A Comprehensive Study on the Microstructure and Mechanical Behavior of Glycoluril–Formaldehyde Polymer-Modified Cement Paste
Abstract
1. Introduction
2. Materials and Methods
2.1. Cement
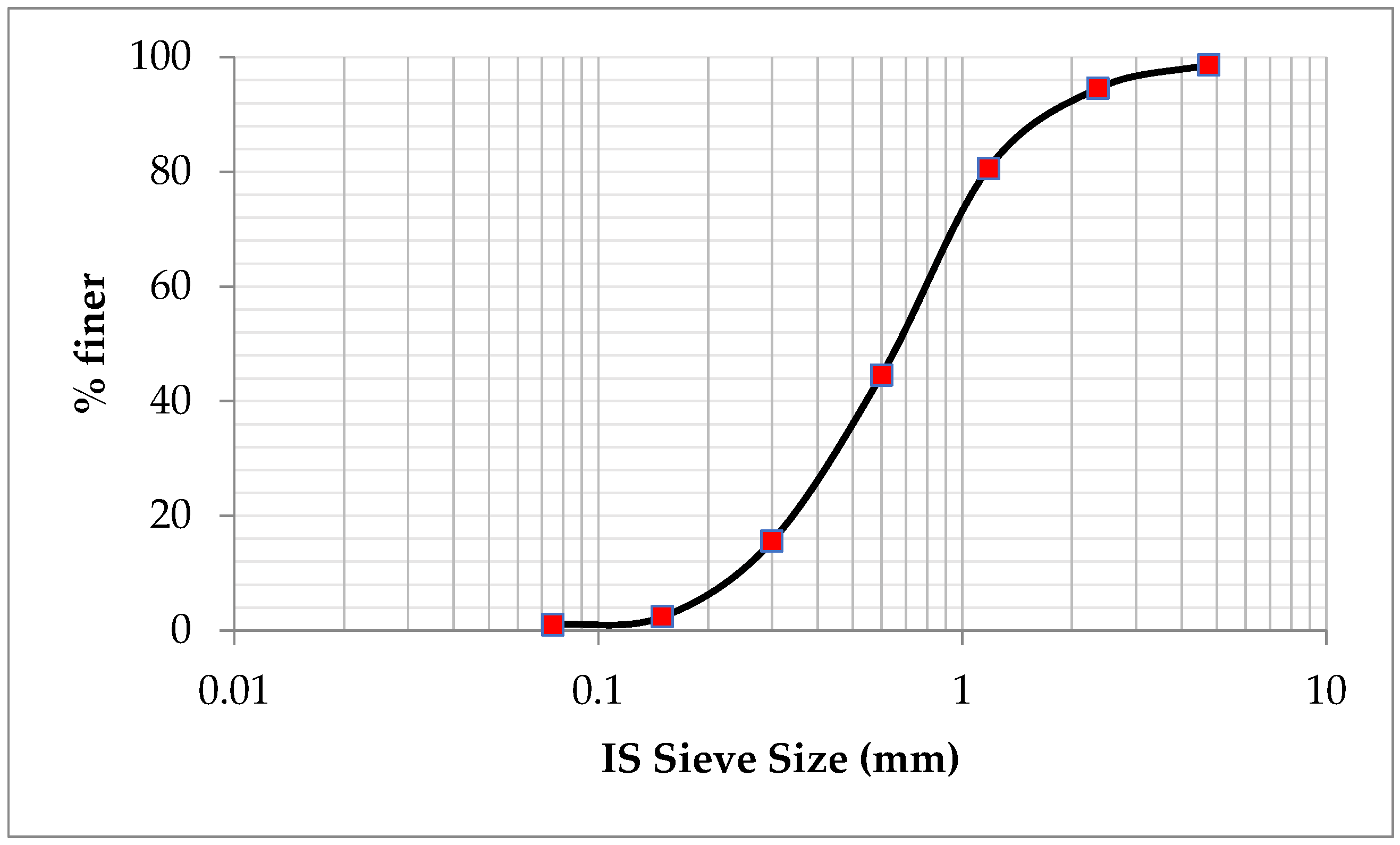
2.2. Fine Aggregate
2.3. Water
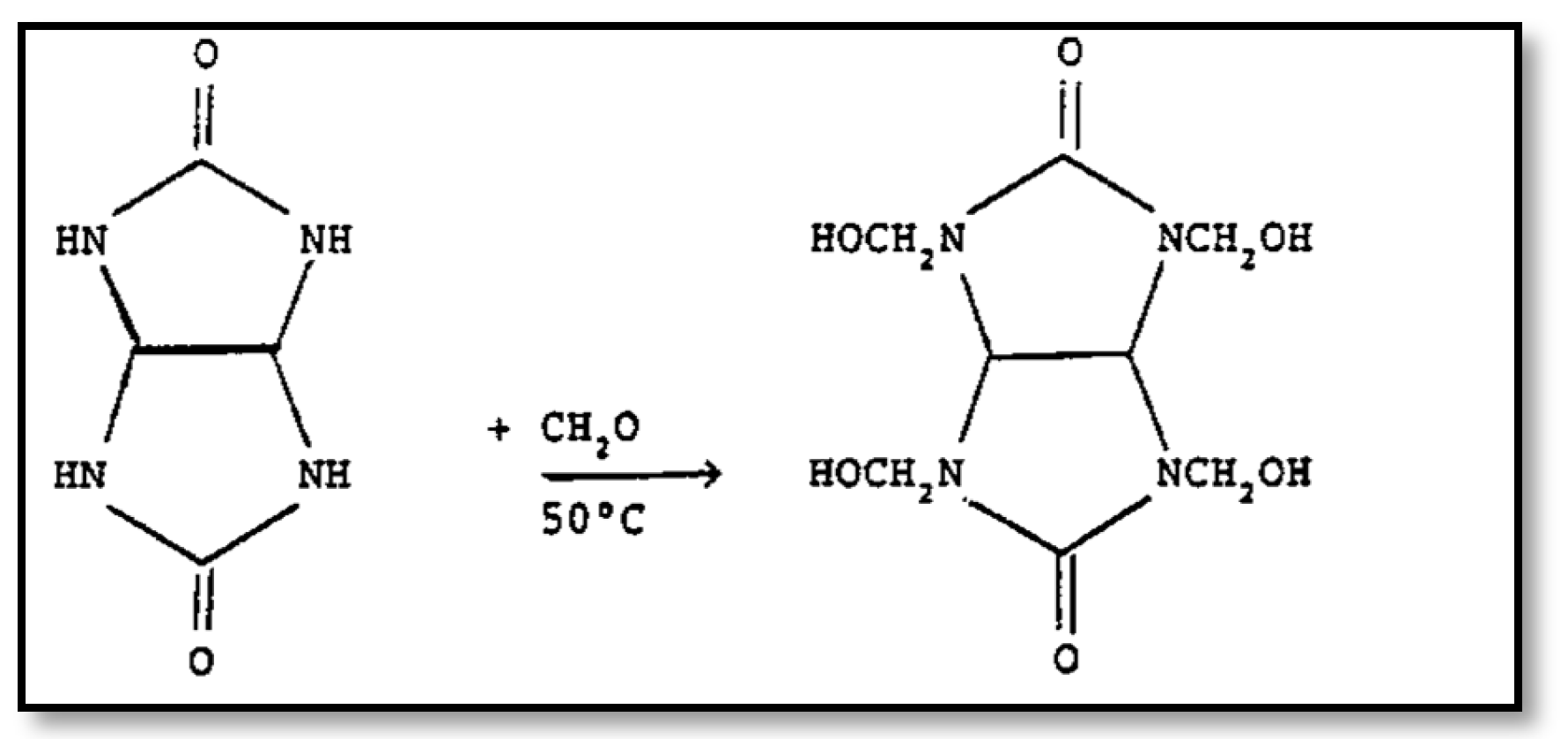
2.4. Glycoluril
2.5. Formaldehyde
2.6. New Approach in Glycoluril–Formaldehyde Polymer Concrete
2.7. Mix Proportions
2.8. Tests on Cement Mortar
2.8.1. Fresh Cement Mortar Properties
2.8.2. Compressive Strength on Mortar Cubes Mix Proportions
2.8.3. Test on Microstructural Behavior
X-Ray Diffraction Analysis
Scanning Electron Microscopy
3. Results and Discussion
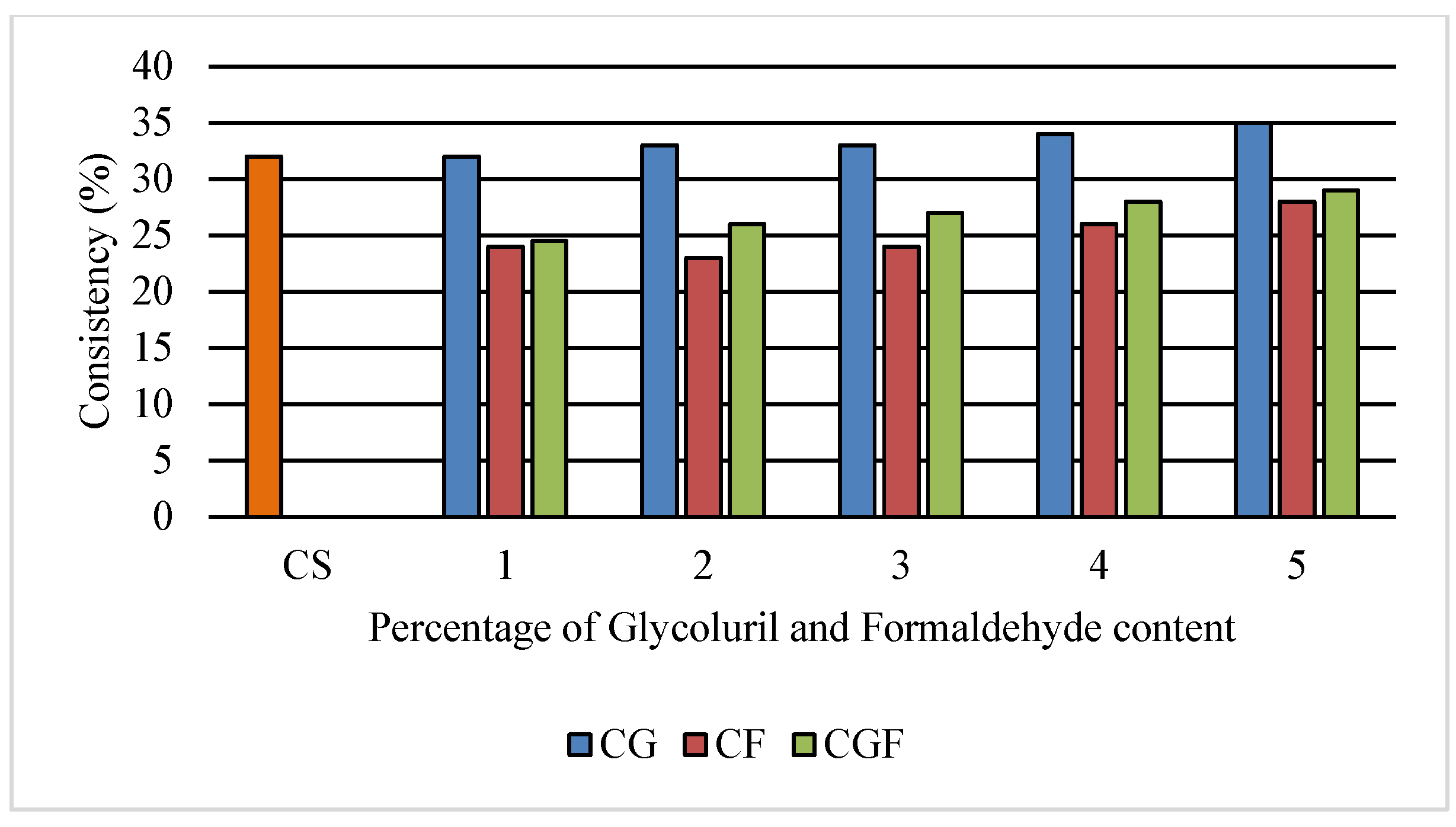
3.1. Consistency of Cement Paste
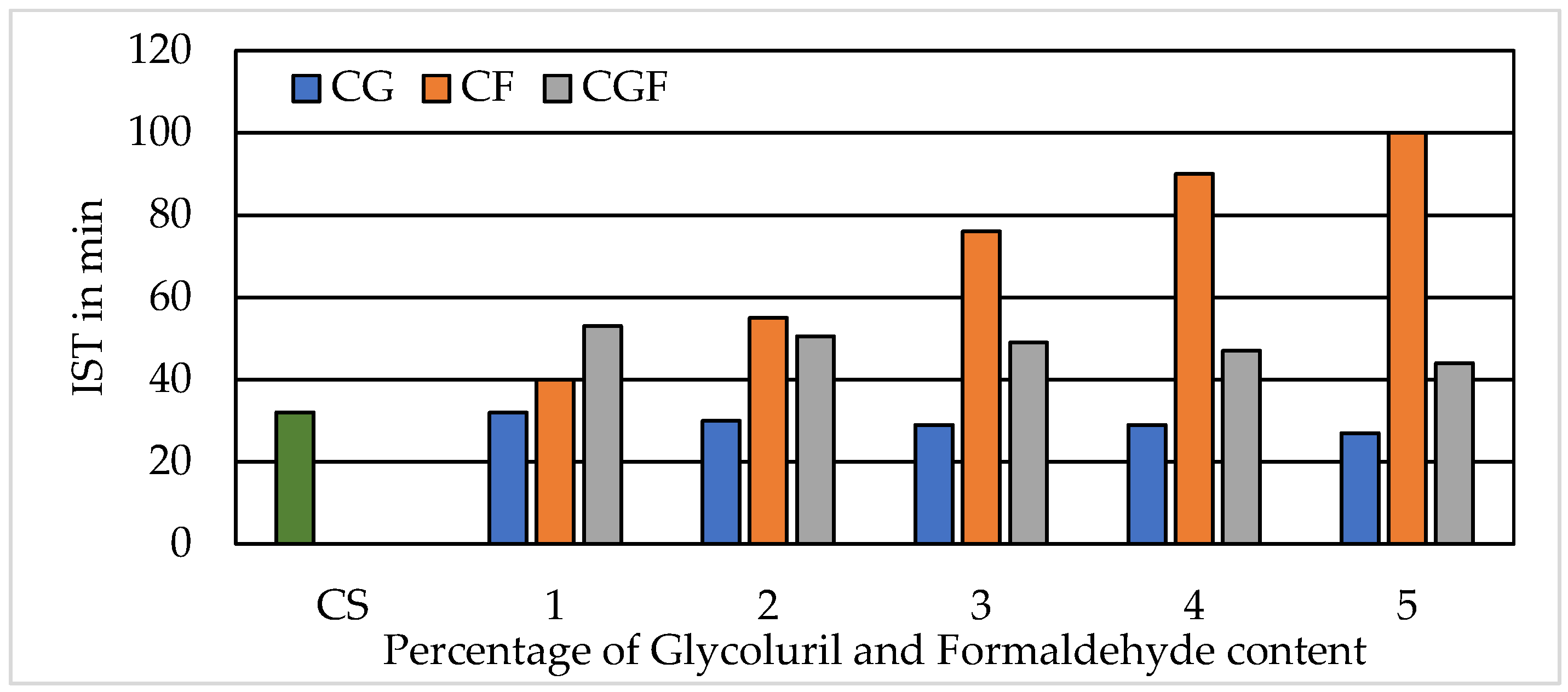
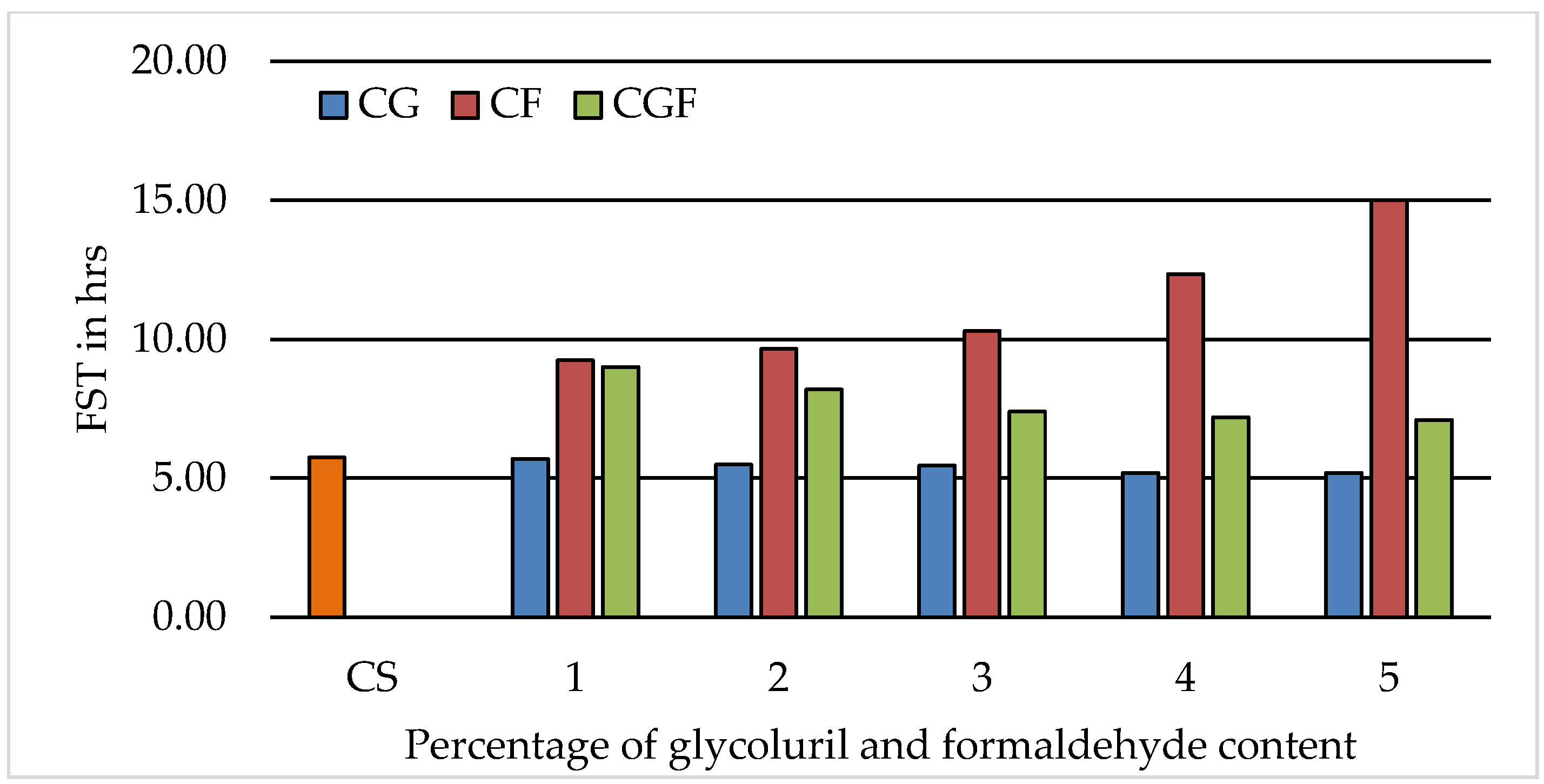
3.2. Initial and Final Setting Time of Cement
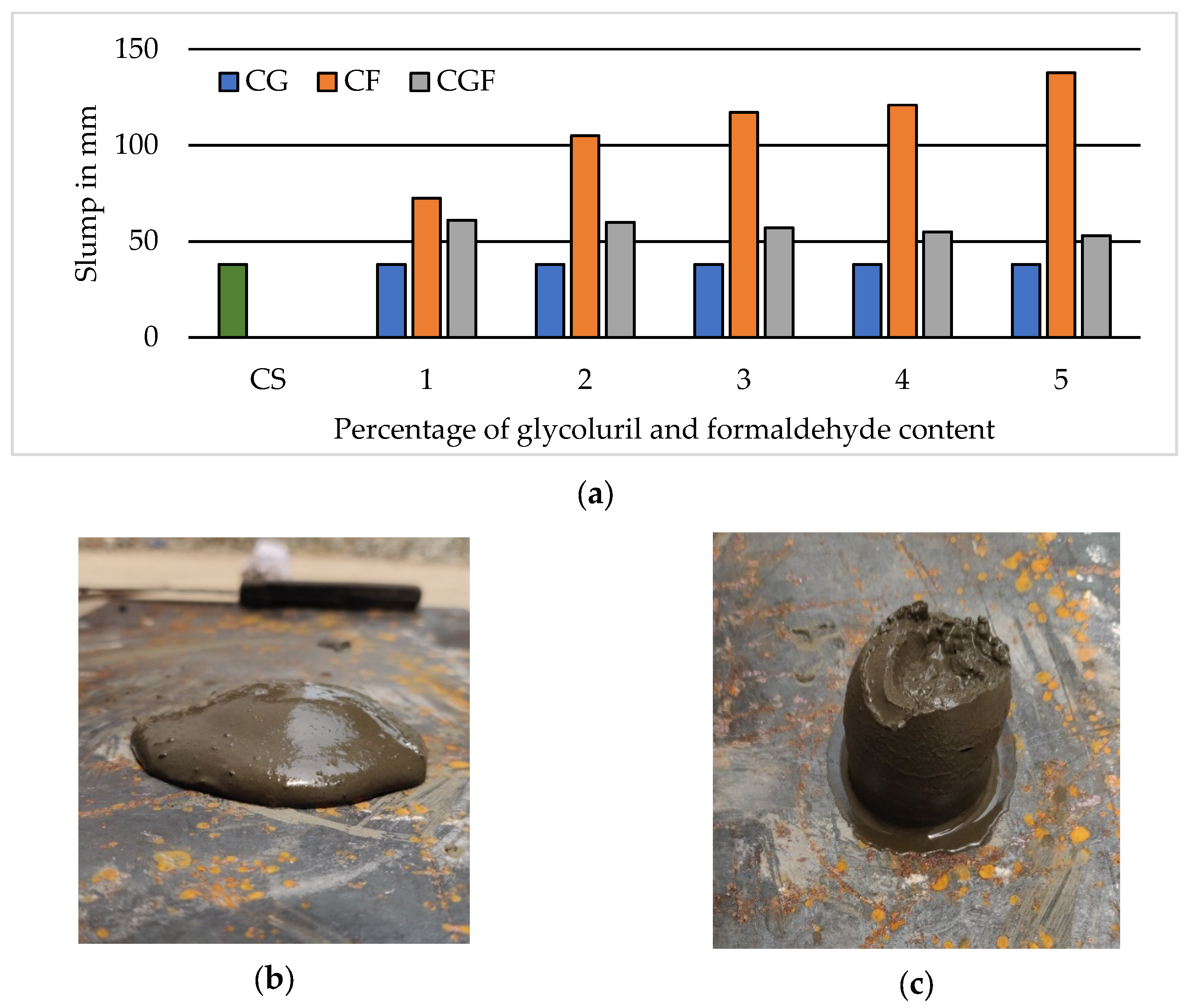
3.3. Miniature Slump Cone Test
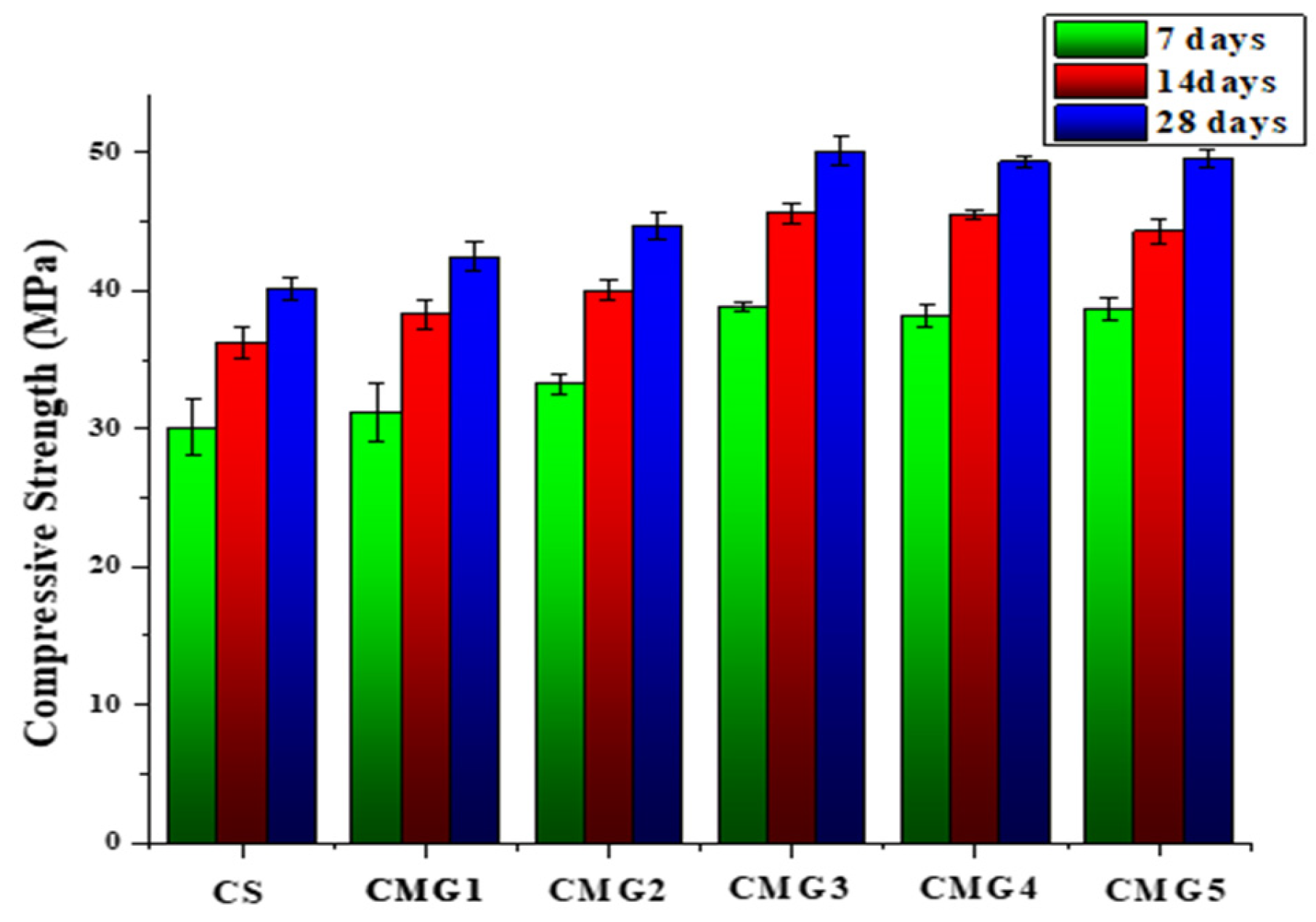
3.4. Compressive Strength of Cement Mortar
3.5. Microstructural Study
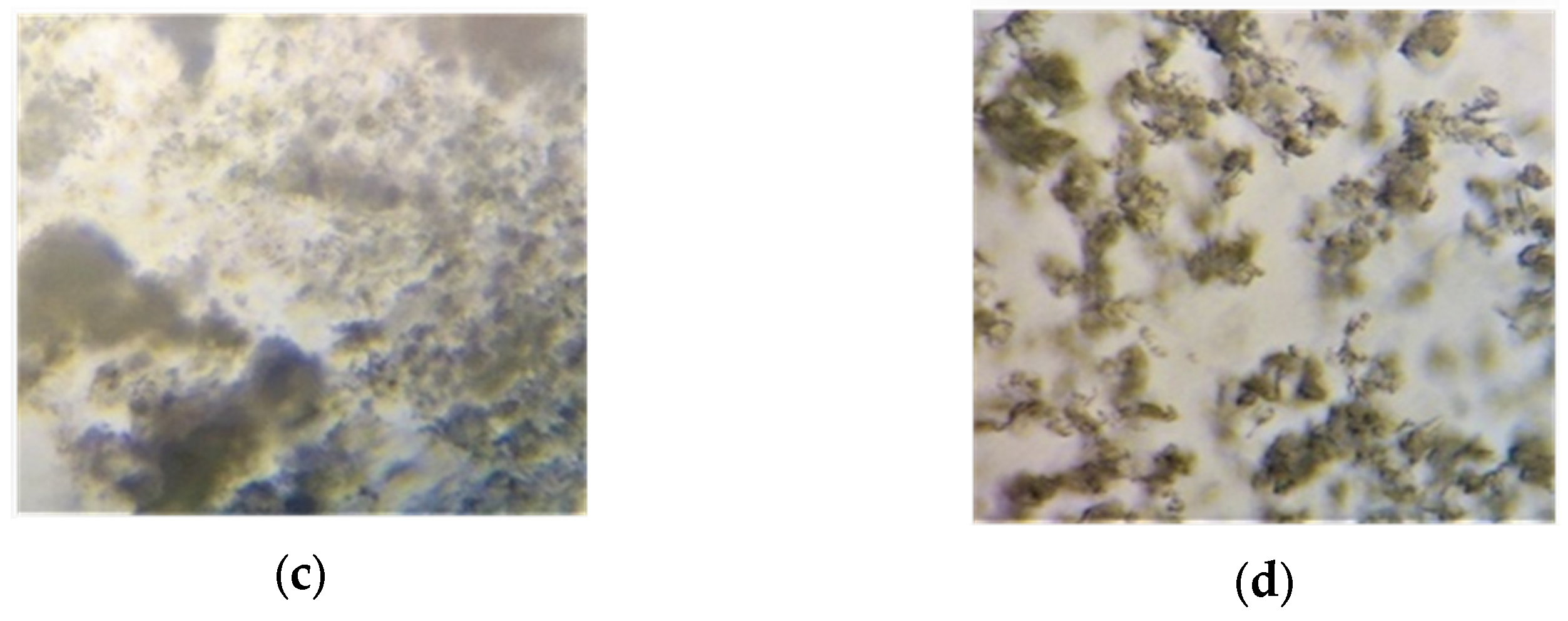
3.5.1. Microscopic Study
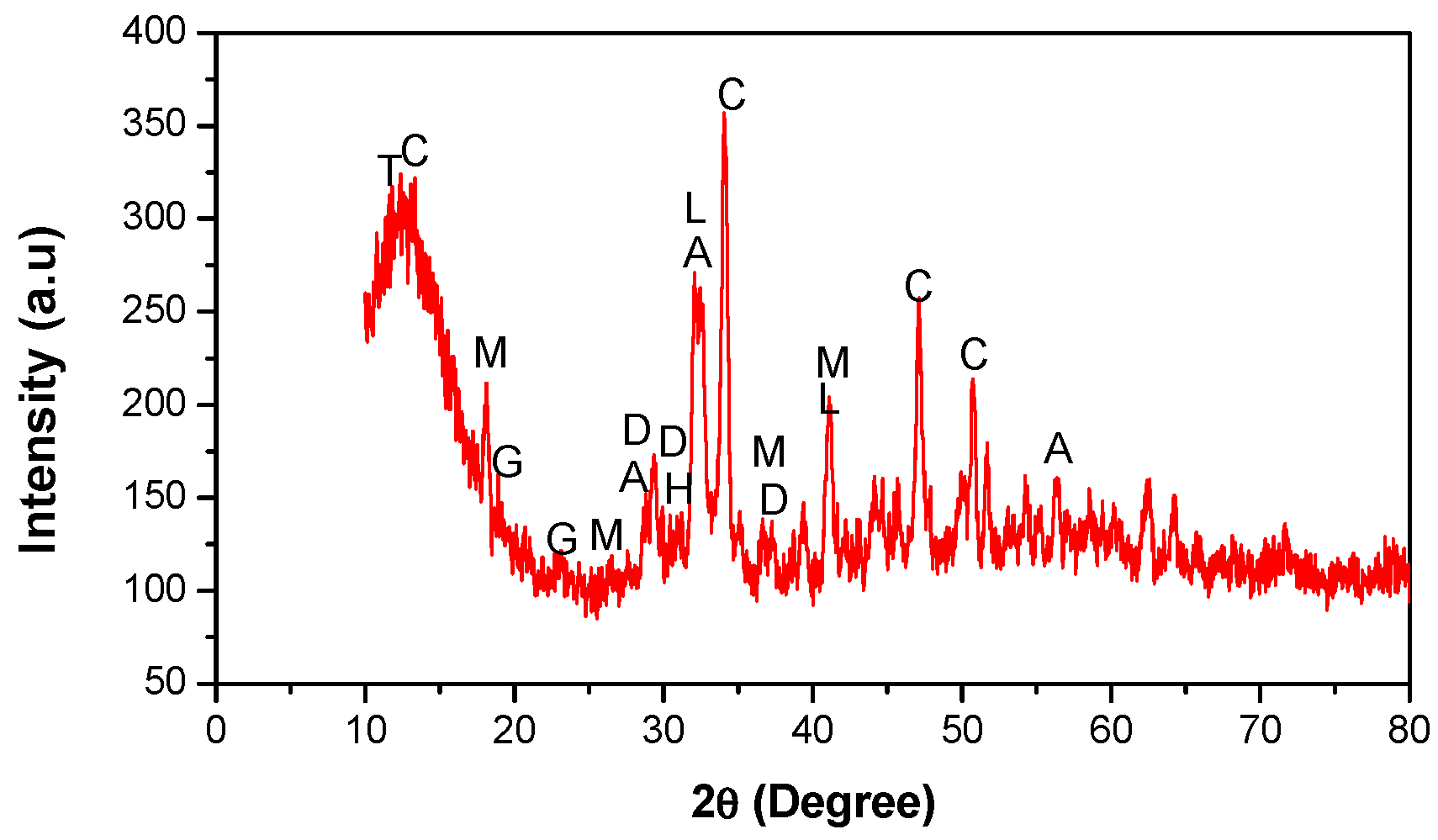
