Analysis of Porcine Pro- and Anti-Inflammatory Cytokine Induction by S. suis In Vivo and In Vitro
Abstract
1. Introduction
2. Results
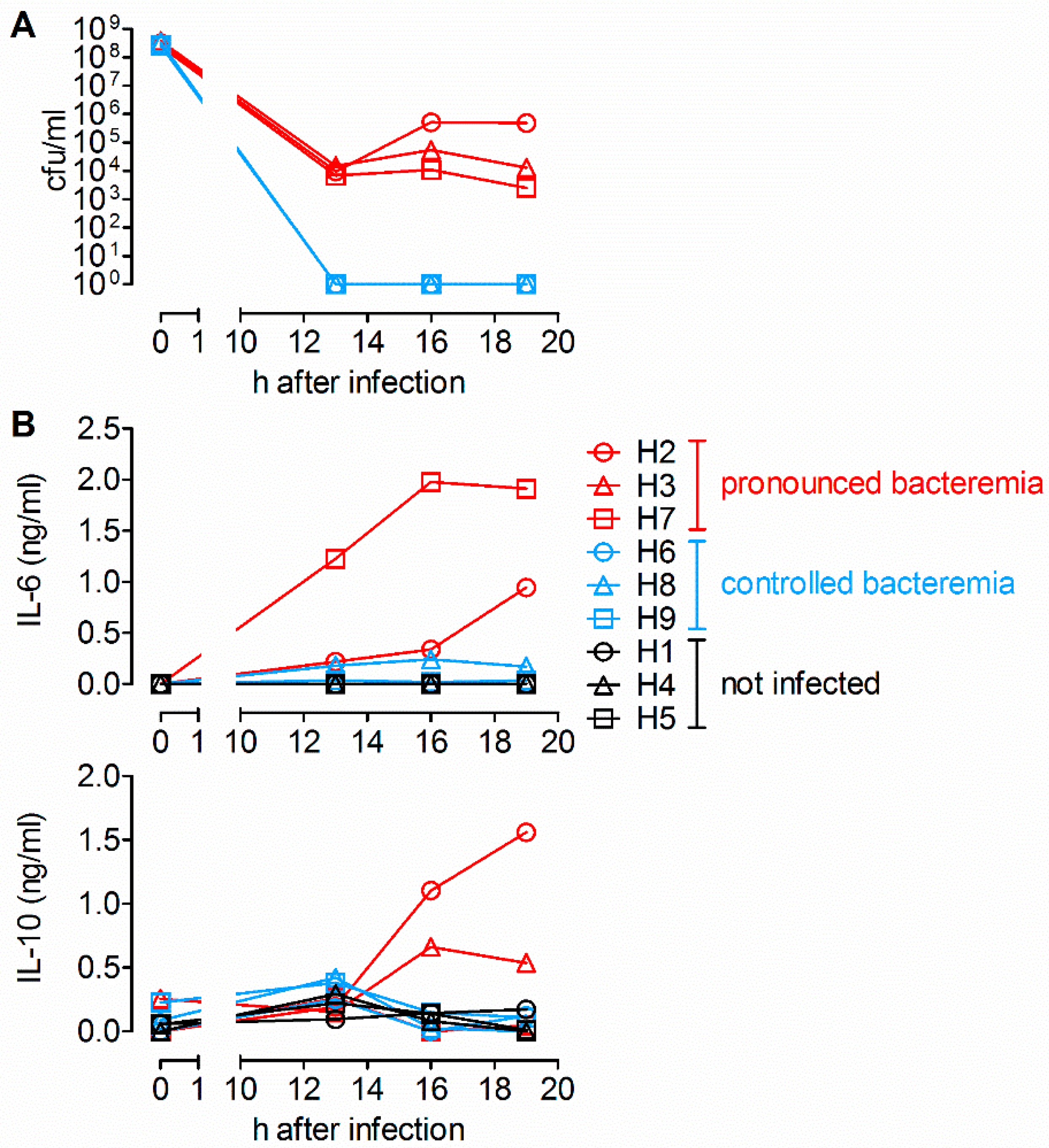
2.1. Detection of IL-6 and IL-10 in Sera of Piglets with Pronounced Bacteremia after Intravenous Infection with S. suis
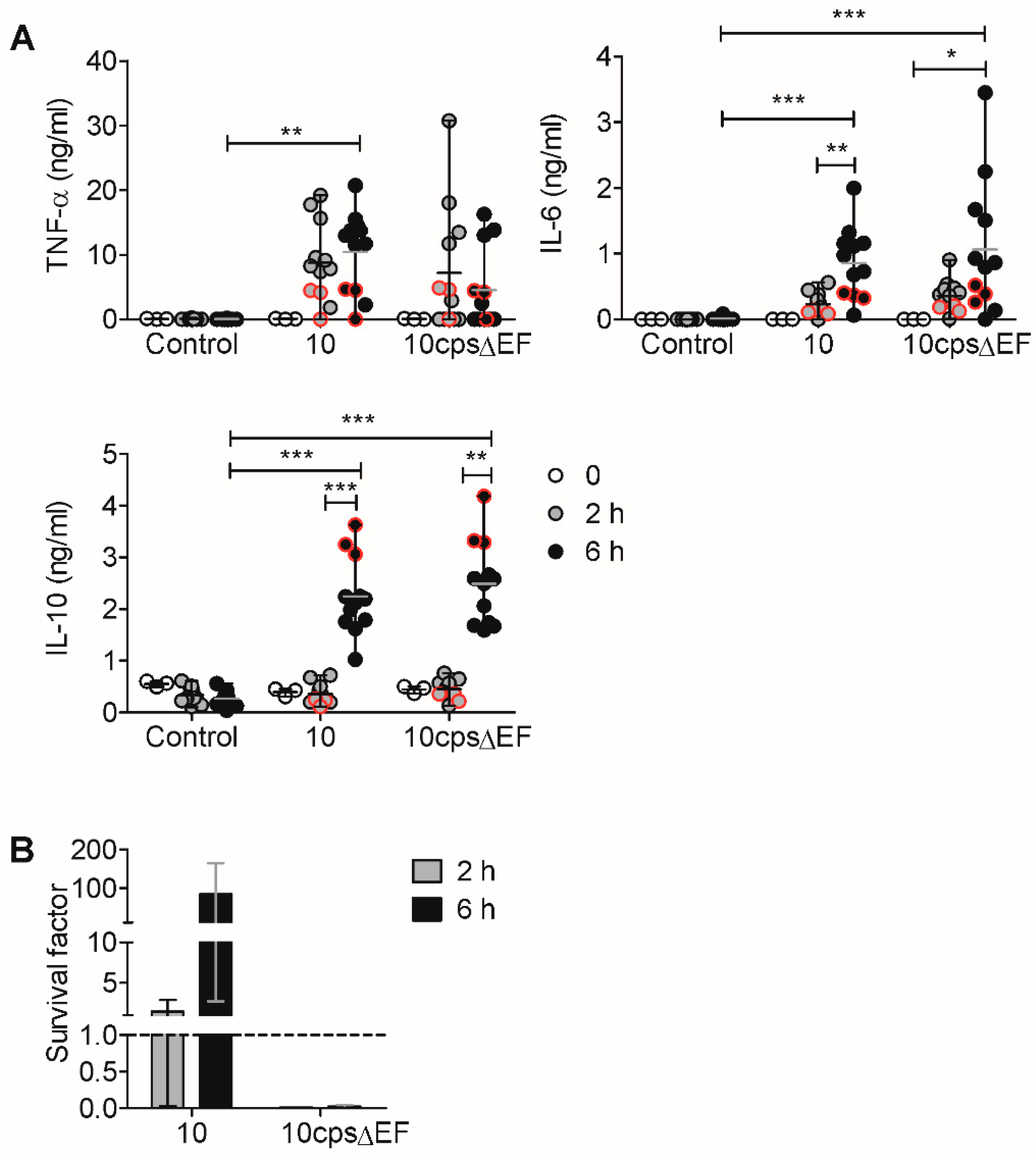
2.2. In Vitro Induction of Pro- and Anti-Inflammatory Cytokines by S. suis is Limited by IL-10
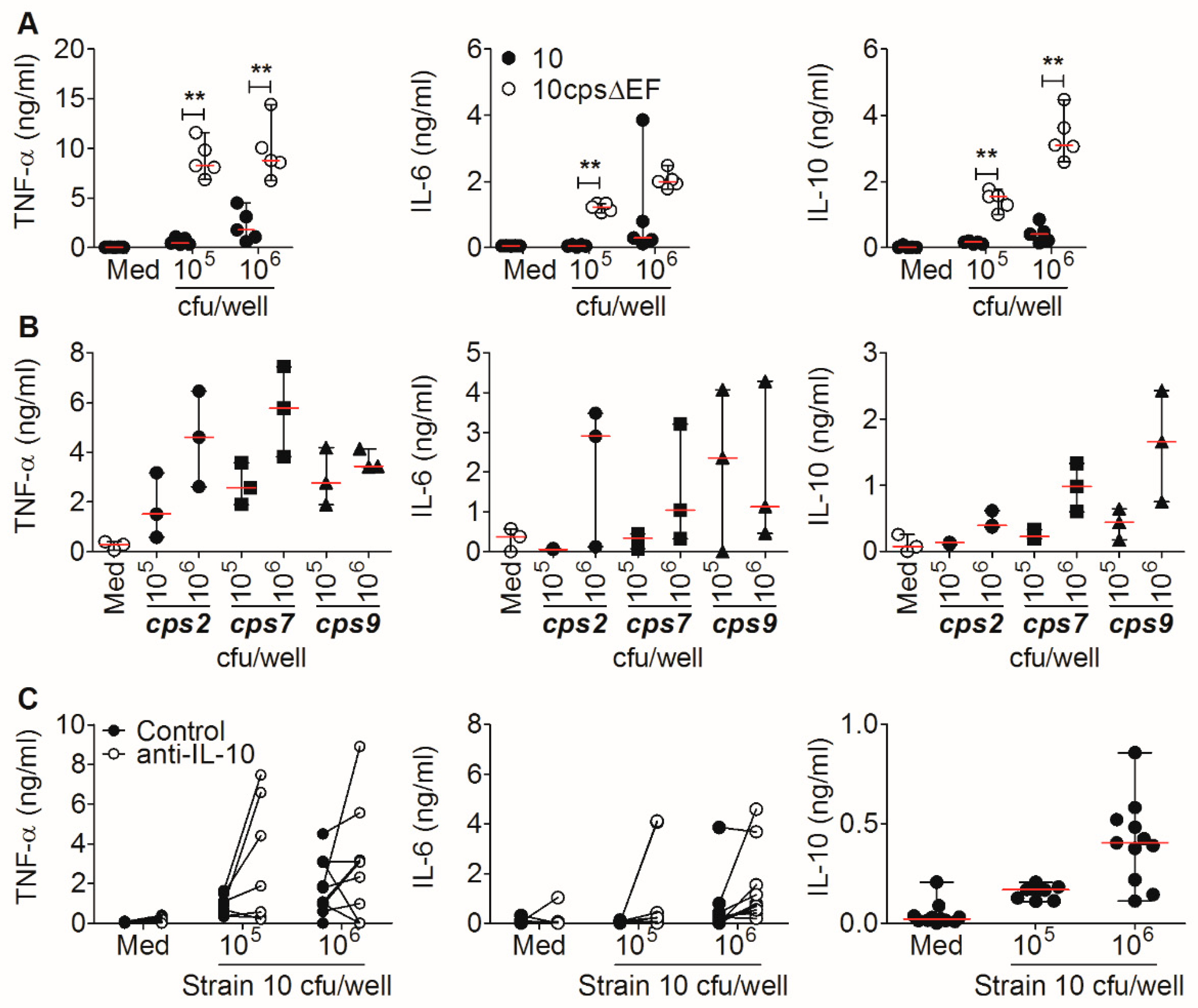
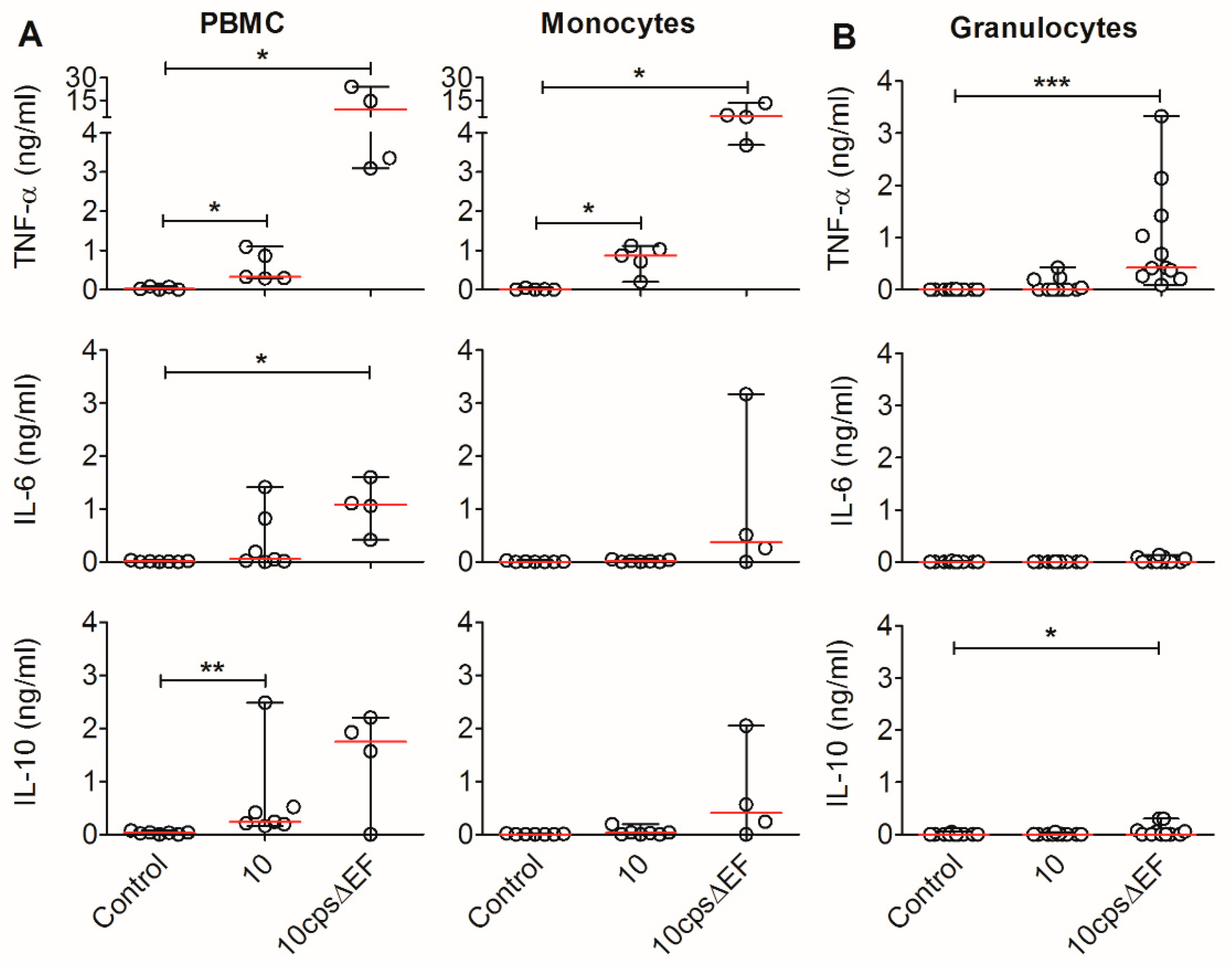
2.3. Encapsulated S. suis Induces TNF-α in Monocytes, but Not in Granulocytes
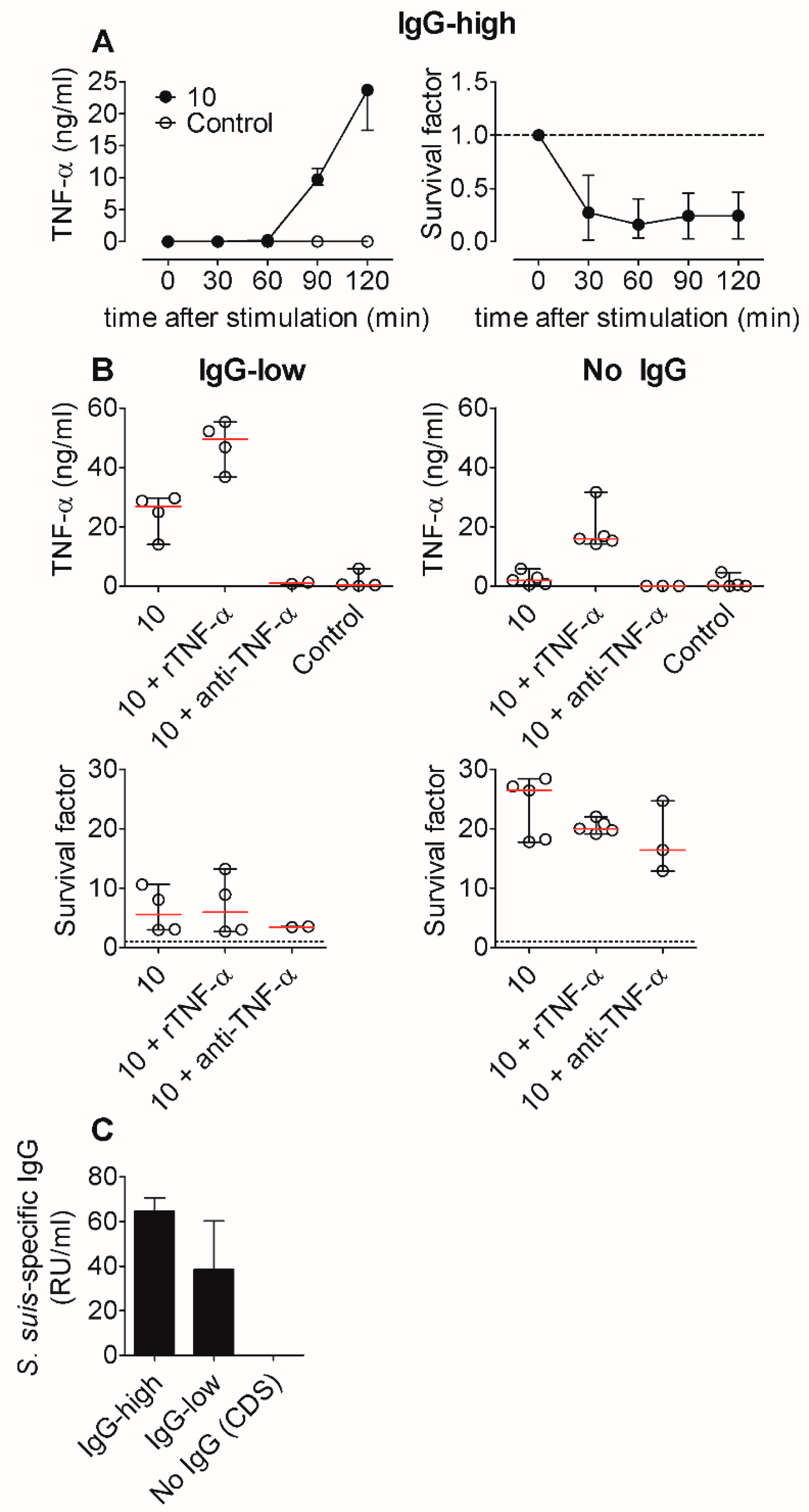
2.4. TNF-α Induction is Not Crucial for Bacterial Killing of S. suis In Vitro, Not Even in the Absence of S. suis-Specific Immunoglobulin (Ig) G
3. Discussion
4. Methods
4.1. Bacterial Strains
4.2. Animal Experiments and Blood Sampling
4.3. Bactericidal Assay
4.4. Isolation of PBMC, Monocytes, and Granulocytes
4.5. Cell Culture and Stimulation
4.6. Flow Cytometry
4.7. Cytokine Quantification
4.8. S. suis-Specific IgG Quantification
4.9. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goyette-Desjardins, G.; Auger, J.P.; Xu, J.; Segura, M.; Gottschalk, M. Streptococcus suis, an important pig pathogen and emerging zoonotic agent-an update on the worldwide distribution based on serotyping and sequence typing. Emerg. Microbes Infect. 2014, 3, 45. [Google Scholar] [CrossRef]
- Kerdsin, A.; Oishi, K.; Sripakdee, S.; Boonkerd, N.; Polwichai, P.; Nakamura, S.; Uchida, R.; Sawanpanyalert, P.; Dejsirilert, S. Clonal dissemination of human isolates of Streptococcus suis serotype 14 in Thailand. J. Med. Microbiol. 2009, 58, 1508–1513. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, C.; Varaldo, P.E.; Facinelli, B. Streptococcus suis, an Emerging Drug-Resistant Animal and Human Pathogen. Front. Microbiol. 2011, 2, 235. [Google Scholar] [CrossRef] [PubMed]
