N-acetyl Cysteine Coated Gallium Particles Demonstrate High Potency against Pseudomonas aeruginosa PAO1
Abstract
1. Introduction
2. Materials and Methods
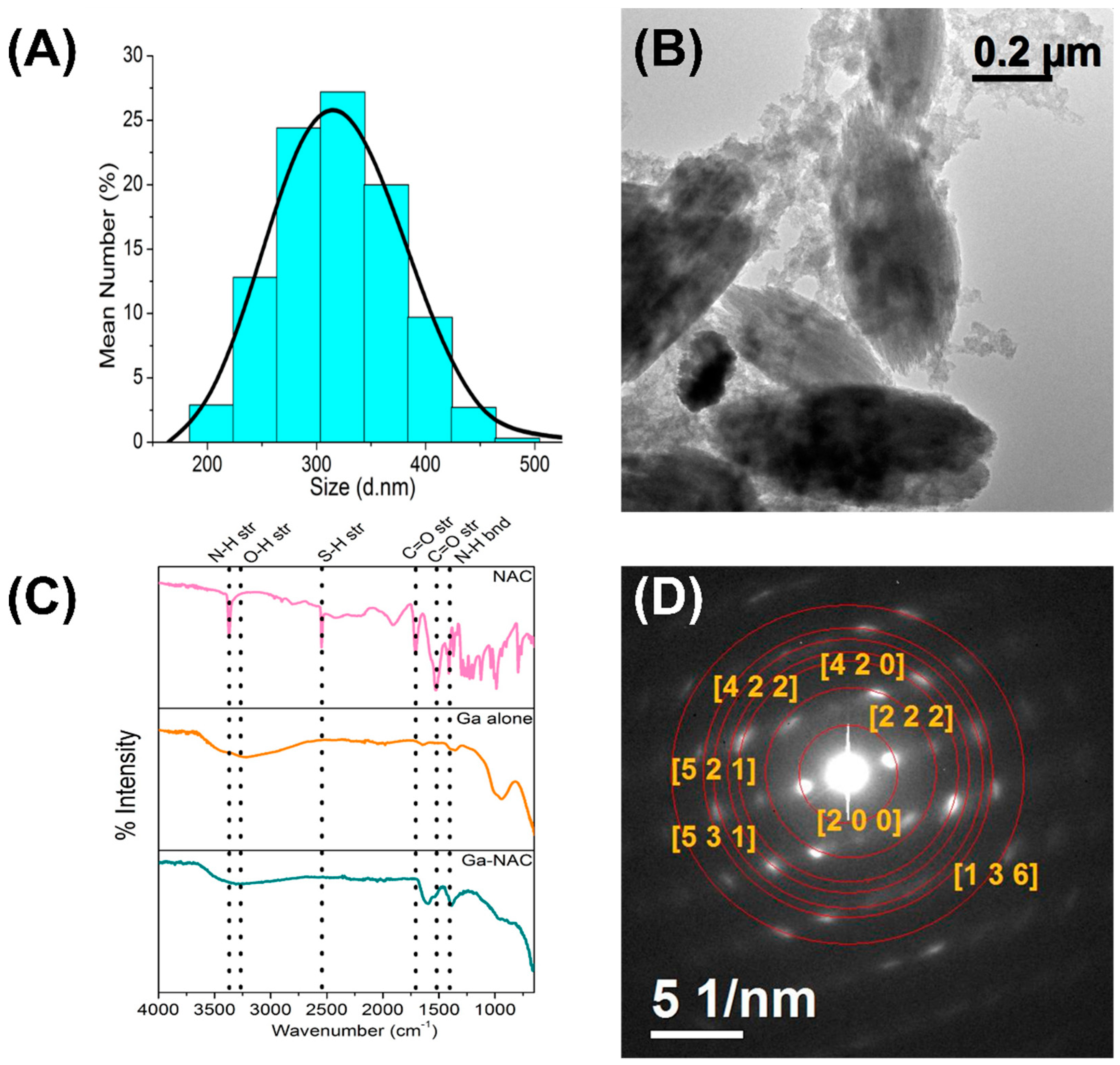
3. Ga-NAC Synthesis and Characterization
4. Ga-NAC Antimicrobial Studies
4.1. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
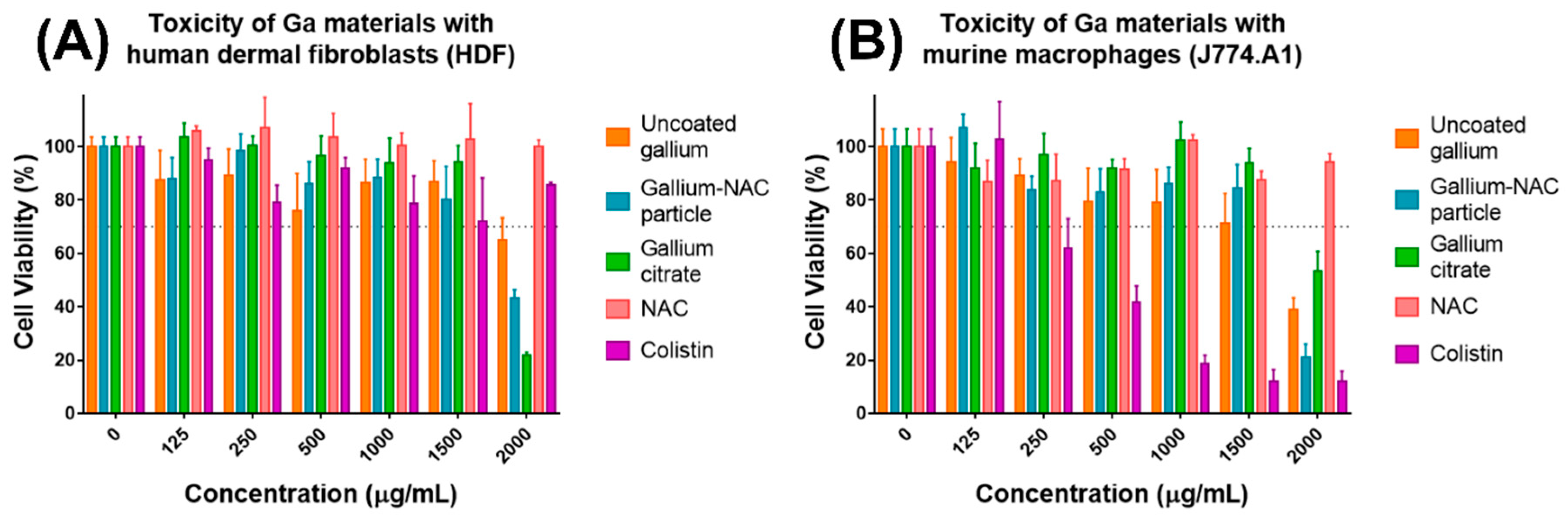
4.2. Cell Culture and Cytotoxicity Study
5. Biofilm Studies
5.1. Bacterial Viability in Established Biofilms after Treatment
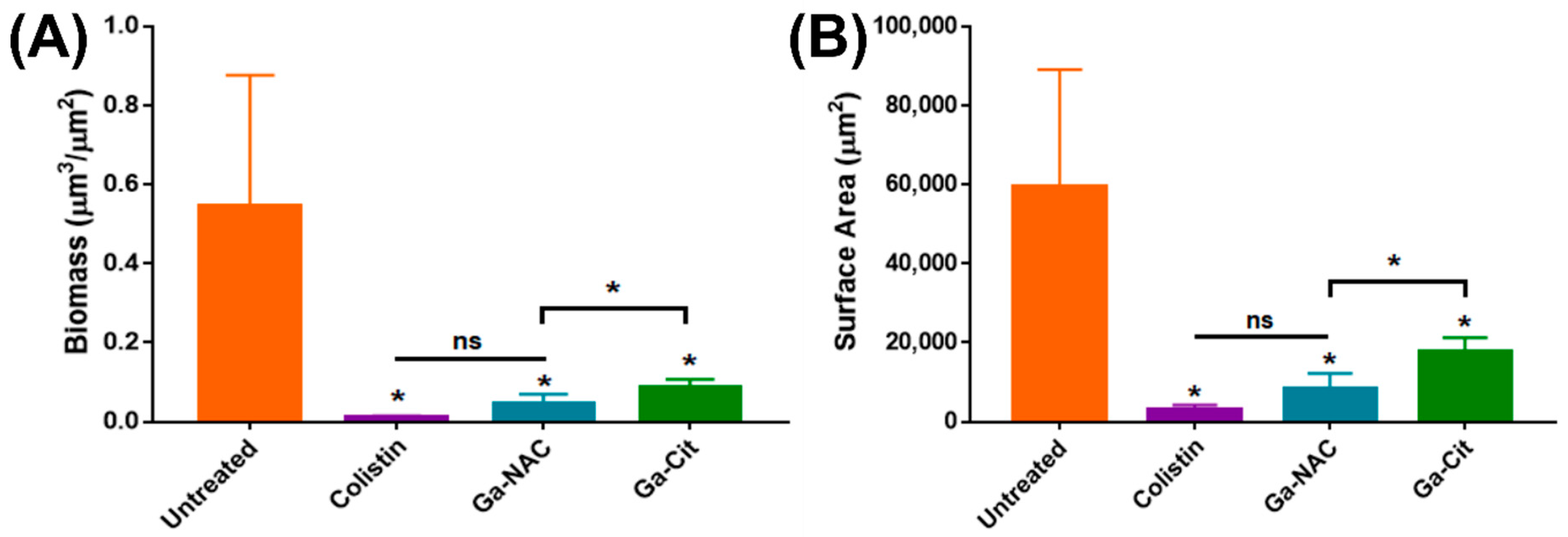
5.2. Inhibition of Biofilm Formation
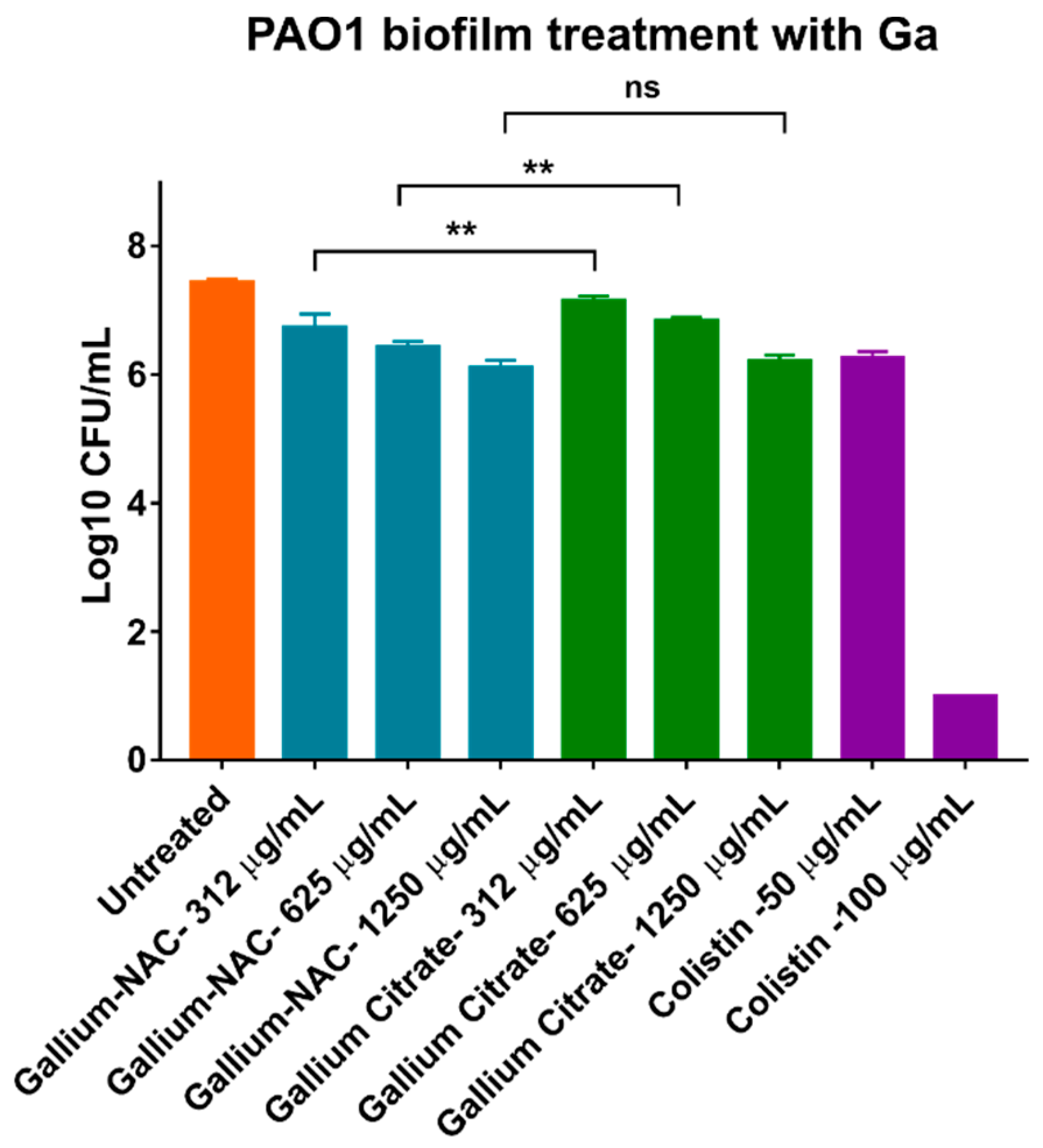
5.3. Treatment of Established Biofilm
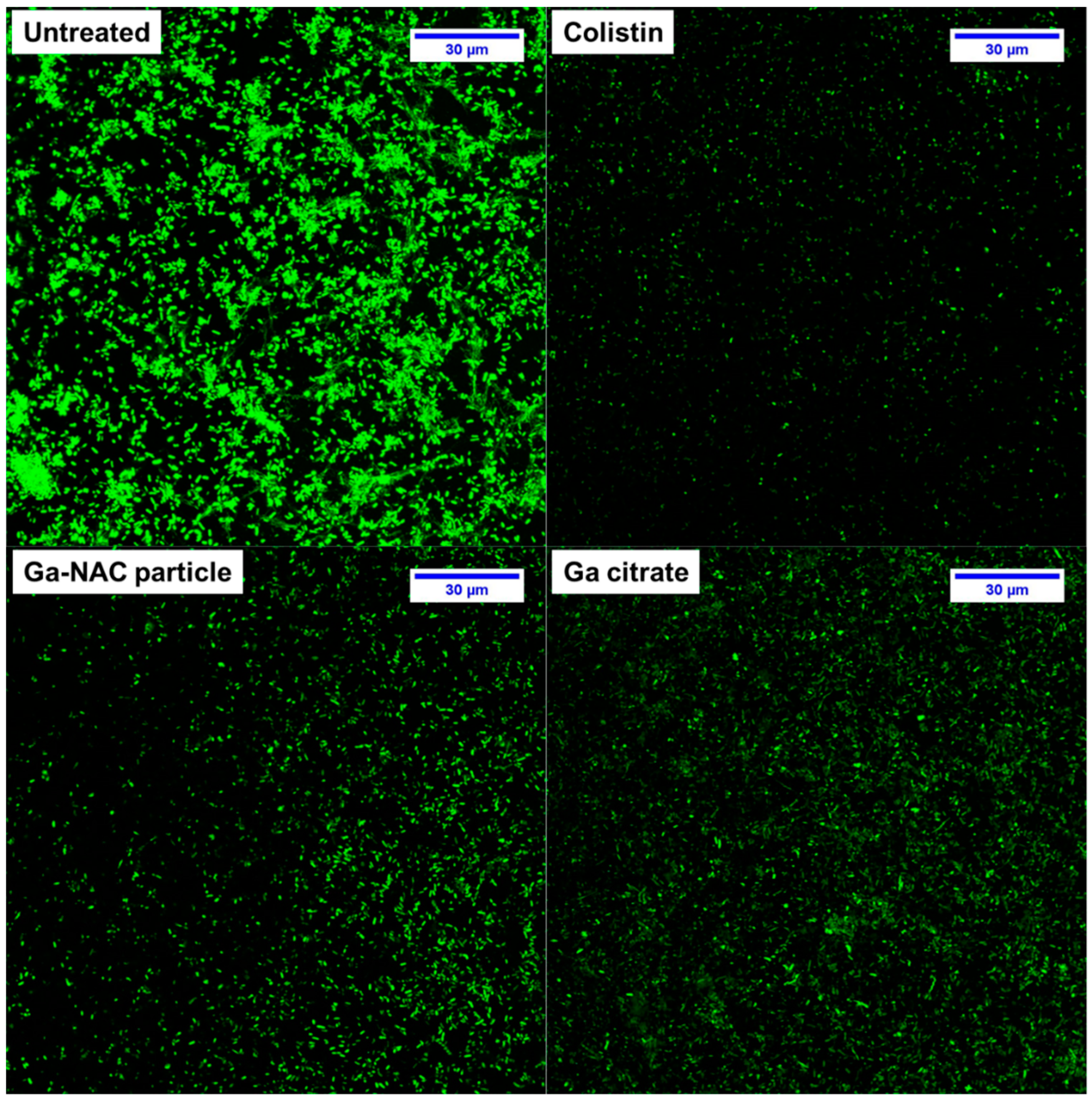
5.4. Confocal Laser Scanning Microscopy (CLSM)
6. Ga-NAC Particle Mode of Action
6.1. Bacterial Sample Preparation
6.2. Cell Membrane Chemistry
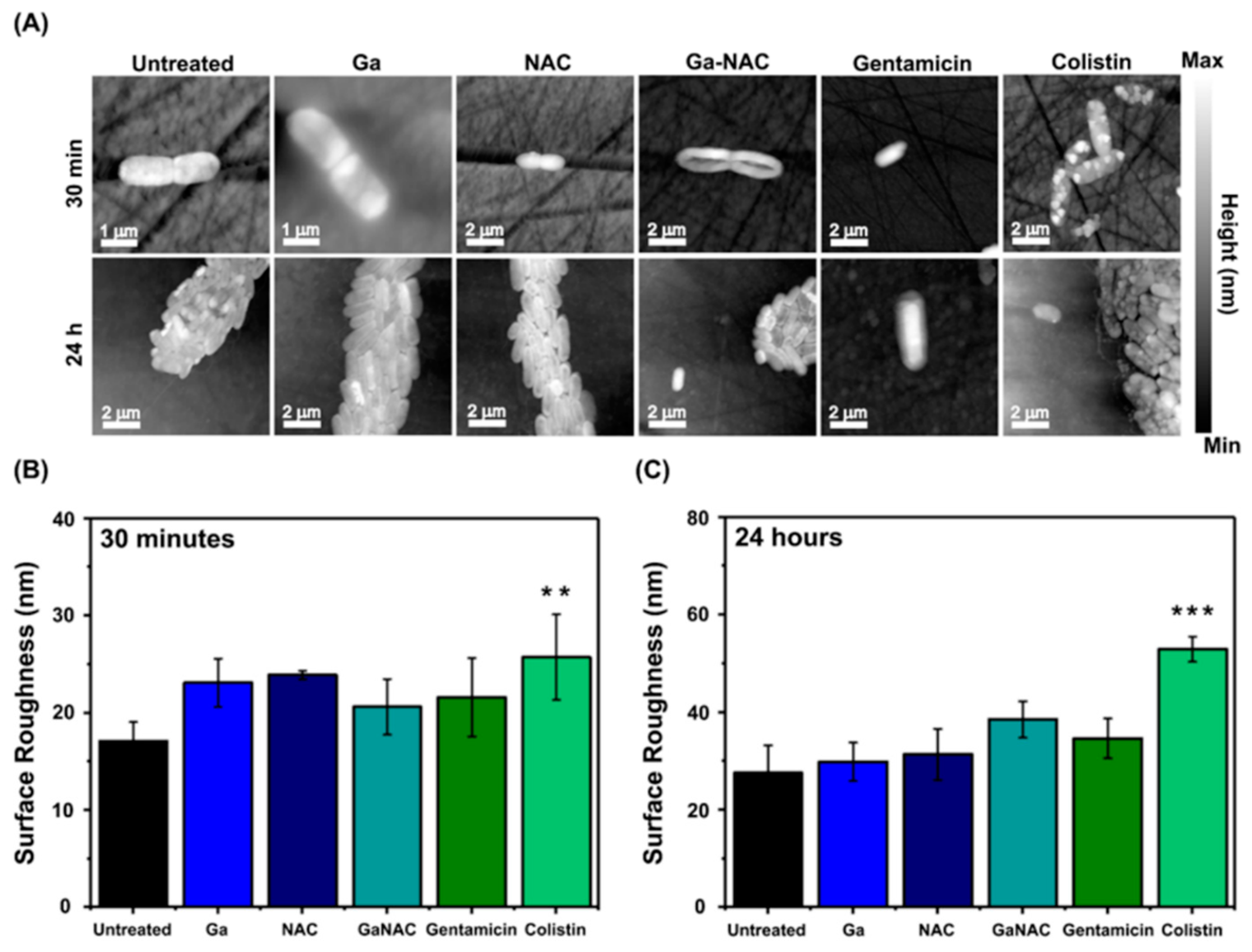
6.3. Cell Membrane Degradation Determined by Atomic Force Microscopy (AFM)
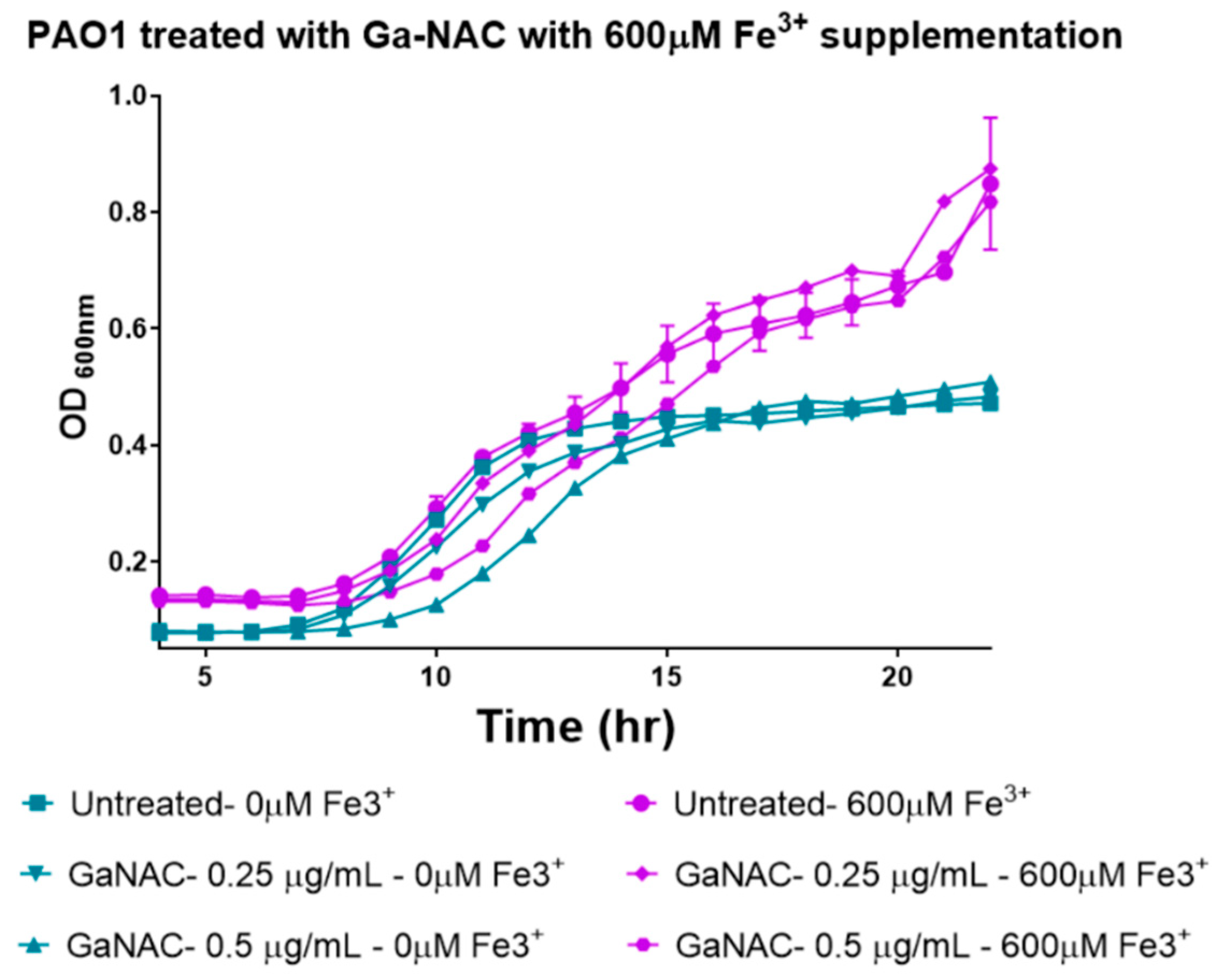
6.4. Time-Dependent PAO1 Growth Curve with Fe Supplementation
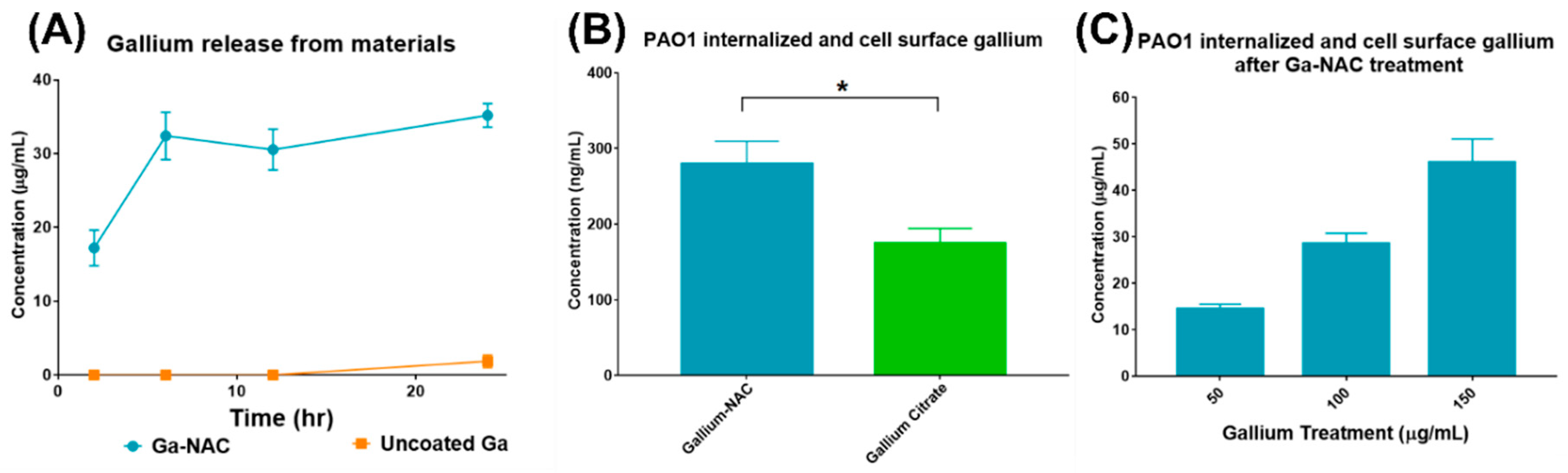
6.5. Ga Release from NAC Coated and Uncoated Ga Particle
6.6. Ga-cell Association with PAO1
7. Results
7.1. Material Characterization
7.2. MIC and MBC
7.3. Cytotoxicity of Ga-NAC Particles
7.4. Biofilm Inhibition and Disruption
7.5. Cell Membrane Chemistry
7.6. Cell Membrane Degradation Determined by Atomic Force Microscopy (AFM)
