SBS-Modified Asphalt Accelerated Swelling Technology and Performance Evaluation
Abstract
1. Introduction
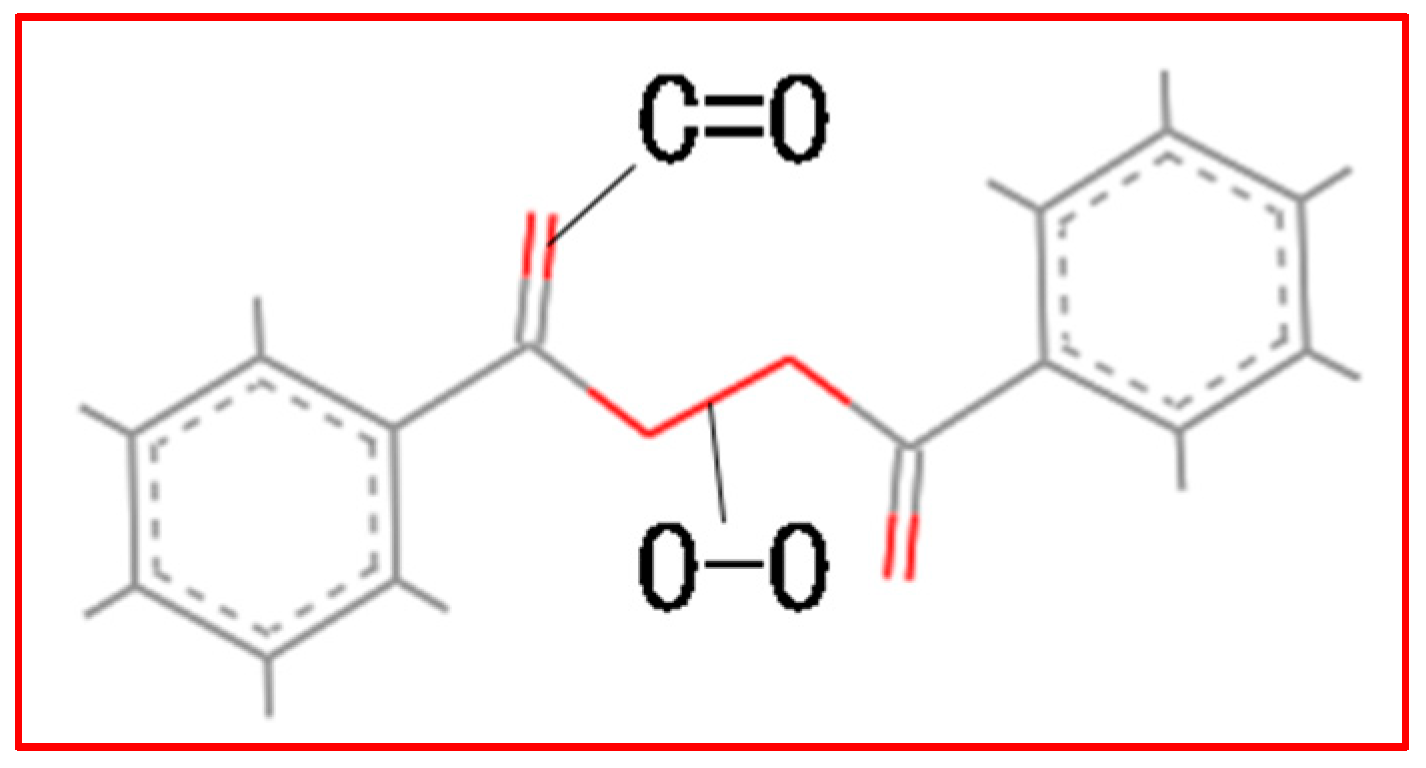
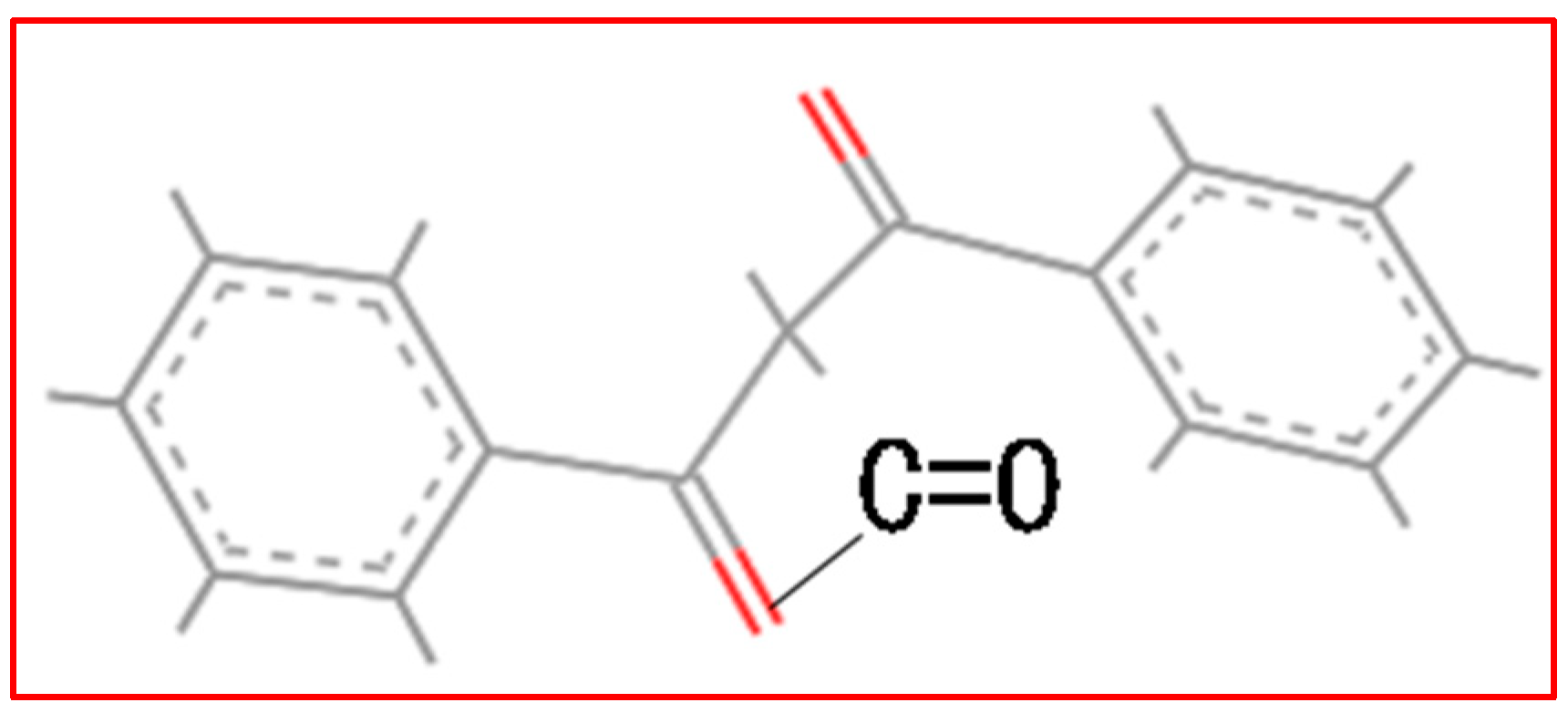
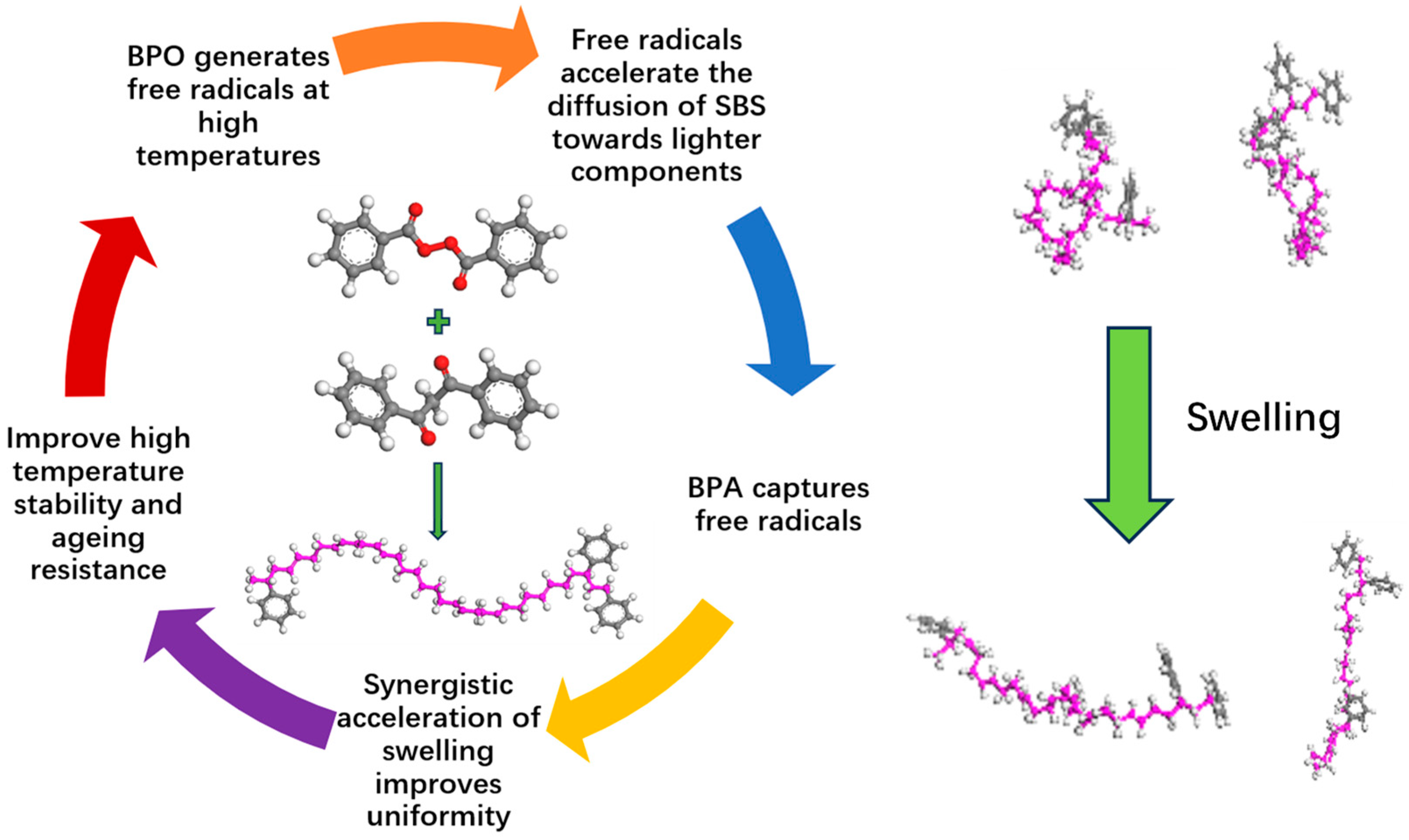
