Seroepidemiological Study of Spotted Fever Group Rickettsiae and Identification of a Putative New Species, Rickesttsia sp. Da-1, in Gongliao, Northeast Taiwan
Abstract
1. Introduction
2. Results
2.1. Demographics of the Participants
2.2. Serology
2.3. Potential Risk Factors for SFG Rickettsiae Exposure
2.4. Collection of Ticks
2.5. Molecular Findings in Ticks
3. Discussion
4. Materials and Methods
4.1. Study Setting and Human Subject
4.2. Serology
4.2.1. Screening of Sera by ELISA for SFG Rickettsiae Exposure
4.2.2. Detection of IgG against SFG Rickettsiae, TG Rickettsiae, and O. tsutsugamushi by IFA
4.3. Collection of Ticks and Tick Species Identification
4.4. Detection of Potential Tick-Borne Pathogens
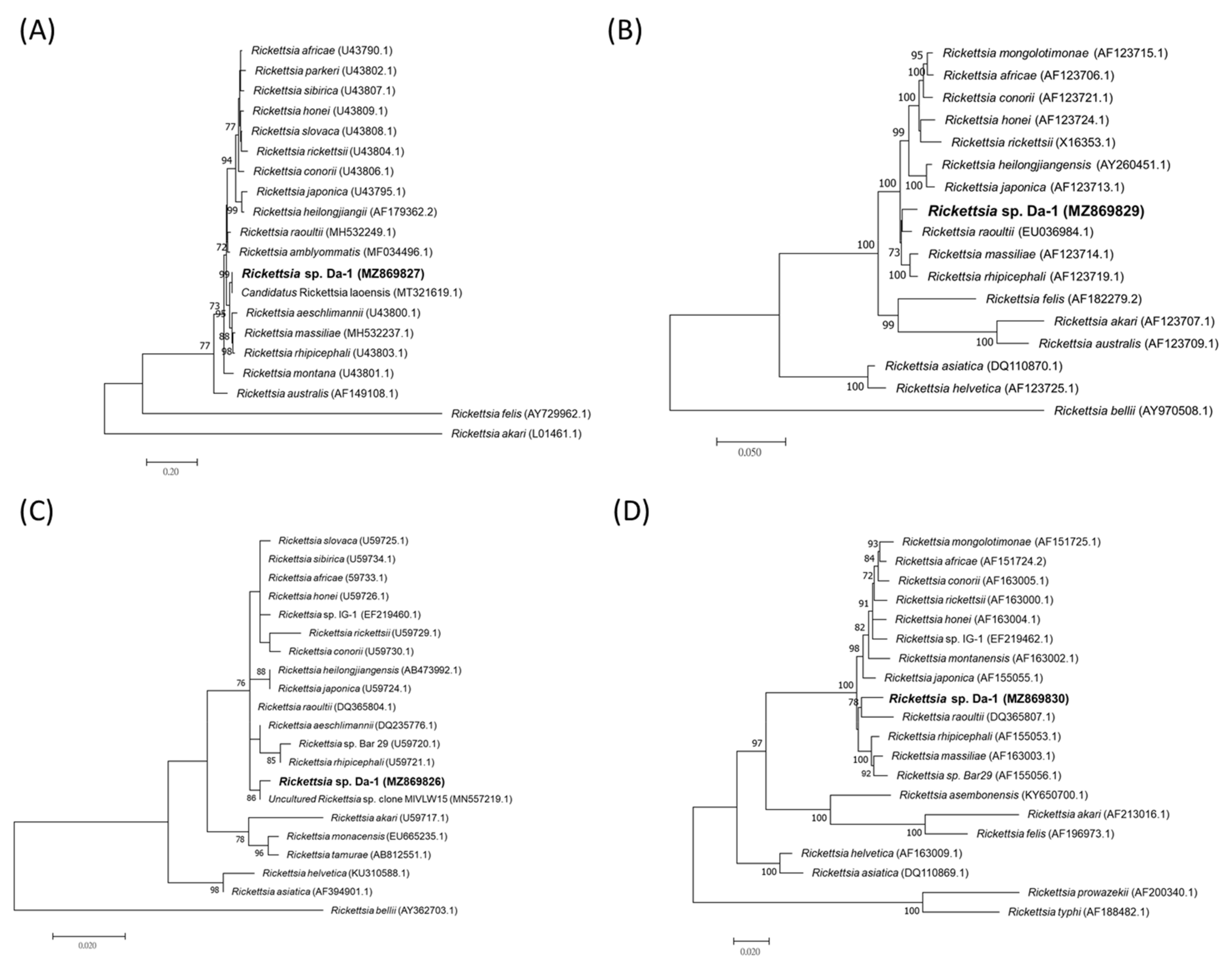
4.5. Phylogenetic Analysis
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walker, D. Rickettsiae. In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Brezina, R.; Murray, E.; Tarizzo, M.; Bögel, K. Rickettsiae and rickettsial diseases. Bull. World Health Organ. 1973, 49, 433–442. [Google Scholar] [PubMed]
- Tamura, A.; Ohashi, N.; Urakami, H.; Miyamura, S. Classification of Rickettsia tsutsugamushi in a new genus, Orientia gen. nov., as Orientia tsutsugamushi comb. nov. Int. J. Syst. Bacteriol. 1995, 45, 589–591. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, J.B.; Beier, M.S.; Rahman, M.; Ammerman, N.; Shallom, J.; Purkayastha, A.; Sobral, B.; Azad, A. Plasmids and rickettsial evolution: Insight from Rickettsia felis. PLoS ONE 2007, 2, e266. [Google Scholar] [CrossRef] [PubMed]
- Sekeyová, Z.; Danchenko, M.; Filipčík, P.; Fournier, P. Rickettsial infections of the central nervous system. PLoS Negl. Trop. Dis. 2019, 13, e0007469. [Google Scholar] [CrossRef]
- Taiwan Centers for Disease Control. Taiwan National Infectious Disease Statistics System. Available online: https://nidss.cdc.gov.tw/Home/Index (accessed on 20 August 2021).
