Obtaining of Composite Cements with Addition of Fly Ash
Abstract
1. Introduction
- Fly ash contains amorphous silicon dioxide (SiO2), which can react with calcium hydrates formed during cement hydration and subsequently participate in the formation of calcium hydrosilicates, which increase the strength of cement and durability of concrete solution;
- Finely dispersed particles of fly ash with a size of less than 45 μm improve the structure of the cement mortar, reducing porosity and increasing density;
- Utilizing fly ash as a partial substitute for Portland cement clinker decreases both the energy demand and the environmental impact of cement manufacturing;
- Replacing the clinker component with up to 30% fly ash can significantly reduce CO2 emissions, making cement production more sustainable.
2. Basic Research Methods
2.1. Materials
2.2. Methods of High-Precision Instrumental Diagnostics
2.2.1. Chemical Analysis
2.2.2. Analysis by X-Ray Fluorescence Spectroscopy (XRF)
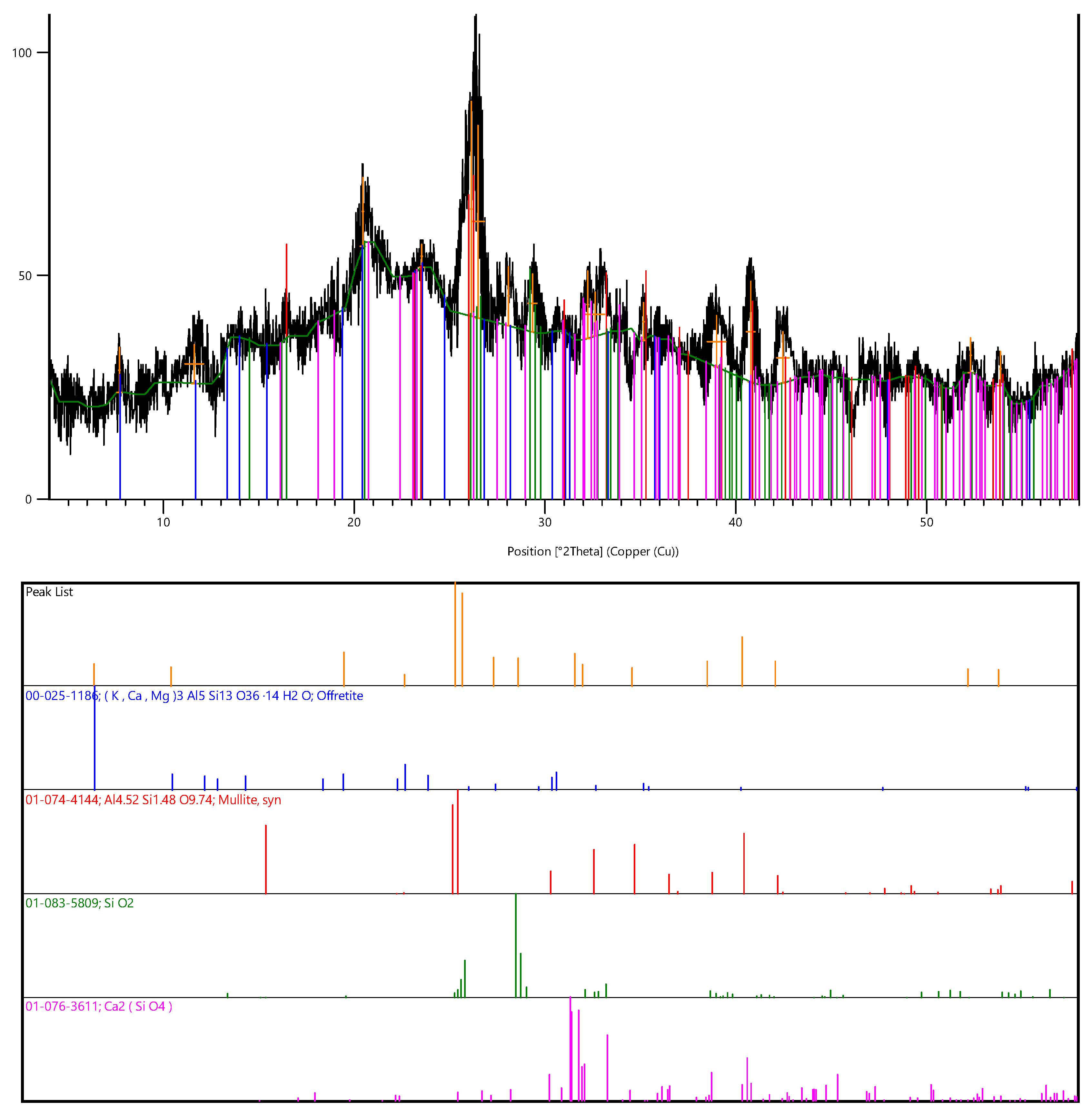
2.2.3. X-Ray Diffraction (XRD) Analysis
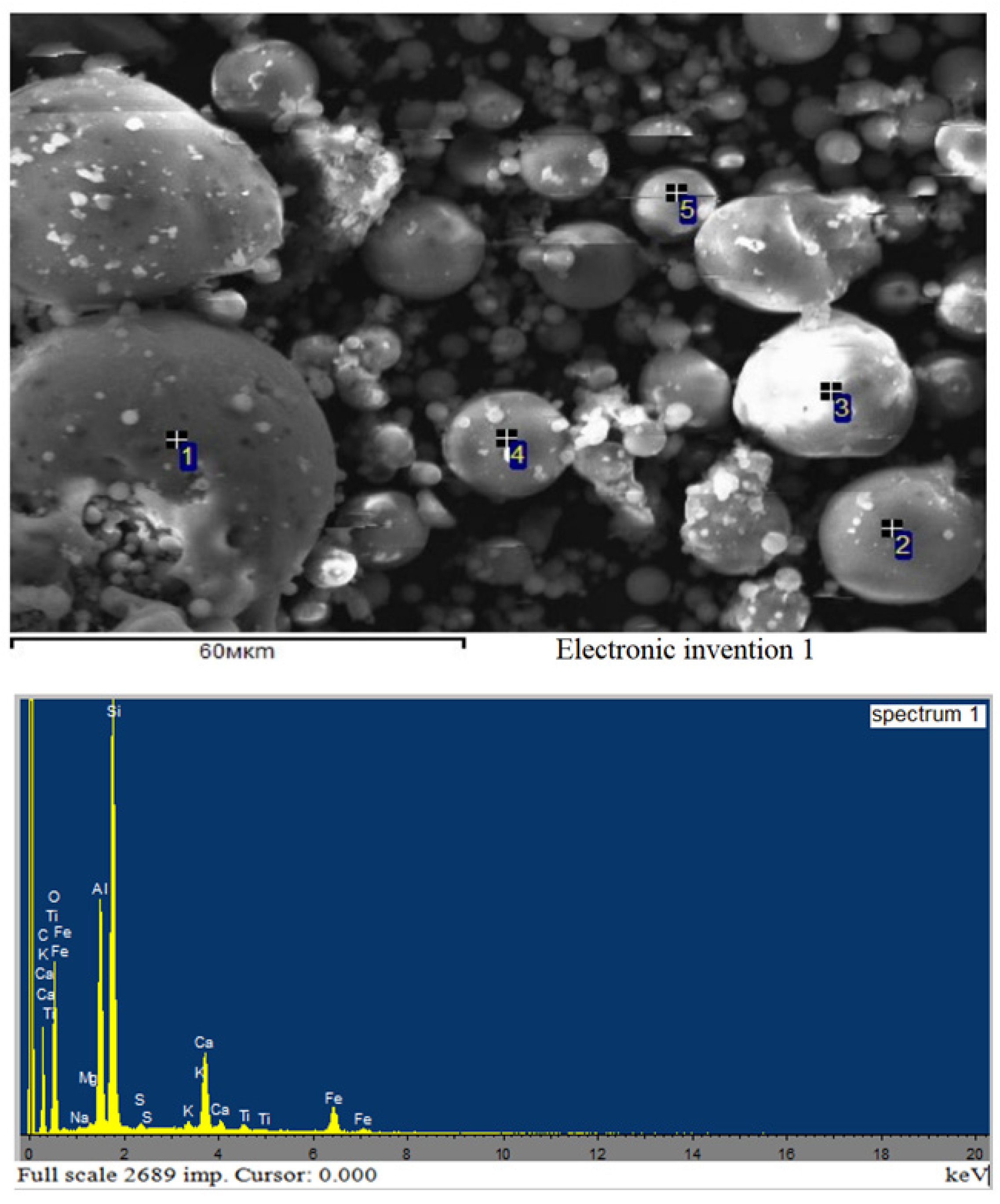
2.2.4. Microscopic Analysis
2.2.5. Research of the Ash Activity Index
- IA90—the ash activity index at the age of 90 days;
- Ro28 and Ro90—the compressive strength of the base composition mortar at the age of 28 and 90 days, respectively;
- Rk28 and Rk90—the compressive strength of the control composition mortar at the age of 28 and 90 days, respectively.
