Antibacterial Evaluation of Tricyclic Antidepressants Against S. aureus and the Possible Pathways of the Mechanism of Action
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Isolates and Drugs
2.2. Antimicrobial Susceptibility Testing (AST) and Breakpoints
2.3. Assessment of Minimum Bactericidal Concentration (MBC) and Tolerance Level
2.4. Analysis of the Interaction Between Antibacterials and TCAs
2.5. Possible Mechanism of Action
2.5.1. Treatment of Cells
2.5.2. Determination of Cell Viability
2.5.3. Assessment of DNA Fragmentation
2.5.4. Alkaline Comet Test
2.5.5. Quantification of Reactive Oxygen Species (ROS)
2.5.6. Analysis of Carbonyl Proteins
2.6. Data Analysis
2.7. Scanning Electron Microscopy (SEM)
3. Results
3.1. TCAs Exhibited Antibacterial Activity Against S. aureus and Had a Bactericidal Action Profile
3.2. The Association Between TCAs and OXA Demonstrated a Synergistic Effect Against S. aureus
3.3. TCAs Reduced MRSA Viability
3.4. Treatment of MRSA with TCAs Resulted in an Increase in TUNEL-Positive Cells
3.5. The Action of TCAs on DNA Evidenced by the Alkaline Comet Assay
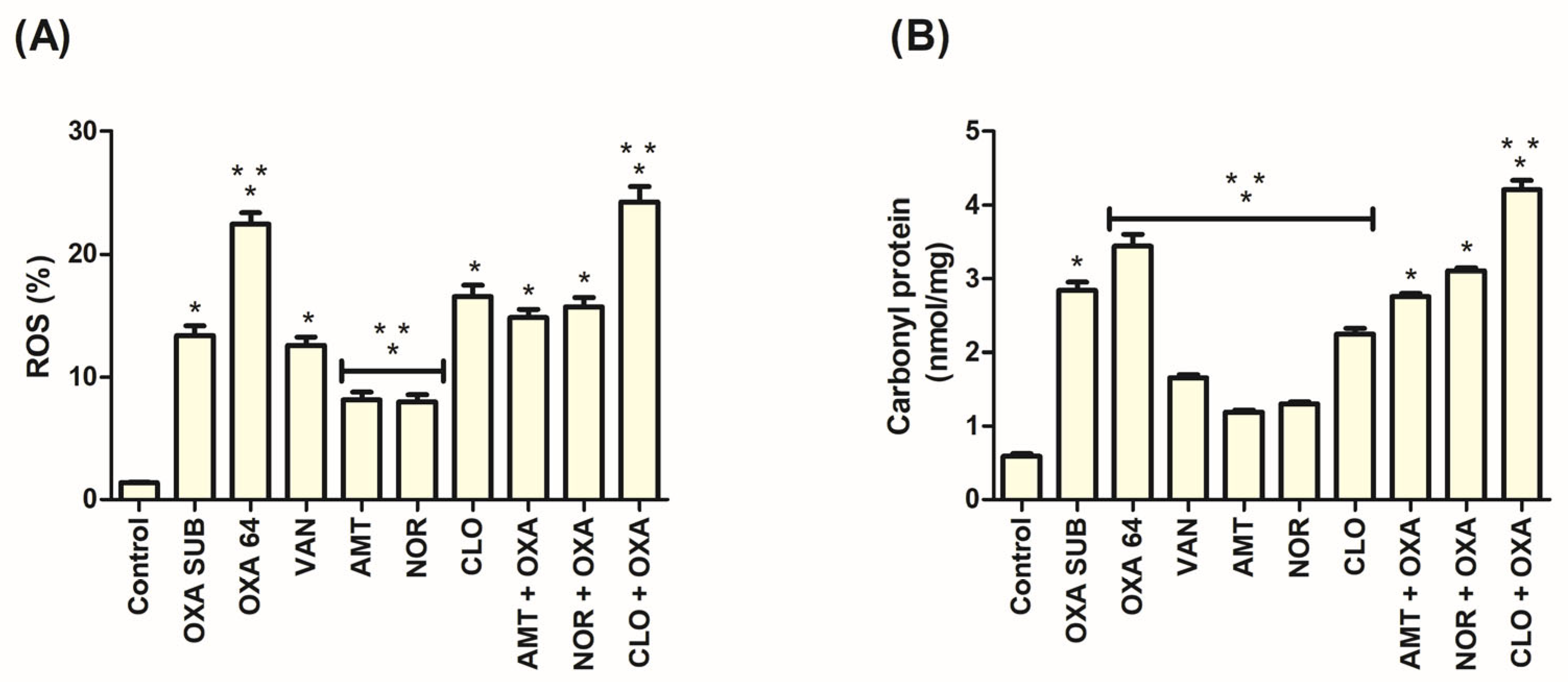
3.6. ROS Production Occurred in the Action of TCAs Against MRSA
3.7. Increased Protein Carbonylation Was Associated with the Action of TCAs in MRSA
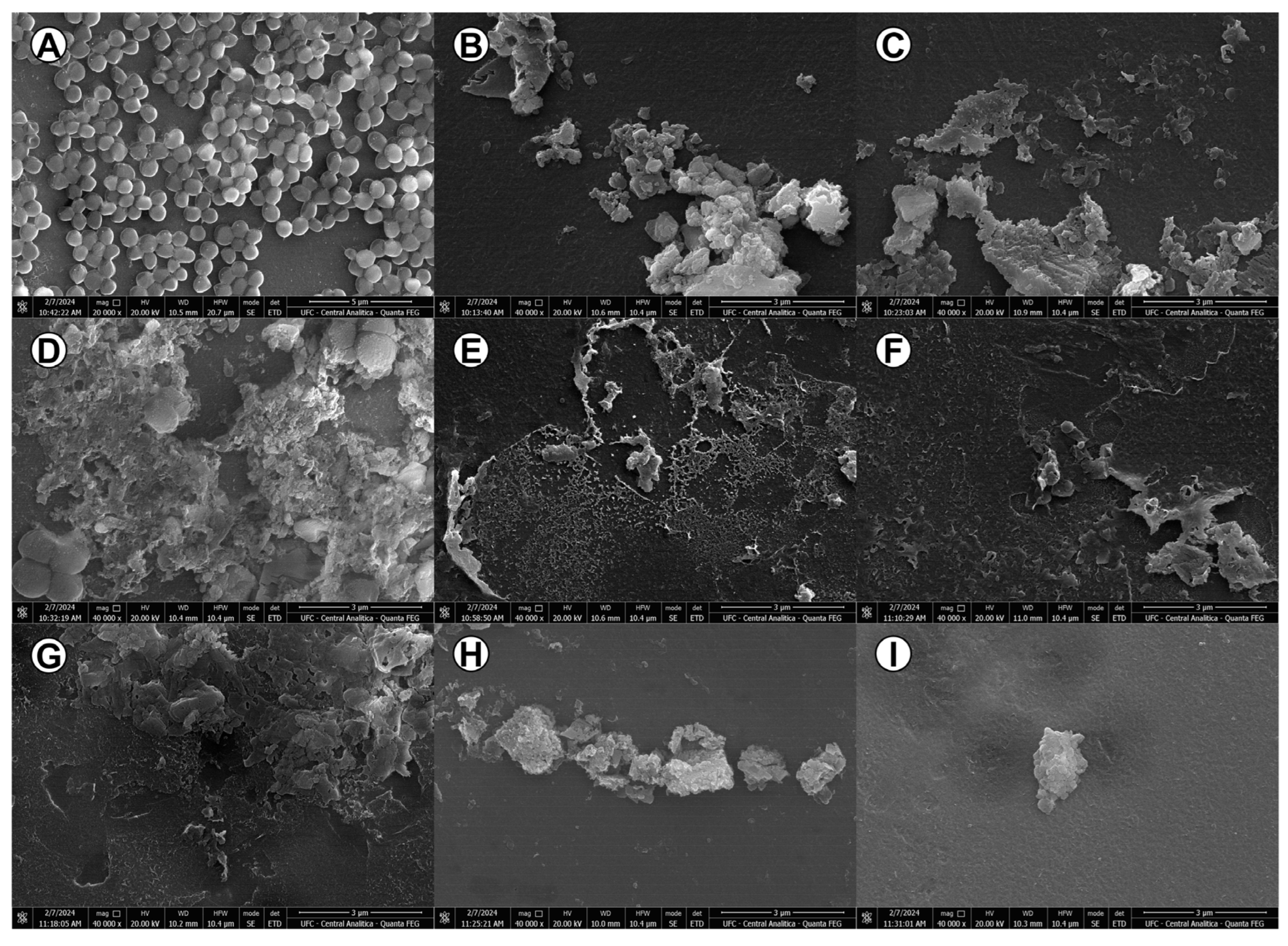
3.8. The Antibacterial Activity of TCAs Generated Considerable Morphological Changes in MRSA Bacterial Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADI | Additive |
| AMT | Amitriptyline |
| ANOVA | Analysis of variance |
| AST | Antimicrobial susceptibility testing |
| BHI | Brain heart infusion |
| CFU | Colony-Forming Units |
| CLSI | Clinical and Laboratory Standards Institute |
| CLO | Clomipramine |
| DNA | Deoxyribonucleic acid |
| DNPH | 2,4-dinitrophenylhydrazine |
| ESKAPE | Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp. |
| FDA | Food and Drug Administration |
| FICI | Fractional Inhibitory Concentration Index |
| IND | Indifferent |
| LABIMAN | Laboratory for Bioprospection of Antimicrobial Molecules |
| MBC | Minimum Bactericidal Concentration |
| MBC/MIC | Tolerance level |
| MIC | Minimum Inhibitory Concentration |
| MRSA | Methicillin-resistant S. aureus |
| MSSA | Methicillin-sensitive S. aureus |
| NOR | Nortriptyline |
| OXA | Oxacillin |
| OXA SUB | Subinhibitory oxacillin |
| PI | Propidium iodide |
| ROS | Reactive oxygen species |
| SEM | Scanning electron microscopy |
| SYN | Synergism |
| TCAs | Tricyclic antidepressants |
| TUNEL | Terminal deoxynucleotidyl transferase dUTP nick-end labeling |
