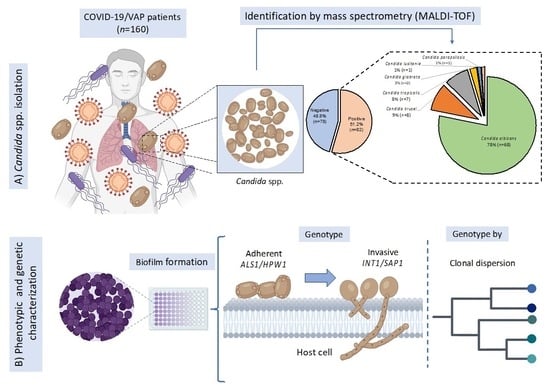
Typing of Candida spp. from Colonized COVID-19 Patients Reveal Virulent Genetic Backgrounds and Clonal Dispersion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Population of COVID-19 Patients
2.3. Intentional Isolation of Yeasts from COVID-19 Patients
2.4. Fungal Identification by MALDI-TOF Mass Spectrometry
2.5. Adherent Phenotype of Candida spp. Strains
2.6. DNA Extraction of Candida spp. Strains
2.7. Detection of Virulence Genes of Adherence and Invasion in Candida spp. Strains
2.8. Search for Clones through Molecular Typing of Candida spp. by T3B Method
3. Results
3.1. Description of Population of COVID-19 Patients
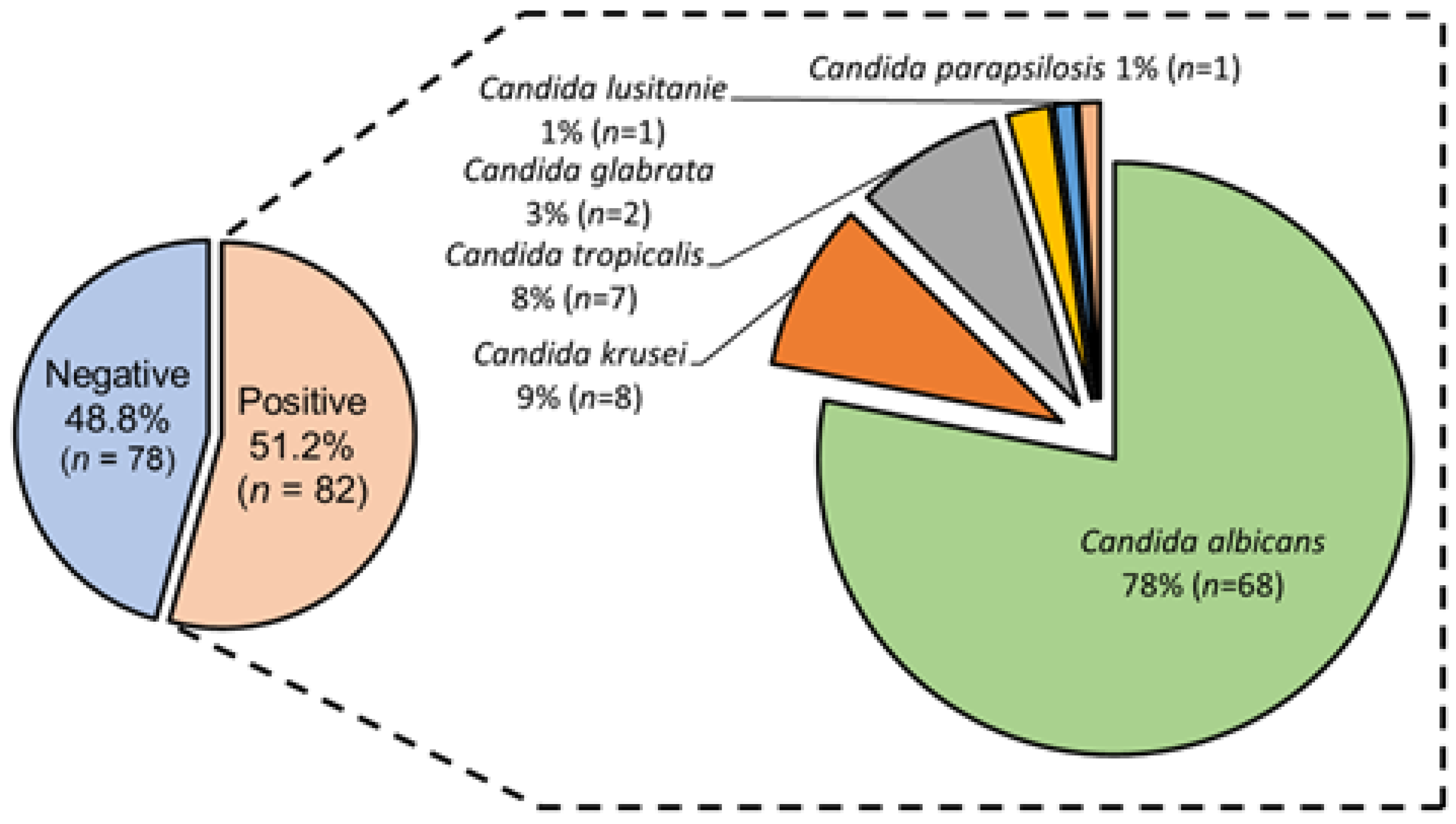
3.2. Candida spp. Isolation from Colonized COVID-19 Patients
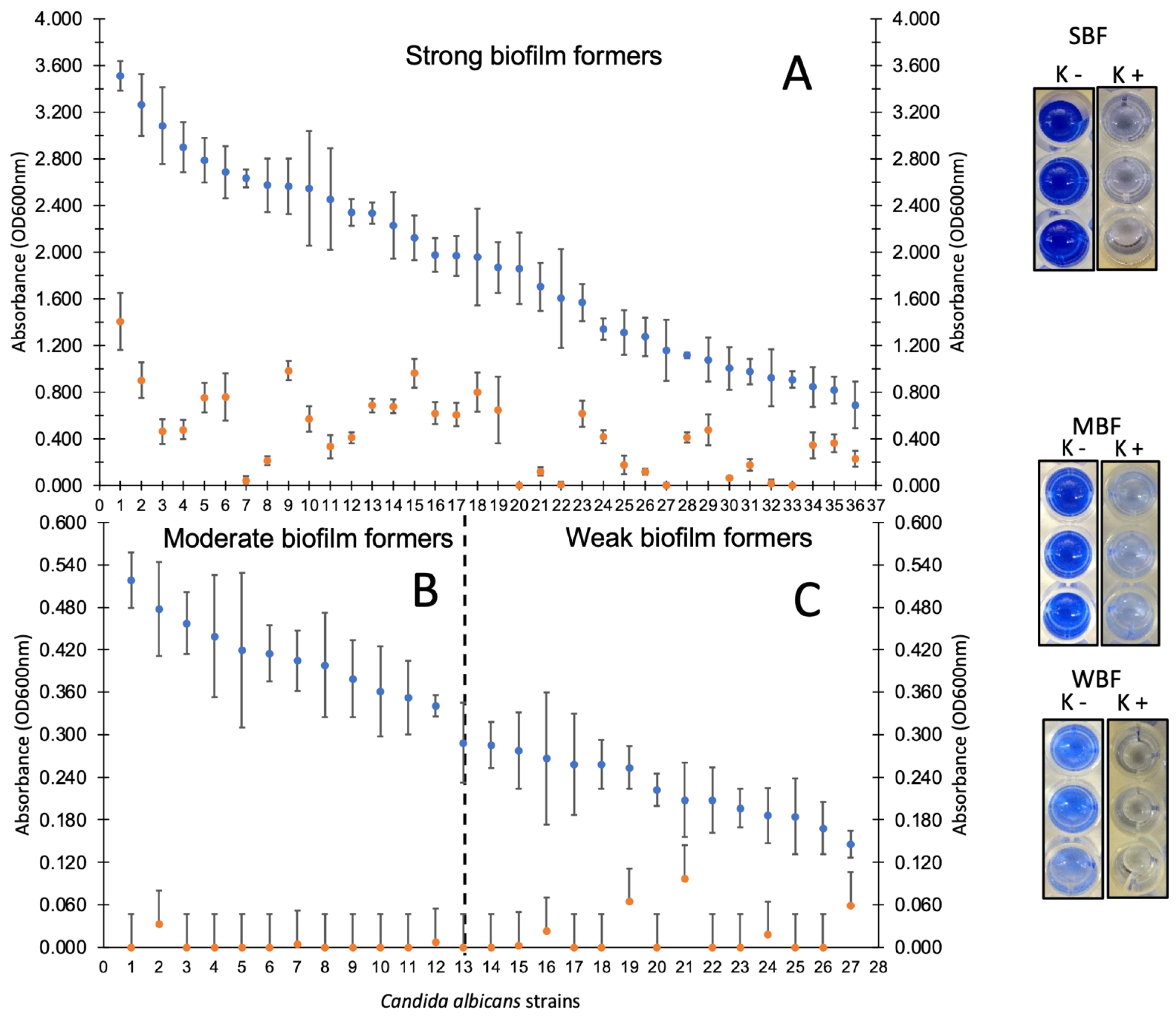
3.3. Candida spp. Strains Showed High Adherence Capacity on Polystyrene
3.4. Disruption of Mature Biofilms of Candida spp. Show a Possible Virulent Genotype
3.5. High Frequency of Candida spp. with Virulent Genetic Background
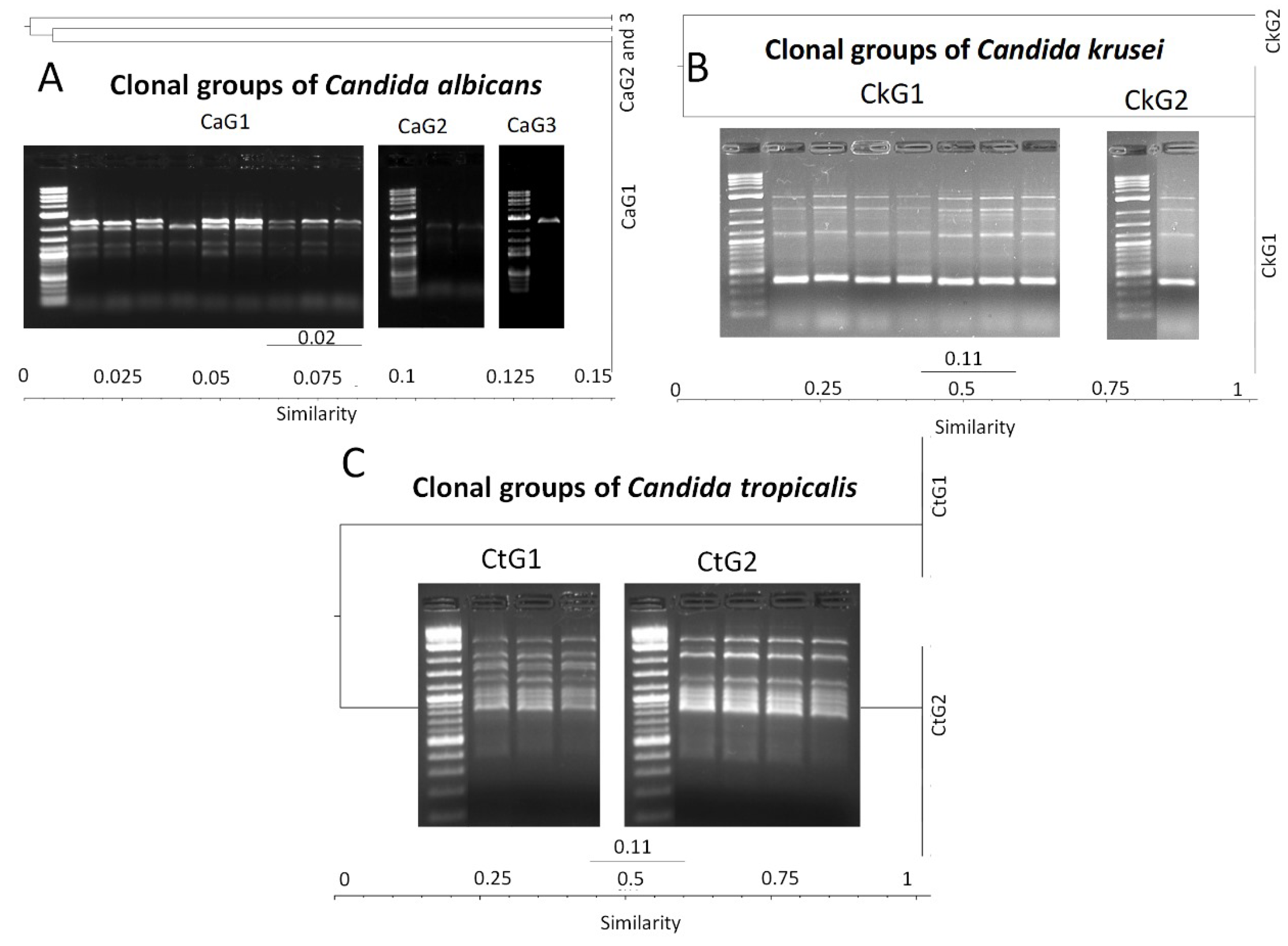
3.6. Molecular Typing of Candida spp. by T3B Method
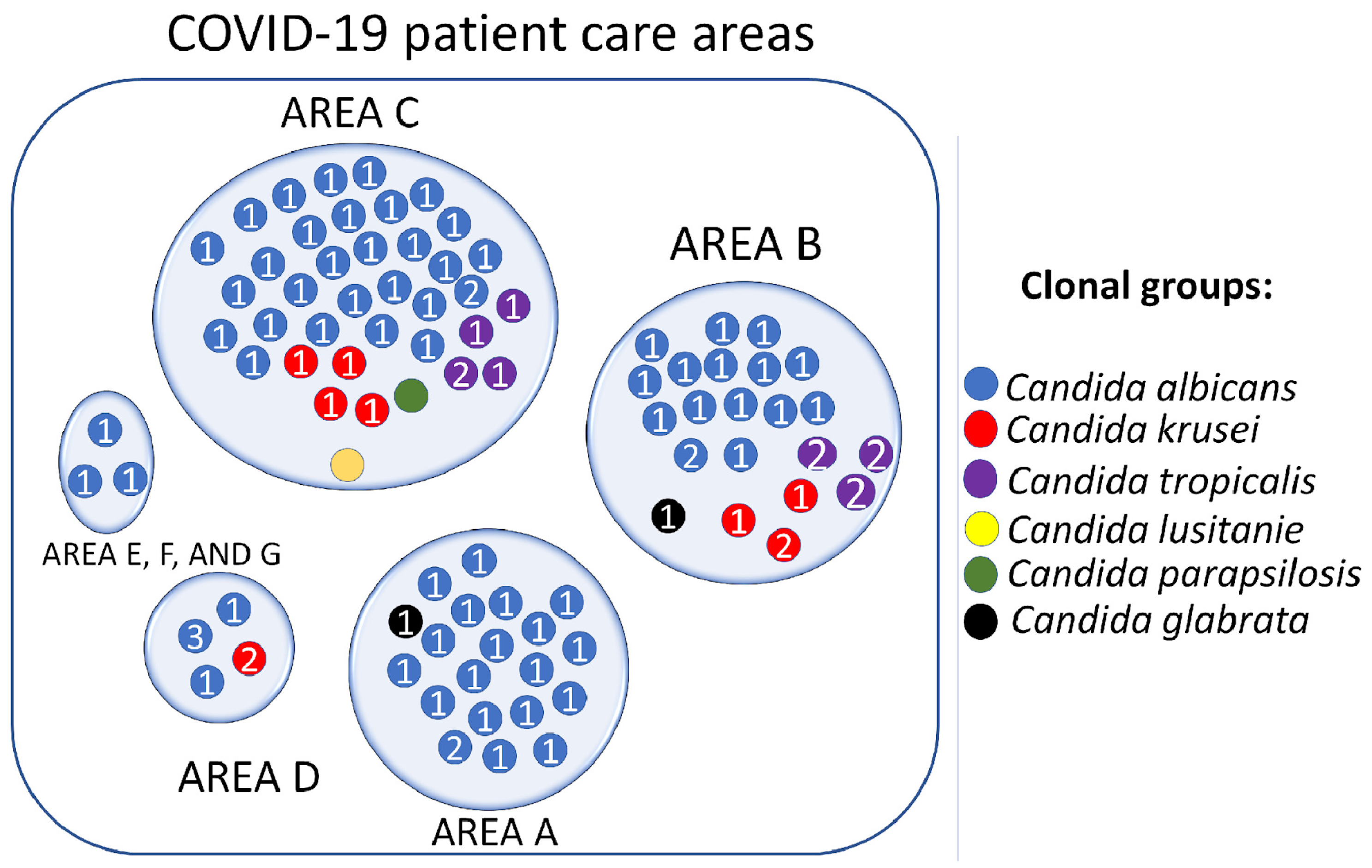
3.7. Candida spp. Clonal Dispersion Investigation in COVID-19 Areas
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baker, M.A.; Sands, K.E.; Huang, S.S.; Kleinman, K.; Septimus, E.J.; Varma, N.; Blanchard, J.; Poland, R.E.; Coady, M.H.; Yokoe, D.S.; et al. The Impact of Coronavirus Disease 2019 (COVID-19) on Healthcare-Associated Infections. Clin. Infect. Dis. 2022, 74, 1748–1754. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Olivadese, V.; Trecarichi, E.M.; Torti, C. Bacterial Ventilator-Associated Pneumonia in COVID-19 Patients: Data from the Second and Third Waves of the Pandemic. J. Clin. Med. 2022, 11, 2279. [Google Scholar] [CrossRef] [PubMed]
- Velásquez-Garcia, L.; Mejia-Sanjuanelo, A.; Viasus, D.; Carratalà, J. Causative Agents of Ventilator-Associated Pneumonia and Resistance to Antibiotics in COVID-19 Patients: A Systematic Review. Biomedicines 2022, 10, 1226. [Google Scholar] [CrossRef]
- Gaspari, R.; Spinazzola, G.; Teofili, L.; Avolio, A.W.; Fiori, B.; Maresca, G.M.; Spanu, T.; Nicolotti, N.; De Pascale, G.; Antonelli, M. Protective effect of SARS-CoV-2 preventive measures against ESKAPE and Escherichia coli infections. Eur. J. Clin. Investig. 2021, 12, e13687. [Google Scholar] [CrossRef]
- Loyola-Cruz, M.Á.; Durán-Manuel, E.M.; Cruz-Cruz, C.; Márquez-Valdelamar, L.M.; Bravata-Alcántara, J.C.; Cortés-Ortíz, I.A.; Cureño-Díaz, M.A.; Ibáñez-Cervantes, G.; Fernández-Sánchez, V.; Castro-Escarpulli, G.; et al. ESKAPE bacteria characterization reveals the presence of Acinetobacter baumannii and Pseudomonas aeruginosa outbreaks in COVID-19/VAP patients. Am. J. Infect. Control 2023, 22, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Meawed, T.E.; Ahmed, S.M.; Mowafy, S.M.S.; Samir, G.M.; Anis, R.H. Bacterial and fungal ventilator associated pneumonia in critically ill COVID-19 patients during the second wave. J. Infect. Public Health 2021, 14, 1375–1380. [Google Scholar] [CrossRef]
