Clinical Characteristics of Neurocysticercosis in a Peruvian Population-Based Epilepsy Cohort: A Descriptive Cross-Sectional Study of Baseline Clinical Intake
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Del Brutto, O.H. Neurocysticercosis. Handb. Clin. Neurol. 2014, 121, 1445–1459. [Google Scholar] [PubMed]
- Del Brutto, O.H. Human Neurocysticercosis: An Overview. Pathogens 2022, 11, 1212. [Google Scholar] [CrossRef] [PubMed]
- White, A.C. Neurocysticercosis: Updates on Epidemiology, Pathogenesis, Diagnosis, and Management. Annu. Rev. Med. 2000, 51, 187–206. Available online: www.annualreviews.org (accessed on 11 November 2022). [CrossRef] [PubMed]
- Moyano, L.M.; Saito, M.; Montano, S.M.; Gonzalvez, G.; Olaya, S.; Ayvar, V.; González, I.; Larrauri, L.; Tsang, V.C.W.; Llanos, F.; et al. Neurocysticercosis as a Cause of Epilepsy and Seizures in Two Community-Based Studies in a Cysticercosis-Endemic Region in Peru. PLoS Neglected Trop. Dis. 2014, 8, e2692. [Google Scholar] [CrossRef] [PubMed]
- Neligan, A.; Bell, G.S.; Shorvon, S.D.; Sander, J.W. Temporal trends in the mortality of people with epilepsy: A review. Epilepsia 2010, 51, 2241–2246. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef]
- Thurman, D.J.; Begley, C.E.; Carpio, A.; Helmers, S.; Hesdorffer, D.C.; Mu, J.; Touré, K.; Parko, K.L.; Newton, C.R. The primary prevention of epilepsy: A report of the Prevention Task Force of the International League Against Epilepsy. Epilepsia 2018, 59, 905–914. [Google Scholar] [CrossRef]
- Commission on Tropical Diseases of the International League Against Epilepsy. Relationship Between Epilepsy and Tropical Diseases. Epilepsia 1994, 35, 89–93. [Google Scholar] [CrossRef]
- Newton, C.R.; Garcia, H.H. Epilepsy in poor regions of the world. Lancet 2012, 380, 1193–1201. [Google Scholar] [CrossRef]
- Garcia, H.H.; Gonzalez, A.E.; Tsang, V.C.; O’Neal, S.E.; Llanos-Zavalaga, F.; Gonzalvez, G.; Romero, J.; Rodriguez, S.; Moyano, L.M.; Ayvar, V.; et al. Elimination of Taenia solium Transmission in Northern Peru. N. Engl. J. Med. 2016, 374, 2335–2344. [Google Scholar] [CrossRef]
