Red Mud in Combination with Construction Waste Red Bricks for the Preparation of Low-Carbon Binder Materials: Design and Material Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
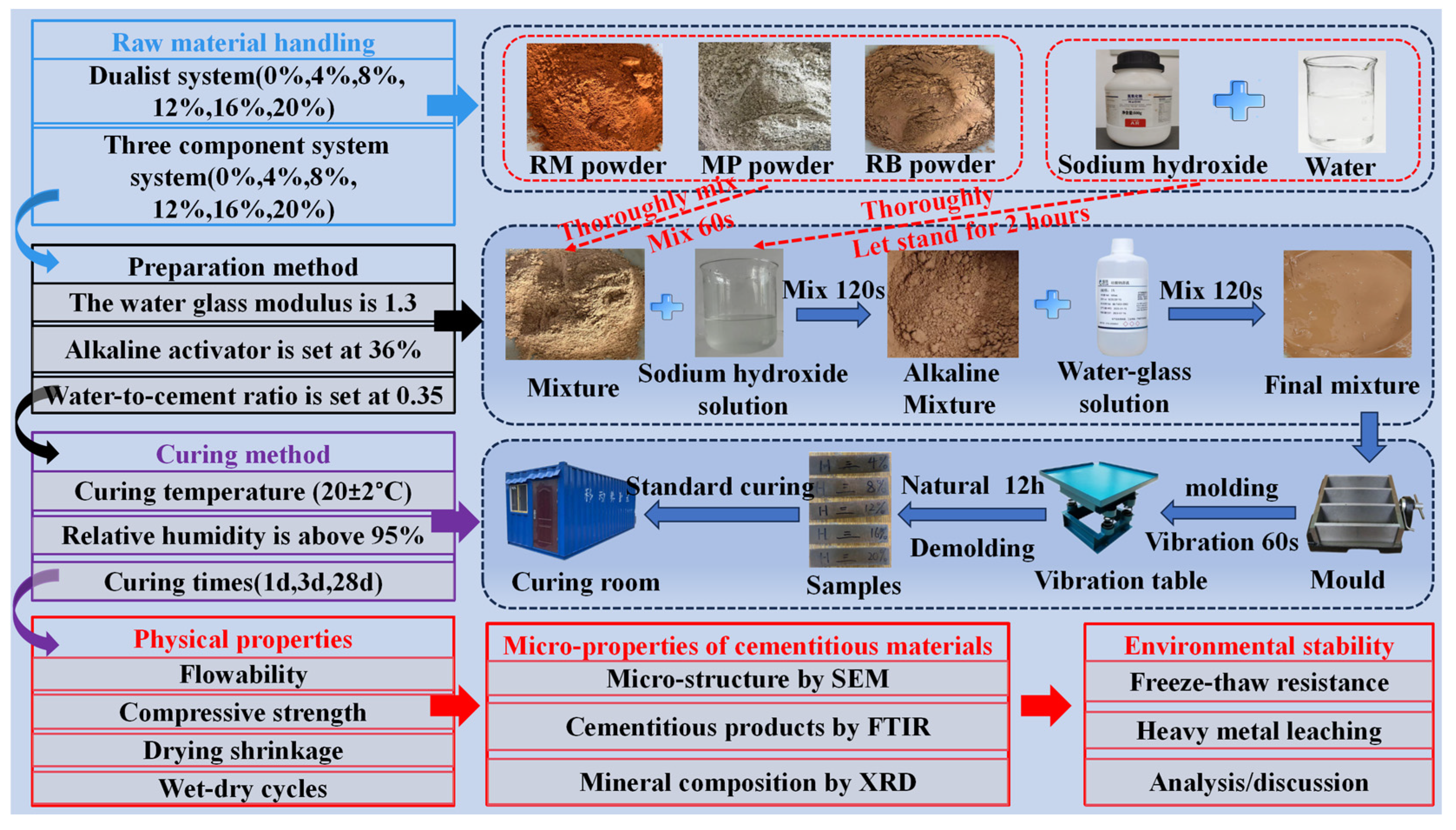
2.2. Preparation Method of Gelling Materials
2.3. Test Methods
2.3.1. Fluidity
2.3.2. Compressive Strength
2.3.3. Drying Shrinkage and Mass Loss
2.3.4. Dry–Wet Cycle
