Vaccination-Challenge Trials in Beagle Dogs Using Whole-Cell Leptospira interrogans Serovar Copenhageni Vaccine: Prevention of Clinical Leptospirosis, Serological, Leptospiremia, Leptospiruria, Cytokines, Hematological, and Pathological Changes
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Dogs Used in the Study
2.3. Ethical Approval
2.4. Strain of Leptospira interrogans Used
2.5. Preparation of Vaccine
2.6. Challenge of Dogs with Virulent Leptospires
2.6.1. Study 1
2.6.2. Study 2
2.7. Clinical Assessment of Dogs and Collection of Samples
2.7.1. Clinical Scoring
2.7.2. Serology
2.7.3. Detection of Leptospires in Blood and Urine
2.7.4. Cytokine Assay
2.7.5. Hematology
2.7.6. Blood Biochemistry
2.8. Euthanasia
2.9. Post-Mortem Examination
2.10. Detection of Leptospires in Organs
2.11. Statistical Analyses
3. Results
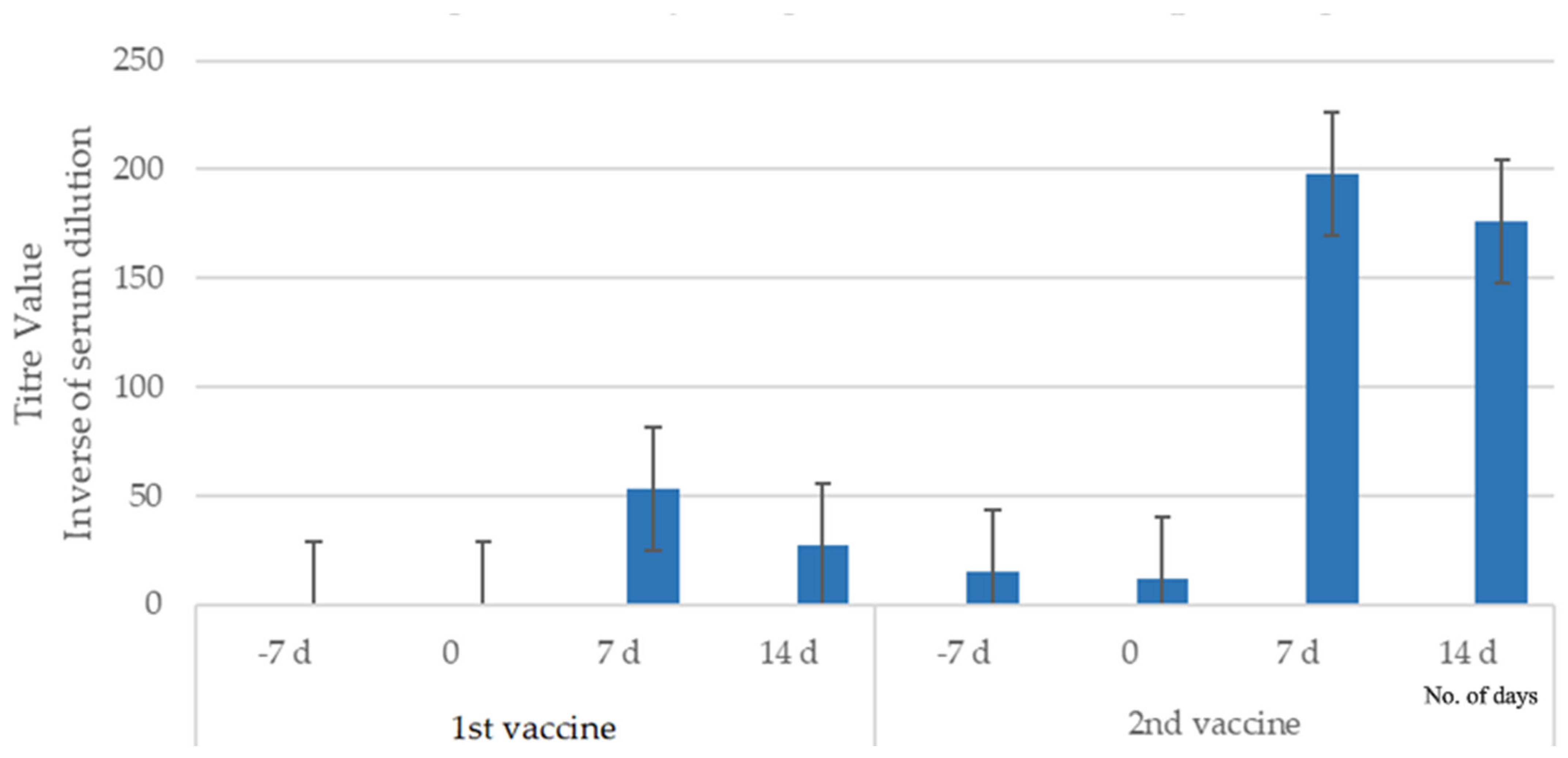
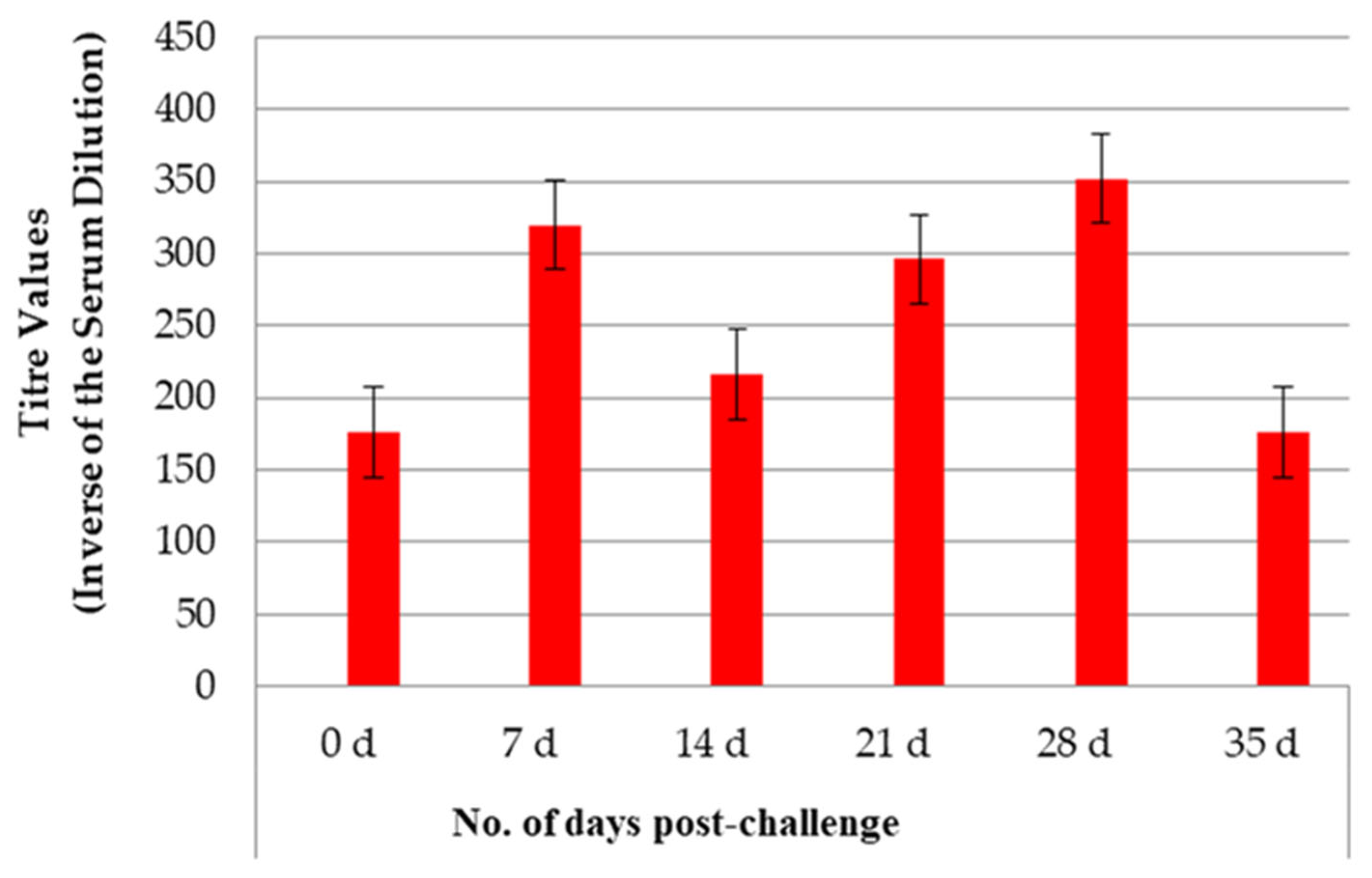
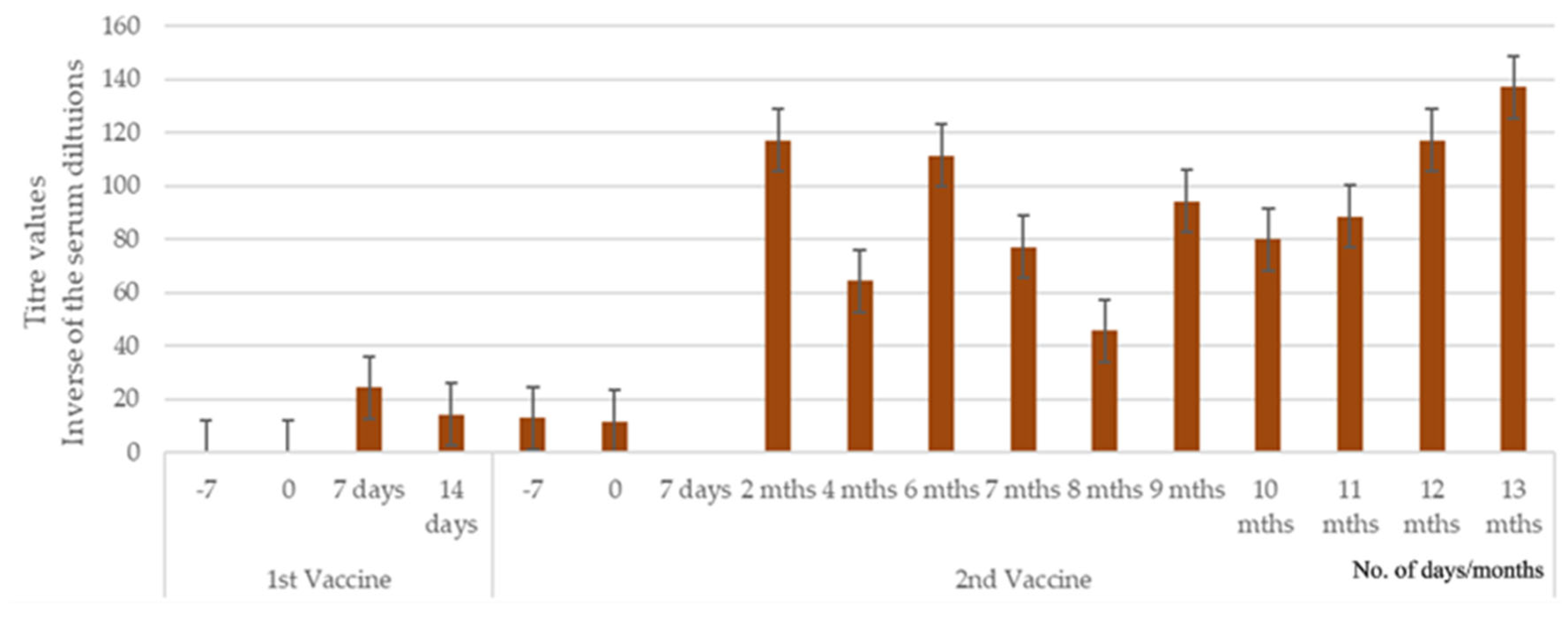
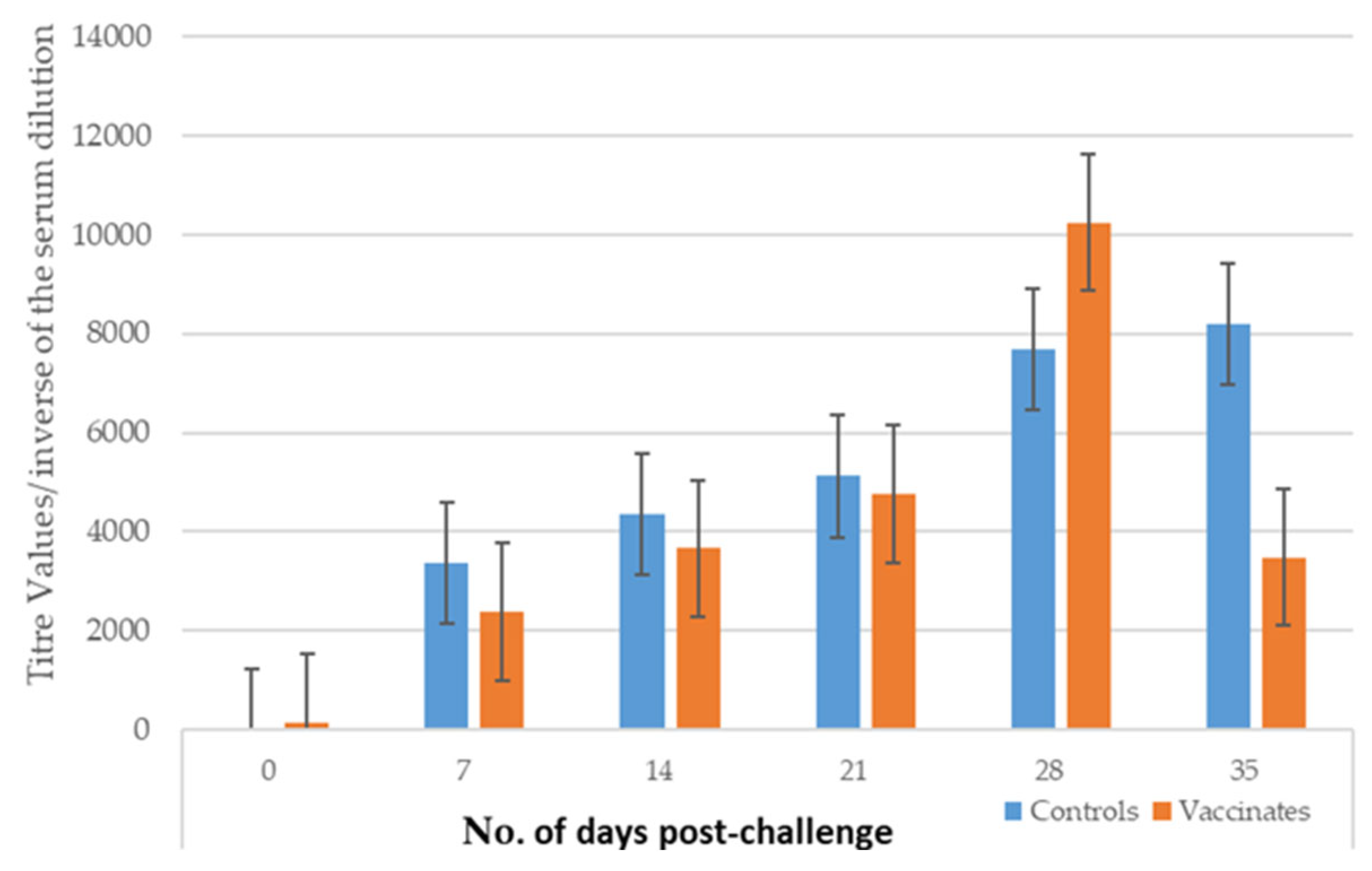
3.1. Antibody Titers After Vaccination and Challenge
3.1.1. Study 1
