, S.B.; Caragata, E.P.; Moreira, L.A. Wolbachia Blocks Currently Circulating Zika Virus Isolates in Brazilian Aedes aegypti Mosquitoes. Cell Host Microbe 2016, 19, 744–771. [Google Scholar] [CrossRef]
- Mariño, Y.; Verle Rodrigues, J.; Bayman, P. Wolbachia Affects Reproduction and Population Dynamics of the Coffee Berry Borer (Hypothenemus hampei): Implications for Biological Control. Insects 2017, 8, 8. [Google Scholar] [CrossRef]
- Chegeni, T.N.; Fakhar, M. Promising Role of Wolbachia as Anti-parasitic Drug Target and Eco-Friendly Biocontrol Agent. Recent Pat. Antiinfect. Drug Discov. 2019, 14, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Bongio, N.J.; Lampe, D.J. Inhibition of Plasmodium berghei Development in Mosquitoes by Effector Proteins Secreted from Asaia sp. Bacteria Using a Novel Native Secretion Signal. PLoS ONE 2015, 10. Available online: https://doi.org/10.1371/journal.pone.0143541 (accessed on 20 April 2020). [CrossRef] [PubMed]
- Wang, S.; Jacobs-Lorena, M. Genetic approaches to interfere with malaria transmission by vector mosquitoes. Trends Biotechnol. 2013, 31, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Rami, A.; Raz, A.; Zakeri, S.; Dinparast Djadid, N. Isolation and identification of Asaia sp. in Anopheles spp. mosquitoes collected from Iranian malaria settings: Steps toward applying paratransgenic tools against malaria. Parasit Vectors 2018, 11, 367. [Google Scholar] [CrossRef] [PubMed]
- Favia, G.; Ricci, I.; Marzorati, M.; Negri, I.; Alma, A.; Sacchi, L.; Bandi, C.; Daffonchio, D. Bacteria of the Genus Asaia: A Potential Paratransgenic Weapon Against Malaria. In Transgenesis and the Management of Vector-Borne Disease; Springer: New York, NY, USA, 2008; pp. 49–59. [Google Scholar]
- Favia, G.; Ricci, I.; Damiani, C.; Raddadi, N.; Crotti, E.; Marzorati, M.; Rizzi, A.; Urso, R.; Brusetti, L.; Borin, S.; et al. Bacteria of the genus Asaia stably associate with Anopheles stephensi, an Asian malarial mosquito vector. Proc. Natl. Acad. Sci. USA 2007, 104, 9047–9051. [Google Scholar] [CrossRef] [PubMed]
- Damiani, C.; Ricci, I.; Crotti, E.; Rossi, P.; Rizzi, A.; Scuppa, P.; Esposito, F.; Bandi, C.; Daffonchio, D.; Favia, G. Paternal transmission of symbiotic bacteria in malaria vectors. Curr. Biol. 2008, 18, R1087–R1088. [Google Scholar] [CrossRef]
- Nugapola, N.W.N.P.; De Silva, W.A.P.P.; Karunaratne, S.H.P.P. Distribution and phylogeny of Wolbachia strains in wild mosquito populations in Sri Lanka. Parasites Vectors 2017, 10, 230. [Google Scholar] [CrossRef]
- Mamlouk, D.; Gullo, M. Acetic Acid Bacteria: Physiology and Carbon Sources Oxidation. Indian J. Microbiol. 2013, 53, 377. [Google Scholar] [CrossRef]
- Cappelli, A.; Damiani, C.; Mancini, M.V.; Valzano, M.; Rossi, P.; Serrao, A.; Ricci, I.; Favia, G. Asaia Activates Immune Genes in Mosquito Eliciting an Anti-Plasmodium Response: Implications in Malaria Control. Front. Genet. 2019, 10, 836. [Google Scholar] [CrossRef]
- Mitraka, E.; Stathopoulos, S.; Siden-Kiamos, I.; Christophides, G.K.; Louis, C. Asaia accelerates larval development of Anopheles gambiae. Pathog. Glob. Health 2013, 107, 305. [Google Scholar] [CrossRef]
- Shane, J.L.; Grogan, C.L.; Cwalina, C.; Lampe, D.J. Blood meal-induced inhibition of vector-borne disease by transgenic microbiota. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mertz, F.P.; YAO, R.C. Saccharopolyspora spinosa sp. nov. Isolated from Soil Collected in a Sugar Mill Rum Still. Int. J. Syst. Bacteriol. 1990, 40, 34–39. [Google Scholar] [CrossRef]
- Hertlein, M.B.; Mavrotas, C.; Jousseaume, C.; Lysandrou, M.; Thompson, G.D.; Jany, W.; Ritchie, S.A. A Review of Spinosad as a Natural Product for Larval Mosquito Control. J. Am. Mosq. Control Assoc. 2010, 26, 67–87. [Google Scholar] [CrossRef]
- Su, T. Resistance and Its Management to Microbial and Insect Growth Regulator Larvicides in Mosquitoes; InTech Europe: Rijeka, Croatia, 2016; pp. 135–154. [Google Scholar] [CrossRef]
- WHO. Spinosad DT in Drinking-Water: Use for Vector Control in Drinking-water Sources and Containers; WHO: Geneva, Switzerland, 2010; Available online: https://www.who.int/water_sanitation_health/dwq/chemicals/spinosadbg.pdf (accessed on 20 April 2020).