- Baele, M.; Chiers, K.; Devriese, L.A.; Smith, H.E.; Wisselink, H.J.; Vaneechoutte, M.; Haesebrouck, F. The Gram-positive tonsillar and nasal flora of piglets before and after weaning. J. Appl. Microbiol. 2001, 91, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Pena Cortes, L.C.; LeVeque, R.M.; Funk, J.; Marsh, T.L.; Mulks, M.H. Development of the tonsillar microbiome in pigs from newborn through weaning. BMC Microbiol. 2018, 18, 35. [Google Scholar] [CrossRef] [PubMed]
- Murase, K.; Watanabe, T.; Arai, S.; Kim, H.; Tohya, M.; Ishida-Kuroki, K.; Võ, T.H.; Nguyễn, T.P.B.; Nakagawa, I.; Osawa, R.; et al. Characterization of pig saliva as the major natural habitat of Streptococcus suis by analyzing oral, fecal, vaginal, and environmental microbiota. PLoS ONE 2019, 14. [Google Scholar] [CrossRef] [PubMed]
- Torremorell, M.; Calsamiglia, M.; Pijoan, C. Colonization of suckling pigs by Streptococcus suis with particular reference to pathogenic serotype 2 strains. Can. J. Vet. Res. 1998, 62, 21–26. [Google Scholar] [PubMed]
- Madsen, L.W.; Bak, H.; Nielsen, B.; Jensen, H.E.; Aalbaek, B.; Riising, H.J. Bacterial colonization and invasion in pigs experimentally exposed to Streptococcus suis serotype 2 in aerosol. J. Vet. Med. Ser. B 2002, 49, 211–215. [Google Scholar] [CrossRef]
- Straw, B.E. (Ed.) Diseases of Swine, 9th ed.; Blackwell Publ: Ames, IA, USA, 2006. [Google Scholar]
- Vasconcelos, D.; Middleton, D.M.; Chirino-Trejo, J.M. Lesions caused by natural infection with Streptococcus suis type 9 in weaned pigs. J. Vet. Diagn. Investig. 1994, 6, 335–341. [Google Scholar] [CrossRef]
- Perch, B.; Pedersen, K.B.; Henrichsen, J. Serology of capsulated streptococci pathogenic for pigs: Six new serotypes of Streptococcus suis. J. Clin. Microbiol. 1983, 17, 993–996. [Google Scholar]
- Gottschalk, M.; Higgins, R.; Jacques, M.; Mittal, K.R.; Henrichsen, J. Description of 14 new capsular types of Streptococcus suis. J. Clin. Microbiol. 1989, 27, 2633–2636. [Google Scholar] [PubMed]
- Gottschalk, M.; Higgins, R.; Jacques, M.; Beaudoin, M.; Henrichsen, J. Characterization of six new capsular types (23 through 28) of Streptococcus suis. J. Clin. Microbiol. 1991, 29, 2590–2594. [Google Scholar] [PubMed]
