1. Introduction
In recent years, rapid industrial and economic development has led to accelerated urbanization and an increasing demand for construction materials, particularly steel and high-performance concrete. The continual rise in steel prices, especially in Asia, has resulted in the frequent theft of cast iron products such as manhole covers, posing serious public safety risks. At the same time, municipal solid waste management has become a pressing environmental concern. Although the government has constructed numerous waste incineration facilities over the past decade, Taiwan still generates approximately 10 million metric tons of waste annually. While incineration effectively reduces the volume of waste, it produces a substantial amount of incineration ash—about 15% of the original waste volume—of which fly ash remains especially problematic due to its high content of heavy metals and toxic substances such as dioxins. Without proper treatment, this fly ash poses a risk of severe secondary pollution. Finding ways to reduce and reuse incineration fly ash is therefore an urgent challenge [
1,
2].
Municipal solid waste incineration generates two types of ash: bottom ash and fly ash. Bottom ash, characterized by a larger particle size and lower concentrations of heavy metals and soluble salts, generally meets the regulatory limits set by the Environmental Protection Administration (EPA) and is thus classified as general industrial waste suitable for resource reuse [
2,
3]. In contrast, fly ash, with finer particle sizes, is collected by air pollution control devices and contains high concentrations of toxic heavy metals such as lead and zinc, as well as soluble salts, often exceeding hazardous waste thresholds and thus classified as hazardous waste [
3]. Tay [
4] reported that fly ash predominantly falls within the clay-size range (86%), with only a small portion in the sand-size fraction (14%), which is influenced by incinerator conditions such as combustion temperature, operating parameters, and APC (air pollution control) system type.
Heavy metal concentrations in fly ash are strongly correlated with particle size—smaller particles tend to contain higher levels of heavy metals [
5,
6,
7]. For instance, Pb content increases with surface area, and is positively correlated with the chlorine and carbon contents [
8,
9]. According to IAWG [
5], elements such as Ca, K, Na, and Cl are predominantly retained in fly ash after incineration, especially volatile heavy metals like Pb, Zn, and Cd, which condense on fly ash surfaces after volatilization. Furthermore, the chemical composition of fly ash varies depending on the waste composition, incinerator design, and the flue gas treatment method (e.g., wet or dry scrubbers, addition of Ca(OH)
2 or activated carbon). The high content of alkali metals like K and Na in fly ash may pose durability concerns when used in cement-based materials [
10].
Several stabilization and solidification techniques—such as cement-based solidification, chemical treatment, plasma vitrification, and wet chemical processes—have been developed for treating incinerator fly ash, each with specific advantages and limitations, as summarized in
Table 1. Among these, the ideal treatment method should aim to (1) completely eliminate dioxins and prevent their reformation; (2) recover heavy metals to avoid secondary leaching; (3) reduce the waste volume to zero, eliminating the need for landfilling; (4) produce valuable end products with broad applicability; (5) avoid secondary pollution and emissions during treatment; and (6) provide environmental, ecological, and energy-saving benefits [
1,
2]. In this context, the dense matrix and high binding capacity of UHPC offer a promising platform for the encapsulation and immobilization of hazardous constituents in incineration fly ash, potentially integrating environmental safety with advanced construction performance [
11,
12].
IFA and WQS are potential by-products that can be reused as partial replacements for cement or quartz powder in concrete materials. While numerous studies have explored the incorporation of such materials in ordinary concrete, their application in UHPC remains in the exploratory stage. Effective utilization of these industrial by-products could reduce carbon emissions, lower material costs, and promote resource recycling. However, due to the high concentrations of hazardous heavy metals in IFA, direct landfilling without proper intermediate treatment can result in significant leaching risks. The environmental impact of heavy metals varies depending on their chemical forms, mobility, and origin. Therefore, pretreatment is essential to stabilize these metals and prevent ecological harm [
10].
Dioxins in waste fly ash are highly toxic, and improper treatment poses serious safety risks. In the study by Yajun Lv et al. [
13], high-temperature treatment reduced dioxin levels to 1/1209 of the original. When 20% of cement was replaced with treated MSWI fly ash, UHPC showed faster hydration, lower fluidity, shorter setting time, and improved 28-day compressive strength. The treated fly ash also produced harmless pores (<20 nm) and effectively bound heavy metals, indicating its potential as a cement substitute in UHPC. Lee et al. [
14] also showed that adding incinerator fly ash to UHPC supports recycling and carbon reduction without harming its mechanical performance, indicating practical application potential.
UHPC is known for its outstanding compressive and flexural strength, extremely low porosity, and superior durability. It has been widely applied in infrastructure such as bridges, roadways, and structural reinforcement [
15,
16,
17]. These superior properties result from the use of fine powders and a low water-to-binder ratio, making UHPC highly sensitive to the quality and characteristics of its constituent materials. Therefore, when incorporating alternative materials such as IFA or WQS, it is crucial to evaluate their effects on workability, mechanical behavior, and long-term durability. In addition, the chemical stability and heavy metal leaching potential of such waste-based materials are essential environmental assessment criteria [
14,
18].
