Taxonomical and Functional Assessment of the Endometrial Microbiota in A Context of Recurrent Reproductive Failure: A Case Report
Abstract
1. Introduction
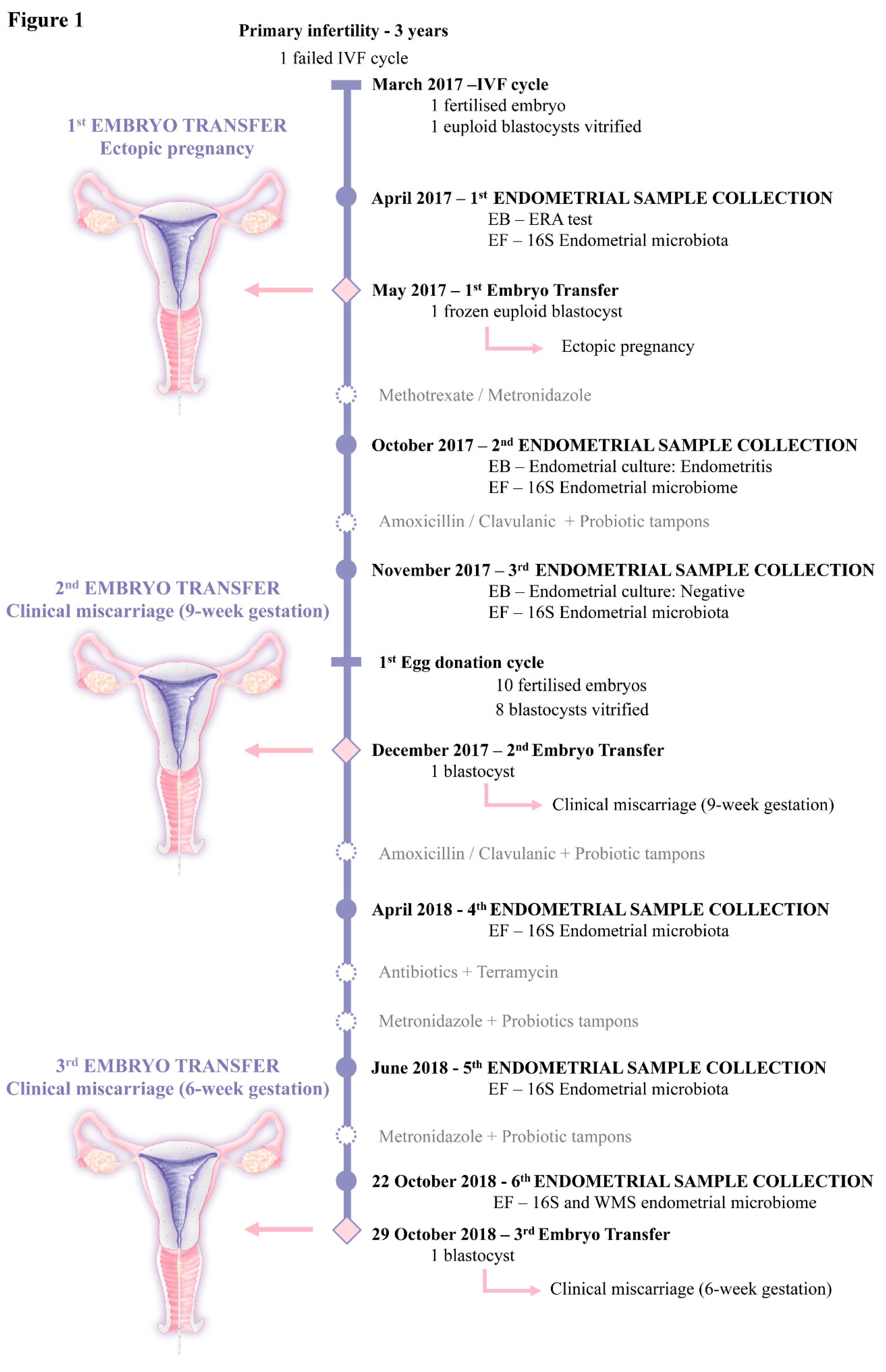
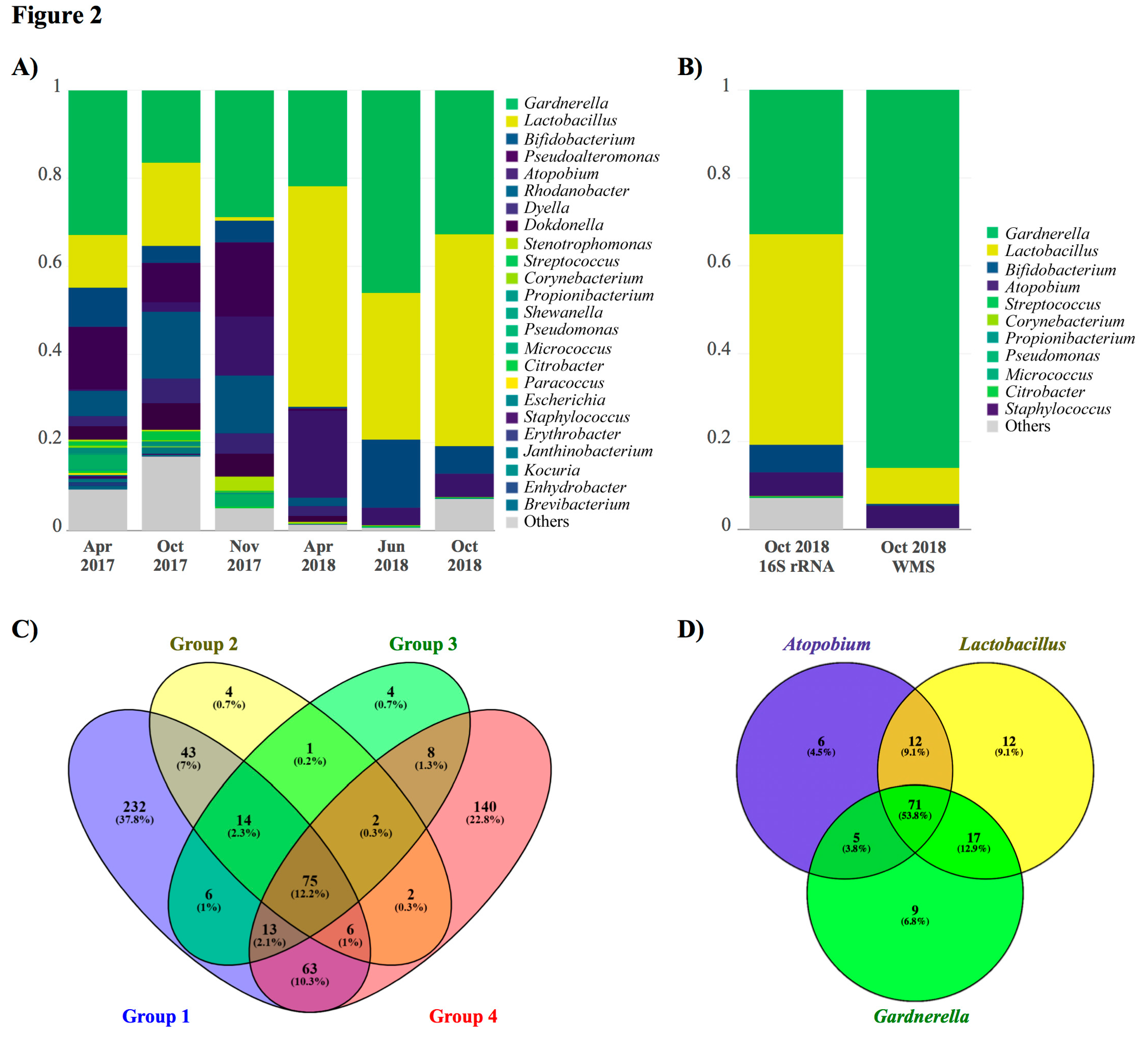
2. Case Presentation
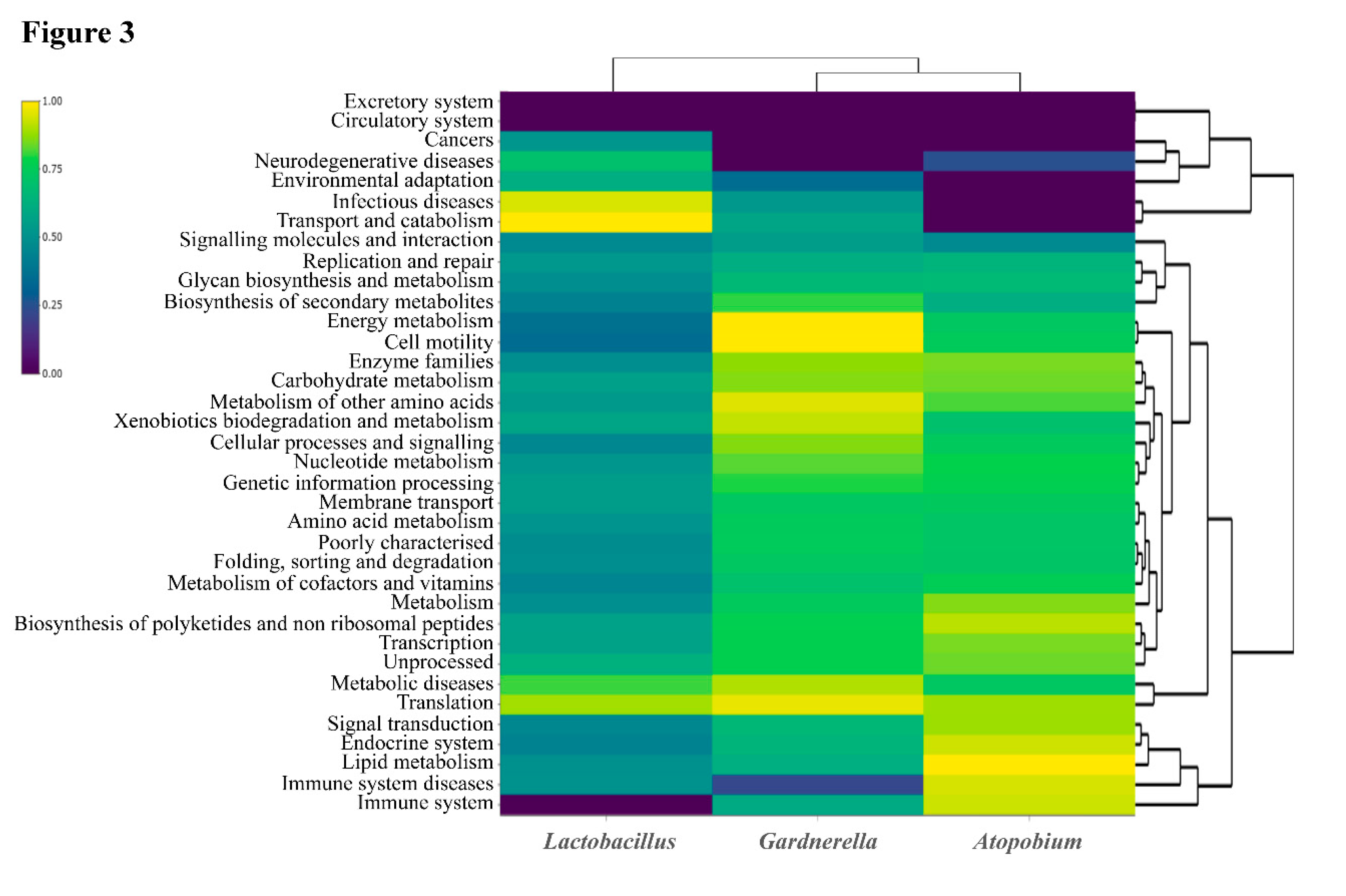
3. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
| 9 categories included exclusively in Gardnerella | RPKM |
| Lipid metabolism-Fatty acid biosynthesis | 729.54 |
| Cell motility-Bacterial chemotaxis | 559.63 |
| Energy metabolism-Sulfur metabolism | 303.44 |
| Cellular processes and signalling-Sporulation | 225.36 |
| Signaling molecules and interaction-Bacterial toxins | 207.02 |
| Cell motility-Bacterial motility proteins | 155.71 |
| Environmental adaptation-Circadian rhythm - Plant | 137.72 |
| Carbohydrate metabolism-Inositol phosphate metabolism | 132.10 |
| Immune system-NOD-like receptor signalling pathway | 115.83 |
| 6 categories included exclusively in Atopobium | RPKM |
| Metabolism-Nucleotide metabolism | 92.61 |
| Metabolism of cofactors and vitamins-Folate biosynthesis | 68.42 |
| Cellular processes and signalling-Cell motility and secretion | 55.19 |
| Immune system-RIG-I-like receptor signalling pathway | 38.13 |
| Metabolism of other amino acids-Beta-alanine metabolism | 34.60 |
| Neurodegenerative diseases-Alzheimer’s disease | 10.19 |
| 12 categories included exclusively in Lactobacillus | RPKM |
| Carbohydrate metabolism-C5-branched dibasic acid metabolism | 34.35 |
| Genetic information processing-Transcription related proteins | 33.44 |
| Metabolic diseases-Type I diabetes mellitus | 25.47 |
| Carbohydrate metabolism-Pentose and glucuronate interconversions | 24.87 |
| Glycan biosynthesis and metabolism-Lipopolysaccharide biosynthesis proteins | 24.49 |
