Membrane Binding, Cellular Cholesterol Content and Resealing Capacity Contribute to Epithelial Cell Damage Induced by Suilysin of Streptococcus suis
Abstract
1. Introduction
2. Results and Discussion
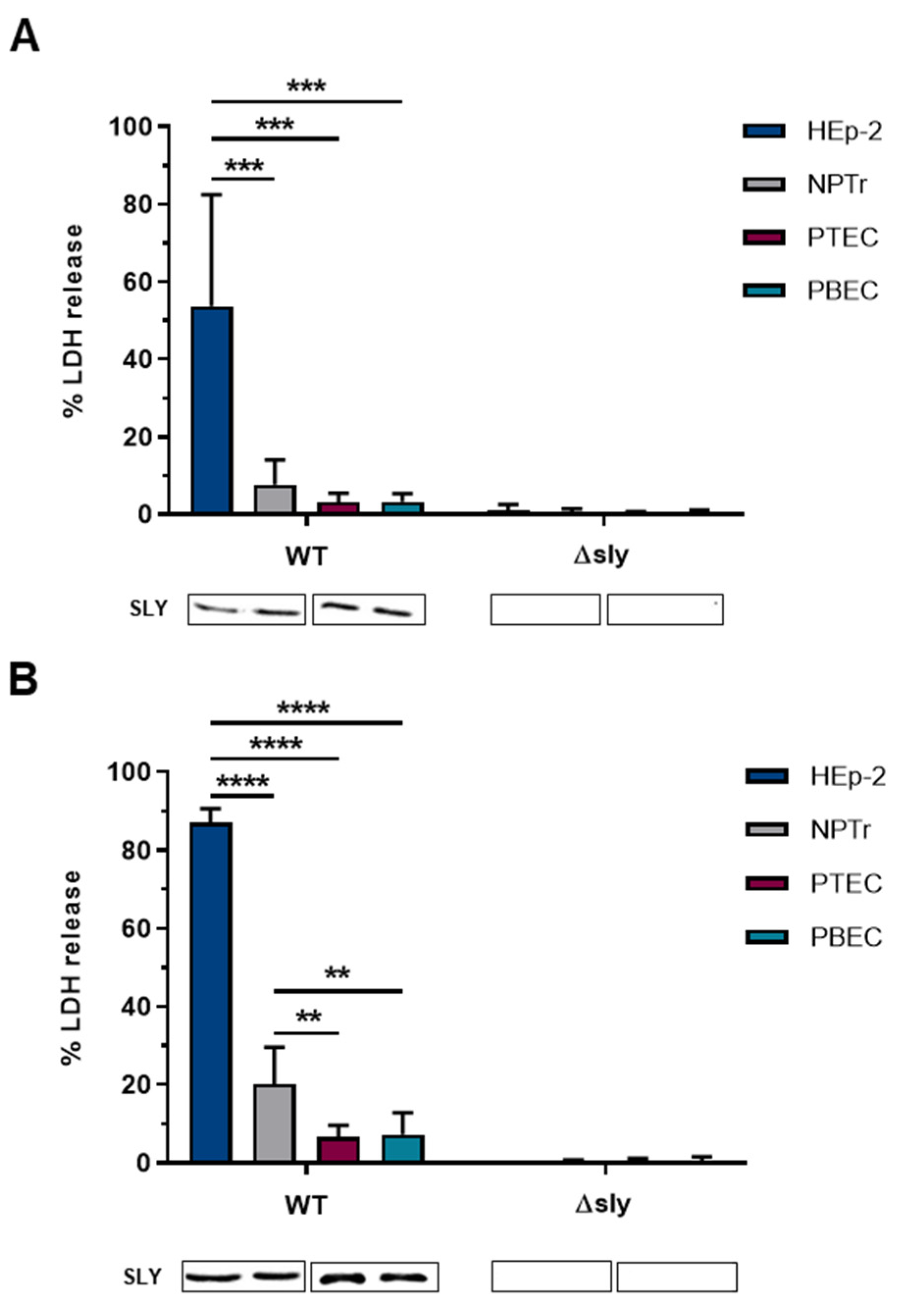
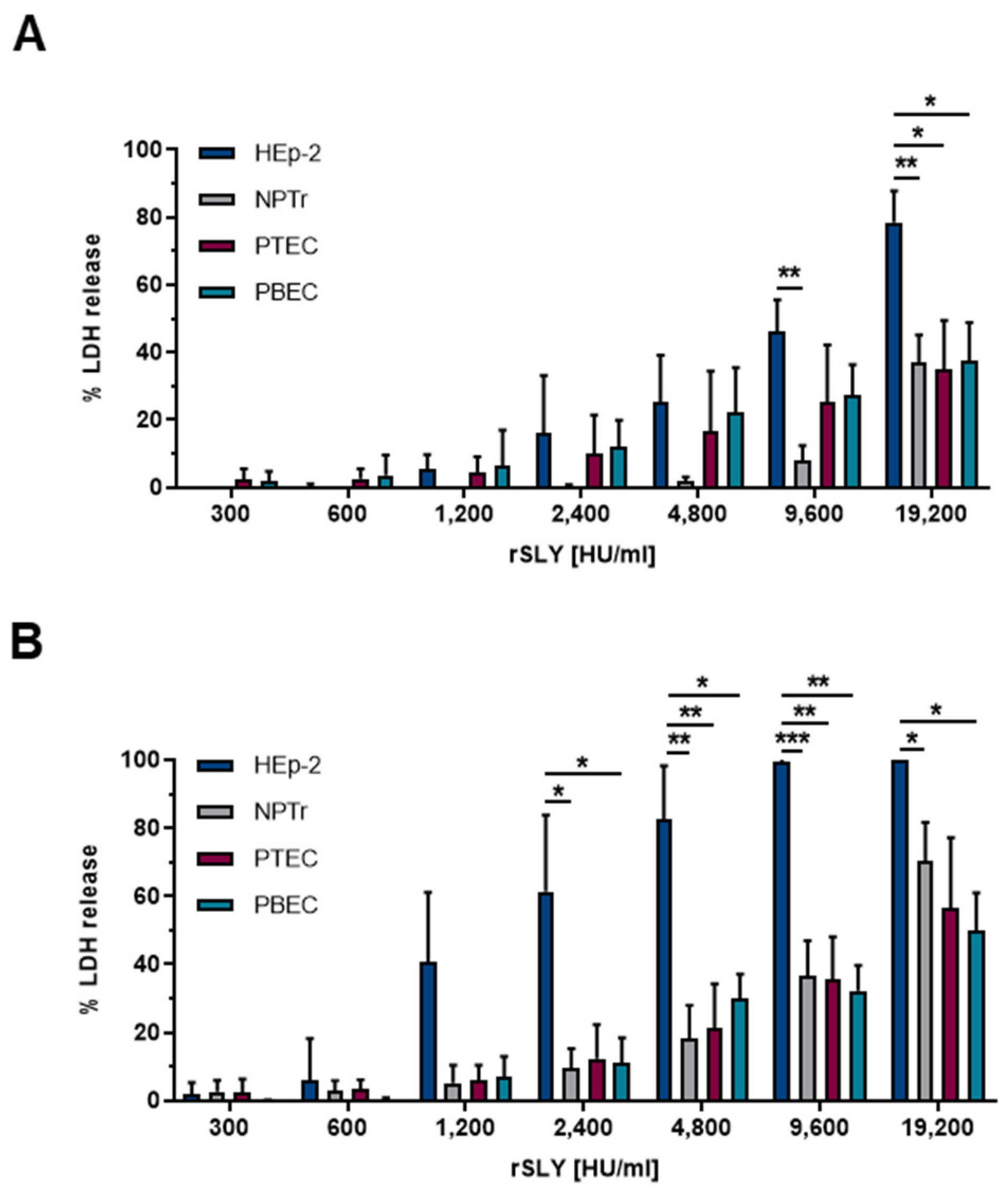
2.1. Time- and Dose-Dependent Damage in Different Respiratory Epithelial Cells Caused by SLY
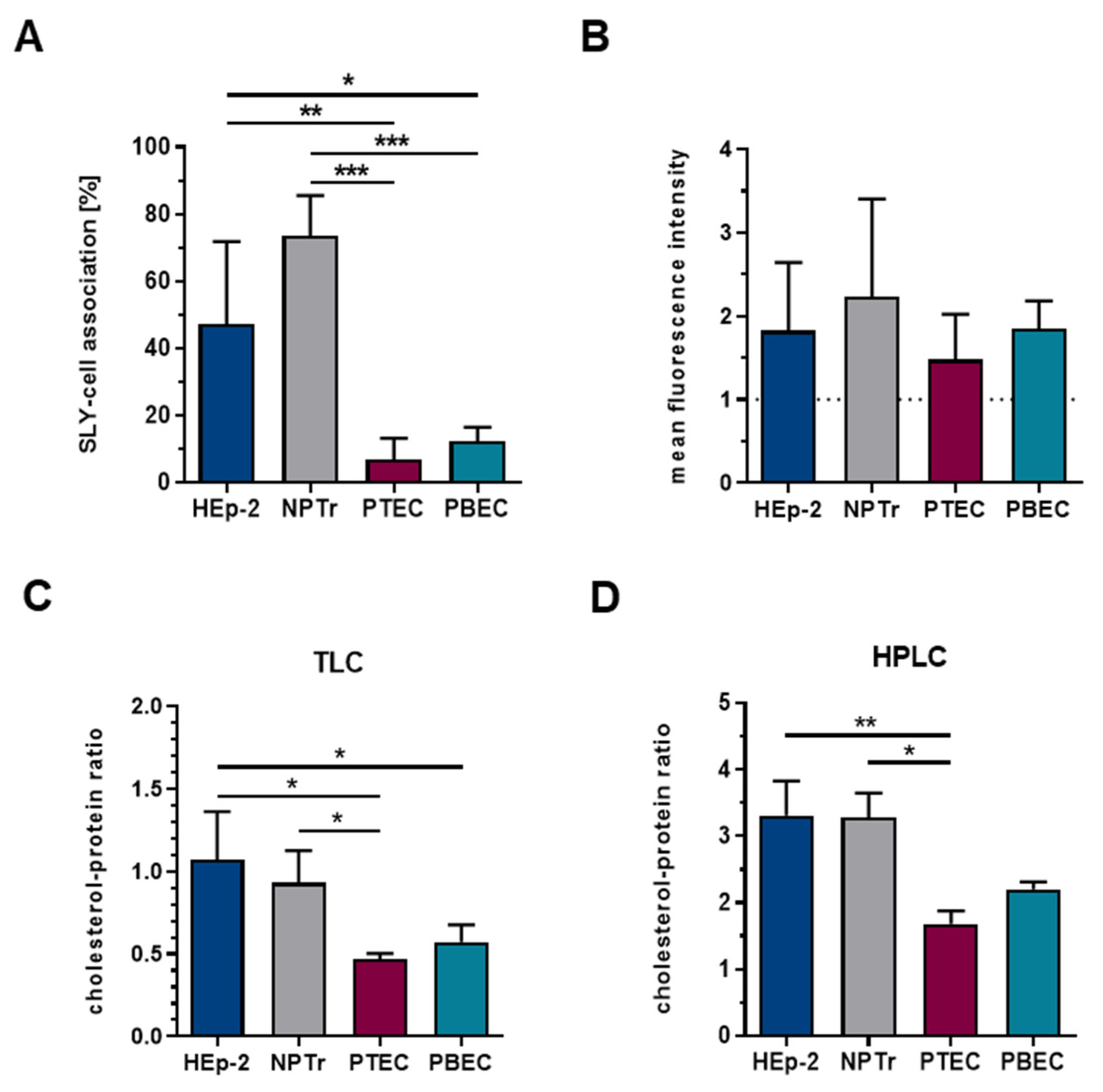
2.2. The Amount of Membrane-Bound SLY Is Not the Sole Factor Contributing to Cellular Damage and Is Not Only Dependent on the Total Cellular Cholesterol Content
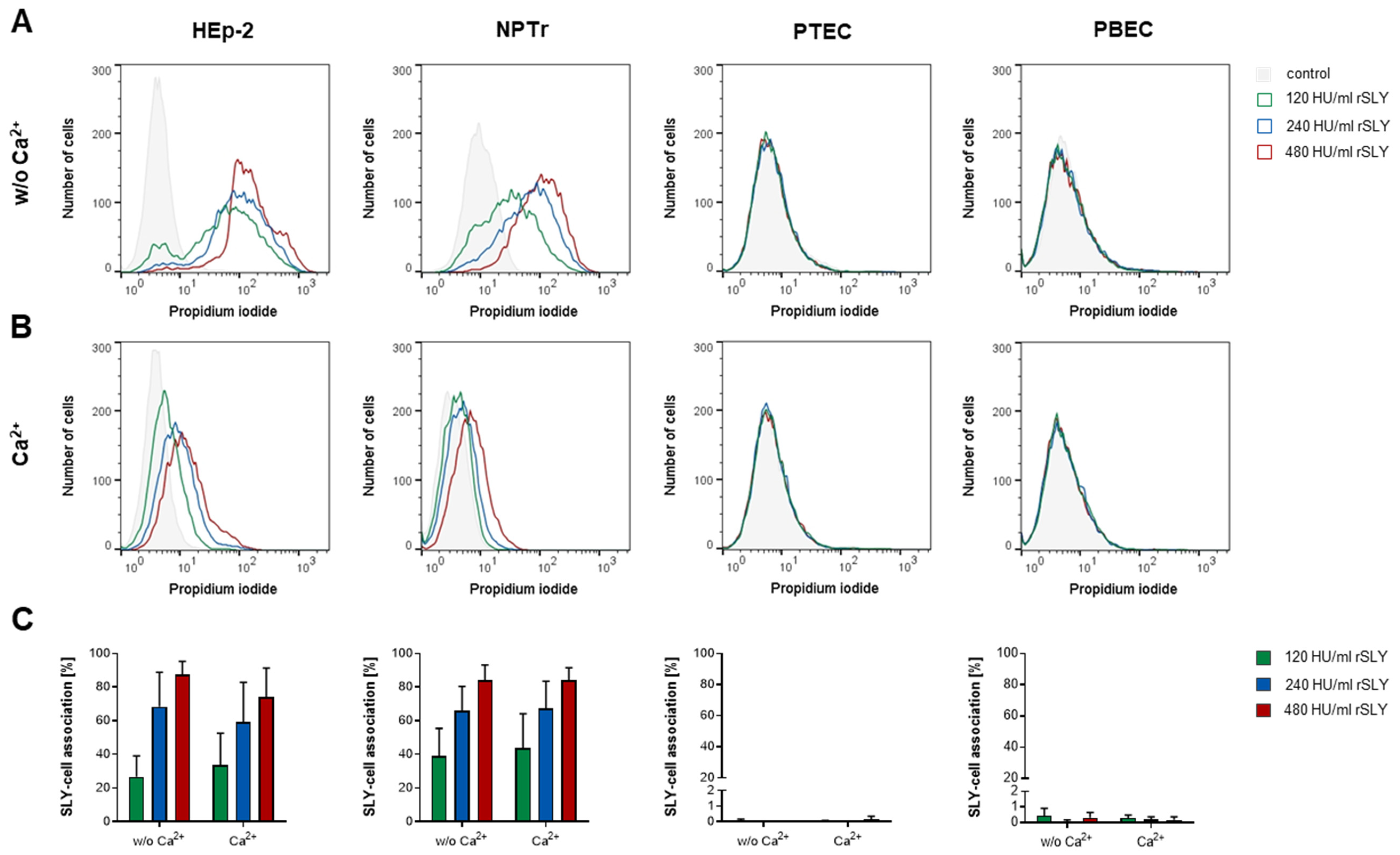
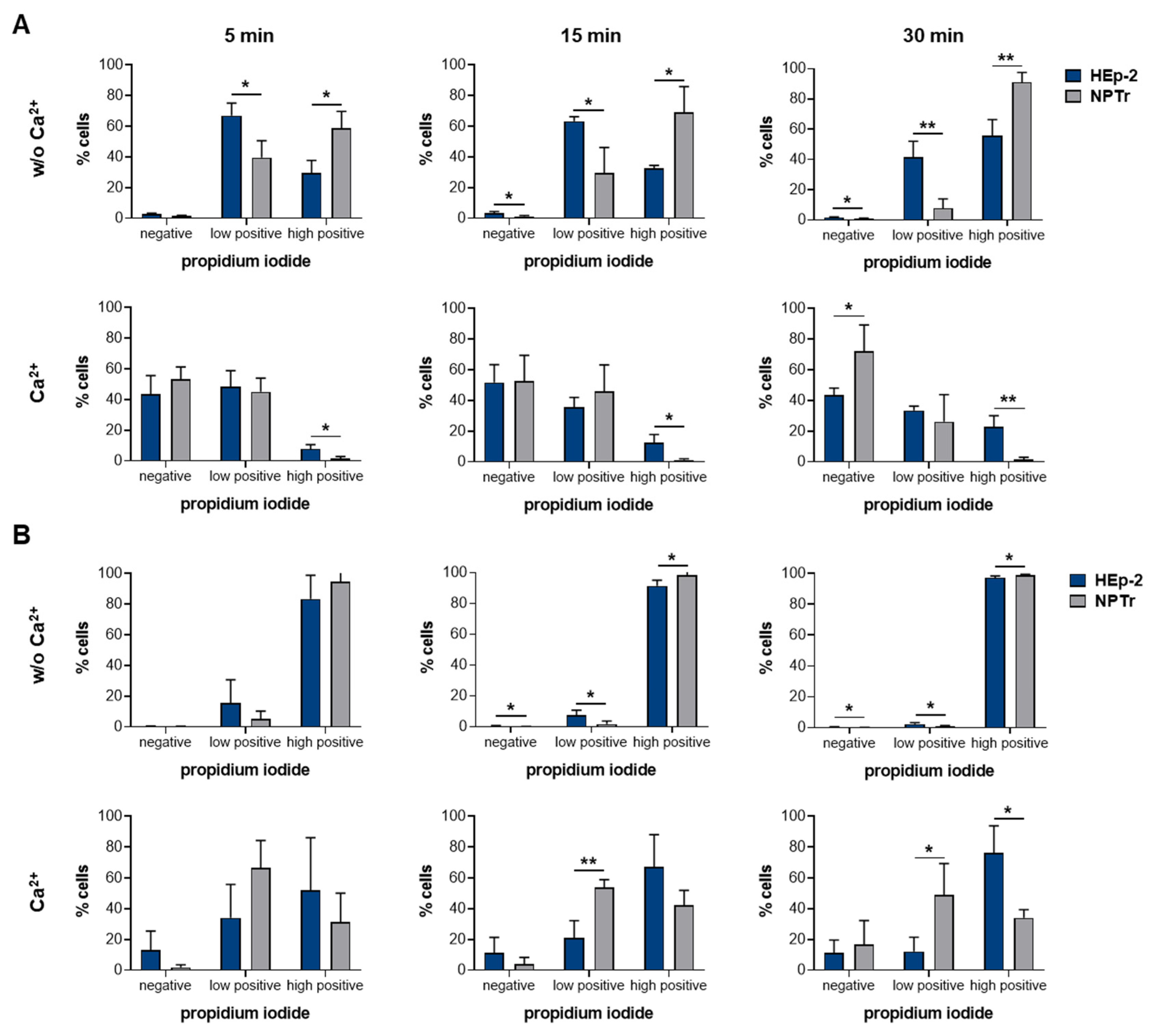
2.3. SLY-Induced Cell Damage Can Be Repaired by Resealing in a Ca2+-Dependent Manner
2.4. NPTr Cells Reseal SLY-Induced Cell Damage More Efficiently Than HEp-2 Cells
3. Conclusions
4. Materials and Methods
4.1. Bacterial Strains and Recombinant Suilysin Protein
4.2. Cell Culture
4.3. Cytotoxicity Assay
4.4. Immunoblot Analysis
4.5. FACS Analysis of SLY-Cell Association
4.6. Lipid Extraction and Quantitative Measurement of the Cellular Cholesterol and Protein Content
4.7. FACS Analysis of Membrane Resealing Capacity
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tweten, R.K. Cholesterol-dependent cytolysins, a family of versatile pore-forming toxins. Infect. Immun. 2005, 73, 6199–6209. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.; Dudkina, N.V.; Lukoyanova, N.; Hodel, A.W.; Farabella, I.; Pandurangan, A.P.; Jahan, N.; Pires Damaso, M.; Osmanovic, D.; Reboul, C.F.; et al. Stepwise visualization of membrane pore formation by suilysin, a bacterial cholesterol-dependent cytolysin. eLife 2014, 3, e04247. [Google Scholar] [CrossRef] [PubMed]
- Tenenbaum, T.; Asmat, T.M.; Seitz, M.; Schroten, H.; Schwerk, C. Biological activities of suilysin: Role in Streptococcus suis pathogenesis. Future Microbiol. 2016, 11, 941–954. [Google Scholar] [CrossRef] [PubMed]
- Korchev, Y.E.; Bashford, C.L.; Pasternak, C.A. Differential sensitivity of pneumolysin-induced channels to gating by divalent cations. J. Membr. Biol. 1992, 127, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Aroian, R.; van der Goot, F.G. Pore-forming toxins and cellular non-immune defenses (CNIDs). Curr. Opin. Microbiol. 2007, 10, 57–61. [Google Scholar] [CrossRef]
- Los, F.C.; Randis, T.M.; Aroian, R.V.; Ratner, A.J. Role of pore-forming toxins in bacterial infectious diseases. Microbiol. Mol. Biol. Rev. 2013, 77, 173–207. [Google Scholar] [CrossRef]
- Farrand, A.J.; LaChapelle, S.; Hotze, E.M.; Johnson, A.E.; Tweten, R.K. Only two amino acids are essential for cytolytic toxin recognition of cholesterol at the membrane surface. Proc. Natl. Acad. Sci. USA 2010, 107, 4341–4346. [Google Scholar] [CrossRef]
- Farrand, A.J.; Hotze, E.M.; Sato, T.K.; Wade, K.R.; Wimley, W.C.; Johnson, A.E.; Tweten, R.K. The Cholesterol-dependent Cytolysin Membrane-binding Interface Discriminates Lipid Environments of Cholesterol to Support beta-Barrel Pore Insertion. J. Biol. Chem. 2015, 290, 17733–17744. [Google Scholar] [CrossRef]
- Giddings, K.S.; Zhao, J.; Sims, P.J.; Tweten, R.K. Human CD59 is a receptor for the cholesterol-dependent cytolysin intermedilysin. Nat. Struct. Mol. Biol. 2004, 11, 1173–1178. [Google Scholar] [CrossRef]