UHPC exhibits exceptional ductility—up to 250 times greater than that of conventional concrete—which, along with its extremely low permeability, makes it resistant to chemical attack and ideal for use in aggressive environments [
19,
20,
21,
22]. According to Lee et al. [
14], UHPC also demonstrates excellent bonding strength, dynamic modulus, and durability, making it suitable for structural repair and strengthening. Moreover, its dense matrix gives UHPC strong solidification capability, suggesting its potential for encapsulating toxic substances effectively.
On the other hand, WQS, a by-product of thermal plasma gasification technology used for municipal solid waste (MSW) treatment, has emerged as a promising sustainable material. In this high-temperature process, organic matter is converted into syngas, while inorganic residues are vitrified into inert slag. This slag retains most of the heavy and alkali metals present in the original waste and can be used as a construction material [
12,
18].
Therefore, this study investigates the effects of incorporating various replacement ratios of IFA or WQS into UHPC as partial substitutes for quartz powder or cement. After 28 days of curing, mechanical and load-bearing tests were conducted on both laboratory specimens and full-scale trench and manhole cover products to assess their performance under practical conditions. This roadmap helps readers follow the structure and purpose of the research.
2. Experiments
The primary objective of this study is to investigate the effects of incorporating different replacement ratios of IFA or WQS into ultra-high-performance concrete (UHPC) as partial substitutes for quartz powder or cement. Basic mechanical tests were conducted, and after the specimens reached 28 days of curing, loading tests were