2.3.5. Microstructure Test
2.3.6. Freeze–Thaw Cycling Test
2.3.7. Heavy Metal Leaching Characterization Test
3. Results and Discussion
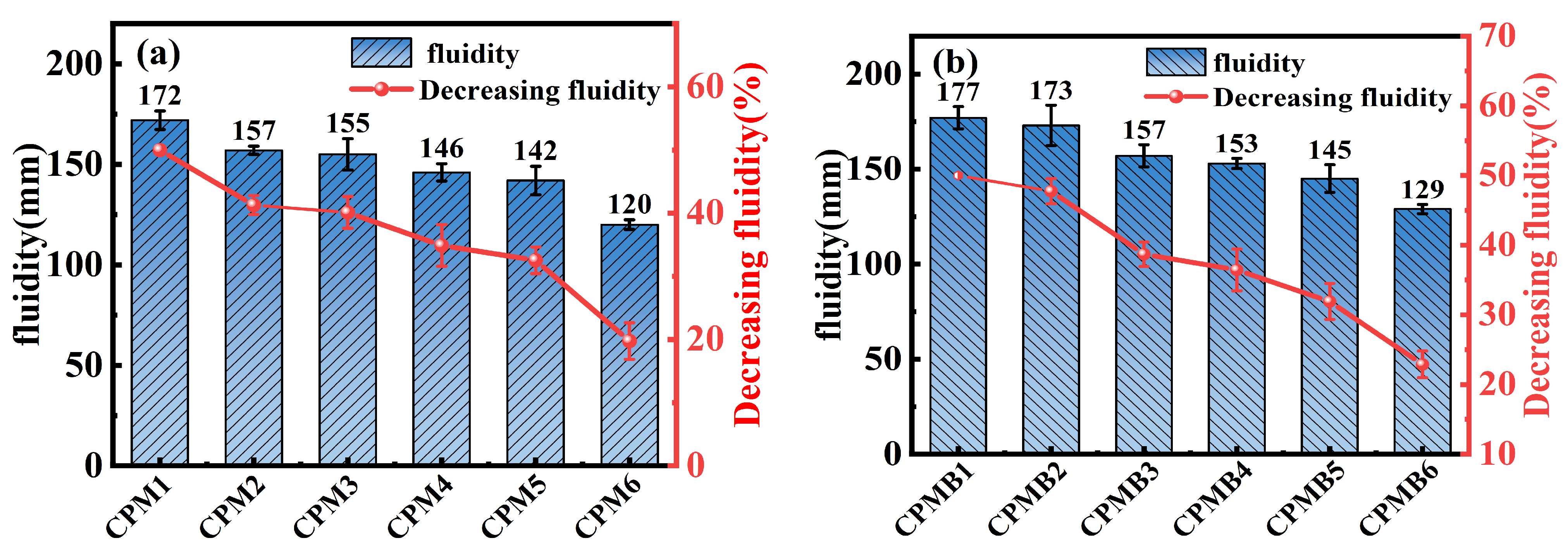
3.1. Fluidity
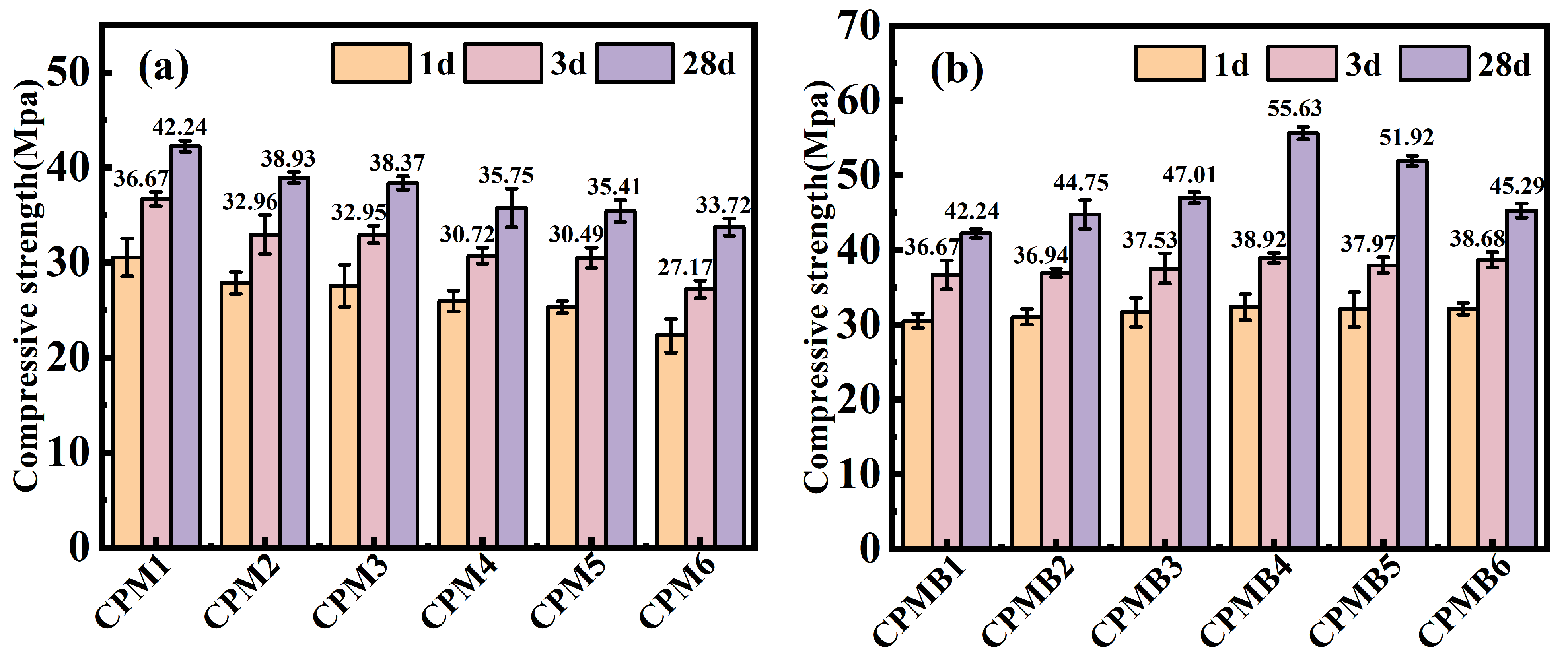
3.2. Compressive Strength
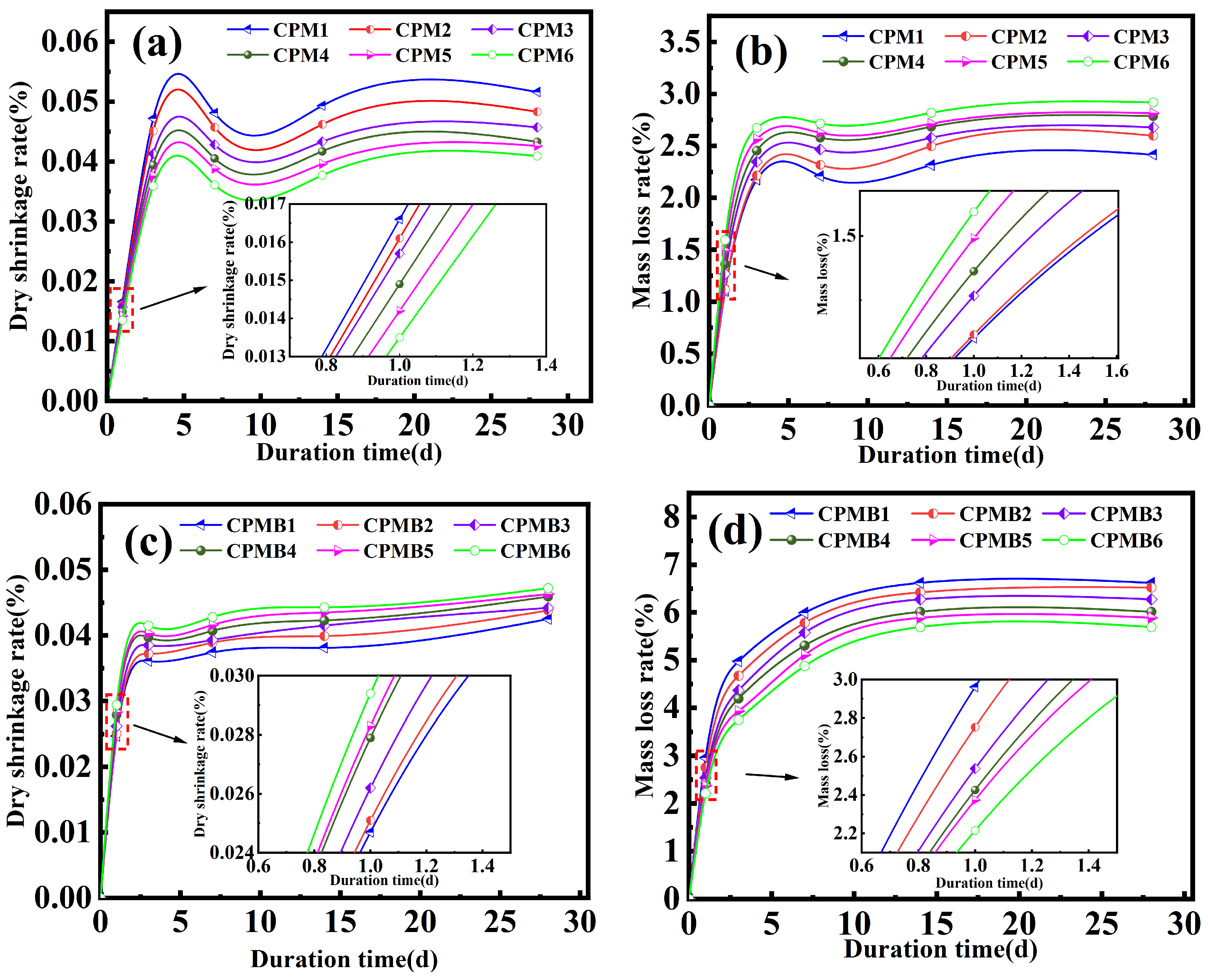
3.3. Drying Shrinkage and Mass Loss
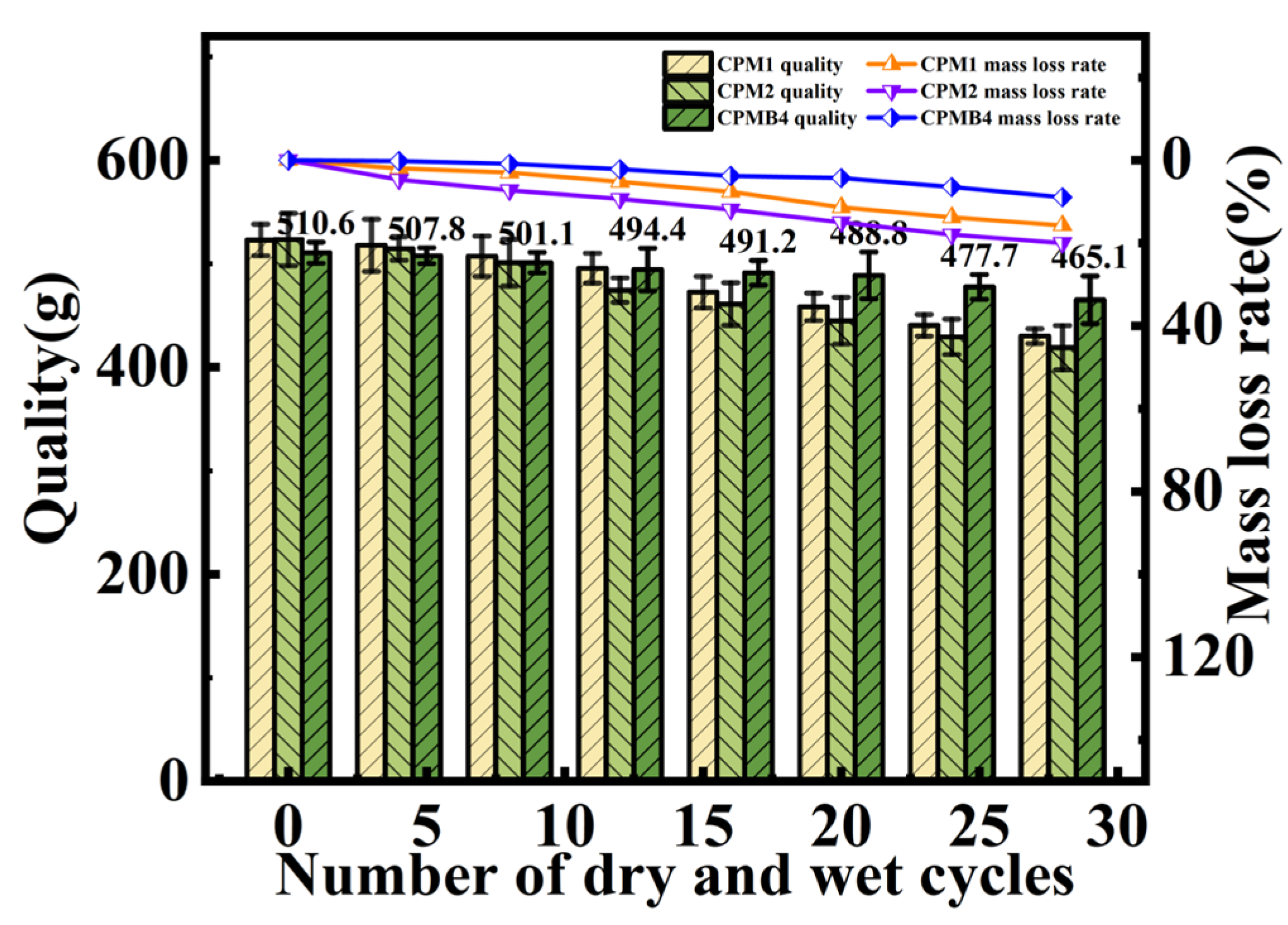