- Higgins, R.; Gottschalk, M.; Boudreau, M.; Lebrun, A.; Henrichsen, J. Description of six new capsular types (29–34) of Streptococcus suis. J. Vet. Diagn. Investig. 1995, 7, 405–406. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.E.; Gottschalk, M.; Brousseau, R.; Harel, J.; Hemmingsen, S.M.; Goh, S.H. Biochemical analysis, cpn60 and 16S rDNA sequence data indicate that Streptococcus suis serotypes 32 and 34, isolated from pigs, are Streptococcus orisratti. Vet. Microbiol. 2005, 107, 63–69. [Google Scholar] [CrossRef]
- Nomoto, R.; Maruyama, F.; Ishida, S.; Tohya, M.; Sekizaki, T.; Osawa, R. Reappraisal of the taxonomy of Streptococcus suis serotypes 20, 22 and 26: Streptococcus parasuis sp. nov. Int. J. Syst. Evol. Microbiol. 2015, 65, 438–443. [Google Scholar] [CrossRef]
- Pan, Z.; Ma, J.; Dong, W.; Song, W.; Wang, K.; Lu, C.; Yao, H. Novel variant serotype of Streptococcus suis isolated from piglets with meningitis. Appl. Environ. Microbiol. 2015, 81, 976–985. [Google Scholar] [CrossRef]
- Tohya, M.; Arai, S.; Tomida, J.; Watanabe, T.; Kawamura, Y.; Katsumi, M.; Ushimizu, M.; Ishida-Kuroki, K.; Yoshizumi, M.; Uzawa YIguchi, S.; et al. Defining the taxonomic status of Streptococcus suis serotype 33: The proposal for Streptococcus ruminantium sp. nov. Int. J. Syst. Evol. Microbiol. 2017, 67, 3660–3665. [Google Scholar] [CrossRef]
- Smith, H.E.; Damman, M.; van der Velde, J.; Wagenaar, F.; Wisselink, H.J.; Stockhofe-Zurwieden, N.; Smits, M.A. Identification and characterization of the cps locus of Streptococcus suis serotype 2: The capsule protects against phagocytosis and is an important virulence factor. Infect. Immun. 1999, 67, 1750–1756. [Google Scholar]
- Segura, M.; Gottschalk, M.; Olivier, M. Encapsulated Streptococcus suis inhibits activation of signaling pathways involved in phagocytosis. Infect. Immun. 2004, 72, 5322–5330. [Google Scholar] [CrossRef]
- Benga, L.; Fulde, M.; Neis, C.; Goethe, R.; Valentin-Weigand, P. Polysaccharide capsule and suilysin contribute to extracellular survival of Streptococcus suis co-cultivated with primary porcine phagocytes. Vet. Microbiol. 2008, 132, 211–219. [Google Scholar] [CrossRef]
