Mechanical Performance Degradation and Microstructural Evolution of Grout-Reinforced Fractured Diorite Under High Temperature and Acidic Corrosion Coupling
Abstract
1. Introduction
2. Test Materials and Procedures
2.1. Preparation of Fractured Diorite Specimens
2.2. Grouting Methods and Test Procedures
3. Results and Analyses
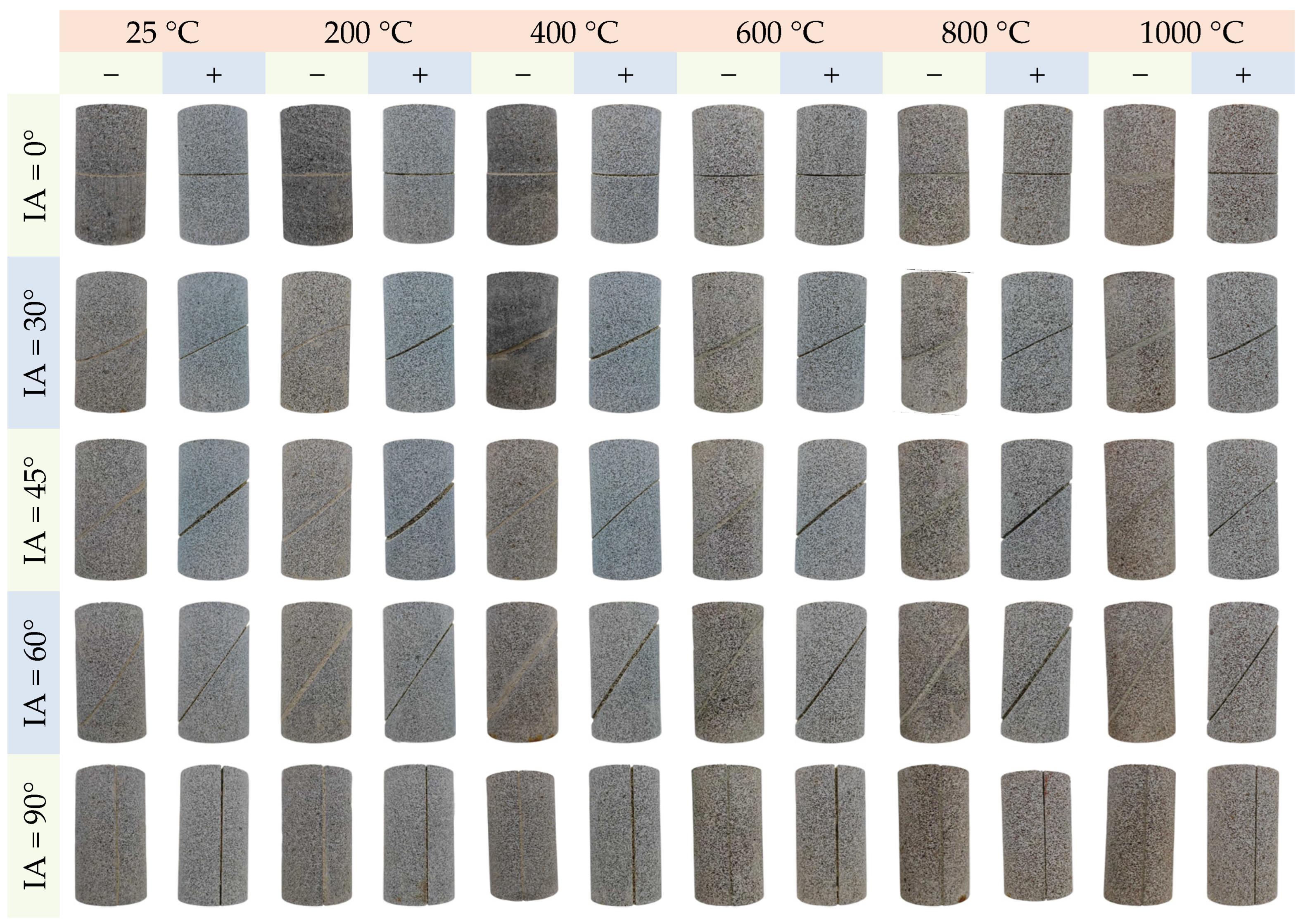