7.7. Time Dependent PAO1 Growth Curve with Fe Supplementation
7.8. Ga release and Association with PAO1
8. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Control CFD and Prevention. Antibiotic Resistance Threats in the United States; Centres for Disease Control and Prevention; US Department of Health and Human Services: Atlanta, GA, USA, 2013.
- Zayyad, H.; Eliakim-Raz, N.; Leibovici, L.; Paul, M. Revival of old antibiotics: needs, the state of evidence and expectations. Int. J. Antimicrob. Agents 2017, 49, 536–541. [Google Scholar] [CrossRef]
- Falagas, M.E.; Kasiakou, S.K.; Saravolatz, L.D. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin. Infect. Dis. 2005, 40, 1333–1341. [Google Scholar] [CrossRef]
- Edwards, C.L.; Hayes, R.L. Tumor scanning with 67Ga citrate. J. Nucl. Med. 1969, 10, 103–105. [Google Scholar]
- Kaneko, Y.; Thoendel, M.; Olakanmi, O.; Britigan, B.E.; Singh, P.K. The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J. Clin. Investig. 2007, 117, 877–888. [Google Scholar] [CrossRef]
- Halwani, M.; Yebio, B.; Suntres, Z.E.; Alipour, M.; Azghani, A.O.; Omri, A. Co-encapsulation of gallium with gentamicin in liposomes enhances antimicrobial activity of gentamicin against Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2008, 62, 1291–1297. [Google Scholar] [CrossRef]