| Environmental adaptation-Plant-pathogen interaction | 22.52 |
| Xenobiotics biodegradation and metabolism-Benzoate degradation via hydroxylation | 16.80 |
| Metabolism of cofactors and vitamins-Ubiquinone and other terpenoid-quinone biosynthesis | 15.18 |
| Carbohydrate metabolism-Citrate cycle (TCA cycle) | 12.50 |
| Metabolic diseases-Type II diabetes mellitus | 10.66 |
| Cellular processes and signalling-Pores ion channels | 6.16 |
| Metabolism-Amino acid metabolism | 4.21 |
References
- Moore, D.E.; Soules, M.R.; Klein, N.A.; Fujimoto, V.Y.; Agnew, K.J.; Eschenbach, D.A. Bacteria in the transfer catheter tip influence the live-birth rate after in vitro fertilization. Fertil. Steril. 2000, 74, 1118–1124. [Google Scholar] [CrossRef]
- Salim, R.; Ben-Shlomo, I.; Colodner, R.; Keness, Y.; Shalev, E. Bacterial colonization of the uterine cervix and success rate in assisted reproduction: Results of a prospective survey. Hum. Reprod. 2002, 17, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Verstraelen, H.; Senok, A.C. Vaginal lactobacilli, probiotics, and IVF. Reprod. Biomed. Online 2005, 11, 674–675. [Google Scholar] [CrossRef]
- Egbase, P.E.; al-Sharhan, M.; al-Othman, S.; al-Mutawa, M.; Udo, E.E.; Grudzinskas, J.G. Incidence of microbial growth from the tip of the embryo transfer catheter after embryo transfer in relation to clinical pregnancy rate following in-vitro fertilization and embryo transfer. Hum. Reprod. 1996, 11, 1687–1689. [Google Scholar] [CrossRef] [PubMed]
- Fanchin, R.; Harmas, A.; Benaoudia, F.; Lundkvist, U.; Olivennes, F.; Frydman, R. Microbial flora of the cervix assessed at the time of embryo transfer adversely affects in vitro fertilization outcome. Fertil. Steril. 1998, 70, 866–870. [Google Scholar] [CrossRef]