3.1. The Change of Apparent Color
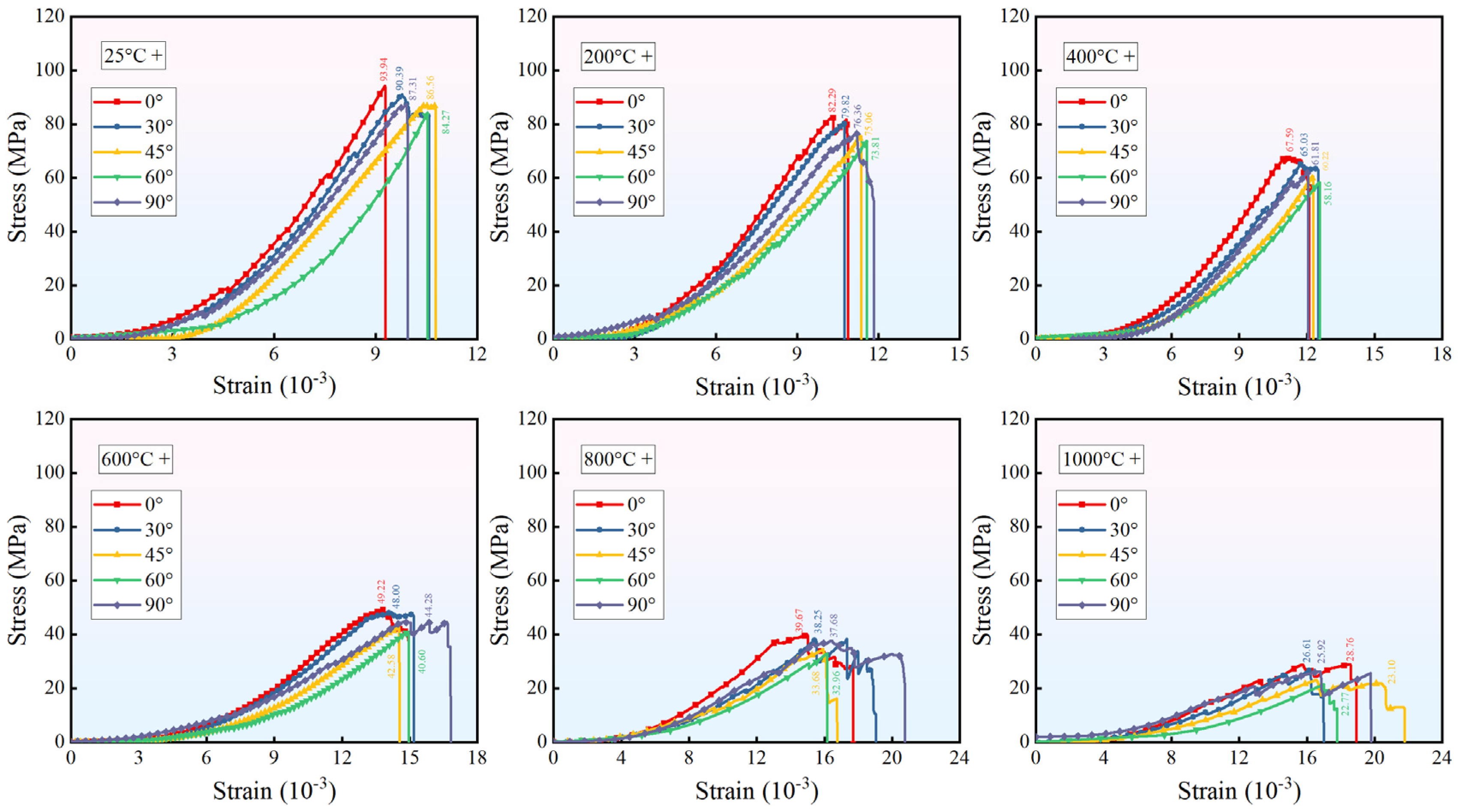
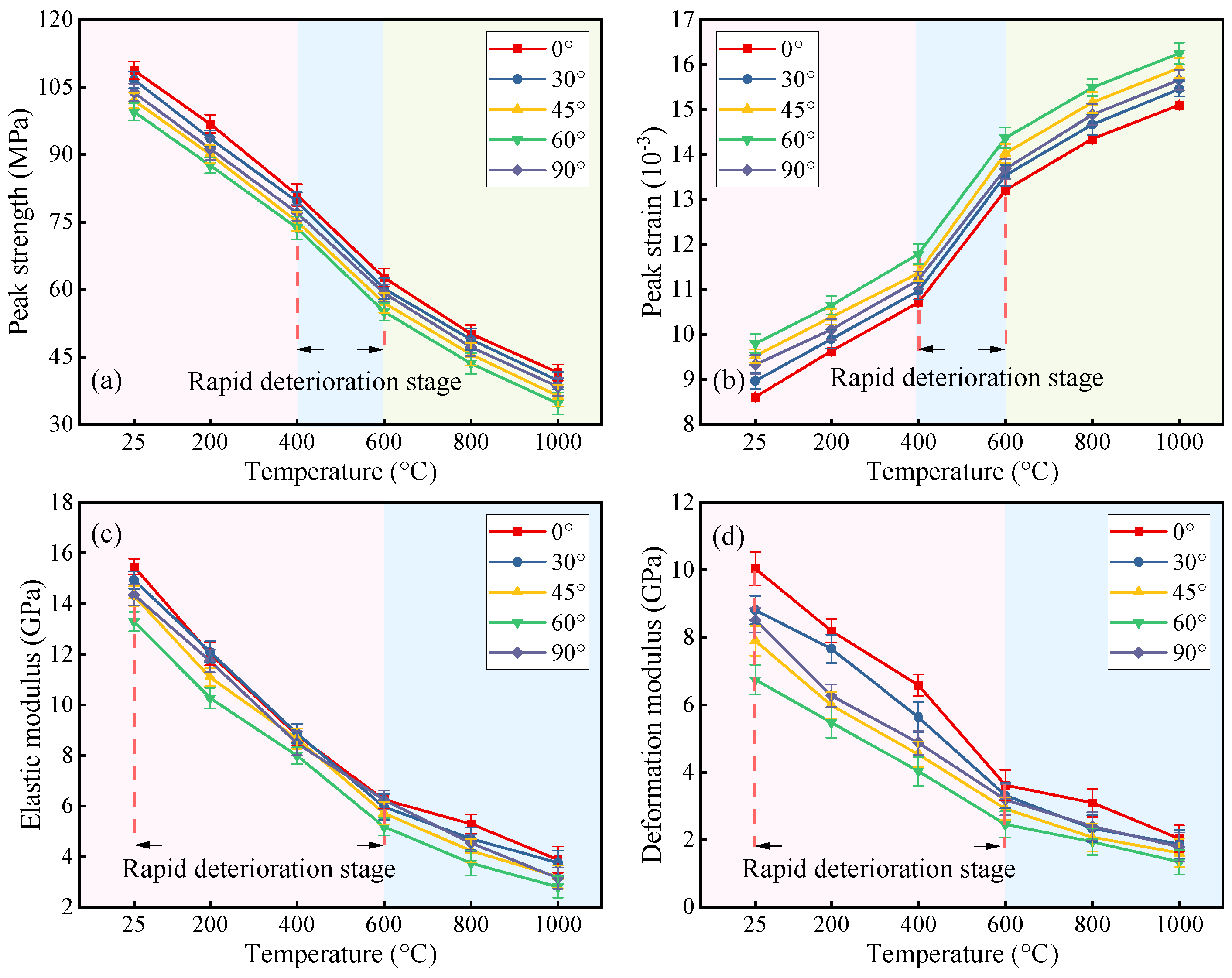
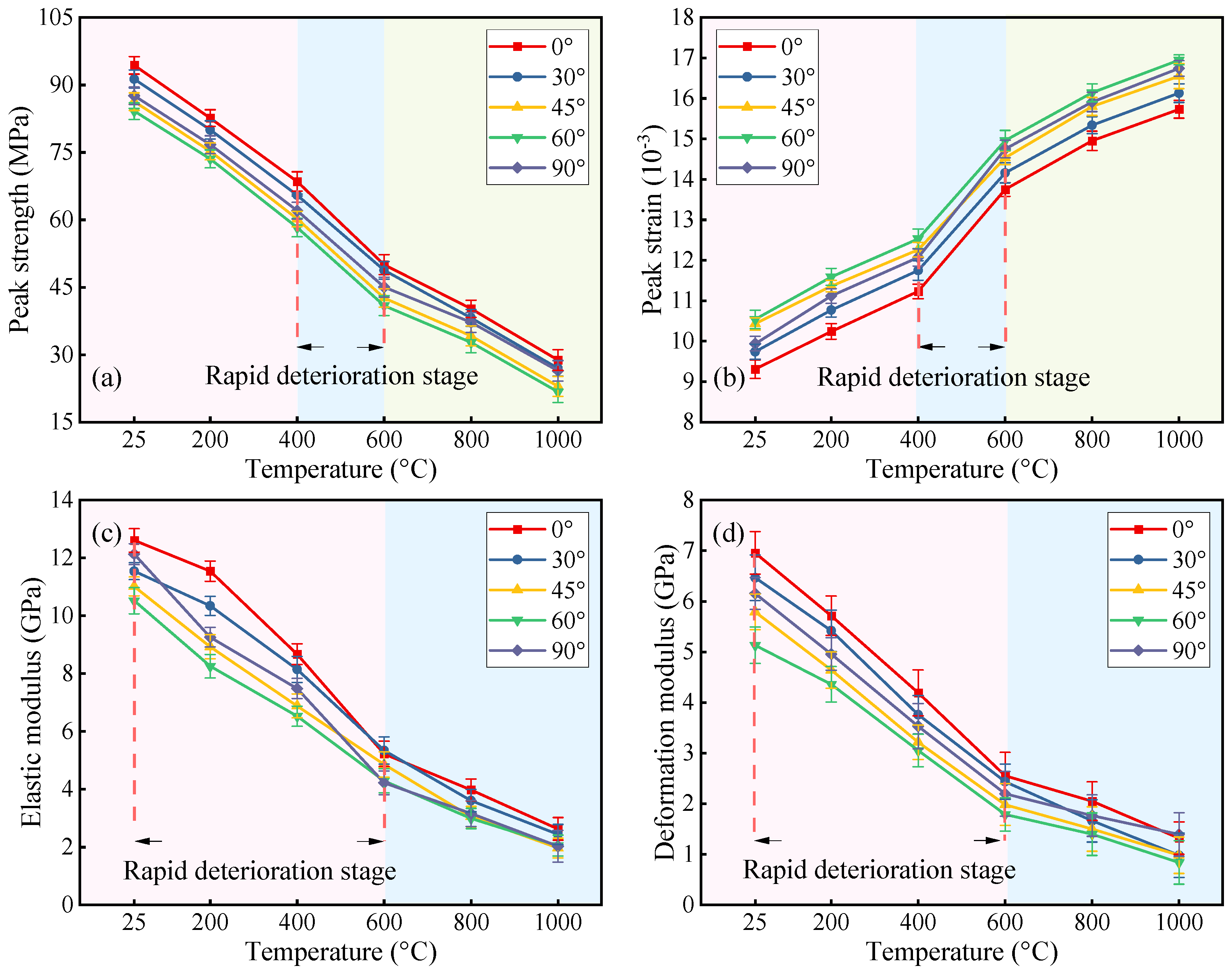
3.2. Mechanical Test Results
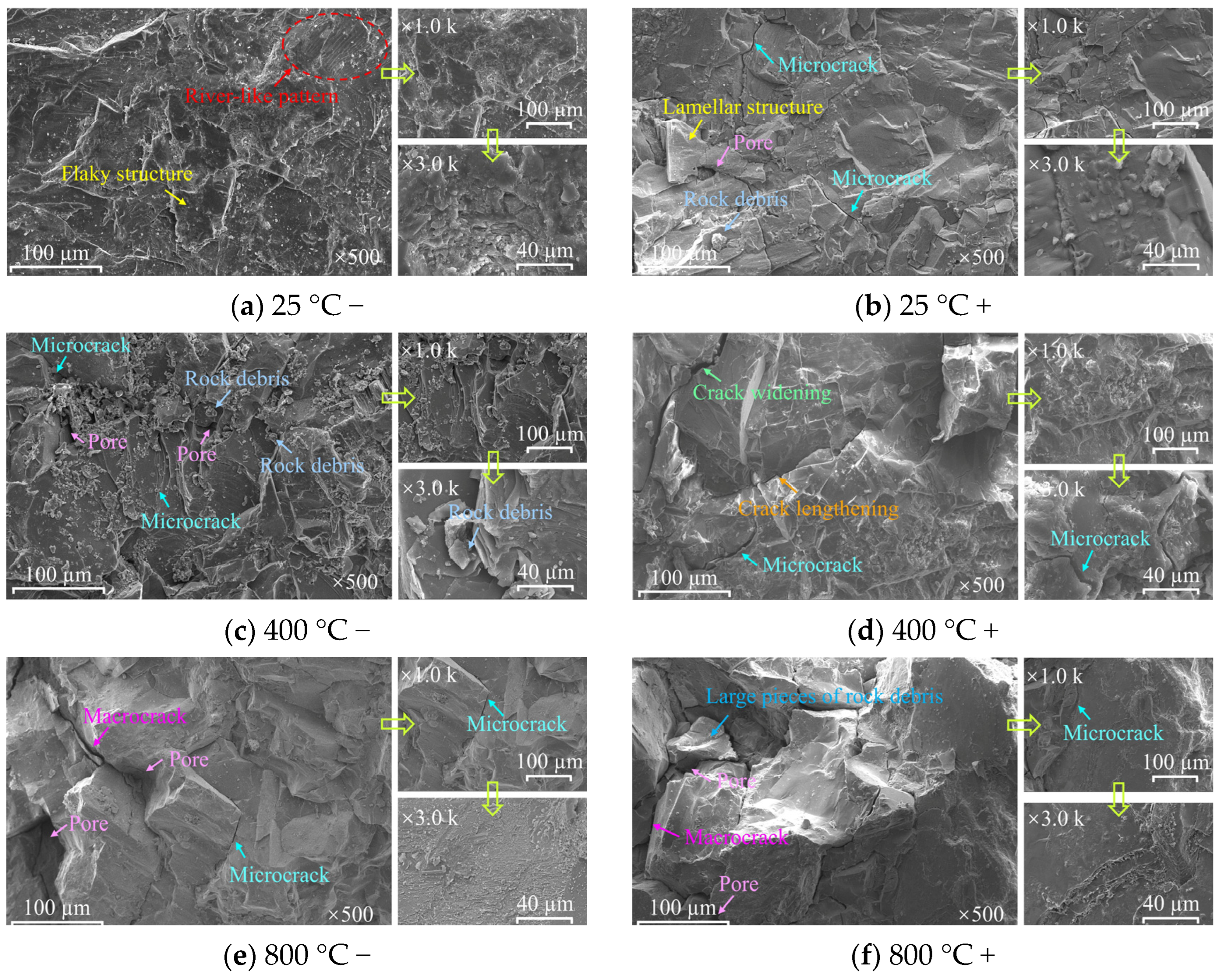
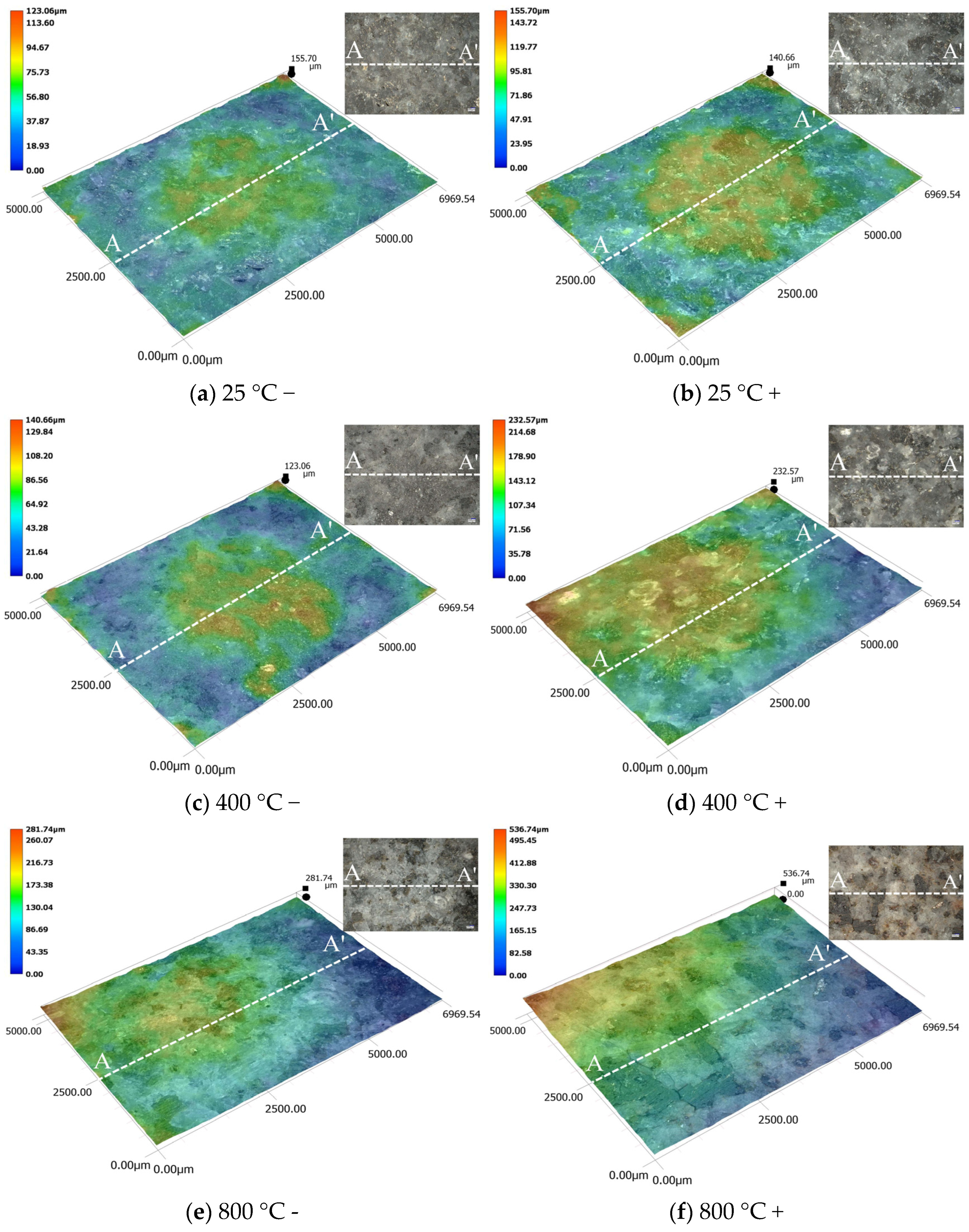
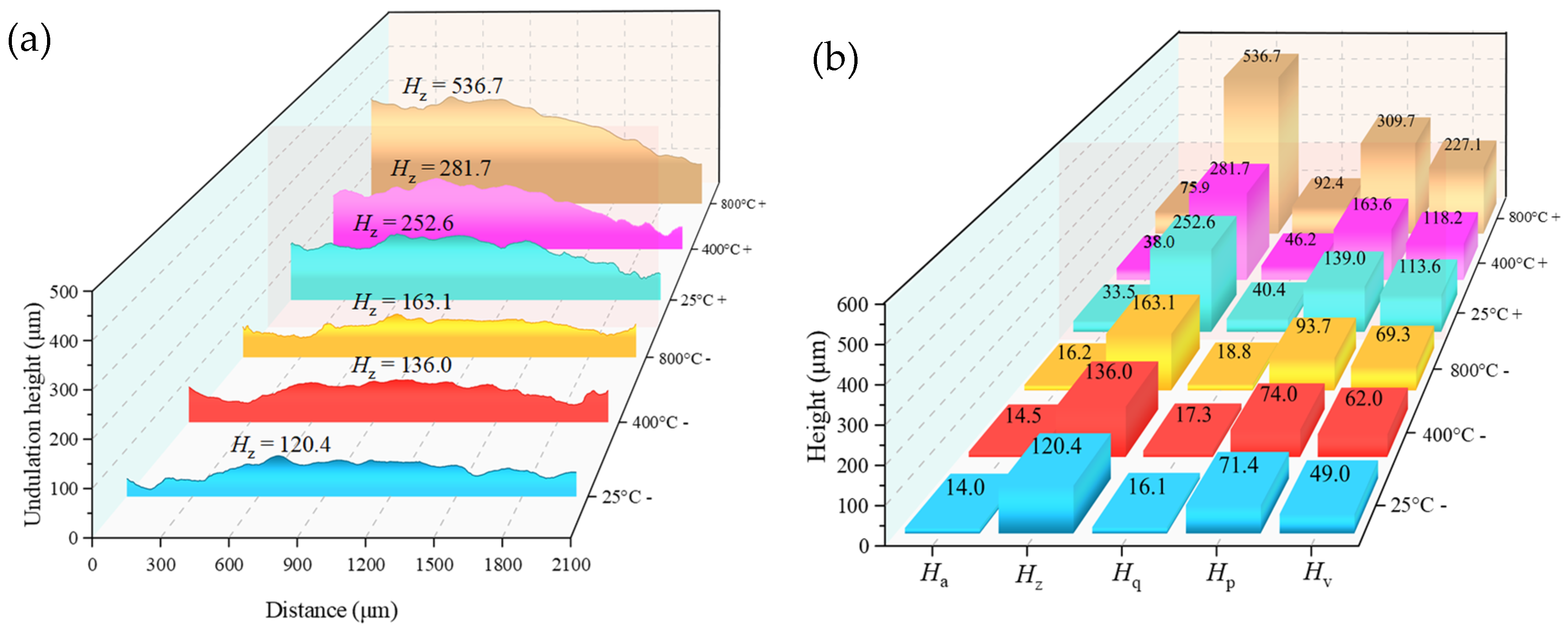
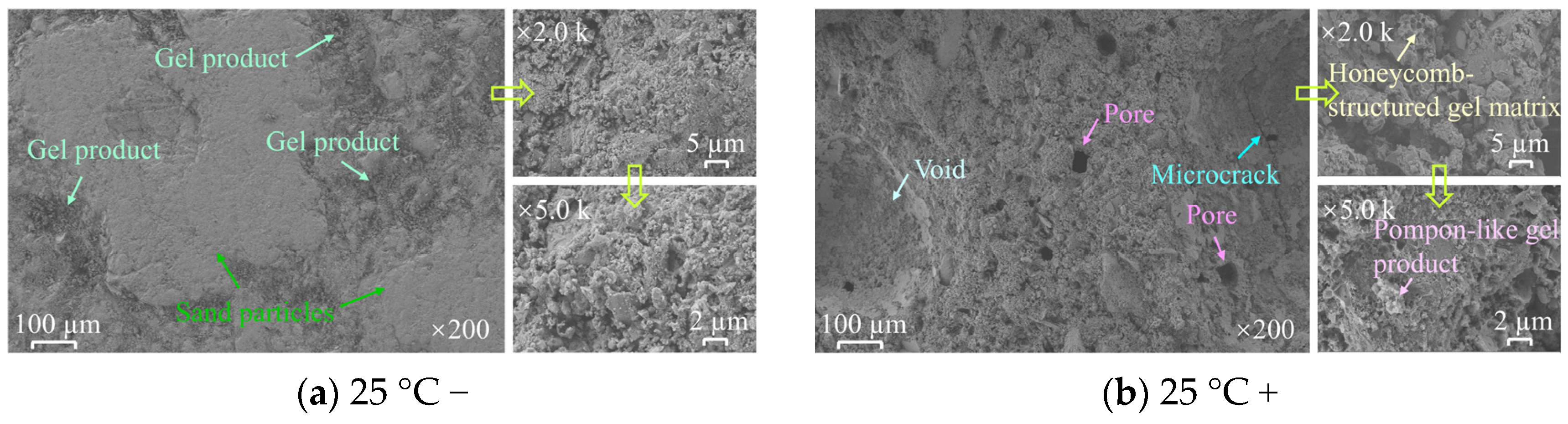