- Richter, K.; Ramezanpour, M.; Thomas, N.; Prestidge, C.A.; Wormald, P.J.; Vreugde, S. Mind De GaPP: In vitro efficacy of deferiprone and gallium—Protoporphyrin against Staphylococcus aureus biofilms. Int. Forum Allergy Rhinol. 2016, 6, 737–743. [Google Scholar] [CrossRef]
- Rzhepishevska, O.; Ekstrand-Hammarström, B.; Popp, M.; Björn, E.; Bucht, A.; Sjöstedt, A.; Antti, H.; Ramstedt, M. The antibacterial activity of Ga3+ is influenced by ligand complexation as well as the bacterial carbon source. Antimicrob. Agents Chemother. 2011, 55, 5568–5580. [Google Scholar] [CrossRef]
- Hijazi, S.; Visaggio, D.; Pirolo, M.; Frangipani, E.; Bernstein, L.; Visca, P. Antimicrobial activity of gallium compounds on ESKAPE pathogens. Front. Cell. Infect. Microbiol. 2018, 8, 316. [Google Scholar] [CrossRef]
- DeLeon, K.; Balldin, F.; Watters, C.; Hamood, A.; Griswold, J.; Sreedharan, S.; Rumbaugh, K.P. Gallium maltolate treatment eradicates Pseudomonas aeruginosa infection in thermally injured mice. Antimicrob. Agents Chemother. 2009, 53, 1331–1337. [Google Scholar] [CrossRef]
- Goss, C.H.; Kaneko, Y.; Khuu, L.; Anderson, G.D.; Ravishankar, S.; Aitken, M.L.; Lechtzin, N.; Zhou, G.; Czyz, D.M.; McLean, K.; et al. Gallium disrupts bacterial iron metabolism and has therapeutic effects in mice and humans with lung infections. Sci. Transl. Med. 2018, 10, eaat7520. [Google Scholar] [CrossRef]