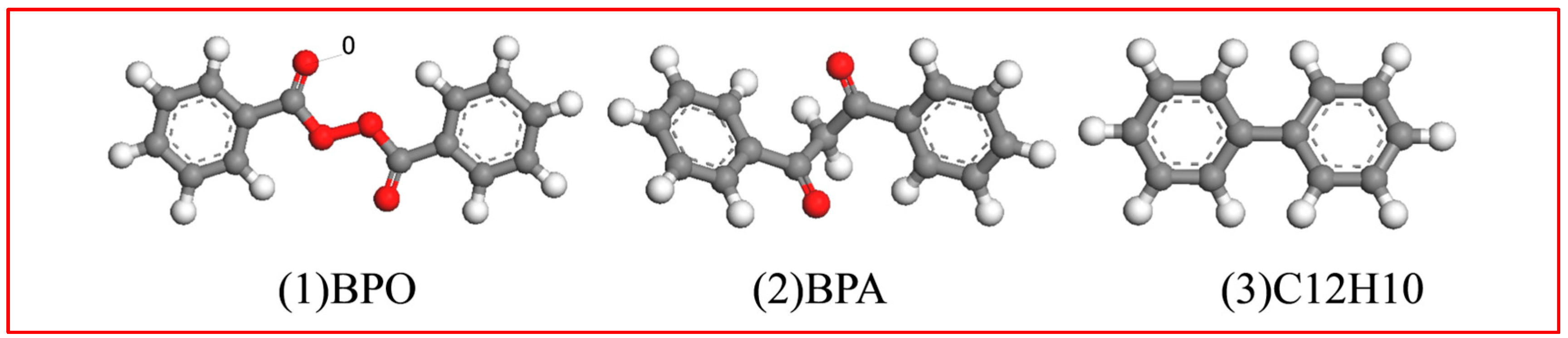
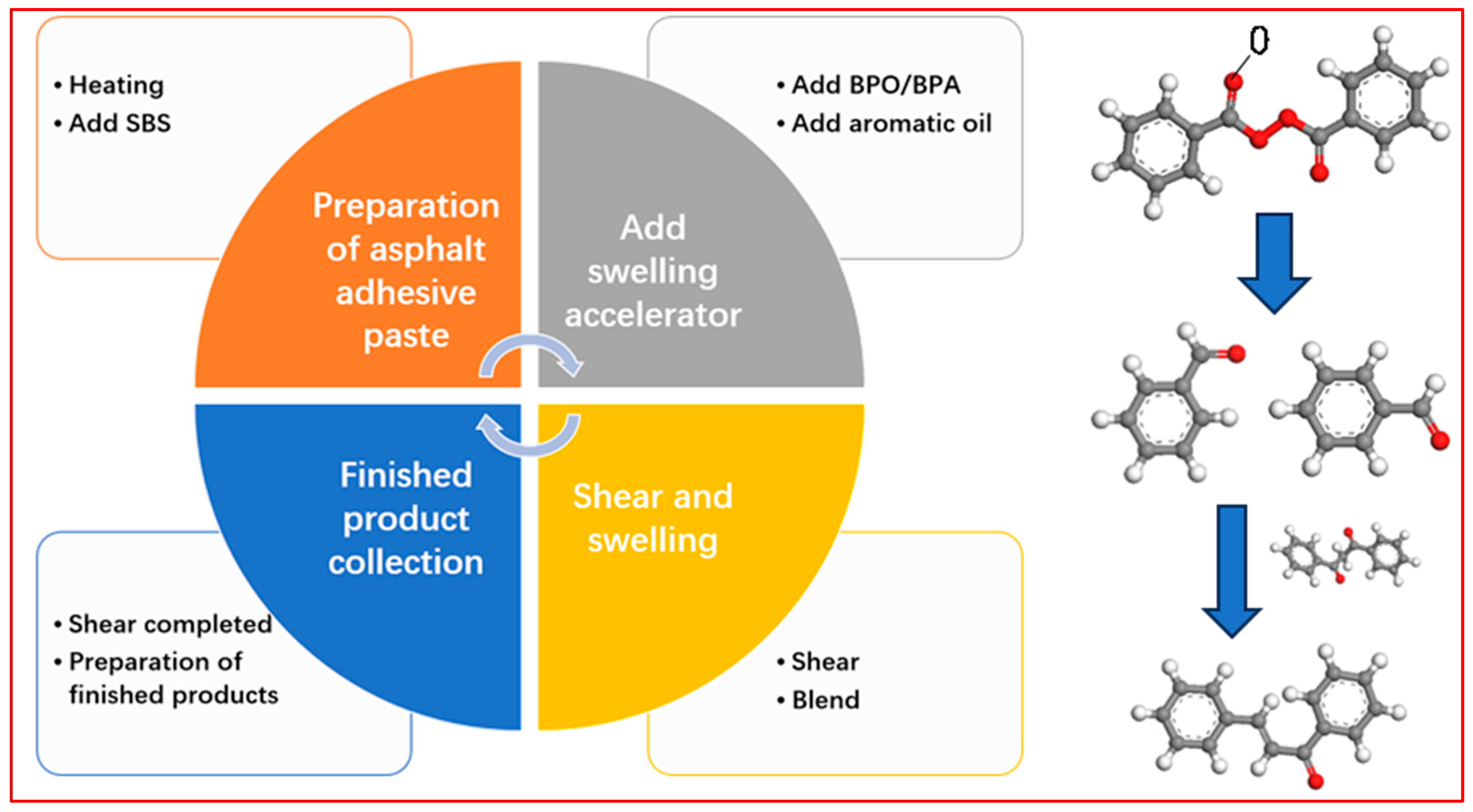
2. Accelerated Swelling Mechanism of BPO and BPA
3. Asphalt Swelling Molecular Dynamics Model Based on BPO and BPA
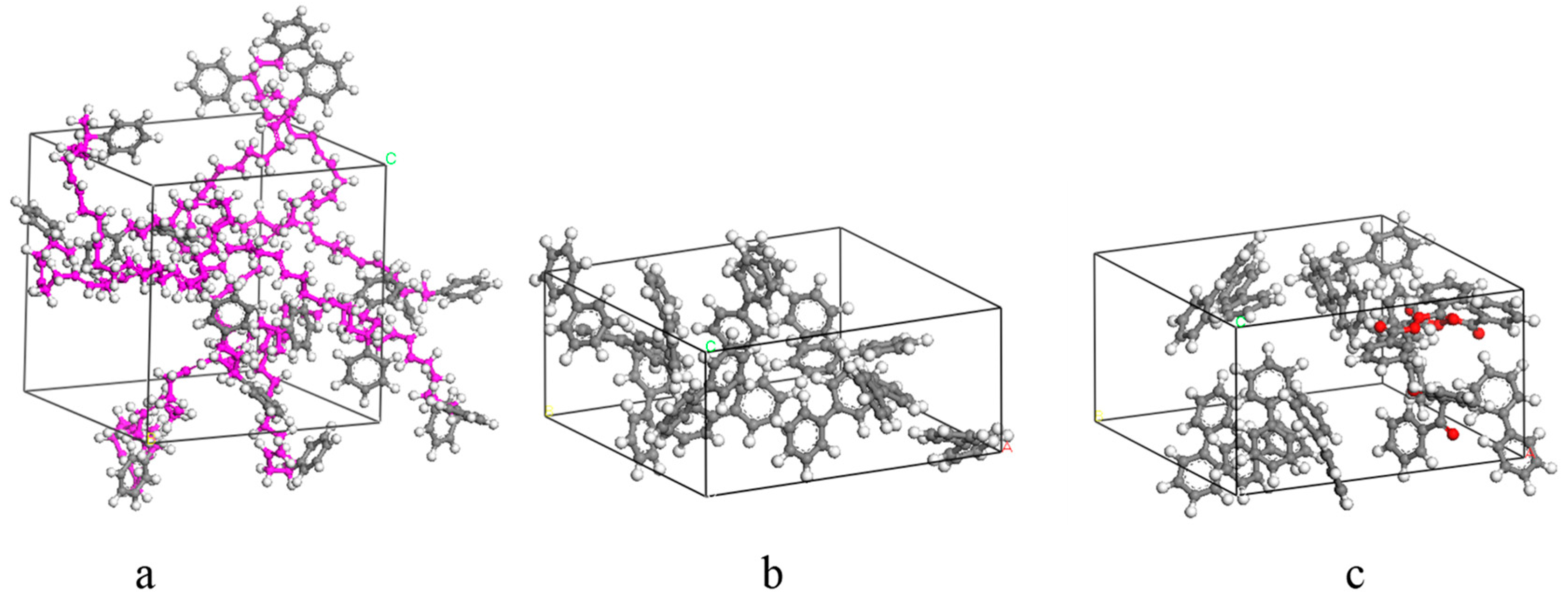