3.1.2. Study 2
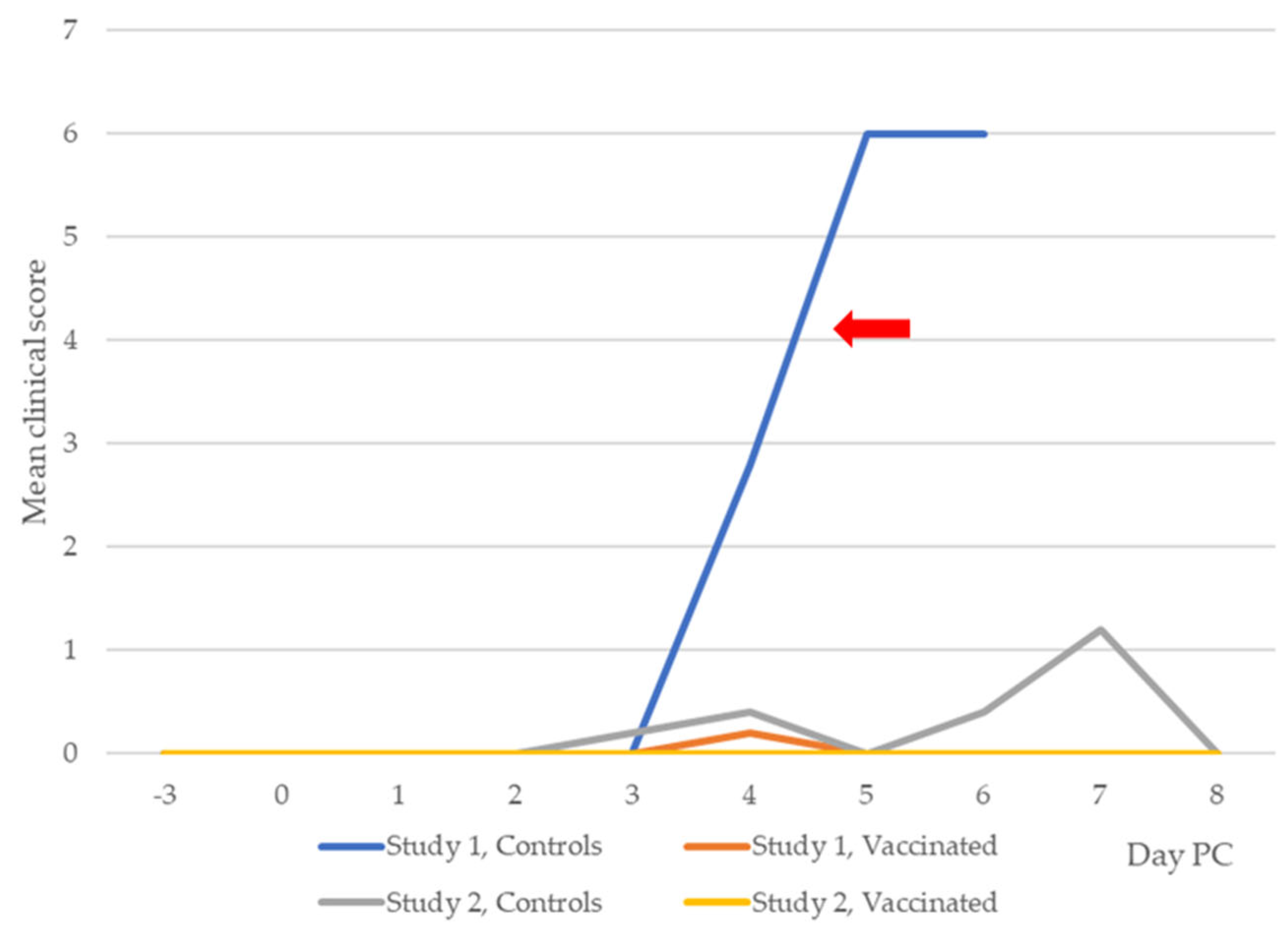
3.2. Clinical Leptospirosis
3.2.1. Study 1
3.2.2. Study 2
3.3. Leptospiremia
3.3.1. Study 1
3.3.2. Study 2
3.4. Leptospiruria
3.4.1. Study 1
3.4.2. Study 2
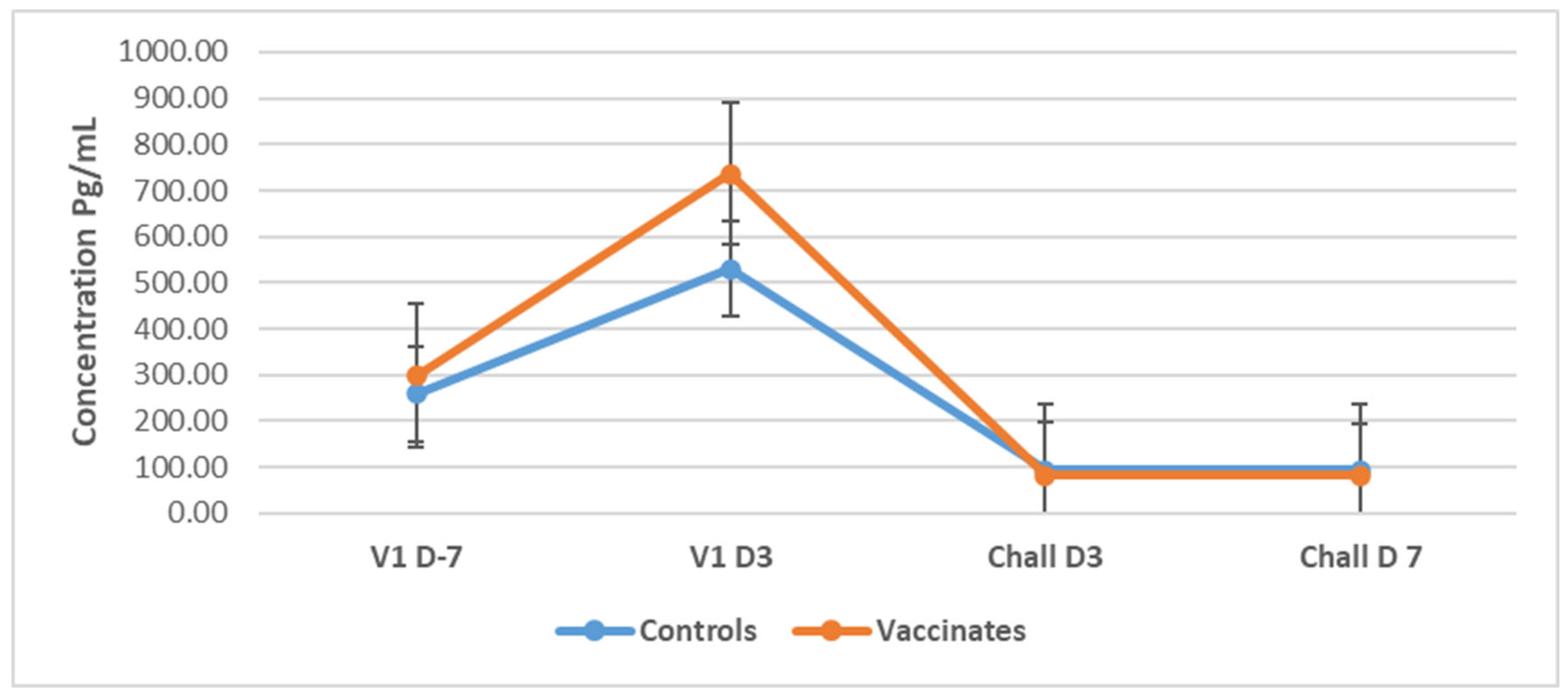
3.5. Cytokines
3.5.1. Tumor Necrosis Factor-Alpha (TNF-α)
3.5.2. Interleukin-4 (IL-4)
3.5.3. Interleukin-10 (IL-10)
3.5.4. Interferon-Gamma (IFN-γ)
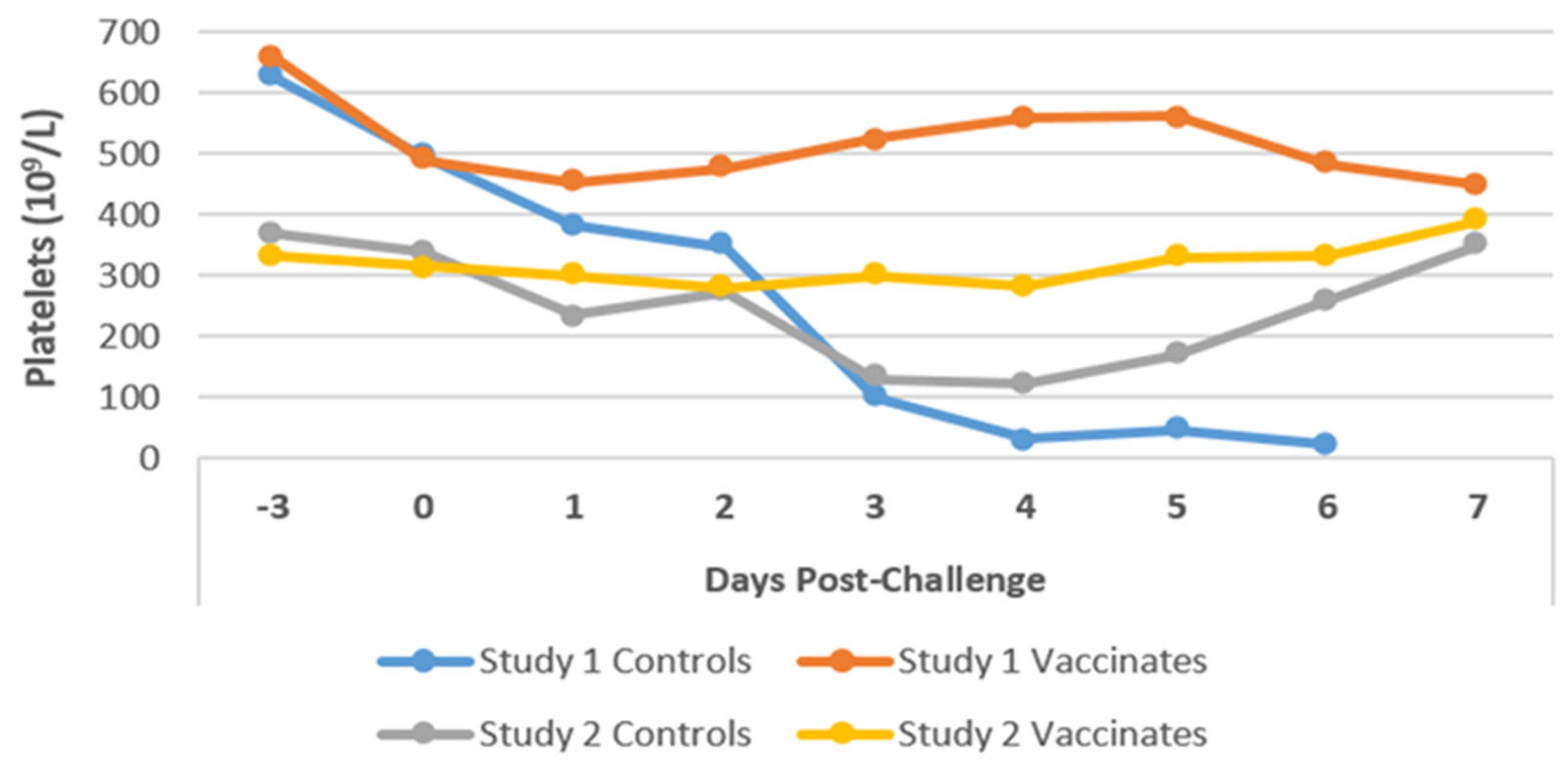
3.6. Hematology
3.6.1. Platelets
3.6.2. White Blood Cell Counts
3.6.3. Biochemistry
Alanine Transaminase (ALT)
Bilirubin
Urea and Creatinine
Serum Protein
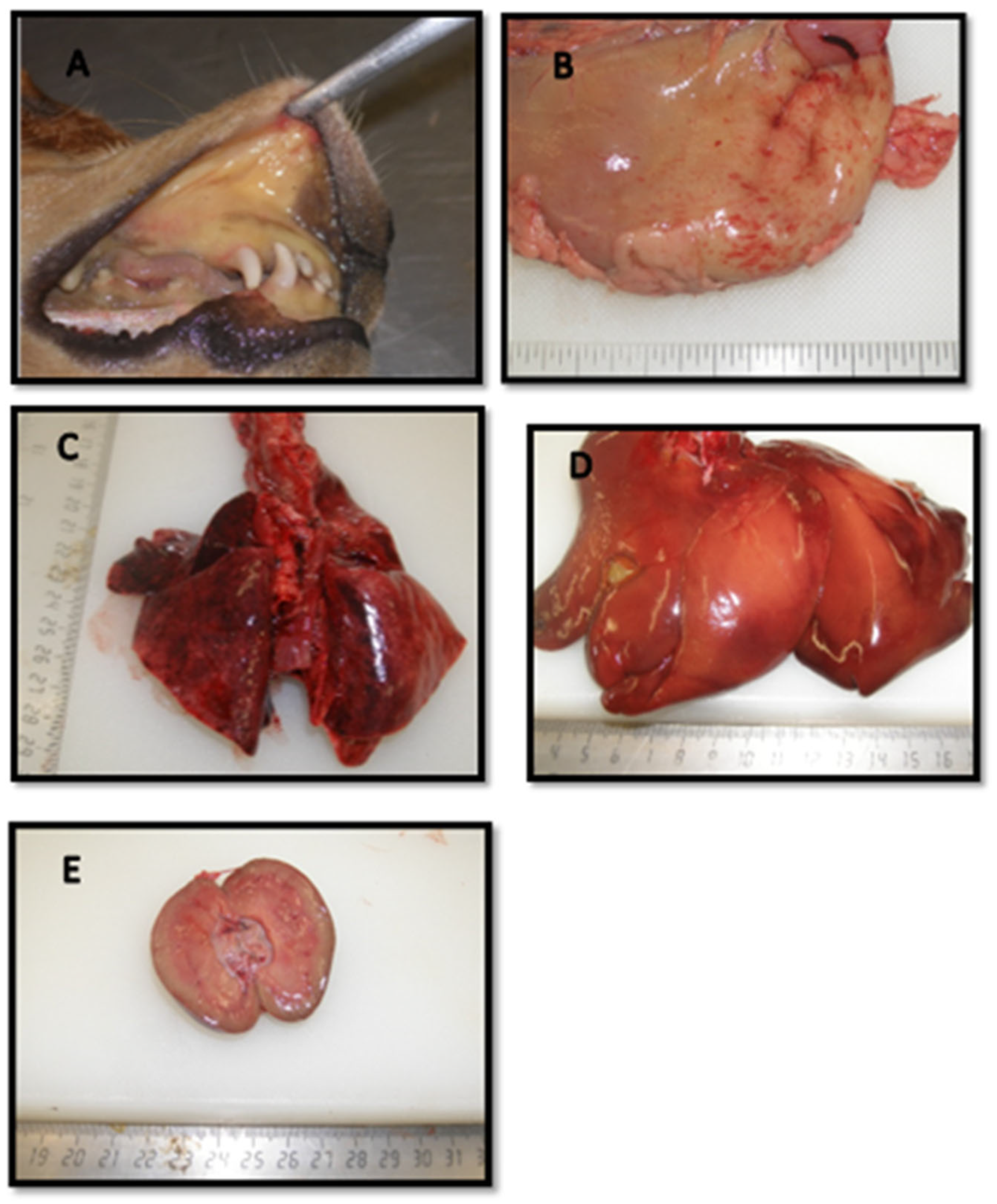
3.7. Pathology
3.7.1. Gross Pathological Findings
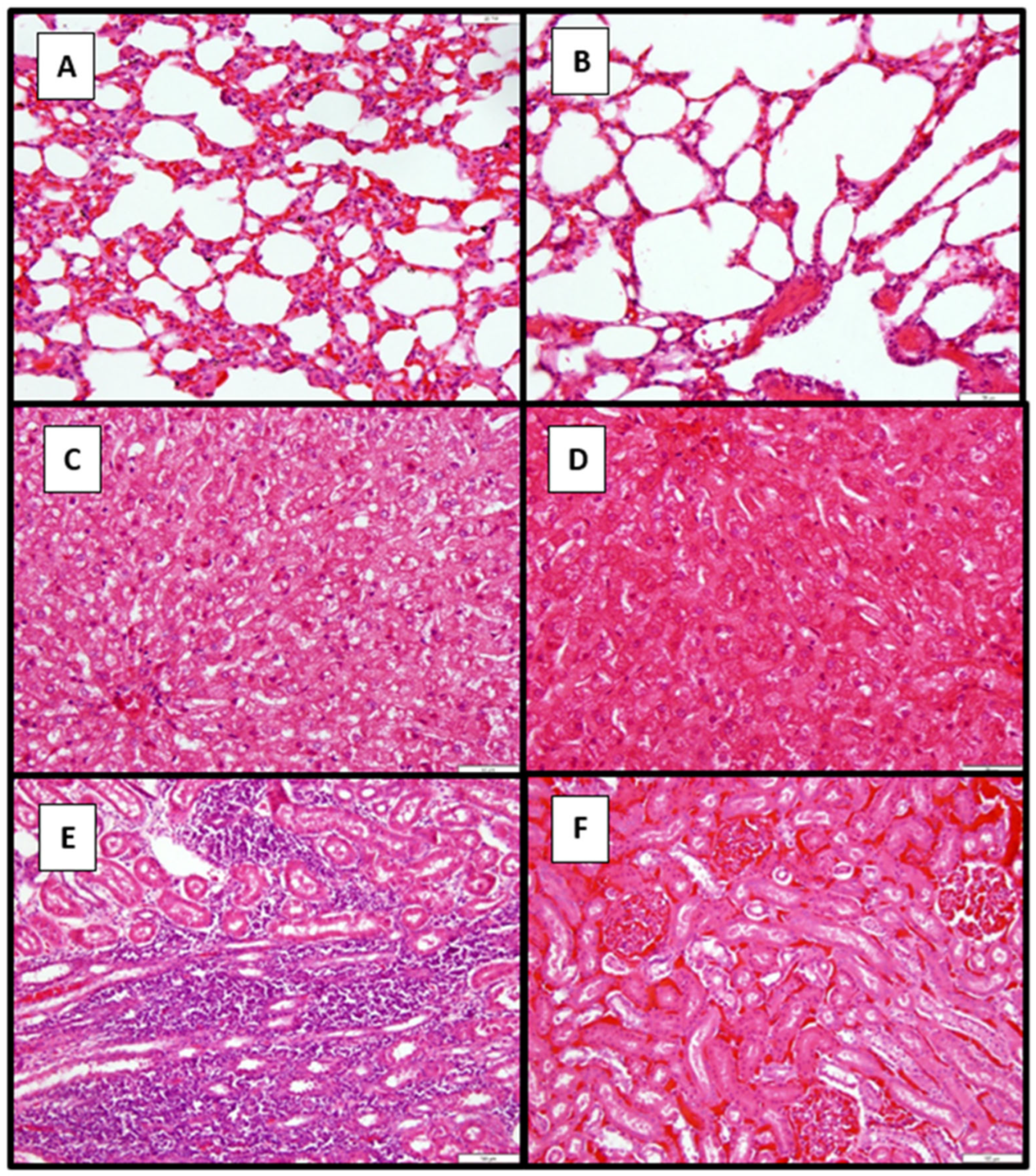
3.7.2. Histopathological Findings in Dogs in Study 2
3.8. Isolation of Leptospires from Organs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gonçalves-de-Albuquerque, C.F.; Burth, P.; Silva, A.R.; Younes-Ibrahim, M.; Castro-Faria-Neto, H.C.; Castro-Faria, M.V. Leptospira and Inflammation. Mediat. Inflamm. 2012, 2012, 317950. [Google Scholar] [CrossRef] [PubMed]