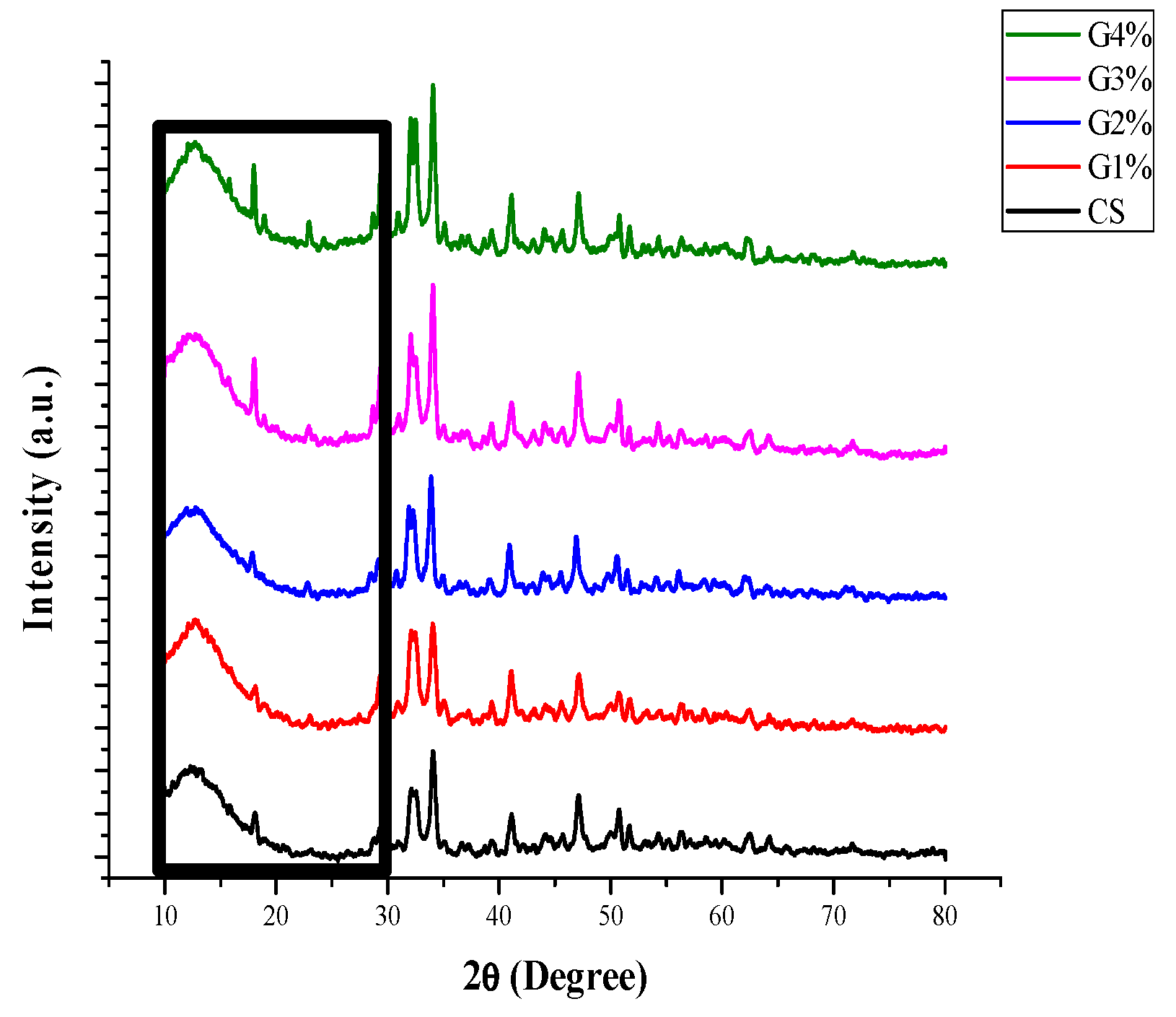
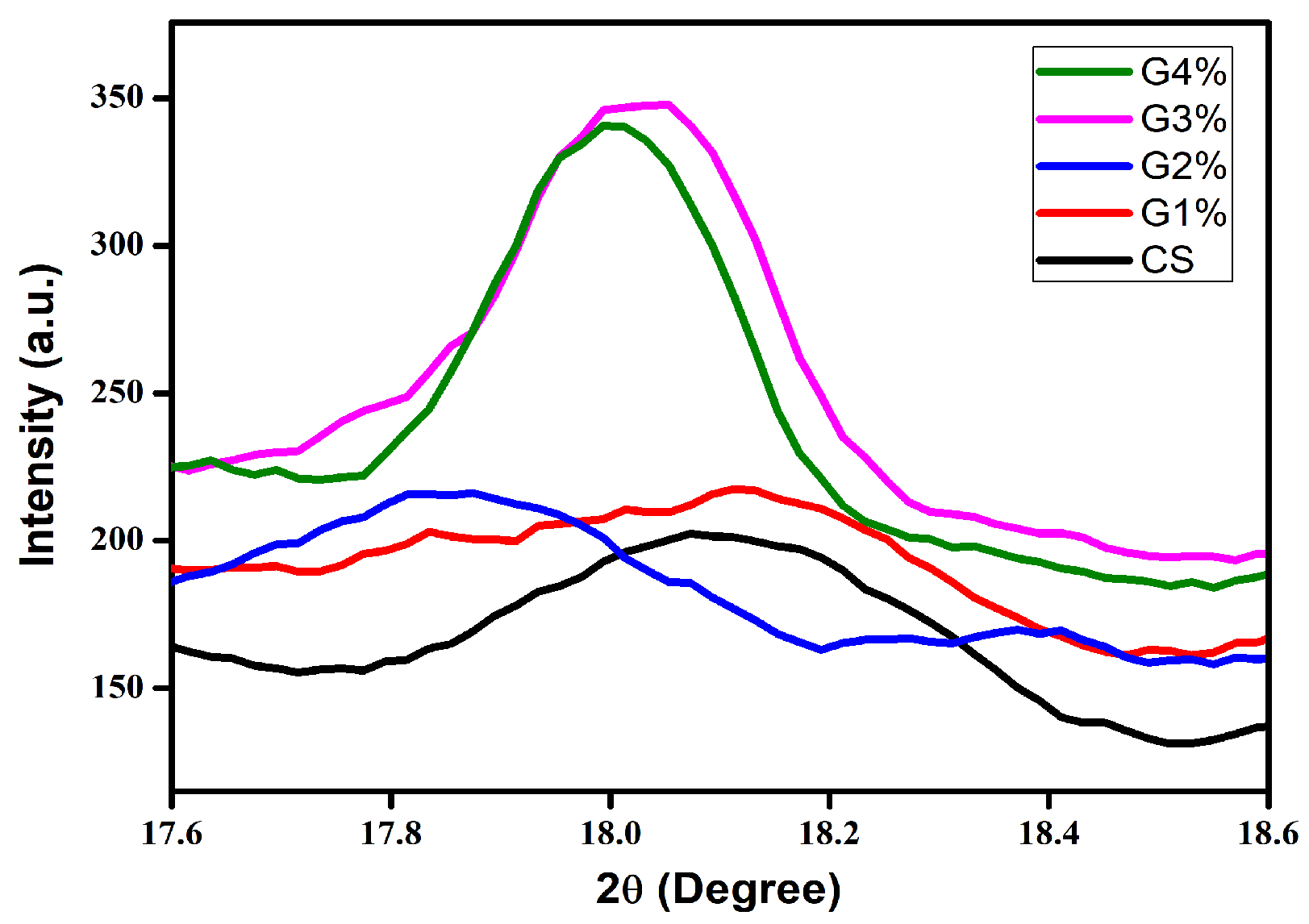
3.5.2. XRD Analysis
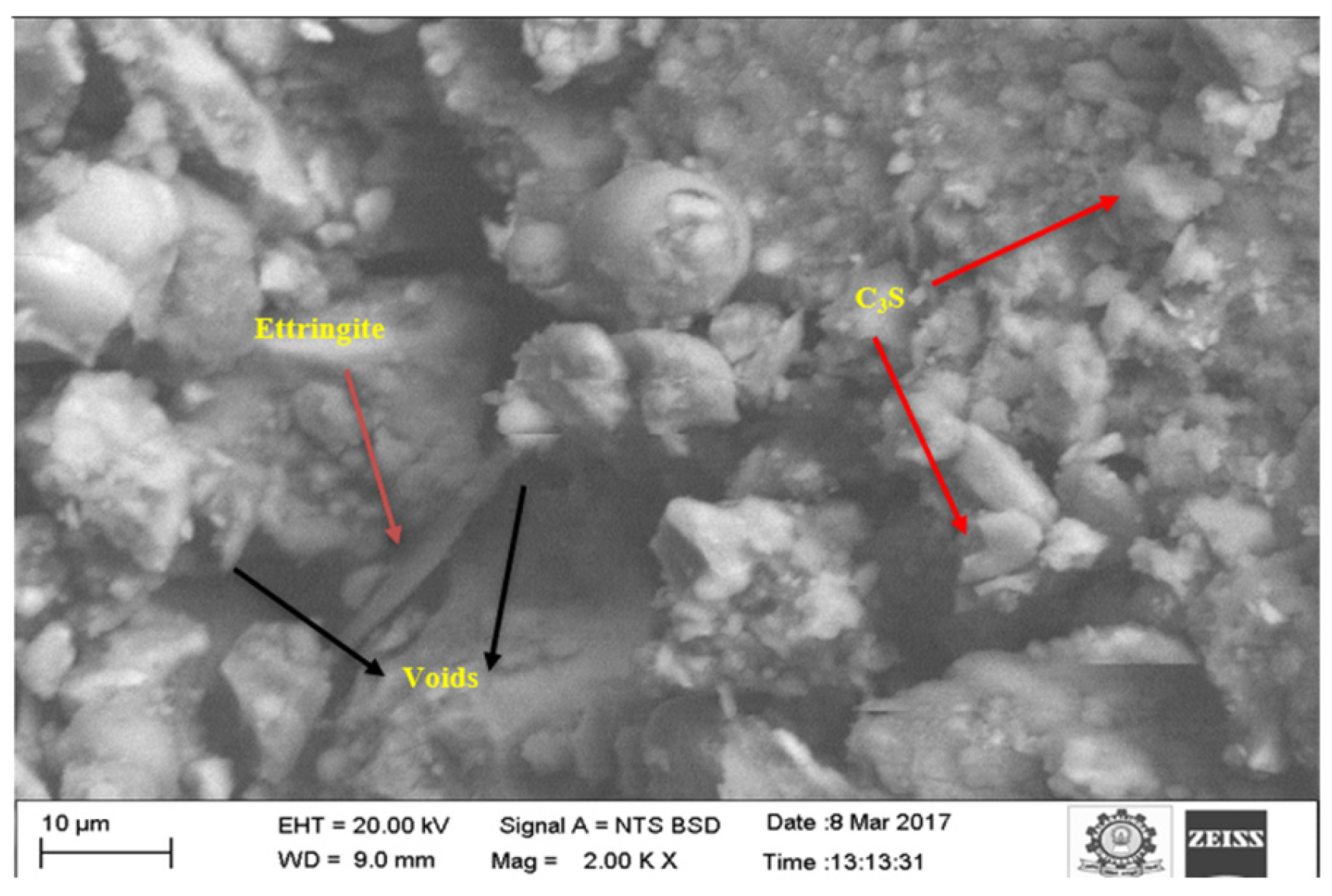
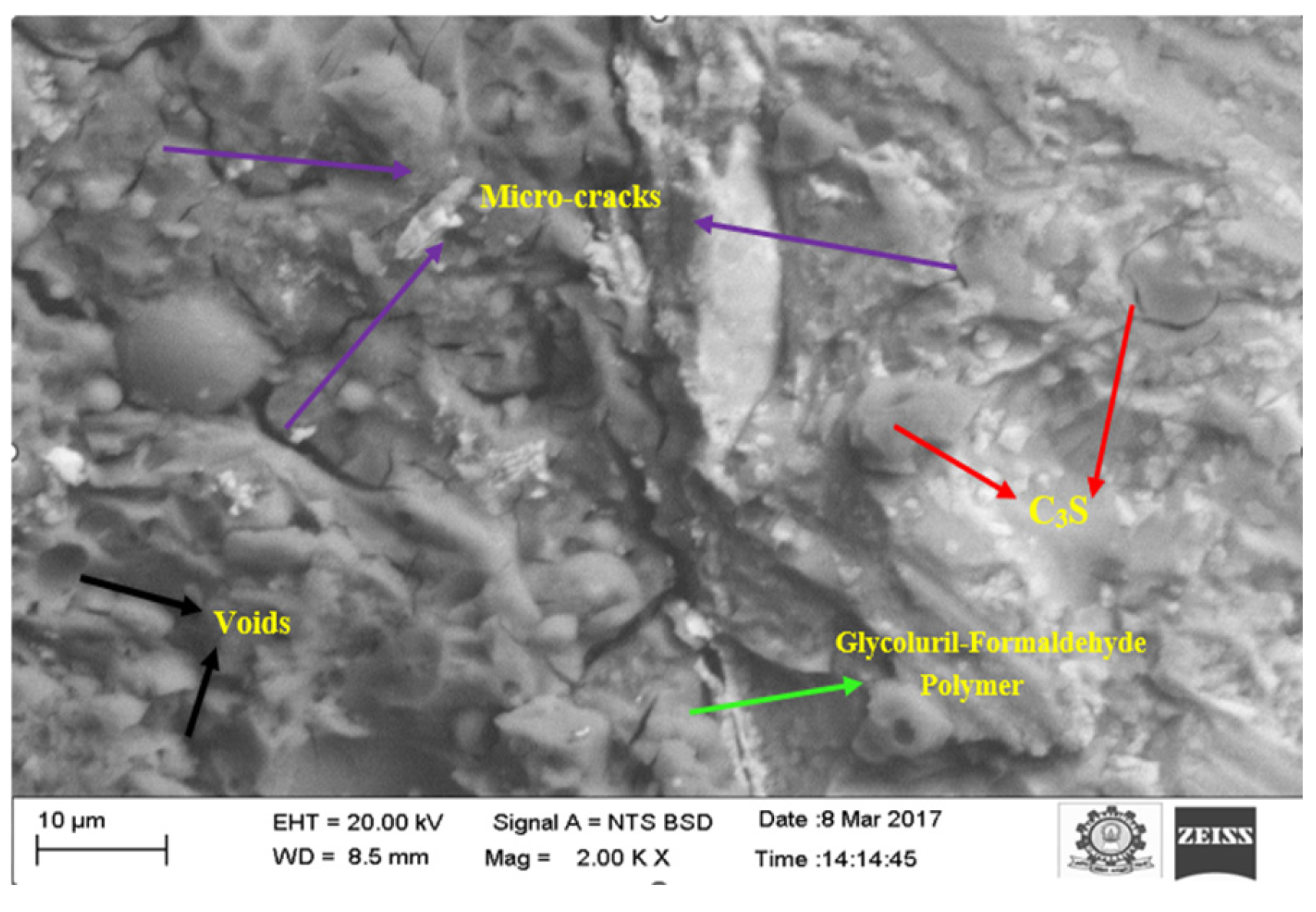
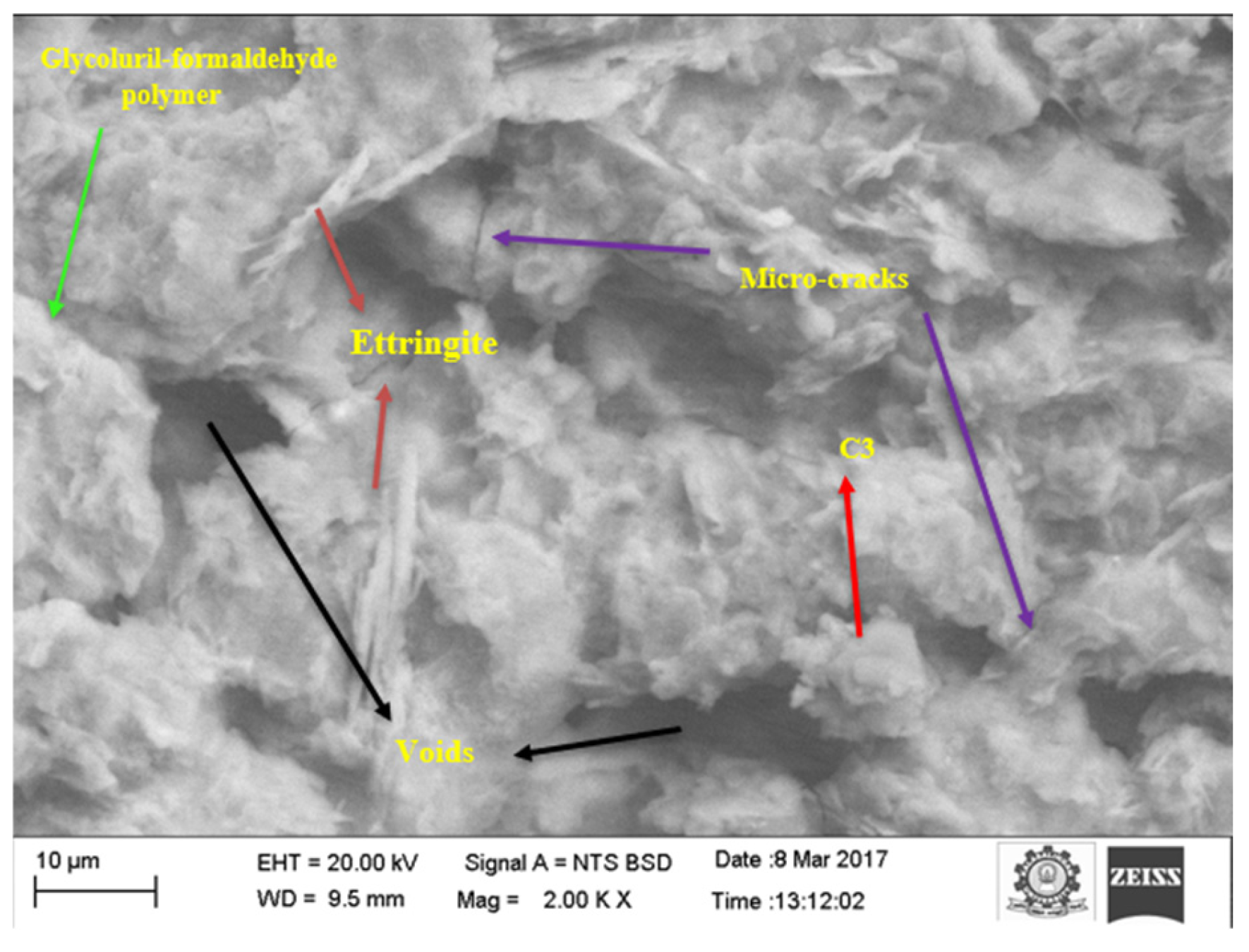
3.5.3. SEM Analysis
3.6. Possible Chemical Interaction Between GF and Cement Paste
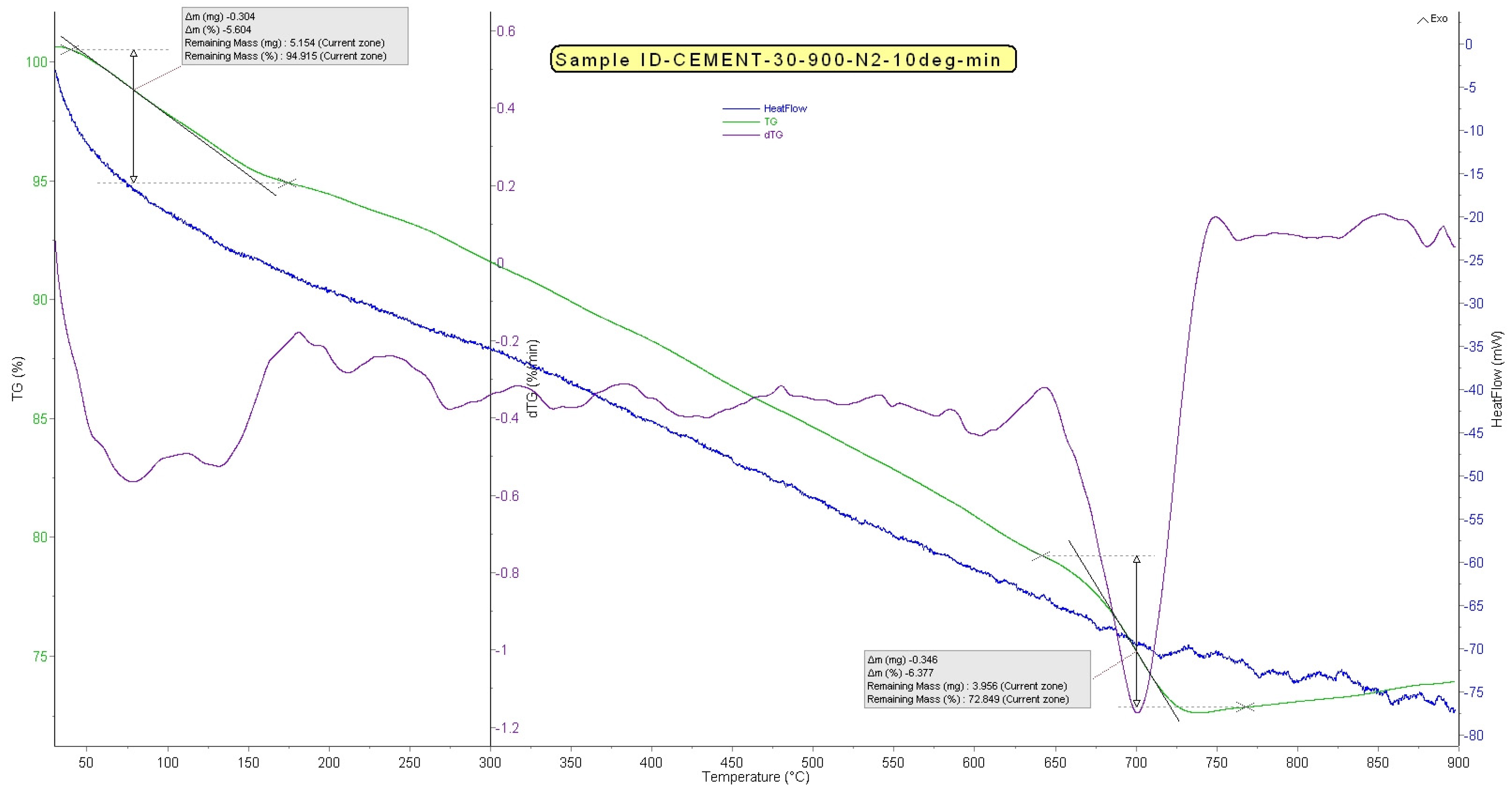
3.7. Thermogravimetric (TG) and Differential Thermogravimetric (DTG) Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pothinathan, S.K.M.; Muthukannan, M.; Selvapalam, N.; Gnanaraj, S.C. Investigation on strength properties of polymer modified concrete using glycoluril. Int. Rev. Appl. Sci. Eng. 2021, 12, 278–284. [Google Scholar] [CrossRef]
- Cabrera, J.G. Deterioration of Concrete Due to Reinforcement Steel Corrosion. Cem. Concr. Compos. 2018, 18, 47–59. [Google Scholar] [CrossRef]
- Fournier, B.; Bérubé, M.-A. Alkali-Aggregate Reaction in Concrete: A Review of Basic Concepts and Engineering Implications. Can. J. Civ. Eng. 2000, 27, 167–191. [Google Scholar] [CrossRef]
- Gu, L.; Li, H.; Yang, X.; Dong, B.; Wen, Z. Leakage behavior of toxic substances of naphthalene sulfonate-formaldehyde condensation from cement based materials. J. Environ. Manag. 2020, 255, 109934. [Google Scholar] [CrossRef]