- Selman, H.; Mariani, M.; Barnocchi, N.; Mencacci, A.; Bistoni, F.; Arena, S.; Pizzasegale, S.; Brusco, G.F.; Angelini, A. Examination of bacterial contamination at the time of embryo transfer, and its impact on the IVF/pregnancy outcome. J. Assist. Reprod. Genet. 2007, 24, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4680–4687. [Google Scholar] [CrossRef]
- Swidsinski, A.; Verstraelen, H.; Loening-Baucke, V.; Swidsinski, S.; Mendling, W.; Halwani, Z. Presence of a polymicrobial endometrial biofilm in patients with bacterial vaginosis. PLoS ONE 2013, 8, e53997. [Google Scholar] [CrossRef]
- Moreno, I.; Cicinelli, E.; Garcia-Grau, I.; Gonzalez-Monfort, M.; Bau, D.; Vilella, F.; De Ziegler, D.; Resta, L.; Valbuena, D.; Simon, C. The diagnosis of chronic endometritis in infertile asymptomatic women: A comparative study of histology, microbial cultures, hysteroscopy, and molecular microbiology. Am. J. Obstet. Gynecol. 2018. [Google Scholar] [CrossRef]
- Moreno, I.; Codoñer, F.M.; Vilella, F.; Valbuena, D.; Martinez-Blanch, J.F.; Jimenez-Almazán, J.; Alonso, R.; Alamá, P.; Remohí, J.; Pellicer, A.; et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am. J. Obstet. Gynecol. 2016, 215, 684–703. [Google Scholar] [CrossRef]
- Swidsinski, A.; Mendling, W.; Loening-Baucke, V.; Swidsinski, S.; Dörffel, Y.; Scholze, J.; Lochs, H.; Verstraelen, H. An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. Am. J. Obstet. Gynecol. 2008, 198, 97.e1–97.e6. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Earl, J.; Retchless, A.; Hillier, S.L.; Rabe, L.K.; Cherpes, T.L.; Powell, E.; Janto, B.; Eutsey, R.; Hiller, N.L.; et al. Comparative genomic analyses of 17 clinical isolates of Gardnerella vaginalis provide evidence of multiple genetically isolated clades consistent with subspeciation into genovars. J. Bacteriol. 2012, 194, 3922–3937. [Google Scholar] [CrossRef] [PubMed]