- Marina, C.F.; Bond, J.G.; Muñoz, J.; Valle, J.; Chirino, N.; Williams, T. Spinosad: A biorational mosquito larvicide for use in car tires in southern Mexico. Parasit. Vectors 2012, 5, 95. [Google Scholar] [CrossRef] [PubMed]
- Marina, C.F.; Bond, J.; Muñoz, J.; Valle, J.; Novelo-Gutiérrez, R.; Williams, T. Efficacy and non-target impact of spinosad, Bti and temephos larvicides for control of Anopheles spp. in an endemic malaria region of southern Mexico. Parasit. Vectors 2014, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, K.; Murugan, K.; Nareshkumar, A.; Bragadeeswaran, S. Larvicidal and pupicidal activity of spinosad against the malarial vector Anopheles stephensi. Asian Pac. J. Trop. Med. 2011, 4, 610–613. [Google Scholar] [CrossRef]
- Qiu, H.; Jun, H.W.; Mccall, J.W. Pharmacokinetics, formulation, and safety of insect repellent N,N-Diethyl-3-methylbenzamide (deet): A review. J. Am. Mosq. Control Assoc. 1998, 14, 12–27. [Google Scholar]
- Stanczyk, N.M.; Brookfield, J.F.Y.; Field, L.M.; Logan, J.G. Aedes aegypti Mosquitoes Exhibit Decreased Repellency by DEET following Previous Exposure. PLoS ONE 2013, 8, e54438. [Google Scholar] [CrossRef]
- Deletre, E.; Martin, T.; Duménil, C.; Chandre, F. Insecticide resistance modifies mosquito response to DEET and natural repellents. Parasit. Vectors 2019, 12, 89. [Google Scholar] [CrossRef] [PubMed]
- Debboun, M.; Frances, S.P.; Strickman, D. Insect Repellents Handbook; CRC Press: Boca Raton, FL, USA, 2014; ISBN 9781466553552. [Google Scholar]
- Van Roey, K.; Sokny, M.; Denis, L.; Van den Broeck, N.; Heng, S.; Siv, S.; Sluydts, V.; Sochantha, T.; Coosemans, M.; Durnez, L. Field evaluation of picaridin repellents reveals differences in repellent sensitivity between Southeast Asian vectors of malaria and arboviruses. PLoS Negl. Trop. Dis. 2014, 8, e3326. [Google Scholar] [CrossRef]
- Carroll, S.P. Prolonged efficacy of IR3535 repellents against mosquitoes and blacklegged ticks in North America. J. Med. Entomol. 2008, 45, 706–714. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, M.Y. Essential Oils as Repellents against Arthropods. Biomed Res. Int. 2018, 2018, 6860271. [Google Scholar] [CrossRef] [PubMed]
- Tilquin, M.; Paris, M.; Reynaud, S.; Despres, L.; Ravanel, P.; Geremia, R.A.; Gury, J. Long lasting persistence of Bacillus thuringiensis Subsp. israelensis (Bti) in mosquito natural habitats. PLoS ONE 2008, 3, e3432. [Google Scholar] [CrossRef] [PubMed]
- Paris, M.; Tetreau, G.; Laurent, F.; Lelu, M.; Despres, L.; David, J.-P. Persistence of Bacillus thuringiensis israelensis (Bti) in the environment induces resistance to multiple Bti toxins in mosquitoes. Pest Manag. Sci. 2011, 67, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Bravo, A.; Soberón, M. How to cope with insect resistance to Bt toxins? Trends Biotechnol. 2008, 26, 573–579. [Google Scholar] [CrossRef]