- Gatto, I.; Biagioni, E.; Coloretti, I.; Farinelli, C.; Avoni, C.; Caciagli, V.; Busani, S.; Sarti, M.; Pecorari, M.; Gennari, W.; et al. Modena COVID-19 Working Group. Cytomegalovirus blood reactivation in COVID-19 critically ill patients: Risk factors and impact on mortality. Intensive Care Med. 2022, 48, 706–713. [Google Scholar] [CrossRef]
- Wolday, D.; Gebrecherkos, T.; Arefaine, Z.G.; Kiros, Y.K.; Gebreegzabher, A.; Tasew, G.; Abdulkader, M.; Abraha, H.E.; Desta, A.A.; Hailu, A.; et al. Effect of co-infection with intestinal parasites on COVID-19 severity: A prospective observational cohort study. eClinicalMedicine 2021, 39, 101054. [Google Scholar] [CrossRef]
- Koehler, P.; von Stillfried, S.; Garcia Borrega, J.; Fuchs, F.; Salmanton-García, J.; Pult, F.; Böll, B.; Eichenauer, D.A.; Shimabukuro-Vornhagen, A.; Kurzai, O.; et al. Aspergillus tracheobronchitis in COVID-19 patients with acute respiratory distress syndrome: A cohort study. Eur. Respir. J. 2022, 59, 2103142. [Google Scholar] [CrossRef]
- Vetrugno, L.; Anzellotti, G.M.; Frontera, R.; Parinisi, Z.; Sessa, B.; Deana, C.; Maggiore, S.M. Severe Recurrent COVID-Associated Pulmonary Aspergillosis: A Challenging Case. Healthcare 2022, 10, 2483. [Google Scholar] [CrossRef]
- Hoenigl, M.; Seidel, D.; Carvalho, A.; Rudramurthy, S.M.; Arastehfar, A.; Gangneux, J.P.; Nasir, N.; Bonifaz, A.; Araiza, J.; Klimko, N.; et al. The emergence of COVID-19 associated mucormycosis: A review of cases from 18 countries. Lancet Microbe 2022, 3, e543–e552. [Google Scholar] [CrossRef] [PubMed]
- Al-Tawfiq, J.A.; Alhumaid, S.; Alshukairi, A.N.; Temsah, M.H.; Barry, M.; Al Mutair, A.; Rabaan, A.A.; Al-Omari, A.; Tirupathi, R.; AlQahtani, M.; et al. COVID-19 and mucormycosis superinfection: The perfect storm. Infection 2021, 49, 833–853. [Google Scholar] [CrossRef]
- Ahmed, N.; Mahmood, M.S.; Ullah, M.A.; Araf, Y.; Rahaman, T.I.; Moin, A.T.; Hosen, M.J. COVID-19-Associated Candidiasis: Possible Patho-Mechanism, Predisposing Factors, and Prevention Strategies. Curr. Microbiol. 2022, 79, 127. [Google Scholar] [CrossRef]
- Benedetti, M.F.; de Abreu, M.S.; Cadena, R.C.; Arias, M.C.; Posse, G.; Capece, P.; Nusblat, A.; Cuestas, M.L. Invasive pulmonary aspergillosis and candidiasis in a critically ill patient with COVID-19. J. Mycol. Med. 2022, 32, 101251. [Google Scholar] [CrossRef]
- Erami, M.; Raiesi, O.; Momen-Heravi, M.; Getso, M.I.; Fakhrehi, M.; Mehri, N.; Yarahmadi, M.; Amiri, S.; Raissi, V.; Hashemi, S.J. Clinical impact of Candida respiratory tract colonization and acute lung infections in critically ill patients with COVID-19 pneumonia. Microb. Pathog. 2022, 166, 105520. [Google Scholar] [CrossRef]
- Avkan-Oğuz, V.; Çelİk, M.; Eren-Kutsoylu, O.Ö.; Nazli, A.; Uğur, Y.L.; Taylan, A.; Ergan, B.; Irmak, Ç.; Duğral, E.; Özkütük, A.A. Fungal colonization and infections in patients with COVID-19 in intensive care units: A real-life experience at a tertiary-care hospital. Respir. Med. Res. 2022, 82, 100937. [Google Scholar] [CrossRef]