3.4. Dry–Wet Cycle
3.5. Mechanistic Analysis
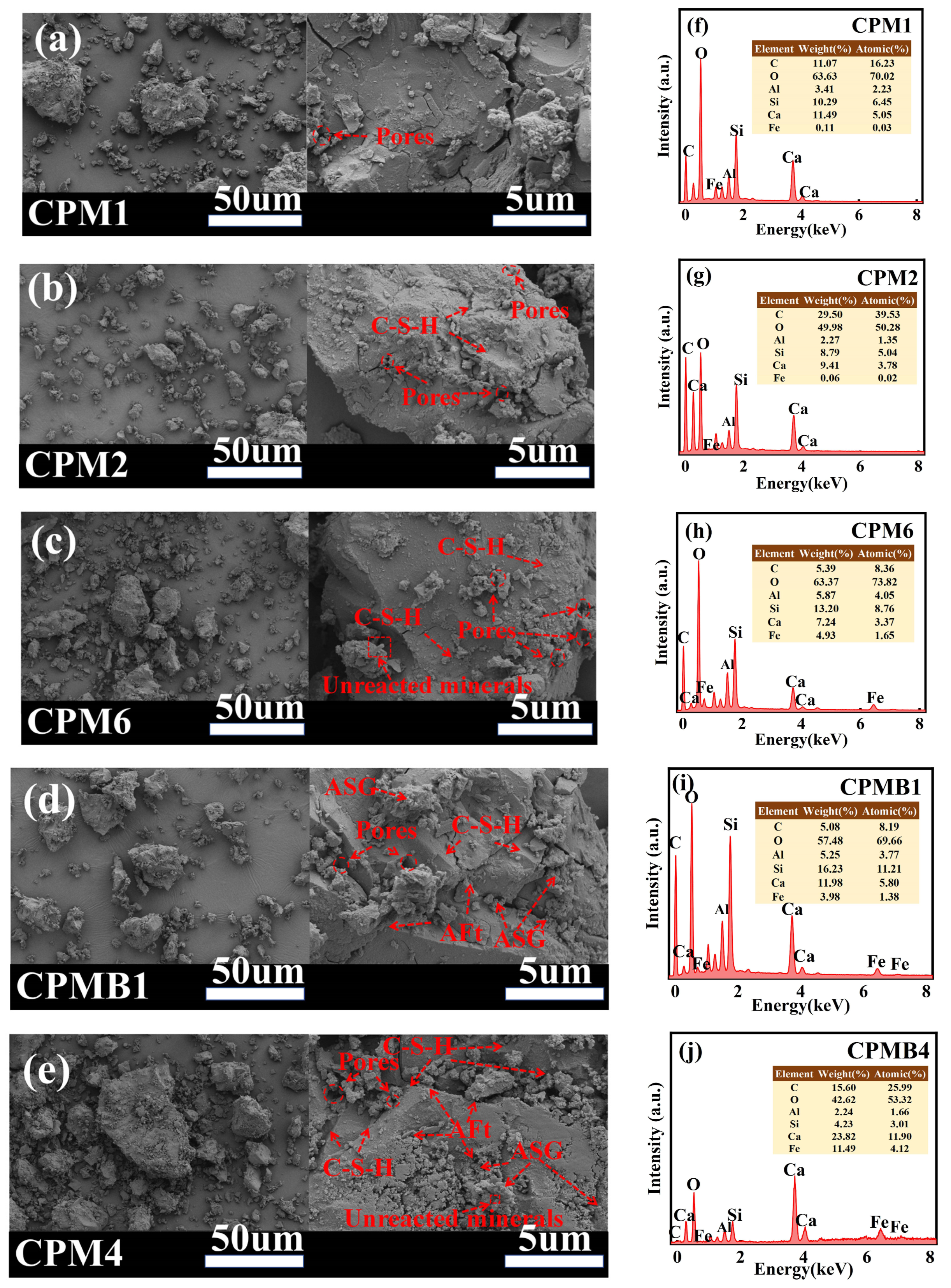
3.5.1. Microstructure Test
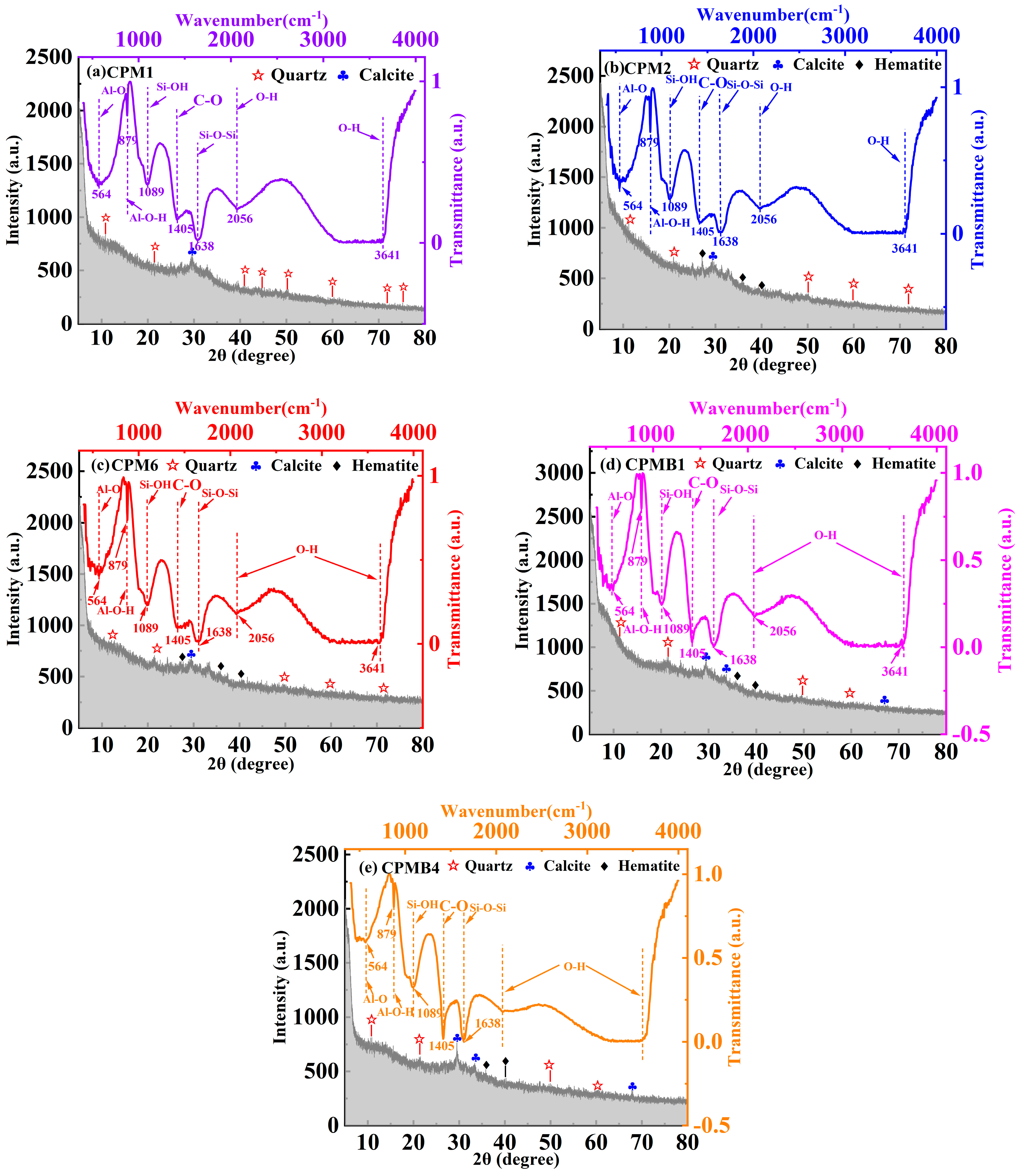
3.5.2. XRD
3.5.3. FTIR
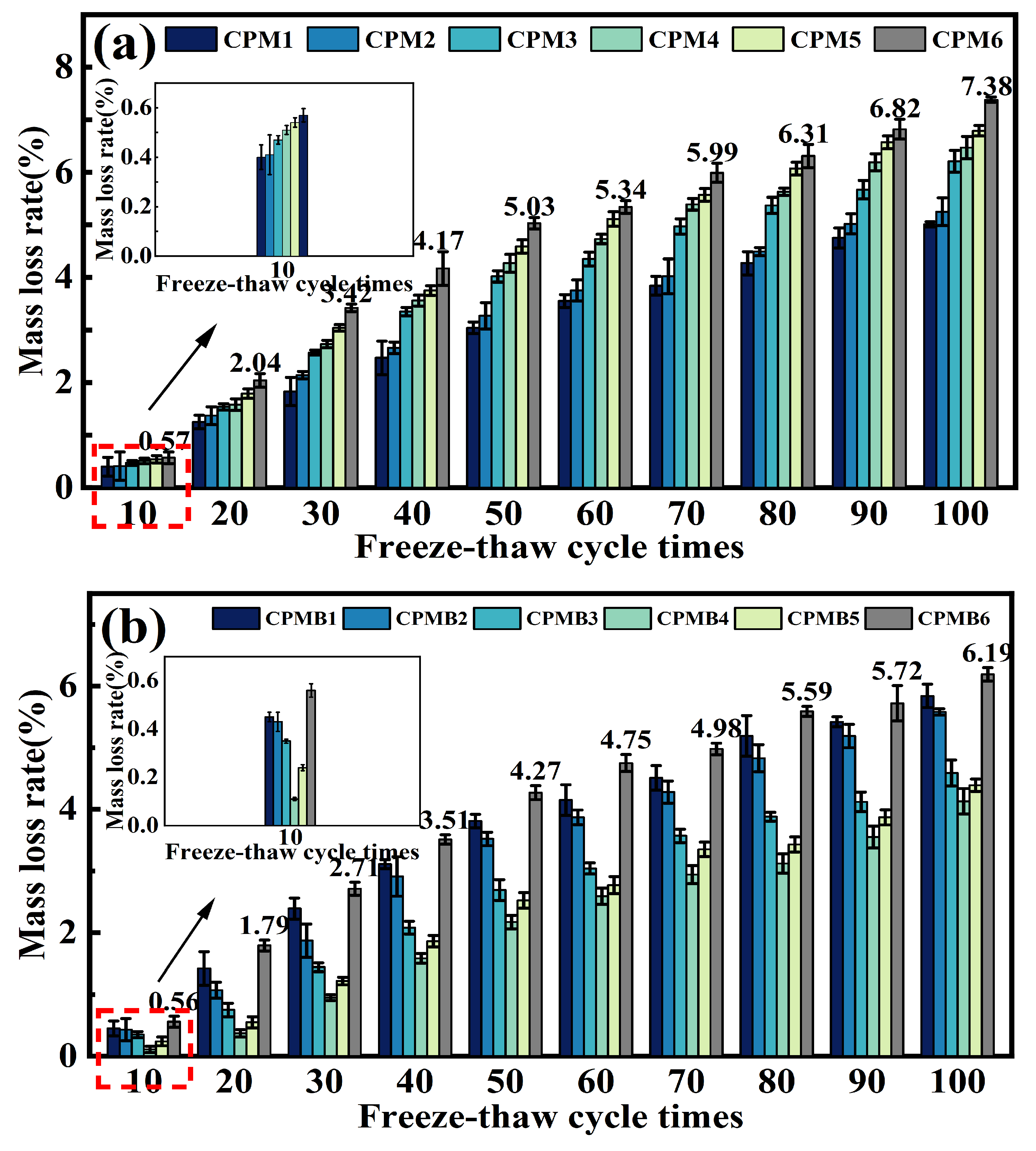
3.6. Freeze–Thaw Resistance
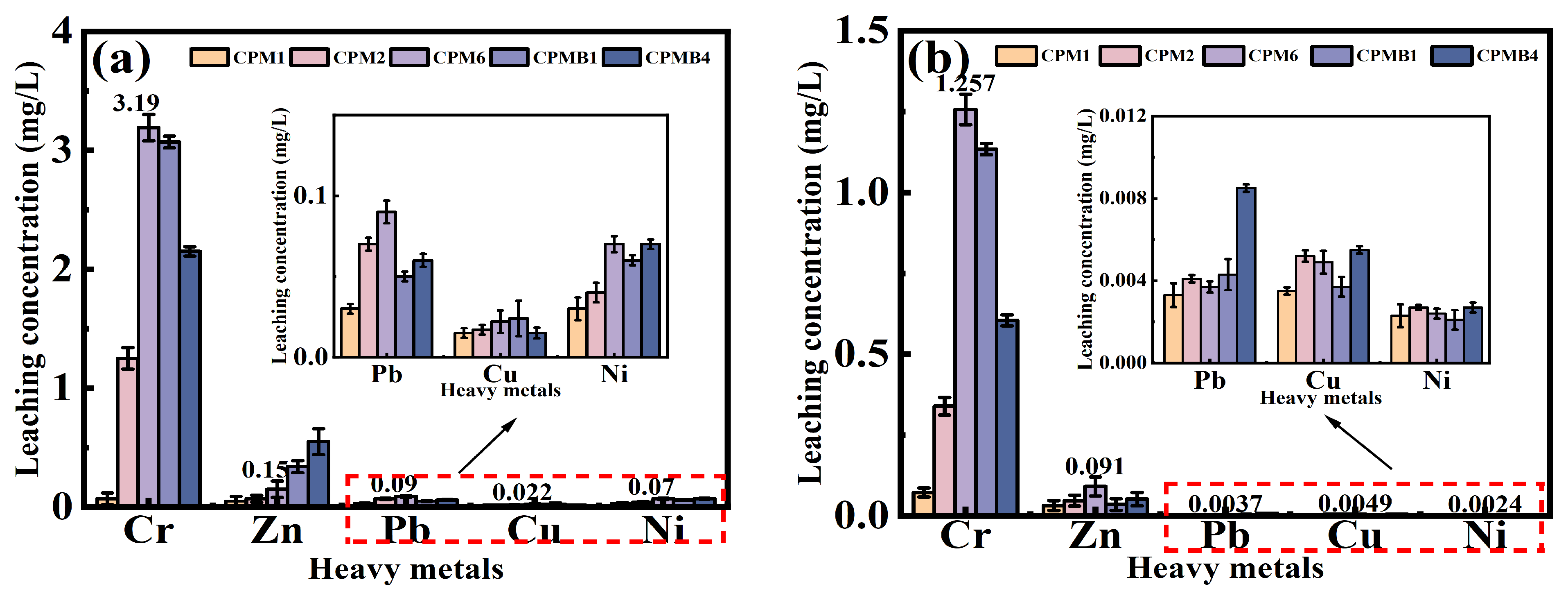
3.7. Heavy Metal Leaching
4. Conclusions
- (1)
- Under standard curing conditions, the ternary cementitious material with 16% RB performed the best, with a 4.5% improvement in fluidity, 18.27% increase in compressive strength, 39.56% increase in drying shrinkage, 45.91% reduction in mass loss, and 11.07% reduction in wet and dry cycle mass loss.
- (2)
- The incorporation of RB stimulates Al-Si activity, generates amorphous alumino-silicate gel phases and C-S-H gels, and also promotes the formation of chalcocite. XRD results show that the incorporation of RB reduces porosity and forms a dense structure, which enhances the mechanical properties of the cementitious materials.
- (3)
- Under standard curing conditions, the ternary cementitious material with 16% RB showed the best freeze–thaw resistance, with a mass loss of only 4.13% after 100 freeze–thaw cycles.