| UFC | Federal University of Ceará |
| VAN | Vancomycin |
References
- Linz, M.S.; Mattappallil, A.; Finkel, D.; Parker, D. Clinical Impact of Staphylococcus aureus Skin and Soft Tissue Infections. Antibiotics 2023, 12, 557. [Google Scholar] [CrossRef]
- Huitema, L.; Phillips, T.; Alexeev, V.; Tomic-Canic, M.; Pastar, I.; Igoucheva, O. Intracellular Escape Strategies of Staphylococcus aureus in Persistent Cutaneous Infections. Exp. Dermatol. 2021, 30, 1428–1439. [Google Scholar] [CrossRef]
- Mitevska, E.; Wong, B.; Surewaard, B.G.J.; Jenne, C.N. The Prevalence, Risk, and Management of Methicillin-Resistant Staphylococcus aureus Infection in Diverse Populations across Canada: A Systematic Review. Pathogens 2021, 10, 393. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.T.; Chen, E.Z.; Yang, L.; Peng, C.; Wang, Q.; Xu, Z.; Chen, D.Q. Emerging Resistance Mechanisms for 4 Types of Common Anti-MRSA Antibiotics in Staphylococcus aureus: A Comprehensive Review. Microb. Pathog. 2021, 156, 104915. [Google Scholar] [CrossRef]
- Shoaib, M.; Aqib, A.I.; Muzammil, I.; Majeed, N.; Bhutta, Z.A.; Kulyar, M.F.e.A.; Fatima, M.; Zaheer, C.N.F.; Muneer, A.; Murtaza, M.; et al. MRSA Compendium of Epidemiology, Transmission, Pathophysiology, Treatment, and Prevention within One Health Framework. Front. Microbiol. 2023, 13, 1067284. [Google Scholar] [CrossRef] [PubMed]
- Salmanov, A.; Shchehlov, D.; Artyomenko, V.; Svyrydiuk, O.; Maliarchuk, R.; Bortnik, I.; Mamonova, M.; Korniyenko, S.; Rud, V.; Gudym, M.; et al. Nosocomial Transmission of Multi-Drug-Resistant Organisms in Ukrainian Hospitals: Results of a Multi-Centre Study (2019–2021). J. Hosp. Infect. 2023, 132, 104–115. [Google Scholar] [CrossRef]
- Yao, Z.; Wu, Y.; Xu, H.; Lei, Y.; Long, W.; Li, M.; Gu, Y.; Jiang, Z.; Cao, C. Prevalence and Clinical Characteristics of Methicillin-Resistant Staphylococcus aureus Infections among Dermatology Inpatients: A 7-Year Retrospective Study at a Tertiary Care Center in Southwest China. Front. Public Health 2023, 11, 1124930. [Google Scholar] [CrossRef] [PubMed]
- Riche, C.V.W.; Cassol, R.; Falci, D.R.; Ramirez, M.; Dias, C.A.G. Epidemiology and Risk Factors for Mortality among Methicillin-Resistant Staphylococcus aureus Bacteremic Patients in Southern Brazil. PLoS ONE 2023, 18, e0283774. [Google Scholar] [CrossRef]
- WHO. WHO Bacterial Priority Pathogens List; WHO: Geneva, Switzerland, 2024; ISBN 978-92-4-009346-1. [Google Scholar]
- Abavisani, M.; Khoshrou, A.; Eshaghian, S.; Karav, S.; Sahebkar, A. Overcoming Antibiotic Resistance: The Potential and Pitfalls of Drug Repurposing. J. Drug Target. 2024, 33, 341–367. [Google Scholar] [CrossRef]