- Georghiou, G.P.; Wirth, M.C. Influence of Exposure to Single versus Multiple Toxins of Bacillus thuringiensis subsp. israelensis on Development of Resistance in the Mosquito Culex quinquefasciatus (Diptera: Culicidae). Appl. Environ. Microbiol. 1997, 63, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Cadavid-Restrepo, G.; Sahaza, J.; Orduz, S. Treatment of an Aedes aegypti colony with the Cry11Aa toxin for 54 generations results in the development of resistance. Mem. Inst. Oswaldo Cruz 2012, 107, 74–79. [Google Scholar] [CrossRef][Green Version]
- Stalinski, R.; Tetreau, G.; Gaude, T.; Després, L. Pre-selecting resistance against individual Bti Cry toxins facilitates the development of resistance to the Bti toxins cocktail. J. Invertebr. Pathol. 2014, 119, 50–53. [Google Scholar] [CrossRef]
- Paris, M.; Melodelima, C.; Coissac, E.; Tetreau, G.; Reynaud, S.; David, J.-P.; Despres, L. Transcription profiling of resistance to Bti toxins in the mosquito Aedes aegypti using next-generation sequencing. J. Invertebr. Pathol. 2012, 109, 201–208. [Google Scholar] [CrossRef]
- Cheong, H.; Dhesi, R.K.; Gill, S.S. Marginal cross-resistance to mosquitocidal Bacillus thuringiensis strains in Cry11A-resistant larvae: Presence of Cry11A-like toxins in these strains. Fems Microbiol. Lett. 2006, 153, 419–424. [Google Scholar] [CrossRef]
- Demissew, A.; Balkew, M.; Girma, M. Larvicidal activities of chinaberry, neem and Bacillus thuringiensis israelensis (Bti) to an insecticide resistant population of Anopheles arabiensis from Tolay, Southwest Ethiopia. Asian Pac. J. Trop. Biomed. 2016, 6, 554–561. [Google Scholar] [CrossRef]
- Boyer, S.; Tilquin, M.; Ravanel, P. differential sensitivity to Bacillus thuringiensis var. israelensis and temephos in field mosquito populations of Ochlerotatus cataphylla (Diptera: Culicidae): Toward resistance? Environ. Toxicol. Chem. 2007, 26, 157. [Google Scholar] [CrossRef] [PubMed]
- Paris, M.; Boyer, S.; Bonin, A.; Collado, A.; David, J.-P.; Despres, L. Genome scan in the mosquito Aedes rusticus: Population structure and detection of positive selection after insecticide treatment. Mol. Ecol. 2010, 19, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Thieme, J.; White, G.S.; Lura, T.; Mayerle, N.; Faraji, A.; Cheng, M.L.; Brown, M.Q. High Resistance to Bacillus sphaericus and Susceptibility to Other Common Pesticides in Culex pipiens (Diptera: Culicidae) from Salt Lake City, UT. J. Med. Entomol. 2019, 56, 506–513. [Google Scholar] [CrossRef]
- Park, H.-W.; Bideshi, D.K.; Federici, B.A. Properties and applied use of the mosquitocidal bacterium, Bacillus sphaericus. J. Asia. Pac. Entomol. 2010, 13, 159–168. [Google Scholar] [CrossRef]
- Ahmed, I.; Yokota, A.; Yamazoe, A.; Fujiwara, T. Proposal of Lysinibacillus boronitolerans gen. nov. sp. nov., and transfer of Bacillus fusiformis to Lysinibacillus fusiformis comb. nov. and Bacillus sphaericus to Lysinibacillus sphaericus comb. nov. Int. J. Syst. Evol. Microbiol. 2007, 57, 1117–1125. [Google Scholar] [CrossRef]
