Can sPD-1 and sPD-L1 Plasma Concentrations Predict Treatment Response among Patients with Extraparenchymal Neurocysticercosis?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Controls
2.2. Plasma Samples
2.3. Evaluation of sPD1 and SPD-L1 in Plasma
2.4. Cytokine Titration and Peripheral Immunologic Profile
2.5. Statistical Analysis
3. Results
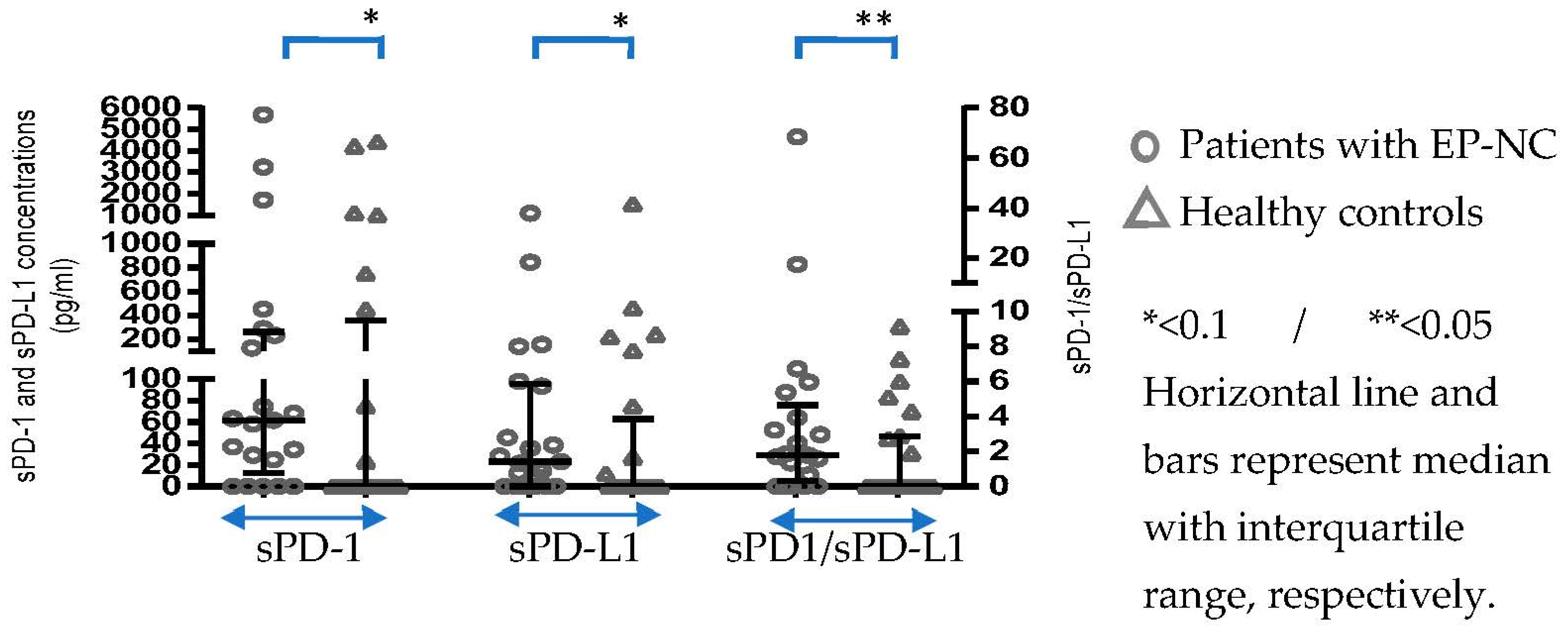
3.1. Differences in sPD-1 and sPD-L1 Presence and Concentration between Patients and Controls
3.2. Correlation between Presence of sPD-1/sPD-L1 and Response to Treatment
3.3. Correlation between Concentration of sPD-1/sPD-L1 and the Specific Peripheral Immunologic Profile
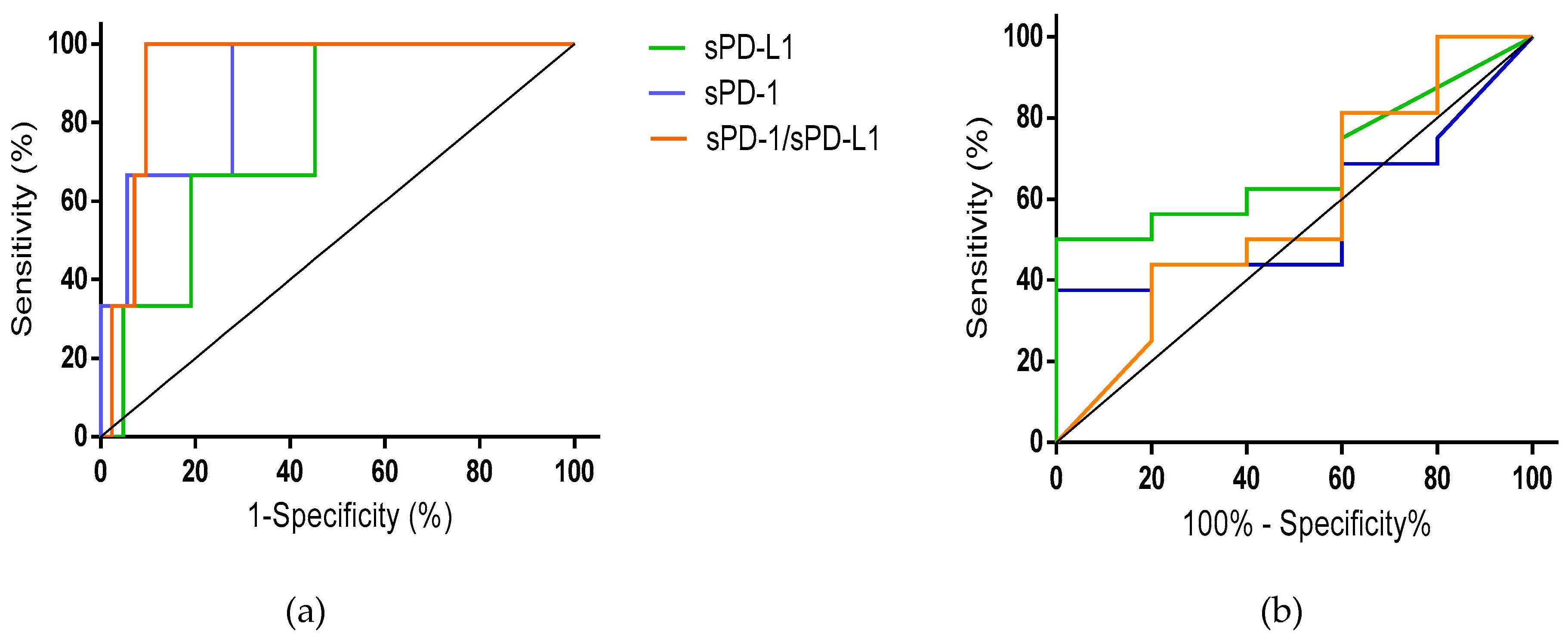
3.4. Sensitivity and Specificity of Pretreatment sPD-1/sPD-L1 to Predict Treatment Response
3.5. Evolution sPD-1 and sPD-L1 Concentrations and sPD-1/sPD-L1 Ratio over Time by Treatment Response
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marcin Sierra, M.; Arroyo, M.; Cadena Torres, M.; Ramírez Cruz, N.; García Hernández, F.; Taboada, D.; Galicia Martínez, Á.; Govezensky, T.; Sciutto, E.; Toledo, A.; et al. Extraparenchymal neurocysticercosis: Demographic, clinicoradiological, and inflammatory features. PloS Negl. Trop. Dis. 2017, 11, e0005646. [Google Scholar] [CrossRef] [PubMed]
- Fleury, A.; Carrillo-Mezo, R.; Flisser, A.; Sciutto, E.; Corona, T. Subarachnoid basal neurocysticercosis: A focus on the most severe form of the disease. Expert. Rev. Anti Infect. Ther. 2011, 9, 123–133. [Google Scholar] [CrossRef]
- Osorio, R.; Carrillo-Mezo, R.; Romo, M.L.; Toledo, A.; Matus, C.; González-Hernández, I.; Jung, H.; Fleury, A. Factors Associated with Cysticidal Treatment Response in Extraparenchymal Neurocysticercosis. J. Clin. Pharmacol. 2019, 59, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Adalid-Peralta, L.; Arce-Sillas, A.; Fragoso, G.; Cárdenas, G.; Rosetti, M.; Casanova-Hernández, D.; Rangel-Escareño, C.; Uribe-Figueroa, L.; Fleury, A.; Sciutto, E. Cysticerci drive dendritic cells to promote in vitro and in vivo Tregs differentiation. Clin. Dev. Immunol. 2013, 2013, 981468. [Google Scholar] [CrossRef]
- Romo, M.L.; Osorio, R.; Toledo, A.; Carrillo-Mezo, R.; Valdez, R.; Romano, M.C.; Sciutto, E.; Fragoso, G.; Fleury, A. Low responsiveness of peripheral lymphocytes in extraparenchymal neurocysticercosis. PLoS Negl. Trop. Dis. 2023, 17, e0011386. [Google Scholar] [CrossRef]