3.3. Results of SEM and 3D Topography Scans
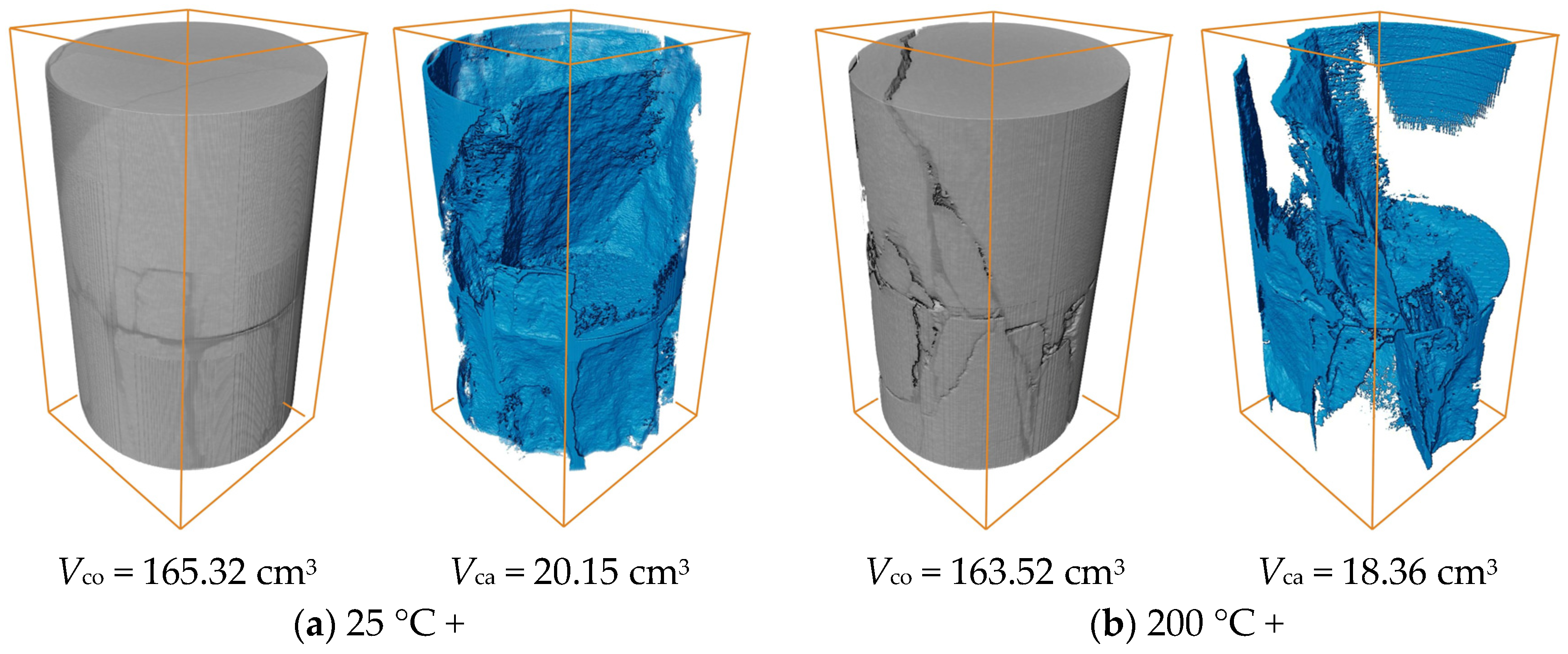
3.4. CT Scan Results
4. Discussion
4.1. Analysis of Damage Mechanism
4.2. Fitting of Strength Parameters
5. Conclusions
- (1)
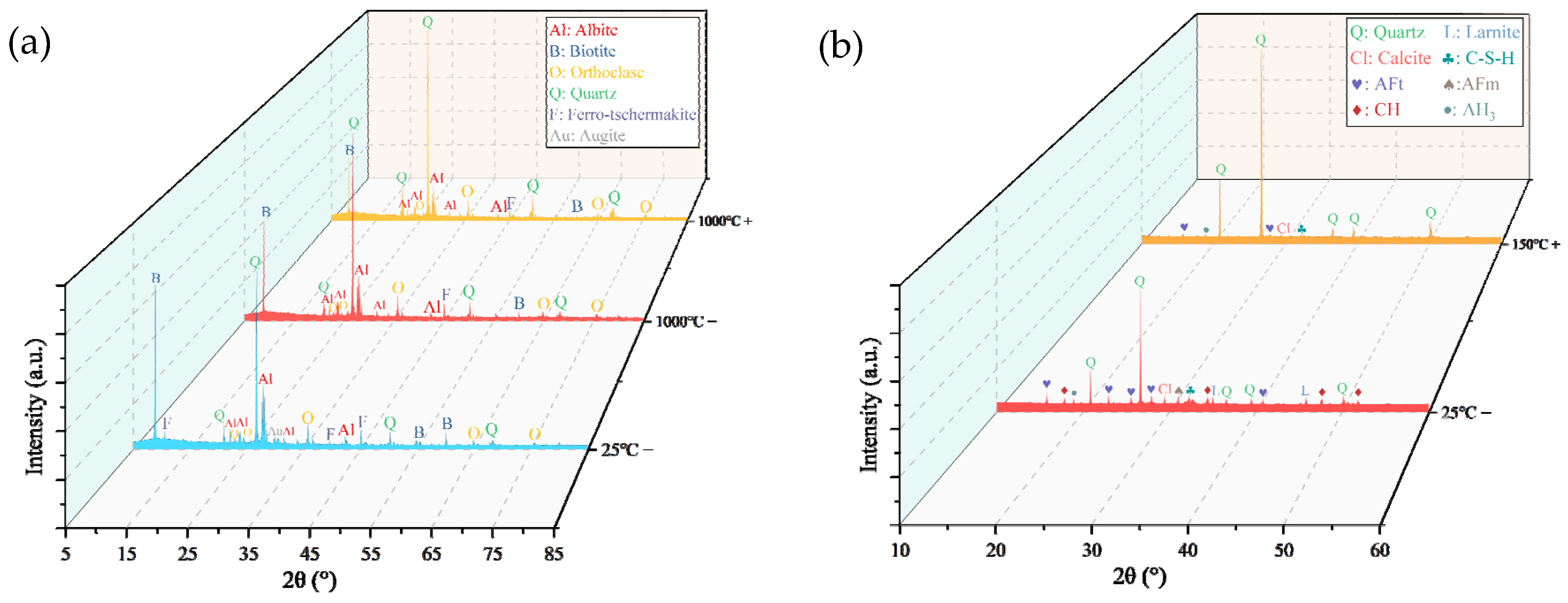
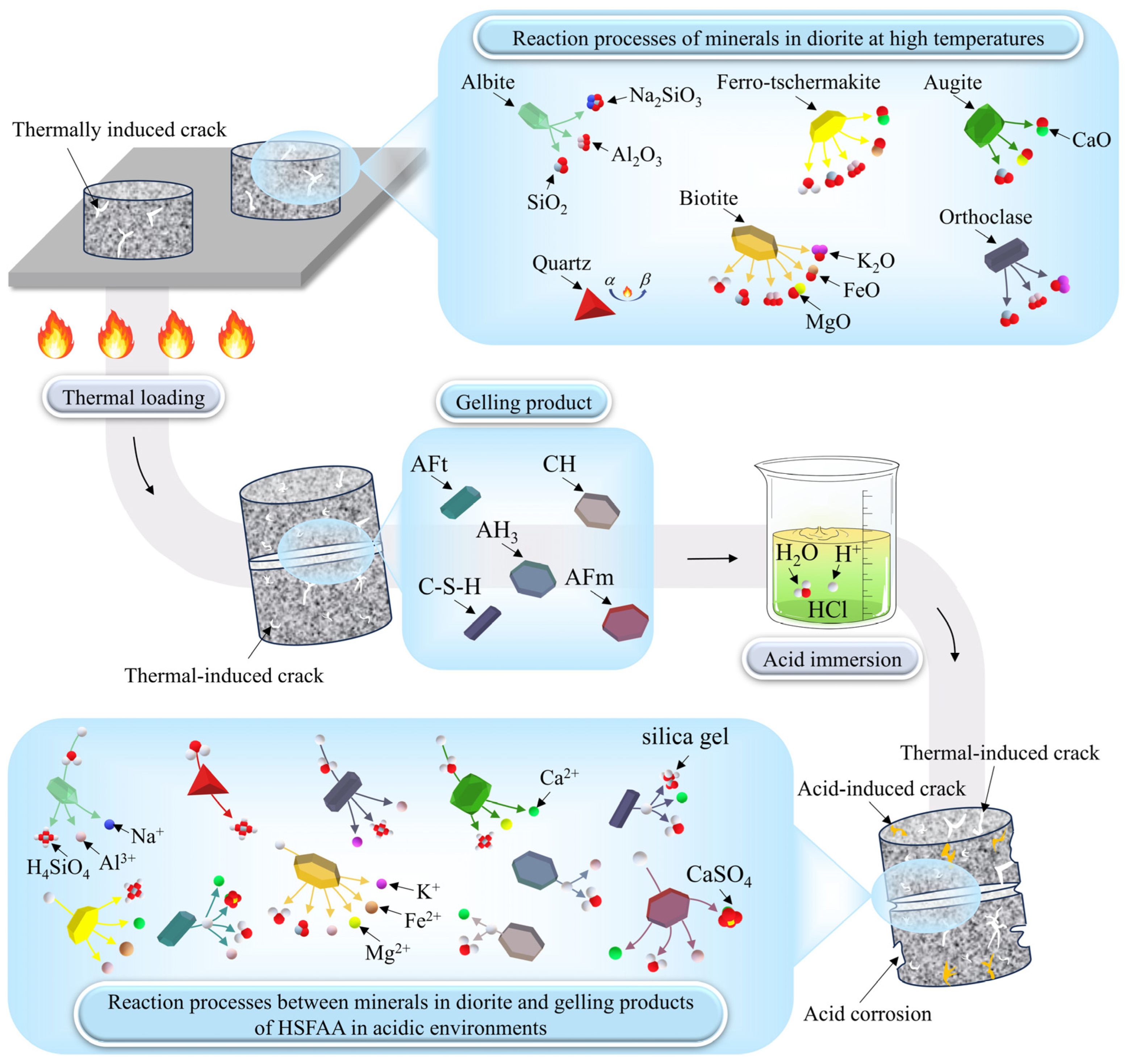
- When thermal treatment exceeded 600 °C, phase reconstruction and oxidation