- Houde, M.; Gottschalk, M.; Gagnon, F.; van Calsteren, M.R.; Segura, M. Streptococcus suis capsular polysaccharide inhibits phagocytosis through destabilization of lipid microdomains and prevents lactosylceramide-dependent recognition. Infect. Immun. 2012, 80, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Segura, M. Fisher scientific award lecture—The capsular polysaccharides of Group B Streptococcus and Streptococcus suis differently modulate bacterial interactions with dendritic cells. Can. J. Microbiol. 2012, 58, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Meijerink, M.; Ferrando, M.L.; Lammers, G.; Taverne, N.; Smith, H.E.; Wells, J.M. Immunomodulatory effects of Streptococcus suis capsule type on human dendritic cell responses, phagocytosis and intracellular survival. PLoS ONE 2012, 7, 35849. [Google Scholar] [CrossRef] [PubMed]
- Charland, N.; Harel, J.; Kobisch, M.; Lacasse, S.; Gottschalk, M. Streptococcus suis serotype 2 mutants deficient in capsular expression. Microbiology 1998, 144, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Segura, M.A.; Cléroux, P.; Gottschalk, M. Streptococcus suis and group B Streptococcus differ in their interactions with murine macrophages. FEMS Immunol. Med. Microbiol. 1998, 21, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Segura, M.; Stankova, J.; Gottschalk, M. Heat-Killed Streptococcus suis Capsular Type 2 Strains Stimulate Tumor Necrosis Factor Alpha and Interleukin-6 Production by Murine Macrophages. Infect. Immun. 1999, 67, 4646–4654. [Google Scholar] [CrossRef]
- Segura, M.; Vadeboncoeur, N.; Gottschalk, M. CD14-dependent and -independent cytokine and chemokine production by human THP-1 monocytes stimulated by Streptococcus suis capsular type 2. Clin. Exp. Immunol. 2002, 127, 243–254. [Google Scholar] [CrossRef]
- Segura, M.; Vanier, G.; Al-Numani, D.; Lacouture, S.; Olivier, M.; Gottschalk, M. Proinflammatory cytokine and chemokine modulation by Streptococcus suis in a whole-blood culture system. FEMS Immunol. Med. Microbiol. 2006, 47, 92–106. [Google Scholar] [CrossRef]
