Neurocysticercosis Diagnosis in a Non-Endemic Country: France
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. T. solium PCR
2.3. Cysticercosis Serology
2.3.1. ELISA
2.3.2. Western Blot
2.4. Criteria for Defining NCC
2.5. Statistical Analysis
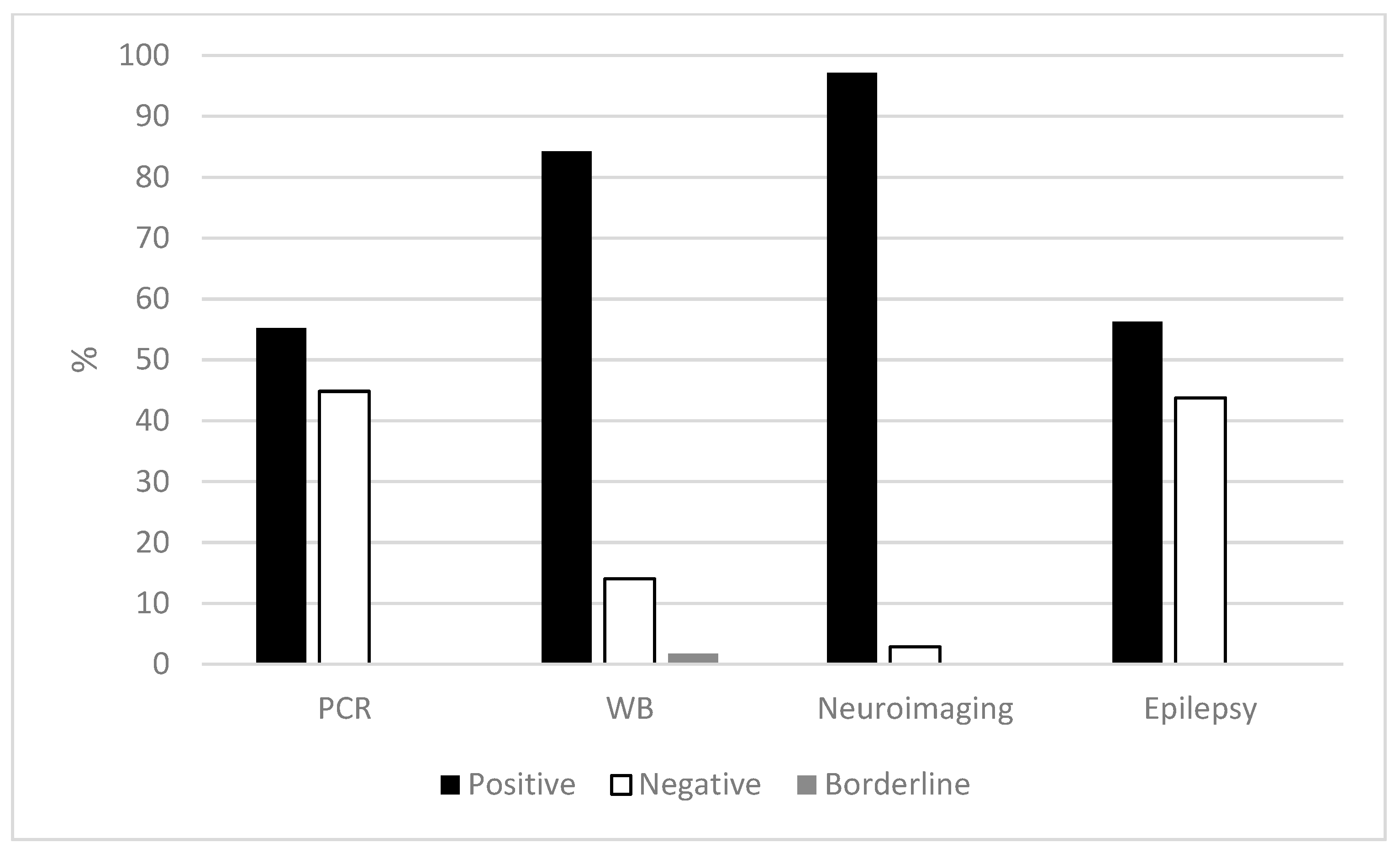
3. Results
3.1. Clinical, Radiological, and Epidemiological Data
3.2. Serology and PCR
3.3. Classification of Cases according to Del Brutto’s Criteria
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Clinical, Radiological, Epidemiological, Serological, and Real-Time PCR Results of Cases
| Case | Clinical Signs | Cerebral Radiology Result | Epidemiology | No. of Samples | Serological Result ELISA Western-Blot | Real-Time PCR Result | Del Brutto [8] Classification | |
| C1 | No information | No information | Togo | 1 serum | Serum negative/CSF Nd | Serum positive/CSF Nd | Nd | Unclassified |
| C2 | No information | No information | Togo | 1 serum, 1 CSF | Serum positive/CSF positive | Serum positive/CSF positive | Nd | Probable NCC |
| C3 | Epilepsy | 1 cystic lesion in the frontal lobe | Haiti | 1 serum, 1 CSF | Serum negative/CSF negative | Serum positive/CSF negative | Nd | Definitive NCC |
| C4 | No information | No information | No information | 1 serum, 1 CSF | Serum positive/CSF negative | Serum positive/CSF positive | Nd | Unclassified |
| C5 | No information | No information | No information | 1 serum | Serum negative/CSF Nd | Serum positive/CSF Nd | Nd | Unclassified |
| C6 | Headaches, visual disorders | 5 cysts with scolex and perilesional edema | Madagascar | 1 serum | Serum negative/CSF Nd | Serum positive/CSF Nd | Serum negative/CSF Nd | Definitive NCC |
| C7 | Epilepsy | 4 bilateral temporal lesions | Travel Ivory Coast, Chad | 1 serum | Serum negative/CSF Nd | Serum positive/CSF Nd | Nd | Definitive NCC |
| C8 | No information | No information | No information | 1 serum, 1 CSF | Serum positive/CSF negative | Serum positive/CSF positive | Serum Nd/CSF positive | Unclassified |
| C9 | Epilepsy, disorders of consciousness, neurological signs | 1 calcification | Portugal | 1 serum, 2 CSF | Nd | Serum positive/CSF positive | Serum Nd/CSF negative X2 | Probable NCC |
| C10 | Visual blur, headaches, epilepsy | Cerebral lesions | No information | 1 serum, 1 CSF, 1 brain biopsy | Serum positive/CSF Nd | Serum positive /CSF Nd | Serum Nd/CSF negative/ brain biopsy positive | Probable NCC |
| C11 | Chronic meningoencephalitis | No anomaly found | Madagascar | 1 serum, 1 CSF | Serum positive/CSF positive | Serum positive/CSF positive | Serum Nd/CSF positive | Probable NCC |
| C12 | Chronic meningitis, intracranial hypertension | 2 microcalcifications (occipital region) | Guatemala, NCC in 2007 | 1 serum, 1 CSF | Serum positive/CSF positive | Serum Nd/CSF positive | Nd | Probable NCC |