performed. In addition to laboratory-scale specimens, two types of trench cover and manhole cover products were fabricated using selected UHPC mixtures and subjected to standardized load-bearing tests in order to evaluate their applicability under practical service conditions. The study aims to explore the relationship between the replacement percentage of IFA or WQS and the resulting strength, thereby determining the optimal replacement content for both structural performance and product-level applications.
2.1. UHPC Materials and Mixtures
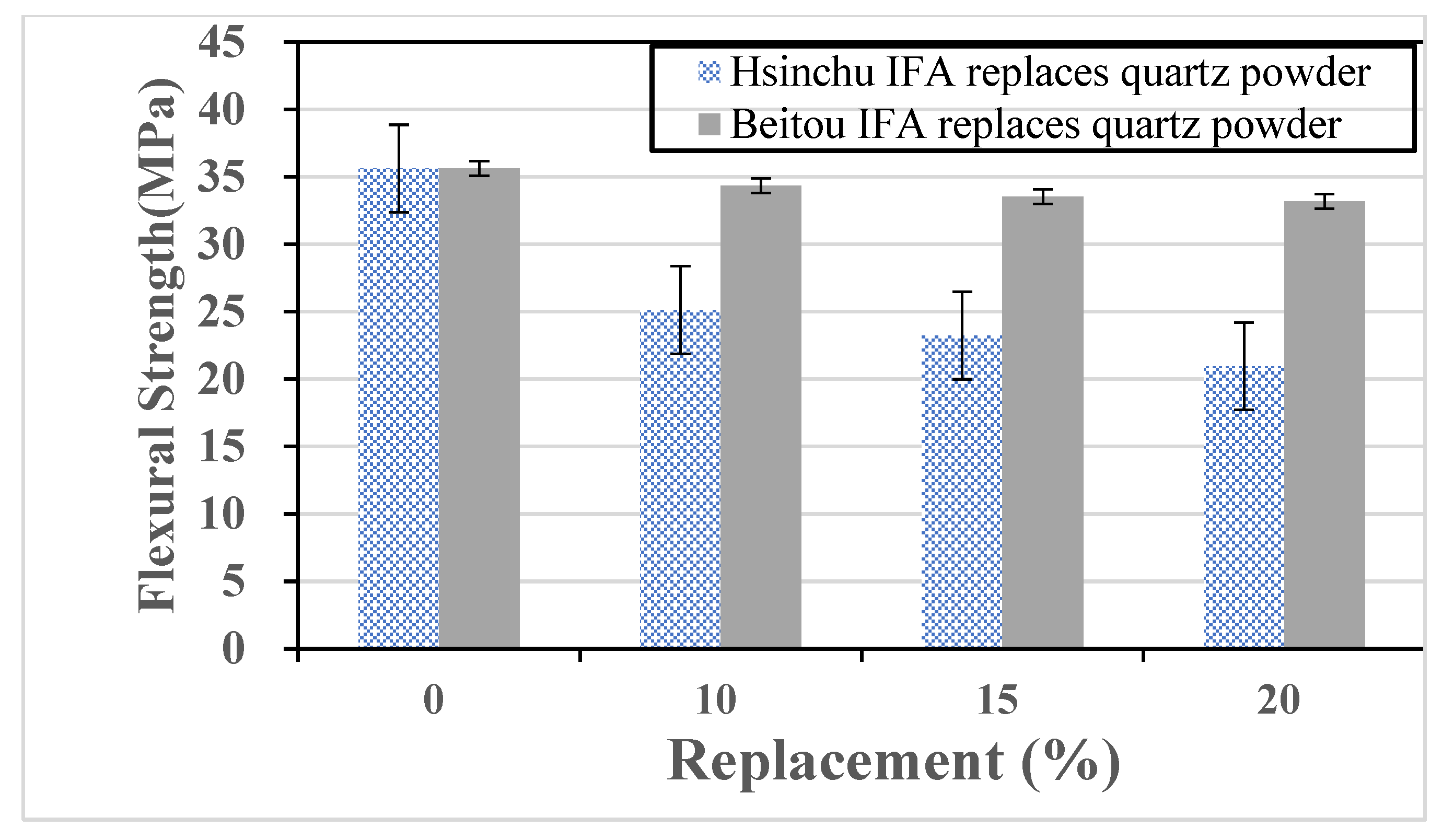
The test specimens in this study were made using ultra-high-performance concrete (UHPC). The primary materials and mixtures included water, cement, silica sand, silica fume, quartz powder, superplasticizer, defoaming agent, and steel fibers. The key variable in the experiment was the partial replacement of quartz powder or cement with either incinerator fly ash (IFA) or water-quenched slag (WQS), as shown in
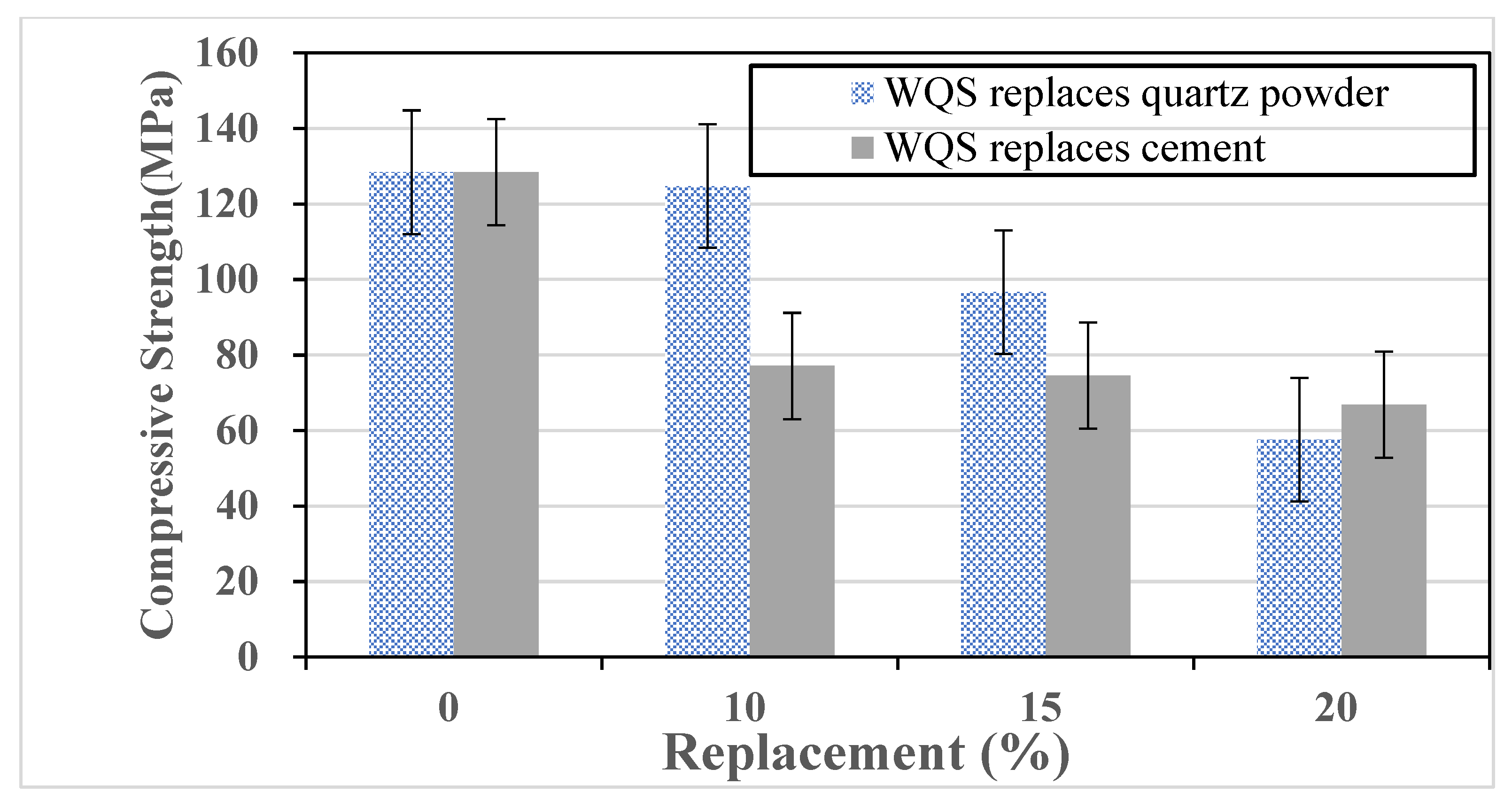
Table 2. WQS and IFA are distinct supplementary materials—WQS is a by-product of molten slag rapidly cooled by water, while IFA is a fine particulate residue collected from municipal solid waste incineration. The UHPC mix designs included two different series of samples: one series incorporated WQS and the other incorporated IFA as partial replacements, rather than a combined mixture of both materials. The variable in each group was the substitution ratio of quartz powder or cement, which was replaced by IFA or WQS by weight (10%, 15%, and 20% levels). This systematic variation allows for a direct comparison between the control group and the modified mixtures to determine the optimal replacement level in terms of mechanical performance and material sustainability.