reactions of minerals like biotite transformed surfaces from gray-black to brown. Acid immersion led to whitening and severe corrosion at HSFAA edges. The “thermal–acid” coupling accelerated mineral dissolution and structural transformation, resulting in a whitening appearance dominated by insoluble mineral residues.
- (2)
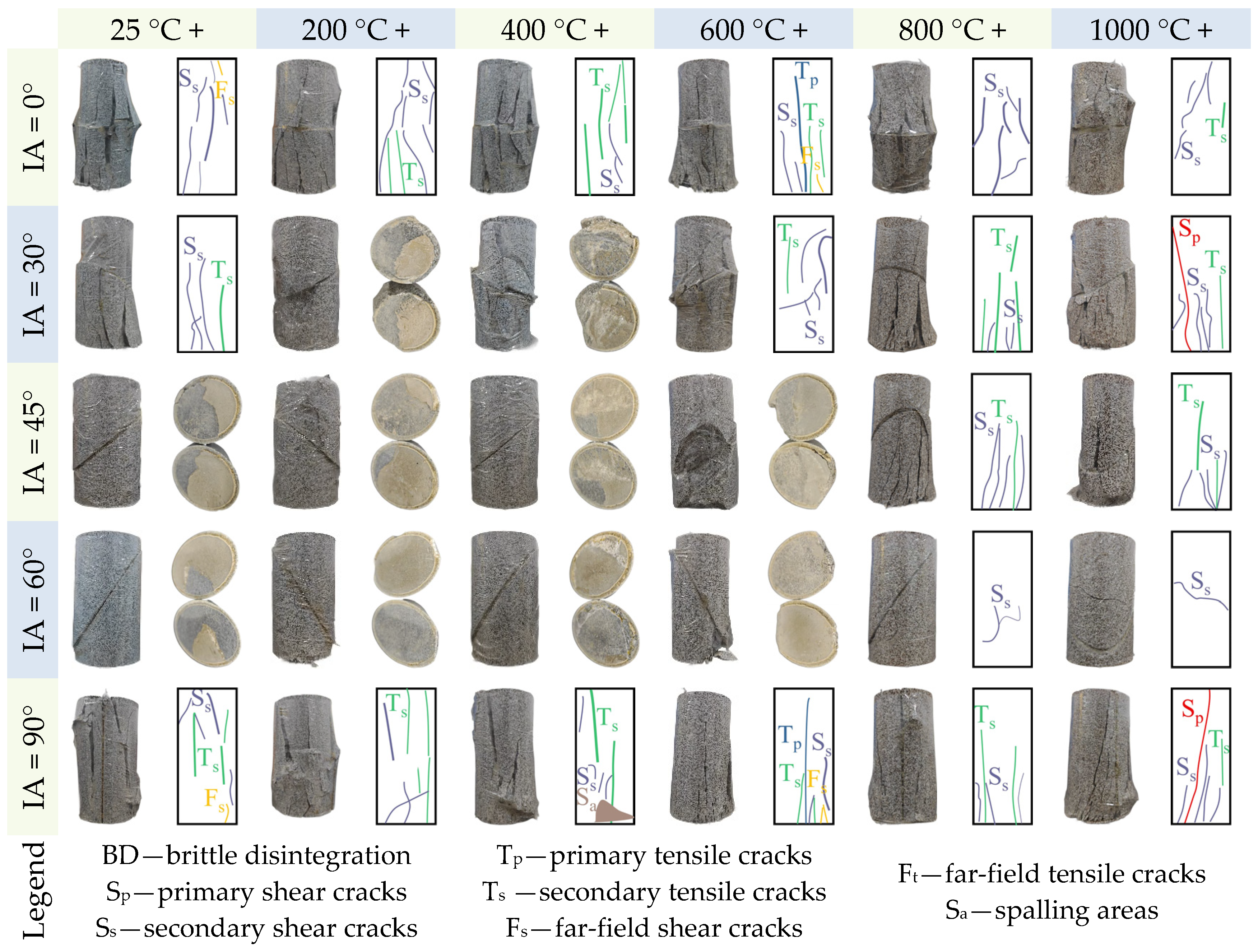
- Increasing fracture angles (0–60°) exacerbated interfacial shear slip and reduced peak strength by up to 69.46% under thermal–acid coupling. Specimens with 90° angles retained higher strength. High temperatures (>600 °C) degraded stiffness and enhanced ductility, while acid corrosion further weakened interfaces and skeletons, amplifying ductile deformation.