- Gelber, S.E.; Aguilar, J.L.; Lewis, K.L.; Ratner, A.J. Functional and phylogenetic characterization of Vaginolysin, the human-specific cytolysin from Gardnerella vaginalis. J. Bacteriol. 2008, 190, 3896–3903. [Google Scholar] [CrossRef]
- Feil, S.C.; Lawrence, S.; Mulhern, T.D.; Holien, J.K.; Hotze, E.M.; Farrand, S.; Tweten, R.K.; Parker, M.W. Structure of the lectin regulatory domain of the cholesterol-dependent cytolysin lectinolysin reveals the basis for its lewis antigen specificity. Structure 2012, 20, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Soltani, C.E.; Hotze, E.M.; Johnson, A.E.; Tweten, R.K. Structural elements of the cholesterol-dependent cytolysins that are responsible for their cholesterol-sensitive membrane interactions. Proc. Natl. Acad. Sci. USA 2007, 104, 20226–20231. [Google Scholar] [CrossRef] [PubMed]
- Giddings, K.S.; Johnson, A.E.; Tweten, R.K. Redefining cholesterol’s role in the mechanism of the cholesterol-dependent cytolysins. Proc. Natl. Acad. Sci. USA 2003, 100, 11315–11320. [Google Scholar] [CrossRef] [PubMed]
- LaChapelle, S.; Tweten, R.K.; Hotze, E.M. Intermedilysin-receptor interactions during assembly of the pore complex: Assembly intermediates increase host cell susceptibility to complement-mediated lysis. J. Biol. Chem. 2009, 284, 12719–12726. [Google Scholar] [CrossRef]
- Jacobs, A.A.C.; Loeffen, P.L.W.; Vandenberg, A.J.G.; Storm, P.K. Identification, Purification, and Characterization of a Thiol-Activated Hemolysin (Suilysin) of Streptococcus-Suis. Infect. Immun. 1994, 62, 1742–1748. [Google Scholar]
- He, Z.; Pian, Y.; Ren, Z.; Bi, L.; Yuan, Y.; Zheng, Y.; Jiang, Y.; Wang, F. Increased production of suilysin contributes to invasive infection of the Streptococcus suis strain 05ZYH33. Mol. Med. Rep. 2014, 10, 2819–2826. [Google Scholar] [CrossRef]
- de Greeff, A.; Wisselink, H.J.; de Bree, F.M.; Schultsz, C.; Baums, C.G.; Thi, H.N.; Stockhofe-Zurwieden, N.; Smith, H.E. Genetic diversity of Streptococcus suis isolates as determined by comparative genome hybridization. BMC Microbiol. 2011, 11, 161. [Google Scholar] [CrossRef]
- Norton, P.M.; Rolph, C.; Ward, P.N.; Bentley, R.W.; Leigh, J.A. Epithelial invasion and cell lysis by virulent strains of Streptococcus suis is enhanced by the presence of suilysin. FEMS Immunol. Med Microbiol. 1999, 26, 25–35. [Google Scholar] [CrossRef]
- Jacobs, A.A.; van den Berg, A.J.; Baars, J.C.; Nielsen, B.; Johannsen, L.W. Production of suilysin, the thiol-activated haemolysin of Streptococcus suis, by field isolates from diseased pigs. Vet. Rec. 1995, 137, 295–296. [Google Scholar] [CrossRef]
- Segers, R.P.; Kenter, T.; de Haan, L.A.; Jacobs, A.A. Characterisation of the gene encoding suilysin from Streptococcus suis and expression in field strains. FEMS Microbiol. Lett. 1998, 167, 255–261. [Google Scholar] [CrossRef][Green Version]