- Caldara, M.; Marmiroli, N. Antimicrobial Properties of Antidepressants and Antipsychotics—Possibilities and Implications. Pharmaceuticals 2021, 14, 915. [Google Scholar] [CrossRef]
- Ding, L.; Chen, X.; Lv, Y.; Peng, Q.; Wang, R.; Yang, B.; Wei, L. Research Progress on the Synergistic Effect and Its Mechanisms of Antidepressants and Antibiotics against Resistant Pathogens. Arch. Microbiol. 2025, 207, 157. [Google Scholar] [CrossRef] [PubMed]
- Vos, C.F.; Aarnoutse, R.E.; Op de Coul, M.J.M.; Spijker, J.; Groothedde-Kuyvenhoven, M.M.; Mihaescu, R.; Wessels-Basten, S.J.W.; Rovers, J.J.E.; ter Hark, S.E.; Schene, A.H.; et al. Tricyclic Antidepressants for Major Depressive Disorder: A Comprehensive Evaluation of Current Practice in the Netherlands. BMC Psychiatry 2021, 21, 481. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Patterson, M.; Jimenez, X.F. Beyond Depression: Other Uses for Tricyclic Antidepressants. Cleve Clin. J. Med. 2019, 86, 807–814. [Google Scholar] [CrossRef]
- Machado, C.d.S.; da Rosa, T.F.; Serafin, M.B.; Bottega, A.; Coelho, S.S.; Foletto, V.S.; Rampelotto, R.F.; Lorenzoni, V.V.; de L. Marion, S.; Hörner, R. In Vitro Evaluation of the Antibacterial Activity of Amitriptyline and Its Synergistic Effect with Ciprofloxacin, Sulfamethoxazole–Trimethoprim, and Colistin as an Alternative in Drug Repositioning. Med. Chem. Res. 2020, 29, 166–177. [Google Scholar] [CrossRef]
- Mandal, A.; Sinha, C.; Jena, A.K.; Ghosh, S.; Samanta, A. An Investigation on In Vitro and In Vivo Antimicrobial Properties of the Antidepressant: Amitriptyline Hydrochloride. Braz. J. Microbiol. 2010, 41, 635–642. [Google Scholar] [CrossRef]
- Ugurel, E.; Turgut, D. Synergistic Combination of Carvedilol, Amlodipine, Amitriptyline, and Antibiotics as an Alternative Treatment Approach for the Susceptible and Multidrug—Resistant A. baumannii Infections via Drug Repurposing. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 42, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Bellido, J.L.; Munoz-Criado, S.; Garcìa-Rodrìguez, J.A. Antimicrobial Activity of Psychotropic Drugs: Selective Serotonin Reuptake Inhibitors. Int. J. Antimicrob. Agents 2000, 14, 177–180. [Google Scholar] [CrossRef]
- Kalaycı, S.; Demirci, S.; Sahin, F. Antimicrobial Properties of Various Psychotropic Drugs Against Broad Range Microorganisms. Curr. Psychopharmacol. 2014, 3, 195–202. [Google Scholar] [CrossRef]