- Tsai, K.; Chung, L.; Chien, C.; Tung, Y.; Wei, H.; Yen, T.; Shu, P.; Wang, H. Human granulocytic anaplasmosis in Kinmen, an offshore island of Taiwan. PLoS Negl. Trop. Dis. 2019, 13, e0007728. [Google Scholar] [CrossRef]
- Yen, T.; Tung, Y.; Wang, H.; Tsai, K. Detection of Ehrlichia chaffeensis in a febrile patient in Kinmen, an offshore island of Taiwan. J. Formos Med. Assoc. 2020, 119, 1329–1330. [Google Scholar] [CrossRef]
- Peng, S.; Yang, S.; Ho, Y.; Chen, H.; Shu, P. Human case of Ehrlichia chaffeensis infection, Taiwan. Emerg. Infect. Dis. 2019, 25, 2141–2143. [Google Scholar] [CrossRef]
- Tsai, K.; Lu, H.; Tsai, J.; Yu, S.; Huang, J.; Shu, P. Human case of Rickettsia felis infection, Taiwan. Emerg. Infect. Dis. 2008, 14, 1970–1972. [Google Scholar] [CrossRef]
- Lai, C.; Chang, L.; Lin, J.; Tsai, K.; Hung, Y.; Kuo, L.; Lin, H.; Chen, Y. Human spotted fever group rickettsioses are underappreciated in southern Taiwan, particularly for the species closely-related to Rickettsia felis. PLoS ONE 2014, 9, e95810. [Google Scholar] [CrossRef]
- Yang, W.; Hsu, M.; Shu, P.; Tsai, K.; Fang, C. Neglected human Rickettsia felis infection in Taiwan: A retrospective seroepidemiological survey of patients with suspected rickettsioses. PLoS Negl. Trop. Dis. 2021, 15, e0009355. [Google Scholar] [CrossRef]
- Tsai, K.; Lu, H.; Huang, J.; Fournier, P.; Mediannikov, O.; Raoult, D.; Shu, P. African tick bite fever in a Taiwanese traveler returning from South Africa: Molecular and serologic studies. Am. J. Trop. Med. Hyg. 2009, 81, 735–739. [Google Scholar] [CrossRef]
- Kuo, C.; Shu, P.; Mu, J.; Lee, P.; Wu, Y.; Chung, C.; Wang, H. Widespread Rickettsia spp. Infections in Ticks (Acari: Ixodoidea) in Taiwan. J. Med. Entomol. 2015, 52, 1096–1102. [Google Scholar] [CrossRef]
- Kuo, C.; Huang, J.; Lin, T.; Wang, H. Detection of Rickettsia spp. and host and habitat associations of fleas (Siphonaptera) in eastern Taiwan. Med. Vet. Entomol. 2012, 26, 341–350. [Google Scholar] [CrossRef]
- Kuo, C.; Lin, Y.; Yao, C.; Shih, H.; Chung, L.; Liao, H.; Hsu, Y.; Wang, H. Tick-borne pathogens in ticks collected from birds in Taiwan. Parasit Vectors 2017, 10, 587. [Google Scholar] [CrossRef]
- Shih, C.; Yang, P.; Chao, L. Molecular Detection and genetic identification of Rickettsia infection in Ixodes granulatus ticks, an incriminated vector for geographical transmission in Taiwan. Microorganisms 2021, 9, 1309. [Google Scholar] [CrossRef]
- Kuo, C.; Shu, P.; Mu, J.; Wang, H. High prevalence of Rickettsia spp. infections in small mammals in Taiwan. Vector Borne Zoonotic Dis. 2015, 15, 13–20. [Google Scholar] [CrossRef]
- Kuo, C.; Huang, C.; Wang, H. Identification of potential hosts and vectors of scrub typhus and tick-borne spotted fever group rickettsiae in eastern Taiwan. Med. Vet. Entomol. 2011, 25, 169–177. [Google Scholar] [CrossRef]
- Hsu, Y.; Lin, C.; Chome, l.B.; Tsai, K.; Wu, W.; Huang, C.; Chang, C. Identification of Rickettsia felis in fleas but not ticks on stray cats and dogs and the evidence of Rickettsia rhipicephali only in adult stage of Rhipicephalus sanguineus and Rhipicephalus haemaphysaloides. Comp. Immunol. Microbiol. Infect. Dis. 2011, 34, 513–518. [Google Scholar] [CrossRef]
- Tsui, P.; Tsai, K.; Weng, M.; Hung, Y.; Liu, Y.; Hu, K.; Lien, J.; Lin, P.; Shaio, M.; Wang, H.; et al. Molecular detection and characterization of spotted fever group rickettsiae in Taiwan. Am. J. Trop. Med. Hyg. 2007, 77, 883–890. [Google Scholar] [CrossRef]