- Fittipaldi, N.; Fuller, T.E.; Teel, J.F.; Wilson, T.L.; Wolfram, T.J.; Lowery, D.E.; Gottschalk, M. Serotype distribution and production of muramidase-released protein, extracellular factor and suilysin by field strains of Streptococcus suis isolated in the United States. Vet. Microbiol. 2009, 139, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, M.; Lacouture, S.; Bonifait, L.; Roy, D.; Fittipaldi, N.; Grenier, D. Characterization of Streptococcus suis isolates recovered between 2008 and 2011 from diseased pigs in Quebec, Canada. Vet. Microbiol. 2013, 162, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Staats, J.J.; Plattner, B.L.; Stewart, G.C.; Changappa, M.M. Presence of the Streptococcus suis suilysin gene and expression of MRP and EF correlates with high virulence in Streptococcus suis type 2 isolates. Vet. Microbiol. 1999, 70, 201–211. [Google Scholar] [CrossRef]
- Meng, F.; Wu, N.H.; Nerlich, A.; Herrler, G.; Valentin-Weigand, P.; Seitz, M. Dynamic Virus-Bacterium Interactions in a Porcine Precision-Cut Lung Slice Coinfection Model: Swine Influenza Virus Paves the Way for Streptococcus suis Infection in a Two-Step Process. Infect. Immun. 2015, 83, 2806–2815. [Google Scholar] [CrossRef]
- Seitz, M.; Baums, C.G.; Neis, C.; Benga, L.; Fulde, M.; Rohde, M.; Goethe, R.; Valentin-Weigand, P. Subcytolytic effects of suilysin on interaction of Streptococcus suis with epithelial cells. Vet. Microbiol. 2013, 167, 584–591. [Google Scholar] [CrossRef]
- Lecours, M.P.; Gottschalk, M.; Houde, M.; Lemire, P.; Fittipaldi, N.; Segura, M. Critical role for Streptococcus suis cell wall modifications and suilysin in resistance to complement-dependent killing by dendritic cells. J. Infect. Dis. 2011, 204, 919–929. [Google Scholar] [CrossRef]
- Chabot-Roy, G.; Willson, P.; Segura, M.; Lacouture, S.; Gottschalk, M. Phagocytosis and killing of Streptococcus suis by porcine neutrophils. Microb. Pathog. 2006, 41, 21–32. [Google Scholar] [CrossRef]
- Lv, Q.; Hao, H.; Bi, L.; Zheng, Y.; Zhou, X.; Jiang, Y. Suilysin remodels the cytoskeletons of human brain microvascular endothelial cells by activating RhoA and Rac1 GTPase. Protein Cell 2014, 5, 261–264. [Google Scholar] [CrossRef]
- Vadeboncoeur, N.; Segura, M.; Al-Numani, D.; Vanier, G.; Gottschalk, M. Pro-inflammatory cytokine and chemokine release by human brain microvascular endothelial cells stimulated by Streptococcus suis serotype 2. FEMS Immunol. Med. Microbiol. 2003, 35, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Vanier, G.; Fittipaldi, N.; Slater, J.D.; de la Cruz Dominguez-Punaro, M.; Rycroft, A.N.; Segura, M.; Maskell, D.J.; Gottschalk, M. New putative virulence factors of Streptococcus suis involved in invasion of porcine brain microvascular endothelial cells. Microb. Pathog. 2009, 46, 13–20. [Google Scholar] [CrossRef]
- Lun, S.; Perez-Casal, J.; Connor, W.; Willson, P.J. Role of suilysin in pathogenesis of Streptococcus suis capsular serotype 2. Microb. Pathog. 2003, 34, 27–37. [Google Scholar] [CrossRef]
- Tanabe, S.; Gottschalk, M.; Grenier, D. Hemoglobin and Streptococcus suis cell wall act in synergy to potentiate the inflammatory response of monocyte-derived macrophages. Innate Immun. 2008, 14, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Lalonde, M.; Segura, M.; Lacouture, S.; Gottschalk, M. Interactions between Streptococcus suis serotype 2 and different epithelial cell lines. Microbiology 2000, 146 Pt 8, 1913–1921. [Google Scholar] [CrossRef]