- Schuyler, J.A.; Mordechai, E.; Adelson, M.E.; Sobel, J.D.; Gygax, S.E.; Hilbert, D.W. Identification of intrinsically metronidazole-resistant clades of Gardnerella vaginalis. Diagn. Microbiol. Infect. Dis. 2016, 84, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Balashov, S.V.; Mordechai, E.; Adelson, M.E.; Gygax, S.E. Identification, quantification and subtyping of Gardnerella vaginalis in noncultured clinical vaginal samples by quantitative PCR. J. Med. Microbiol. 2014, 63, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Yeoman, C.J.; Yildirim, S.; Thomas, S.M.; Durkin, A.S.; Torralba, M.; Sutton, G.; Buhay, C.J.; Ding, Y.; Dugan-Rocha, S.P.; Muzny, D.M.; et al. Comparative genomics of Gardnerella vaginalis strains reveals substantial differences in metabolic and virulence potential. PLoS ONE 2010, 5, e12411. [Google Scholar] [CrossRef]
- Koumans, E.H.; Markowitz, L.E.; Hogan, V.; Group, C.B.W. Indications for therapy and treatment recommendations for bacterial vaginosis in nonpregnant and pregnant women: A synthesis of data. Clin. Infect. Dis. 2002, 35, S152–S172. [Google Scholar] [CrossRef]
- Bradshaw, C.S.; Morton, A.N.; Hocking, J.; Garland, S.M.; Morris, M.B.; Moss, L.M.; Horvath, L.B.; Kuzevska, I.; Fairley, C.K. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J. Infect. Dis. 2006, 193, 1478–1486. [Google Scholar] [CrossRef]
- De Backer, E.; Verhelst, R.; Verstraelen, H.; Claeys, G.; Verschraegen, G.; Temmerman, M.; Vaneechoutte, M. Antibiotic susceptibility of Atopobium vaginae. BMC Infect. Dis. 2006, 6, 51. [Google Scholar] [CrossRef]