- Richter, K.; Thomas, N.; Claeys, J.; McGuane, J.; Prestidge, C.A.; Coenye, T.; Wormald, P.J.; Vreugde, S. A topical hydrogel with deferiprone and gallium-protoporphyrin targets bacterial iron metabolism and has antibiofilm activity. Antimicrob. Agents Chemother. 2017, 61, e00481-17. [Google Scholar] [CrossRef]
- Arivett, B.A.; Fiester, S.E.; Ohneck, E.J.; Penwell, W.F.; Kaufman, C.M.; Relich, R.F.; Actis, L.A. Antimicrobial activity of gallium protoporphyrin IX against Acinetobacter baumannii strains displaying different antibiotic resistance phenotypes. Antimicrob. Agents Chemother. 2015, 59, 7657–7665. [Google Scholar] [CrossRef]
- Ooi, M.L.; Richter, K.; Drilling, A.J.; Thomas, N.; Prestidge, C.A.; James, C.; Moratti, S.; Vreugde, S.; Psaltis, A.J.; Wormald, P.J. Safety and Efficacy of Topical Chitogel-Deferiprone-Gallium Protoporphyrin in Sheep Model. Front. Microbiol. 2018, 9, 917. [Google Scholar] [CrossRef]
- Minandri, F.; Bonchi, C.; Frangipani, E.; Imperi, F.; Visca, P. Promises and failures of gallium as an antibacterial agent. Future Microbiol. 2014, 9, 379–397. [Google Scholar] [CrossRef]
- Oglesby-Sherrouse, A.G.; Djapgne, L.; Nguyen, A.T.; Vasil, A.I.; Vasil, M.L. The complex interplay of iron, biofilm formation, and mucoidy affecting antimicrobial resistance of Pseudomonas aeruginosa. Pathog. Dis. 2014, 70, 307–320. [Google Scholar] [CrossRef]