- American Type Culture Collection Standards Development Organization Workgroup ASN-0002. Cell line misidentification: The beginning of the end. Nat. Rev. Cancer 2010, 10, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Lorsch, J.R.; Collins, F.S.; Lippincott-Schwartz, J. Cell Biology. Fixing problems with cell lines. Science 2014, 346, 1452–1453. [Google Scholar] [CrossRef]
- Pan, C.; Kumar, C.; Bohl, S.; Klingmueller, U.; Mann, M. Comparative proteomic phenotyping of cell lines and primary cells to assess preservation of cell type-specific functions. Mol. Cell. Proteom. 2009, 8, 443–450. [Google Scholar] [CrossRef]
- Alge, C.S.; Hauck, S.M.; Priglinger, S.G.; Kampik, A.; Ueffing, M. Differential protein profiling of primary versus immortalized human RPE cells identifies expression patterns associated with cytoskeletal remodeling and cell survival. J. Proteome Res. 2006, 5, 862–878. [Google Scholar] [CrossRef]
- Ray, S.; Thapa, R.; Keyel, P.A. Multiple Parameters Beyond Lipid Binding Affinity Drive Cytotoxicity of Cholesterol-Dependent Cytolysins. Toxins 2018, 11. [Google Scholar] [CrossRef]
- Gottschalk, M.G.; Lacouture, S.; Dubreuil, J.D. Characterization of Streptococcus suis capsular type 2 haemolysin. Microbiology 1995, 141 Pt 1, 189–195. [Google Scholar] [CrossRef]
- Vanier, G.; Segura, M.; Friedl, P.; Lacouture, S.; Gottschalk, M. Invasion of porcine brain microvascular endothelial cells by Streptococcus suis serotype 2. Infect. Immun. 2004, 72, 1441–1449. [Google Scholar] [CrossRef]
- Tenenbaum, T.; Adam, R.; Eggelnpohler, I.; Matalon, D.; Seibt, A.; GE, K.N.; Galla, H.J.; Schroten, H. Strain-dependent disruption of blood-cerebrospinal fluid barrier by Streptoccocus suis in vitro. FEMS Immunol. Med. Microbiol. 2005, 44, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Auger, J.P.; Christodoulides, M.; Segura, M.; Xu, J.; Gottschalk, M. Interactions of Streptococcus suis serotype 2 with human meningeal cells and astrocytes. BMC Res. Notes 2015, 8, 607. [Google Scholar] [CrossRef] [PubMed]
- Segura, M.; Gottschalk, M. Streptococcus suis interactions with the murine macrophage cell line J774: Adhesion and cytotoxicity. Infect. Immun. 2002, 70, 4312–4322. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Wu, N.H.; Seitz, M.; Herrler, G.; Valentin-Weigand, P. Efficient suilysin-mediated invasion and apoptosis in porcine respiratory epithelial cells after streptococcal infection under air-liquid interface conditions. Sci. Rep. 2016, 6, 26748. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Tong, J.; Votsch, D.; Peng, J.Y.; Cai, X.; Willenborg, M.; Herrler, G.; Wu, N.H.; Valentin-Weigand, P. Viral Coinfection Replaces Effects of Suilysin on Streptococcus suis Adherence to and Invasion of Respiratory Epithelial Cells Grown under Air-Liquid Interface Conditions. Infect. Immun. 2019, 87. [Google Scholar] [CrossRef]
- Charland, N.; Nizet, V.; Rubens, C.E.; Kim, K.S.; Lacouture, S.; Gottschalk, M. Streptococcus suis serotype 2 interactions with human brain microvascular endothelial cells. Infect. Immun. 2000, 68, 637–643. [Google Scholar] [CrossRef]