The UHPC used in this experiment incorporated Type II Portland cement (moderate heat cement) produced by Taiwan Cement Corporation. Type II Portland cement generates a lower heat of hydration and has a slower heat release rate compared to Type I Portland cement. It also offers resistance to moderate sulfate attack, making it suitable for mass concrete structures. In Taiwan’s highly corrosive environment, the sulfate resistance of Type II cement enhances the durability of materials. The chemical composition and properties of the cement are shown in
Table 3 [
14].
The silica fume used in this study was produced by Sanlin Trading Co. (Taichung, Taiwan) and had a particle size ranging from 0.1 to 1 μm. Its chemical composition and physical properties are shown in
Table 4. The main component is silicon dioxide (SiO
2), which accounts for approximately 90%.
Quartz powder not only promotes the formation of C-S-H gel through the reaction with calcium hydroxide (CH), but also enhances the strength of ultra-high-performance concrete (UHPC) after high-temperature curing. The quartz powder used in this study was produced by Chih Chun Industrial Co., Ltd. (Taipei, Taiwan) and had a particle size ranging from 5 to 20 μm. The optimal addition amount of quartz powder is generally 15–35% by weight of cement [
1]. The silica sand used in this study was also produced by Chih Chun Industrial Co., Ltd., model #3. The particle size distribution obtained from sieve analysis is shown in
Table 5. The fineness modulus (F.M.) of 2.54 indicates that the silica sand has a medium-fine grading, suitable for achieving dense particle packing in UHPC mixtures.
A high-range water-reducing admixture imported from Japan, produced by TAKEMOTO OIL & FAT Co., Ltd. (Gamagori, Japan), was used in this study. The product model is CHUPOL SSP104 and had a solid content of 30%. It functions effectively as a water-reducing agent. The main chemical component is an anionic surfactant based on acrylic acid graft copolymer, with a specific gravity ranging from 1.07 to 1.13.
The WQS (water-quenched slag) is a vitrified material composed of inorganic components from the CaO–SiO2–Al2O3 system. The major crystalline phases in this system are anorthite (CaO·Al2O3·2SiO2) and wollastonite (CaO·SiO2), both of which are needle-like crystals. These crystals interweave in a complex pattern within the glassy matrix, resulting in high strength, making the material highly suitable for use in civil engineering and construction. The properties of the molten slag can be classified according to its cooling method into air-cooled (dry) slag and water-cooled (quenched) slag.
In this study, all WQS used was ground to pass through a #200 sieve. The results of the toxicity characteristic leaching procedure (TCLP) for the WQS (unit: mg/L) are shown in
Table 6, and the chemical composition analysis is shown in
Table 7.