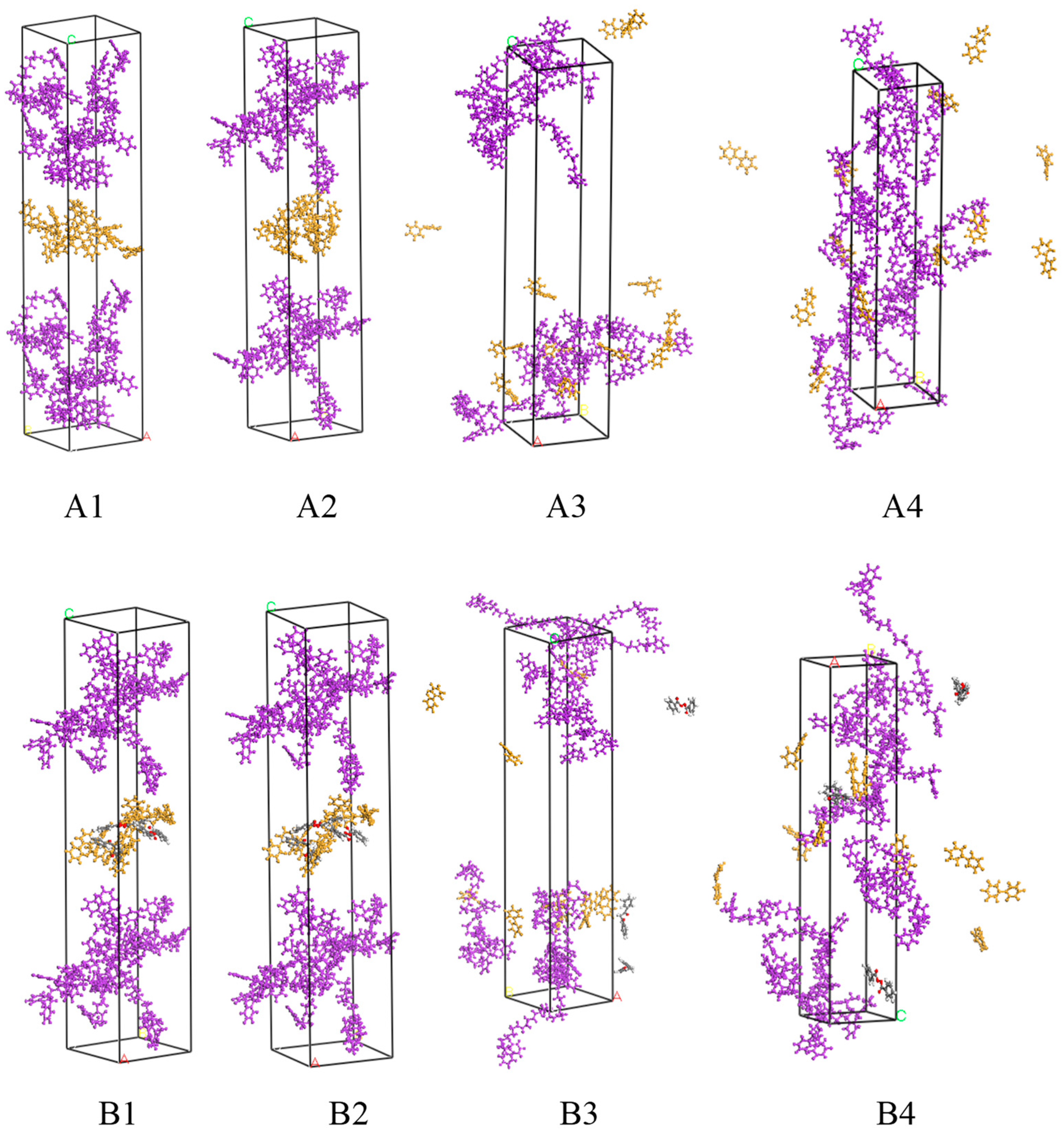
3.1. Establishment of Molecular Dynamics Model for SBS–Pre-Swelling Agent Blend System
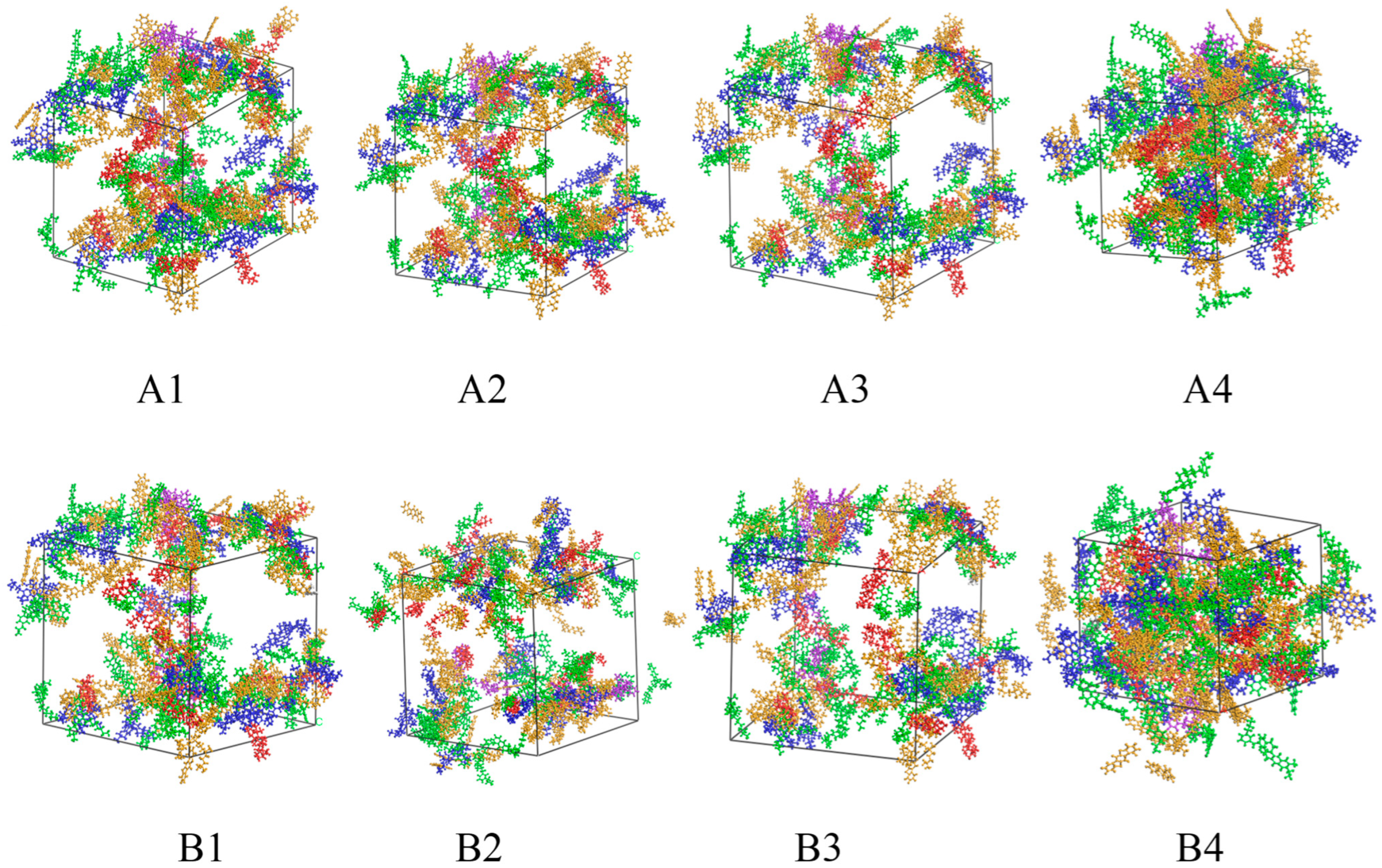
3.2. Establishment of a Molecular Dynamics Model for Modified Asphalt Systems