- Müller-Alouf, H.; Alouf, J.E.; Gerlach, D.; Ozegowski, J.H.; Fitting, C.; Cavaillon, J.M. Comparative study of cytokine release by human peripheral blood mononuclear cells stimulated with Streptococcus pyogenes superantigenic erythrogenic toxins, heat-killed streptococci, and lipopolysaccharide. Infect. Immun. 1994, 62, 4915–4921. [Google Scholar]
- Dominguez-Punaro, M.C.; Segura, M.; Plante, M.M.; Lacouture, S.; Rivest, S.; Gottschalk, M. Streptococcus suis Serotype 2, an Important Swine and Human Pathogen, Induces Strong Systemic and Cerebral Inflammatory Responses in a Mouse Model of Infection. J. Immunol. 2007, 179, 1842–1854. [Google Scholar] [CrossRef]
- Graveline, R.; Segura, M.; Radzioch, D.; Gottschalk, M. TLR2-dependent recognition of Streptococcus suis is modulated by the presence of capsular polysaccharide which modifies macrophage responsiveness. Int. Immunol. 2007, 19, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Chabot-Roy, G.; Willson, P.; Segura, M.; Lacouture, S.; Gottschalk, M. Phagocytosis and killing of Streptococcus suis by porcine neutrophils. Microb. Pathog. 2006, 41, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Rieckmann, K.; Seydel, A.; Szewczyk, K.; Klimke, K.; Rungelrath, V.; Baums, C.G. Streptococcus suis cps7: An emerging virulent sequence type (ST29) shows a distinct, IgM-determined pattern of bacterial survival in blood of piglets during the early adaptive immune response after weaning. Vet. Res. 2018, 49, 405. [Google Scholar] [CrossRef] [PubMed]
- Rieckmann, K.; Seydel, A.; Klose, K.; Alber, G.; Baums, C.G.; Schütze, N. Vaccination with the immunoglobulin M-degrading enzyme of Streptococcus suis, Ide, leads to protection against a highly virulent serotype 9 strain. Vaccine X 2019, 3, 100046. [Google Scholar] [CrossRef] [PubMed]
- Tecchio, C.; Micheletti, A.; Cassatella, M.A. Neutrophil-Derived Cytokines: Facts Beyond Expression. Front. Immunol. 2014, 5, 508. [Google Scholar] [CrossRef] [PubMed]
- Raymond, C.R.; Wilkie, B.N. Toll-like receptor, MHC II, B7 and cytokine expression by porcine monocytes and monocyte-derived dendritic cells in response to microbial pathogen-associated molecular patterns. Vet. Immunol. Immunopathol. 2005, 107, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Sipos, W.; Duvigneau, C.J.; Hartl, R.T.; Schwendenwein, I. Exploratory reference intervals on hematology and cellular immune system of multiparous Large White sows. Vet. Immunol. Immunopathol. 2011, 141, 307–311. [Google Scholar] [CrossRef][Green Version]