- Prestel, C.; Anderson, E.; Forsberg, K.; Lyman, M.; de Perio, M.A.; Kuhar, D.; Edwards, K.; Rivera, M.; Shugart, A.; Walters, M.; et al. Candida auris Outbreak in a COVID-19 Specialty Care Unit-Florida, July-August 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 56–57. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Lozano, H.; Treviño-Rangel, R.J.; González, G.M.; Ramírez-Elizondo, M.T.; Lara-Medrano, R.; Aleman-Bocanegra, M.C.; Guajardo-Lara, C.E.; Gaona-Chávez, N.; Castilleja-Leal, F.; Torre-Amione, G.; et al. Outbreak of Candida auris infection in a COVID-19 hospital in Mexico. Clin. Microbiol. Infect. 2021, 27, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.S.; Lee, S.S.; Chen, W.C.; Tseng, C.H.; Lee, N.Y.; Chen, P.L.; Li, M.C.; Syue, L.S.; Lo, C.L.; Ko, W.C.; et al. COVID-19-associated candidiasis and the emerging concern of Candida auris infections. J. Microbiol. Immunol. Infect. 2022, 14, 672–679. [Google Scholar] [CrossRef]
- Durán-Manuel, E.M.; Cruz-Cruz, C.; Ibáñez-Cervantes, G.; Bravata-Alcantará, J.C.; Sosa-Hernández, O.; Delgado-Balbuena, L.; León-García, G.; Cortés-Ortíz, I.A.; Cureño-Díaz, M.A.; Castro-Escarpulli, G.; et al. Clonal dispersion of Acinetobacter baumannii in an intensive care unit designed to patients COVID-19. J. Infect. Dev. Ctries. 2021, 15, 58–68. [Google Scholar] [CrossRef]
- Bandara, H.M.H.N.; Samaranayake, L.P. Emerging strategies for environmental decontamination of the nosocomial fungal pathogen Candida auris. J. Med. Microbiol. 2022, 71, 001548. [Google Scholar] [CrossRef] [PubMed]
- Moser, D.; Biere, K.; Han, B.; Hoerl, M.; Schelling, G.; Choukér, A.; Woehrle, T. COVID-19 Impairs Immune Response to Candida albicans. Front. Immunol. 2021, 12, 640644. [Google Scholar] [CrossRef] [PubMed]
- Johanson, W.G.; Pierce, A.K.; Sanford, J.P.; Thomas, G.D. Nosocomial respiratory infections with gram-negative bacilli. The significance of colonization of the respiratory tract. Ann. Intern. Med. 1972, 77, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Sharma, S.; Parwez; Saxena, S. Changing panorama for surveillance of device-associated healthcare infections: Challenges faced in implementation of current guidelines. Indian J. Med. Microbiol. 2018, 36, 18–25. [Google Scholar] [CrossRef]
- Mehta, A.; Bhagat, R. Preventing Ventilator-Associated Infections. Clin. Chest Med. 2016, 37, 683–692. [Google Scholar] [CrossRef]
- Nickerson, W.J. Reduction of inorganic substances by yeasts. I. Extracellular reduction of sulfite by species of Candida. J. Infect. Dis. 1953, 93, 43–56. [Google Scholar] [CrossRef]
- Stepanović, S.; Vuković, D.; Hola, V.; Di Bonaventura, G.; Djukić, S.; Cirković, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef]
- Karygianni, L.; Paqué, P.N.; Attin, T.; Thurnheer, T. Single DNase or Proteinase Treatment Induces Change in Composition and Structural Integrity of Multispecies Oral Biofilms. Antibiotics 2021, 10, 400. [Google Scholar] [CrossRef]
- Hernandez-Cuellar, E.; Guerrero-Barrera, A.L.; Avelar-Gonzalez, F.J.; Díaz, J.M.; Santiago, A.S.; Chávez-Reyes, J.; Poblano-Sánchez, E. Characterization of Candida albicans and Staphylococcus aureus polymicrobial biofilm on different surfaces. Rev. Iberoam. Micol. 2022, 39, 36–43. [Google Scholar] [CrossRef]