- (3)
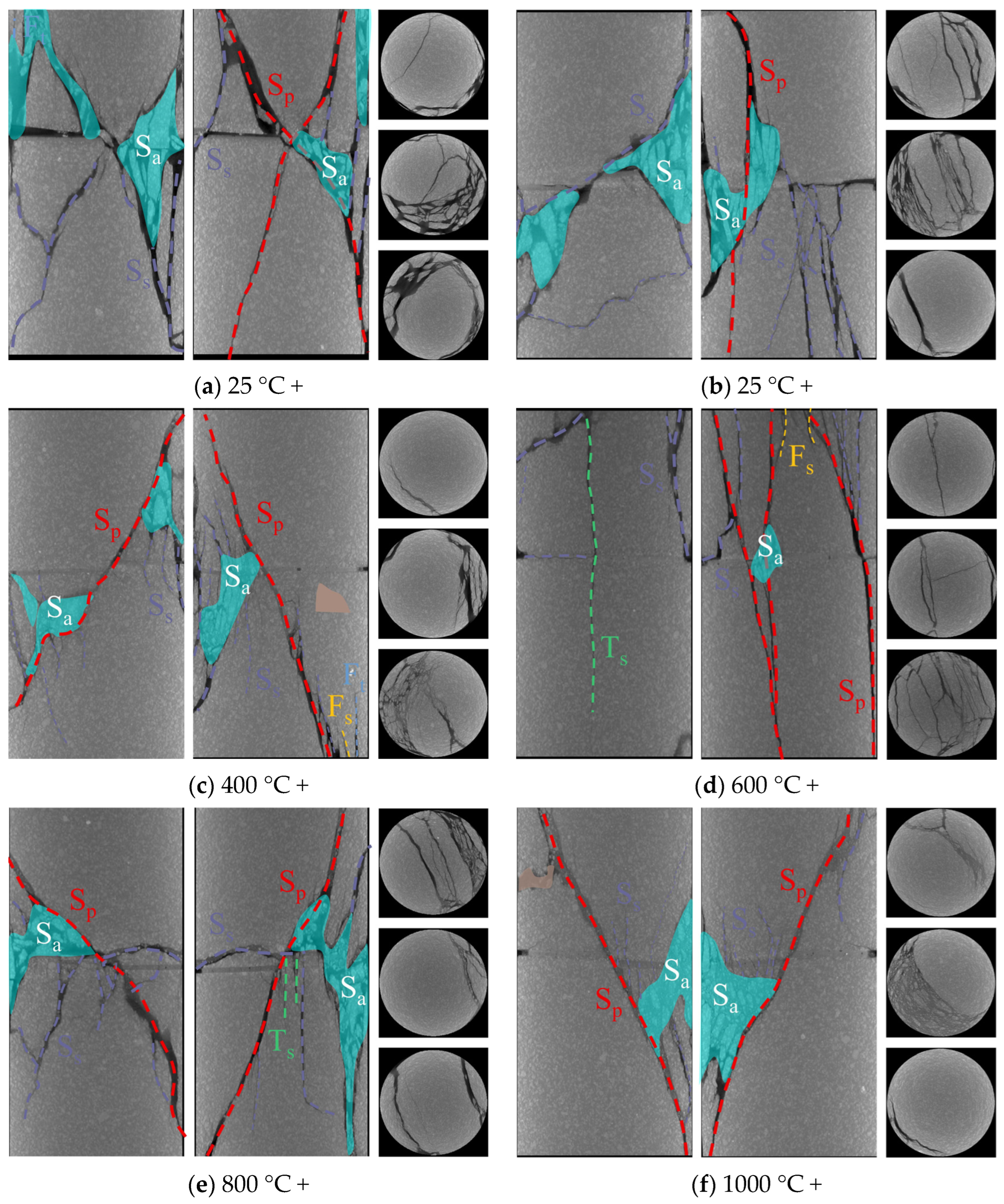
- Specimens exposed to uncoupled acid solutions at lower temperatures (25–600 °C) exhibited high brittleness, and some specimens disintegrated under uniaxial compression. Shear-dominated failures along prefabricated fractures prevailed with increasing angles. Acid corrosion suppressed brittle disintegration by forming non-uniform notches that enabled gradual energy release, promoting shear slip along fractures. Acidic environments significantly increased Ts crack density and shifted failure modes from shear-dominant to tensile-shear hybrid patterns.
- (4)
- 3D CT showed a reduction in Vca with increasing heat treatment temperature, indicating a transition from macro-scale crack propagation to micro-scale coalescence. Acid corrosion further reduced Vca by 34.64% at 1000 °C. SEM revealed surface roughness increase, microcracks, and crushed rock chips under acid treatment. Acid preferentially dissolved active mineral components in diorite, while hydration products of HSFAA decomposed significantly.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tomar, M.S.; Khurana, S. Impact of passive fire protection on heat release rates in road tunnel fire: A review. Tunn. Undergr. Space Technol. 2019, 85, 149–159. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, T.; Cui, Y.; Wang, W.; Yang, X.; Huang, X. Strength deterioration and damage mechanism of grout-reinforced fractured sandstone under the coupled effects of acidic erosion and freeze-thaw cycles. Constr. Build. Mater. 2024, 443, 137761. [Google Scholar] [CrossRef]
- Ma, S.J.; Ma, D.P. Grouting Material for Broken Surrounding Rock and its Mechanical Properties of Grouting Reinforcement. Geotech. Geol. Eng. 2021, 39, 3785–3793. [Google Scholar] [CrossRef]
- Huang, Z.; Zeng, W.; Gu, Q.; Wu, Y.; Zhong, W.; Zhao, K. Investigations of variations in physical and mechanical properties of granite, sandstone, and marble after temperature and acid solution treatments. Constr. Build. Mater. 2021, 307, 124943. [Google Scholar] [CrossRef]
- Zhang, J.P.; Liu, L.M.; Li, Y. Mechanism and experiment of self-stress grouting reinforcement for fractured rock mass of underground engineering. Tunn. Undergr. Space Technol. 2023, 131, 104826. [Google Scholar] [CrossRef]
- Mehdiabadi, N.; Abdi, Y.; Khanlari, G. An experimental investigation on the durability of clay-bearing rocks in different pH conditions. Geotech. Geol. Eng. 2023, 41, 2645–2663. [Google Scholar] [CrossRef]
- Wu, C.; Wang, S.Q.; Li, D.L.; Wang, X.K. NMR Experimental Study on Dynamic Process of Pore Structure and Damage Mechanism of Sandstones with Different Grain Sizes under Acid Erosion. Shock Vib. 2020, 2020, 3819507. [Google Scholar] [CrossRef]
- He, J.W.; Li, H.; Yang, W.; Lu, J.X.; Lu, Y.; Liu, T.; Shi, S.L. Experimental study on erosion mechanism and pore structure evolution of bituminous and anthracite coal under matrix acidification and its significance to coalbed methane recovery. Energy 2023, 283, 128485. [Google Scholar] [CrossRef]
- Feng, M.M.; Cao, X.X.; Yuan, K.S.; Zhang, M.W.; Li, Z.J. Influence of acidic environment on damage mechanism of sandstone under different climatic temperatures. Bull. Eng. Geol. Environ. 2022, 81, 452. [Google Scholar] [CrossRef]
- Yu, H.D.; Zhang, C.G.; Chen, H.H.; Canbulat, I.; Saydam, S. Effect of Anion Concentration in the Acidic Environment on Rock’s Mechanical Properties. Rock Mech. Rock Eng. 2025, 58, 4569–4583. [Google Scholar] [CrossRef]
- An, H.M.; Zeng, T.S.; Zhang, Z.H.; Liu, L. Experimental Study of the Rock Mechanism under Coupled High Temperatures and Dynamic Loads. Adv. Civ. Eng. 2020, 2020, 8866621. [Google Scholar] [CrossRef]
- Hu, J.J.; Xie, H.P.; Li, C.B.; Liu, G.K. Evolution mechanism of permeability of hot dry rock under coupled effect of thermal fatigue and seawater interaction during coastal geothermal development. Renew. Sustain. Energy Rev. 2024, 189, 114061. [Google Scholar] [CrossRef]
- Xie, H.P.; Lu, J.; Li, C.B.; Li, M.H.; Gao, M.Z. Experimental study on the mechanical and failure behaviors of deep rock subjected to true triaxial stress: A review. Int. J. Min. Sci. Technol. 2022, 32, 915–950. [Google Scholar] [CrossRef]
- Tian, H.; Mei, G.; Jiang, G.-S.; Qin, Y. High-Temperature Influence on Mechanical Properties of Diorite. Rock Mech. Rock Eng. 2017, 50, 1661–1666. [Google Scholar] [CrossRef]
- Li, M.; Mao, X.; Cao, L.; Pu, H.; Lu, A. Influence of heating rate on the dynamic mechanical performance of coal measure rocks. Int. J. Geomech. 2017, 17, 04017020. [Google Scholar] [CrossRef]