2.2.6. Physical and Mechanical Tests
- pneumatic sieving device
- scales (error no more than 0.01 g).
3. Results and Discussion
3.1. Composition of the Source Materials Used in This Research
- SiO2 d/n spectra—4.24; 3.36; 1.59 Å;
- 3Al2O3·2SiO2 d/n spectra—5.34; 2.87; 2.68; 2.53; 2.28; 2.20; 2.11; 1.59; 1.52 Å;
- FeO(OH) d/n spectra—7.37; 2.28; 1.38 Å.
- the ash particles are spherical, glassy and hollow, ranging in size from 1 µm to 50 µm;
- large particles contain smaller spherical particles within their cavities (shown by the arrow);
- tiny loose beads are typically firmly “glued” to the surface of large particles.
- The mechanism of fly ash particle formation can be represented as follows:
- the ash carried out of the furnace is at a high temperature; therefore, upon rapid cooling (during interaction with water), tiny glass beads are formed;
- very hot small glass beads stick together upon collision, trapping air bubbles with them; this can form large beads containing many smaller beads;
- balls of different sizes cool at different rates, so hotter small balls attach to the surface of colder large balls and create a dense outer shell.
- CEM II class A-Z—6–20%;
- CEM II class B-Z—21–35%;
- CEM V class A—18–30%;
- CEM V class B—31–49%.
3.2. Properties of Composite Cements
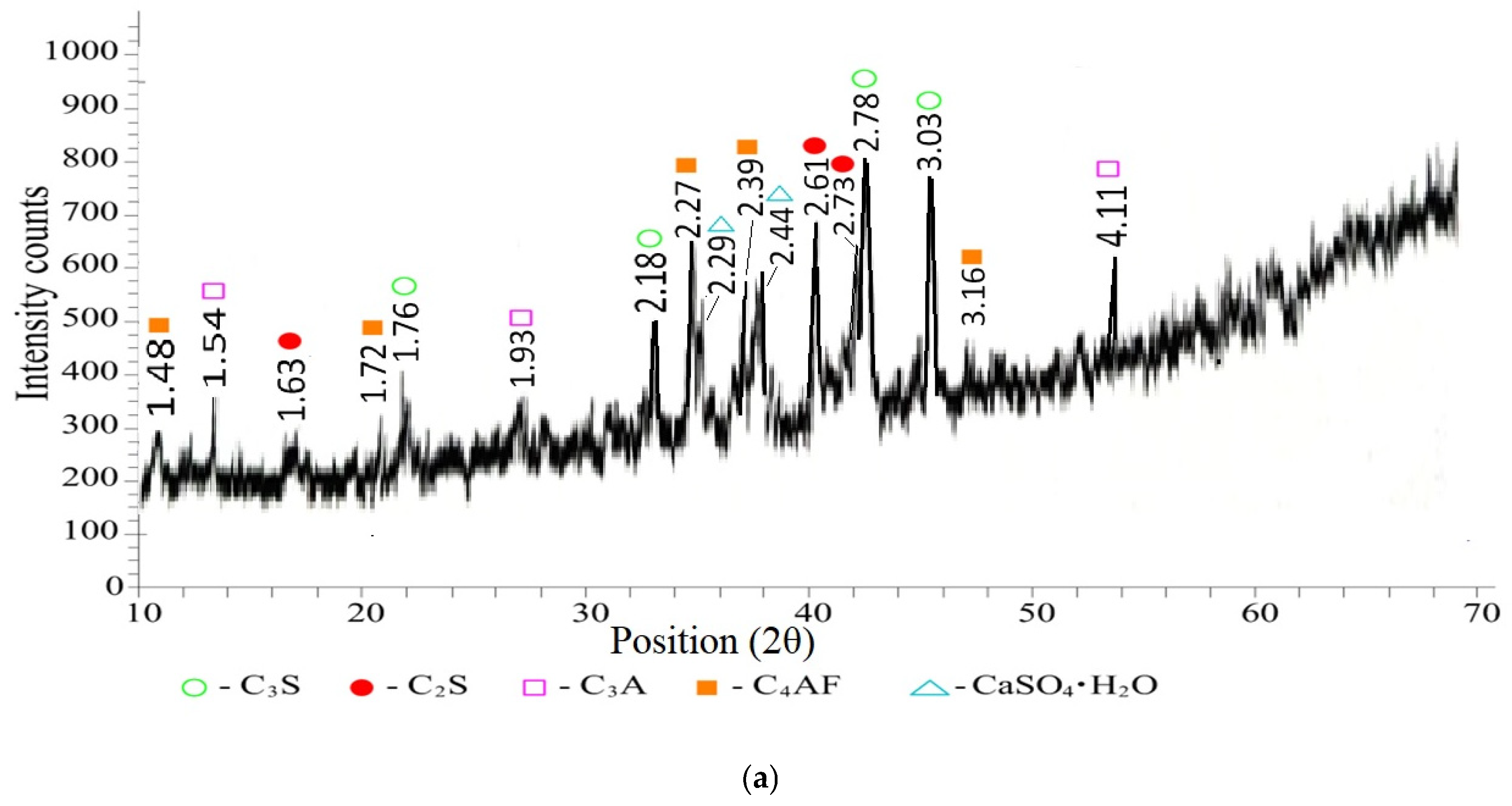
- Hydration of cement minerals alite and belite without ash:
- 2
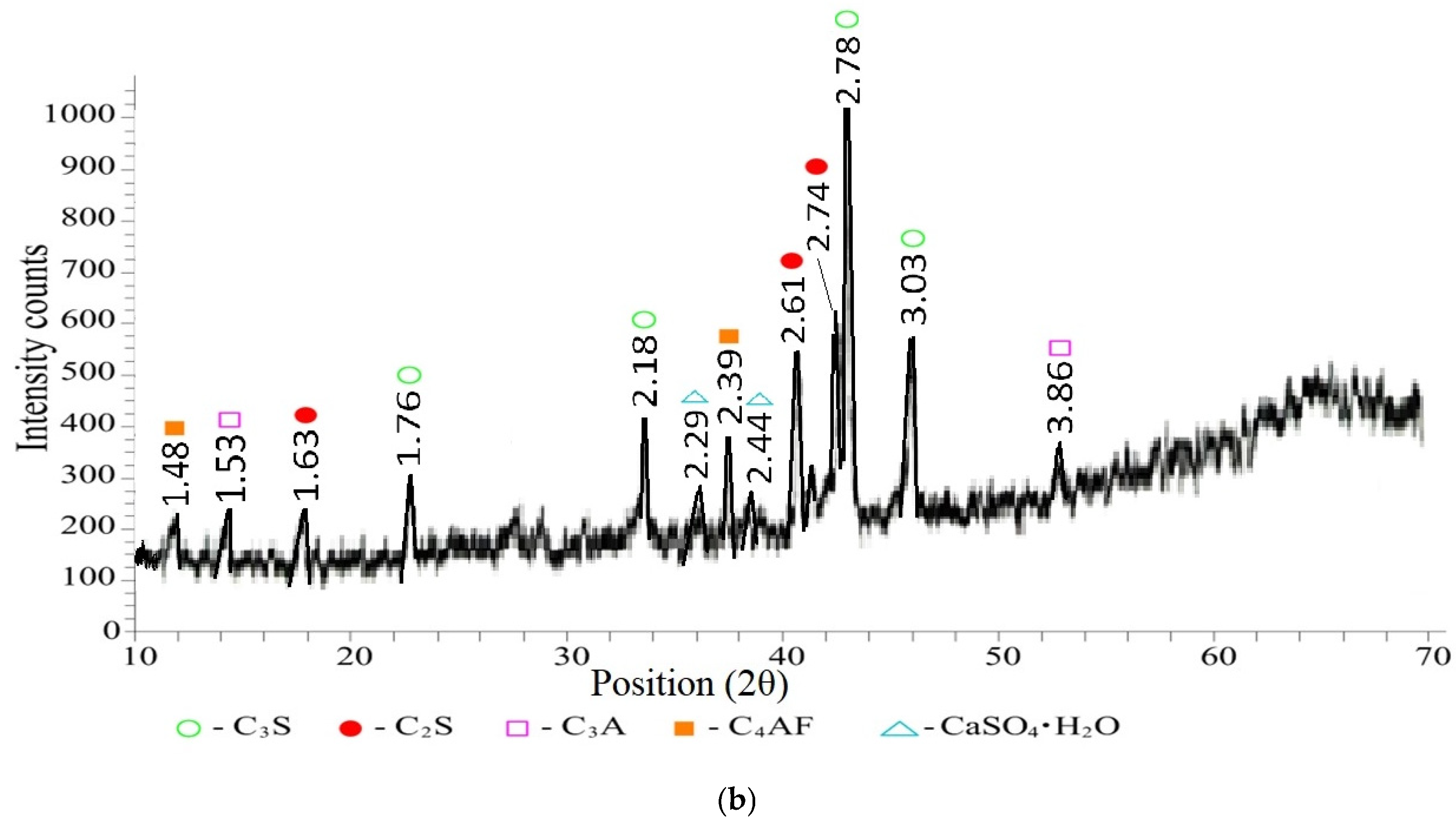
- Pozzolanic activity resulting from the inclusion of fly ash:
- 3
- Reaction with other components of fly ash, in particular Al2O3:
- 4
- Reaction of ettringite formation:
- -
- calcium hydroxide (Ca(OH)2) d/n—4.9386; 3.1192; 2.6345; 1.9298; 1.7978; 1.4816 Å;
- -
- calcium hydrosilicates (C-S-H) d/n—7.8237; 5.2264; 2.8585; 1.7212; 1.6783; 1.617; 1.512; 1.4494 Å;
- -
- alite (C3S—3CaO·SiO2) d/n—3.0432; 2.9836; 2.7818; 1.9816; 1.8242; 1.7665; 1.5412 Å;
- -
- belite (C2S—2CaO·SiO2) d/n—3.2454; 2.7529; 2.288; 2.21; 2.1334; 2.0566 Å;
- -
- ettringite (Ca6Al2(SO4)3(OH)12·26H2O) d/n—8.7513; 5.6607; 4.6988; 3.6671; 3.4901; 2.5654; 2.4769 Å;
- -
- tricalcium aluminate (C3A—3CaO·Al2O3) d/n—6.2998; 4.0642; 2.3573 Å;
- -
- tetracalcium aluminopherite (4CaO·Al2O3·Fe2O3) d/n—4.3293; 1.878; 1.6468; 1.5776; 1.4195; 1.3892; 1.366 Å.
- -
- calcium hydroxide (Ca(OH)2) d/n—4.9143; 3.1157; 1.9263; 1.7976; 1.4848 Å;
- -
- calcium hydrosilicates (C-S-H) d/n—7.2842; 1.6881; 1.5267; 1.4475 Å;
