Effect of Sugars on Chlamydia trachomatis Infectivity
Abstract
1. Introduction
2. Results
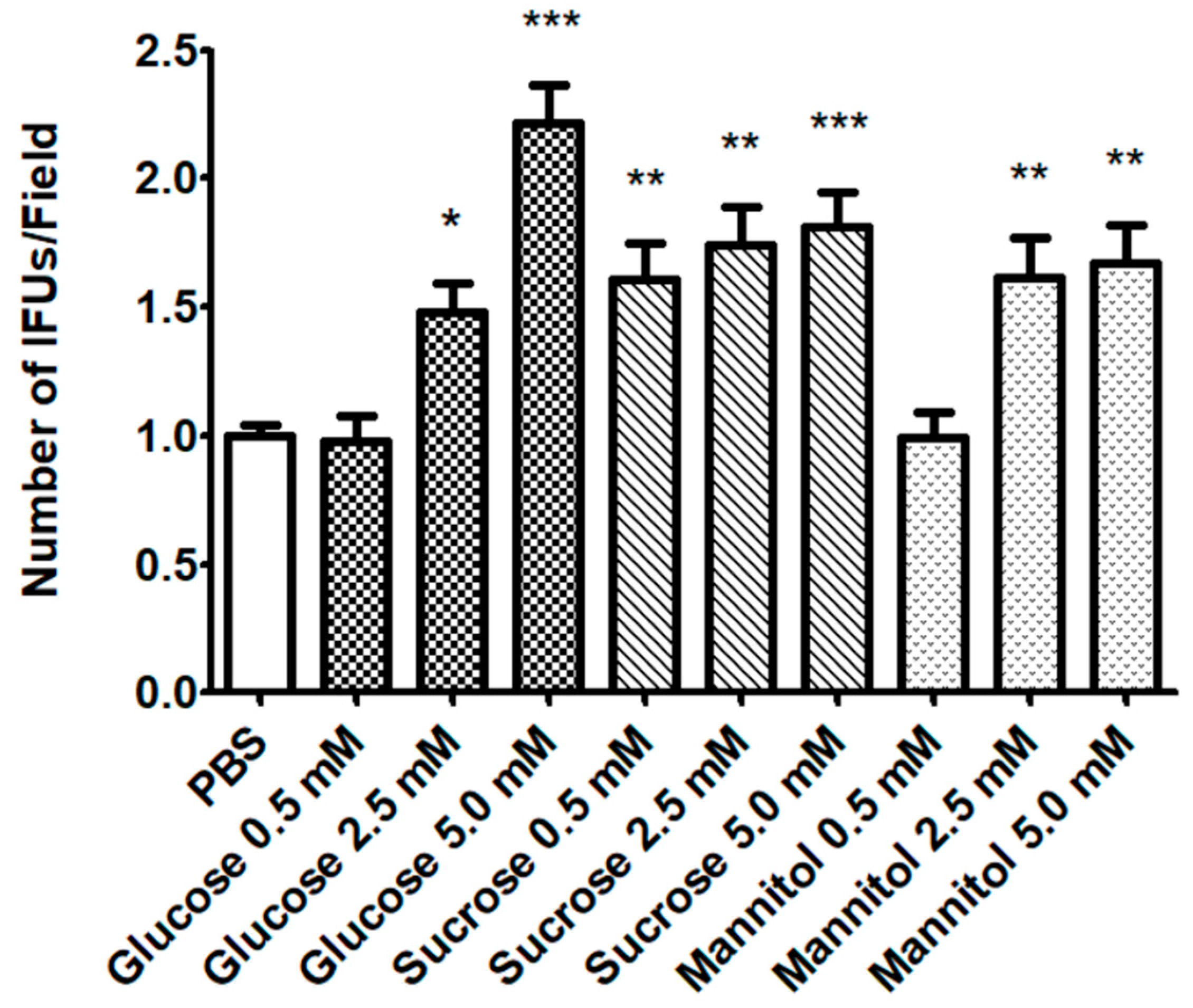
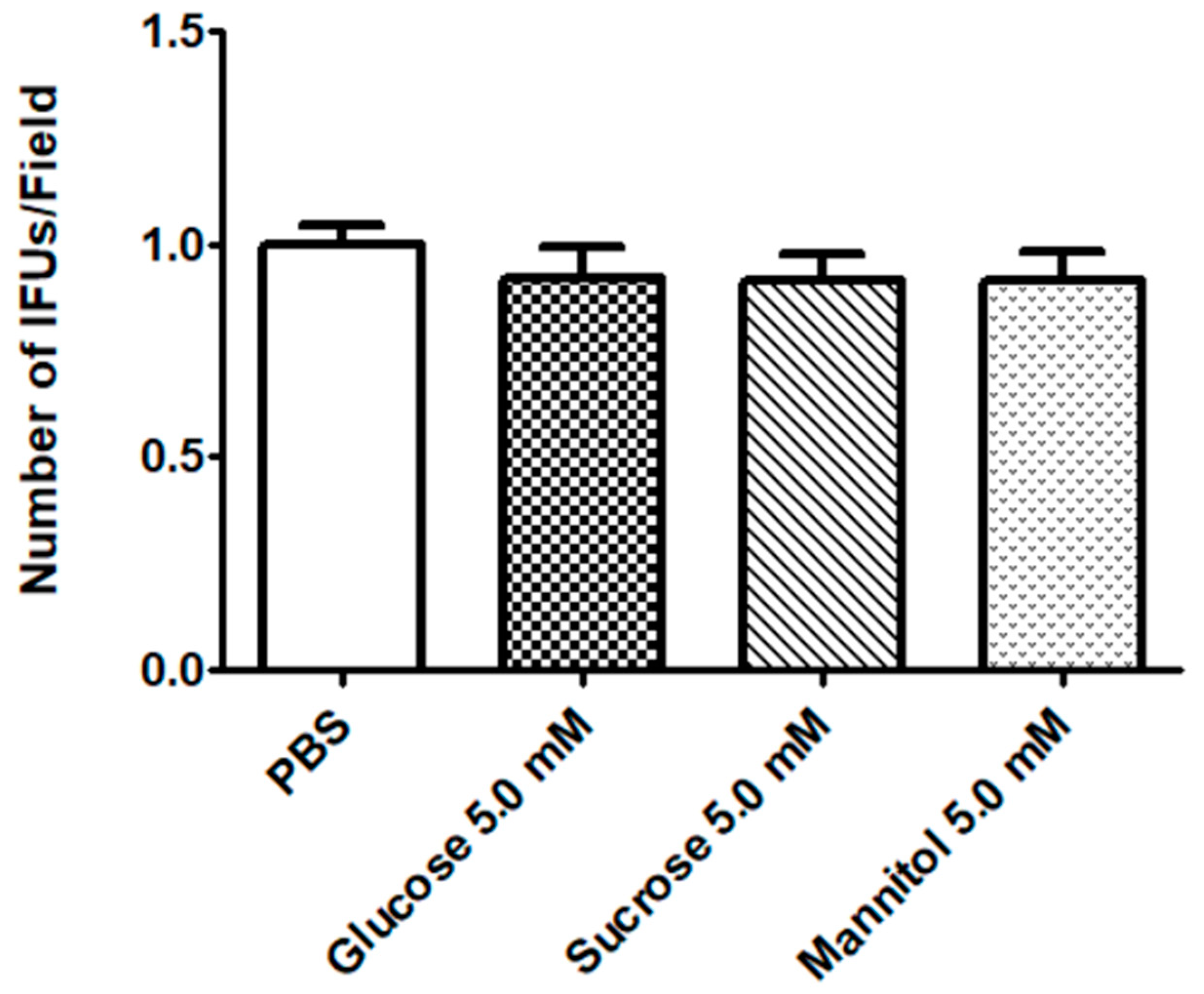
2.1. Effect of Sugar Solutions on CT Infectivity
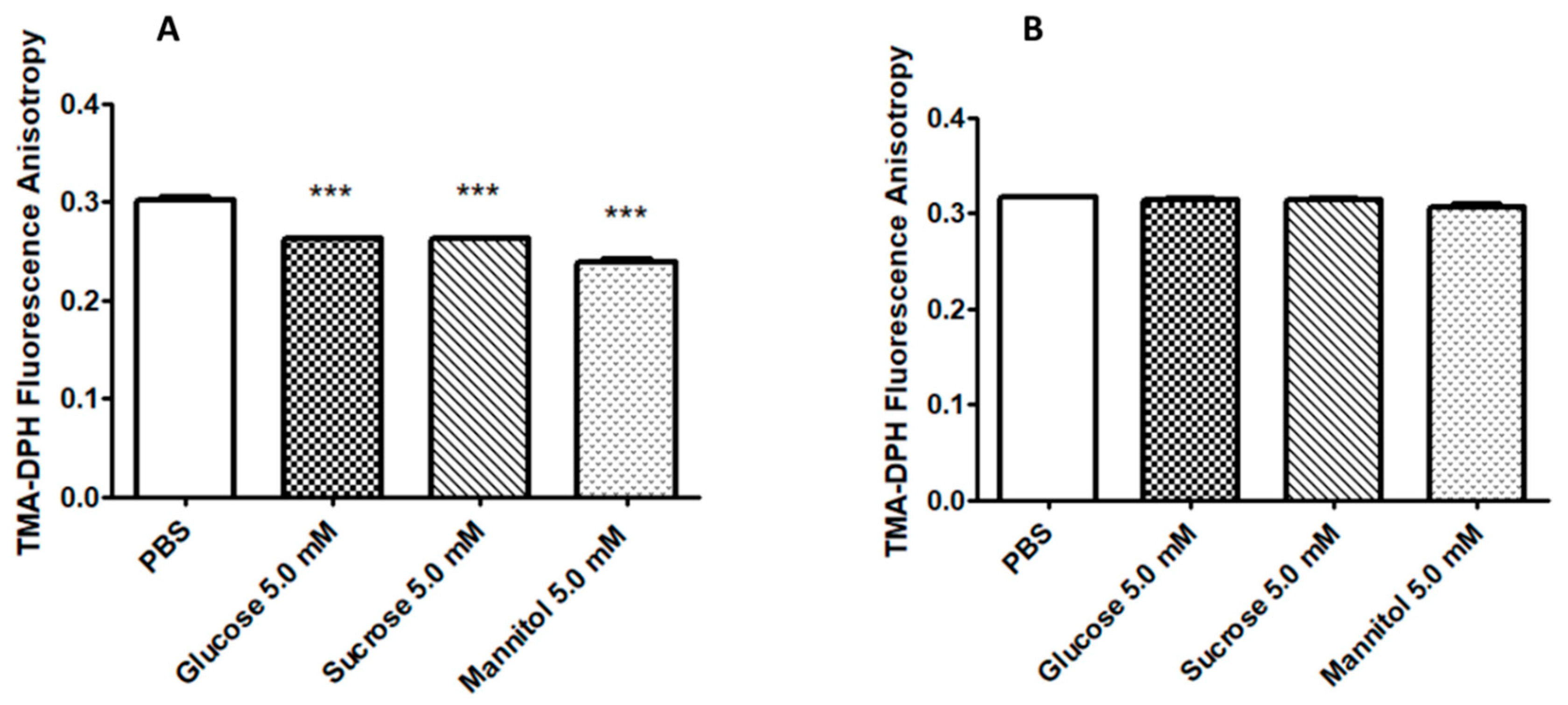
2.2. Sugar Solutions Increase CT EB Membrane Fluidity
2.3. CT EBs Incubated with Sugars Induce FAK Phosphorylation in HeLa Cells.
3. Discussion
4. Materials and Methods
4.1. Chlamydia trachomatis Strain and Cell Culture
4.2. Evaluation of CT Infectivity after EB Incubation with Sugar Solutions
4.3. Evaluation of CT Infectivity after HeLa incubation with Sugar Solutions
4.4. Fluorescence Anisotropy Measurements
4.5. Flow Cytometry
4.6. Cell Lysis and Western Blot Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- European Centre for Disease Prevention and Control. Sexually Transmitted Infection in Europe 2013; ECDC: Stockholm, Sweden, 2015. [Google Scholar]
- Menon, S.; Timms, P.; Allan, J.A.; Alexander, K.; Rombauts, L.; Horner, P.; Keltz, M.; Hocking, J.; Huston, W.M. Human and Pathogen Factors Associated with Chlamydia trachomatis-Related Infertility in Women. Clin. Microbiol. Rev. 2015, 28, 969–985. [Google Scholar] [CrossRef] [PubMed]
- Moulder, J.W. Interaction of chlamydiae and host cells in vitro. Microbiol. Rev. 1991, 55, 143–190. [Google Scholar] [CrossRef] [PubMed]
- Elwell, C.; Mirrashidi, K.; Engel, J. Chlamydia cell biology and pathogenesis. Nat. Rev. Microbiol. 2016, 14, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Omsland, A.; Sager, J.; Nair, V.; Sturdevant, D.E.; Hackstadt, T. Developmental stage-specific metabolic and transcriptional activity of Chlamydia trachomatis in an axenic medium. Proc. Natl. Acad. Sci. USA 2012, 109, 19781–19785. [Google Scholar] [CrossRef] [PubMed]