- Tanigawa, T.; Suzuki, J.; Ueta, T.; Katsumoto, T.; Tanaka, Y. Different sensitivity to streptolysin-O of cells in macrophage lineage. Microbiol. Immunol. 1996, 40, 81–84. [Google Scholar] [CrossRef]
- Hirst, R.A.; Yesilkaya, H.; Clitheroe, E.; Rutman, A.; Dufty, N.; Mitchell, T.J.; O’Callaghan, C.; Andrew, P.W. Sensitivities of human monocytes and epithelial cells to pneumolysin are different. Infect. Immun. 2002, 70, 1017–1022. [Google Scholar] [CrossRef][Green Version]
- Wade, K.R.; Hotze, E.M.; Briles, D.E.; Tweten, R.K. Mouse, but not human, ApoB-100 lipoprotein cholesterol is a potent innate inhibitor of Streptococcus pneumoniae pneumolysin. PLoS Pathog. 2014, 10, e1004353. [Google Scholar] [CrossRef]
- Ferrari, M.; Scalvini, A.; Losio, M.N.; Corradi, A.; Soncini, M.; Bignotti, E.; Milanesi, E.; Ajmone-Marsan, P.; Barlati, S.; Bellotti, D.; et al. Establishment and characterization of two new pig cell lines for use in virological diagnostic laboratories. J. Virol. Methods 2003, 107, 205–212. [Google Scholar] [CrossRef]
- Delgado-Ortega, M.; Olivier, M.; Sizaret, P.Y.; Simon, G.; Meurens, F. Newborn pig trachea cell line cultured in air-liquid interface conditions allows a partial in vitro representation of the porcine upper airway tissue. BMC Cell Biol. 2014, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Idone, V.; Tam, C.; Goss, J.W.; Toomre, D.; Pypaert, M.; Andrews, N.W. Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. J. Cell Biol. 2008, 180, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Wolfmeier, H.; Schoenauer, R.; Atanassoff, A.P.; Neill, D.R.; Kadioglu, A.; Draeger, A.; Babiychuk, E.B. Ca2+-dependent repair of pneumolysin pores: A new paradigm for host cellular defense against bacterial pore-forming toxins. Biochim. Biophys. Acta 2015, 1853, 2045–2054. [Google Scholar] [CrossRef] [PubMed]
- Walev, I.; Bhakdi, S.C.; Hofmann, F.; Djonder, N.; Valeva, A.; Aktories, K.; Bhakdi, S. Delivery of proteins into living cells by reversible membrane permeabilization with streptolysin-O. Proc. Natl. Acad. Sci. USA 2001, 98, 3185–3190. [Google Scholar] [CrossRef]
- Gonzalez, M.R.; Bischofberger, M.; Freche, B.; Ho, S.; Parton, R.G.; van der Goot, F.G. Pore-forming toxins induce multiple cellular responses promoting survival. Cell. Microbiol. 2011, 13, 1026–1043. [Google Scholar] [CrossRef]
- Cassidy, S.K.; O’Riordan, M.X. More than a pore: The cellular response to cholesterol-dependent cytolysins. Toxins 2013, 5, 618–636. [Google Scholar] [CrossRef]
- Bhakdi, S.; Tranum-Jensen, J.; Sziegoleit, A. Mechanism of membrane damage by streptolysin-O. Infect. Immun. 1985, 47, 52–60. [Google Scholar]
- Palmer, M.; Harris, R.; Freytag, C.; Kehoe, M.; Tranum-Jensen, J.; Bhakdi, S. Assembly mechanism of the oligomeric streptolysin O pore: The early membrane lesion is lined by a free edge of the lipid membrane and is extended gradually during oligomerization. EMBO J. 1998, 17, 1598–1605. [Google Scholar] [CrossRef]
- Gilbert, R.J.; Mikelj, M.; Dalla Serra, M.; Froelich, C.J.; Anderluh, G. Effects of MACPF/CDC proteins on lipid membranes. Cell. Mol. Life Sci. 2013, 70, 2083–2098. [Google Scholar] [CrossRef]