- -
- alite (C3S—3CaO·SiO2) d/n—3.0379; 2.9688; 2.7795; 1.8263 Å;
- -
- belite (C2S—2CaO·SiO2) d/n—3.2433; 2.7795; 2.7463; 2.2859; 2.2106 Å;
- -
- ettringite (Ca6Al2(SO4)3(OH)12·26H2O) d/n—8.1539; 5.5912; 3.8917; 2.6324 Å;
- -
- quartz* (SiO2) d/n—4.2806; 3.453 Å.
- Reduction in the amount of free Ca(OH)2;
- Compaction of the microstructure due to crystallization of calcium hydrosilicates;
- Increased durability due to reduced porosity;
- Modification of the ash-gel and ash-cement matrix interfaces.
4. Conclusions
- Compositions of composite cements based on Portland cement of CEM I class 42.5 and CEM II class 32.5 grades with the addition of fly ash in the amount of 5 to 25% were developed. The optimal composition for composite cement is CEM I 42.5—90%, fly ash—10%.
- The determination of fly ash showed that fly ash has a fairly stable composition. The majority of the fly ash is present in the form of glassy spheres; small amounts of phases are present—quartz (SiO2), mullite (3Al2O3·2SiO2), and goethite (FeO(OH)). In addition to the primary oxides SiO2 and Al2O3, the ash also contains trace elements including As, Ni, Cr, P, Sc, Mn, Pb, Ti, Zr, Ge, Ga, and W. These elements do not create separate compounds but are integrated within minerals and the glass phase.
- During the grinding process, the incorporation of 5–25 wt. % fly ash to the structure of the cement composition helps to improve the dispersion of the system, increase the homogeneity of the mixture and activate the pozzolanic reaction. The use of fly ash as a joint additive leads to an increase in the grindability of composite cements. The grindability of the obtained compositions improved compared to the control by 10.6–15.8%.
- When assessing the strength properties of the cements studied, it was observed that incorporating fly ash enhances the strength of the composite mixtures compared to cement without any additives. In particular, when adding 5% fly ash, the strength increases by 2.7–15.6% compared to cement without additives; 10%—by 9.4–19.6%; 15%—by 7.6–18.9%; 20%—by 6.1–10.0%; 25%—by 1.4–3.6%. The test results showed that the compressive strength of the produced composite cements after 28 days ranged from 42.1 to 54.2 MPa, which fully meets the requirements for cement grades 32.5 and 42.5 according to the strength class specified in GOST 31108-2020.