- Dikmen, N.; Duran, N.; Ay, E.; Cimen, F.; Tek, E. Genotyping, drug resistance and virulence factors of Candida species isolated from patients using long-term inhaled steroids. Int. J. Clin. Pract. 2021, 75, e14820. [Google Scholar] [CrossRef]
- Lim, Y.H.; Lee, D.H. Multiplex Polymerase Chain Reaction Assay for Simultaneous Detection of Candida albicans and Candida dublinensis. J. Microbiol. 2002, 40, 146–150. [Google Scholar]
- Inci, M.; Atalay, M.A.; Özer, B.; Evirgen, O.; Duran, N.; Motor, V.K.; Koç, A.N.; Önlen, Y.; Kilinç, Ç.; Durmaz, S. Investigations of ALS1 and HWP1 genes in clinical isolates of Candida Albicans. Turk. J. Med. Sci. 2013, 43, 125–130. [Google Scholar]
- Thanos, M.; Schonian, G.; Meyer, W.; Schweynoch, C.; Graser, Y.; Mitchell, T.G.; Presber, W.; Tietz, H.J. Rapid identification of Candida species by DNA fingerprinting with PCR. J. Clin. Microbiol. 1996, 34, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Tenover, F.C.; Arbeit, R.D.; Goering, R.V.; Mickelsen, P.A.; Murray, B.E.; Persing, D.H.; Swaminathan, B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: Criteria for bacterial strain typing. J. Clin. Microbiol. 1995, 33, 2233–2239. [Google Scholar] [CrossRef] [PubMed]
- Prokop, M.; van Everdinge, W.; van Rees Vellinga, T.; Quarles van Ufford, H.; Stöger, L.; Beenen, L.; Geurts, B.; Gietema, H.; Krdzalic, J.; Schaefer-Prokop, C.; et al. COVID-19 Standardized Reporting Working Group of the Dutch Radiological Society. CO-RADS: A Categorical CT Assessment Scheme for Patients Suspected of Having COVID-19-Definition and Evaluation. Radiology 2020, 296, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Lee, C.M.; Chang, E.; Kang, C.K.; Park, W.B.; Kim, N.J.; Choe, P.G.; Oh, M.D. Clinical Impact of Empirical Antibiotic Therapy in Patients With Coronavirus Disease 2019 Requiring Oxygen Therapy. J. Korean Med. Sci. 2022, 25, e238. [Google Scholar] [CrossRef]
- Bienvenu, A.L.; Bestion, A.; Pradat, P.; Richard, J.C.; Argaud, l.; Guichon, C.; Roux, S.; Piriou, V.; Paillet, C.; Leboucher, G.; et al. Impact of COVID-19 pandemic on antifungal consumption: A multicenter retrospective analysis. Crit. Care 2022, 13, 384. [Google Scholar] [CrossRef]
- Riche, C.V.W.; Cassol, R.; Pasqualotto, A.C. Is the Frequency of Candidemia Increasing in COVID-19 Patients Receiving Corticosteroids? J. Fungi 2020, 6, 286. [Google Scholar] [CrossRef]
- Hoenigl, M.; Seidel, D.; Sprute, R.; Cunha, C.; Oliverio, M.; Goldman, G.H.; Ibrahim, A.S.; Carvalho, A. COVID-19-associated fungal infections. Nat. Microbiol. 2022, 7, 1127–1140. [Google Scholar] [CrossRef]
- Seyedjavadi, S.S.; Bagheri, P.; Nasiri, M.J.; Razzaghi-Abyaneh, M.; Goudarzi, M. Fungal Infection in Co-infected Patients With COVID-19: An Overview of Case Reports/Case Series and Systematic Review. Front. Microbiol. 2022, 13, 888452. [Google Scholar] [CrossRef]
- Shirvani, F.; Fattahi, A. Pulmonary Candidiasis Associated with COVID-19: Evaluation of Causative Agents and their Antifungal Susceptibility Patterns. Tanaffos 2021, 20, 29–35. [Google Scholar] [PubMed]
- Nucci, M.; Barreiros, G.; Guimarães, L.F.; Deriquehem, V.A.S.; Castiñeiras, A.C.; Nouér, S.A. Increased incidence of candidemia in a tertiary care hospital with the COVID-19 pandemic. Mycoses 2021, 64, 152–156. [Google Scholar] [CrossRef]