- Sonnen, A.F.; Plitzko, J.M.; Gilbert, R.J. Incomplete pneumolysin oligomers form membrane pores. Open Biol. 2014, 4, 140044. [Google Scholar] [CrossRef]
- Waheed, A.A.; Shimada, Y.; Heijnen, H.F.; Nakamura, M.; Inomata, M.; Hayashi, M.; Iwashita, S.; Slot, J.W.; Ohno-Iwashita, Y. Selective binding of perfringolysin O derivative to cholesterol-rich membrane microdomains (rafts). Proc. Natl. Acad. Sci. USA 2001, 98, 4926–4931. [Google Scholar] [CrossRef] [PubMed]
- Ohno-Iwashita, Y.; Iwamoto, M.; Ando, S.; Iwashita, S. Effect of lipidic factors on membrane cholesterol topology—Mode of binding of theta-toxin to cholesterol in liposomes. Biochim. Biophys. Acta 1992, 1109, 81–90. [Google Scholar] [CrossRef]
- Heuck, A.P.; Hotze, E.M.; Tweten, R.K.; Johnson, A.E. Mechanism of membrane insertion of a multimeric beta-barrel protein: Perfringolysin O creates a pore using ordered and coupled conformational changes. Mol. Cell 2000, 6, 1233–1242. [Google Scholar] [CrossRef]
- Alving, C.R.; Habig, W.H.; Urban, K.A.; Hardegree, M.C. Cholesterol-dependent tetanolysin damage to liposomes. Biochim. Biophys. Acta 1979, 551, 224–228. [Google Scholar] [CrossRef]
- Rottem, S.; Cole, R.M.; Habig, W.H.; Barile, M.F.; Hardegree, M.C. Structural characteristics of tetanolysin and its binding to lipid vesicles. J. Bacteriol. 1982, 152, 888–892. [Google Scholar]
- Nelson, L.D.; Johnson, A.E.; London, E. How interaction of perfringolysin O with membranes is controlled by sterol structure, lipid structure, and physiological low pH: Insights into the origin of perfringolysin O-lipid raft interaction. J. Biol. Chem. 2008, 283, 4632–4642. [Google Scholar] [CrossRef]
- Flanagan, J.J.; Tweten, R.K.; Johnson, A.E.; Heuck, A.P. Cholesterol exposure at the membrane surface is necessary and sufficient to trigger perfringolysin O binding. Biochemistry 2009, 48, 3977–3987. [Google Scholar] [CrossRef]
- Savinov, S.N.; Heuck, A.P. Interaction of Cholesterol with Perfringolysin O: What Have We Learned from Functional Analysis? Toxins 2017, 9. [Google Scholar] [CrossRef]
- Bavdek, A.; Gekara, N.O.; Priselac, D.; Gutierrez Aguirre, I.; Darji, A.; Chakraborty, T.; Macek, P.; Lakey, J.H.; Weiss, S.; Anderluh, G. Sterol and pH interdependence in the binding, oligomerization, and pore formation of Listeriolysin O. Biochemistry 2007, 46, 4425–4437. [Google Scholar] [CrossRef]
- Jacobs, T.; Darji, A.; Frahm, N.; Rohde, M.; Wehland, J.; Chakraborty, T.; Weiss, S. Listeriolysin O: Cholesterol inhibits cytolysis but not binding to cellular membranes. Mol. Microbiol. 1998, 28, 1081–1089. [Google Scholar] [CrossRef]
- Johnson, M.K.; Geoffroy, C.; Alouf, J.E. Binding of cholesterol by sulfhydryl-activated cytolysins. Infect. Immun. 1980, 27, 97–101. [Google Scholar] [PubMed]
- Ohno-Iwashita, Y.; Iwamoto, M.; Mitsui, K.; Kawasaki, H.; Ando, S. Cold-labile hemolysin produced by limited proteolysis of theta-toxin from Clostridium perfringens. Biochemistry 1986, 25, 6048–6053. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, M.; Ohno-Iwashita, Y.; Ando, S. Role of the essential thiol group in the thiol-activated cytolysin from Clostridium perfringens. Eur. J. Biochem. 1987, 167, 425–430. [Google Scholar] [CrossRef]