- Frangipani, E.; Bonchi, C.; Minandri, F.; Imperi, F.; Visca, P. Pyochelin potentiates the inhibitory activity of gallium on Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2014, 58, 5572–5575. [Google Scholar] [CrossRef]
- Bonchi, C.; Frangipani, E.; Imperi, F.; Visca, P. Pyoverdine and proteases affect the response of Pseudomonas aeruginosa to gallium in human serum. Antimicrob. Agents Chemother. 2015, 59, 5641–5646. [Google Scholar] [CrossRef]
- Zhao, T.; Liu, Y. N-acetylcysteine inhibit biofilms produced by Pseudomonas aeruginosa. BMC Microbiol. 2010, 10, 140. [Google Scholar] [CrossRef]
- Suk, J.S.; Boylan, N.J.; Trehan, K.; Tang, B.C.; Schneider, C.S.; Lin, J.M.; Boyle, M.P.; Zeitlin, P.L.; Lai, S.K.; Cooper, M.J.; et al. N-acetylcysteine enhances cystic fibrosis sputum penetration and airway gene transfer by highly compacted DNA nanoparticles. Mol. Ther. 2011, 19, 1981–1989. [Google Scholar] [CrossRef]
- Dinicola, S.; De Grazia, S.; Carlomagno, G.; Pintucci, J.P. N-acetylcysteine as powerful molecule to destroy bacterial biofilms. A systematic review. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2942–2948. [Google Scholar]
- Costa, F.; Sousa, D.M.; Parreira, P.; Lamghari, M.; Gomes, P.; Martins, M.C.L. N-acetylcysteine-functionalized coating avoids bacterial adhesion and biofilm formation. Sci. Rep. 2017, 7, 17374. [Google Scholar] [CrossRef]