- Placencia, M.; Sander, J.W.A.S.; Shorvon, S.D.; Ellison, R.H.; Cascante, S.M. Validation of a screening questionnaire for the detection of epileptic seizures in epidemiological studies. Brain 1992, 115, 783–794. Available online: https://academic.oup.com/brain/article/115/3/783/326323 (accessed on 21 July 2023). [CrossRef] [PubMed]
- Bonnet, G.; Pizzitutti, F.; Gonzales-Gustavson, E.A.; Gabriël, S.; Pan, W.K.; Garcia, H.H.; Bustos, J.A.; Vilchez, P.; O’neal, S.E.; for the Cysticercosis Working Group in Peru. CystiHuman: A model of human neurocysticercosis. PLoS Comput. Biol. 2022, 18, e1010118. [Google Scholar] [CrossRef] [PubMed]
- Scharf, R.J.; Scharf, G.J.; Stroustrup, A. Developmental Milestones Institute for Advanced Studies in Culture. Pediatr. Rev. 2016, 37, 25–38. Available online: http://publications.aap.org/pediatricsinreview/article-pdf/37/1/25/824665/pedsinreview_20140103.pdf (accessed on 20 October 2023). [CrossRef]
- Tsang, V.C.W.; Brand, J.A.; Boyer, A.E. An enzyme-linked immunoelectrotransfer blot assay and glycoprotein antigens for diagnosing human cysticercosis (Taenia solium). J. Infect. Dis. 1989, 159, 50–59. [Google Scholar] [CrossRef]
- Rodriguez, S.; Dorny, P.; Tsang, V.C.; Pretell, E.J.; Brandt, J.; Lescano, A.G.; Gonzalez, A.E.; Gilman, R.H.; Garcia, H.H. Detection of taenia solium antigens and anti-t. solium antibodies in paired serum and cerebrospinal fluid samples from patients with intraparenchymal or extraparenchymal neurocysticercosis. J. Infect. Dis. 2009, 199, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Del Brutto, O.H. Twenty-five years of evolution of standard diagnostic criteria for neurocysticercosis. How have they impacted diagnosis and patient outcomes? Expert Rev. Neurother. 2019, 20, 147–155. [Google Scholar] [CrossRef]
- Del Brutto, O.H.; Nash, T.E.; White, A.C.; Rajshekhar, V.; Wilkins, P.P.; Singh, G.; Vasquez, C.M.; Salgado, P.; Gilman, R.H.; Garcia, H.H. Revised diagnostic criteria for neurocysticercosis. J. Neurol. Sci. 2017, 372, 202–210. [Google Scholar] [CrossRef]