- Nielsen-Leroux, C.; Pasquier, F.; Charles, J.F.; Sinègre, G.; Gaven, B.; Pasteur, N. Resistance to Bacillus sphaericus involves different mechanisms in Culex pipiens (Diptera:Culicidae) larvae. J. Med. Entomol. 1997, 34, 321–327. [Google Scholar] [CrossRef]
- Rao, D.R.; Mani, T.R.; Rajendran, R.; Joseph, A.S.; Gajanana, A.; Reuben, R. Development of a high level of resistance to Bacillus sphaericus in a field population of Culex quinquefasciatus from Kochi, India. J. Am. Mosq. Control Assoc. 1995, 11, 1–5. [Google Scholar]
- Rodcharoen, J.; Mulla, M.S. Biological Fitness of Culex quinquefasciatus (Diptera: Culicidae) Susceptible and Resistant to Bacillus sphaericus. J. Med. Entomol. 1997, 34, 5–10. [Google Scholar] [CrossRef]
- De Oliveira, C.M.F.; Filho, F.C.; Beltràn, J.E.N.; Silva-Filha, M.H.; Regis, L. Biological fitness of a Culex quinquefasciatus population and its resistance to Bacillus sphaericus. J. Am. Mosq. Control Assoc. 2003, 19, 125–129. [Google Scholar]
- Amorim, L.B.; de Barros, R.A.; de Melo Chalegre, K.D.; de Oliveira, C.M.F.; Narcisa Regis, L.; Silva-Filha, M.H.N.L. Stability of Culex quinquefasciatus resistance to Bacillus sphaericus evaluated by molecular tools. Insect Biochem. Mol. Biol. 2010, 40, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.E.; Mazzarri, M.; Sojo, M.; García, A.G.Y. Effectiveness of Bacillus sphaericus strain 2362 on larvae of Anopheles nuñeztovari. Investig. Clin. 2001, 42, 131–146. [Google Scholar] [PubMed]
- Nicolas, L.; Darriet, F.; Hougard, J.M. Efficacy of Bacillus sphaericus 2362 against larvae of Anopheles gambiae under laboratory and field conditions in West Africa. Med. Vet. Entomol. 1987, 1, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Derua, Y.A.; Kahindi, S.C.; Mosha, F.W.; Kweka, E.J.; Atieli, H.E.; Zhou, G.; Lee, M.-C.; Githeko, A.K.; Yan, G. Susceptibility of Anopheles gambiae complex mosquitoes to microbial larvicides in diverse ecological settings in western Kenya. Med. Vet. Entomol. 2019, 33, 220–227. [Google Scholar] [CrossRef]
- Skovmand, O.; Bauduin, S. Efficacy of a granular formulation of Bacillus sphaericus against Culex quinquefasciatus and Anopheles gambiae in West African countries. J. Vector Ecol. 1997, 22, 43–51. [Google Scholar]
- Poopathi, S.; Mani, T.R.; Rao, D.R.; Kabilan, L. Evaluation of Synergistic Interaction between Bacillus sphaericus and a Neem-based Biopesticide on Bsph-Susceptible Culex quinquefasciatus Say Larvae. Int. J. Trop. Insect Sci. 2011, 22, 303–306. [Google Scholar] [CrossRef]
- Raoult, D.; Abat, C. Developing new insecticides to prevent chaos: The real future threat. Lancet Infect. Dis. 2017, 17, 804–805. [Google Scholar] [CrossRef]
- IVCC Annual Report 2017–2018. 2018. Available online: http://www.ivcc.com/about/governance/annual-reports (accessed on 20 April 2020).