- (4)
- The results of the heavy metal leaching characterization test showed that the leaching concentrations of Cr, As, Pb, Ni, and Cu in the specimens were below the specified limits for the release of non-hazardous substances to the environment under freeze–thaw cycles and non-freeze–thaw conditions.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zeng, Q.; Mao, T.; Li, H.; Peng, Y. Thermally insulating lightweight cement-based composites incorporating glass beads and nano-silica aerogels for sustainably energy-saving buildings. Energy Build. 2018, 174, 97–110. [Google Scholar] [CrossRef]
- Amasyali, K.; El-Gohary, N.M. A review of data-driven building energy consumption prediction studies. Renew. Sustain. Energy Rev. 2018, 81, 1192–1205. [Google Scholar] [CrossRef]
- Samal, S.; Ray, A.K.; Bandopadhyay, A. Proposal for resources, utilization and processes of red mud in India—A review. Miner. Eng. 2013, 118, 43–55. [Google Scholar] [CrossRef]
- Deng, B.; Wang, X.; Luong, D.X.; Carter, R.A.; Wang, Z.; Tomson, M.B.; Tour, J.M. Rare earth elements from waste. Sci. Adv. 2022, 8, eabm3132. [Google Scholar] [CrossRef]
- Guo, X.; Deng, Z.; Li, Z.; Zhang, X.; Zhang, L.; Zhao, Q. The effect of different activators on the performance of an inorganic solidifiable gel plugging fluid. Gas Sci. Eng. 2016, 34, 253–264. [Google Scholar] [CrossRef]
- Liu, Y.; Naidu, R.; Ming, H. Red mud as an amendment for pollutants in solid and liquid phases. Geoderma 2011, 163, 1–12. [Google Scholar] [CrossRef]
- Rivera, R.M.; Ulenaers, B.; Ounoughene, G.; Binnemans, K.; Van Gerven, T. Extraction of rare earths from bauxite residue (red mud) by dry digestion followed by water leaching. Miner. Eng. 2018, 119, 82–92. [Google Scholar] [CrossRef]
- Khairul, M.; Zanganeh, J.; Moghtaderi, B. The composition, recycling and utilisation of Bayer red mud. Resour. Conserv. Recycl. 2019, 141, 483–498. [Google Scholar] [CrossRef]
- Mukiza, E.; Zhang, L.; Liu, X.; Zhang, N. Utilization of red mud in road base and subgrade materials: A review. Resour. Conserv. Recycl. 2019, 141, 187–199. [Google Scholar] [CrossRef]
- Li, X.; Ji, M.; Nghiem, L.D.; Zhao, Y.; Liu, D.; Yang, Y.; Wang, Q.; Trinh, Q.T.; Vo, D.-V.N.; Pham, V.Q. A novel red mud adsorbent for phosphorus and diclofenac removal from wastewater. J. Mol. Liq. 2020, 303, 112286. [Google Scholar] [CrossRef]
- Zaharaki, D.; Galetakis, M.; Komnitsas, K. Valorization of construction and demolition (C&D) and industrial wastes through alkali activation. Constr. Build. Mater. 2016, 121, 686–693. [Google Scholar]
- Couperthwaite, S.J.; Costa de Jesus, C.P.; Kiyohara, P.K.; Coelho, A.C.V.; Frost, R.L. Red Mud from Brazil: Thermal Behavior and Physical Properties. Ind. Eng. Chem. Res. 2012, 51, 775–779. [Google Scholar]
- Robert, M.J.G.; Philip, R.; Katalin, G. Behavior of Aluminum, Arsenic, and Vanadium during the Neutralization of Red Mud Leachate by HCl, Gypsum, or Seawater. Environ. Sci. Technol. 2013, 47, 6527–6535. [Google Scholar]
- Sahu, M.K.; Mandal, S.; Dash, S.S.; Badhai, P.; Patel, R.K. Removal of Pb (II) from aqueous solution by acid activated red mud. J. Environ. Chem. Eng. 2013, 1, 1315–1324. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Li, S.; Lin, C.; Gao, Y.; Liu, C. Feasibility of preparing red mud-based cementitious materials: Synergistic utilization of industrial solid waste, waste heat, and tail gas. J. Clean. Prod. 2021, 285, 124896. [Google Scholar] [CrossRef]
- Lemougna, P.N.; Wang, K.-t.; Tang, Q.; Cui, X.-m. Study on the development of inorganic polymers from red mud and slag system: Application in mortar and lightweight materials. Constr. Build. Mater. 2017, 156, 486–495. [Google Scholar] [CrossRef]
- Qiu, K.; Zeng, G.; Shu, B.; Luo, D. Study on the performance and solidification mechanism of multi-source solid-waste-based soft soil solidification materials. Materials 2023, 16, 4517. [Google Scholar] [CrossRef]
- Taki, G.; Grierson, P.F.; Scullett-Dean, G.; Brand, H.E.; Murphy, D.V.; Santini, T.C. Blending bauxite residues with multiple byproducts improves capping materials for tailings storage facilities. J. Environ. Manag. 2023, 338, 117852. [Google Scholar] [CrossRef]
- Kaya-Özkiper, K.; Uzun, A.; Soyer-Uzun, S. Red mud-and metakaolin-based geopolymers for adsorption and photocatalytic degradation of methylene blue: Towards self-cleaning construction materials. J. Clean. Prod. 2021, 288, 125120. [Google Scholar] [CrossRef]
- Hao, X.; Liu, X.; Zhang, Z.; Zhang, W.; Lu, Y.; Wang, Y.; Yang, T. In-depth insight into the cementitious synergistic effect of steel slag and red mud on the properties of composite cementitious materials. J. Build. Eng. 2022, 52, 104449. [Google Scholar] [CrossRef]
- Pan, Z.; Li, D.; Yu, J.; Yang, N. Properties and microstructure of the hardened alkali-activated red mud–slag cementitious material. Cem. Concr. Res. 2003, 33, 1437–1441. [Google Scholar] [CrossRef]
- Hou, S.M.; Gao, S.; Li, Q. Preparation technology and mechanism of alkali-activated red mud based cementitious materials. J. Concr. 2020, 1, 27–31. [Google Scholar]
- Krivenko, P.; Kovalchuk, O.; Pasko, A.; Croymans, T.; Hult, M.; Lutter, G.; Vandevenne, N.; Schreurs, S.; Schroeyers, W. Development of alkali activated cements and concrete mixture design with high volumes of red mud. Constr. Build. Mater. 2017, 151, 819–826. [Google Scholar] [CrossRef]
- Kang, S.-P.; Kwon, S.-J. Effects of red mud and alkali-activated slag cement on efflorescence in cement mortar. Constr. Build. Mater. 2017, 133, 459–467. [Google Scholar] [CrossRef]
- Chen, K.; Lin, W.-T.; Liu, Q.; Chen, B.; Tam, V.W. Micro-characterizations and geopolymerization mechanism of ternary cementless composite with reactive ultra-fine fly ash, red mud and recycled powder. Constr. Build. Mater. 2022, 343, 128091. [Google Scholar] [CrossRef]
- Luo, L.; Ma, C.; Ma, Y.; Zhang, S.; Lv, J.; Cui, M. New insights into the sorption mechanism of cadmium on red mud. Environ. Pollut. 2011, 159, 1108–1113. [Google Scholar] [CrossRef]
- CCES 01-2004; Guide for Design and Construction of Concrete Structures for Durability. China Civil Engineering Society; China Architecture & Building Press: Beijing, China, 2005.
- Brooks, A.L.; He, Y.; Farzadnia, N.; Seyfimakrani, S.; Zhou, H. Incorporating PCM-enabled thermal energy storage into 3D printable cementitious composites. Cem. Concr. Compos. 2022, 129, 104492. [Google Scholar] [CrossRef]
- ASTM C109; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars. American Society for Testing and Materials; ASTM International: West Conshohocken, PA, USA, 2020.