- Tsai, K.; Wang, H.; Chen, C.; Huang, J.; Lu, H.; Su, C.; Shu, P. Isolation and identification of a novel spotted fever group rickettsia, strain IG-1, from Ixodes granulatus ticks collected on Orchid Island (Lanyu), Taiwan. Am. J. Trop. Med. Hyg. 2008, 79, 256–261. [Google Scholar] [CrossRef]
- Rochlin, I.; Toledo, A. Emerging tick-borne pathogens of public health importance: A mini-review. J. Med. Microbiol. 2020, 69, 781–791. [Google Scholar] [CrossRef]
- Guglielmone, A.; Robbins, R.; Apanaskevich, D.; Petney, T.; Estrasa-Pena, A.; Horak, I.; Shao, R.; Barker, S. The Argasidae, Ixodidae and Nuttalliellidae (Acari: Ixodida) of the world: A list of valid species names. Zootaxa 2010, 2528, 1–28. [Google Scholar] [CrossRef]
- Robbins, R. The ticks (Acari: Ixodida: Argasidae, Ixodidae)of Taiwan: A synonymic checklist. Proc. Entomol. Soc. Wash. 2005, 107, 245–253. [Google Scholar]
- Tsai, Y.; Shyu, C.; Yao, C.; Lin, J. The ixodid ticks collected from dogs and other animals in Taiwan and Kinmen Island. Int. J. Acarol. 2012, 38, 110–115. [Google Scholar] [CrossRef]
- Kwak, M.; Kuo, C.; Chu, H. First record of the sea snake tick Amblyomma nitidum Hirst and Hirst, 1910 (Acari: Ixodidae) from Taiwan. Ticks Tick Borne Dis. 2020, 11, 101383. [Google Scholar] [CrossRef]
- Kuo, C.; Huang, J.; Chien, C.; Shih, H.; Wang, H. First molecular detection of Anaplasma phagocytophilum in the hard tick Rhipicephalus haemaphysaloides in Taiwan. Exp. Appl. Acarol. 2018, 75, 437–443. [Google Scholar] [CrossRef]
- Chao, L.; Shih, C. Molecular analysis of Rhipicephalus sanguineus (Acari: Ixodidae), an incriminated vector tick for Babesia vogeli in Taiwan. Exp. Appl. Acarol. 2016, 70, 469–481. [Google Scholar] [CrossRef]
- Chao, L.; Liu, L.; Ho, T.; Shih, C. First detection and molecular identification of Borrelia garinii spirochete from Ixodes ovatus tick ectoparasitized on stray cat in Taiwan. PLoS ONE 2014, 9, e110599. [Google Scholar] [CrossRef]
- Shih, C.; Wang, J.; Chao, L.; Wu, T. Lyme disease in Taiwan: First human patient with characteristic erythema chronicum migrans skin lesion. J. Clin. Microbiol. 1998, 36, 807–808. [Google Scholar] [CrossRef]
- Shih, C.; Liu, L.; Chung, W.; Ong, S.; Wang, C. Human babesiosis in Taiwan: Asymptomatic infection with a Babesia microti-like organism in a Taiwanese woman. J. Clin. Microbiol. 1997, 35, 450–454. [Google Scholar] [CrossRef]
- Lin, T.; Ou, S.; Maeda, K.; Shimoda, H.; Chan, J.; Tu, W.; Hsu, W.; Chou, C. The first discovery of severe fever with thrombocytopenia syndrome virus in Taiwan. Emerg. Microbes Infect. 2020, 9, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Yang, S.; Tang, S.; Wang, T.; Hsu, T.; Su, C.; Chen, M.; Shimojima, M.; Yoshikawa, T.; Shu, P. Human case of severe fever with thrombocytopenia syndrome virus infection, Taiwan, 2019. Emerg. Infect. Dis. 2020, 26, 1612–1614. [Google Scholar] [CrossRef] [PubMed]
- Takada, N.; Fujita, H.; Yano, Y.; Huang, W.; Khamboonruang, C. Serosurveys of spotted fever and murine typhus in local residents of Taiwan and Thailand compared with Japan. Southeast. Asian J. Trop. Med. Public Health 1993, 24, 354–356. [Google Scholar] [PubMed]