- Babamahmoodi, F.; Rezai, M.S.; Ahangarkani, F.; Mohammadi, K.A.; Alizadeh-Navaei, R.; Alishahi, A.; Najafi, N.; Haddadi, A.; Davoudi, A.; Azargon, L.; et al. Multiple Candida strains causing oral infection in COVID-19 patients under orticosteroids and antibiotic therapy: An observational study. Front. Cell. Infect. Microbiol. 2022, 12, 1103226. [Google Scholar] [CrossRef] [PubMed]
- Salehi, M.; Ahmadikia, K.; Mahmoudi, S.; Kalantari, S.; Jamalimoghadamsiahkali, S.; Izadi, A.; Kord, M.; Dehghan Manshadi, S.A.; Seifi, A.; Ghiasvand, F.; et al. Oropharyngeal candidiasis in hospitalised COVID-19 patients from Iran: Species identification and antifungal susceptibility pattern. Mycoses 2020, 63, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Carvalhaes, C.G.; DeVries, S.; Rhomberg, P.R.; Castanheira, M. Impact of COVID-19 on the antifungal susceptibility profiles of isolates collected in a global surveillance program that monitors invasive fungal infections. Med. Mycol. 2022, 60, myac028. [Google Scholar] [CrossRef] [PubMed]
- Jayant, S.; Patel, K.; Priya, P.; Verma, A.N.; Singh, B.; Dahariya, R. Prevalence of Candida infection in Covid-19 pandemic: A study from a tertiary care center in Central India. Asian J. Med. Sci. 2021, 12, 3–7. [Google Scholar] [CrossRef]
- Davari, A.; Jafarzadeh, J.; Hedayati, M.T.; Shokohi, T.; Abastabar, M.; Nikmanesh, B.; Moazeni, M. High frequency of Candida krusei colonization in critically ill pediatrics: A cross-sectional study in children’s medical center, Tehran, Iran. Curr. Med. Mycol. 2022, 8, 25–31. [Google Scholar] [CrossRef]
- Allaw, F.; Kara Zahreddine, N.; Ibrahim, A.; Tannous, J.; Taleb, H.; Bizri, A.R.; Dbaibo, G.; Kanj, S.S. First Candida auris Outbreak during a COVID-19 Pandemic in a Tertiary-Care Center in Lebanon. Pathogens 2021, 10, 157. [Google Scholar] [CrossRef]
- Sathyapalan, D.T.; Antony, R.; Nampoothiri, V.; Kumar, A.; Shashindran, N.; James, J.; Thomas, J.; Prasanna, P.; Sudhir, A.S.; Philip, J.M.; et al. Evaluating the measures taken to contain a Candida auris outbreak in a tertiary care hospital in South India: An outbreak investigational study. BMC Infect. Dis. 2021, 21, 425. [Google Scholar] [CrossRef]
- Contreras, D.A.; Morgan, M.A. Surveillance diagnostic algorithm using real-time PCR assay and strain typing method development to assist with the control of C. auris amid COVID-19 pandemic. Front. Cell. Infect. Microbiol. 2022, 12, 887754. [Google Scholar] [CrossRef]
- Gorton, R.L.; Jones, G.L.; Kibbler, C.C.; Collier, S. Candida nivariensis isolated from a renal transplant patient with persistent candiduria-Molecular identification using ITS PCR and MALDI-TOF. Med. Mycol. Case Rep. 2013, 2, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Ceballos-Garzón, A.; Cortes, G.; Morio, F.; Zamora-Cruz, E.L.; Linares, M.Y.; Ariza, B.E.; Valderrama, S.L.; Garzón, J.R.; Alvarez-Moreno, C.A.; Le Pape, P.; et al. Comparison between MALDI-TOF MS and MicroScan in the identification of emerging and multidrug resistant yeasts in a fourth-level hospital in Bogotá, Colombia. BMC Microbiol. 2019, 19, 106. [Google Scholar] [CrossRef]