- In further work, particular emphasis should be paid to studying the processes of interaction of composite cements with fly ash with various modifying additives. First of all, this applies to plasticizers, which are actively used in concrete compositions.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, H.F.W. Cement Chemistry; Thomas Telford: London, UK, 1997; p. 459. [Google Scholar]
- Stark, J.; Viht, B. Cement and Lime; [Unknown publisher]: Kiev, Ukraine, 2008; p. 469. [Google Scholar]
- On Approval of the Best Available Techniques Guide ‘Cement and Lime Production’ Resolution of the Government of the Republic of Kazakhstan. Available online: https://adilet.zan.kz/rus/docs/P2300000941 (accessed on 24 October 2023).
- Mahasenan, N.; Smith, S.; Humphreys, K. The cement industry and global climate change: Current and potential future cement industry CO2 emissions. In Proceedings of the Greenhouse Gas Control Technologies—6th International Conference, Kyoto, Japan, 1–4 October 2002; pp. 995–1000. [Google Scholar]
- Rehan, R.; Nehdi, M. Carbon dioxide emissions and climate change: Policy implications for the cement industry. Environ. Sci. Policy 2005, 8, 105–114. [Google Scholar] [CrossRef]
- Sousa, V.; Bogas, J.A.; Real, S.; Meireles, I. Industrial production of recycled cement: Energy consumption and carbon dioxide emission estimation. Environ. Sci. Pollut. Res. 2023, 30, 8778–8789. [Google Scholar] [CrossRef]
- Salim, B.; Fragkoulis, K.; Bibhuti, B.D.; Maria, I. Decarbonising cement and concrete production: Strategies, challenges and pathways for sustainable development. J. Build. Eng. 2024, 86, 108861. [Google Scholar] [CrossRef]
- Cement and Concrete Companies Reveal ‘2050 Climate Ambition’. World Cement. Available online: https://www.worldcement.com/europe-cis/02092020/cement-and-concrete-companies-reveal-2050-climate-ambition/ (accessed on 2 September 2020).
- Griffiths, S.; Sovacool, K.; Rio, D.D.F.D.; Foley, A.M.; Bazilian, M.D.; Kim, J.; Uratani, J.M. Decarbonizing the cement and concrete industry: A systematic review of socio-technical systems, technological innovations, and policy options. Renew. Sustain. Energy Rev. 2023, 180, 113291. [Google Scholar] [CrossRef]
- Shah, I.H.; Miller, S.A.; Jiang, D.; Myers, R.J. Cement substitution with secondary materials can reduce annual global CO2 emissions by up to 1.3 gigatons. Nat. Commun. 2022, 13, 5758. [Google Scholar] [CrossRef] [PubMed]
- GCCA Sustainability Guidelines for the Monitoring and Reporting of CO2 Emissions from Cement Manufacturing. Available online: https://gccassociation.org/wp-content/uploads/2019/10/GCCA_Guidelines_CO2Emissions_v04_AMEND.pdf (accessed on 24 September 2025).
- Report of the World Commission on Environment and Development: Our Common Future. Available online: https://sustainabledevelopment.un.org/content/documents/5987our–common–future.pdf. (accessed on 24 September 2025).
- Mandicak, T.; Mesaros, P.; Kanalikova, A.; Spak, M. Supply Chain Management and Big Data Concept Effects on Economic Sustainability of Building Design and Project Planning. Appl. Sci. 2021, 11, 11512. [Google Scholar] [CrossRef]
- Hjort, M.; Skobelev, D.; Almgren, R.; Guseva, T.; Koh, T. Best Available Techniques and Sustainable Development Goals. In Proceedings of the 19th International Multidisciplinary Scientific Geo Conference SGEM GREEN 2019, Sofia, Bulgaria, 9–11 December 2019; pp. 185–192. [Google Scholar]
- CEMBUREAU. Activity Report; CEMBUREAU: Brussels, Belgium, 2021; p. 52. Available online: https://www.cembureau.eu/media/1sjf4sk4/cembureau-activity-report-2020.pdf (accessed on 27 June 2022).
- Çimen, Ö. Construction and built environment in circular economy: A comprehensive literature review. J. Clean. Prod. 2021, 305, 127180. [Google Scholar] [CrossRef]
- Arora, M.; Raspall, F.; Cheah, L.; Silva, A. Buildings and the circular economy: Estimating urban mining, recovery and reuse potential of building components. Resour. Conserv. Recycl. 2020, 154, 104581. [Google Scholar] [CrossRef]
- Guerra, B.C.; Leite, F. Circular economy in the construction industry: An overview of United States stakeholders’ awareness, major challenges, and enablers. Resour. Conserv. Recycl. 2021, 170, 105617. [Google Scholar] [CrossRef]
- Ren, L.; Zhou, S.; Peng, T.; Ou, X. A review of CO2 emissions reduction technologies and low-carbon development in the iron and steel industry focusing on China. Renew. Sustain. Energy Rev. 2021, 143, 110846. [Google Scholar] [CrossRef]
- Potapova, E.N.; Guseva, T.V.; Tolstykh, T.O.; Bubnov, A.G. Technological, technical, organizational and managerial solutions for the sustainable development and decarbonization of cement sector. Tech. Technol. Silic. 2023, 30, 104–115. [Google Scholar]
- Kuandykova, A.; Taimasov, B.; Potapova, E.; Sarsenbaev, B.; Kolesnikov, A.; Begentayev, M.; Kuldeyev, E.; Dauletiyarov, M.; Zhanikulov, N.; Amiraliyev, B.; et al. Production of Composite cement clinker based on industrial waste. J. Compos. Sci. 2024, 8, 257. [Google Scholar] [CrossRef]
- Bashmakov, I.A.; Potapova, E.N.; Borisov, K.B.; Lebedev, O.V.; Guseva, T.V. Cement Sector Decarbonization and Development of Environmental and Energy Management Systems. Build. Mater. 2023, 9, 4–12. [Google Scholar]
- Circularity Indicators. Available online: www.ellenmacarthurfoundation.org (accessed on 31 July 2019).
- Circular Economy: Definition, Importance and Benefits—European Parliament. Available online: www.europarl.europa.eu (accessed on 12 February 2015).
- Iacovidou, E.; Hahladakis, J.N.; Purnell, P. A systems thinking approach to understanding the challenges of achieving the circular economy. Environ. Sci. Pollut. Res. 2021, 28, 24785–24806. [Google Scholar] [CrossRef]
- Kirchherr, J.; Reike, D.; Hekkert, M. Conceptualizing the circular economy: An analysis of 114 definitions. Resour. Conserv. Recycl. 2017, 127, 221–232. [Google Scholar] [CrossRef]
- Korchunov, I.; Dmitrieva, E.; Potapova, E.; Sivkov, S.; Morozov, A. Frost Resistance of The Hardened Cement with Calcined Clays. Iran. J. Mater. Sci. Eng. 2022, 19, 1–9. [Google Scholar]
- Amiraliyev, B.; Taimasov, B.; Potapova, E.; Sarsenbaev, B.; Begentayev, M.; Dauletiyarov, M.; Kuandykova, A.; Abdullin, A.; Ainabekov, N.; Auyesbek, S. Heat Treatment of Clay Shales and Their Utilization as Active Mineral Additives for the Production of Composite Cements. J. Compos. Sci. 2025, 9, 269. [Google Scholar] [CrossRef]
- Juenger, M.C.; Snellings, R.; Bernal, S.A. Supplementary cementitious materials: New sources, characterization, and performance insights. Cem. Concr. Res. 2019, 122, 257–273. [Google Scholar] [CrossRef]
- Smolskaya, E.A.; Potapova, E.N.; Volosatova, A.A.; Rudomazin, V.V. Creating low-carbon cement—A step towards a green transformation of the cement industry. Tech. Technol. Silic. 2025, 32, 16–28. [Google Scholar]
- On Approval of the Rules for Development, Application, Monitoring and Revision of Best Available Techniques Guides. Available online: https://adilet.zan.kz/rus/docs/P2100000775 (accessed on 28 October 2021).