- Grieshaber, S.; Grieshaber, N.; Yang, H.; Baxter, B.; Hackstadt, T.; Omsland, A. Impact of Active Metabolism on Chlamydia trachomatis Elementary Body Transcript Profile and Infectivity. J. Bacteriol. 2018, 200, e00065-18. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hybiske, K.; Stephens, R.S. Orchestration of the mammalian host cell glucose transporter proteins-1 and 3 by Chlamydia contributes to intracellular growth and infectivity. Pathog. Dis. 2017, 75, ftx108. [Google Scholar] [CrossRef][Green Version]
- Mehlitz, A.; Eylert, E.; Huber, C.; Lindner, B.; Vollmuth, N.; Karunakaran, K.; Goebel, W.; Eisenreich, W.; Rudel, T. Metabolic adaptation of Chlamydia trachomatis to mammalian host cells. Mol. Microbiol. 2017, 103, 1004–1019. [Google Scholar] [CrossRef]
- Nardini, P.; Ñahui Palomino, R.A.; Parolin, C.; Laghi, L.; Foschi, C.; Cevenini, R.; Vitali, B.; Marangoni, A. Lactobacillus crispatus inhibits the infectivity of Chlamydia trachomatis elementary bodies, in vitro study. Sci. Rep. 2016, 6, 29024. [Google Scholar] [CrossRef]
- Foschi, C.; Laghi, L.; D’Antuono, A.; Gaspari, V.; Zhu, C.; Dellarosa, N.; Salvo, M.; Marangoni, A. Urine metabolome in women with Chlamydia trachomatis infection. PLoS ONE 2018, 13, e0194827. [Google Scholar] [CrossRef]
- Boehm, M.; Krause-Gruszczynska, M.; Rohde, M.; Tegtmeyer, N.; Takahashi, S.; Oyarzabal, O.A.; Backert, S. Major host factors involved in epithelial cell invasion of Campylobacter jejuni: Role of fibronectin, integrin β1, FAK, Tiam-1, and DOCK180 in activating Rho GTPase Rac1. Front. Cell. Infect. Microbiol. 2011, 1, 17. [Google Scholar] [CrossRef]
- Caccia, D.; Miccichè, F.; Cassinelli, G.; Mondellini, P.; Casalini, P.; Bongarzone, I. Dasatinib reduces FAK phosphorylation increasing the effects of RPI-1 inhibition in a RET/PTC1-expressing cell line. Mol. Cancer 2010, 9, 278. [Google Scholar] [CrossRef]