- Taylor, A.; Vongphayloth, K.; Vongsouvath, M.; Grandadam, M.; Brey, P.; Newton, P.; Sutherland, I.; Dittrich, S. Large-scale survey for tickborne bacteria, Khammouan Province, Laos. Emerg. Infect. Dis. 2016, 22, 1635–1639. [Google Scholar] [CrossRef]
- Fournier, P.; Dumler, J.; Greub, G.; Zhang, J.; Wu, Y.; Raoult, D. Gene sequence-based criteria for identification of new Rickettsia isolates and description of Rickettsia heilongjiangensis sp. nov. J. Clin. Microbiol. 2003, 41, 5456–5465. [Google Scholar] [CrossRef]
- Ormsbee, R.; Peacock, M.; Philip, R.; Casper, E.; Plorde, J.; Gabre-Kidan, T.; Wright, L. Antigenic relationships between the typhus and spotted fever groups of rickettsiae. Am. J. Epidemiol. 1978, 108, 53–59. [Google Scholar]
- Pérez-Arellano, J.; Fenollar, F.; Angel-Moreno, A.; Bolaños, M.; Hernández, M.; Santana, E.; Hemmersbach-Miller, M.; Martín, A.; Raoult, D. Human Rickettsia felis infection, Canary Islands, Spain. Emerg. Infect. Dis. 2005, 11, 1961–1964. [Google Scholar] [CrossRef]
- Raoult, D.; La Scola, B.; Enea, M.; Fournier, P.; Roux, V.; Fenollar, F.; Galvao, M.; de Lamballerie, X. A flea-associated Rickettsia pathogenic for humans. Emerg. Infect. Dis. 2001, 7, 73–81. [Google Scholar] [CrossRef]
- Znazen, A.; Rolain, J.; Hammami, A.; Jemaa, M.; Raoult, D. Rickettsia felis infection, Tunisia. Emerg. Infect. Dis. 2006, 12, 138–140. [Google Scholar] [CrossRef]
- Chao, L.; Hsieh, C.; Ho, T.; Shih, C. First zootiological survey of hard ticks (Acari: Ixodidae) infesting dogs in northern Taiwan. Exp. Appl. Acarol. 2019, 77, 105–115. [Google Scholar] [CrossRef]
- Gehrke, F.; Gazeta, G.; Souza, E.; Ribeiro, A.; Marrelli, M.; Schumaker, T. Rickettsia rickettsii, Rickettsia felis and Rickettsia sp. TwKM03 infecting Rhipicephalus sanguineus and Ctenocephalides felis collected from dogs in a Brazilian spotted fever focus in the State of Rio De Janeiro/Brazil. Clin. Microbiol. Infect. 2009, 15 Suppl. 2, 267–268. [Google Scholar] [CrossRef][Green Version]
- Wang, X.; Sun, X.; Sun, Y.; Chen, K.; Zhang, K.; Xu, W.; Fan, K.; Lin, W.; Chen, T.; Lin, X.; et al. Identification and molecular analysis of Ixodid ticks (Acari: Ixodidae) infesting wild boars (Sus scrofa) and tick-borne pathogens at the Meihua mountain of southwestern Fujian, China. Vet. Parasitol. Reg. Stud. Rep. 2020, 22, 100492. [Google Scholar] [CrossRef]
- Ajithkumar, K.; Ravindran, R.; Ghosh, S. Dermacentor auratus Supino, 1897 (Acarina, Ixodidae) reported from Wayanad, Kerala. Indian J. Med. Res. 2012, 135, 435–436. [Google Scholar]
- Kwak, M.; Chavatte, J.; Chew, K.; Lee, B. Emergence of the zoonotic tick Dermacentor (Indocentor) auratus Supino, 1897 (Acari: Ixodidae) in Singapore. Ticks Tick Borne Dis 2021, 12, 101574. [Google Scholar] [CrossRef]
- Chao, L.; Wu, W.; Shih, C. Molecular analysis of Ixodes granulatus, a possible vector tick for Borrelia burgdorferi sensu lato in Taiwan. Exp. Appl Acarol 2009, 48, 329–344. [Google Scholar] [CrossRef]
- Guglielmone, A.; Robbins, R. Hard ticks (Acari: Ixodida: Ixodidae) parasitizing humans: A global overview; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- McNabb, S.; Jajosky, R.; Hall-Baker, P.; Adams, D.; Sharp, P.; Worshams, C.; Anderson, W.; Javier, A.; Jones, G.; Nitschke, D.; et al. Summary of notifiable diseases—United States, 2006. MMWR Morb Mortal Wkly. Rep. 2008, 55, 1–92. [Google Scholar]