- Adesina, A. Recent advances in the concrete industry to reduce its carbon dioxide emissions. Environ. Chall. 2020, 1, 100004. [Google Scholar] [CrossRef]
- Rissman, J.; Bataille, C.; Masanet, E.; Aden, N.; Morrow, W.R., III; Zhou, N.; Elliott, N.; Dell, R.; Heeren, N.; Huckestein, B.; et al. Technologies and policies to decarbonize global industry: Review and assessment of mitigation drivers through 2070. Appl. Energy 2020, 266, 114848. [Google Scholar] [CrossRef]
- Arezoumandi, M.; Looney, T.J.; Volz, J.S. Effect of fly ash replacement level on the bond strength of reinforcing steel in concrete beams. J. Clean. Prod. 2015, 87, 745–751. [Google Scholar] [CrossRef]
- Ismael Jaf, D.K.; Abdulrahman, P.I.; Mohammed, A.S.; Kurda, R.; Qaidi, S.M.A.; Asteris, P.G. Machine learning techniques and multi-scale models to evaluate the impact of silicon dioxide (SiO2) and calcium oxide (CaO) in fly ash on the compressive strength of green concrete. Constr. Build. Mater. 2023, 400, 132604. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Volli, V.; Shu, C. Progressive utilisation prospects of coal fly ash: A review. Sci. Total Environ. 2019, 672, 951–989. [Google Scholar] [CrossRef]
- Kashem, A.; Karim, R.; Das, P.; Datta, S.D.; Alharthai, M. Compressive strength prediction of sustainable concrete incorporating rice husk ash (RHA) using hybrid machine learning algorithms and parametric analyses. Case Stud. Constr. Mater. 2024, 20, e03030. [Google Scholar] [CrossRef]
- Li, C.; Mei, X.; Dias, D.; Cui, Z.; Zhou, J. Compressive strength prediction of rice husk ash concrete using a hybrid artificial neural network model. Materials 2023, 16, 3135. [Google Scholar] [CrossRef] [PubMed]
- Jittin, V.; Bahurudeen, A.; Ajinkya, S.D. Utilisation of rice husk ash for cleaner production of different construction products. J. Clean. Prod. 2020, 263, 121578. [Google Scholar] [CrossRef]
- Rashad, A.M. A brief on high-volume Class F fly ash as cement replacement—A guide for Civil Engineer. Int. J. Sustain. Built Environ. 2015, 4, 278–306. [Google Scholar] [CrossRef]
- Klassen, V.K.; Taimasov, B.T. Cementology. In Structure, Properties of Cements and Optimization of Technological Processes; Vologda: Moscow, Russia, 2024; p. 264. [Google Scholar]
- Chatterjee, A.K. Cement Production Technology. In Principles and Practice; CRC Press: Boca Raton, FL, USA, 2018; p. 439. [Google Scholar]
- Thomas, M.D.A.; Shehata, M.H.; Shashiprakash, S.; Hopkins, D.S.; Cail, K. Properties of concrete containing high levels of fly ash: Influence of curing and age at testing. Cem. Concr. Res. 2007, 27, 1019–1029. [Google Scholar]
- Siddique, R. Utilization of coal combustion by-products in sustainable construction materials. Resour. Conserv. Recycl. 2010, 54, 1060–1066. [Google Scholar] [CrossRef]
- Hemalatha, T.; Ananth, R. A review on fly ash characteristics—Towards promoting high volume utilization in developing sustainable concrete. J. Clean. Prod. 2017, 147, 546–559. [Google Scholar] [CrossRef]
- Kumar Mehta, P.; Paulo, J.M. Monteiro. In Concrete, Microstructure, Properties and Materials, 4th ed.; McGraw-Hill Education: New York, NY, USA, 2014; p. 675. [Google Scholar]
- Cordeiro, G.C.; Toledo Filho, R.D.; Fairbairn, E.M.R. Ultrafine sugar cane bagasse ash: High potential pozzolanic material for tropical countries. Rev. IBRACON De Estrut. E Mater. 2010, 3, 50–67. [Google Scholar] [CrossRef]
- Kumar, R. Research methodology. In A Step-By-Step Guide for Beginners, 5th ed.; C&M Digitals(P) Ltd.: Chennai, India, 2019; p. 503. [Google Scholar]
- Biernacki, J.J.; Chaudhari, O.A. Leaching behavior of Hazardous heavy metals from lime fly ash cements. J. Environ. Eng. 2012, 139, 633–641. [Google Scholar]
- Chindaprasirt, P.; Kanchanda, P.; Sathonsaowaphak, A.; Cao, H.T. Sulfate resistance of blended cements containing fly ash and rice husk ash. Constr. Build. Mater. 2007, 21, 1356–1361. [Google Scholar] [CrossRef]
- Zhang, Z.; Su, T.; Zhang, L.; Zheng, R.; Ma, K.; Zhang, L.; Amaechi, C.V.; Wang, C. The Influence of Fly Ash and Slag on the Mechanical Properties of Geopolymer Concrete. Buildings 2024, 14, 2720. [Google Scholar] [CrossRef]
- Provis, J.L.; Van Deventer, J.S.L. Alkali-Activated Materials. In State of the Art Report, RILEM TC 224-AAM; Springer: London, UK, 2014; p. 388. [Google Scholar]
- Niyazbekova, R.; Mukhambetov, G.; Tlegenov, R.; Aldabergenova, S.; Shansharova, L.; Mikhalchenko, V.; Bembenek, M. The Influence of Addition of Fly Ash from Astana Heat and Power Plants on the Properties of the Polystyrene Concrete. Energies 2023, 16, 4092. [Google Scholar] [CrossRef]
- Wang, L.; He, Z.; Cai, X. Characterization of pozzolanic reaction and its effect on the C-S-H Gel in fly Ash-cement paste. J. Wuhan Univ. Technol. Mater. Sci. 2011, 26, 319–324. [Google Scholar] [CrossRef]
- GOST 31108-2020; Cements for General Construction. Technical Conditions. Publishing House Standard—Inform: Moscow, Russia, 2019.
- GOST 5382-2019; Cements and Materials for Cement Production. Methods of Chemical Analysis. Publishing House of Standards: Moscow, Russia, 2019.
- Yesimov, B.O.; Adyrbaeva, T.A.; Zhakipbaev, B.E. X-ray determinant of minerals V.I. Mikheeva. In Methodological Instructions for Universities; M. Auezov SKSU: Shymkent, Kazakhstan, 2012; p. 164. [Google Scholar]
- Rad, S.J.B. Electron Probe Microanalysis and Scanning Electron Microscopy in Geology; Tekhnosfera: Moscow, Russia, 2008; p. 232. [Google Scholar]
- GOST 30744-2001; Cements. Test Methods Using Polyfractional Sand. Official Publishing: Moscow, Russia, 2001.