- Ma, P.C.; Tretiakova, M.S.; Nallasura, V.; Jagadeeswaran, R.; Husain, A.N.; Salgia, R. Downstream signalling and specific inhibition of c-MET/HGF pathway in small cell lung cancer: Implications for tumour invasion. Br. J. Cancer 2007, 97, 368–377. [Google Scholar] [CrossRef]
- Hazel, J.R.; Williams, E.E. The role of alterations in membrane lipid composition in enabling physiological adaptation of organisms to their physical environment. Prog. Lipid. Res. 1990, 29, 167–227. [Google Scholar] [CrossRef]
- Sajbidor, J. Effect of some environmental factors on the content and composition of microbial membrane lipids. Crit. Rev. Biotechnol. 1997, 17, 87–103. [Google Scholar] [CrossRef]
- Rose, A.H. Influence of the environment on microbial lipid composition. In Microbial Lipids; Ratledge, C., Wilkinson, S.G., Eds.; Academic Press: Toronto, ON, Canada, 1990; Volume 2, pp. 255–279. [Google Scholar]
- Cai, S.; He, F.; Samra, H.S.; de la Maza, L.M.; Bottazzi, M.E.; Joshi, S.B.; Middaugh, C.R. Biophysical and stabilization studies of the Chlamydia trachomatis mouse pneumonitis major outer membrane protein. Mol. Pharm. 2009, 6, 1553–1561. [Google Scholar] [CrossRef]
- Hussain, H.; Du, Y.; Scull, N.J.; Mortensen, J.S.; Tarrasch, J.; Bae, H.E.; Loland, C.J.; Byrne, B.; Kobilka, B.K.; Chae, P.S. Accessible Mannitol-Based Amphiphiles (MNAs) for Membrane Protein Solubilisation and Stabilisation. Chemistry 2016, 22, 7068–7073. [Google Scholar] [CrossRef] [PubMed]
- Savini, M.; Cecchini, C.; Verdenelli, M.C.; Silvi, S.; Orpianesi, C.; Cresci, A. Pilot-scale production and viability analysis of freeze-dried probiotic bacteria using different protective agents. Nutrients 2010, 2, 330–339. [Google Scholar] [CrossRef]