- ECDC. Epidemiological Situation of Rickettsioses in EU/EFTA Countries; ECDC: Stockholm, Sweeden, 2013. [Google Scholar]
- Satoh, H.; Tsuneki, A.; Inokuma, H.; Kumazawa, N.; Jahana, Y.; Kiyuuna, T.; Okabayashi, T.; Muramatsu, Y.; Ueno, H.; Morita, C. Seroprevalence of antibodies against spotted fever group rickettsia among dogs and humans in Okinawa, Japan. Microbiol. Immunol. 2001, 45, 85–87. [Google Scholar] [CrossRef]
- Ando, S.; Kurosawa, M.; Sakata, A.; Fujita, H.; Sakai, K.; Sekine, M.; Katsumi, M.; Saitou, W.; Yano, Y.; Takada, N.; et al. Human Rickettsia heilongjiangensis infection, Japan. Emerg. Infect. Dis. 2010, 16, 1306–1308. [Google Scholar] [CrossRef]
- Imaoka, K.; Kaneko, S.; Tabara, K.; Kusatake, K.; Morita, E. The first human case of Rickettsia tamurae infection in Japan. Case Rep. Dermatol. 2011, 3, 68–73. [Google Scholar] [CrossRef]
- Fujita, H.; Fournier, P.; Takada, N.; Saito, T.; Raoult, D. Rickettsia asiatica sp. nov., isolated in Japan. Int. J. Syst. Evol. Microbiol. 2006, 56, 2365–2368. [Google Scholar] [CrossRef][Green Version]
- Inokuma, H.; Ohashi, M.; Jilintai; Tanabe, S.; Miyahara, K. Prevalence of tick-borne Rickettsia and Ehrlichia in Ixodes persulcatus and Ixodes ovatus in Tokachi district, Eastern Hokkaido, Japan. J. Vet. Med. Sci. 2007, 69, 661–664. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fournier, P.; Takada, N.; Fujita, H.; Raoult, D. Rickettsia tamurae sp. nov., isolated from Amblyomma testudinarium ticks. Int. J. Syst. Evol. Microbiol. 2006, 67, 1673–1675. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.; Kim, J.; Choi, Y.; Jung, K.; Kim, Y.; Lee, S.; Choi, M.; Kim, I.; Walker, D.; Park, K. First serologic evidence of human spotted fever group rickettsiosis in Korea. J. Clin. Microbiol. 2004, 42, 2310–2313. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jang, W.; Choi, Y.; Kim, J.; Jung, K.; Ryu, J.; Lee, S.; Yoo, C.; Paik, H.; Choi, M.; Park, K.; et al. Seroepidemiology of spotted fever group and typhus group rickettsioses in humans. Microbiol. Immunol. 2005, 49, 17–24. [Google Scholar] [CrossRef]
- Strickman, D.; Tanskul, P.; Eamsila, C.; Kelly, D. Prevalence of antibodies to rickettsiae in the human population of suburban Bangkok. Am. J. Trop. Med. Hyg. 1994, 51, 149–153. [Google Scholar] [CrossRef]
- Bhengsri, S.; Baggett, H.; Edouard, S.; Dowell, S.; Dasch, G.; Fisk, T.; Raoult, D.; Parola, P. Sennetsu neorickettsiosis, spotted fever group, and typhus group rickettsioses in three provinces in Thailand. Am. J. Trop. Med. Hyg. 2016, 95, 43–49. [Google Scholar] [CrossRef]
- Tay, S.; Ho, T.; Rohani, M.; Devi, S. Antibodies to Orientia tsutsugamushi, Rickettsia typhi and spotted fever group rickettsiae among febrile patients in rural areas of Malaysia. Trans. R Soc. Trop. Med. Hyg. 2000, 94, 280–284. [Google Scholar] [CrossRef]
- Phongmany, S.; Rolain, J.; Phetsouvanh, R.; Blacksell, S.; Soukkhaseum, V.; Rasachack, B.; Phiasakha, K.; Soukkhaseum, S.; Frichithavong, K.; Chu, V.; et al. Rickettsial infections and fever, Vientiane, Laos. Emerg. Infect. Dis. 2006, 12, 256–262. [Google Scholar] [CrossRef]
- Trung, N.; Hoi, L.; Thuong, N.; Toan, T.; Huong, T.; Hoa, T.; Fox, A.; Kinh, N.; van Doorn, H.; Wertheim, H.; et al. Seroprevalence of scrub typhus, typhus, and spotted fever among rural and urban populations of Northern Vietnam. Am. J. Trop. Med. Hyg. 2017, 96, 1084–1087. [Google Scholar] [CrossRef]