| C13 * | No information | 1 abscess | India | 1 serum, 1 CSF, 1 brain biopsy | Nd | Nd | Serum negative/CSF negative/brain biopsy positive | Definitive NCC |
| C14 | Headaches, mental changes, basal meningitis | 1 cyst above planum sphenoidal, small frontotemporal cysts, hydrocephalus, arachnoiditis | Bosnia and Herzegovina | 1 serum, 1 CSF | Serum positive/CSF positive | Serum positive/CSF positive | Serum Nd/CSF positive | Definitive NCC |
| C15 | Mental changes | Hydrocephalus, 1 intraventricular cyst | Serbia | 1 serum, 1 CSF | Serum positive/CSF positive | Serum positive/CSF positive | Serum Nd/CSF positive | Definitive NCC |
| C16 | Epilepsy | 1 calcified lesion, multiple intracerebral lesions | Congo | 1 serum, 2 CSF | Nd | Serum negative/CSF negative X2 | Serum Nd/CSF negative then positive | Probable NCC |
| C17 | Meningitis, neurological signs | 1 active arachnoidal ventricular lesion | Guyane, Haiti | 1 CSF | Nd | Serum Nd/CSF positive | Serum Nd/CSF positive | Probable NCC |
| C18 | Behavioral disorder, mutism | Tetraventricular dilatation, hydrocephalus | Madagascar | 1 serum, 1 CSF | Nd | Serum positive/CSF positive | Serum Nd/CSF positive | Probable NCC |
| C19 | Chronic meningitis, right hemiparesis | Normal scanner | Cape Verde | 1 CSF | Nd | Serum Nd/CSF positive | Serum Nd/CSF positive | Unclassified |
| C20 | Headaches, chronic meningitis | Cysts | Haiti | 1 serum, 2 CSF | Nd | Serum positive/CSF positive X2 | Serum Nd/CSF positive X2 | Definitive NCC |
| C21 | Eosinophilic meningitis, headache | 1 brain lesion, 1 calcification | Mexico | 1 CSF | Nd | Serum Nd/CSF positive | Serum Nd/CSF positive | Probable NCC |
| C22 * | No information | Compression and bypass of the 4th ventricle | No information | 1 CSF | Nd | Nd | Serum Nd/CSF positive | Definitive NCC |
| C23 | No information | 1 intracranial lesion, 1 cyst, hydrocephalus | Cape Verde | 1 CSF | Nd | Serum Nd/CSF positive | Serum Nd/CSF positive | Probable NCC |
| C24 | Epilepsy | Multiple cystic lesions (12) | Congo | 1 serum, 1 CSF | Nd | Serum positive/CSF Nd | Serum Nd/CSF positive | Definitive NCC |
| C25 | Chronic meningitis | Lepto-meningitis | No information | 1 CSF | Nd | Serum Nd/CSF limit | Serum Nd/CSF negative | Unclassified |
| C26 | Hemiplegia, epilepsy | 1 cerebral lesion with per-lesion edema | DRC | 2 CSF | Serum Nd/CSF positive | Serum Nd/CSF positive X2 | Serum Nd/CSF negative X2 | Probable NCC |
| C27 | Epilepsy | Multifocal cysts | No information | 1 CSF | Nd | Serum Nd/CSF positive | Serum Nd/CSF negative | Unclassified |
| C28 | No information | No information | Cape Verde | 1 CSF | Nd | Serum Nd/CSF positive | Serum Nd/CSF negative | Unclassified |