- Brindha, U.; Maheswaran, J.; Chellapandian, M.; Arunachelam, N. Quantitative Assessment of Strengthening Strategies and Design Recommendations for the Repair of Corrosion-Damaged Reinforced Concrete Members. Buildings 2023, 13, 1080. [Google Scholar] [CrossRef]
- Andersen, R.; Negendahl, K. Lifespan prediction of existing building typologies. J. Build. Eng. 2023, 65, 105696. [Google Scholar] [CrossRef]
- Darvell, B.W. Polymers; Woodhead Publishing: Sawston, UK, 2018. [Google Scholar] [CrossRef]
- Kardon, J.B. Polymer-Modified Concrete: Review. J. Mater. Civ. Eng. 2018, 9, 85–92. [Google Scholar] [CrossRef]
- Ghassemi, P.; Toufigh, V. Durability of Epoxy Polymer and Ordinary Cement Concrete in Aggressive Environments. Constr. Build. Mater. 2020, 234, 117887. [Google Scholar] [CrossRef]
- Bhutta, M.A.R.; Maruya, T.; Tsuruta, K. Use of Polymer-Impregnated Concrete Permanent form in Marine Environment: 10-year Outdoor Exposure in Saudi Arabia. Constr. Build. Mater. 2013, 43, 50–57. [Google Scholar] [CrossRef]