- Richards, A.; Ratiwayanto, S.; Rahardjo, E.; Kelly, D.; Dasch, G.; Fryauff, D.; Bangs, M. Serologic evidence of infection with ehrlichiae and spotted fever group rickettsiae among residents of Gag Island, Indonesia. Am. J. Trop. Med. Hyg. 2003, 68, 480–484. [Google Scholar] [CrossRef]
- Li, J.; Hu, W.; Wu, T.; Li, H.; Hu, W.; Sun, Y.; Chen, Z.; Shi, Y.; Zong, J.; Latif, A.; et al. Japanese Spotted Fever in Eastern China, 2013. Emerg. Infect. Dis. 2018, 24, 2107–2109. [Google Scholar] [CrossRef]
- Fournier, P.; Gouriet, F.; Brouqui, P.; Lucht, F.; Raoult, D. Lymphangitis-associated rickettsiosis, a new rickettsiosis caused by Rickettsia sibirica mongolotimonae: Seven new cases and review of the literature. Clin. Infect. Di.s 2005, 40, 1435–1444. [Google Scholar] [CrossRef]
- Yen, T.; Zhang, Z.; Chao, C.; Ching, W.; Shu, P.; Tseng, L.; Carvalho, A.; Tsai, K. Serologic evidence for Orientia exposure in the Democratic Republic of Sao Tome and Principe. Vector Borne Zoonotic Dis. 2019, 19, 821–827. [Google Scholar] [CrossRef]
- Demma, L.; McQuiston, J.; Nicholson, W.; Murphy, S.; Marumoto, P.; Sengebau-Kingzio, M.; Kuartei, S.; Durand, A.; Swerdlow, D. Scrub typhus, Republic of Palau. Emerg. Infect. Dis. 2006, 12, 290–295. [Google Scholar] [CrossRef]
- Walker, A. Ticks-Ixodida. In The Arthropods of Humans and Domestic Animals; Walker, A., Ed.; Chapman & Hall: London, UK, 1994; pp. 25–48. [Google Scholar]
- Walker, J.; Keirans, J.; Horak, I. The Genus Rhipicephalus (Acari, Ixodidae): A Guide to the Brown Ticks of the World; Cambridge University: Cambridge, UK, 2000. [Google Scholar]
- Yamaguti, N.; Tipton, V.; Keegan, H.; Toshiaoka, S. Ticks of Japan, Korea, and the Ryukyu islands. Brigh Young Univ. Sci. Bull. Biol. Ser. 1971, 15, 1–225. [Google Scholar]
- Teng, K.; Jiang, Z. Acari: Ixodidae; Science Press: Beijing, China, 1991; Volume 39. (in Chinese) [Google Scholar]
- Black, W.t.; Piesman, J. Phylogeny of hard- and soft-tick taxa (Acari: Ixodida) based on mitochondrial 16S rDNA sequences. Proc. Natl. Acad. Sci. USA 1994, 91, 10034–10038. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Fournier, P.; Roux, V.; Raoult, D. Phylogenetic analysis of spotted fever group rickettsiae by study of the outer surface protein rOmpA. Int. J. Syst. Bacteriol. 1998, 48 Pt. 3, 839–849. [Google Scholar] [CrossRef]
- Roux, V.; Raoult, D. Phylogenetic analysis of members of the genus Rickettsia using the gene encoding the outer-membrane protein rOmpB (ompB). Int. J. Syst. Evol. Microbiol. 2000, 50 Pt. 4, 1449–1455. [Google Scholar] [CrossRef]
- Roux, V.; Rydkina, E.; Eremeeva, M.; Raoult, D. Citrate synthase gene comparison, a new tool for phylogenetic analysis, and its application for the rickettsiae. Int. J. Syst. Bacteriol. 1997, 47, 252–261. [Google Scholar] [CrossRef]
- Sekeyova, Z.; Roux, V.; Raoult, D. Phylogeny of Rickettsia spp. inferred by comparing sequences of ‘gene D’, which encodes an intracytoplasmic protein. Int. J. Syst. Evol. Microbiol. 2001, 51, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Hsi, T.; Hsiao, S.; Minahan, N.; Yen, T.; de Assunção Carvalho, A.; Raoult, D.; Fournier, P.; Tsai, K. Seroepidemiological and molecular investigation of spotted fever group rickettsiae and Coxiella burnetii in Sao Tome Island: A One Health approach. Transbound Emerg. Dis. 2020, 67 Suppl. S2, 36–43. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