- Oliver, J.C.; Bland, L.A.; Oettinger, C.W.; Arduino, M.J.; McAllister, S.K.; Aguero, S.M.; Favero, M.S. Cytokine kinetics in an in vitro whole blood model following an endotoxin challenge. Lymphokine Cytok. Res. 1993, 12, 115–120. [Google Scholar]
- DeForge, L.E.; Kenney, J.S.; Jones, M.L.; Warren, J.S.; Remick, D.G. Biphasic production of IL-8 in lipopolysaccharide (LPS)-stimulated human whole blood. Separation of LPS- and cytokine-stimulated components using anti-tumor necrosis factor and anti-IL-1 antibodies. J. Immunol. 1992, 148, 2133–2141. [Google Scholar]
- Baums, C.G.; Valentin-Weigand, P. Surface-associated and secreted factors of Streptococcus suis in epidemiology, pathogenesis and vaccine development. Anim. Health Res. Rev. 2009, 10, 65–83. [Google Scholar] [CrossRef]
- Segura, M.; Gottschalk, M. Streptococcus suis interactions with the murine macrophage cell line J774: Adhesion and cytotoxicity. Infect. Immun. 2002, 70, 4312–4322. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Heitbrock, H.W.; Passlick, B.; Käfferlein, E.; Coulie, P.G.; Izbicki, J.R. Protection against lethal pneumococcal septicemia in pigs is associated with decreased levels of interleukin-6 in blood. Infect. Immun. 1992, 60, 1692–1694. [Google Scholar] [PubMed]
- Auray, G.; Lachance, C.; Wang, Y.; Gagnon, C.A.; Segura, M.; Gottschalk, M. Transcriptional Analysis of PRRSV-Infected Porcine Dendritic Cell Response to Streptococcus suis Infection Reveals Up-Regulation of Inflammatory-Related Genes Expression. PLoS ONE 2016, 11, 0156019. [Google Scholar] [CrossRef] [PubMed]
- Van der Sluijs, K.F.; van Elden, L.J.R.; Nijhuis, M.; Schuurman, R.; Pater, J.M.; Florquin, S.; Goldman, M.; Jansen, H.M.; Lutter, R.; van der Poll, T.; et al. IL-10 is an important mediator of the enhanced susceptibility to pneumococcal pneumonia after influenza infection. J. Immunol. 2004, 172, 7603–7609. [Google Scholar] [CrossRef] [PubMed]