- Allyn, J.; Miailhe, A.F.; Delmas, B.; Marti, L.; Allou, N.; Jabot, J.; Reignier, J. Severe leptospirosis in tropical and non-tropical areas: A comparison of two french, multicentre, retrospective cohorts. PLoS Neglected Trop. Dis. 2024, 18, e0012084. [Google Scholar] [CrossRef] [PubMed]
- Paz, L.N.; Dias, C.S.; Almeida, D.S.; Balassiano, I.T.; Medeiros, M.A.; Costa, F.; Silva, D.N.; Reis, J.N.; Estrela-Lima, A.; Hamond, C.; et al. Multidisciplinary approach in the diagnosis of acute leptospirosis in dogs naturally infected by Leptospira interrogans serogroup Icterohaemorrhagiae: A prospective study. Comp. Immunol. Microbiol. Infect. Dis. 2021, 77, 101664. [Google Scholar] [CrossRef]
- Adler, B.; de la Peña Moctezuma, A. Leptospira and leptospirosis. Vet. Microbiol. 2010, 140, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Dehkordi, A.G.; Al-Tamary, S.R.; Al-Tamary, A.R.; Al-Tamary, H.R.; Albarari, S.S.; Assad, A.M.; Hamdan, G.M.; Mikadze, I. Atypical Cases of Leptospirosis: Insights From Georgia. Case Rep. Infect. Dis. 2024, 2024, 1414417. [Google Scholar] [CrossRef]