- ISO 9277:2022; Determination of the Specific Surface Area of Solids by Gas Adsorption—Bet Method. ISO: Geneva, Switzerland, 2022.
- ASTM C204-2017; Standard Test Methods for Fineness of Hydraulic Cement by Air-Permeability Apparatus. ASTM: West Conshohocken, PA, USA, 2017.
- Abdullin, A.; Zhanikulov, N.; Taimasov, B.; Potapova, E.; Alfereva, Y.; Ksenofontov, D.; Zhakipbayev, B. Obtaining Composite Zinc Phosphate Cement with the Addition of Phosphoric Slag. J. Compos. Sci. 2025, 9, 200. [Google Scholar] [CrossRef]
- GOST 310.1–81; Cements. Test Methods. Standards Publisher: Moscow, USSR, 1981.
- GOST 310.3–81; Cements. Methods for Determination of Standard Consistency, Times of Setting and Soundness. Standards Publisher: Moscow, USSR, 1981.
- GOST 310.4–81; Cements. Methods of Bending and Compression Strength Determination. Standards Publisher: Moscow, USSR, 1981.
- Makarova, I.A.; Lokhova, N.A. Physicochemical Methods for Studying Building Materials; BrSU Publishing House: Barnaul, Russia, 2011; p. 139. [Google Scholar]
- Forsgren, J.; Frykstrand, S.; Grandfield, K.; Mihranyan, A.; Stromme, M. A Template-Free, Ultra-Adsorbing, High Surface Area Carbonate nanostructure. PLoS ONE 2014, 8, e68486. [Google Scholar] [CrossRef]
- Van Riessen, A.; Jamieson, E.; Gildenhuys, H.; Skane, R.; Allery, J. Using XRD to Assess the Strength of Fly-Ash- and Metakaolin-Based Geopolymers. Materials 2025, 18, 2093. [Google Scholar] [CrossRef] [PubMed]
- Nurpeissova, M.B.; Yestemesov, Z.A.; Bek, A.A.; Kim, V.S.; Syndarbekova, G.K. Main characteristics of fly ash from Ekibastuz SRPP-2. News Acad. Sci. Repub. Kazakhstan Ser. Geol. Tech. Sci. 2023, 2, 168–176. [Google Scholar]
- GOST 25592–91; Mixes of Fly-Ash and Slag of Thermal Plants for Conctetes. Specification. Standards Publisher: Moscow, USSR, 1991.
- Giergicny, Z. Fly ash as a component of cement. Cement and Its Application. № 4. 2014. Available online: https://jcement.ru/magazine/vypusk-4-417/zola-unos-kak-komponent-tsementa/ (accessed on 29 August 2014).
- Tkaczewska, E. Mechanical properties of cement mortar containing fine-grained fraction of fly ashes. Open J. Civ. Eng. 2013, 3, 54–68. [Google Scholar] [CrossRef]
- Li, G.; Zhou, C.; Ahmad, W.; Usanova, K.I.; Karelina, M.; Mohamed, A.M.; Khallaf, R. Fly Ash Application as Supplementary Cementitious Material: A Review. Materials 2022, 15, 2664. [Google Scholar] [CrossRef] [PubMed]
- Schulze, S.E.; Rickert, J. Influence of the chemical composition of fly ash on its reactivity. Cement and Its Application. № 1. 2012. Available online: https://jcement.ru/magazine/vypusk-1-/vliyanie-khimicheskogo-sostava-zoly-unosa-na-ee-reaktsionnuyu-sposobnost/ (accessed on 28 February 2012).
- Akmalaiuly, K.; Berdikul, N.; Pundienė, I.; Pranckevičienė, J. The Effect of Mechanical Activation of Fly Ash on Cement-Based Materials Hydration and Hardened State Properties. Materials 2023, 16, 2959. [Google Scholar] [CrossRef] [PubMed]