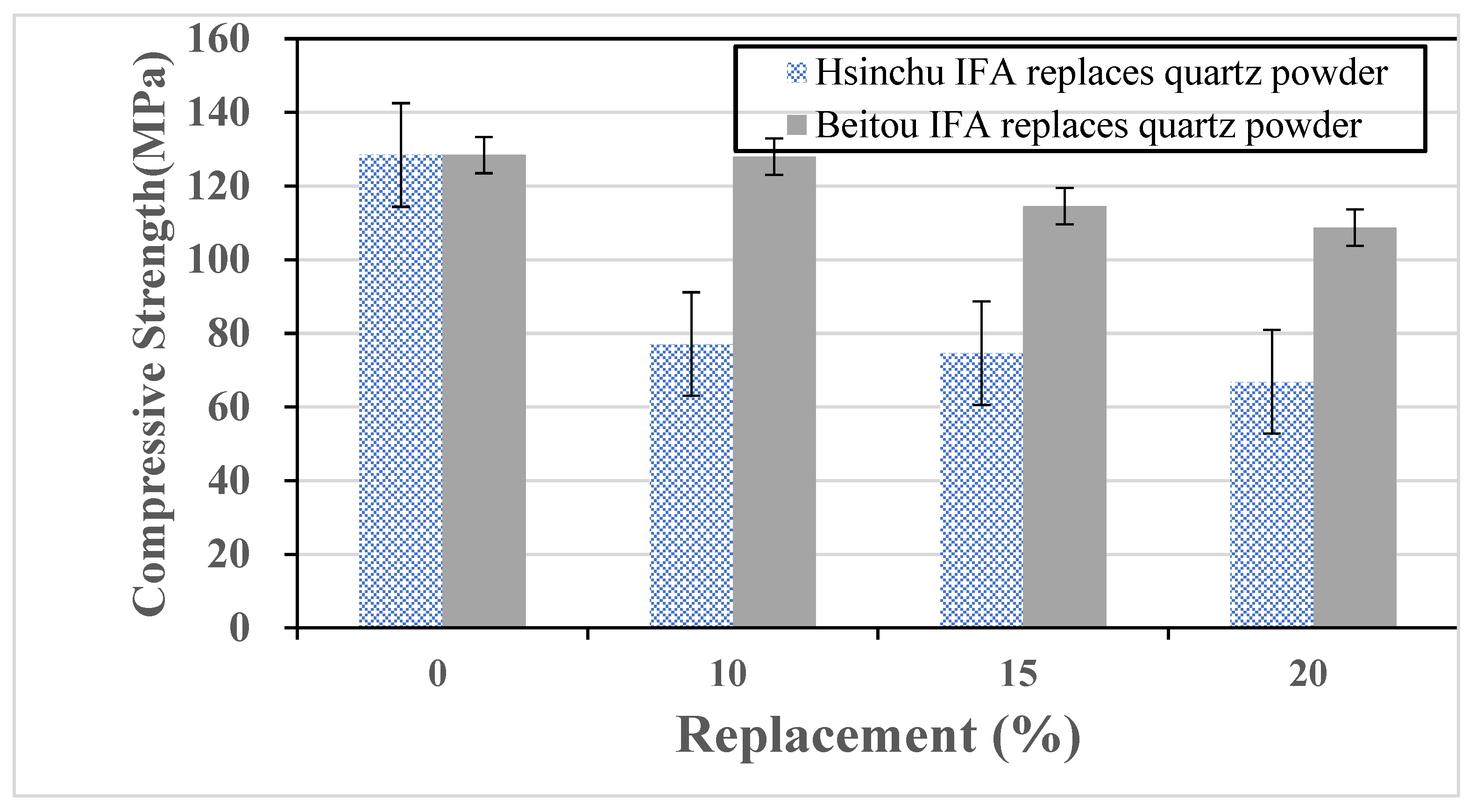
The study obtained the toxicity characteristic leaching results (unit: ppm) and chemical properties of municipal solid waste incineration fly ash from Hsinchu and Beitou, as shown in
Table 8 and
Table 9. According to the data, incineration fly ash exhibits high alkalinity. The particle size distribution of the fly ash is comparable to coarse fine aggregate. Due to its porous nature, the fly ash has a lower specific gravity and significantly higher water absorption than conventional aggregates. All incineration fly ash used in this study was ground to pass through a #200 sieve. The chemical composition of water-quenched slag (
Table 7) and incineration fly ash (
Table 9) was determined using X-ray fluorescence (XRF, PANalytical Axios, National Central University, Taoyuan, Taiwan). The concentrations of heavy metals presented in
Table 6 and
Table 8 were obtained through the Toxicity Characteristic Leaching Procedure (TCLP) in accordance with U.S. EPA Method 1311 [
23], followed by analysis using Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES, PerkinElmer Optima 8000, National Taiwan University, Taipei, Taiwan).
2.2. Test Method and Concrete Samples
The main testing items in this study involved utilizing the material characteristics of water-quenched slag and incineration fly ash to replace quartz powder or cement in UHPC. The test specimens included 4 cm × 4 cm × 16 cm prismatic specimens and Φ5 cm × 10 cm cylindrical samples. Basic mechanical tests were conducted, along with heavy metal content analysis within the specimens. By evaluating the strength variations in the UHPC, the optimal replacement ratio and the most suitable type of incineration ash residue were determined, aiming to reduce production costs and achieve the goal of resource reuse.
The compressive strength tests of cylindrical UHPC specimens were conducted in accordance with ASTM C39/C39M [
24]. Flexural performance of prismatic specimens followed ASTM C1609/C1609M [
25] for fiber-reinforced concrete. The chemical composition of the incineration fly ash and molten slag was analyzed according to ASTM C114 [
26], and heavy metal contents were examined following EPA Method 1311 (TCLP).