- Nakashiro, H.; Umakoshi, K.; Tanaka, K.; Tachibana, N. Leptospirosis transmitted from a pet dog. BMJ Case Rep. 2024, 17, e261369. [Google Scholar] [CrossRef]
- Orlando, S.A.; Mora-Jaramillo, N.; Paredes-Núñez, D.; Rodriguez-Pazmiño, A.S.; Carvajal, E.; Sosa, A.L.; Rivera, A.; Calderon, J.; Herrera, D.G.; Arcos, F.; et al. Leptospirosis outbreak in Ecuador in 2023: A pilot study for surveillance from a One Health perspective. One Health 2024, 19, 100948. [Google Scholar] [CrossRef]
- Vyn, C.M.; Libera, K.C.; Jardine, C.M.; Grant, L.E. Canine leptospirosis: A One Health approach for improved surveillance, prevention, and interdisciplinary collaboration. Can. Vet. J. 2024, 65, 609–612. [Google Scholar]
- Waranius, B. Human Case of Leptospirosis During a Canine Disease Outbreak—Wyoming, 2023. MMWR. Morb. Mortal. Wkly. Rep. 2024, 73, 602–606. [Google Scholar] [CrossRef]
- Baer, R.; Turnberg, W.; Yu, D.; Wohrle, R. Leptospirosis in a Small Animal Veterinarian: Reminder to Follow Standardized Infection Control Procedures. Zoonoses Public Health 2010, 57, 281–284. [Google Scholar] [CrossRef]
- Smith, A.M.; Stull, J.W.; Moore, G.E. Potential Drivers for the Re-Emergence of Canine Leptospirosis in the United States and Canada. Trop. Med. Infect. Dis. 2022, 7, 377. [Google Scholar] [CrossRef] [PubMed]
- Ellis, W.A. Control of canine leptospirosis in Europe: Time for a change? Vet. Rec. 2010, 167, 602–605. [Google Scholar] [CrossRef]
- Griebsch, C.; Kirkwood, N.; Norris, J.M.; Ward, M.P. A comparison of risk factors for canine leptospirosis and seropositivity in New South Wales, Australia. Aust. Vet. J. 2025, 103, 106–111. [Google Scholar] [CrossRef]
- Klaasen, H.L.; Adler, B. Recent advances in canine leptospirosis: Focus on vaccine development. Vet. Med. 2015, 6, 245–260. [Google Scholar]
- Adler, B. (Ed.) Leptospira and Leptospirosis, 1st ed.; Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2015; Volume 387, Available online: https://link.springer.com/10.1007/978-3-662-45059-8 (accessed on 8 April 2023).