- Draeger, A.; Monastyrskaya, K.; Babiychuk, E.B. Plasma membrane repair and cellular damage control: The annexin survival kit. Biochem. Pharmacol. 2011, 81, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Wippel, C.; Fortsch, C.; Hupp, S.; Maier, E.; Benz, R.; Ma, J.; Mitchell, T.J.; Iliev, A.I. Extracellular calcium reduction strongly increases the lytic capacity of pneumolysin from streptococcus pneumoniae in brain tissue. J. Infect. Dis. 2011, 204, 930–936. [Google Scholar] [CrossRef]
- Babiychuk, E.B.; Monastyrskaya, K.; Potez, S.; Draeger, A. Intracellular Ca2+ operates a switch between repair and lysis of streptolysin O-perforated cells. Cell Death Differ. 2009, 16, 1126–1134. [Google Scholar] [CrossRef]
- Potez, S.; Luginbuhl, M.; Monastyrskaya, K.; Hostettler, A.; Draeger, A.; Babiychuk, E.B. Tailored protection against plasmalemmal injury by annexins with different Ca2+ sensitivities. J. Biol. Chem. 2011, 286, 17982–17991. [Google Scholar] [CrossRef]
- Keyel, P.A.; Loultcheva, L.; Roth, R.; Salter, R.D.; Watkins, S.C.; Yokoyama, W.M.; Heuser, J.E. Streptolysin O clearance through sequestration into blebs that bud passively from the plasma membrane. J. Cell Sci. 2011, 124, 2414–2423. [Google Scholar] [CrossRef]
- Wolfmeier, H.; Radecke, J.; Schoenauer, R.; Koeffel, R.; Babiychuk, V.S.; Drucker, P.; Hathaway, L.J.; Mitchell, T.J.; Zuber, B.; Draeger, A.; et al. Active release of pneumolysin prepores and pores by mammalian cells undergoing a Streptococcus pneumoniae attack. Biochim. Biophys. Acta 2016, 1860, 2498–2509. [Google Scholar] [CrossRef]
- Maurer, J.; Hupp, S.; Pillich, H.; Mitchell, T.J.; Chakraborty, T.; Iliev, A.I. Missing elimination via membrane vesicle shedding contributes to the diminished calcium sensitivity of listeriolysin O. Sci. Rep. 2018, 8, 15846. [Google Scholar] [CrossRef]
- Romero, M.; Keyel, M.; Shi, G.; Bhattacharjee, P.; Roth, R.; Heuser, J.E.; Keyel, P.A. Intrinsic repair protects cells from pore-forming toxins by microvesicle shedding. Cell Death Differ. 2017, 24, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Husmann, M.; Beckmann, E.; Boller, K.; Kloft, N.; Tenzer, S.; Bobkiewicz, W.; Neukirch, C.; Bayley, H.; Bhakdi, S. Elimination of a bacterial pore-forming toxin by sequential endocytosis and exocytosis. FEBS Lett. 2009, 583, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.G.; Saka, H.A.; Chinen, I.; Zoppino, F.C.; Yoshimori, T.; Bocco, J.L.; Colombo, M.I. Protective role of autophagy against Vibrio cholerae cytolysin, a pore-forming toxin from V. cholerae. Proc. Natl. Acad. Sci. USA 2007, 104, 1829–1834. [Google Scholar] [CrossRef] [PubMed]
- Atanassoff, A.P.; Wolfmeier, H.; Schoenauer, R.; Hostettler, A.; Ring, A.; Draeger, A.; Babiychuk, E.B. Microvesicle shedding and lysosomal repair fulfill divergent cellular needs during the repair of streptolysin O-induced plasmalemmal damage. PLoS ONE 2014, 9, e89743. [Google Scholar] [CrossRef]
- Takeuchi, D.; Akeda, Y.; Nakayama, T.; Kerdsin, A.; Sano, Y.; Kanda, T.; Hamada, S.; Dejsirilert, S.; Oishi, K. The Contribution of Suilysin to the Pathogenesis of Streptococcus suis Meningitis. J. Infect. Dis. 2014, 209, 1509–1519. [Google Scholar] [CrossRef]