- Cárdenas, G.; Fragoso, G.; Rosetti, M.; Uribe-Figueroa, L.; Rangel-Escareño, C.; Saenz, B.; Hernández, M.; Sciutto, E.; Fleury, A. Neurocysticercosis: The effectiveness of the cysticidal treatment could be influenced by the host immunity. Med. Microbiol. Immunol. 2014, 203, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Francisco, L.M.; Sage, P.T.; Sharpe, A.H. PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010, 236, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, A.H.; Pauken, K.E. The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. 2018, 18, 153–167. [Google Scholar] [CrossRef]
- Chen, R.Y.; Zhu, Y.; Shen, Y.Y.; Xu, Q.Y.; Tang, H.Y.; Cui, N.X.; Jiang, L.; Dai, X.M.; Chen, W.Q.; Lin, Q.; et al. The role of PD-1 signaling in health and immune-related diseases. Front. Immunol. 2023, 14, 1163633. [Google Scholar] [CrossRef]
- Dai, S.; Jia, R.; Zhang, X.; Fang, Q.; Huang, L. The PD-1/PD-Ls pathway and autoimmune diseases. Cell Immunol. 2014, 290, 72–79. [Google Scholar] [CrossRef]
- Sari, M.I.; Ilyas, S. The Expression Levels and Concentrations of PD-1 and PD-L1 Proteins in Septic Patients: A Systematic Review. Diagnostics 2022, 12, 2004. [Google Scholar] [CrossRef]
- Carpio, A.; Fleury, A.; Romo, M.L.; Abraham, R.; Fandiño, J.; Durán, J.C.; Cárdenas, G.; Moncayo, J.; Leite Rodrigues, C.; San-Juan, D.; et al. New diagnostic criteria for neurocysticercosis: Reliability and validity. Ann. Neurol. 2016, 80, 434–442. [Google Scholar] [CrossRef]
- Konar, S.; Kandregula, S.; Sashidhar, A.; Prabhuraj, A.R.; Saini, J.; Shukla, D.; Srinivas, D.; Indira Devi, B.; Somanna, S.; Arimappamagan, A. Endoscopic intervention for intraventricular neurocysticercal cyst: Challenges and outcome analysis from a single institute experience. Clin. Neurol. Neurosurg. 2020, 198, 106179. [Google Scholar] [CrossRef] [PubMed]
- Nash, T.E.; O’Connell, E.M. Subarachnoid neurocysticercosis: Emerging concepts and treatment. Curr. Opin. Infect. Dis. 2020, 33, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Garcia, H.H.; Lescano, A.G.; Gonzales, I.; Bustos, J.A.; Pretell, E.J.; Horton, J.; Saavedra, H.; Gonzalez, A.E.; Gilman, R.H.; Cysticercosis Working Group in Peru. Cysticidal Efficacy of Combined Treatment With Praziquantel and Albendazole for Parenchymal Brain Cysticercosis. Clin. Infect. Dis. 2016, 62, 1375–1379. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, M.; Mezawa, H.; Kawai, T.; Urashima, M. Elevated Soluble PD-L1 in Pregnant Women’s Serum Suppresses the Immune Reaction. Front. Immunol. 2019, 10, 86. [Google Scholar] [CrossRef]