- García, H.H.; Del Brutto, O.H. Imaging findings in neurocysticercosis. Acta Trop. 2003, 87, 71–78. [Google Scholar] [CrossRef]
- Del Brutto, O.H.; Arroyo, G.; Del Brutto, V.J.; Zambrano, M.; García, H.H. On the relationship between calcified neurocysticercosis and epilepsy in an endemic village: A large-scale, computed tomography–based population study in rural Ecuador. Epilepsia 2017, 58, 1955–1961. [Google Scholar] [CrossRef]
- Nicoletti, A.; Bartoloni, A.; Sofia, V.; Bartalesi, F.; Chavez, J.R.; Osinaga, R.; Paradisi, F.; Dumas, J.-L.; Tsang, V.C.W.; Reggio, A.; et al. Epilepsy and neurocysticercosis in rural Bolivia: A population-based survey. Epilepsia 2005, 46, 1127–1132. [Google Scholar] [CrossRef]
- Brutto, O.H.D.; Santibáñez, R.; Idrovo, L.; Rodrìguez, S.; Díaz-Calderón, E.; Navas, C.; Gilman, R.H.; Cuesta, F.; Mosquera, A.; Gonzalez, A.E.; et al. Epilepsy and Neurocysticercosis in Atahualpa: A Door-to-Door Survey in Rural Coastal Ecuador. Epilepsia 2005, 46, 583–587. [Google Scholar] [CrossRef]
- Yemadje, L.; Houinato, D.; Quet, F.; Druet-Cabanac, M.; Preux, P. Understanding the differences in prevalence of epilepsy in tropical regions. Epilepsia 2011, 52, 1376–1381. [Google Scholar] [CrossRef]
- Kwon, C.; Wagner, R.G.; Carpio, A.; Jetté, N.; Newton, C.R.; Thurman, D.J. The worldwide epilepsy treatment gap: A systematic review and recommendations for revised definitions–A report from the ILAE Epidemiology Commission. Epilepsia 2022, 63, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Bruno, E.; Bartoloni, A.; Zammarchi, L.; Strohmeyer, M.; Bartalesi, F.; Bustos, J.A.; Santivañez, S.; García, H.H.; Nicoletti, A.; the COHEMI Project Study Group. Epilepsy and Neurocysticercosis in Latin America: A Systematic Review and Meta-analysis. PLoS Neglected Trop. Dis. 2013, 7, e2480. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.-C.; Dua, T.; Ma, J.; Saxena, S.; Birbeck, G. Global disparities in the epilepsy treatment gap: A systematic review. Bull. World Health Organ. 2009, 88, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Kanner, A.M.; Bicchi, M.M. Antiseizure Medications for Adults with Epilepsy: A Review. JAMA 2022, 327, 1269–1281. [Google Scholar] [CrossRef] [PubMed]