- Liu, D.; Li, J.; Pan, H.; He, F.; Liu, Z.; Wu, Q.; Bai, C.; Yu, S.; Yang, X. Potential advantages of a novel chitosan-N-acetylcysteine surface modified nanostructured lipid carrier on the performance of ophthalmic delivery of curcumin. Sci. Rep. 2016, 6, 28796. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard-Ninth Edition, in CLSI Document M07-A9; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Visca, P.; Ciervo, A.; Sanfilippo, V.; Orsi, N. Iron-regulated salicylate synthesis by Pseudomonas spp. Microbiology 1993, 139, 1995–2001. [Google Scholar] [CrossRef]
- Hackel, M.A.; Tsuji, M.; Yamano, Y.; Echols, R.; Karlowsky, J.A.; Sahm, D.F. In vitro activity of the siderophore cephalosporin, cefiderocol, against carbapenem-nonsusceptible and multidrug-resistant isolates of Gram-negative bacilli collected worldwide in 2014 to 2016. Antimicro. Agents Chemother. 2018, 62, e01968-17. [Google Scholar] [CrossRef]
- Standardization, I.O.F. Biological Evaluation of Medical Devices in Part 5: Tests for in Vitro Cytotoxicity; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- Nguyen, U.T.; Wenderska, I.B.; Chong, M.A.; Koteva, K.; Wright, G.D.; Burrows, L.L. Small-molecule modulators of Listeria monocytogenes biofilm development. Appl. Environ. Microbiol. 2012, 78, 1454–1465. [Google Scholar] [CrossRef]
- Harrison, J.J.; Turner, R.J.; Ceri, H. High-throughput metal susceptibility testing of microbial biofilms. BMC Microbiol. 2005, 5, 53. [Google Scholar] [CrossRef]