- Furlanello, T.; Mazzotta, E.; Bertasio, C.; D’Incau, M.; Bellinati, L.; Lucchese, L.; Natale, A. The Challenge of Bacterial Strain Identification: Leptospira interrogans Serovars Australis in a Dog and Long-Term Clinical Follow-Up. Trop. Med. Infect. Dis. 2024, 9, 285. [Google Scholar] [CrossRef]
- Sarangi, S.; Vijaya Bharathi, M.; Madhanmohan, M.; Meenambigai, T.V.; Soundararajan, C.; Manimaran, K.; Senthilkumar, T.M. Occurrence of leptospirosis in dogs with renal and/or hepatic disease in Chennai, India. Indian J. Anim. Health 2024, 63, 256–262. [Google Scholar] [CrossRef]
- Grosenbaugh, D.A.; Pardo, M.C. Fifteen-month duration of immunity for the serovar Grippotyphosa fraction of a tetravalent canine leptospirosis vaccine. Vet. Rec. 2018, 182, 665. [Google Scholar] [CrossRef] [PubMed]
- Surendran, Y.; Nandikha, M.; Amin-Nordin, S.; Dhanda, S.K.; Ali, M.R.; Joseph, N.M. Vaccine development for leptospirosis: A systematic review. Asian Pac. J. Trop. Med. 2023, 16, 533–545. [Google Scholar] [CrossRef]
- Teixeira, A.F.; Cavenague, M.F.; Kochi, L.T.; Fernandes, L.G.; Souza, G.O.; de Souza Filho, A.F.; Vasconcellos, S.A.; Heinemann, M.B.; Nascimento, A.L. Immunoprotective activity induced by leptospiral outer membrane proteins in hamster model of acute leptospirosis. Front. Immunol. 2020, 11, 568694. [Google Scholar] [CrossRef]
- Sant’Anna da Costa, R.; Di Azevedo, M.I.N.; dos Santos Baptista Borges, A.L.; Aymée, L.; Martins, G.; Lilenbaum, W. Effect of Vaccination against Leptospira on Shelter Asymptomatic Dogs Following a Long-Term Study. Animals 2022, 12, 1788. [Google Scholar] [CrossRef]
- Bergmann Esteves, S.; Moreira Santos, C.; Ferreira Salgado, F.; Paldês Gonçales, A.; Gil Alves Guilloux, A.; Marinelli Martins, C.; Kuribaiashi Hagiwara, M.; Alonso Miotto, B. Efficacy of commercially available vaccines against canine leptospirosis: A systematic review and meta-analysis. Vaccine 2022, 40, 1722–1740. [Google Scholar] [CrossRef]
- Sonrier, C.; Branger, C.; Michel, V.; Ruvoën-Clouet, N.; Ganière, J.; André-Fontaine, G. Evidence of cross-protection within Leptospira interrogans in an experimental model. Vaccine 2000, 19, 86–94. [Google Scholar] [CrossRef]
- Srikram, A.; Zhang, K.; Bartpho, T.; Lo, M.; Hoke, D.E.; Sermswan, R.W.; Adler, B.; Murray, G.L. Cross-protective immunity against leptospirosis elicited by a live, attenuated lipopolysaccharide mutant. J. Infect. Dis. 2011, 203, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Maia, M.A.C.; Bettin, E.B.; Barbosa, L.N.; de Oliveira, N.R.; Bunde, T.T.; Pedra, A.C.K.; Rosa, G.A.; da Rosa, E.E.B.; Seixas Neto, A.C.P.; Grassmann, A.A.; et al. Challenges for the development of a universal vaccine against leptospirosis revealed by the evaluation of 22 vaccine candidates. Front. Cell Infect. Microbiol. 2022, 12, 940966. [Google Scholar] [CrossRef] [PubMed]
- Cagliero, J.; Villanueva, S.Y.A.M.; Matsui, M. Leptospirosis Pathophysiology: Into the Storm of Cytokines. Front. Cell Infect. Microbiol. 2018, 8, 204. [Google Scholar] [CrossRef] [PubMed]
- Limothai, U.; Lumlertgul, N.; Sirivongrangson, P.; Kulvichit, W.; Tachaboon, S.; Dinhuzen, J.; Chaisuriyong, W.; Peerapornratana, S.; Chirathaworn, C.; Praditpornsilpa, K.; et al. The role of leptospiremia and specific immune response in severe leptospirosis. Sci. Rep. 2021, 11, 14630. [Google Scholar] [CrossRef]
- Heeb, L.E.; Egholm, C.; Boyman, O. Evolution and function of interleukin-4 receptor signaling in adaptive immunity and neutrophils. Genes Immun. 2020, 21, 143–149. [Google Scholar] [CrossRef]
- Xie, X.; Lv, T.; Wu, D.; Shi, H.; Zhang, S.; Xian, X.; Liu, G.; Zhang, W.; Cao, Y. IL-10 Deficiency Protects Hamsters from Leptospira Infection. Infect. Immun. 2022, 90, e00584-21. [Google Scholar] [CrossRef]
- Bandara, K.; Gunasekara, C.; Weerasekera, M.; Marasinghe, C.; Ranasinghe, N.; Fernando, N. Do the Th17 cells play a role in the pathogenesis of leptospirosis? Can. J. Infect. Dis. Med. Microbiol. 2018, 2018, 9704532. [Google Scholar] [CrossRef]
- Maissen-Villiger, C.A.; Schweighauser, A.; van Dorland, H.A.; Morel, C.; Bruckmaier, R.M.; Zurbriggen, A.; Francey, T. Expression Profile of Cytokines and Enzymes mRNA in Blood Leukocytes of Dogs with Leptospirosis and Its Associated Pulmonary Hemorrhage Syndrome. PLoS ONE 2016, 11, e0148029. [Google Scholar] [CrossRef]
- Novak, A.; Hindriks, E.; Hoek, A.; Veraart, C.; Broens, E.M.; Ludwig, I.; Rutten, V.; Sloots, A.; Broere, F. Cellular and humoral immune responsiveness to inactivated Leptospira interrogans in dogs vaccinated with a tetravalent Leptospira vaccine. Vaccine 2023, 41, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Sykes, J.E.; Francey, T.; Schuller, S.; Stoddard, R.A.; Cowgill, L.D.; Moore, G.E. Updated ACVIM consensus statement on leptospirosis in dogs. J. Vet. Intern. Med. 2023, 37, 1966–1982. [Google Scholar] [CrossRef]
- Hilbe, M.; Posthaus, H.; Paternoster, G.; Schuller, S.; Imlau, M.; Jahns, H. Exudative glomerulonephritis associated with acute leptospirosis in dogs. Vet. Pathol. 2023, 61, 453. [Google Scholar] [CrossRef]
- André-Fontaine, G.; Triger, L. MAT cross-reactions or vaccine cross-protection: Retrospective study of 863 leptospirosis canine cases. Heliyon 2018, 4, e00869. [Google Scholar] [CrossRef]
- Chou, L.F.; Yang, H.Y.; Hung, C.C.; Tian, Y.C.; Hsu, S.H.; Yang, C.W. Leptospirosis kidney disease: Evolution from acute to chronic kidney disease. Biomed. J. 2023, 46, 100595. [Google Scholar] [CrossRef] [PubMed]