- Harmuth, H. Investigation of the Adherence and the Fracture Behaviour of Polymer Cement Concrete. Cem. Concr. Res. 1995, 25, 497–502. [Google Scholar] [CrossRef]
- Erhard, D.R.; Chorinsky, G.F. Repair of Concrete Floors with Polymer Modified Cement Mortars; Springer: Berlin/Heidelberg, Germany, 1986. [Google Scholar] [CrossRef]
- Agavriloaie, L.; Oprea, S.; Barbuta, M.; Luca, F. Characterisation of Polymer Concrete with Epoxy Poly-urethane Acryl Matrix. Constr. Build. Mater. 2012, 37, 190–196. [Google Scholar] [CrossRef]
- Akihama, S.; Morita, H.; Chida, H. Improvement of Mechanical Properties of Concrete Through the Addition of Polymer Latex; American Concrete Institute: Farmington Hills, MI, USA, 1973; Volume 40, pp. 319–338. [Google Scholar]
- Wang, J.; Jiang, N.; Jiang, H. Effect of the Evolution of Phenol–Formaldehyde Resin on the High-Temperature Bonding. Int. J. Adhes. Adhes. 2009, 29, 718–723. [Google Scholar] [CrossRef]
- Pothinathan, S.K.; Muthukannan, M.; Selvapalam, N. Comparison of bond strength analysis on the in-terfacial layer of old and new concrete using latex, epoxy and glycoluril. IOP Conf. Ser. Mater. Sci. Eng. 2020, 983, 012006. [Google Scholar] [CrossRef]