- Campbell, R.; Tasevska, N.; Jackson, K.G.; Sagi-Kiss, V.; di Paolo, N.; Mindell, J.S.; Lister, S.J.; Khaw, K.T.; Kuhnle, G.G.C. Association between urinary biomarkers of total sugar intake and measures of obesity in a cross-sectional study. PLoS ONE 2017, 12, e0179508. [Google Scholar] [CrossRef]
- Bouatra, S.; Aziat, F.; Mandal, R.; Guo, A.C.; Wilson, M.R.; Knox, C.; Bjorndahl, T.C.; Krishnamurthy, R.; Saleem, F.; Liu, P.; et al. The human urine metabolome. PLoS ONE 2013, 8, e73076. [Google Scholar] [CrossRef] [PubMed]
- Fadel, S.; Eley, A. Is lipopolysaccharide a factor in infectivity of Chlamydia trachomatis? J. Med. Microbiol. 2008, 57, 261–266. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ajonuma, L.C.; Fok, K.L.; Ho, L.S.; Chan, P.K.; Chow, P.H.; Tsang, L.L.; Wong, C.H.; Chen, J.; Li, S.; Rowlands, D.K.; et al. CFTR is required for cellular entry and internalization of Chlamydia trachomatis. Cell. Biol. Int. 2010, 34, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.D.; Cunningham, D.; Liang, X.; Chen, X.; Toone, E.J.; Raetz, C.R.; Zhou, P.; Valdivia, R.H. Lipooligosaccharide is required for the generation of infectious elementary bodies in Chlamydia trachomatis. Proc. Natl. Acad. Sci. USA 2011, 108, 10284–10289. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Briones, M.; Chiou, J.; Lei, L.; Patton, M.J.; Ma, L.; McClarty, G.; Caldwell, H.D. Chlamydia trachomatis Lipopolysaccharide Evades the Canonical and Noncanonical Inflammatory Pathways To Subvert Innate Immunity. mBio 2019, 10, e00595-19. [Google Scholar] [CrossRef] [PubMed]
- Heine, H.; Gronow, S.; Zamyatina, A.; Kosma, P.; Brade, H. Investigation on the agonistic and antagonistic biological activities of synthetic Chlamydia lipid A and its use in in vitro enzymatic assays. J. Endotoxin Res. 2007, 13, 126–132. [Google Scholar] [CrossRef]