| C29 * | Temporal headaches | 1 cyst | Bolivia | 1 serum, 1 CSF | Nd | Serum Nd/CSF limit | Serum negative/CSF negative | Definitive NCC |
| C30 | Headaches | 1 frontal cystic lesion, perilesional edema | No information | 1 CSF | Nd | Serum Nd/CSF limit | Serum Nd/CSF negative | Unclassified |
| C31 | Epilepsy | 1 active and inflammatory lesion | Madagascar | 1 CSF | Nd | Serum Nd/CSF positive | Serum Nd/CSF negative | Probable NCC |
| C32 | Epilepsy, intracranial hypertension, motor deficit | 1 calcified frontal cortico-subcortical nodular lesion | India | 1 serum, 1 CSF | Nd | Serum positive/CSF Nd | Serum Nd/CSF negative | Definitive NCC |
| C33 | Epilepsy | 1 left temporal cerebral lesion | Cameroon | 1 serum, 1 CSF, 1 brain biopsy | Serum negative/CSF negative | Serum Nd/CSF negative | Serum Nd/CSF negative/brain biopsy positive | Probable NCC |
| C34 | Epilepsy, fever | Hypodense calcified parieto-occipital brain abscess | Congo Kinshasa | 1 serum, 1 brain biopsy | Serum positive/CSF Nd | Serum negative/CSF Nd | Serum Nd/CSF Nd/brain biopsy positive | Probable NCC |
| C35 | Epilepsy, balance disorder, intracranial hypertension | Ventricular dilatation | Madagascar | 1 serum, 1 CSF | Serum positive/CSF positive | Serum positive/CSF Nd | Serum Nd/CSF positive | Probable NCC |
| C36 * | Headaches, epilepsy | 1 temporal cystic lesion | No travel, consumption of raw sausage | 1 serum | Serum negative/CSF Nd | Serum negative/CSF Nd | Nd | Definitive NCC |
| C37 | Psychiatric and memory disorders, spatial disorientation aphasia | 3 IP lesions | Africa | 1 serum, 1 CSF | Nd | Serum positive/CSF positive | Serum negative/CSF positive | Definitive NCC |
| C38 | Headaches, neck ache, nausea, vomiting | Multiple intraventricular lesions, ventricular dilatation, foramen of Monro obstruction | Central Africa | 1 serum, 1 CSF | Nd | Serum positive/CSF positive | Serum negative/CSF positive | Definitive NCC |
| C39 | Headaches, nausea, vomiting | IP calcifications, hydrocephalus, arachnoiditis | Haiti | 1 serum, 2 CSF | Nd | Serum positive /CSF positive | Serum negative/CSF negative then positive | Definitive NCC |
| C40 | Epilepsy, neuralgia, paresthesia | IP calcifications, hydrocephalus, arachnoiditis | Haiti | 1 serum, 1 CSF | Nd | Serum positive/CSF positive | Serum negative/CSF positive | Definitive NCC |
| C41 | Epilepsy, headaches, vomiting | 2 IP lesions, chronic meningitis | Cape Verde | 1 serum, 3 CSF | Nd | Serum positive/CSF positive | Serum negative/CSF positive X3 | Definitive NCC |
| C42 | Epilepsy, confusion | Multiple IP lesions, hydrocephalus, vasculitis | Cape Verde | 1 serum, 6 CSF | Nd | Serum positive/CSF positive | Serum negative/CSF positive X6 | Definitive NCC |