- Chang, B.; Huang, T.; Wei, H.; Shen, L.; Zhu, D.; He, W.; Chen, Q.; Zhang, H.; Li, Y.; Huang, R.; et al. The correlation and prognostic value of serum levels of soluble programmed death protein 1 (sPD-1) and soluble programmed death-ligand 1 (sPD-L1) in patients with hepatocellular carcinoma. Cancer Immunol. Immunother. 2019, 68, 353–363. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Sasaki, T.; Kano, M.; Shiraishi, T.; Suito, H.; Murakami, K.; Toyozumi, T.; Otsuka, R.; Kinoshita, K.; Iida, S.; et al. Soluble PD L1 reflects cachexia status in patients with gastric cancer and is an independent prognostic marker for relapse free survival after radical surgery. Mol. Clin. Oncol. 2023, 18, 39. [Google Scholar] [CrossRef]
- Okuma, Y.; Wakui, H.; Utsumi, H.; Sagawa, Y.; Hosomi, Y.; Kuwano, K.; Homma, S. Soluble Programmed Cell Death Ligand 1 as a Novel Biomarker for Nivolumab Therapy for Non-Small-cell Lung Cancer. Clin. Lung Cancer. 2018, 19, 410–417. [Google Scholar] [CrossRef]
- Mazzaschi, G.; Minari, R.; Zecca, A.; Cavazzoni, A.; Ferri, V.; Mori, C.; Squadrilli, A.; Bordi, P.; Buti, S.; Bersanelli, M.; et al. Soluble PD-L1 and Circulating CD8+PD-1+ and NK Cells Enclose a Prognostic and Predictive Immune Effector Score in Immunotherapy Treated NSCLC patients. Lung Cancer. 2020, 148, 1–11. [Google Scholar] [CrossRef]
- Scirocchi, F.; Strigari, L.; Di Filippo, A.; Napoletano, C.; Pace, A.; Rahimi, H.; Botticelli, A.; Rughetti, A.; Nuti, M.; Zizzari, I.G. Soluble PD-L1 as a Prognostic Factor for Immunotherapy Treatment in Solid Tumors: Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 14496. [Google Scholar] [CrossRef]
- Széles, Á.; Fazekas, T.; Váncsa, S.; Váradi, M.; Kovács, P.T.; Krafft, U.; Grünwald, V.; Hadaschik, B.; Csizmarik, A.; Hegyi, P.; et al. Pre-treatment soluble PD-L1 as a predictor of overall survival for immune checkpoint inhibitor therapy: A systematic review and meta-analysis. Cancer Immunol. Immunother. 2023, 72, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Song, M.Y.; Park, S.H.; Nam, H.J.; Choi, D.H.; Sung, Y.C. Enhancement of vaccine-induced primary and memory CD8(+) T-cell responses by soluble PD-1. J. Immunother. 2011, 34, 297–306. [Google Scholar] [CrossRef] [PubMed]