- Otto, R.G.; Van Gorp, E.; Kloezen, W.; Meletiadis, J.; Van den Berg, S.; Mouton, J.W. An Alternative Strategy for Combination Therapy: Interactions between Polymyxin B and Non-Antibiotics. Int. J. Antimicrob. Agents 2019, 53, 34–39. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard, 10th ed.; CLSI Document M07-A10; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015; Volume 35, pp. 1–110. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 31st ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021; pp. 1–352. [Google Scholar]
- Cabral, V.P.d.F.; Rodrigues, D.S.; Barbosa, A.D.; Moreira, L.E.A.; Sá, L.G.d.A.V.; da Silva, C.R.; de Andrade Neto, J.B.; Silva, J.; Marinho, E.S.; dos Santos, H.S.; et al. Antibacterial Activity of Paroxetine against Staphylococcus aureus and Possible Mechanisms of Action. Future Microbiol. 2023, 18, 415–426. [Google Scholar] [CrossRef]
- Piasecki, B.; Biernasiuk, A.; Skiba, A.; Skalicka-Woźniak, K.; Ludwiczuk, A. Composition, Anti-MRSA Activity and Toxicity of Essential Oils from Cymbopogon Species. Molecules 2021, 26, 7542. [Google Scholar] [CrossRef] [PubMed]
- Odds, F.C. Synergy, Antagonism, and What the Chequerboard Puts between Them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- Jorge, P.; Grzywacz, D.; Kamysz, W.; Lourenço, A.; Pereira, M.O. Searching for New Strategies against Biofilm Infections: Colistin-AMP Combinations against Pseudomonas aeruginosa and Staphylococcus aureus Single- and Double-Species Biofilms. PLoS ONE 2017, 12, e0174654. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.C.; Hong, Y.; Danavall, D.C.A.; Howard-Jones, M.H.; Gibson, D.; Frischer, M.E.; Verity, P.G. Distinguishing between Living and Nonliving Bacteria: Evaluation of the Vital Stain Propidium Iodide and Its Combined Use with Molecular Probes in Aquatic Samples. J. Microbiol. Methods 1998, 32, 225–236. [Google Scholar] [CrossRef]
- Shi, L.; Günther, S.; Hübschmann, T.; Wick, L.Y.; Harms, H.; Müller, S. Limits of Propidium Iodide as a Cell Viability Indicator for Environmental Bacteria. Cytom. Part A 2007, 71, 592–598. [Google Scholar] [CrossRef]
- Do Av Sá, L.G.; Da Silva, C.R.; De A Neto, J.B.; Cândido, T.M.; De Oliveira, L.C.; Do Nascimento, F.B.S.A.; Barroso, F.D.D.; Da Silva, L.J.; De Mesquita, J.R.L.; De Moraes, M.O.; et al. Etomidate Inhibits the Growth of MRSA and Exhibits Synergism with Oxacillin. Future Microbiol. 2020, 15, 1611–1619. [Google Scholar] [CrossRef] [PubMed]
- Andrade Neto, J.B.; Alexandre Josino, M.A.; Rocha da Silva, C.; de Sousa Campos, R.; Aires do Nascimento, F.B.S.; Sampaio, L.S.; Gurgel do Amaral Valente Sá, L.; de Sá Carneiro, I.; Dias Barroso, F.D.; Juvêncio da Silva, L.; et al. A Mechanistic Approach to the in-vitro Resistance Modulating Effects of Fluoxetine against Meticillin Resistant Staphylococcus aureus Strains. Microb. Pathog. 2019, 127, 335–340. [Google Scholar] [CrossRef]