In addition to laboratory-scale specimens, two types of cover products were fabricated, namely trench covers (60 × 35 × 4 cm) and manhole covers (120 × 60 × 5 cm), as summarized in
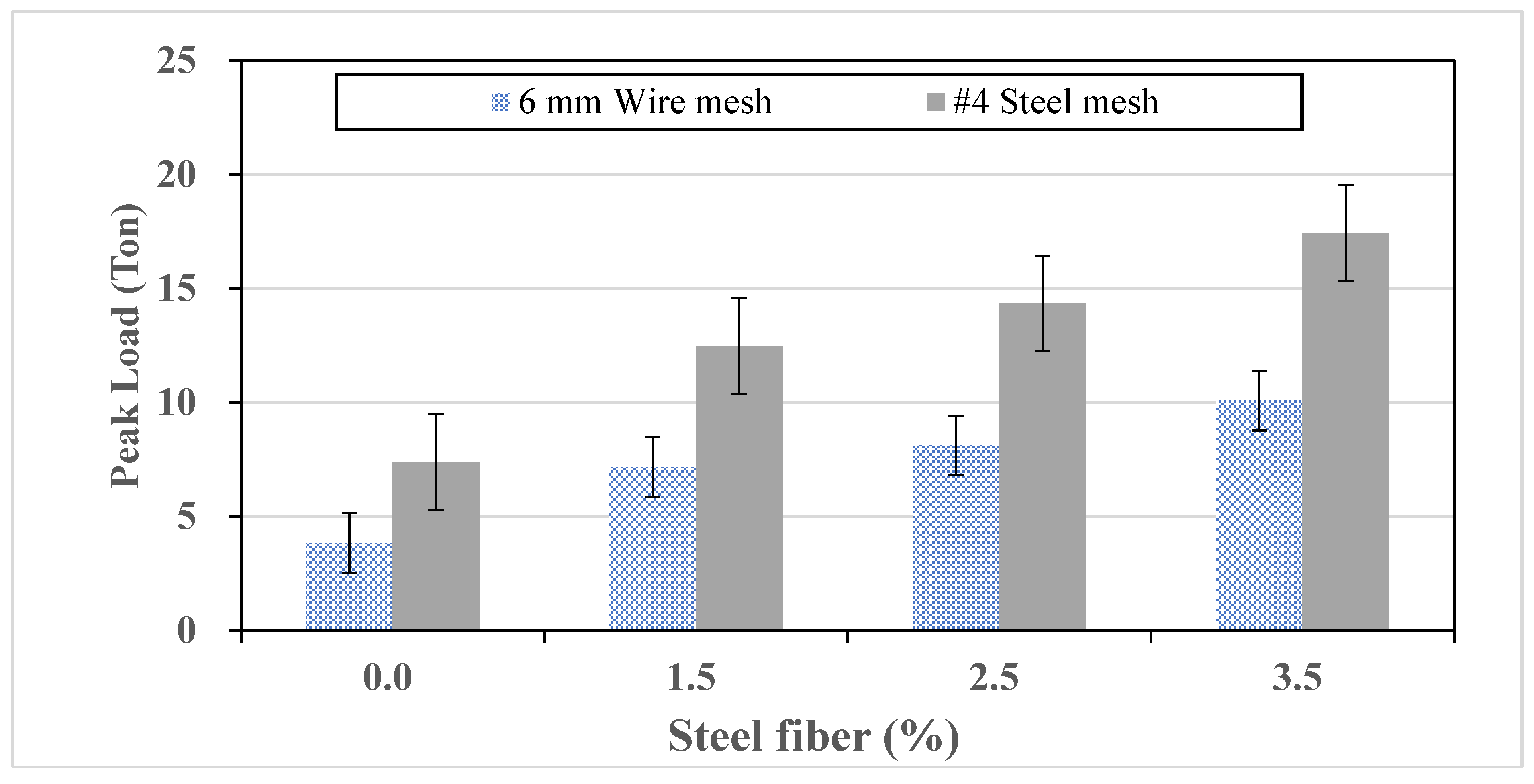
Table 10. The number of specimens prepared for each mix test included three cylindrical specimens, three prismatic specimens, three trench covers, and two manhole covers. Each cover was produced using selected UHPC mixtures with steel fibers at volume fractions of 0%, 1.5%, 2.5%, and 3.5%. To further enhance the toughness and load-bearing capacity, welded 6 mm wire mesh or #4 steel mesh was embedded at thicknesses ranging from 2.0 cm to 3.0 cm. The load-bearing test procedure, including equipment, boundary conditions, and load application points, followed the method reported by Lee et al. [
14]. Finally, full-scale load-bearing tests were conducted to evaluate the structural performance and service applicability of these UHPC-based cover products.