- Caraballo, R.; Fejerman, N. Management of epilepsy in resource-limited settings. Epileptic Disord. 2015, 17, 13–18. [Google Scholar] [CrossRef]
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef]
- Wilson, M.; Bryan, R.T.; Fried, J.A.; Ware, D.A.; Schantz, P.M.; Pilcher, J.B.; Tsang, V.C. Clinical Evaluation of the Cysticercosis Enzyme-Linked Immunoelectrotransfer Blot in Patients with Neurocysticercosis. J. Infect. Dis. 1991, 164, 1007–1009. Available online: https://www.jstor.org/stable/30112023 (accessed on 20 October 2023). [CrossRef]
- Sako, Y.; Takayanagui, O.M.; Odashima, N.S.; Ito, A. Comparative study of paired serum and cerebrospinal fluid samples from neurocysticercosis patients for the detection of specific antibody to taenia solium immunodiagnostic antigen. Trop. Med. Health 2015, 43, 171–176. [Google Scholar] [CrossRef]
- HGarcia, H.H.; E Nash, T.; Del Brutto, O.H. Clinical symptoms, diagnosis, and treatment of neurocysticercosis. Lancet Neurol. 2014, 13, 1202–1215. [Google Scholar] [CrossRef] [PubMed]
| N = 1975 | ||
|---|---|---|
| n | % | |
| Demographics | ||
| Age, years | ||
| Average (SD) | 29.4 (16.9) | |
| Sex | ||
| Male | 966 | 48.9 |
| Female | 1009 | 51.1 |
| Educational attainment | ||
| No formal education | 83 | 5.1 |
| Primary | 707 | 40.7 |
| Secondary | 634 | 39.2 |
| Trade or other vocational | 30 | 1.9 |
| Tertiary | 165 | 10.2 |
| Health Characteristics | ||
| BMI, kg/m2 | ||
| Underweight | 50 | 2.5 |
| Healthy | 626 | 31.7 |
| Overweight or obese | 526 | 26.6 |
| Missing | 773 | 39.1 |
| Current substance use | ||
| Tobacco † | 78 | 4.0 |
| Alcohol † | 408 | 20.7 |
| Drugs † | 6 | 0.3 |
| Previously diagnosed medical comorbidities | ||
| Diabetes † | 23 | 1.6 |
| Hypertension † | 56 | 2.8 |
| Dyslipidemia † | 34 | 1.7 |
| Heart Disease † | 14 | 0.7 |
| Psychiatric diagnosis † | 38 | 1.9 |
| Epilepsy risk factors | ||
| Head Trauma † | 12 | 0.6 |
| Febrile seizure or fever lasting > 15 days † | 725 | 36.7 |