| C43 | Headaches, nausea, memory disorders, dysphasia, spatial disorientation, muscular cysticercosis | IP calcifications, parasagittal meningioma, then hydrocephalus, meningoencephalitis | Madagascar | 1 serum, 2 CSF | Nd | Serum positive/CSF positive | Serum negative/CSF positive X2 | Definitive NCC |
| C44 | Headaches, epilepsy | 4 IP lesions | Cape Verde | 1 serum, 1 CSF | Nd | Serum positive/CSF positive | Serum negative/CSF negative | Definitive NCC |
| C45 | Headaches, epilepsy | 1 IP lesion | India | 1 serum, 1 CSF | Nd | Serum positive/CSF negative | Serum negative/CSF negative | Definitive NCC |
Appendix B. Collaborators Groups
References
- Salavracos, M. Diagnostic et Prise en Charge d’un Cas de Neurocysticercose en Belgique. Louvain Med. 2019, 138, 239–245. [Google Scholar]
- Sotelo, J. Neurocysticercosis. BMJ 2003, 326, 511–512. [Google Scholar] [CrossRef] [PubMed]
- Hawk, M.W.; Shahlaie, K.; Kee, D.; Theis, J.H. Neurocysticercosis: A Review. Surg. Neurol. 2005, 63, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Garcia, H.H. Neurocysticercosis. Neurol. Clin. 2018, 36, 851–864. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.T.; Dubón-Murcia, S.A.; Aguilar-Estrada, R.L.; Chaves-Sell, F. Bu Neurocysticercose et Épilepsie. Epilepsies 2010, 22, 126–133. [Google Scholar] [CrossRef]
- Carpio, A.; Romo, M.L.; Hauser, W.A.; Kelvin, E.A. New understanding about the relationship among neurocysticercosis, seizures, and epilepsy. Seizure 2021, 90, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Carabin, H.; Ndimubanzi, P.; Budke, C.; Nguyen, H.; Qian, Y.; Cowan, L.; Stoner, J.; Rainwater, E.; Dickey, M. Clinical Manifestations Associated with Neurocysticercosis: A Systematic Review. PLoS Negl. Trop. Dis. 2011, 5, e1152. [Google Scholar] [CrossRef] [PubMed]
- Del Brutto, O.H. Diagnostic Criteria for Neurocysticercosis, Revisited. Pathog. Glob. Health 2012, 106, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Michelet, L.; Fleury, A.; Sciutto, E.; Kendjo, E.; Fragoso, G.; Paris, L.; Bouteille, B. Human Neurocysticercosis: Comparison of Different Diagnostic Tests Using Cerebrospinal Fluid. J. Clin. Microbiol. 2011, 49, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Yera, H.; Dupont, D.; Houze, S.; Ben M’Rad, M.; Pilleux, F.; Sulahian, A.; Gatey, C.; Andrieu, F.G.; Dupouy-Camet, J. Confirmation and Follow-Up of Neurocysticercosis by Real-Time PCR in Cerebrospinal Fluid Samples of Patients Living in France. J. Clin. Microbiol. 2011, 49, 4338–4340. [Google Scholar] [CrossRef] [PubMed]
- ANOFEL_rare analyses of Parasitology. Available online: https://anofel.net/wp-content/uploads/2022/09/ANOFEL_analyses-rares-Parasitologie-v2022-09-05.pdf (accessed on 5 September 2022).