- Cormack, B.P.; Ghori, N.; Falkow, S. An adhesin of the yeast pathogen Candida glabrata mediating adherence to human epithelial cells. Science 1999, 285, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Moyes, D.L.; Richardson, J.P.; Naglik, J.R. Candida albicans-epithelial interactions and pathogenicity mechanisms: Scratching the surface. Virulence 2015, 6, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Tripathi, A.H.; Gautam, P.; Gahtori, R.; Pande, A.; Singh, Y.; Madan, T.; Upadhyay, S.K. Adhesins in the virulence of opportunistic fungal pathogens of human. Mycology 2021, 12, 296–324. [Google Scholar] [CrossRef]
- Kelly, M.T.; MacCallum, D.M.; Clancy, S.D.; Odds, F.C.; Brown, A.J.; Butler, G. The Candida albicans CaACE2 gene affects morphogenesis, adherence and virulence. Mol. Microbiol. 2004, 53, 969–983. [Google Scholar] [CrossRef]
- Argimón, S.; Wishart, J.A.; Leng, R.; Macaskill, S.; Mavor, A.; Alexandris, T.; Nicholls, S.; Knight, A.W.; Enjalbert, B.; Walmsley, R.; et al. Developmental regulation of an adhesin gene during cellular morphogenesis in the fungal pathogen Candida albicans. Eukaryot. Cell 2007, 6, 682–692. [Google Scholar] [CrossRef]
- Zhu, W.; Filler, S.G. Interactions of Candida albicans with epithelial cells. Cell Microbiol. 2010, 12, 273–282. [Google Scholar] [CrossRef]
- Naglik, J.R.; Moyes, D.L.; Wächtler, B.; Hube, B. Candida albicans interactions with epithelial cells and mucosal immunity. Microbes Infect. 2011, 13, 963–976. [Google Scholar] [CrossRef]
- Monroy-Pérez, E.; Sáinz-Espuñes, T.; Paniagua-Contreras, G.; Negrete-Abascal, E.; Rodríguez-Moctezuma, J.R.; Vaca, S. Frequency and expression of ALS and HWP1 genotypes in Candida albicans strains isolated from Mexican patients suffering from vaginal candidosis. Mycoses 2012, 55, e151–e157. [Google Scholar] [CrossRef]
- Ardehali, S.H.; Azimi, T.; Fallah, F.; Aghamohammadi, N.; Alimehr, S.; Karimi, A.M.; Azimi, L. Molecular detection of ALS1, ALS3, HWP1 and SAP4 genes in Candida Genus isolated from hospitalized patients in Intensive Care Unit, Tehran, Iran. Cell Mol. Biol. 2019, 65, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Kinneberg, K.M.; Bendel, C.M.; Jechorek, R.P.; Cebelinski, E.A.; Gale, C.A.; Berman, J.G.; Erlandsen, S.L.; Hostetter, M.K.; Wells, C.L. Effect of INT1 gene on Candida albicans murine intestinal colonization. J. Surg. Res. 1999, 87, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Arita, G.S.; Faria, D.R.; Capoci, I.R.G.; Kioshima, E.S.; Bonfim-Mendonça, P.S.; Svidzinski, T.I.E. Cell wall associated proteins involved in filamentation with impact on the virulence of Candida albicans. Microbiol. Res. 2022, 258, 126996. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.H.; Damasceno, J.L.; Casemiro, L.A.; Martins, C.H.G.; Pires, R.H.; Candido, R.C. Susceptibility to Oral Antiseptics and Virulence Factors Ex Vivo Associated with Candida spp. Isolated from Dental Prostheses. J. Prosthodont. 2019, 28, 398–408. [Google Scholar] [CrossRef]