| Family history of epilepsy † | 1127 | 57.1 |
| History of neurosurgery † | 15 | 0.8 |
| Developmental abnormality † | 225 | 11.4 |
| Epilepsy Characteristics | ||
| Active epilepsy * | ||
| Active | 1691 | 85.6 |
| Inactive | 181 | 9.2 |
| Missing | 103 | 5.2 |
| Age of onset | ||
| <18 years | 1073 | 54.4 |
| 18 or older | 586 | 29.7 |
| Missing | 316 | 16.0 |
| Duration of epilepsy, years | ||
| Average (SD) | 13.2 (13.4) | |
| Median (IQR) | 9.0 (2–20) | |
| Missing | 303 | 15.4 |
| Seizure types by history | ||
| Generalized | 400 | 20.3 |
| Focal | 1084 | 54.9 |
| Indeterminate | 38 | 1.9 |
| Missing | 453 | 22.9 |
| EEG results (n = 252) ** | ||
| Normal | 102 | 40.5 |
| Non-specific abnormality | 73 | 29.0 |
| Focal epileptiform | 34 | 13.5 |
| Generalized epileptiform | 21 | 8.3 |
| Epileptiform uncertain focal versus generalized | 22 | 8.7 |
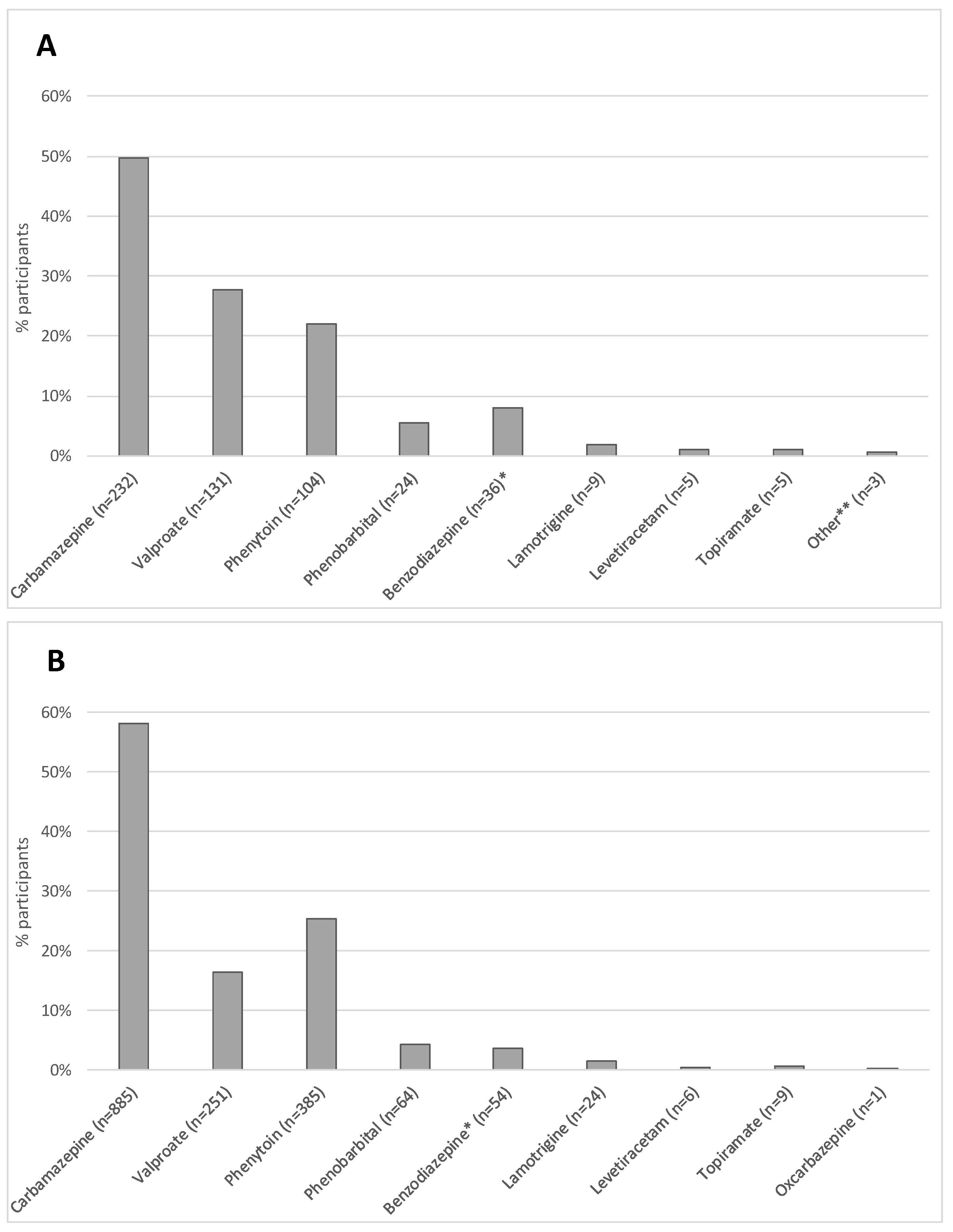
| ASM Characteristics (n = 1691) *** | ||
| History of any prior ASM usage reported | ||
| Yes | 442 | 26.1 |
| Number of prior ASM(s) used **** | ||
| Monotherapy | 372 | 84.2 |
| Dual therapy or more | 70 | 15.8 |
| ASM prescribed at baseline intake | ||
| Yes | 1523 | 96.0 |
| Number of ASM(s) prescribed at baseline intake **** | ||
| Monotherapy | 1377 | 90.4 |
| Dual therapy or more | 146 | 9.6 |
| n | % | |
|---|---|---|
| Diagnostics | ||
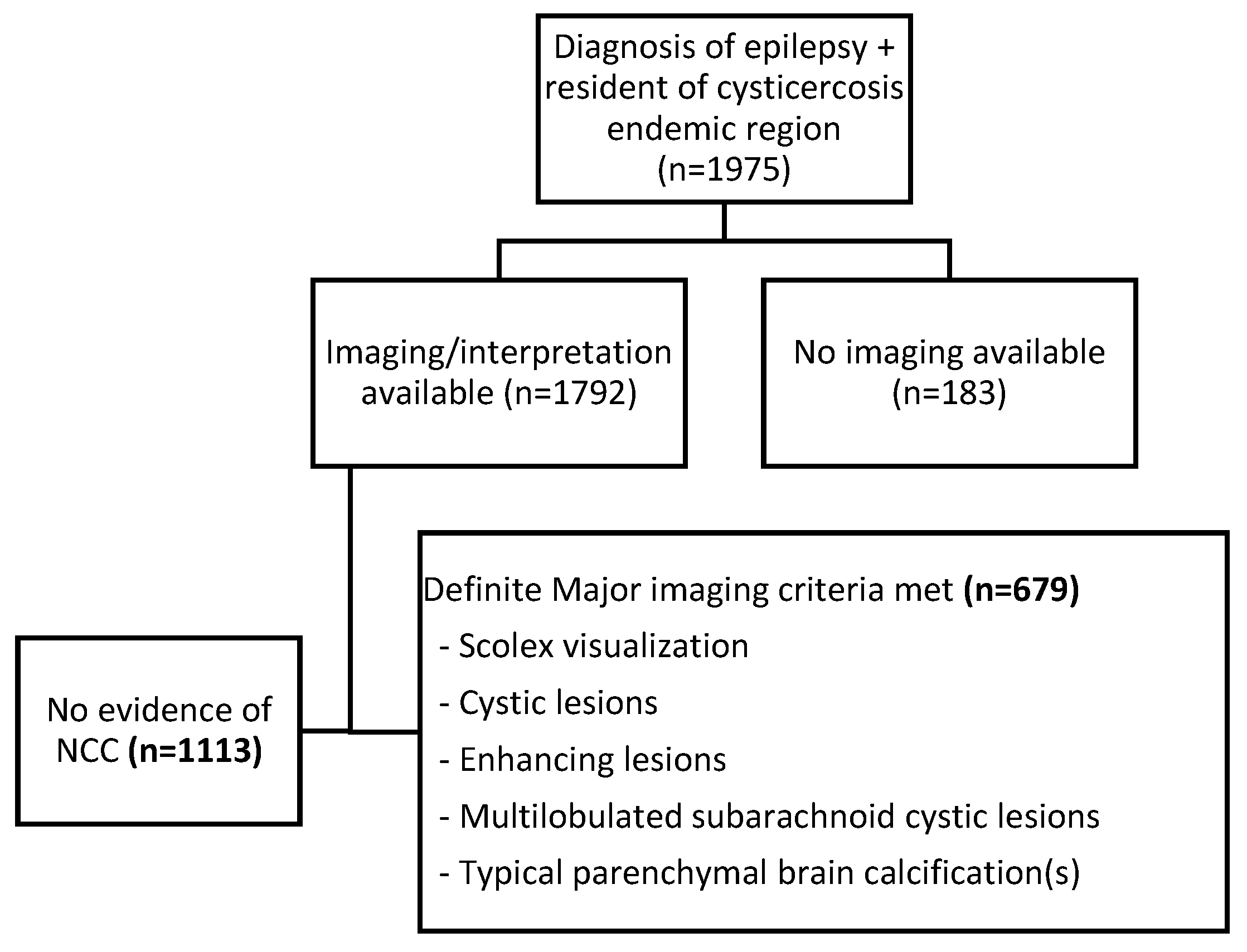
| CT Scan Result (n = 1792) | ||
| No NCC | 1113 | 62.1 |
| Calcified NCC | 586 | 32.7 |
| Viable/colloidal NCC cysts | 42 | 2.3 |
| Cysts and calcifications | 51 | 2.9 |
| Serologies | ||
| EITB assay results (n = 1745) | ||
| 0 bands | 1095 | 62.8 |
| 1 or more | 650 | 37.3 |
| Antigen ELISA results (n = 176) | ||
| Negative | 122 | 30.7 |
| Positive | 54 | 69.3 |
| NCC N = 679 | Non-NCC N = 1113 | ||||
|---|---|---|---|---|---|
| n | % | n | % | p Value | |
| Demographics | |||||
| Age, years | |||||
| Average with SD | 34.3 (16.9) | 27.0 (16.3) | <0.001 | ||
| Sex | |||||
| Male | 333 | 49.0 | 545 | 49.0 | 0.975 |
| Female | 346 | 51.0 | 568 | 51.0 | |
| Health Characteristics | |||||