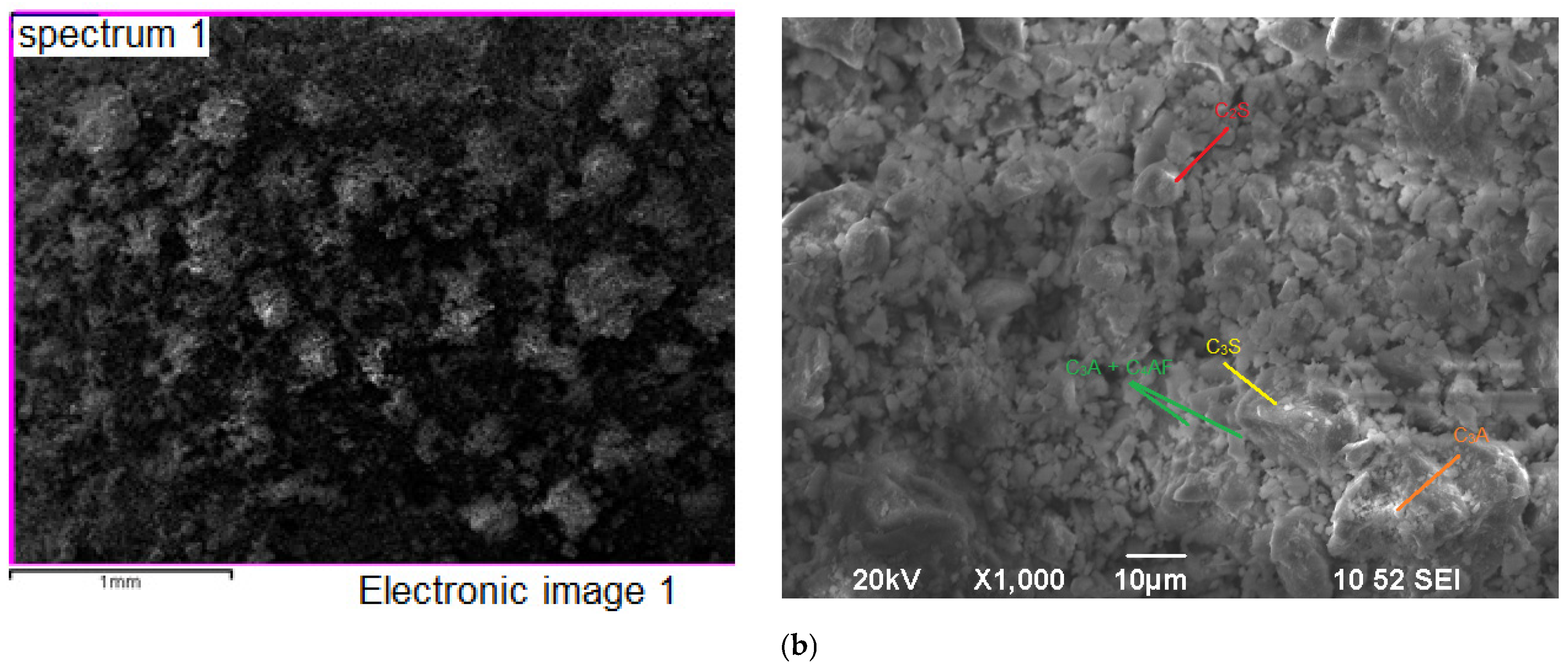

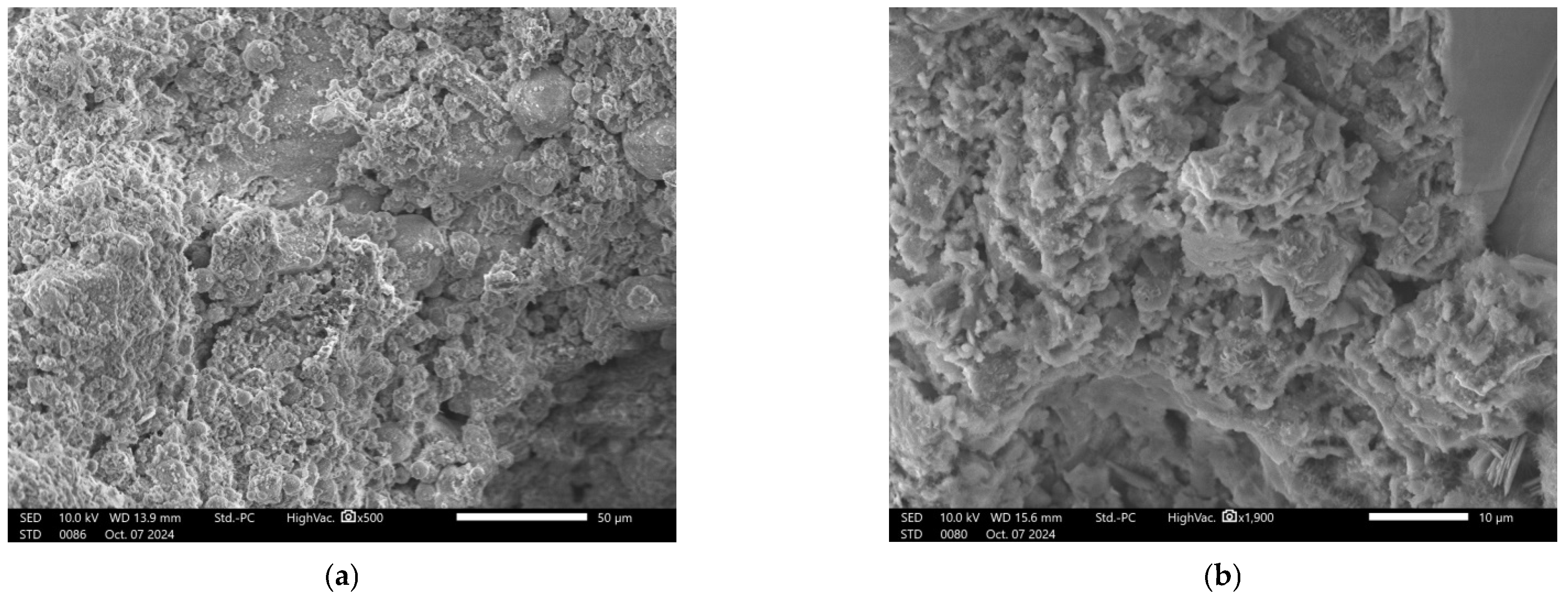
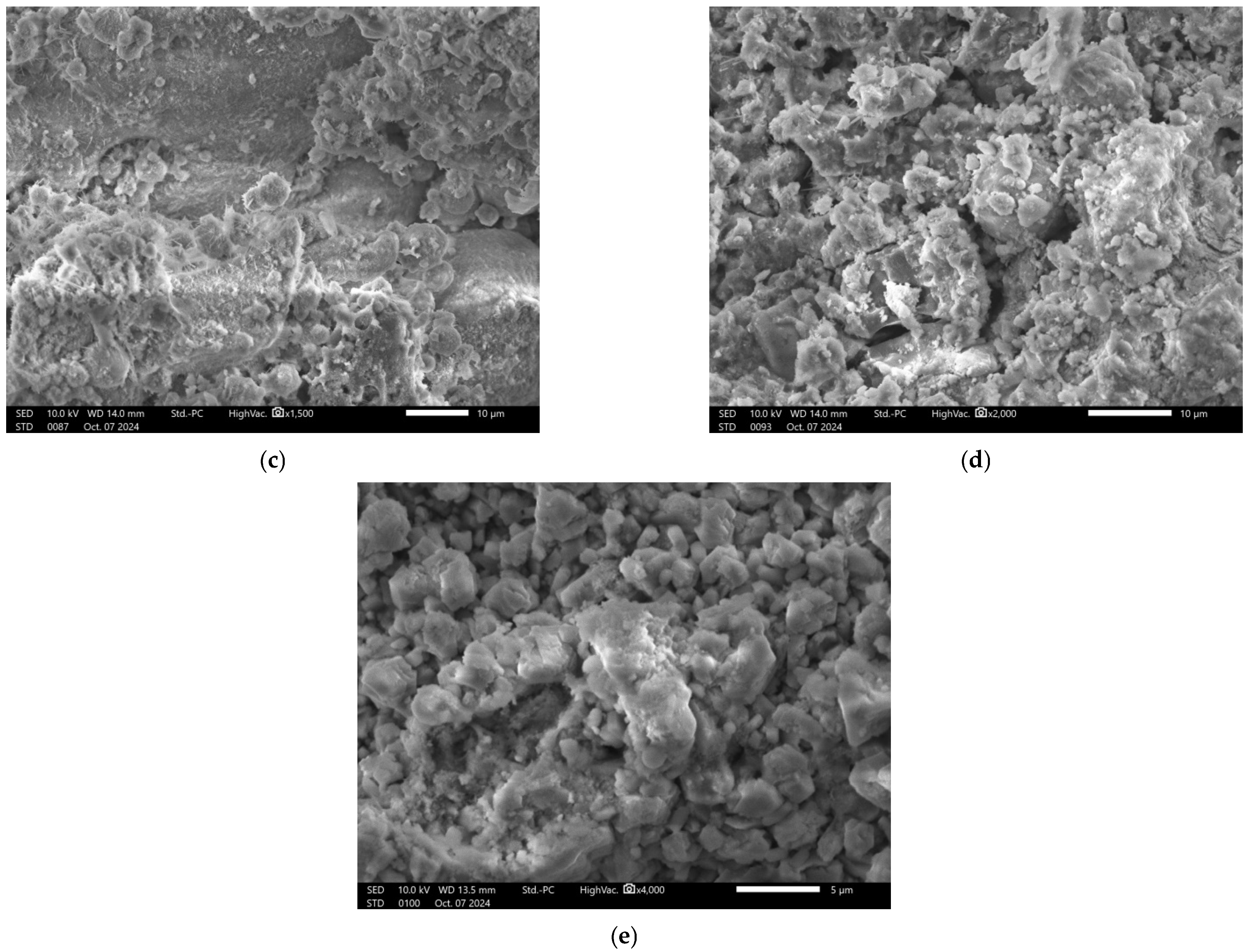
| Material | Chemical Composition, wt. % | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Na2O | K2O | TiO2 | MnO | P2O5 | Loss on Ignition | Total | |
| Fly ash | 69.66 | 21.34 | 2.78 | 1.57 | 0.39 | 0.23 | 0.45 | 0.54 | 0.96 | 0.06 | 0.33 | 1.69 | 100.0 |
| 64.28 | 22.85 | 4.69 | 1.34 | 0.63 | 1.47 | 0.44 | 0.69 | 1.03 | 0.18 | 0.39 | 2.01 | 100.0 | |
| 64.94 | 21.49 | 4.71 | 1.13 | 0.72 | 1.77 | 0.59 | 0.69 | 1.30 | 0.28 | 0.39 | 1.99 | 100.0 | |
| № | Name of Indicators | Designation of Regulatory Documentation for Test Methods | Standards for Regulatory Documentation | Actual Results | Note |
|---|---|---|---|---|---|
| 1 | Activity index at the age of 28 days, %, not less | GOST 25592-91 [70] GOST 30744-2001 | 75 | 72.0 | Fly ash from the Ekibastuz State District Power Plant-2 |
| 2 | Activity index at the age of 90 days, %, not less | GOST 25592-91 GOST 30744-2001 | 85 | 87.7 |
| Material | Chemical Composition, wt. % | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Na2O | K2O | TiO2 | MnO | P2O5 | Loss on Ignition | Total | |
| CEM I 42.5 | 20.79 | 3.96 | 3.70 | 63.88 | 0.55 | 2.56 | 0.29 | 0.63 | 0.10 | 0.16 | 0.10 | 3.48 | 100.0 |
| CEM II 32.5 | 18.25 | 5.84 | 3.27 | 62.40 | 0.59 | 2.30 | 0.36 | 0.69 | 0.48 | 0.10 | 0.10 | 5.82 | 100.0 |
| № | Name of Tests | Requirements According to GOST 30744-2001 | CEM II 32.5 | Requirements According to GOST 30744-2001 | CEM I 42.5 |
|---|---|---|---|---|---|
| 1 | Fineness of grinding by passing through sieve No. 008, % | not standardized | 97.0 | not standardized | 97.4 |
| 2 | Normal density, % | not standardized | 27.5 | not standardized | 28.0 |
| 3 | Start of setting, minutes, not earlier than | 75 | 210 | 60 | 220 |
| 4 | Uniformity of volume change (expansion), mm, not more than | 10 | not changes | 10 | not changes |
| 5 | Compressive strength, MPa, at the age of 7 days, not less than | 16 | 17.0 | 10 | 29.7 |
| 6 | Compressive strength, MPa, at the age of 28 days, | 41.0 | 45.3 | ||
| not less than | 32.5 | 42.5 | |||
| not more than | 52.5 | 62.5 | |||
| 7 | Ultimate compressive strength after heat treatment, MPa | more than 25.5 | 28.0 | more than 27.0 | 29.3 |
| 8 | Specific effective activity of natural radionuclides, Bq/kg | up to 370 | 103.1 | up to 370 | 100.7 |
| 9 | Tricalcium silicate (C3S), % | not standardized | 54.19 | not standardized | 59.09 |
| 10 | Dicalcium silicate (C2S), % | not standardized | 16.65 | not standardized | 10.74 |
| 11 | Tricalcium aluminate (C3A), % | not standardized | 7.37 | not standardized | 9.93 |
| 12 | Calcium alumoferrite (C4AF), % | not standardized | 15.76 | not standardized | 15.02 |
| 13 | Free calcium oxide (CaO), % | to 2% | 1.44 | to 2% | 0.41 |
| 14 | Periclase (MgO), % | to 5% | 1.43 | to 5% | 1.47 |