- Waqas, U.; Ahmed, M.F. Prediction Modeling for the Estimation of Dynamic Elastic Young’s Modulus of Thermally Treated Sedimentary Rocks Using Linear–Nonlinear Regression Analysis, Regularization, and ANFIS. Rock Mech. Rock Eng. 2020, 53, 5411–5428. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, T.; Cui, Y.; Zheng, J.; Wang, W.; Li, Y. Experimental study on the deterioration mechanisms of physical and mechanical properties of red sandstone after thermal-acid coupling treatment. Constr. Build. Mater. 2024, 455, 139106. [Google Scholar] [CrossRef]
- Leroy, M.N.L.; Marius, F.W.; Francois, N. Experimental and Theoretical Investigations of Hard Rocks at High Temperature: Applications in Civil Engineering. Adv. Civ. Eng. 2021, 2021, 8893944. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, Z.; Pu, H.; Li, X. Effect of Thermal Treatment on Brazilian Tensile Strength of Granites with Different Grain Size Distributions. Rock Mech. Rock Eng. 2018, 51, 1293–1303. [Google Scholar] [CrossRef]
- Bao, H.; Xu, G.; Wang, Q.; Peng, Y.; Liu, J. Study on the deterioration mechanism of cement-based materials in acid water containing aggressive carbon dioxide. Constr. Build. Mater. 2020, 243, 118233. [Google Scholar] [CrossRef]
- Araghi, H.J.; Nikbin, I.; Reskati, S.R.; Rahmani, E.; Allahyari, H. An experimental investigation on the erosion resistance of concrete containing various PET particles percentages against sulfuric acid attack. Constr. Build. Mater. 2015, 77, 461–471. [Google Scholar] [CrossRef]
- Huang, X.; Pang, J.Y.; Zou, J.Q. Study on the Effect of Dry-Wet Cycles on Dynamic Mechanical Properties of Sandstone Under Sulfuric Acid Solution. Rock Mech. Rock Eng. 2022, 55, 1253–1269. [Google Scholar] [CrossRef]
- Huang, Z.; Zeng, W.; Wu, Y.; Li, S.J.; Gu, Q.X.; Zhao, K. Effects of temperature and acid solution on the physical and tensile mechanical properties of red sandstones. Environ. Sci. Pollut. Res. 2021, 28, 20608–20623. [Google Scholar] [CrossRef]
- Li, S.G.; Wu, Y.M.; Huo, R.K.; Song, Z.P.; Fujii, Y.; Shen, Y.J. Mechanical Properties of Acid-corroded Sandstone Under Uniaxial Compression. Rock Mech. Rock Eng. 2021, 54, 289–302. [Google Scholar] [CrossRef]
- Zhao, J.X.; Lu, C.H.; Deng, L.M.; Liu, G.C. Impacts of simulated acid solution on the disintegration and cation release of purple rock (mudstone) in Southwest China. Geomorphology 2018, 316, 35–43. [Google Scholar] [CrossRef]
- Shang, D.L.; Zhao, Z.H.; Dou, Z.H.; Yang, Q. Shear behaviors of granite fractures immersed in chemical solutions. Eng. Geol. 2020, 279, 105869. [Google Scholar] [CrossRef]
- Chen, W.H.; Yang, J.; Li, L.; Wang, H.C.; Huang, L.; Jia, Y.C.; Hu, Q.Y.; Jiang, X.W.; Tang, J.Z. Investigation of Mechanical Properties Evolution and Crack Initiation Mechanisms of Deep Carbonate Rocks Affected by Acid Erosion. Sustainability 2023, 15, 11807. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, W.; Qiu, X.; Zheng, J.; Liu, T. Mechanical properties of fracture-grouted prefabricated sandstone after thermal-acid coupling treatment: An experimental study. Constr. Build. Mater. 2024, 411, 134552. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Du, M.R.; Fang, H.Y.; Zhang, C.; Li, M.J.; Shi, M.S. Influence of different corrosion environments on mechanical properties of a roadbed rehabilitation polyurethane grouting material under uniaxial compression. Constr. Build. Mater. 2021, 301, 124092. [Google Scholar] [CrossRef]
- Manquehual, C.J.; Jakobsen, P.D.; Bruland, A. Corrosion Level of Rock Bolts Exposed to Aggressive Environments in Nordic Road Tunnels. Rock Mech. Rock Eng. 2021, 54, 5903–5920, Erratum in Rock Mech. Rock Eng. 2021, 54, 5921–5922. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhang, W.Q.; Pan, X.H.; Zhang, Y.L. The effect of heating/cooling cycles on chrominance, wave velocity, thermal conductivity and tensile strength of diorite. Environ. Earth Sci. 2019, 78, 403. [Google Scholar] [CrossRef]
- Corkum, A.G.; Asiri, Y.; El Naggar, H.; Kinakin, D. The Leeb Hardness Test for Rock: An Updated Methodology and UCS Correlation. Rock Mech. Rock Eng. 2018, 51, 665–675. [Google Scholar] [CrossRef]
- Sha, S.; Rong, G.; Chen, Z.H.; Li, B.W.; Zhang, Z.Y. Experimental Evaluation of Physical and Mechanical Properties of Geothermal Reservoir Rock after Different Cooling Treatments. Rock Mech. Rock Eng. 2020, 53, 4967–4991. [Google Scholar] [CrossRef]
- Mehta, P. Stability of ettringite on heating. J. Am. Ceram. Soc. 1972, 55, 55–57. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, T.; Cui, Y.; Wang, W.; Qing, C. Experimental study on the performance of basalt fiber combined with cement-based material solidified shield waste mud under the coupled effects of acid corrosion and dry-wet cycles. Constr. Build. Mater. 2025, 463, 140110. [Google Scholar] [CrossRef]