- IS 12269; Ordinary Portland Cement, 53 Grade—Specification. Bureau of Indian Standards: New Delhi, India, 2013.
- IS 4031; Method of Physical Test for Hydraulic Cement: Part 11. Determination of Density and Specific Gravity of Cement. Bureau of Indian Standards: New Delhi, India, 2005.
- IS 4031; Method of Physical Test for Hydraulic cement: Part 4. Determination of Consistency of Cement Paste. Bureau of Indian Standards: New Delhi, India, 2005.
- IS 2386; Methods of Test for Aggregates for Concrete, Part 3: Specific Gravity, Density, Voids, Absorption and Bulking. Bureau of Indian Standards: New Delhi, India, 2002.
- IS 383; Indian Standards Specification for Coarse and Fine Aggregates from Natural Sources for Con-crete. Bureau of Indian Standards: New Delhi, India, 2016.
- IS 4031; Method of Physical Test for Hydraulic cement: Part 6. Determination of Compressive Strength of Cement (Other than Masonry Cement). Bureau of Indian Standards: New Delhi, India, 2005.
- IS 10086; Specification for Moulds for Use in Tests of Cement and Concrete. Bureau of Indian Standards: New Delhi, India, 2004.
- DIN 1048-1; Testing Concrete; Testing of Fresh Concrete. International Organization for Standardization: Geneva, Switzerland, 1991.