- Sergeant, M.; Baxter, L.; Jarrett, P.; Shaw, E.; Ousley, M.; Winstanley, C.; Morgan, J.A.W. Identification, typing, and insecticidal activity of Xenorhabdus isolates from entomopathogenic nematodes in United Kingdom soil and characterization of the xpt toxin loci. Appl. Environ. Microbiol. 2006, 72, 5895–5907. [Google Scholar] [CrossRef]
- Pineda-Castellanos, M.L.; Rodríguez-Segura, Z.; Villalobos, F.J.; Hernández, L.; Lina, L.; Nuñez-Valdez, M.E. Pathogenicity of Isolates of Serratia Marcescens towards Larvae of the Scarab Phyllophaga Blanchardi (Coleoptera). Pathogens 2015, 4, 210–228. [Google Scholar] [CrossRef]
- Niu, H.; Wang, N.; Liu, B.; Xiao, L.; Wang, L.; Guo, H. Synergistic and additive interactions of Serratia marcescens S-JS1 to the chemical insecticides for controlling Nilaparvata lugens (Hemiptera: Delphacidae). J. Econ. Entomol. 2018, 111, 823–828. [Google Scholar] [CrossRef]
- Wei, G.; Lai, Y.; Wang, G.; Chen, H.; Li, F.; Wang, S. Insect pathogenic fungus interacts with the gut microbiota to accelerate mosquito mortality. Proc. Natl. Acad. Sci. USA 2017, 114, 5994–5999. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Mubarak, M.S. Diterpenes and their derivatives as promising agents against dengue virus and dengue vectors: A literature-based review. Phyther. Res. 2019. Available online: https://doi.org/10.1002/ptr.6562 (accessed on 20 April 2020).
- Itoh, T.; Kawada, H.; Abe, A.; Eshita, Y.; Rongsriyam, Y.; Igarashi, A. Utilization of bloodfed females of Aedes aegypti as a vehicle for the transfer of the insect growth regulator pyriproxyfen to larval habitats. J. Am. Mosq. Control Assoc. 1994, 10, 344–347. [Google Scholar] [PubMed]
- Revay, E.E.; Müller, G.C.; Qualls, W.A.; Kline, D.L.; Naranjo, D.P.; Arheart, K.L.; Kravchenko, V.D.; Yefremova, Z.; Hausmann, A.; Beier, J.C.; et al. Control of Aedes albopictus with attractive toxic sugar baits (ATSB) and potential impact on non-target organisms in St. Augustine, Florida. Parasitol. Res. 2014, 113, 73–79. [Google Scholar] [CrossRef]
- Naranjo, D.P.; Qualls, W.A.; Müller, G.C.; Samson, D.M.; Roque, D.; Alimi, T.; Arheart, K.; Beier, J.C.; Xue, R.-D. Evaluation of boric acid sugar baits against Aedes albopictus (Diptera: Culicidae) in tropical environments. Parasitol. Res. 2013, 112, 1583–1587. [Google Scholar] [CrossRef]
- Müller, G.C.; Beier, J.C.; Traore, S.F.; Toure, M.B.; Traore, M.M.; Bah, S.; Doumbia, S.; Schlein, Y. Successful field trial of attractive toxic sugar bait (ATSB) plant-spraying methods against malaria vectors in the Anopheles gambiae complex in Mali, West Africa. Malar. J. 2010, 9, 210. [Google Scholar] [CrossRef] [PubMed]
- Allan, S.A. Susceptibility of adult mosquitoes to insecticides in aqueous sucrose baits. J. Vector Ecol. 2011, 36, 59–67. [Google Scholar] [CrossRef]
- George, O. Poinar Nematodes for Biological Control of Insects; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar] [CrossRef]
- Petersen, J.J. Role of mermithid nematodes in biological control of mosquitoes. Exp. Parasitol. 1973, 33, 239–247. [Google Scholar] [CrossRef]
- Abagli, A.Z.; Alavo, T.B.; Platzer, E.G. Efficacy of the insect parasitic nematode, Romanomermis iyengari, for malaria vector control in Benin West Africa. Malar. J. 2012, 11, P5. [Google Scholar] [CrossRef]
- Paily, K.P.; Balaraman, K. Susceptibility of ten species of mosquito larvae to the parasitic nematode Romanomermis iyengari and its development. Med. Vet. Entomol. 2000, 14, 426–429. [Google Scholar] [CrossRef]
- Abagli, A.Z.; Alavo, T.B.C.; Perez-Pacheco, R.; Platzer, E.G. Efficacy of the mermithid nematode, Romanomermis iyengari, for the biocontrol of Anopheles gambiae, the major malaria vector in sub-Saharan Africa. Parasit. Vectors 2019, 12, 253. [Google Scholar] [CrossRef]
- Abagli, A.Z.; Alavo. Biocontrol of Culex quinquefasciatus using the insect parasitic nematode, Romanomermis iyengari (Nematoda: Mermithidae). Trop. Biomed. 2019, 36, 1003–1013. Available online: http://msptm.org/files/Vol36No4/1003-1013-Alavo-TBC.pdf (accessed on 20 April 2020).