- Dwyer, D.J.; Camacho, D.M.; Kohanski, M.A.; Callura, J.M.; Collins, J.J. Antibiotic-Induced Bacterial Cell Death Exhibits Physiological and Biochemical Hallmarks of Apoptosis. Mol. Cell 2012, 46, 561–572. [Google Scholar] [CrossRef]
- Dong, Y.; Zhu, H.; Shen, Y.; Zhang, W.; Zhang, L. Antibacterial Activity of Silver Nanoparticles of Different Particle Size against Vibrio natriegens. PLoS ONE 2019, 14, e0222322. [Google Scholar] [CrossRef]
- Leitão, A.C.; Ferreira, T.L.; do Amaral Valente Sá, L.G.; Rodrigues, D.S.; de Souza, B.O.; Barbosa, A.D.; Moreira, L.E.A.; de Andrade Neto, J.B.; de Farias Cabral, V.P.; Rios, M.E.F.; et al. Antibacterial Activity of Menadione Alone and in Combination with Oxacillin against Methicillin-Resistant Staphylococcus aureus and Its Impact on Biofilms. J. Med. Microbiol. 2023, 72, 001751. [Google Scholar] [CrossRef]
- Da Silva, C.R.; De Andrade Neto, J.B.; Costa Sidrim, J.J.; Ferreira Ângelo, M.R.; Ferreira Magalhães, H.I.; Cavalcanti, B.C.; Nogueira Brilhante, R.S.; MacEdo, D.S.; De Moraes, M.O.; Pinto Lobo, M.D.; et al. Synergistic Effects of Amiodarone and Fluconazole on Candida tropicalis Resistant to Fluconazole. Antimicrob. Agents Chemother. 2013, 57, 1691–1700. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Fernández, P.; López-Romero, E.; Cuéllar-Cruz, M. A Comparative Proteomic Analysis of Candida Species in Response to the Oxidizing Agent Cumene Hydroperoxide. Arch. Microbiol. 2021, 203, 2219–2228. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.R.; Arias, C.A. ESKAPE Pathogens: Antimicrobial Resistance, Epidemiology, Clinical Impact and Therapeutics. Nat. Rev. Microbiol. 2024, 22, 598–616. [Google Scholar] [CrossRef] [PubMed]
- Lorente-torres, B.; Llano-verdeja, J.; Castañera, P.; Ferrero, H.Á.; Fern, S.; Javadimarand, F.; Mateos, L.M.; Letek, M.; Mourenza, Á. Innovative Strategies in Drug Repurposing to Tackle Intracellular Bacterial Pathogens. Antibiotics 2024, 13, 834. [Google Scholar] [CrossRef]
- Rajamohan, R.; Subramania, A.; Lee, Y.R. Polymer-Mediated Electrospun Nanofibrous Mats on Supramolecular Assembly of Nortriptyline in the β-Cyclodextrin Medium for Antibacterial Study. J. Biomater. Sci. Polym. Ed. 2022, 33, 1256–1268. [Google Scholar] [CrossRef]