- Slanina, H.; Hebling, S.; Hauck, C.R.; Schubert-Unkmeir, A. Cell invasion by Neisseria meningitidis requires a functional interplay between the focal adhesion kinase, Src and cortactin. PLoS ONE 2012, 7, e39613. [Google Scholar] [CrossRef]
- Dowd, G.C.; Bhalla, M.; Kean, B.; Thomas, R.; Ireton, K. Role of Host Type IA Phosphoinositide 3-Kinase Pathway Components in Invasin-Mediated Internalization of Yersinia enterocolitica. Infect. Immun. 2016, 84, 1826–1841. [Google Scholar] [CrossRef]
- Shi, J.; Casanova, J.E. Invasion of host cells by Salmonella typhimurium requires focal adhesion kinase and p130Cas. Mol. Biol. Cell 2006, 17, 4698–4708. [Google Scholar] [CrossRef]
- Parolin, C.; Frisco, G.; Foschi, C.; Giordani, B.; Salvo, M.; Vitali, B.; Marangoni, A.; Calonghi, N. Lactobacillus crispatus BC5 Interferes With Chlamydia trachomatis Infectivity Through Integrin Modulation in Cervical Cells. Front. Microbiol. 2018, 9, 2630. [Google Scholar] [CrossRef]
- Coombes, B.K.; Mahony, J.B. Identification of MEK- and phosphoinositide 3-kinase-dependent signalling as essential events during Chlamydia pneumoniae invasion of HEp2 cells. Cell. Microbiol. 2002, 4, 447–460. [Google Scholar] [CrossRef]
- Thwaites, T.; Nogueira, A.T.; Campeotto, I.; Silva, A.P.; Grieshaber, S.S.; Carabeo, R.A. The Chlamydia effector TarP mimics the mammalian leucine-aspartic acid motif of paxillin to subvert the focal adhesion kinase during invasion. J. Biol. Chem. 2014, 289, 30426–30442. [Google Scholar] [CrossRef]