- Brigatti, M.F.; Guggenheim, S. Mica crystal chemistry and the influence of pressure, temperature, and solid solution on atomistic models. Rev. Mineral. Geochem. 2002, 46, 1–97. [Google Scholar]
- Fukuhara, M.; Sampei, A. Effects on high-temperature-elastic properties on α-/β-quartz phase transition of fused quartz. J. Mater. Sci. Lett. 1999, 18, 751–753. [Google Scholar] [CrossRef]
- Vidana Pathiranagei, S.; Gratchev, I.; Kong, R. Engineering properties of four different rocks after heat treatment. Geomech. Geophys. Geo-Energy Geo-Resour. 2021, 7, 16. [Google Scholar] [CrossRef]
- Martyushev, D.A.; Govindarajan, S.K.; Li, Y.W.; Yang, Y.F. Experimental study of the influence of the content of calcite and dolomite in the rock on the efficiency of acid treatment. J. Pet. Sci. Eng. 2022, 208, 109770. [Google Scholar] [CrossRef]
- Yang, S.Q.; Jing, H.W.; Huang, Y.H.; Ranjith, P.G.; Jiao, Y.Y. Fracture mechanical behavior of red sandstone containing a single fissure and two parallel fissures after exposure to different high temperature treatments. J. Struct. Geol. 2014, 69, 245–264. [Google Scholar] [CrossRef]
- Li, M.; Wang, D.M.; Shao, Z.L. Experimental study on changes of pore structure and mechanical properties of sandstone after high-temperature treatment using nuclear magnetic resonance. Eng. Geol. 2020, 275, 105739. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, T.; Cui, Y.; Wang, Z.; Wang, W.; Zheng, J. Compression and shear properties of OPC-MCA and basalt fiber cured shield waste mud after dry-wet cycles. Constr. Build. Mater. 2024, 426, 136153. [Google Scholar] [CrossRef]
- Zhang, H.G.; Liu, T.; Wang, W.H.; Cui, Y.X.; Wu, Y.K.; Qiu, X.Y. Effective curing of waste mud from slurry shield tunnels using OPC-MCA: Experimental investigations and microstructural analysis. Tunn. Undergr. Space Technol. 2025, 161, 106554. [Google Scholar] [CrossRef]
- Damion, T.; Chaunsali, P. Evaluating acid resistance of Portland cement, calcium aluminate cement, and calcium sulfoaluminate based cement using acid neutralisation. Cem. Concr. Res. 2022, 162, 107000. [Google Scholar] [CrossRef]
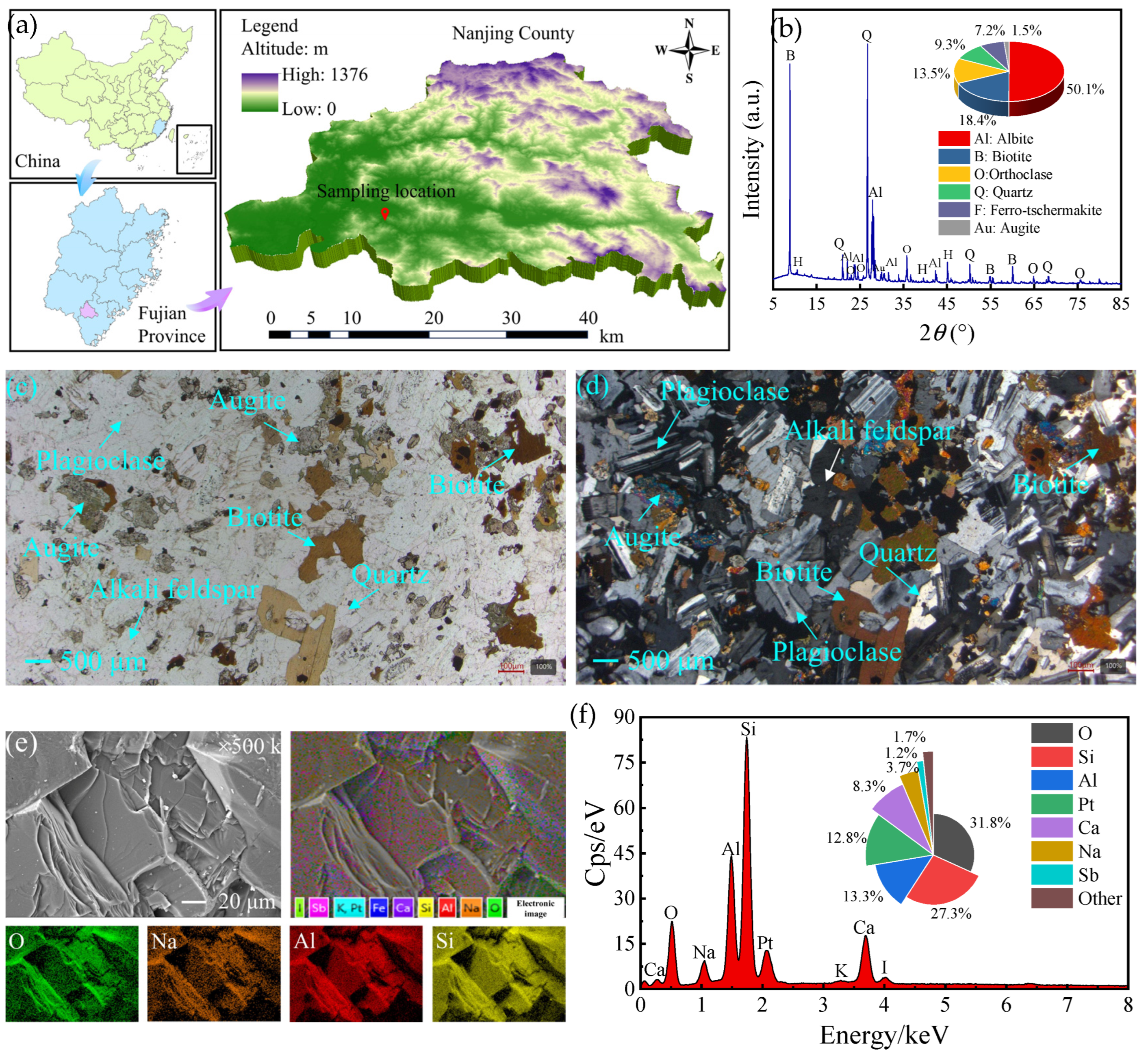






| Materials | SiO2 | CaO | Al2O3 | SO3 | MgO | Fe2O3 | TiO2 | K2O | P2O5 | Na2O | Lol |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HSFAA | 36.85 | 33.46 | 14.40 | 6.67 | 1.78 | 1.58 | 0.65 | 0.29 | 0.11 | 0.10 | 3.03 |
| Minerals | Reaction Equations |
|---|---|
| Albite | |
| Biotite | |
| Orthoclase | |
| Ferro-tschermakite | |
| Quartz | |
| Augite |
| Minerals | Reaction Equations |
|---|---|
| Albite | |
| Biotite | |
| Orthoclase | |
| Ferro-tschermakite | |
| Quartz | |
| Augite |
| Hydration Products | Reaction Equations |
|---|---|
| CH | |
| AFt | |
| AFm | |
| AH3 | |
| C-S-H |
| IA | Specimens Subjected to the “Thermal Treatment-Grouting Reinforcement” Protocol | Specimens Subjected to the “Thermal Treatment-Grouting Reinforcement-Acid Immersion” Protocol | ||
|---|---|---|---|---|
| Equations | R2 | Equations | R2 | |
| 0 | 1.00 | 1.00 | ||
| 30 | 0.99 | 1.00 | ||
| 45 | 0.99 | 1.00 | ||
| 60 | 0.99 | 0.99 | ||
| 90 | 1.00 | 0.99 | ||
| IA | Specimens Subjected to the “Thermal Treatment-Grouting reinforcement” Protocol | Specimens Subjected to the “Thermal Treatment-Grouting Reinforcement-Acid Immersion” Protocol | ||
|---|---|---|---|---|
| Equations | R2 | Equations | R2 | |
| 0 | 1.00 | 0.98 | ||
| 30 | 0.99 | 0.99 | ||
| 45 | 0.99 | 1.00 | ||
| 60 | 0.99 | 1.00 | ||
| 90 | 1.00 | 0.99 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Zhang, H.; Liu, T.; Yang, Z.; Zhang, Y.; Ling, X. Mechanical Performance Degradation and Microstructural Evolution of Grout-Reinforced Fractured Diorite Under High Temperature and Acidic Corrosion Coupling. Buildings 2025, 15, 3547. https://doi.org/10.3390/buildings15193547
Cui Y, Zhang H, Liu T, Yang Z, Zhang Y, Ling X. Mechanical Performance Degradation and Microstructural Evolution of Grout-Reinforced Fractured Diorite Under High Temperature and Acidic Corrosion Coupling. Buildings. 2025; 15(19):3547. https://doi.org/10.3390/buildings15193547
Chicago/Turabian StyleCui, Yuxue, Henggen Zhang, Tao Liu, Zhongnian Yang, Yingying Zhang, and Xianzhang Ling. 2025. "Mechanical Performance Degradation and Microstructural Evolution of Grout-Reinforced Fractured Diorite Under High Temperature and Acidic Corrosion Coupling" Buildings 15, no. 19: 3547. https://doi.org/10.3390/buildings15193547
APA StyleCui, Y., Zhang, H., Liu, T., Yang, Z., Zhang, Y., & Ling, X. (2025). Mechanical Performance Degradation and Microstructural Evolution of Grout-Reinforced Fractured Diorite Under High Temperature and Acidic Corrosion Coupling. Buildings, 15(19), 3547. https://doi.org/10.3390/buildings15193547