- JGJ/T 70-2009; Standard for Test Method of Basic Properties of Construction Mortar. Ministry of Housing and Urban-Rural Development of PRC; China Architecture & Building Press: Beijing, China, 2009.
- GB/T 50082-2009; Standard for Test Methods of Long-Term Performance and Durability of Ordinary Concrete. Ministry of Housing and Urban-Rural Development of PRC; China Architecture & Building Press: Beijing, China, 2009.
- HJ557-2010; Solid Waste. Extraction Procedure for Leaching Toxicity. Horizontal Vibration Method. Ministry of Environment Protection; China Environmental Science Press: Beijing, China, 2010.
- Li, S.; Zhang, J.; Li, Z.; Liu, C.; Chen, J. Feasibility study on grouting material prepared from red mud and metallurgical wastewater based on synergistic theory. J. Hazard. Mater. 2021, 407, 124358. [Google Scholar] [CrossRef]
- Cheng, X.; Long, D.; Zhang, C.; Gao, X.; Yu, Y.; Mei, K.; Zhang, C.; Guo, X.; Chen, Z. Utilization of red mud, slag and waste drilling fluid for the synthesis of slag-red mud cementitious material. J. Clean. Prod. 2019, 238, 117902. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Lei, Z.; Gao, M.; Sun, J.; Tong, L.; Chen, S.; Wang, Y. Designing low-carbon fly ash based geopolymer with red mud and blast furnace slag wastes: Performance, microstructure and mechanism. J. Environ. Manag. 2024, 354, 120362. [Google Scholar] [CrossRef]
- Ruyters, S.; Mertens, J.; Vassilieva, E.; Dehandschutter, B.; Poffijn, A.; Smolders, E. The red mud accident in Ajka (Hungary): Plant toxicity and trace metal bioavailability in red mud contaminated soil. Environ. Sci. Technol. 2011, 45, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- Khatib, J.M. Properties of concrete incorporating fine recycled aggregate. Cem. Concr. Res. 2005, 35, 763–769. [Google Scholar] [CrossRef]
- Li, Z.; You, H.; Gao, Y.; Wang, C.; Zhang, J. Effect of ultrafine red mud on the workability and microstructure of blast furnace slag-red mud based geopolymeric grouts. Powder Technol. 2021, 392, 610–618. [Google Scholar] [CrossRef]
- Muraleedharan, M.; Nadir, Y. Factors affecting the mechanical properties and microstructure of geopolymers from red mud and granite waste powder: A review. Ceram. Int. 2021, 47, 13257–13279. [Google Scholar] [CrossRef]
- Pan, Z.; Cheng, L.; Lu, Y.; Yang, N. Hydration products of alkali-activated slag–red mud cementitious material. Cem. Concr. Res. 2002, 32, 357–362. [Google Scholar] [CrossRef]
- Wong, C.L.; Mo, K.H.; Yap, S.P.; Alengaram, U.J.; Ling, T.-C. Potential use of brick waste as alternate concrete-making materials: A review. J. Clean. Prod. 2018, 195, 226–239. [Google Scholar] [CrossRef]
- Ge, Z.; Sun, R.J.; Zheng, L. Mechanical properties of concrete with recycled clay-brick-powder. Adv. Mater. Res 2011, 250, 360–364. [Google Scholar] [CrossRef]
- Ma, S.; Cao, Z.; Wei, C.; Shao, Y.; Wu, P.; Zhang, Z.; Liu, X. Red mud-modified magnesium potassium phosphate cement used as rapid repair materials: Durability, bonding property, volume stability and environment performance optimization. Constr. Build. Mater. 2024, 415, 135144. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, Z.; Liu, X.; Li, Y.; Zeng, Q.; Zhang, W. Reuse of red mud in magnesium potassium phosphate cement: Reaction mechanism and performance optimization. J. Build. Eng. 2022, 61, 105290. [Google Scholar] [CrossRef]
- Van Jaarsveld, J.; Van Deventer, J.S.; Lukey, G. The effect of composition and temperature on the properties of fly ash-and kaolinite-based geopolymers. Chem. Eng. J. 2002, 89, 63–73. [Google Scholar] [CrossRef]
- Elzeadani, M.; Bompa, D.; Elghazouli, A. One part alkali activated materials: A state-of-the-art review. J. Build. Eng. 2022, 57, 104871. [Google Scholar] [CrossRef]
- Xu, J.; Chen, P. Synergistic effect of iron tailings, steel slag and red mud cementitious materials on mechanical and microstructure properties. J. Build. Eng. 2024, 95, 110131. [Google Scholar] [CrossRef]
- Statkauskas, M.; Vaičiukynienė, D.; Grinys, A.; Borg, R.P. Mechanical properties and microstructure of ternary alkali activated system: Red brick waste, metakaolin and phosphogypsum. Constr. Build. Mater. 2023, 387, 131648. [Google Scholar] [CrossRef]
- Grellier, A.; Bulteel, D.; Bouarroudj, M.E.K.; Rémond, S.; Zhao, Z.; Courard, L. Alternative hydraulic binder development based on brick fines: Influence of particle size and substitution rate. J. Build. Eng. 2021, 39, 102263. [Google Scholar] [CrossRef]
- Kerru, N.; Gummidi, L.; Bhaskaruni, S.V.; Maddila, S.N.; Jonnalagadda, S.B. Green synthesis and characterization of novel 1, 2, 4, 5-tetrasubstituted imidazole derivatives with eco-friendly red brick clay as efficacious catalyst. Mol. Divers. 2020, 24, 889–901. [Google Scholar] [CrossRef]
- Sharma, G.; Jeevanandam, P. Synthesis of self-assembled prismatic iron oxide nanoparticles by a novel thermal decomposition route. RSC Adv. 2013, 3, 189–200. [Google Scholar] [CrossRef]
- Xu, C.; Ni, W.; Li, K.; Zhang, S.; Xu, D. Activation mechanisms of three types of industrial by-product gypsums on steel slag–granulated blast furnace slag-based binders. Constr. Build. Mater. 2021, 288, 123111. [Google Scholar] [CrossRef]
- Sedira, N.; Castro-Gomes, J.; Magrinho, M. Red clay brick and tungsten mining waste-based alkali-activated binder: Microstructural and mechanical properties. Constr. Build. Mater. 2018, 190, 1034–1048. [Google Scholar] [CrossRef]
- GB5085.3-2007; Identification Standards for Hazardous Wastes. Identification for Extraction Toxicity. National Standards of People’s Republic of China; China Standards Press: Beijing, China, 2007.