- Suradhat, S.; Thanawongnuwech, R.; Poovorawan, Y. Upregulation of IL-10 gene expression in porcine peripheral blood mononuclear cells by porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 2003, 84, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Greenberger, M.J.; Strieter, R.M.; Kunkel, S.L.; Danforth, J.M.; Goodman, R.E.; Standiford, T.J. Neutralization of IL-10 increases survival in a murine model of Klebsiella pneumonia. J. Immunol. 1995, 155, 722–729. [Google Scholar]
- Lehmann, A.K.; Halstensen, A.; Sørnes, S.; Røkke, O.; Waage, A. High levels of interleukin 10 in serum are associated with fatality in meningococcal disease. Infect. Immun. 1995, 63, 2109–2112. [Google Scholar]
- Laichalk, L.L.; Danforth, J.M.; Standiford, T.J. Interleukin-10 inhibits neutrophil phagocytic and bactericidal activity. FEMS Immunol Med. Microbiol. 1996, 15, 181–187. [Google Scholar] [CrossRef]
- Sneath, P.H.A.; Bergey, D.H.; Holt, J.G. (Eds.) Bergey’s Manual of Systematic Bacteriology; Williams & Wilkins: Baltimore, MD, USA, 1992. [Google Scholar]
- Gawad, K.A.; Schneider, C.; Brinken, B.; Hufländer, A.; Diederichs, B.; Mack, D.; Gutensohn, K.; Izbicki, J.R. Anti-TNF antibody treatment has no positive effect on survival in a model of pneumococcal sepsis in pigs. J. Investig. Surg. 2001, 14, 291–297. [Google Scholar] [CrossRef]
- Lin, L.; Xu, L.; Lv, W.; Han, L.; Xiang, Y.; Fu, L.; Jin, M.; Zhou, R.; Chen, H.; Zhang, A. An NLRP3 inflammasome-triggered cytokine storm contributes to Streptococcal toxic shock-like syndrome (STSLS). PLoS Pathog. 2019, 15, 1007795. [Google Scholar] [CrossRef]
- Vecht, U.; Wisselink, H.J.; Stockhofe-Zurwieden, N.; Smith, H.E. Characterization of virulence of the Streptococcus suis serotype 2 reference strain Henrichsen S 735 in newborn gnotobiotic pigs. Vet. Microbiol. 1996, 51, 125–136. [Google Scholar] [CrossRef]
- Seele, J.; Singpiel, A.; Spoerry, C.; Pawel-Rammingen, U.; von Valentin-Weigand, P.; Baums, C.G. Identification of a novel host-specific IgM protease in Streptococcus suis. J. Bacteriol. 2013, 195, 930–940. [Google Scholar] [CrossRef] [PubMed]