- Ferraris, C.F.; Obla, K.H.; Hill, R. The Influence of Mineral Admixtures on the Rheology of Cement Paste and Concrete. Cem. Concr. Res. 2001, 31, 245–255. [Google Scholar] [CrossRef]
- Nazari, A.; Riahi, S.; Riahi, S.; Shamekhi, S.F.; Khademno, A. Assessment of the Effects of the Cement Paste Composite in Presence TiO2 Nano Particles. J. Am. Sci. 2010, 6, 43–46. [Google Scholar]
- Quercia, G.; Hüsken, G.; Brouwers, H.J.H. Water Demand of Amorphous Nano Silica and its Impact on the Workability of Cement Paste. Cem. Concr. Res. 2012, 42, 344–357. [Google Scholar] [CrossRef]
- Mehdipour, I.; Khayat, K.H. Effect of Particle-Size Distribution and Specific Surface Area of Different Binder Systems on Packing Density and Flow Characteristics of Cement Paste. Cem. Concr. Compos. 2017, 78, 120–131. [Google Scholar] [CrossRef]
- Givi, A.N.; Rashid, S.A.; Aziz, F.N.A.; Salleh, M.A.M. Assessment of the Effects of Rice Husk Ash Parti-cle Size on Strength, Water Permeability and Workability of Binary Blended Concrete. Constr. Build. Mater. 2010, 24, 2145–2150. [Google Scholar] [CrossRef]