- Chen, X.; Guo, Y.; Ding, S.; Zhang, H.; Xia, F.; Wang, J.; Zhou, M. Utilization of red mud in geopolymer-based pervious concrete with function of adsorption of heavy metal ions. J. Clean. Prod. 2019, 207, 789–800. [Google Scholar] [CrossRef]
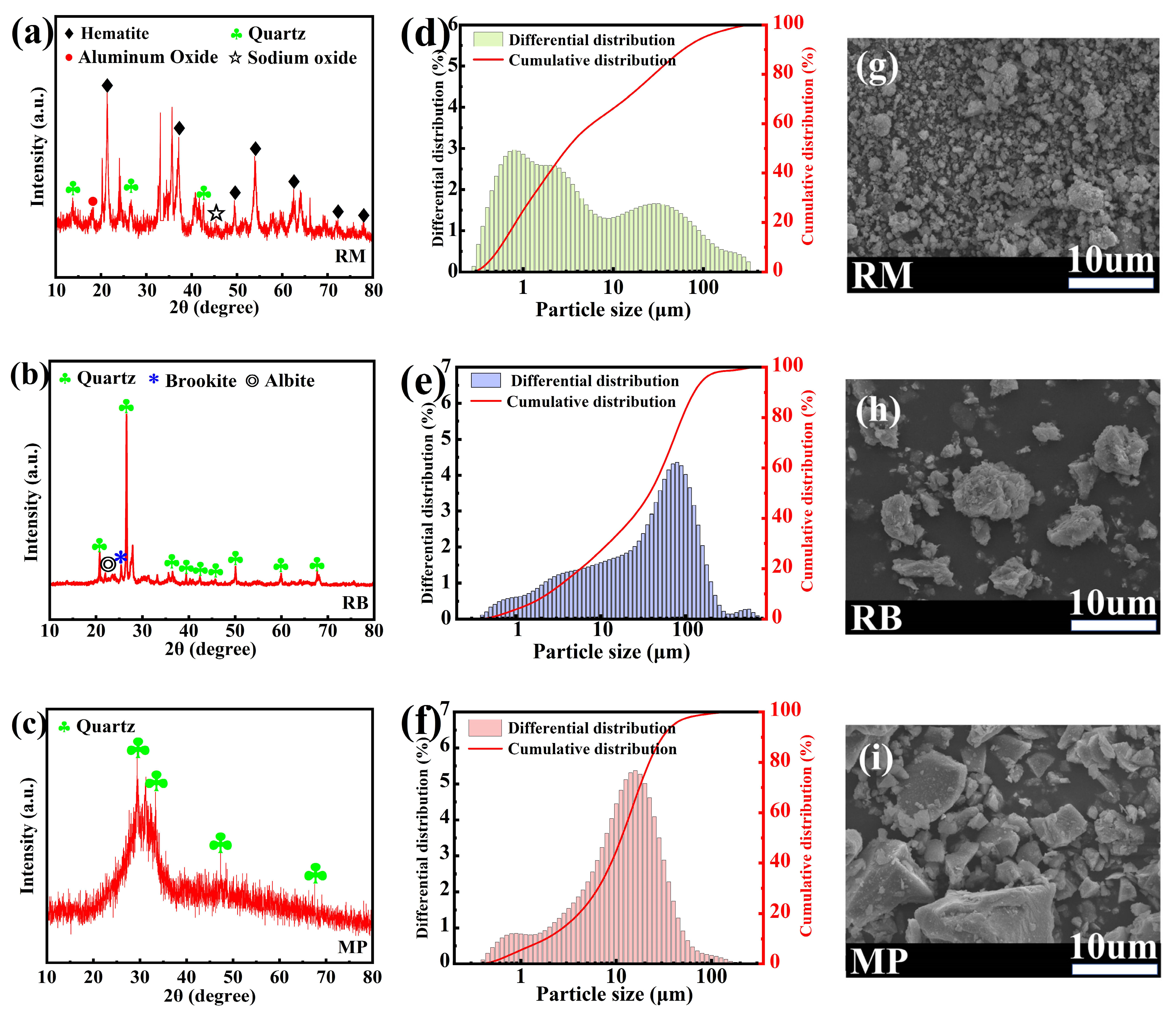
| Composition (wt%) | CaO | SiO2 | Al2O3 | Fe2O3 | SO3 | MgO | TiO2 | Na2O | LOI |
|---|---|---|---|---|---|---|---|---|---|
| RM | 1.17 | 18.23 | 23.97 | 38.96 | 0.72 | 0.17 | 4.71 | 10.95 | 9.42 |
| RB | 10.11 | 52.37 | 24.43 | 2.14 | 2.43 | 2.18 | 0.79 | 2.04 | 0.58 |
| MP | 25.17 | 40.67 | 16.52 | 0.28 | 2.74 | 8.75 | 1.09 | 0.77 | - |
| Sample | Name | MP (%) | RM | MP (%) | Alkaline Activator Module (%) | Water-to-Cement Ratio |
|---|---|---|---|---|---|---|
| Dualist system | CPM1 | 100 | - | - | 1.3 | 0.35 |
| CPM2 | 96 | 4 | - | |||
| CPM3 | 92 | 8 | - | |||
| CPM4 | 88 | 12 | - | |||
| CPM5 | 84 | 16 | - | |||
| CPM6 | 80 | 20 | - | |||
| Three-component system | CPMB1 | 84 | 15.36 | 0.64 | 1.3 | 0.35 |
| CPMB2 | 84 | 14.72 | 1.28 | |||
| CPMB3 | 84 | 14.08 | 1.92 | |||
| CPMB4 | 84 | 13.44 | 2.56 | |||
| CPMB5 | 84 | 12.80 | 3.20 | |||
| CPMB6 | 84 | 12.16 | 3.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, T.; Luo, H.; Han, R.; Zhao, Y.; Chen, L.; Liu, M.; Gui, Z.; Xing, J.; Chen, D.; He, B.-J. Red Mud in Combination with Construction Waste Red Bricks for the Preparation of Low-Carbon Binder Materials: Design and Material Characterization. Buildings 2024, 14, 3982. https://doi.org/10.3390/buildings14123982
Qin T, Luo H, Han R, Zhao Y, Chen L, Liu M, Gui Z, Xing J, Chen D, He B-J. Red Mud in Combination with Construction Waste Red Bricks for the Preparation of Low-Carbon Binder Materials: Design and Material Characterization. Buildings. 2024; 14(12):3982. https://doi.org/10.3390/buildings14123982
Chicago/Turabian StyleQin, Teng, Hui Luo, Rubin Han, Yunrui Zhao, Limin Chen, Meng Liu, Zhihang Gui, Jiayao Xing, Dongshun Chen, and Bao-Jie He. 2024. "Red Mud in Combination with Construction Waste Red Bricks for the Preparation of Low-Carbon Binder Materials: Design and Material Characterization" Buildings 14, no. 12: 3982. https://doi.org/10.3390/buildings14123982
APA StyleQin, T., Luo, H., Han, R., Zhao, Y., Chen, L., Liu, M., Gui, Z., Xing, J., Chen, D., & He, B.-J. (2024). Red Mud in Combination with Construction Waste Red Bricks for the Preparation of Low-Carbon Binder Materials: Design and Material Characterization. Buildings, 14(12), 3982. https://doi.org/10.3390/buildings14123982