4. Selection of Parameters for Evaluating Accelerated Swelling Effects
4.1. Microscopic Evaluation Parameters for Accelerated Swelling Effect
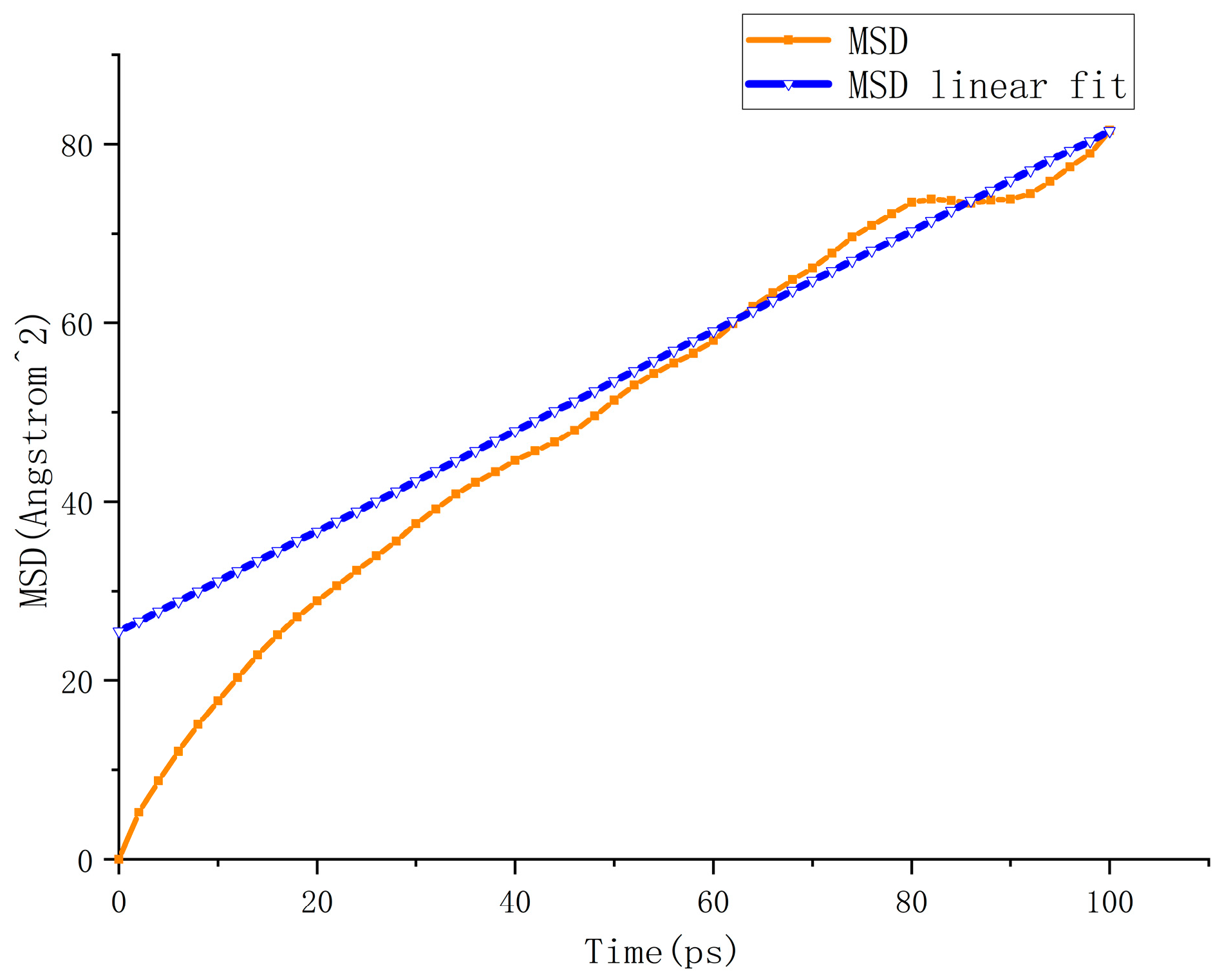
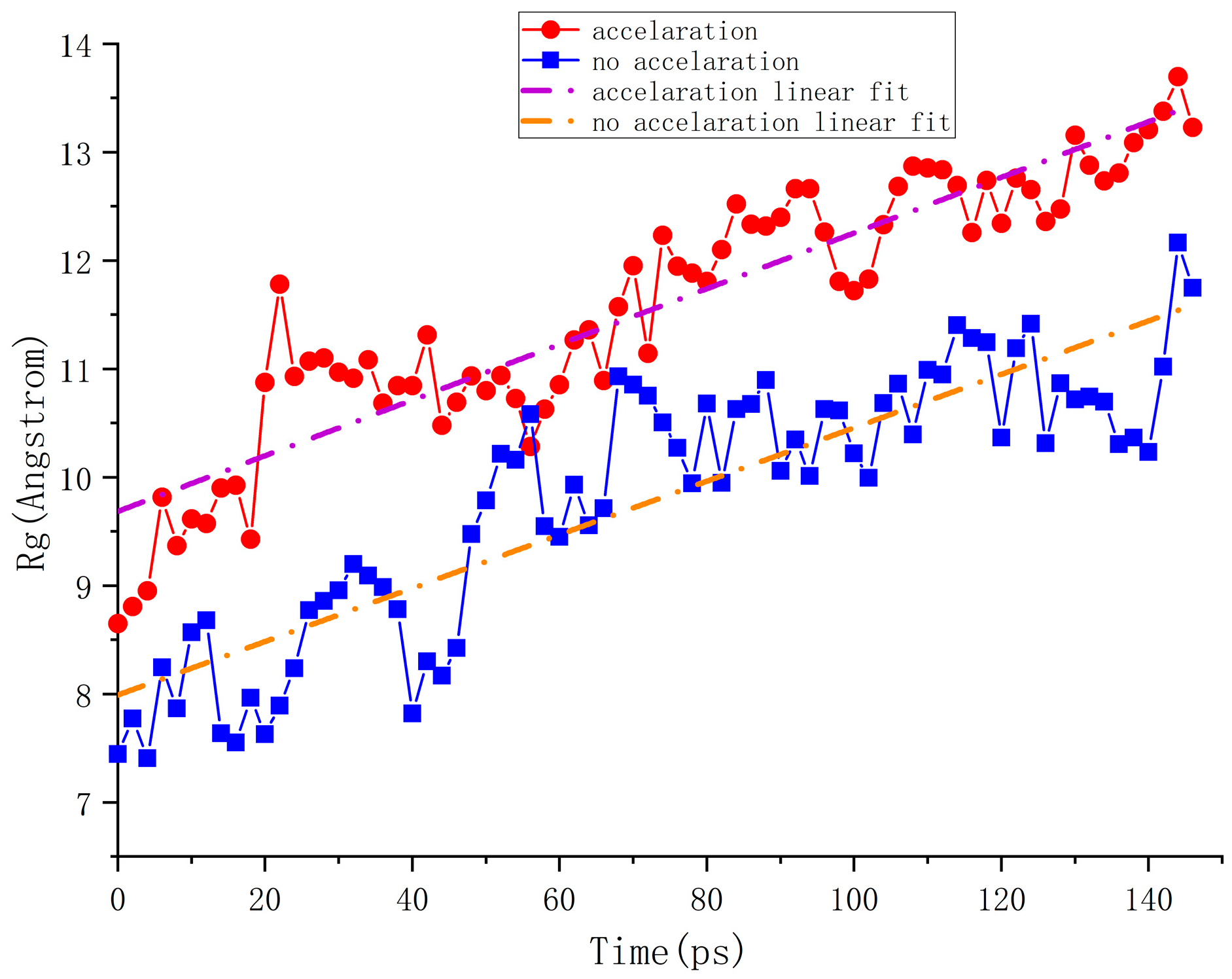