- da Fonseca-Martins, A.M.; Ramos, T.D.; Pratti, J.E.S.; Firmino-Cruz, L.; Gomes, D.C.O.; Soong, L.; Saraiva, E.M.; de Matos Guedes, H.L. Immunotherapy using anti-PD-1 and anti-PD-L1 in Leishmania amazonensis-infected BALB/c mice reduce parasite load. Sci. Rep. 2019, 9, 20275. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, A.; Kumar, S.; Bodhale, N.; Jafarzadeh, S.; Nemati, M.; Sharifi, I.; Sarkar, A.; Saha, B. The expression of PD-1 and its ligands increases in Leishmania infection and its blockade reduces the parasite burden. Cytokine 2022, 153, 155839. [Google Scholar] [CrossRef]
- Lasso, P.; Mateus, J.; Pavía, P.; Rosas, F.; Roa, N.; Thomas, M.C.; López, M.C.; González, J.M.; Puerta, C.J.; Cuéllar, A. Inhibitory Receptor Expression on CD8+ T Cells Is Linked to Functional Responses against Trypanosoma cruzi Antigens in Chronic Chagasic Patients. J. Immunol. 2015, 195, 3748–3758. [Google Scholar] [CrossRef]
- Pérez-Antón, E.; Egui, A.; Thomas, M.C.; Simón, M.; Segovia, M.; López, M.C. Immunological exhaustion and functional profile of CD8+ T lymphocytes as cellular biomarkers of therapeutic efficacy in chronic Chagas disease patients. Acta Trop. 2020, 202, 105242. [Google Scholar] [CrossRef]
- Arana, Y.; Gálvez, R.I.; Jacobs, T. Role of the PD-1/PD-L1 Pathway in Experimental Trypanosoma cruzi Infection and Potential Therapeutic Options. Front. Immunol. 2022, 13, 866120. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, Y.; Su, M.; Zhang, R.; Ding, J.; Hao, X.; Ma, Y. Role of soluble programmed death-1 (sPD-1) and sPD-ligand 1 in patients with cystic echinococcosis. Exp. Ther. Med. 2016, 11, 251–256. [Google Scholar] [CrossRef]
- Ben Salah, E.; Sakly, W.; Barrera, C.; Mosbahi, S.; Bellanger, A.P.; Farhani, R.; Ksia, A.; Gottstein, B.; Nouri, A.; Babba, H.; et al. Soluble programmed death-1 (sPD-1) as predictor of early surgical outcomes of paediatric cystic echinococcosis. Parasite Immunol. 2021, 43, e12809. [Google Scholar] [CrossRef]