| BMI, kg/m2 | |||||
| Underweight | 11 | 1.6 | 32 | 2.9 | 0.027 |
| Healthy | 189 | 27.8 | 368 | 33.1 | |
| Overweight or obese | 188 | 27.7 | 281 | 25.3 | |
| Substance Use | |||||
| Tobacco † | 36 | 5.3 | 35 | 3.1 | 0.02 |
| Alcohol † | 163 | 24.0 | 201 | 18.1 | 0.002 |
| Epilepsy Risk Factors | |||||
| Head Trauma † | 1 | 0.2 | 11 | 1.0 | 0.037 + |
| Febrile seizure or fever lasting > 15 days † | 197 | 29.0 | 447 | 40.2 | <0.001 |
| FH of epilepsy † | 380 | 56.0 | 646 | 58.0 | 0.329 |
| History of neurosurgery † | 3 | 0.4 | 8 | 0.7 | 0.549 + |
| Developmental abnormality † | 51 | 7.5 | 136 | 12.2 | 0.002 |
| Epilepsy Characteristics | |||||
| Active epilepsy (n = 1713) * | |||||
| Active | 572 | 88.0 | 971 | 91.4 | 0.025 |
| Inactive | 78 | 12.0 | 92 | 8.7 | |
| Age of Onset (n = 1541) | |||||
| <18 years | 338 | 57.6 | 661 | 69.3 | <0.001 |
| 18 or older | 249 | 42.4 | 293 | 30.7 | |
| Duration of Epilepsy (n = 1553) | |||||
| Average (SD) | 15.8 (15.0) | 12.1 (12.3) | <0.001 | ||
| Median (IQR) | 12.0 (3–25) | 8.0 (2–19) | |||
| Seizure types by history (n = 1412) | |||||
| Generalized | 112 | 21.3 | 250 | 28.1 | 0.009 |
| Focal | 397 | 75.5 | 623 | 70.0 | |
| Indeterminate | 17 | 3.2 | 17 | 2.0 | |
| EEG results (n = 238) ** | |||||
| Normal | 35 | 46.7 | 60 | 36.8 | 0.331+ |
| Non-specific abnormality | 20 | 26.7 | 49 | 30.1 | |
| Focal epileptiform | 12 | 16.0 | 21 | 12.9 | |
| Generalized epileptiform | 3 | 4.0 | 16 | 9.8 | |
| Epileptiform uncertain focal versus generalized | 5 | 6.7 | 7 | 10.4 | |
| ASM Characteristics (n = 1543) *** | |||||
| History of any prior ASM usage reported | |||||
| Yes | 131 | 22.9 | 266 | 27.4 | 0.051 |
| History of number of prior ASM(s) used † | |||||
| Monotherapy | 111 | 84.7 | 221 | 83.1 | 0.676 |
| Dual therapy or more | 20 | 15.3 | 45 | 16.9 | |
| ASM prescribed at baseline intake | |||||
| Yes | 539 | 94.2 | 859 | 88.5 | <0.001 |
| Number of ASM(s) prescribed at baseline † | |||||
| Monotherapy | 503 | 93.3 | 763 | 88.8 | 0.005 |
| Dual therapy or more | 36 | 6.7 | 96 | 11.2 | |
| Cysticercosis Diagnostic Serologies | |||||