- Bradshaw, C.S.; Tabrizi, S.N.; Fairley, C.K.; Morton, A.N.; Rudland, E.; Garland, S.M. The association of Atopobium vaginae and Gardnerella vaginalis with bacterial vaginosis and recurrence after oral metronidazole therapy. J. Infect. Dis. 2006, 194, 828–836. [Google Scholar] [CrossRef]
- Sfakianoudis, K.; Simopoulou, M.; Nikas, Y.; Rapani, A.; Nitsos, N.; Pierouli, K.; Pappas, A.; Pantou, A.; Markomichali, C.; Koutsilieris, M.; et al. Efficient treatment of chronic endometritis through a novel approach of intrauterine antibiotic infusion: A case series. BMC Womens Health 2018, 18, 197. [Google Scholar] [CrossRef]
- MacPhee, R.A.; Hummelen, R.; Bisanz, J.E.; Miller, W.L.; Reid, G. Probiotic strategies for the treatment and prevention of bacterial vaginosis. Expert Opin. Pharmacother. 2010, 11, 2985–2995. [Google Scholar] [CrossRef] [PubMed]
- Vilella, F.; Ramirez, L.; Berlanga, O.; Martínez, S.; Alamá, P.; Meseguer, M.; Pellicer, A.; Simon, C. PGE2 and PGF2α concentrations in human endometrial fluid as biomarkers for embryonic implantation. J. Clin. Endocrinol. Metab. 2013, 98, 4123–4132. [Google Scholar] [CrossRef] [PubMed]
- Van der Gaast, M.H.; Beier-Hellwig, K.; Fauser, B.C.; Beier, H.M.; Macklon, N.S. Endometrial secretion aspiration prior to embryo transfer does not reduce implantation rates. Reprod. Biomed. Online 2003, 7, 105–109. [Google Scholar] [CrossRef]
- Schmieder, R.; Edwards, R. Quality control and preprocessing of metagenomic datasets. Bioinformatics 2011, 27, 863–864. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 2010, 26, 589–595. [Google Scholar] [CrossRef]
- Tamames, J.; Puente-Sánchez, F. SqueezeMeta, a highly portable, fully automatic metagenomic analysis pipeline. Front. Microbiol. 2018, 9, 3349. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2008; Available online: www.R-project.org (accessed on 10 September 2019).