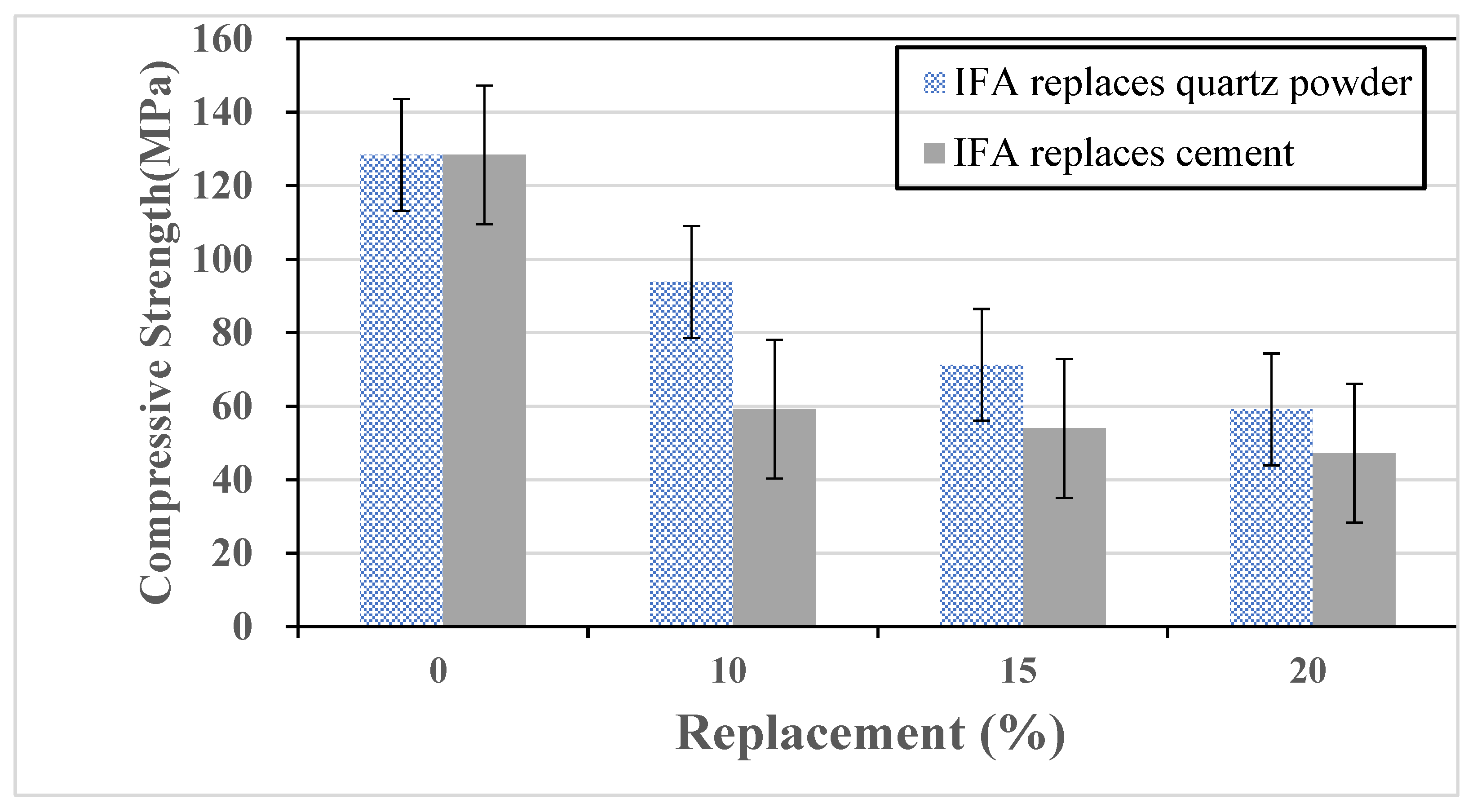
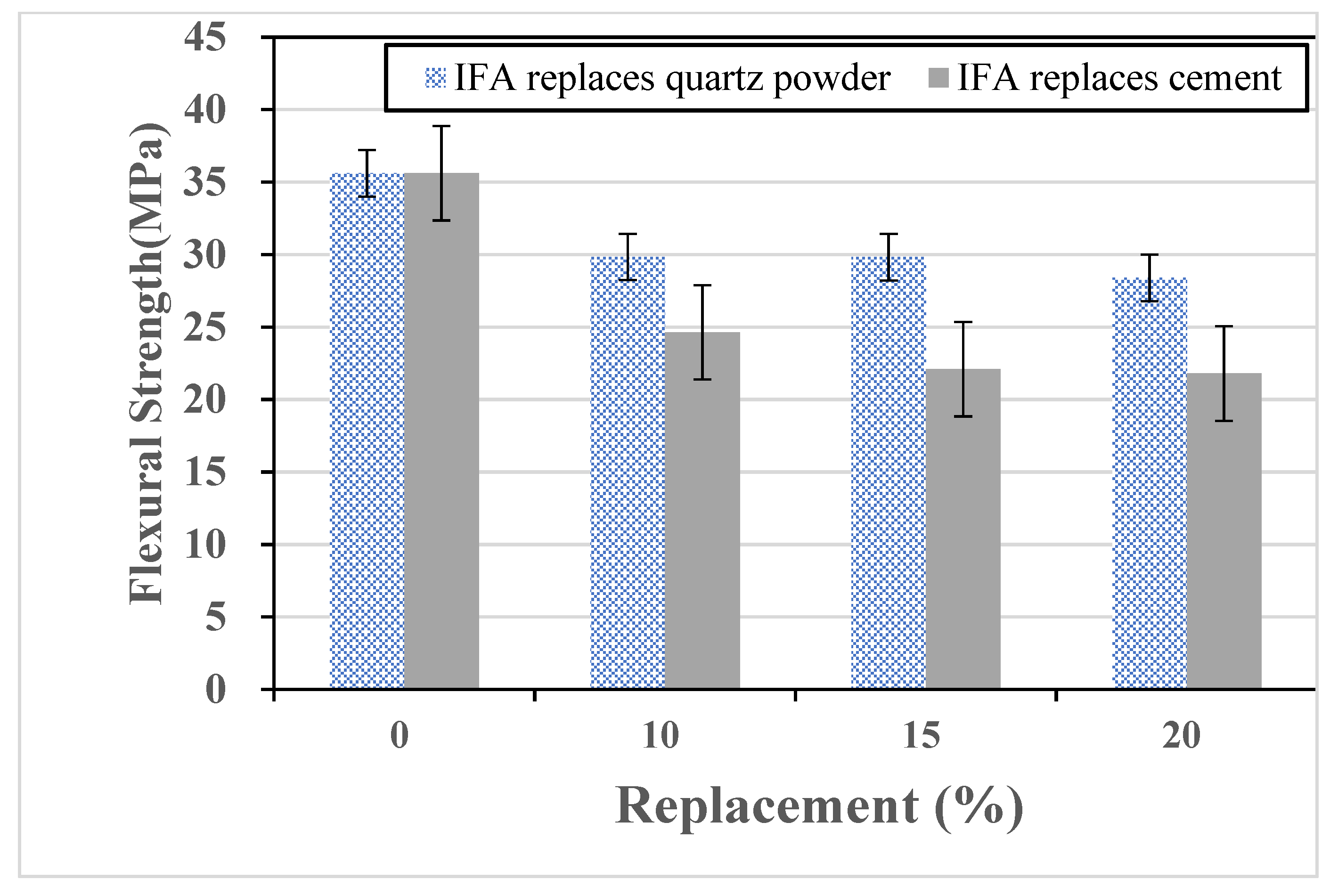
Fibers are incorporated into concrete to mitigate shrinkage-induced surface cracking and provide tensile resistance under compression, thereby delaying crack initiation and propagation. In this study, ultra-high-performance concrete (UHPC) incorporating 2.5% steel fibers by volume was evaluated for mechanical performance with partial cement replacement by IFA or WQS at 0%, 10%, 15%, and 20% by weight. Cylindrical specimens (Φ5 cm × 10 cm) were tested in compression, and prismatic specimens (4 cm × 4 cm × 16 cm) in flexure. After demolding, the UHPC specimens were placed in a high-temperature steam curing cabinet at a constant temperature of 90 °C for four days to accelerate hydration and decomposition reactions within the concrete, as shown in
Figure 1. Subsequently, the specimens were oven-dried at 90 °C for two days (
Figure 2), followed by immersion in a saturated limewater curing tank at room temperature. All tests were performed after the specimens reached 28 days of age. High-temperature curing was employed to refine the UHPC microstructure, enhance the early-age strength, and assess the strength development using varying fly ash replacement levels.
2.3. Analysis of the Heavy Metals in Leachate
Analysis of the heavy metals in the leachate was conducted following the TCLP method (EPA Method 1311) to evaluate the environmental suitability of incineration residues [