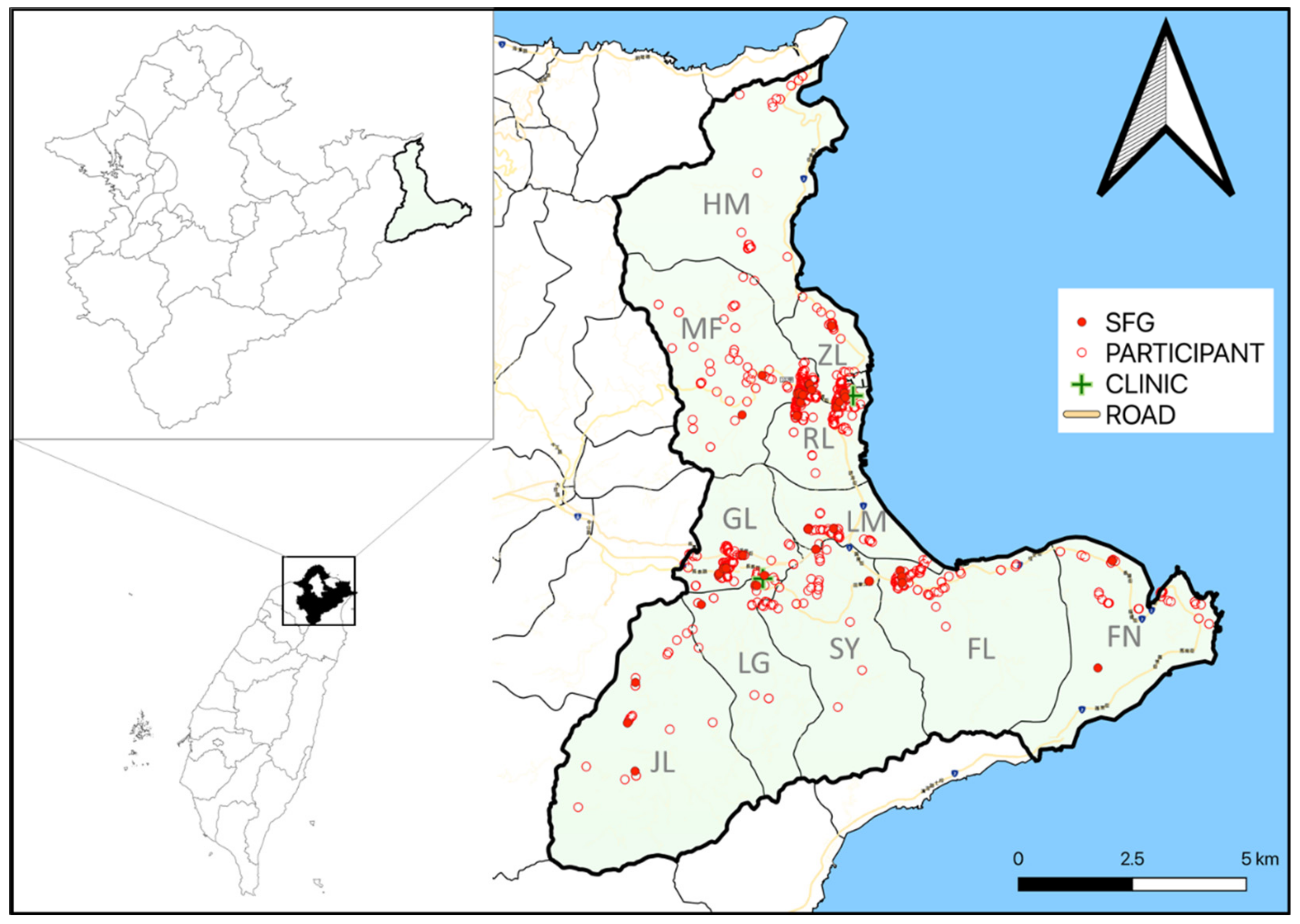
| Variables | Annual Health Exam (n = 260) | Patients Visiting the Group Practice Center (n = 557) | Patients Visiting Dr. Enjoy’s Clinic (n = 291) | p Value |
|---|---|---|---|---|
| Gender | <0.01 | |||
| Male | 104 | 327 | 106 | |
| Female | 156 | 230 | 185 | |
| Age (mean ± SD) | 55.1 ± 15.2 | 58.2 ± 20.4 | 63.3 ± 16.1 | <0.01 |
| Village | <0.01 | |||
| Gongliao (GL) | 12 | 24 | 106 | |
| Jilin (JL) | 1 | 9 | 24 | |
| Shuangyu (SY) | 10 | 29 | 41 | |
| Longgang (LG) | 0 | 4 | 46 | |
| Longmen (LM) | 8 | 25 | 14 | |
| Fulong (FL) | 23 | 65 | 21 | |
| Renli (RL) | 83 | 137 | 18 | |
| Zhenli (ZL) | 78 | 149 | 11 | |
| Fulian (FN) | 14 | 31 | 6 | |
| Meifeng (MF) | 29 | 38 | 3 | |
| Hemei (HM) | 2 | 46 | 1 |
| SFGR ELISA (n = 118) | IFA | |||||
|---|---|---|---|---|---|---|
| R. rickettsia (n = 77) | R. conorii (n = 68) | R. typhi (n = 45) | O. tsutsugamushi (n = 155) | R. rickettsii + R. typhi (n = 23) | ||
| SFGR ELISA | - | 75 | 68 | 26 | 15 | 23 |
| IFA | ||||||
| R. rickettsii | 75 | - | 62 | 23 | 5 | - |
| R. conorii | 68 | 62 | - | 24 | 9 | 23 |
| R. typhi | 26 | 23 | 24 | - | 5 | - |
| O. tsutsugamushi | 15 | 5 | 9 | 5 | - | 10 |
| Variables | No. of Samples Tested | No. (%) of Positive Samples | Univariate Regression Analysis | Multiple Regression Analysis |
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | |||
| Gender | ||||
| Male | 537 | 39 (7.3) | Reference | ND |
| Female | 571 | 36 (6.3) | 0.9 (0.5–1.4) | ND |
| Age | ||||
| <65 yr | 564 | 21 (3.7) | Reference | Reference |
| ≥65 yr | 544 | 54 (9.9) | 2.9 (1.7–4.8) *** | 2.1 (1.2–3.8) * |
| Sampling site | ||||
| Annual health exam | 260 | 10 (3.8) | Reference | Reference |
| Group practice center | 557 | 41 (7.4) | 2.4 (1.1–5.2) * | 1.7 (0.7–3.9) |
| Dr. Enjoy’s Clinic | 291 | 24 (8.2) | 3.4 (1.5–7.5) ** | 2.2 (0.8–5.8) |
| Village | ||||
| Gongliao (GL) | 142 | 9 (6.3) | Reference | Reference |
| Jilin (JL) | 34 | 7 (20.6) | 3.8 (1.3–11.2) * | 3.3 (1.1–10.1) * |
| Shuangyu (SY) | 80 | 8 (10.0) | 1.6 (0.6–4.4) | 1.5 (0.5–4.1) |
| Longgang (LG) | 50 | 5 (10.0) | 1.6 (0.5–5.2) | 1.4 (0.4–4.5) |
| Longmen (LM) | 47 | 4 (8.5) | 1.4 (0.4–4.7) | 1.6 (0.4–5.7) |
| Fulong (FL) | 109 | 7 (6.4) | 1.0 (0.4–2.8) | 1.2 (0.4–3.7) |
| Renli (RL) | 238 | 11 (4.6) | 0.7 (0.3–1.8) | 0.9 (0.3–2.7) |
| Zhenli (ZL) | 238 | 10 (4.2) | 0.6 (0.3–1.6) | 0.8 (0.3–2.4) |
| Fulian (FN) | 51 | 4 (7.8) | 1.3 (0.4–4.3) | 1.9 (0.5–7.3) |
| Meifeng (MF) | 70 | 6 (8.6) | 1.4 (0.5–4.1) | 1.6 (0.5–5.3) |
| Hemei (HM) | 49 | 4 (8.2) | 1.3 (0.4–4.5) | 1.8 (0.5–7.2) |
| Occupation | ||||
| NA | 239 | 22 (9.2) | Reference | Reference |
| Agricultural worker | 67 | 6 (9.0) | 1.0 (0.4–2.5) | 0.7 (0.2–1.9) |
| Housemaker | 331 | 29 (8.8) | 0.9 (0.5–1.7) | 0.9 (0.4–1.8) |
| Industrial laborer | 191 | 7 (3.7) | 0.4 (0.2–0.9) * | 0.6 (0.2–1.5) |
| Businessman | 57 | 0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
| Government official | 27 | 1 (3.7) | 0.4 (0.0–2.9) | 0.5 (0.1–4.4) |
| Teacher | 7 | 1 (14.3) | 1.6 (0.2–14.3) | 2.7 (0.3–25.9) |
| Armed force occupation | 1 | 0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
| Student | 15 | 1 (6.7) | 0.7 (0.1–5.6) | 0.8 (0.1–7.2) |
| Other | 173 | 8 (4.6) | 0.5 (0.2–1.2) | 0.6 (0.2–1.4) |