| ADAPTATION TO ENVIRONMENT | |
| Mobile elements and horizontal gene transfer: | Recombinase (RecA); Transposase IS3509a; HK97 family phage major capsid protein; Site-specific recombinase phage integrase family; Helicase UvrD/REP; Phage related protein. |
| Competence: | Putative competence-damage inducible protein (CinA); ABC-type antimicrobial peptide transporter permease component; Glycoside hydrolase (GH) family; Lysozyme; Penicillin-binding protein; Fic-family protein; M13 family peptidase; ATP-binding subunit of Clp protease. |
| Toxin-antitoxin system: | RelB toxin/antitoxin family; Antitoxin/DNA-binding transcriptional repressor DinJ. |
| VIRULENCE | |
| Biofilm formation and exopolysaccharide formation: | Glycosyltransferase (GT) type II; Sortases; LPxTG domain; Actinobacterial surface anchored protein domain. |
| Epithelial adhesion: | Type I fimbrial major subunit precursor; Pilus assembly protein (PilY1); Tfp pilus. |
| Antimicrobial resistance: | Efflux transporter; ABC-type multidrug transport system; ABC-type bacteriocin/lantibiotic; Multidrug resistance transporter EmrB/QacA; Bleomycin hydrolase; SalY-type ABC-antimicrobial peptide transport system; Cadmium resistance transporter CadD family protein. |
| Mucin degradation: | Alpha-mannosidase; Beta-galactosidase. |
| Cytotoxicity and hemolysis: | Hemolysin-like protein. |
| Iron intake and utilisation: | FTR1-family iron permease; FtsK/SpoIIIE family protein. |
| Other virulence factors: | G-related albumin-binding (GA) modules; Virulence-associated E family; Oxygen-insensitive NAPDH nitroreductase (RdxA). |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Grau, I.; Perez-Villaroya, D.; Bau, D.; Gonzalez-Monfort, M.; Vilella, F.; Moreno, I.; Simon, C. Taxonomical and Functional Assessment of the Endometrial Microbiota in A Context of Recurrent Reproductive Failure: A Case Report. Pathogens 2019, 8, 205. https://doi.org/10.3390/pathogens8040205
Garcia-Grau I, Perez-Villaroya D, Bau D, Gonzalez-Monfort M, Vilella F, Moreno I, Simon C. Taxonomical and Functional Assessment of the Endometrial Microbiota in A Context of Recurrent Reproductive Failure: A Case Report. Pathogens. 2019; 8(4):205. https://doi.org/10.3390/pathogens8040205
Chicago/Turabian StyleGarcia-Grau, Iolanda, David Perez-Villaroya, Davide Bau, Marta Gonzalez-Monfort, Felipe Vilella, Inmaculada Moreno, and Carlos Simon. 2019. "Taxonomical and Functional Assessment of the Endometrial Microbiota in A Context of Recurrent Reproductive Failure: A Case Report" Pathogens 8, no. 4: 205. https://doi.org/10.3390/pathogens8040205
APA StyleGarcia-Grau, I., Perez-Villaroya, D., Bau, D., Gonzalez-Monfort, M., Vilella, F., Moreno, I., & Simon, C. (2019). Taxonomical and Functional Assessment of the Endometrial Microbiota in A Context of Recurrent Reproductive Failure: A Case Report. Pathogens, 8(4), 205. https://doi.org/10.3390/pathogens8040205