- Dassanayake, R.S.; Samaranayake, Y.H.; Yau, J.; Samaranayake, L.P. DNA fingerprinting elicited evolutionary trend of oral Candida tropicalis isolates from diverse geographic locales. Indian J. Med. Microbiol. 2006, 24, 186–194. [Google Scholar] [CrossRef]
- Garzillo, C.; Bagattini, M.; Bogdanović, L.; Di Popolo, A.; Iula, V.D.; Catania, M.R.; Raimondi, F.; Triassi, M.; Zarrilli, R. Risk factors for Candida parapsilosis bloodstream infection in a neonatal intensive care unit: A case-control study. Ital. J. Pediatr. 2017, 43, 10. [Google Scholar] [CrossRef]
- Corzo-Leon, D.E.; Peacock, M.; Rodriguez-Zulueta, P.; Salazar-Tamayo, G.J.; MacCallum, D.M. General hospital outbreak of invasive candidiasis due to azole-resistant Candida parapsilosis associated with an Erg11 Y132F mutation. Med. Mycol. 2021, 59, 664–671. [Google Scholar] [CrossRef]
- Cureño-Díaz, M.A.; Durán-Manuel, E.M.; Cruz-Cruz, C.; Ibáñez-Cervantes, G.; Rojo-Gutiérrez, M.I.; Moncayo-Coello, C.V.; Loyola-Cruz, M.Á.; Castro-Escarpulli, G.; Hernández, D.M.R.; Bello-López, J.M. Impact of the modification of a cleaning and disinfection method of mechanical ventilators of COVID-19 patients and ventilator-associated pneumonia: One year of experience. Am. J. Infect. Control 2021, 49, 1474–1480. [Google Scholar] [CrossRef]
- Durán-Manuel, E.M.; Loyola-Cruz, M.Á.; Cruz-Cruz, C.; Ibáñez-Cervantes, G.; Gaytán-Cervantes, J.; González-Torres, C.; Quiroga-Vargas, E.; Calzada-Mendoza, C.C.; Cureño-Díaz, M.A.; Fernández-Sánchez, V.; et al. Massive sequencing of the V3-V4 hypervariable region of bronchoalveolar lavage from patients with COVID-19 and VAP reveals the collapse of the pulmonary microbiota. J. Med. Microbiol. 2022, 71, 12. [Google Scholar] [CrossRef]
- Xie, L.; Chen, L.; Li, X.; Zhou, J.; Tian, H.; Zhao, J.; Li, Z.; Li, Y. Analysis of Lung Microbiome in COVID-19 Patients during Time of Hospitalization. Pathogens 2023, 12, 944. [Google Scholar] [CrossRef]
| Gene | Target | Sequence (5′→3′) | Size Amplicon (bp) | Reference |
|---|---|---|---|---|
| ALS1 | Agglutinin-like sequence type 1 | ALS1F: GAC TAG TGA ACC AAC AAA TAC CAG A | 318 | [30] |
| ALS1R: CCA GAA GAA ACA GCA GGT GA | ||||
| INT1 | Integrin-like protein alpha | INT1FP: AAG CTC TGA TAC CTA CAC TAG CGA | 239 | [31] |
| INT1RP: GTT AGG TCT AAA GTC GAA GTC ATC | ||||
| HWP1 | Hyphal wall protein 1 | HWP1F: ATG ACT CCA GCT GGT TC | 572 | [32] |
| HWP1R: TAG ATC AAGAAT GCA GC | ||||
| SAP1 | Secreted aspartyl protease 1 | SAP1F: GCT CTT GCT ATT GCT TTA TTA | 253 | [30] |
| SAP1R: CAT CAG GAA CCC ATA AAT CAG | ||||
| T3B | Intergenic spacer regions | AGG TCG CGG GTT CGA ATC C | Variable | [33] |
| Patient | Gender | Corticosteroid | Associated Comorbidities, n (%) | Other | ||||
|---|---|---|---|---|---|---|---|---|
| Classification | n (%) | Therapy | n (%) | |||||
| n (%) | Diabetes | Hypertension | Renal | Obesity | Cancer | |||
| Male | Female | Insufficiency | ||||||
| Pediatric a | 2 (1.8) | 5 (9.8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 5 (3.6) | 3 (100) |
| Young adults b | 10 (9.2) | 6 (11.8) | 8 (5.8) | 5 (3.9) | 7 (6.1) | 0 (0) | 15 (10.7) | 0 (0) |
| Adults c | 79 (72.5) | 29 (56.9) | 102 (73.4) | 96 (75) | 85 (74.6) | 12 (85.7) | 97 (69.3) | 0 (0) |