- de Farias Cabral, V.P.; Rodrigues, D.S.; Sá, L.G.d.A.V.; Moreira, L.E.A.; da Silva, C.R.; de Andrade Neto, J.B.; da Costa, É.R.M.; Ferreira, T.L.; de Oliveira, L.C.; de Souza, B.O.; et al. Analysis of the Anti-Candida Activity of Tricyclic Antidepressants in Association with Amphotericin B and Their Antifungal Mechanisms. Braz. J. Microbiol. 2024, 55, 3617–3628. [Google Scholar] [CrossRef]
- Peng, H.; Li, C.; Kadow, S.; Henry, B.D.; Steinmann, J.; Becker, K.A.; Riehle, A.; Beckmann, N.; Wilker, B.; Li, P.; et al. Acid Sphingomyelinase Inhibition Protects Mice from Lung Edema and Lethal Staphylococcus aureus Sepsis. J. Mol. Med. 2015, 93, 675–689. [Google Scholar] [CrossRef]
- Cho, H.; Uehara, T.; Bernhardt, T.G. Article Beta-Lactam Antibiotics Induce a Lethal Malfunctioning of the Bacterial Cell Wall Synthesis Machinery. Cell 2014, 159, 1300–1311. [Google Scholar] [CrossRef]
- Sanganahalli, B.G.; Joshi, P.G.; Joshi, N.B. Differential Effects of Tricyclic Antidepressant Drugs on Membrane Dynamics—A Fluorescence Spectroscopic Study. Life Sci. 2000, 68, 81–90. [Google Scholar] [CrossRef]
- Climo, M.W.; Patron, R.L.; Archer, G.L. Combinations of Vancomycin and B-Lactams Are Synergistic against Staphylococci with Reduced Susceptibilities to Vancomycin. Antimicrob. Agents Chemother. 1999, 43, 1747–1753. [Google Scholar] [CrossRef]
- Macedo, D.; Chaves Filho, A.J.; De Sousa, C.N.S.; Quevedo, J.; Barichello, T.; Nobre Júnior, H.V.; Lucena, D.F. De Antidepressants, Antimicrobials or Both? Gut Microbiota Dysbiosis in Depression and Possible Implications of the Antimicrobial Effects of Antidepressant Drugs for Antidepressant Effectivenes. J. Affect. Disord. 2017, 208, 22–32. [Google Scholar] [CrossRef]
- de Silva Rodrigues, J.H.; Stein, J.; Strauss, M.; Rivarola, H.W.; Ueda-Nakamura, T.; Nakamura, C.V.; Duszenko, M. Clomipramine Kills Trypanosoma brucei by Apoptosis. Int. J. Med. Microbiol. 2016, 306, 196–205. [Google Scholar] [CrossRef]
- da Silva Rodrigues, J.H.; Miranda, N.; Volpato, H.; Ueda-Nakamura, T.; Nakamura, C.V. The Antidepressant Clomipramine Induces Programmed Cell Death in Leishmania amazonensis through a Mitochondrial Pathway. Parasitol. Res. 2019, 118, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Mourenza, Á.; Gil, J.A.; Mateos, M.; Letek, M. Oxidative Stress-Generating Antimicrobials, a Novel Strategy to Overcome Antibacterial Resistance. Antioxidants 2020, 9, 361. [Google Scholar] [CrossRef]
- Thangamani, S.; Mohammad, H.; Younis, W.; Seleem, M.N. Drug Repurposing for the Treatment of Staphylococcal Infections. Curr. Pharm. Des. 2015, 21, 2089–2100. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.A.; Gupta, A.K. The Use of Antidepressant Drugs in Dermatology. J. Eur. Acad. Dermatol. Venereol. 2001, 15, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.A.; Letrent, K.J.; Hager, K.L.; Burch, J.L. Use of Transdermal Amitriptyline Gel in a Patient with Chronic Pain and Depression. Pharmacotherapy 1999, 19, 236–239. [Google Scholar] [CrossRef]