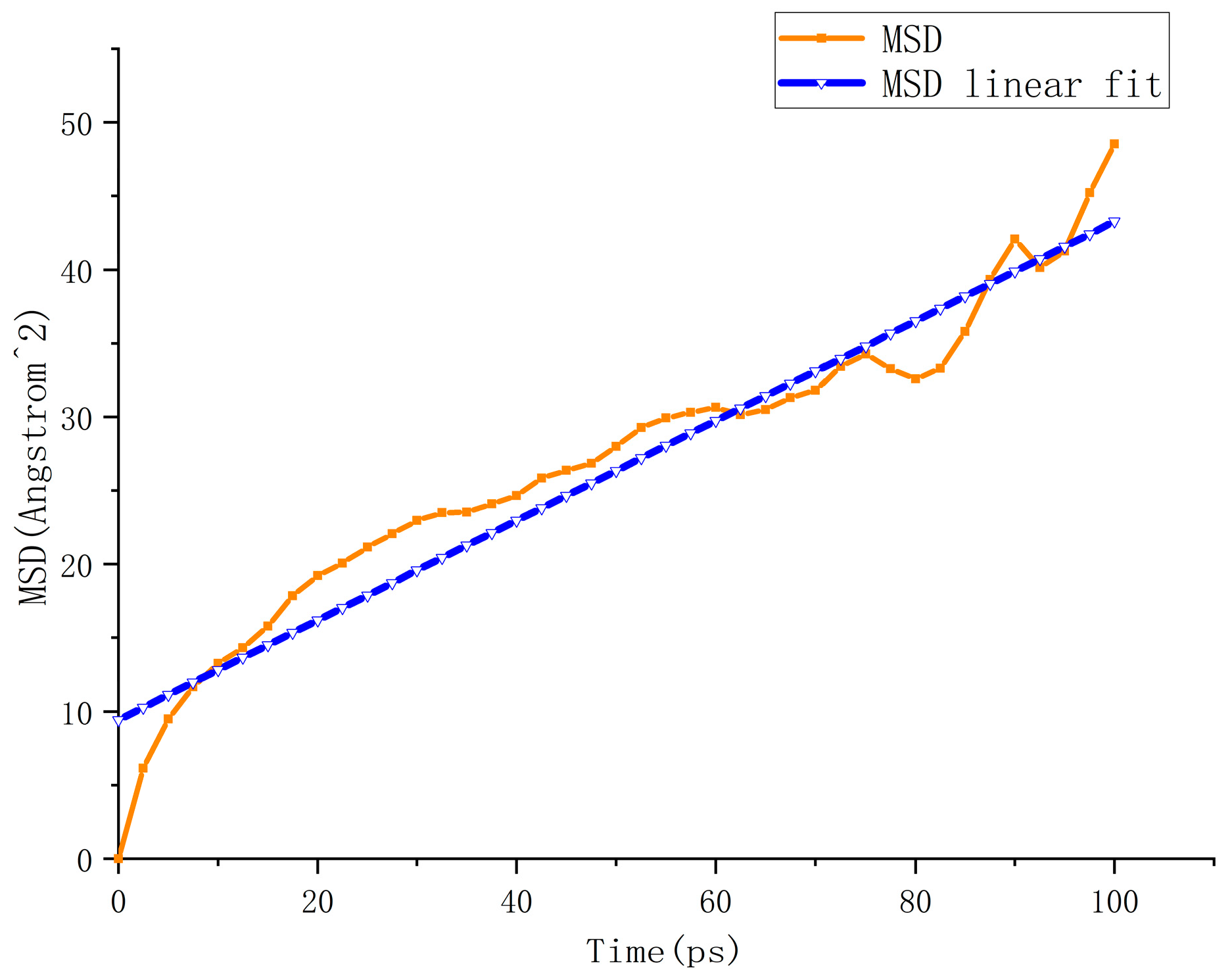
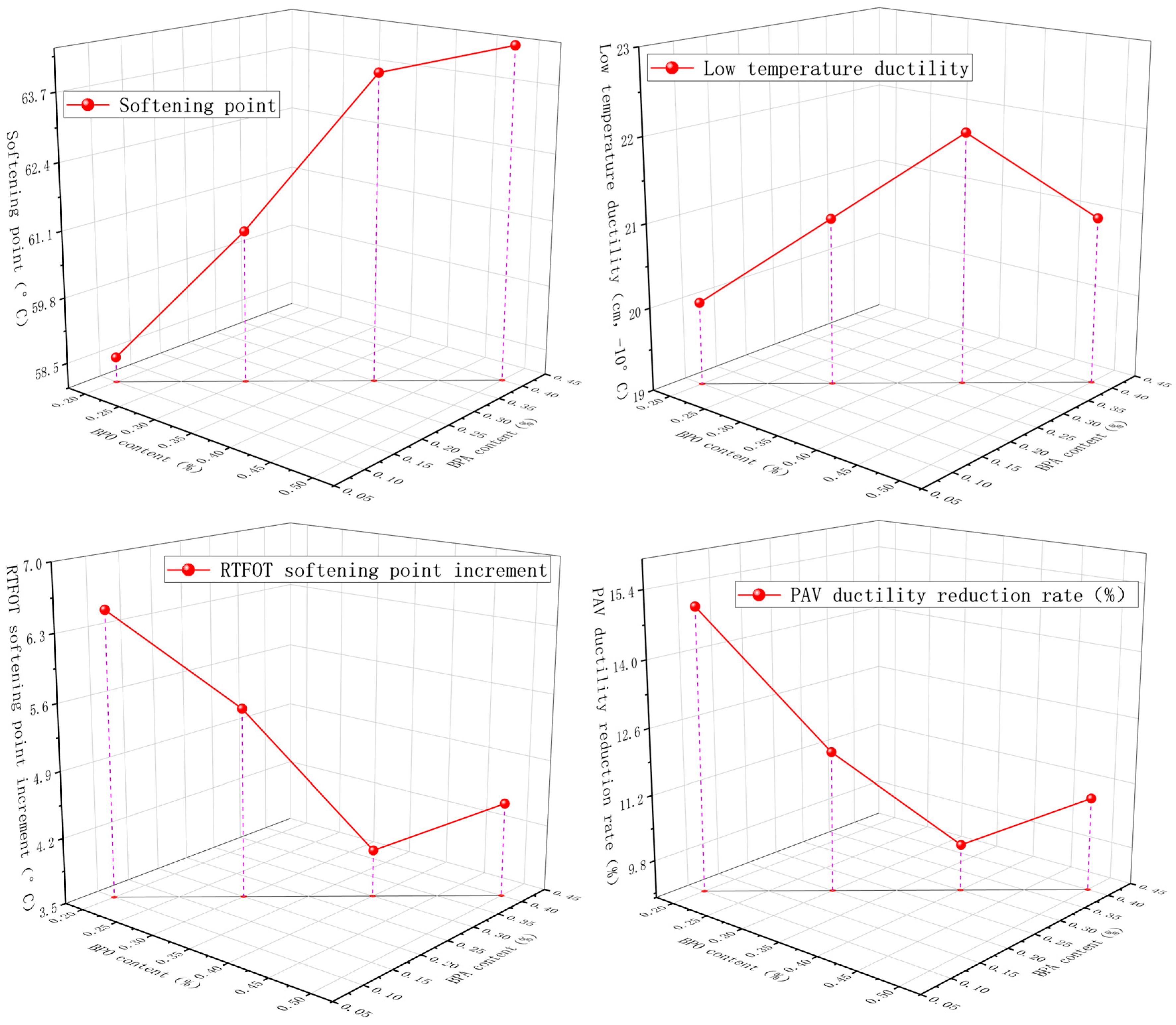
4.2. Macro Evaluation Parameters for Accelerated Swelling Effect
5. Analysis of Accelerated Swelling Effects Based on BPO and BPA
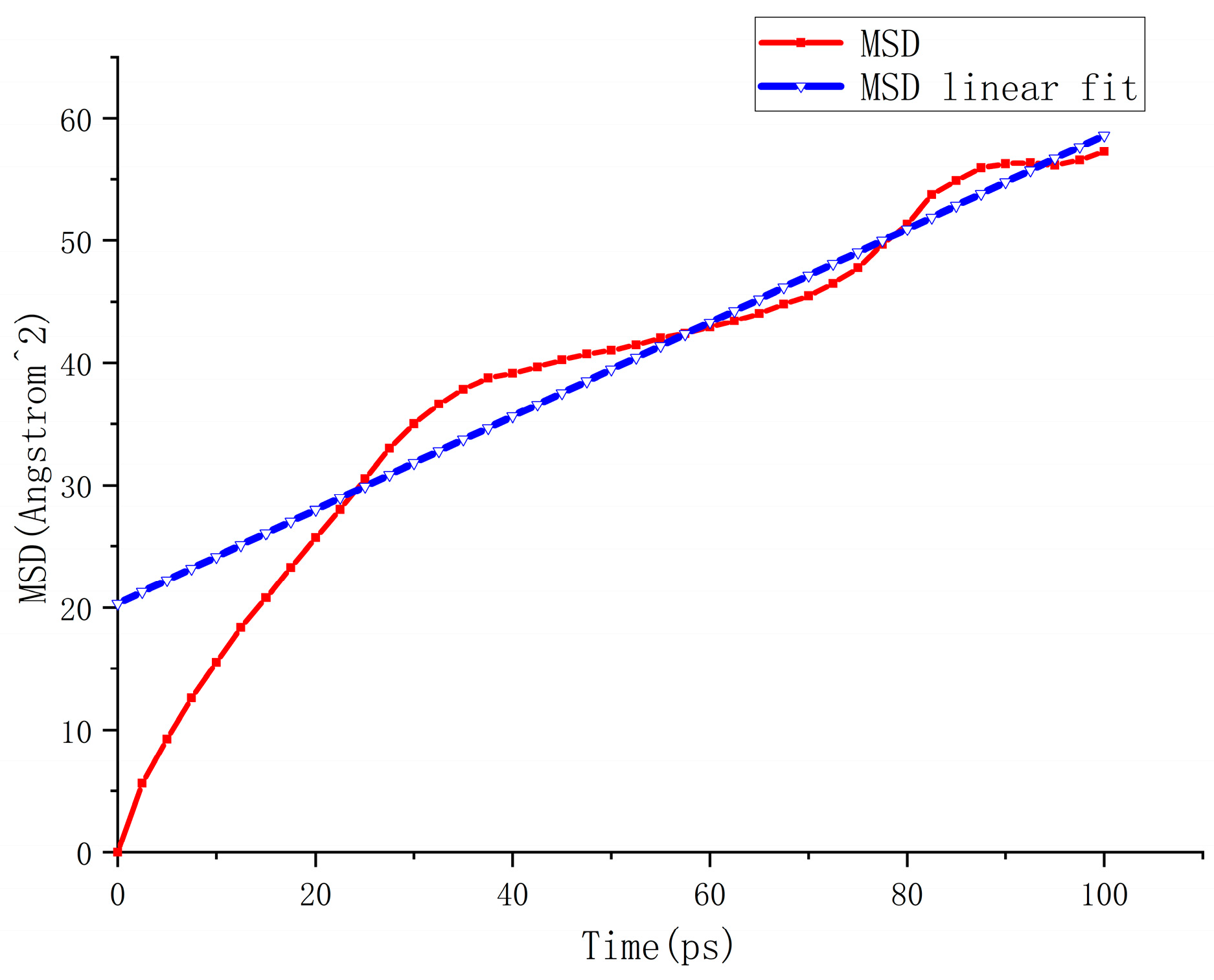