| Elderly d | 18 (16.5) | 11 (21.6) | 29 (20.8) | 27 (21.1) | 22 (19.3) | 2 (14.3) | 23 (16.4) | 0 (0) |
| Total | 109 (100) | 51 (100) | 139 (100) | 128 (100) | 114 (100) | 14 (100) | 140 (100) | 3 (100) |
| Species of Candida | Classification of Biofilm Forming, n (%) | |||
|---|---|---|---|---|
| Non-Forming | Weak | Moderate | Strong | |
| C. albicans (n = 68) | 3 (4.4) | 18 (26.5) | 11 (16.2) | 36 (52.9) |
| C. krusei (n = 8) | 0 (0) | 4 (50) | 4 (50) | 0 (0) |
| C. tropicalis (n = 7) | 0 (0) | 0 (0) | 0 (0) | 7 (100) |
| C. glabrata (n = 2) | 1 (50) | 0 (0) | 0 (0) | 1 (50) |
| C. lusitanie (n = 1) | 0 (0) | 0 (0) | 0 (0) | 1 (100) |
| C. parapsilosis (n = 1) | 0 (0) | 0 (0) | 0 (0) | 1 (100) |
| Total (n = 87) | 4 (4.6) | 22 (25.3) | 15 (17.2) | 46 (52.9) |
| Adherent genotype, n (%) | Candida spp. | Invasive Genotype, n (%) | ||
| INT1 | SAP1 | |||
| ALS1 | C. albicans (n = 68) | 68 (100) | 68 (100) | |
| HWP1 | 68 (100) | 68 (100) | ||
| ALS1 | C. krusei (n = 8) | 6 (75) | 0 (0) | |
| HWP1 | 4 (50) | 0 (0) | ||
| ALS1 | C. tropicalis (n = 7) | 1 (14.2) | 1 (14.2) | |
| HWP1 | 1 (14.2) | 1 (14.2) | ||
| ALS1 | C. glabrata (n = 2) | 2 (100) | 0 (0) | |
| HWP1 | 0 (0) | 0 (0) | ||
| ALS1 | C. lusitanie (n = 1) | 0 (0) | 0 (0) | |
| HWP1 | 0 (0) | 0 (0) | ||
| ALS1 | C. parapsilosis (n = 1) | 0 (0) | 0 (0) | |
| HWP1 | 1 (100) | 0 (0) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quiroga-Vargas, E.; Loyola-Cruz, M.Á.; Rojas-Bernabé, A.; Moreno-Eutimio, M.A.; Pastelin-Palacios, R.; Cruz-Cruz, C.; Durán-Manuel, E.M.; Calzada-Mendoza, C.; Castro-Escarpulli, G.; Hernández-Hernández, G.; et al. Typing of Candida spp. from Colonized COVID-19 Patients Reveal Virulent Genetic Backgrounds and Clonal Dispersion. Pathogens 2023, 12, 1206. https://doi.org/10.3390/pathogens12101206
Quiroga-Vargas E, Loyola-Cruz MÁ, Rojas-Bernabé A, Moreno-Eutimio MA, Pastelin-Palacios R, Cruz-Cruz C, Durán-Manuel EM, Calzada-Mendoza C, Castro-Escarpulli G, Hernández-Hernández G, et al. Typing of Candida spp. from Colonized COVID-19 Patients Reveal Virulent Genetic Backgrounds and Clonal Dispersion. Pathogens. 2023; 12(10):1206. https://doi.org/10.3390/pathogens12101206
Chicago/Turabian StyleQuiroga-Vargas, Edith, Miguel Ángel Loyola-Cruz, Araceli Rojas-Bernabé, Mario Adán Moreno-Eutimio, Rodolfo Pastelin-Palacios, Clemente Cruz-Cruz, Emilio Mariano Durán-Manuel, Claudia Calzada-Mendoza, Graciela Castro-Escarpulli, Geovanni Hernández-Hernández, and et al. 2023. "Typing of Candida spp. from Colonized COVID-19 Patients Reveal Virulent Genetic Backgrounds and Clonal Dispersion" Pathogens 12, no. 10: 1206. https://doi.org/10.3390/pathogens12101206
APA StyleQuiroga-Vargas, E., Loyola-Cruz, M. Á., Rojas-Bernabé, A., Moreno-Eutimio, M. A., Pastelin-Palacios, R., Cruz-Cruz, C., Durán-Manuel, E. M., Calzada-Mendoza, C., Castro-Escarpulli, G., Hernández-Hernández, G., Cureño-Díaz, M. A., Fernández-Sánchez, V., & Bello-López, J. M. (2023). Typing of Candida spp. from Colonized COVID-19 Patients Reveal Virulent Genetic Backgrounds and Clonal Dispersion. Pathogens, 12(10), 1206. https://doi.org/10.3390/pathogens12101206