- Levett, P.N. Leptospirosis. Clin. Microbiol. Rev. 2001, 14, 296–326. [Google Scholar] [CrossRef]
- Adesiyun, A.A.; Hull-Jackson, C.; Mootoo, N.; Halsall, S.; Bennett, R.; Clarke, N.R.; Whittington, C.U.; Seepersadsingh, N. Sero-epidemiology of Canine Leptospirosis in Trinidad: Serovars, Implications for Vaccination and Public Health. J. Vet. Med. Ser. B 2006, 53, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Suepaul, S.M.; Carrington, C.V.; Campbell, M.; Borde, G.; Adesiyun, A.A. Seroepidemiology of leptospirosis in dogs and rats in Trinidad. Trop. Biomed. 2014, 31, 853–861. [Google Scholar]
- Suepaul, S.M.; Carrington, C.V.; Campbell, M.; Borde, G.; Adesiyun, A.A. Study the efficacy of Leptospira vaccines developed from serovars isolated from Trinidad and comparison with commercial vaccines using a hamster model. Vaccine 2010, 28, 5421–5426. [Google Scholar] [CrossRef]
- Arjoonsingh, V.; Suepaul, R.; Adesiyun, A.A. Immune response at a vaccine-challenge study using beagle dogs and locally isolated Leptospira spp. Vet. Immunol. Immunopathol. 2023, 255, 110522. [Google Scholar] [CrossRef]
- Suepaul, S.M.; Carrington, C.V.F.; Campbell, M.; Borde, G.; Adesiyun, A.A. Serovars of Leptospira isolated from dogs and rodents. Epidemiol. Infect. 2010, 138, 1059–1070. [Google Scholar] [CrossRef] [PubMed]
- Srikram, A.; Wongratanacheewin, S.; Puapairoj, A.; Wuthiekanun, V.; Sermswan, R.W. Analyses of vaccination protocols for Leptospira interrogans serovar autumnalis in hamsters. Am. J. Trop. Med. Hyg. 2008, 79, 779–786. [Google Scholar] [CrossRef]
- Minke, J.M.; Bey, R.; Tronel, J.P.; Latour, S.; Colombet, G.; Yvorel, J.; Cariou, C.; Guiot, A.L.; Cozette, V.; Guigal, P.M. Onset and duration of protective immunity against clinical disease and renal carriage in dogs provided by a bi-valent inactivated leptospirosis vaccine. Vet. Microbiol. 2009, 137, 137–145. [Google Scholar] [CrossRef]
- Terpstra, W.J. Human Leptospirosis: Guidance for Diagnosis, Surveillance and Control, 1st ed.; World Health Organization: Geneva, Switzerland, 2003; p. 63. [Google Scholar]
- Plotkin, S.A.; Orenstein, W.A.; Offit, P.A. Vaccines, 6th ed.; Elsevier Saunders: Amsterdam, The Netherlands, 2008; 1748p. [Google Scholar]
- Vernel-Pauillac, F.; Murray, G.L.; Adler, B.; Boneca, I.G.; Werts, C. Anti-Leptospira immunoglobulin profiling in mice reveals strain specific IgG and persistent IgM responses associated with virulence and renal colonization. PLoS Neglected. Trop. Dis. 2021, 15, e0008970. [Google Scholar] [CrossRef]
- Klaasen, H.L.B.M.; Molkenboer, M.J.C.H.; Vrijenhoek, M.P.; Kaashoek, M.J. Duration of immunity in dogs vaccinated against leptospirosis with a bivalent inactivated vaccine. Vet. Microbiol. 2003, 95, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Bouvet, J.; Cariou, C.; Valfort, W.; Villard, S.; Hilaire, F.; Oberli, F.; Cupillard, L.; Guigal, P.M. Efficacy of a multivalent DAPPi-Lmulti canine vaccine against mortality, clinical signs, infection, bacterial excretion, renal carriage and renal lesions caused by Leptospira experimental challenges. Vaccine Rep. 2016, 6, 23–28. [Google Scholar] [CrossRef]
- Moreno-Torres, A.; Malvido-Jiménez, I.R.; De la Peña-Moctezuma, A.; Castillo Sánchez, L.O.; Fraga, T.R.; Barbosa, A.S.; Isaac, L.; Sahagún-Ruiz, A. Culture-attenuated pathogenic Leptospira lose the ability to survive to complement-mediated-killing due to lower expression of factor H binding proteins. Microbes Infect. 2019, 21, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Philip, N.; Garba, B.; Neela, V.K. Long-term preservation of Leptospira spp.: Challenges and prospects. Appl. Microbiol. Biotechnol. 2018, 102, 5427–5435. [Google Scholar] [CrossRef]
- Philip, N.; Jani, J.; Azhari, N.N.; Sekawi, Z.; Neela, V.K. In vivo and in silico virulence analysis of Leptospira species isolated from environments and rodents in leptospirosis outbreak areas in Malaysia. Front. Microbiol. 2021, 12, 753328. [Google Scholar] [CrossRef]
- Bouvet, J.; Segouffin Cariou, C.; Oberli, F.; Guiot, A.L.; Cupillard, L. A New Licensed Quadrivalent Antileptospiral Canine Vaccine Prevents Mortality, Clinical Signs, Infection, Bacterial Excretion, Renal Carriage, and Renal Lesions Caused by Leptospira Australis Experimental Challenge. Vaccines 2024, 12, 1104. [Google Scholar] [CrossRef]
- Klaasen, H.L.; Van der Veen, M.; Sutton, D.; Molkenboer, M.J. A new tetravalent canine leptospirosis vaccine provides at least 12 months immunity against infection. Vet. Immunol. Immunopathol. 2014, 158, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.; Stirling, C.; Thomas, A.; King, V.; Plevová, E.; Chromá, L.; Siedek, E.; Illambas, J.; Salt, J.; Sture, G. A new multivalent (DHPPi/L4R) canine combination vaccine prevents infection, shedding and clinical signs following experimental challenge with four Leptospira serovars. Vaccine 2013, 31, 3131–3134. [Google Scholar] [CrossRef] [PubMed]
- Klaasen, H.L.B.M.; van der Veen, M.; Molkenboer, M.J.C.H.; Sutton, D. A novel tetravalent Leptospira bacterin protects against infection and shedding following challenge in dogs. Vet. Rec. 2013, 172, 181. [Google Scholar] [CrossRef] [PubMed]
- Dib, C.C.; Gonçales, A.P.; Morais, Z.M.D.; Souza, G.O.D.; Miraglia, F.; Abreu, P.A.E.; Vasconcellos, S.A. Cross-protection between experimental anti-leptospirosis bacterins. Braz. J. Microbiol. 2014, 45, 1083–1088. [Google Scholar] [CrossRef]