- Foschi, C.; Bortolotti, M.; Marziali, G.; Polito, L.; Marangoni, A.; Bolognesi, A. Survival and death of intestinal cells infected by Chlamydia trachomatis. PLoS ONE 2019, 14, e0215956. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, A.; Fiorino, E.; Gilardi, F.; Aldini, R.; Scotti, E.; Nardini, P.; Foschi, C.; Donati, M.; Montagnani, M.; Cevenini, M.; et al. Chlamydia pneumoniae acute liver infection affects hepatic cholesterol and triglyceride metabolism in mice. Atherosclerosis 2015, 241, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, A.; Foschi, C.; Nardini, P.; D’Antuono, A.; Banzola, N.; Di Francesco, A.; Ostanello, F.; Russo, I.; Donati, M.; Cevenini, R. Chlamydia trachomatis serovar distribution and other sexually transmitted coinfections in subjects attending an STD outpatients clinic in Italy. New Microbiol. 2012, 35, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R.; Prendergast, F.G.; Hogen, D. Differential polarized phase fluorometric investigations of diphenylhexatriene in lipid bilayers. Quantitation of hindered depolarizing rotations. Biochemistry 1979, 18, 508–519. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marziali, G.; Marangoni, A.; Foschi, C.; Re, M.C.; Calonghi, N. Effect of Sugars on Chlamydia trachomatis Infectivity. Pathogens 2020, 9, 298. https://doi.org/10.3390/pathogens9040298
Marziali G, Marangoni A, Foschi C, Re MC, Calonghi N. Effect of Sugars on Chlamydia trachomatis Infectivity. Pathogens. 2020; 9(4):298. https://doi.org/10.3390/pathogens9040298
Chicago/Turabian StyleMarziali, Giacomo, Antonella Marangoni, Claudio Foschi, Maria Carla Re, and Natalia Calonghi. 2020. "Effect of Sugars on Chlamydia trachomatis Infectivity" Pathogens 9, no. 4: 298. https://doi.org/10.3390/pathogens9040298
APA StyleMarziali, G., Marangoni, A., Foschi, C., Re, M. C., & Calonghi, N. (2020). Effect of Sugars on Chlamydia trachomatis Infectivity. Pathogens, 9(4), 298. https://doi.org/10.3390/pathogens9040298