- Chen, H.; Wubbolts, R.W.; Haagsman, H.P.; Veldhuizen, E.J.A. Inhibition and eradication of Pseudomonas aeruginosa biofilms by host defence peptides. Sci. Rep. 2018, 8, 10446. [Google Scholar] [CrossRef]
- Heydorn, A.; Nielsen, A.T.; Hentzer, M.; Sternberg, C.; Givskov, M.; Ersbøll, B.K.; Molin, S. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 2000, 146, 2395–2407. [Google Scholar] [CrossRef]
- Vorregaard, M. Comstat2-a Modern 3D Image Analysis Environment for Biofilms; Citeseer: Lyngby, Denmark, 2008. [Google Scholar]
- Nečas DKlapetek, P. Gwyddion: An open-source software for SPM data analysis. Open Phys. 2012, 10, 181–188. [Google Scholar]
- Smani, Y.; Canturri, A.M.; Algaba, R.A. Drug repurposing for the treatment of bacterial and fungal infections. Front. Microbiol. 2019, 10, 41. [Google Scholar]
- Warrell, R.P., Jr.; Israel, R.; Frisone, M.; Snyder, T.; Gaynor, J.J.; Bockman, R.S. Gallium nitrate for acute treatment of cancer-related hypercalcemia: A randomized, double-blind comparison to calcitonin. Ann. Intern. Med. 1988, 108, 669–674. [Google Scholar] [CrossRef]


| Treatment | TSB | D-TSB | MHB | |||
|---|---|---|---|---|---|---|
| MIC (µg/mL) | MBC (µg/mL) | MIC (µg/mL) | MBC (µg/mL) | MIC (µg/mL) | MBC (µg/mL) | |
| Gallium citrate | 2 | 4 | 2 | 2 | 8 | 16 |
| Uncoated Ga particle | 8 | 16 | 4 | 8 | 16 | 32 |