- Liebregts, R.; Kopsky, D.J.; Keppel Hesselink, J.M. Topical Amitriptyline in Post-Traumatic Neuropathic Pain. J. Pain. Symptom Manag. 2011, 41, e6–e7. [Google Scholar] [CrossRef]
- Escobar-Chávez, J.J.; Merino, V.; Díez-Sales, O.; Nácher-Alonso, A.; Ganem-Quintanar, A.; Herráez, M.; Merino-Sanjuán, M. Transdermal Nortriptyline Hydrocloride Patch Formulated within a Chitosan Matrix Intended to Be Used for Smoking Cessation. Pharm. Dev. Technol. 2011, 16, 162–169. [Google Scholar] [CrossRef]
- Kopsky, D.J.; Keppel Hesselink, J.M. High Doses of Topical Amitriptyline in Neuropathic Pain: Two Cases and Literature Review. Pain Pract. 2012, 12, 148–153. [Google Scholar] [CrossRef]

| Strains a | OXA b | VAN c | AMT d | NOR e | CLO f | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC g | MIC g | MIC g | MBC h | MBC h/MIC g | Interpretation | MIC g | MBC h | MBC h/MIC g | Interpretation | MIC g | MBC h | MBC h/MIC g | Interpretation | |
| MSSA ATCC 6538p | 0.125 | 1 | 256 | 256 | 1 | Bactericide | 128 | 256 | 2 | Bactericide | 64 | 64 | 1 | Bactericide |
| MSSA 1 | 0.5 | 1 | 256 | 256 | 1 | Bactericide | 128 | 128 | 1 | Bactericide | 64 | 64 | 1 | Bactericide |
| MSSA 2 | 1 | 1 | 256 | 256 | 1 | Bactericide | 128 | 128 | 1 | Bactericide | 64 | 128 | 2 | Bactericide |
| MSSA 3 | 1 | 1 | 256 | 256 | 1 | Bactericide | 128 | 256 | 2 | Bactericide | 64 | 64 | 1 | Bactericide |
| MRSA 1 | 64 | 1 | 256 | 256 | 1 | Bactericide | 128 | 256 | 2 | Bactericide | 64 | 64 | 1 | Bactericide |
| MRSA 2 | 64 | 2 | 256 | 256 | 1 | Bactericide | 128 | 256 | 2 | Bactericide | 128 | 128 | 1 | Bactericide |
| MRSA 3 | 64 | 2 | 256 | 256 | 1 | Bactericide | 128 | 128 | 1 | Bactericide | 64 | 64 | 1 | Bactericide |
| MRSA 4 | 64 | 1 | 256 | 256 | 1 | Bactericide | 128 | 256 | 2 | Bactericide | 64 | 64 | 1 | Bactericide |
| Strains a | MIC100% Combination b (µg/mL) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMT c/OXA d | FICI e | INT f | AMT c/VAN g | FICI e | INT f | NOR h/OXA d | FICI e | INT f | NOR h/VAN g | FICI e | INT f | CLO i/OXA d | FICI e | INT f | CLO i/VAN g | FICI e | INT f | |
| MSSA ATCC 6538p | 128/0.0625 | 1 | ADI | 128/0.5 | 1 | ADI | 64/0.0625 | 1 | ADI | 64/0.5 | 1 | ADI | 64/0.125 | 2 | IND | 32/0.5 | 1 | ADI |
| MSSA 1 | 128/0.25 | 1 | ADI | 128/0.5 | 1 | ADI | 64/0.25 | 1 | ADI | 64/0.5 | 1 | ADI | 32/0.25 | 1 | ADI | 64/1 | 2 | IND |