- Britch, S.C.; Nyberg, H.; Aldridge, R.L.; Swan, T.; Linthicum, K.J. Acoustic Control of Mosquito Larvae In Artificial Drinking Water Containers. J. Am. Mosq. Control Assoc. 2016, 32, 341–344. [Google Scholar] [CrossRef]
- Fredregill, C.L.; Motl, G.C.; Dennett, J.A.; Bueno, R.; Debboun, M. Efficacy of Two LarvasonicTM Units Against Culex Larvae and Effects on Common Aquatic Nontarget Organisms in Harris County, Texas 1. J. Am. Mosq. Control Assoc. 2015, 31, 366–370. [Google Scholar] [CrossRef]
- Mukundarajan, H.; Hol, F.J.H.; Castillo, E.A.; Newby, C.; Prakash, M. Using mobile phones as acoustic sensors for high-throughput mosquito surveillance. Elife 2017, 6, e27854. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.J.; Rohde, B.B.; Zeak, N.; Staunton, K.M.; Prachar, T.; Ritchie, S.A. A low-cost, battery-powered acoustic trap for surveilling male Aedes aegypti during rear-and-release operations. PLoS ONE 2018, 13, e0201709. [Google Scholar] [CrossRef] [PubMed]
- Whyard, S.; Erdelyan, C.N.G.; Partridge, A.L.; Singh, A.D.; Beebe, N.W.; Capina, R. Silencing the buzz: A new approach to population suppression of mosquitoes by feeding larvae double-stranded RNAs. Parasit. Vectors 2015, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- Jesus, T.; Wanner, E.; Cardoso, R. A receding horizon control approach for integrated vector management of Aedes aegypti using chemical and biological control: A mono and a multiobjective approach. Math. Methods Appl. Sci. 2019. Available online: https://doi.org/10.1002/mma.6115 (accessed on 20 April 2020).


| Bacteria | Mosquito | Site | Type of Study | Number of Studied Regions | Date | Reference |
|---|---|---|---|---|---|---|
| Bti + Bs | Culex pipiens–complex | Onondaga County, USA | Field | 2 | June 2003 | [140] |
| Bti | Ochlerotatuscataphylla | Rhône-Alpes, France | Field | 4 | April 2003 | [197] |
| Bti | Aedes rusticus | Rhône-Alpes, France | Field | 13 | Winters 2005 and 2006 | [198] |
| Bti | Culex quinquefasciatus | USA | Laboratory | 1 | Summer 1990 | [191] |
| Bti | Aedes aegypti | USA | Laboratory | 1 | 2011 | [192] |
| Bs | Culex pipiens–complex | Utah, USA | Field | 3 | September 2016 | [199] |
| Bti | Aedes aegypti | France | Laboratory | 1 | 2010 | [189] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahmana, H.; Mediannikov, O. Mosquito-Borne Diseases Emergence/Resurgence and How to Effectively Control It Biologically. Pathogens 2020, 9, 310. https://doi.org/10.3390/pathogens9040310
Dahmana H, Mediannikov O. Mosquito-Borne Diseases Emergence/Resurgence and How to Effectively Control It Biologically. Pathogens. 2020; 9(4):310. https://doi.org/10.3390/pathogens9040310
Chicago/Turabian StyleDahmana, Handi, and Oleg Mediannikov. 2020. "Mosquito-Borne Diseases Emergence/Resurgence and How to Effectively Control It Biologically" Pathogens 9, no. 4: 310. https://doi.org/10.3390/pathogens9040310
APA StyleDahmana, H., & Mediannikov, O. (2020). Mosquito-Borne Diseases Emergence/Resurgence and How to Effectively Control It Biologically. Pathogens, 9(4), 310. https://doi.org/10.3390/pathogens9040310