- Martin, J.W. Materials for Engineering; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Mathur, R.; Sharma, S.K. Effect of Formaldehyde as an Additive on Some Properties of Magnesium Oxysulphate Cement. Mater. Sci. Res. India 2008, 5, 313–320. [Google Scholar]
- Kumar, R. A comparative Study on Dry Lean Concrete Manufactured with OPC vis-a-vis PPC to be Used for the Construction of Concrete Roads. Indian Concr. J. 2016, 90, 70–76. [Google Scholar]
- Pothinathan, S.K.M.; Muthukannan, M.; Selvapalam, N.; Gnanaraj, S.C. Development of high strength mortar by utilizing glycoluril-formaldehyde as an additive. Struct. Concr. 2022, 23, 4058–4066. [Google Scholar] [CrossRef]
- Chen, B.; Qiao, G.; Hou, D.; Wang, M.; Li, Z. Cement-Based Material Modified by In-Situ Polymerization: From Experiments to Molecular Dynamics Investigation. Compos. B Eng. 2020, 194, 108036. [Google Scholar] [CrossRef]
- Pothinathan, S.K.M.; Muthukannan, M.; Selvapalam, N.; Gnanaraj, S.C. Bond Strength Analysis of Re-paired Concrete based on Polymerization of Glycoluril Formaldehyde. J. Eng. Res. 2021, 10, 72–85. [Google Scholar] [CrossRef]
- IS 516; Method of Tests for Strength of Concrete. Bureau of Indian Standards: New Delhi, India, 2004.