| 15 | Gypsum (CaSO4·2H2O) | not standardized | 3.16 | not standardized | 3.34 |
| № | Composition of Composite Cements, % | Grinding Time, Minutes | Specific Surface Area, S, cm2/g | Residue on Sieve No. 008, % | |
|---|---|---|---|---|---|
| CEM II 32.5 | Fly Ash Ekibastuz State District Power Plant-2 | ||||
| 1 | 95 | 5 | 60 | 3227 | 11.37 |
| 2 | 90 | 10 | 60 | 3439 | 10.36 |
| 3 | 85 | 15 | 60 | 3532 | 10.22 |
| 4 | 80 | 20 | 60 | 3620 | 9.48 |
| 5 | 75 | 25 | 60 | 3738 | 8.77 |
| № | Composition of Composite Cements, % | Grinding Time, Minutes | Specific Surface Area, S, cm2/g | Residue on Sieve No. 008, % | |
|---|---|---|---|---|---|
| CEM I 42.5 | Fly Ash Ekibastuz State District Power Plant-2 | ||||
| 1 | 95 | 5 | 60 | 4077 | 6.84 |
| 2 | 90 | 10 | 60 | 4169 | 6.39 |
| 3 | 85 | 15 | 60 | 4289 | 5.84 |
| 4 | 80 | 20 | 60 | 4373 | 5.19 |
| 5 | 75 | 25 | 60 | 4509 | 4.92 |
| Addition of Fly Ash, % | Water Cement Ratio | Setting Time, Hour–Minutes | Compressive Strength, MPa | Average Density, g/cm3 | |||
|---|---|---|---|---|---|---|---|
| Start | End | 3 Days | 7 Days | 28 Days | |||
| CEM II 32.5 | |||||||
| 0 | 0.264 | 3–30 | 4–30 | 12.94 | 17.00 | 41.00 | 1.812 |
| 5 | 0.272 | 3–40 | 4–45 | 17.25 | 27.94 | 42.10 | 1.946 |
| 10 | 0.276 | 3–45 | 4–55 | 18.44 | 28.55 | 44.85 | 1.953 |
| 15 | 0.278 | 3–35 | 4–20 | 18.12 | 28.15 | 44.10 | 1.906 |
| 20 | 0.282 | 2–30 | 3–50 | 17.08 | 27.80 | 43.52 | 1.857 |
| 25 | 0.286 | 2–25 | 3–40 | 16.94 | 27.41 | 42.48 | 1.798 |
| CEM I 42.5 | |||||||
| 0 | 0.274 | 3–40 | 4–35 | 16.60 | 29.70 | 45.30 | 1.974 |
| 5 | 0.276 | 3–45 | 4–40 | 20.46 | 33.74 | 52.36 | 2.133 |
| 10 | 0.279 | 3–50 | 4–50 | 20.87 | 34.45 | 54.18 | 2.160 |
| 15 | 0.284 | 3–25 | 4–10 | 19.94 | 33.20 | 53.84 | 2.108 |
| 20 | 0.288 | 2–15 | 3–45 | 19.67 | 33.08 | 49.81 | 2.057 |
| 25 | 0.295 | 2–10 | 3–30 | 18.99 | 32.85 | 45.93 | 1.994 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakhimova, G.; Syndarbekova, G.; Zhanikulov, N.; Yerkebayeva, B.; Potapova, E.; Rakhimov, M. Obtaining of Composite Cements with Addition of Fly Ash. Buildings 2025, 15, 3523. https://doi.org/10.3390/buildings15193523
Rakhimova G, Syndarbekova G, Zhanikulov N, Yerkebayeva B, Potapova E, Rakhimov M. Obtaining of Composite Cements with Addition of Fly Ash. Buildings. 2025; 15(19):3523. https://doi.org/10.3390/buildings15193523
Chicago/Turabian StyleRakhimova, Galiya, Gulim Syndarbekova, Nurgali Zhanikulov, Bakytkul Yerkebayeva, Ekaterina Potapova, and Murat Rakhimov. 2025. "Obtaining of Composite Cements with Addition of Fly Ash" Buildings 15, no. 19: 3523. https://doi.org/10.3390/buildings15193523
APA StyleRakhimova, G., Syndarbekova, G., Zhanikulov, N., Yerkebayeva, B., Potapova, E., & Rakhimov, M. (2025). Obtaining of Composite Cements with Addition of Fly Ash. Buildings, 15(19), 3523. https://doi.org/10.3390/buildings15193523