5.1. Analysis of Accelerated Swelling Micro-Effects
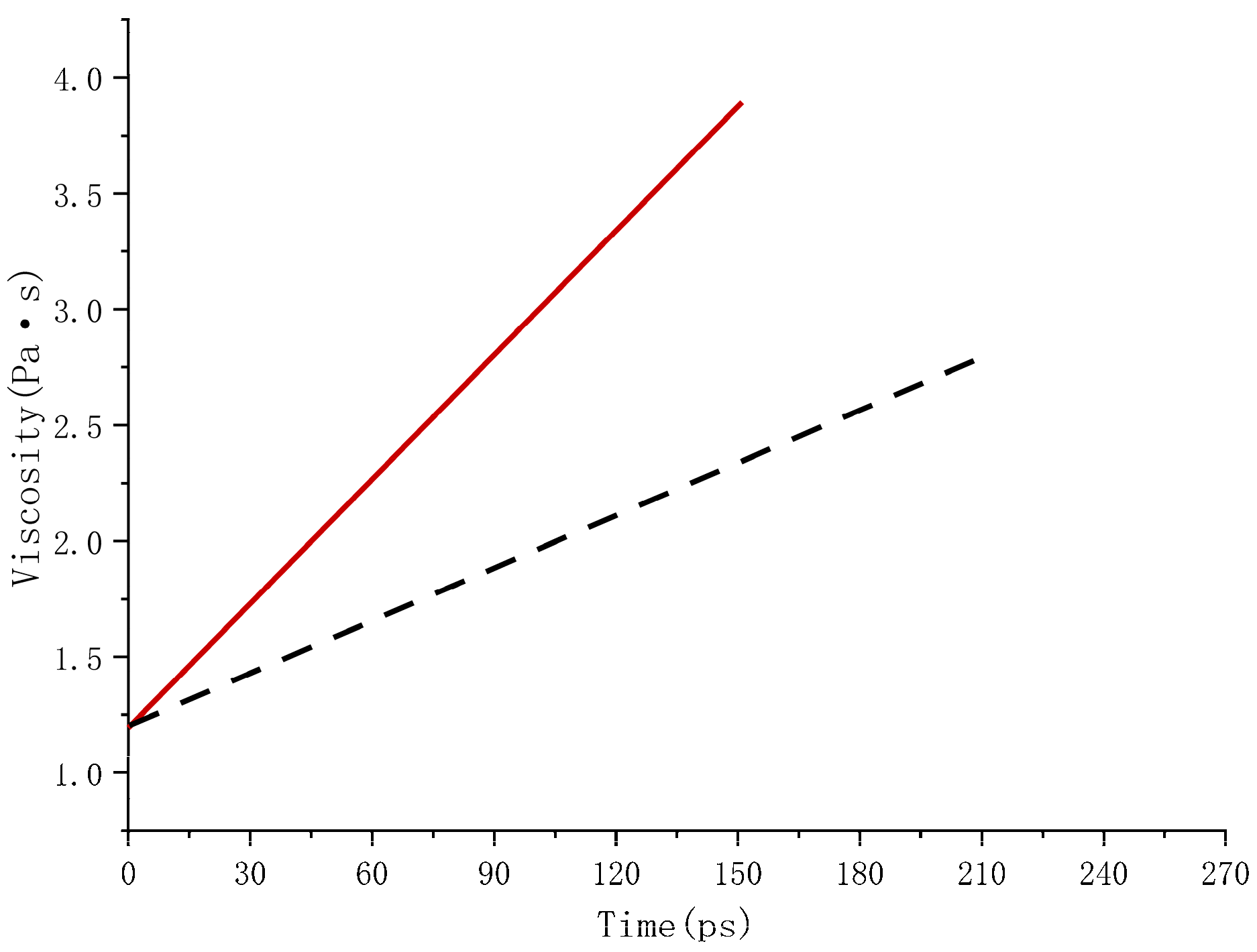
5.2. Analysis of the Macro Effects of Accelerated Swelling
- Δη—viscosity increment;
- Δt—time increment.
6. Consideration of New Processes for the Preparation of Modified Asphalt Using BPO and BPA
6.1. Materials and Reagents
6.2. Preparation Process
- (1)
- Asphalt pre-heating;
- (2)
- Addition of modifiers and accelerators;
- (3)
- High-shear swelling;
- (4)
- Product collection.