- Gnanaraj, S.C.; Chokkalingam, R.B.; Thankam, G.L.; Pothinathan, S.K.M. Influence of Steatite and Fly Ash on the Fresh-Hardened Properties and Micromorphology of Self-Compacting Concrete. Adv. Mater. Sci. Eng. 2021, 2021, 6627450. [Google Scholar] [CrossRef]
- Bedi, R.; Chandra, R.; Singh, S.P. Mechanical Properties of Polymer Concrete. J. Compos. 2013, 2013, 948745. [Google Scholar] [CrossRef]
- Ma, H.; Li, Z. Microstructures and Mechanical Properties of Polymer Modified Mortars Under Distinct Mechanism. Constr. Build. Mater. 2013, 47, 579–587. [Google Scholar] [CrossRef]
- Pascal, S.; Alliche, A.; Pilvin, P. Mechanical Behaviour of Polymer Modified Mortars. Mater. Sci. Eng. A 2004, 380, 1–8. [Google Scholar] [CrossRef]
- Ariffin, N.F.; Hussin, M.W.; Sam, A.R.M.; Bhutta, M.A.R.; Nur, N.H.; Mirza, J. Strength Properties and Molecular Composition of Epoxy-Modified Mortars. Constr. Build. Mater. 2015, 94, 315–322. [Google Scholar] [CrossRef]
- Susilorini, R.M.I.R.; Hardjasaputra, H.; Tudjono, S.; Hapsari, G.; Wahyu, S.R.; Hadikusumo, G.; Sucipto, J. The Advantage of Natural Polymer Modified Mortar with Seaweed: Green Construction Material Innovation for Sustainable Concrete. Procedia Eng. 2014, 95, 419–425. [Google Scholar] [CrossRef]
- Liu, C.; Luo, J.; Li, X.; Gao, Q.; Li, J. Effects of Compounded Curing Agents on Properties and Performance of Urea Formaldehyde Resin. J. Polym. Environ. 2018, 26, 158–165. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, J.; Peng, Z.; Feng, Q.; Lou, Y. Study on the properties of urea-formaldehyde resin in repairing microcracks of cement stone in oil well under CO2 acid environment. React. Funct. Polym. 2023, 191, 105688. [Google Scholar] [CrossRef]
- Elshaboury, N.; Al-Sakkaf, A.; Abdelkader, E.M.; Alfalah, G. Construction and Demolition Waste Management Research: A Science Mapping Analysis. Int. J. Environ. Res. Public Health 2022, 19, 4496. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, R.A.; Lima, N.B.; Lima, V.M.E.; Estolano, A.M.L.; Póvoas, Y.V.; Lima, N.B.D. The role of hydrogen bonds on the mechanical properties of cement-based mortars applied to concrete surfaces. Cem. Concr. Compos. 2021, 115, 103848. [Google Scholar] [CrossRef]
- Jing, Z. Hydrogen Bonding Arrangements at Si–SiO2 Interfaces. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 1995, 13, 1613–1617. [Google Scholar] [CrossRef]
- de Oliveira, A.M.; Oliveira, A.P.; Vieira, J.D.; Junior, A.N.; Cascudo, O. Study of the Development of Hydration of Ternary Cement Pastes Using X-Ray Computed Microtomography, XRD-Rietveld Method, TG/DTG, DSC, Calorimetry and FTIR Techniques. J. Build. Eng. 2023, 64, 105616. [Google Scholar] [CrossRef]
- Pugalenthi, S.; Chellapandian, M.; Dharmaraj, J.J.J.; Devaraj, J.; Arunachelam, N.; Bright Singh, S. Enhancing the Thermal Transport Property of Eutectic Lauric-Stearic Acid Based Phase Change Material with Silicon Carbide Nanoparticles for Usage in Battery Thermal Management System. J. Energy Storage 2024, 84, 110890. [Google Scholar] [CrossRef]
- Alarcon-Ruiz, L.; Platret, G.; Massieu, E.; Ehrlacher, A. The Use of Thermal Analysis in Assessing the Effect of Temperature on a Cement Paste. Cem. Concr. Res. 2005, 35, 609–613. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, X.; Liu, X.; He, Z.; Cao, X.; Guan, B. Evaluation of Workability and Mechanical Properties in Cement Mortar after Compounding Igneous Rock Powder and Silica Fume. Buildings 2024, 14, 359. [Google Scholar] [CrossRef]
- Getachew, E.M.; Yifru, B.W.; Taffese, W.Z.; Yehualaw, M.D. Enhancing Mortar Properties through Thermoactivated Recycled Concrete Cement. Buildings 2023, 13, 2209. [Google Scholar] [CrossRef]
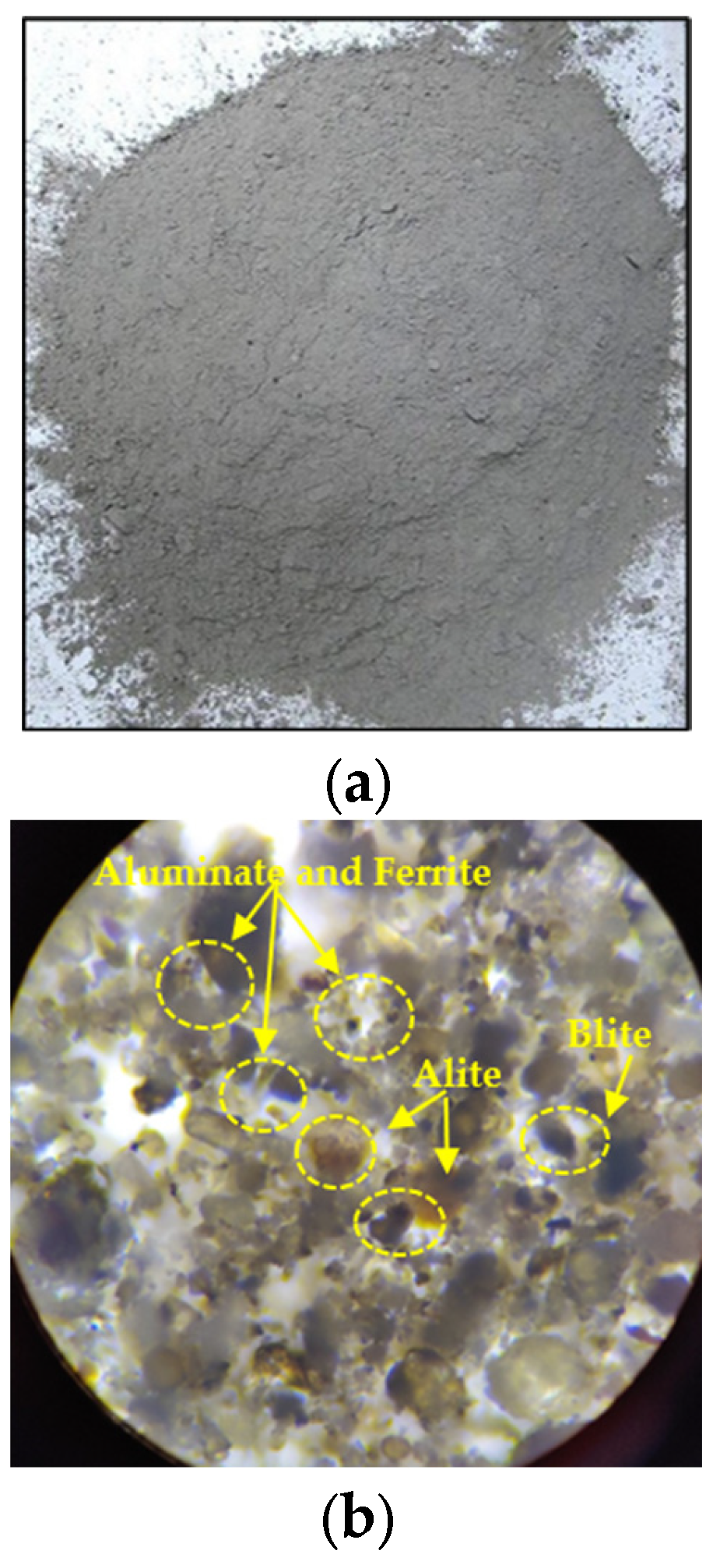





| Physical Properties | Results |
|---|---|
| Mean diameter of particle (µm) | Less than 30 |
| Blaine surface area (cm2/gm) | 3420 |
| Loss of ignition | 3.17% |
| Chemical Composition | Results |
|---|---|
| Calcium oxide (CaO) | 63.23% |
| Silicon dioxide (SiO2) | 21.41% |
| Aluminium oxide (Al2O3) | 4.98% |
| Sulphur trioxide (SO3) | 2.25% |
| Ferric oxide (Fe2O3) | 3.18% |
| Magnesium oxide (MgO) | 1.03% |
| Other mineral Oxides | 1.78% |
| Properties of River Sand | Results |
|---|---|
| Specific gravity | 2.64 |
| Percentage of voids | 44.23% |
| Fineness modulus | 2.73 |
| Water absorption | 2.64% |
| Bulk density | 1.43 kg/m3 |
| Sieve analysis | Zone II |
| Set | Mix ID | Cement (gm) | Glycoluril (gm) | Formaldehyde (gm) |
|---|---|---|---|---|
| CS | 400 | 0 | - | |
| Set-1 | CG1 | 400 | 4 | - |
| CG2 | 400 | 8 | - | |
| CG3 | 400 | 12 | - | |
| CG4 | 400 | 16 | - | |
| CG5 | 400 | 20 | - | |
| Set-2 | CF1 | 400 | - | 4 |
| CF2 | 400 | - | 8 | |
| CF3 | 400 | - | 12 | |
| CF4 | 400 | - | 16 | |
| CF5 | 400 | - | 20 | |
| Set-3 | CGF1 | 400 | 4 | 8 |
| CGF2 | 400 | 8 | 8 | |
| CGF3 | 400 | 12 | 8 | |
| CGF4 | 400 | 16 | 8 | |
| CGF5 | 400 | 20 | 8 |
| Mix ID | Cement (g) | FA (g) | Water (ml) | Glycoluril (g) |
|---|---|---|---|---|
| CS | 600 | 1800 | 270 | - |
| CMG1 | 600 | 1800 | 270 | 0.006 |
| CMG2 | 600 | 1800 | 270 | 0.012 |
| CMG3 | 600 | 1800 | 270 | 0.018 |
| CMG4 | 600 | 1800 | 270 | 0.024 |
| CMG5 | 600 | 1800 | 270 | 0.03 |
| Cu K-Beta Radiation with 2Ɵ Scanning Is Used | |
|---|---|
| Step size | 0.020 |
| Measuring time | 10.00 Deg/min |
| Voltage | 40 kV |
| Current | 15 Ma |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arunachelam, N.; Pothinathan, S.K.M.; Gifta, C.C.; Vignesh, N.P. A Comprehensive Study on the Microstructure and Mechanical Behavior of Glycoluril–Formaldehyde Polymer-Modified Cement Paste. Buildings 2025, 15, 1598. https://doi.org/10.3390/buildings15101598
Arunachelam N, Pothinathan SKM, Gifta CC, Vignesh NP. A Comprehensive Study on the Microstructure and Mechanical Behavior of Glycoluril–Formaldehyde Polymer-Modified Cement Paste. Buildings. 2025; 15(10):1598. https://doi.org/10.3390/buildings15101598
Chicago/Turabian StyleArunachelam, Nakarajan, S. K. M. Pothinathan, C. Chella Gifta, and N. P. Vignesh. 2025. "A Comprehensive Study on the Microstructure and Mechanical Behavior of Glycoluril–Formaldehyde Polymer-Modified Cement Paste" Buildings 15, no. 10: 1598. https://doi.org/10.3390/buildings15101598
APA StyleArunachelam, N., Pothinathan, S. K. M., Gifta, C. C., & Vignesh, N. P. (2025). A Comprehensive Study on the Microstructure and Mechanical Behavior of Glycoluril–Formaldehyde Polymer-Modified Cement Paste. Buildings, 15(10), 1598. https://doi.org/10.3390/buildings15101598