| Serologies | |||||
| EITB assay results (n = 1619) | |||||
| 0 bands | 281 | 43.9 | 745 | 76.1 | <0.001 |
| 1–2 bands | 95 | 14.8 | 110 | 11.2 | |
| 3+ bands | 264 | 41.3 | 124 | 12.7 | |
| Antigen ELISA results (n = 157) | |||||
| Negative | 59 | 59.0 | 49 | 86.0 | <0.001 |
| Positive | 41 | 41.0 | 8 | 14.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allen, S.E.; Moyano, L.M.; Wardle, M.T.; Guzman, C.; Sanchez-Boluarte, S.S.; Bonnet, G.; Bustos, J.A.; O’Neal, S.; Garcia, H.H., on behalf of The Cysticercosis Working Group in Peru. Clinical Characteristics of Neurocysticercosis in a Peruvian Population-Based Epilepsy Cohort: A Descriptive Cross-Sectional Study of Baseline Clinical Intake. Pathogens 2023, 12, 1313. https://doi.org/10.3390/pathogens12111313
Allen SE, Moyano LM, Wardle MT, Guzman C, Sanchez-Boluarte SS, Bonnet G, Bustos JA, O’Neal S, Garcia HH on behalf of The Cysticercosis Working Group in Peru. Clinical Characteristics of Neurocysticercosis in a Peruvian Population-Based Epilepsy Cohort: A Descriptive Cross-Sectional Study of Baseline Clinical Intake. Pathogens. 2023; 12(11):1313. https://doi.org/10.3390/pathogens12111313
Chicago/Turabian StyleAllen, Samantha E., Luz M. Moyano, Melissa T. Wardle, Carolina Guzman, Sofia S. Sanchez-Boluarte, Gabrielle Bonnet, Javier A. Bustos, Seth O’Neal, and Hector H. Garcia on behalf of The Cysticercosis Working Group in Peru. 2023. "Clinical Characteristics of Neurocysticercosis in a Peruvian Population-Based Epilepsy Cohort: A Descriptive Cross-Sectional Study of Baseline Clinical Intake" Pathogens 12, no. 11: 1313. https://doi.org/10.3390/pathogens12111313
APA StyleAllen, S. E., Moyano, L. M., Wardle, M. T., Guzman, C., Sanchez-Boluarte, S. S., Bonnet, G., Bustos, J. A., O’Neal, S., & Garcia, H. H., on behalf of The Cysticercosis Working Group in Peru. (2023). Clinical Characteristics of Neurocysticercosis in a Peruvian Population-Based Epilepsy Cohort: A Descriptive Cross-Sectional Study of Baseline Clinical Intake. Pathogens, 12(11), 1313. https://doi.org/10.3390/pathogens12111313