23]. The reuse potential of incineration bottom ash depends largely on its heavy metal content. If the leachate concentrations obtained through the Toxicity Characteristic Leaching Procedure (TCLP) are below national environmental standards, the material may be considered suitable for recycling. Therefore, accurate heavy metal analysis is essential.
In this study, TCLP was conducted on bottom ash samples, and the leachate was analyzed for its heavy metal contents. Most analyses were performed using atomic absorption spectroscopy (AAS), while lead (Pb) was also tested using the Merck Nova 60 (Chung Hua University, Hsinchu, Taiwan), a portable spectrophotometric device. Compared to AAS or ICP-AES, the Nova 60 offers faster and more convenient testing using reagent kits, making it suitable for on-site applications. The lead detection procedure using the Merck Nova 60 is as follows [
27]:
- a.
Prepare the lead test kit.
- b.
Adjust leachate pH to 3–6 using ammonia or nitric acid.
- c.
Measure total hardness:
- ○
If <70 mL, proceed with Step A only.
- ○
If ≥70 mL, perform Steps A and B.
Step A: Add 5 drops of reagent PK-1 and 5 mL of leachate. Mix.
Insert tube into the Nova 60 and record Reading A, as shown in
Figure 1.
Step B: Add one scoop of PK-2 to the same tube. Mix.
Insert again and record Reading B.
- d.
Final concentration:
- ○
If hardness < 70 mL → result = A;
- ○
If hardness ≥ 70 mL → result = A – B.