- Franzoni, G.; Graham, S.P.; Sanna, G.; Angioi, P.; Fiori, M.S.; Anfossi, A.; Amadori, M.; Dei Giudici, S.; Oggiano, A. Interaction of porcine monocyte-derived dendritic cells with African swine fever viruses of diverse virulence. Vet Microbiol. 2018, 216, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-González, S.; Ruggli, N.; Rosell, R.; Pérez, L.J.; Frías-Leuporeau, M.T.; Fraile, L.; Montoya, M.; Cordoba, L.; Domingo, M.; Ehrensperger, F.; et al. Postnatal persistent infection with classical Swine Fever virus and its immunological implications. PLoS ONE 2015, 10, e0125692. [Google Scholar] [CrossRef]
- Baums, C.G.; Kock, C.; Beineke, A.; Bennecke, K.; Goethe, R.; Schroder, C.; Waldmann, K.H.; Valentin-Weigand, P. Streptococcus suis bacterin and subunit vaccine immunogenicities and protective efficacies against serotypes 2 and 9. Clin. Vaccine Immunol. 2009, 16, 200–208. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hohnstein, F.S.; Meurer, M.; de Buhr, N.; von Köckritz-Blickwede, M.; Baums, C.G.; Alber, G.; Schütze, N. Analysis of Porcine Pro- and Anti-Inflammatory Cytokine Induction by S. suis In Vivo and In Vitro. Pathogens 2020, 9, 40. https://doi.org/10.3390/pathogens9010040
Hohnstein FS, Meurer M, de Buhr N, von Köckritz-Blickwede M, Baums CG, Alber G, Schütze N. Analysis of Porcine Pro- and Anti-Inflammatory Cytokine Induction by S. suis In Vivo and In Vitro. Pathogens. 2020; 9(1):40. https://doi.org/10.3390/pathogens9010040
Chicago/Turabian StyleHohnstein, Florian S., Marita Meurer, Nicole de Buhr, Maren von Köckritz-Blickwede, Christoph G. Baums, Gottfried Alber, and Nicole Schütze. 2020. "Analysis of Porcine Pro- and Anti-Inflammatory Cytokine Induction by S. suis In Vivo and In Vitro" Pathogens 9, no. 1: 40. https://doi.org/10.3390/pathogens9010040
APA StyleHohnstein, F. S., Meurer, M., de Buhr, N., von Köckritz-Blickwede, M., Baums, C. G., Alber, G., & Schütze, N. (2020). Analysis of Porcine Pro- and Anti-Inflammatory Cytokine Induction by S. suis In Vivo and In Vitro. Pathogens, 9(1), 40. https://doi.org/10.3390/pathogens9010040