- Jebbawi, F.; Bellanger, A.P.; Lunström-Stadelmann, B.; Rufener, R.; Dosch, M.; Goepfert, C.; Gottstein, B.; Millon, L.; Grandgirard, D.; Leib, S.L.; et al. Innate and adaptive immune responses following PD-L1 blockade in treating chronic murine alveolar echinococcosis. Parasite Immunol. 2021, 43, e12834. [Google Scholar] [CrossRef] [PubMed]
- Terrazas, L.I.; Montero, D.; Terrazas, C.A.; Reyes, J.L.; Rodríguez-Sosa, M. Role of the programmed Death-1 pathway in the suppressive activity of alternatively activated macrophages in experimental cysticercosis. Int. J. Parasitol. 2005, 35, 1349–1358. [Google Scholar] [CrossRef]
- Arce-Sillas, A.; Álvarez-Luquín, D.D.; Cárdenas, G.; Casanova-Hernández, D.; Fragoso, G.; Hernández, M.; Proaño Narváez, J.V.; García-Vázquez, F.; Fleury, A.; Sciutto, E.; et al. Interleukin 10 and dendritic cells are the main suppression mediators of regulatory T cells in human neurocysticercosis. Clin. Exp. Immunol. 2016, 183, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Hamamoto Filho, P.T.; Singh, G.; Winkler, A.S.; Carpio, A.; Fleury, A. Could Differences in Infection Pressure Be Involved in Cysticercosis Heterogeneity? Trends Parasitol. 2020, 36, 826–834. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toledo, A.; Fragoso, G.; Carrillo-Mezo, R.; Romo, M.L.; Sciutto, E.; Fleury, A. Can sPD-1 and sPD-L1 Plasma Concentrations Predict Treatment Response among Patients with Extraparenchymal Neurocysticercosis? Pathogens 2023, 12, 1116. https://doi.org/10.3390/pathogens12091116
Toledo A, Fragoso G, Carrillo-Mezo R, Romo ML, Sciutto E, Fleury A. Can sPD-1 and sPD-L1 Plasma Concentrations Predict Treatment Response among Patients with Extraparenchymal Neurocysticercosis? Pathogens. 2023; 12(9):1116. https://doi.org/10.3390/pathogens12091116
Chicago/Turabian StyleToledo, Andrea, Gladis Fragoso, Roger Carrillo-Mezo, Matthew L. Romo, Edda Sciutto, and Agnès Fleury. 2023. "Can sPD-1 and sPD-L1 Plasma Concentrations Predict Treatment Response among Patients with Extraparenchymal Neurocysticercosis?" Pathogens 12, no. 9: 1116. https://doi.org/10.3390/pathogens12091116
APA StyleToledo, A., Fragoso, G., Carrillo-Mezo, R., Romo, M. L., Sciutto, E., & Fleury, A. (2023). Can sPD-1 and sPD-L1 Plasma Concentrations Predict Treatment Response among Patients with Extraparenchymal Neurocysticercosis? Pathogens, 12(9), 1116. https://doi.org/10.3390/pathogens12091116