| Ga-NAC particle | 1 | 2 | 0.5 | 1 | 4 | 8 |
| NAC | 2500 | 5000 | 2500 | 5000 | 5000 | 5000 |
| Colistin | 0.5 | 0.5 | 0.5 | 0.5 | 1 | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Young, M.; Ozcan, A.; Lee, B.; Maxwell, T.; Andl, T.; Rajasekaran, P.; Beazley, M.J.; Tetard, L.; Santra, S. N-acetyl Cysteine Coated Gallium Particles Demonstrate High Potency against Pseudomonas aeruginosa PAO1. Pathogens 2019, 8, 120. https://doi.org/10.3390/pathogens8030120
Young M, Ozcan A, Lee B, Maxwell T, Andl T, Rajasekaran P, Beazley MJ, Tetard L, Santra S. N-acetyl Cysteine Coated Gallium Particles Demonstrate High Potency against Pseudomonas aeruginosa PAO1. Pathogens. 2019; 8(3):120. https://doi.org/10.3390/pathogens8030120
Chicago/Turabian StyleYoung, Mikaeel, Ali Ozcan, Briana Lee, Tyler Maxwell, Thomas Andl, Parthiban Rajasekaran, Melanie J. Beazley, Laurene Tetard, and Swadeshmukul Santra. 2019. "N-acetyl Cysteine Coated Gallium Particles Demonstrate High Potency against Pseudomonas aeruginosa PAO1" Pathogens 8, no. 3: 120. https://doi.org/10.3390/pathogens8030120
APA StyleYoung, M., Ozcan, A., Lee, B., Maxwell, T., Andl, T., Rajasekaran, P., Beazley, M. J., Tetard, L., & Santra, S. (2019). N-acetyl Cysteine Coated Gallium Particles Demonstrate High Potency against Pseudomonas aeruginosa PAO1. Pathogens, 8(3), 120. https://doi.org/10.3390/pathogens8030120