- Allen, A.G.; Bolitho, S.; Lindsay, H.; Khan, S.; Bryant, C.; Norton, P.; Ward, P.; Leigh, J.; Morgan, J.; Riches, H.; et al. Generation and characterization of a defined mutant of Streptococcus suis lacking suilysin. Infect. Immun. 2001, 69, 2732–2735. [Google Scholar] [CrossRef]
- Smith, H.E.; Damman, M.; van der Velde, J.; Wagenaar, F.; Wisselink, H.J.; Stockhofe-Zurwieden, N.; Smits, M.A. Identification and characterization of the cps locus of Streptococcus suis serotype 2: The capsule protects against phagocytosis and is an important virulence factor. Infect. Immun. 1999, 67, 1750–1756. [Google Scholar]
- Benga, L.; Fulde, M.; Neis, C.; Goethe, R.; Valentin-Weigand, P. Polysaccharide capsule and suilysin contribute to extracellular survival of Streptococcus suis co-cultivated with primary porcine phagocytes. Vet. Microbiol. 2008, 132, 211–219. [Google Scholar] [CrossRef]
- Willenborg, J.; Fulde, M.; de Greeff, A.; Rohde, M.; Smith, H.E.; Valentin-Weigand, P.; Goethe, R. Role of glucose and CcpA in capsule expression and virulence of Streptococcus suis. Microbiology 2011, 157, 1823–1833. [Google Scholar] [CrossRef]
- Fulcher, M.L.; Gabriel, S.; Burns, K.A.; Yankaskas, J.R.; Randell, S.H. Well-differentiated human airway epithelial cell cultures. Methods Mol. Med. 2005, 107, 183–206. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Pirson, C.; Engel, R.; Jones, G.J.; Holder, T.; Holst, O.; Vordermeier, H.M. Highly purified mycobacterial phosphatidylinositol mannosides drive cell-mediated responses and activate NKT cells in cattle. Clin. Vaccine Immunol. 2015, 22, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Brogden, G.; Shammas, H.; Maalouf, K.; Naim, S.L.; Wetzel, G.; Amiri, M.; von Kockritz-Blickwede, M.; Das, A.M.; Naim, H.Y. Case study on the pathophysiology of Fabry disease: Abnormalities of cellular membranes can be reversed by substrate reduction in vitro. Biosci. Rep. 2017, 37. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vötsch, D.; Willenborg, M.; Oelemann, W.M.R.; Brogden, G.; Valentin-Weigand, P. Membrane Binding, Cellular Cholesterol Content and Resealing Capacity Contribute to Epithelial Cell Damage Induced by Suilysin of Streptococcus suis. Pathogens 2020, 9, 33. https://doi.org/10.3390/pathogens9010033
Vötsch D, Willenborg M, Oelemann WMR, Brogden G, Valentin-Weigand P. Membrane Binding, Cellular Cholesterol Content and Resealing Capacity Contribute to Epithelial Cell Damage Induced by Suilysin of Streptococcus suis. Pathogens. 2020; 9(1):33. https://doi.org/10.3390/pathogens9010033
Chicago/Turabian StyleVötsch, Désirée, Maren Willenborg, Walter M.R. Oelemann, Graham Brogden, and Peter Valentin-Weigand. 2020. "Membrane Binding, Cellular Cholesterol Content and Resealing Capacity Contribute to Epithelial Cell Damage Induced by Suilysin of Streptococcus suis" Pathogens 9, no. 1: 33. https://doi.org/10.3390/pathogens9010033
APA StyleVötsch, D., Willenborg, M., Oelemann, W. M. R., Brogden, G., & Valentin-Weigand, P. (2020). Membrane Binding, Cellular Cholesterol Content and Resealing Capacity Contribute to Epithelial Cell Damage Induced by Suilysin of Streptococcus suis. Pathogens, 9(1), 33. https://doi.org/10.3390/pathogens9010033