| MSSA 2 | 64/0.25 | 0.5 | SYN | 128/0.5 | 1 | ADI | 32/0.25 | 0.5 | SYN | 64/0.5 | 1 | ADI | 32/0.5 | 1 | ADI | 64/1 | 2 | IND |
| MSSA 3 | 64/0.25 | 0.5 | SYN | 128/0.5 | 1 | ADI | 64/0.5 | 1 | ADI | 64/0.5 | 1 | ADI | 16/0.25 | 0.5 | SYN | 64/1 | 2 | IND |
| MRSA 1 | 64/16 | 0.5 | SYN | 128/0.5 | 1 | ADI | 32/16 | 0.5 | SYN | 64/0.5 | 1 | ADI | 16/16 | 0.5 | SYN | 64/1 | 2 | IND |
| MRSA 2 | 64/16 | 0.5 | SYN | 128/1 | 1 | ADI | 32/16 | 0.5 | SYN | 64/1 | 1 | ADI | 32/16 | 0.5 | SYN | 64/1 | 1 | ADI |
| MRSA 3 | 32/8 | 0.25 | SYN | 128/1 | 1 | ADI | 32/16 | 0.5 | SYN | 64/1 | 1 | ADI | 16/16 | 0.5 | SYN | 32/1 | 1 | ADI |
| MRSA 4 | 32/8 | 0.25 | SYN | 128/0.5 | 1 | ADI | 32/16 | 0.5 | SYN | 64/0.5 | 1 | ADI | 16/16 | 0.5 | SYN | 64/1 | 2 | IND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Farias Cabral, V.P.; Rodrigues, D.S.; do Amaral Valente Sá, L.G.; Moreira, L.E.A.; da Silva, C.R.; de Andrade Neto, J.B.; da Costa, É.R.M.; Ferreira, T.L.; de Oliveira, L.C.; de Souza, B.O.; et al. Antibacterial Evaluation of Tricyclic Antidepressants Against S. aureus and the Possible Pathways of the Mechanism of Action. Pathogens 2025, 14, 613. https://doi.org/10.3390/pathogens14070613
de Farias Cabral VP, Rodrigues DS, do Amaral Valente Sá LG, Moreira LEA, da Silva CR, de Andrade Neto JB, da Costa ÉRM, Ferreira TL, de Oliveira LC, de Souza BO, et al. Antibacterial Evaluation of Tricyclic Antidepressants Against S. aureus and the Possible Pathways of the Mechanism of Action. Pathogens. 2025; 14(7):613. https://doi.org/10.3390/pathogens14070613
Chicago/Turabian Stylede Farias Cabral, Vitória Pessoa, Daniel Sampaio Rodrigues, Lívia Gurgel do Amaral Valente Sá, Lara Elloyse Almeida Moreira, Cecília Rocha da Silva, João Batista de Andrade Neto, Érica Rayanne Mota da Costa, Thais Lima Ferreira, Leilson Carvalho de Oliveira, Beatriz Oliveira de Souza, and et al. 2025. "Antibacterial Evaluation of Tricyclic Antidepressants Against S. aureus and the Possible Pathways of the Mechanism of Action" Pathogens 14, no. 7: 613. https://doi.org/10.3390/pathogens14070613
APA Stylede Farias Cabral, V. P., Rodrigues, D. S., do Amaral Valente Sá, L. G., Moreira, L. E. A., da Silva, C. R., de Andrade Neto, J. B., da Costa, É. R. M., Ferreira, T. L., de Oliveira, L. C., de Souza, B. O., Pinheiro, D. R. S., Cavalcanti, B. C., Magalhães, I. L., de Moraes, M. O., & Nobre Júnior, H. V. (2025). Antibacterial Evaluation of Tricyclic Antidepressants Against S. aureus and the Possible Pathways of the Mechanism of Action. Pathogens, 14(7), 613. https://doi.org/10.3390/pathogens14070613