6.3. Characterisation Methods
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, H.; Zhang, Z.; Xie, J.; Gui, Z.; Li, N.; Xu, Y. Analysis of OMMT strengthened UV aging-resistance of Sasobit/SBS modified asphalt: Its preparation, characterization and mechanism. J. Clean. Prod. 2021, 315, 128139. [Google Scholar] [CrossRef]
- Kok, B.V.; Yetkin, Z.U.; Yalcin, E.; Yilmaz, M. Comparison of the preparation conditions for the modification of crumb rubber in laboratory and asphalt plant in terms of rheological properties. Constr. Build. Mater. 2024, 419, 135461. [Google Scholar] [CrossRef]
- Yan, K.; Zhang, H.; Xu, H. Effect of polyphosphoric acid on physical properties, chemical composition and morphology of bitumen. Constr. Build. Mater. 2013, 47, 92–98. [Google Scholar] [CrossRef]
- Han, X.; Mao, S.; Zeng, S.; Duan, H.; Liu, Q.; Xue, L.; Yu, J. Effect of reactive flexible rejuvenators on thermal-oxidative aging resistance of regenerated SBS modified asphalt. J. Clean. Prod. 2022, 380, 135027. [Google Scholar] [CrossRef]
- Li, J.; Yang, J.; Liu, Y.; Zhao, Z.; Tang, X.; Luo, J.; Muhammad, Y. Fabrication of IPDI-LDHs/SBS modified asphalt with enhanced thermal aging and UV aging resistance. Constr. Build. Mater. 2021, 302, 124131. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, H.; Chen, Z.; Li, Q.; Ye, S.; Liu, Q. Novel Multidimensional Composite Development for Aging Resistance of SBS-Modified Asphalt by Attaching Zinc Oxide on Expanded Vermiculite. Energy Fuels 2024, 38, 16772–16781. [Google Scholar] [CrossRef]
- Zhang, F.; Kaloush, K.; Underwood, S.; Hu, C. Preparation and performances of SBS compound modified asphalt mixture by acidification and vulcanization. Constr. Build. Mater. 2021, 296, 123693. [Google Scholar] [CrossRef]
- Wei, J.; Shi, S.; Zhou, Y.; Chen, Z.; Yu, F.; Peng, Z.; Duan, X. Research on Performance of SBS-PPA and SBR-PPA Compound Modified Asphalts. Materials 2022, 15, 2112. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yi, H.; Cui, Y.; Li, S.; Zhou, S.; Yan, C. Research on the Preparation Process of SBS-Modified Asphalt Using Early Shearing Instead of High-Speed Shearing of Modifier. Appl. Sci. 2023, 13, 10335. [Google Scholar] [CrossRef]
- Guo, L.; Xu, W.; Zhang, Y.; Ji, W.; Wu, S. Selecting the Best Performing Modified Asphalt Based on Rheological Properties and Microscopic Analysis of RPP/SBS Modified Asphalt. Materials 2022, 15, 8616. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Shen, Z.; Jiang, L.; Long, Q.; Yu, Y. Study on the Performance of Asphalt Modified with Bio-Oil, SBS and the Crumb Rubber Particle Size Ratio. Polymers 2024, 16, 1929. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, R.; Ghaffari, M.; Shokrollahi, P. Evaluation of polymer blend compatibility based on solubility parameters and molecular dynamics simulations. J. Mol. Liq. 2020, 308, 113064. [Google Scholar]
- Wang, Y.; He, L.; Liu, Z. Energy-efficient preparation of SBS-modified asphalt by optimizing swelling kinetics. J. Clean. Prod. 2022, 380, 135078. [Google Scholar]
- Lee, S.H.; Kim, H.M.; Kim, D.H. Prediction of polymer–solvent compatibility using Hansen solubility parameters and molecular simulations. Polym. J. 2017, 49, 509–516. [Google Scholar]
- Liang, M.; Li, P.; Fan, W. Compatibility and phase behavior of SBS-modified asphalt based on Hansen solubility parameters and molecular dynamics. Fuel 2021, 304, 121463. [Google Scholar]
- Guo, R.; Zhang, X.; Zhou, J. Reactive modification of SBS asphalt using organic peroxides: Mechanism and performance evaluation. Fuel 2023, 341, 127755. [Google Scholar]
- Zhao, K.; Sun, Y.; Zhang, Y. Study on the swelling kinetics and morphology of SBS-modified asphalt using rheological analysis. Constr. Build. Mater. 2022, 329, 127152. [Google Scholar]




| 12 Components | Number of Molecules | Mass Fraction | |
|---|---|---|---|
| saturation fraction | Squalane | 8 | 4.90 |
| Hopane | 8 | 5.02 | |
| aromatic fraction | DOCHN | 24 | 16.55 |
| PHON | 24 | 14.48 | |
| gelatine | Benzobisbenzothiophene | 30 | 12.93 |
| Pyridinohopane | 10 | 6.15 | |
| Trimethylbenzene-oxane | 8 | 6.80 | |
| Quinolinohopane | 8 | 5.58 | |
| Thio-isorenieratane | 8 | 4.82 | |
| asphalt | Phenol | 6 | 5.12 |
| Thiophene | 4 | 5.29 | |
| Pyrrole | 6 | 6.31 | |
| modifying agent | SBS | 5 | 5.13 |
| System Name | Cohesive Energy Density CED (MJ/m3) | Solubility Parameter δ (J/cm3)0.5 | Solubility Parameter Difference (J/cm3)0.5 |
|---|---|---|---|
| SBS–aromatic oil blending conventional system | 256.2 | 15.947 | 0.205 |
| SBS–aromatic oil blend accelerated swelling system | 250.0 | 15.742 | |
| Conventional modified asphalt system | 236.2 | 15.301 | 0.711 |
| Modified asphalt accelerated swelling system | 218.4 | 14.590 |
| Test Metrics | Softening Point | Penetration at 25 °C (dmm) | −10 °C Ductility (cm) |
|---|---|---|---|
| Addition of BPA and BPO | 62 | 78 | 18 |
| Normal conditions | 56 | 84 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, Z.; Yin, Z.; Lin, J.; Bu, X.; Yang, J.; Zheng, C. SBS-Modified Asphalt Accelerated Swelling Technology and Performance Evaluation. Buildings 2025, 15, 3927. https://doi.org/10.3390/buildings15213927
Lv Z, Yin Z, Lin J, Bu X, Yang J, Zheng C. SBS-Modified Asphalt Accelerated Swelling Technology and Performance Evaluation. Buildings. 2025; 15(21):3927. https://doi.org/10.3390/buildings15213927
Chicago/Turabian StyleLv, Zhifeng, Zeran Yin, Jianghai Lin, Xiaohui Bu, Jiahao Yang, and Chuanfeng Zheng. 2025. "SBS-Modified Asphalt Accelerated Swelling Technology and Performance Evaluation" Buildings 15, no. 21: 3927. https://doi.org/10.3390/buildings15213927
APA StyleLv, Z., Yin, Z., Lin, J., Bu, X., Yang, J., & Zheng, C. (2025). SBS-Modified Asphalt Accelerated Swelling Technology and Performance Evaluation. Buildings, 15(21), 3927. https://doi.org/10.3390/buildings15213927