| Tick Species (Accession No.) | No. Ticks (Female, Male, Nymph) | Rickettsia spp. | |
|---|---|---|---|
| Positive Rate %(Positive/Tested) | Accession No. | ||
| Dermacentor auratus (MZ823781) | 1 (1, 0, 0) | 100.0 (1/1) | MZ869826 MZ869827 MZ869828 MZ869829 MZ869830 |
| Haemaphysalis hystricis (MZ823778) | 3 (2, 1, 0) | 0.0 (0/3) | |
| Haemaphysalis ornithophila (MZ823776) | 1 (0, 1, 0) | 0.0 (0/1) | |
| Rhipicephalus sanguineus | 155 (52, 37, 66) | 4.5 (7/155) | AY445819 1 AF540555 1 EF219467.1 1 |
| Rhipicephalus haemaphysaloides | 24 (4, 7, 13) | 16.7 (4/24) | AY445819 1 |
| Total | 184 | 6.5 (12/184) | |
| Gene | % Pairwise Nucleotide Sequence Identity to Closest Neighbors (Accession No.) | No. Matching Nucleotides/Total | Cutoff Values [37] |
|---|---|---|---|
| gltA | 99.74% to Uncultured Rickettsia sp. clone MIVLW15/2017 (MN557219.1) 99.74% to Uncultured bacterium clone HHMJ7 (KC566999.1) 99.48% to R. raoultii isolate N42 (MN550897.1) 98.41% to “Candidatus R. laoensis” (KT753290.1) | 383/384 381/382 382/384 124/126 | 99.9% |
| 5ʹ end of ompA | 100.0% to “Candidatus R. laoensis” isolate MHS2019/12 (MT321619) 98.55% to “Candidatus R. laoensis” isolate MIVLW15/2017 (MK905251.1) 97.64% to R. raoultii isolate z164 (MH532249.1) | 551/551 543/551 538/551 | 98.8% |
| 3ʹ end of ompA | 98.49% to Rickettssia sp. RpA4 (AH009131.2) 98.49% to R. raoultii strain Marne (AH015609.2) 98.40% to R. raoultii isolate Tomsk (MK304548.1) | 3134/3182 3134/3182 3132/3183 | |
| ompB | 97.99% to R. raoultii strain Khabarovsk (CP010969.1) 97.96% to R. raoultii strain IM16 (CP019435.1) 97.94% to R. raoultii strain Khabarovsk (DQ365798.1) 99.28% to “Candidatus R. laoensis” (KT753294.1) | 4333/4422 4332/4422 4334/4425 1101/1109 | 99.2% |
| sca4 | 98.01% to R. montanensis str. OSU 85-930 (CP003340.1) 97.82% to R. raoultii isolate Tomsk (MK304550.1) 97.73% to R. raoultii isolate Nsk862 (MT253668.1) 98.80% to “Candidatus R. laoensis” (KT753292.1) | 2417/2466 2418/2472 2416/2472 820/830 | 99.3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yen, T.-Y.; Wang, H.-C.; Chang, Y.-C.; Su, C.-L.; Chang, S.-F.; Shu, P.-Y.; Tsai, K.-H. Seroepidemiological Study of Spotted Fever Group Rickettsiae and Identification of a Putative New Species, Rickesttsia sp. Da-1, in Gongliao, Northeast Taiwan. Pathogens 2021, 10, 1434. https://doi.org/10.3390/pathogens10111434
Yen T-Y, Wang H-C, Chang Y-C, Su C-L, Chang S-F, Shu P-Y, Tsai K-H. Seroepidemiological Study of Spotted Fever Group Rickettsiae and Identification of a Putative New Species, Rickesttsia sp. Da-1, in Gongliao, Northeast Taiwan. Pathogens. 2021; 10(11):1434. https://doi.org/10.3390/pathogens10111434
Chicago/Turabian StyleYen, Tsai-Ying, Hsi-Chieh Wang, Yin-Chao Chang, Chien-Ling Su, Shu-Fen Chang, Pei-Yun Shu, and Kun-Hsien Tsai. 2021. "Seroepidemiological Study of Spotted Fever Group Rickettsiae and Identification of a Putative New Species, Rickesttsia sp. Da-1, in Gongliao, Northeast Taiwan" Pathogens 10, no. 11: 1434. https://doi.org/10.3390/pathogens10111434
APA StyleYen, T.-Y., Wang, H.-C., Chang, Y.-C., Su, C.-L., Chang, S.-F., Shu, P.-Y., & Tsai, K.-H. (2021). Seroepidemiological Study of Spotted Fever Group Rickettsiae and Identification of a Putative New Species, Rickesttsia sp. Da-1, in Gongliao, Northeast Taiwan. Pathogens, 10(11), 1434. https://doi.org/10.3390/pathogens10111434