- Adler, B.; Ballard, S.A.; Miller, S.J.; Faine, S. Monoclonal antibodies reacting with serogroup and serovar. specific epitopes on different lipopolysaccharide subunits of Leptospira interrogans Serovar Pomona. FEMS Microbiol. Immunol. 1989, 1, 213–218. [Google Scholar] [CrossRef]
- de Oliveira, N.R.; Jorge, S.; Maia, M.A.C.; Bunde, T.T.; Pedra, A.C.K.; Neto, A.C.P.S.; Oliveira, T.L.; Dellagostin, O.A. Protective efficacy of whole-cell inactivated Leptospira vaccines made using virulent or avirulent strains in a hamster model. Vaccine 2021, 39, 5626–5634. [Google Scholar] [CrossRef]
- Vernel-Pauillac, F.; Goarant, C. Differential cytokine gene expression according to outcome in a hamster model of leptospirosis. PLoS Neglected Trop. Dis. 2010, 4, e582. [Google Scholar] [CrossRef]
- Oliver, J.C.; Bland, L.A.; Oettinger, C.W.; Arduino, M.J.; McAllister, S.K.; Aguero, S.M.; Favero, M.S. Cytokine kinetics in an in vitro whole blood model following an endotoxin challenge. Lymphokine Cytokine Res. 1993, 12, 115–120. [Google Scholar]
- Vernel-Pauillac, F.; Merien, F. Proinflammatory and Immunomodulatory Cytokine mRNA Time Course Profiles in Hamsters Infected with a Virulent Variant of Leptospira interrogans. Infect. Immun. 2006, 74, 4172–4179. [Google Scholar] [CrossRef]
- Reis, E.A.; Hagan, J.E.; Ribeiro, G.S.; Teixeira-Carvalho, A.; Martins-Filho, O.A.; Montgomery, R.R.; Shaw, A.C.; Ko, A.I.; Reis, M.G. Cytokine response signatures in disease progression and development of severe clinical outcomes for leptospirosis. PLoS Neglected Trop. Dis. 2013, 7, e2457. [Google Scholar] [CrossRef]
- Volz, M.S.; Moos, V.; Allers, K.; Luge, E.; Mayer-Scholl, A.; Nöckler, K.; Loddenkemper, C.; Jansen, A.; Schneider, T. Specific CD4+ T-cell reactivity and cytokine release in different clinical presentations of leptospirosis. Clin. Vaccine Immunol. 2015, 22, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Mikulski, M.; Boisier, P.; Lacassin, F.; Soupe-Gilbert, M.E.; Mauron, C.; Bruyere-Ostells, L.; Goarant, C. Severity markers in severe leptospirosis: A cohort study. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Wagenaar, J.F.; Goris, M.G.; Partiningrum, D.L.; Isbandrio, B.; Hartskeerl, R.A.; Brandjes, D.P.; Meijers, J.C.; Gasem, M.H.; Van Gorp, E.C. Coagulation disorders in patients with severe leptospirosis are associated with severe bleeding and mortality. Trop. Med. Int. Health 2010, 15, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Spiri, A.M.; Rodriguez-Campos, S.; Matos, J.M.; Glaus, T.M.; Riond, B.; Reusch, C.E.; Hofmann-Lehmann, R.; Willi, B. Clinical, serological and echocardiographic examination of healthy field dogs before and after vaccination with a commercial tetravalent leptospirosis vaccine. BMC Vet. Res. 2017, 13, 138. [Google Scholar] [CrossRef]
- Vázquez-Manzanilla, C.A.; Cárdenas-Marrufo, M.F.; Gutiérrez-Blanco, E.; Jiménez-Coello, M.; Pech-Sosa, N.R.; Ortega-Pacheco, A. Clinical features of chronic kidney disease in dogs with the serological presence of Leptospira spp., Ehrlichia canis, and Anaplasma phagocytophilum. Anim. Dis. 2023, 3, 37. [Google Scholar] [CrossRef]



| Study | Type | Group | No. of Dogs | Challenge (Time After V2 *) |
|---|---|---|---|---|
| 1 | Onset of immunity | Control | 5 | 2 weeks |
| Vaccinated | 5 | |||
| 2 | Duration of immunity | Control | 5 | 14 months |
| Vaccinated | 7 |
| Study | Group | No. of Dogs | No. (%) with Clinical Disease, PC | |||
|---|---|---|---|---|---|---|
| None | Mild | Moderate | Severe a | |||
| 1 | Controls | 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (100.0) |
| Vaccinates | 5 | 4 (80.0) | 1 (20.0) | 0 (0.0) | 0 (0.0) | |
| 2 | Controls | 5 | 0 (0.0) | 5 (100.0) | 0 (0.0) | 0 (0.0) |
| 6-week-old dog b | 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) | |
| Vaccinates | 7 | 7 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noel, T.; Suepaul, R.; Adesiyun, A.A. Vaccination-Challenge Trials in Beagle Dogs Using Whole-Cell Leptospira interrogans Serovar Copenhageni Vaccine: Prevention of Clinical Leptospirosis, Serological, Leptospiremia, Leptospiruria, Cytokines, Hematological, and Pathological Changes. Pathogens 2025, 14, 611. https://doi.org/10.3390/pathogens14070611
Noel T, Suepaul R, Adesiyun AA. Vaccination-Challenge Trials in Beagle Dogs Using Whole-Cell Leptospira interrogans Serovar Copenhageni Vaccine: Prevention of Clinical Leptospirosis, Serological, Leptospiremia, Leptospiruria, Cytokines, Hematological, and Pathological Changes. Pathogens. 2025; 14(7):611. https://doi.org/10.3390/pathogens14070611
Chicago/Turabian StyleNoel, Teola, Rod Suepaul, and Abiodun A. Adesiyun. 2025. "Vaccination-Challenge Trials in Beagle Dogs Using Whole-Cell Leptospira interrogans Serovar Copenhageni Vaccine: Prevention of Clinical Leptospirosis, Serological, Leptospiremia, Leptospiruria, Cytokines, Hematological, and Pathological Changes" Pathogens 14, no. 7: 611. https://doi.org/10.3390/pathogens14070611
APA StyleNoel, T., Suepaul, R., & Adesiyun, A. A. (2025). Vaccination-Challenge Trials in Beagle Dogs Using Whole-Cell Leptospira interrogans Serovar Copenhageni Vaccine: Prevention of Clinical Leptospirosis, Serological, Leptospiremia, Leptospiruria, Cytokines, Hematological, and Pathological Changes. Pathogens, 14(7), 611. https://doi.org/10.3390/pathogens14070611