- Simac, C.; Michel, P.; Andriantsimahavandy, A.; Esterre, P.; Michault, A. Use of Enzyme-Linked Immunosorbent Assay and Enzyme-Linked Immunoelectrotransfer Blot for the Diagnosis and Monitoring of Neurocysticercosis. Parasitol. Res. 1995, 81, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Fargeot, G.; Guey, S.; Hobeika, L.; Girard, P.; Alamowitch, S. Une neurocysticercose parenchymateuse Séronégative–l’Intérêt de la PCR. Rev. Neurol. 2015, 171, A139. [Google Scholar] [CrossRef]
- Carpio, A.; Campoverde, A.; Romo, M.L.; García, L.; Piedra, L.M.; Pacurucu, M.; López, N.; Aguilar, J.; López, S.; Vintimilla, L.C.; et al. Validity of a PCR Assay in CSF for the Diagnosis of Neurocysticercosis. Neurol. Neuroimmunol. Neuroinflammation 2017, 4, e324. [Google Scholar] [CrossRef] [PubMed]
- Symeonidou, I. Human Taeniasis/Cysticercosis: A Potentially Emerging Parasitic Disease in Europe. Ann. Gastroenterol. 2018, 31, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, S.; Bruschi, F. Neurocysticercosis in Europe: Still a Public Health Concern Not Only for Imported Cases. Acta Trop. 2013, 128, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Šoba, B.; Beović, B.; Lužnik, Z.; Skvarč, M.; Logar, J. Evidence of Human Neurocysticercosis in Slovenia. Parasitology 2014, 141, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Brunet, J.; Benoilid, A.; Kremer, S.; Dalvit, C.; Lefebvre, N.; Hansmann, Y.; Chenard, M.-P.; Mathieu, B.; Grimm, F.; Deplazes, P.; et al. First Case of Human Cerebral Taenia Martis Cysticercosis. J. Clin. Microbiol. 2015, 53, 2756–2759. [Google Scholar] [CrossRef] [PubMed]
- Goetghebeur, E.; Liinev, J. Diagnostic Test Analyses in Search of Their Gold Standard: Latent Class Analyses with Random Effects. Stat. Methods Med. Res. 2000, 9, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Hui, S.; Zhou, X. Evaluation of Diagnostic Tests without Gold Standards. Stat. Methods Med. Res. 1998, 7, 354–370. [Google Scholar] [CrossRef] [PubMed]
| Result | Serum | CSF (a) | |
|---|---|---|---|
| ELISA | positive | 10 | 7 |
| negative | 7 | 4 | |
| WB | positive | 27 | 28 |
| negative | 3 | 5 | |
| borderline | 0 | 3 | |
| PCR | positive | 0 | 30 (4) |
| negative | 12 | 18 |
| Result | Definitive NCC Serum/CSF (a) | Probable NCC Serum/CSF (a) | |
|---|---|---|---|
| WB | positive | 17/12 | 6/11 |
| negative | 1/2 | 2/3 | |
| borderline | 0/1 | 0/0 | |
| PCR | positive | 0/21 (1) | 0/7 (3) |
| negative | 12/6 | 0/8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zemmour, I.; Durieux, M.-F.; Herault, E.; Rouges, C.; Šoba, B.; Mercier, A.; Ariey, F.; Preux, P.-M.; Yera, H.; on behalf of Collaborators Group. Neurocysticercosis Diagnosis in a Non-Endemic Country: France. Pathogens 2023, 12, 1205. https://doi.org/10.3390/pathogens12101205
Zemmour I, Durieux M-F, Herault E, Rouges C, Šoba B, Mercier A, Ariey F, Preux P-M, Yera H, on behalf of Collaborators Group. Neurocysticercosis Diagnosis in a Non-Endemic Country: France. Pathogens. 2023; 12(10):1205. https://doi.org/10.3390/pathogens12101205
Chicago/Turabian StyleZemmour, Ines, Marie-Fleur Durieux, Etienne Herault, Célia Rouges, Barbara Šoba, Aurélien Mercier, Frédéric Ariey, Pierre-Marie Preux, Hélène Yera, and on behalf of Collaborators Group. 2023. "Neurocysticercosis Diagnosis in a Non-Endemic Country: France" Pathogens 12, no. 10: 1205. https://doi.org/10.3390/pathogens12101205
APA StyleZemmour, I., Durieux, M.-F., Herault, E., Rouges, C., Šoba, B., Mercier, A., Ariey, F., Preux, P.-M., Yera, H., & on behalf of Collaborators Group. (2023). Neurocysticercosis Diagnosis in a Non-Endemic Country: France. Pathogens, 12(10), 1205. https://doi.org/10.3390/pathogens12101205






