Abstract
Due to the urgent need for a more sustainable built environment and actions against climate change, this paper presents a literature review about photocatalytic TiO2-based thin layers to be applied on mortars in facades. Photocatalysis may be a potential strategy against current environmental and climate challenges by transforming or eliminating hazardous greenhouse gases from the atmosphere. The main subjects researched were the coatings’ efficiency (which encompassed their self-cleaning ability, depolluting effect, and antimicrobial properties), durability, and sustainability. The method was based on the systematic literature review approach. Self-cleaning ability was the most recurrent topic retrieved from published studies, followed by depolluting effect and durability. There are few investigations about antimicrobial properties considering TiO2-coated mortars in facades. However, sustainability studies through Life Cycle Assessment and Life Cycle Costing represented the most significant gap, even requiring broader surveys. The photocatalytic activity of the coatings is well-proven in the literature, although specific evaluations may be needed for each coating composition and testing condition to understand their performance. The type of contamination agents, TiO2 dispersion and characteristics, dopants, nanocomposites, and substrate are among the principal agents influencing the results; therefore, caution must be taken when comparing research. Mainly, adhesion and photocatalytic efficiency after ageing were studied on durability. More field exposures may be recommended. Regarding the trade-offs concerning the environmental impacts of TiO2-based coatings, it is urgent to clarify whether their overall outcome is indeed advantageous and to investigate their resilience regarding climate change scenarios.
1. Introduction
Some major problems that threaten the environmental balance nowadays are urban air pollution and global warming [1]. Cities are challenged due to low air quality [2], involving the emission of pollutants such as nitrogen oxides (NOx), sulphur oxides (SOx) and Volatile Organic Compounds (VOCs) [3], together with organic particulate matter, like soot [4]. Communities, human health, and the infrastructure to which they are related are affected by global warming [5]. To protect the planet and pursue more sustainable decisions, “Sustainable cities and communities” and “Climate action” are among the 17 Sustainable Development Goals adopted by the United Nations to be implemented by 2030 [6].
Regarding the sustainability of buildings, it is fundamental to adapt to the climate change scenario, even for retrofit interventions [7]. Abolhassani et al. [5], Farahani et al. [8], and Kharbouch [9], for example, studied the demands and performance involved with buildings considering future climate conditions.
In this context, photocatalysis may be assigned as a potential strategy against current environmental and climate challenges by transforming or eliminating hazardous greenhouse gases from the atmosphere; TiO2 might be the most suitable alternative to convert mineral and organic compounds [10] and, thus, to assist in climate change actions.
Nano-titanium dioxide (TiO2) is recognized as a relevant alternative due to its photocatalytic behaviour and the potential to purify air and water, associated with self-cleaning performance, chemical stability [11], and antimicrobial properties [1]. Nanomaterials and their use in construction are indicated as ways to seek better environmental performance for buildings [12].
The photocatalytic process by which the TiO2 operates may degrade or transform organic and inorganic substances into less harmful components, for example, degrading or mineralizing microorganisms such as viruses, bacteria, fungi, algae, and other organic compounds to CO2, H2O, and harmless inorganic anions [13]. TiO2 is a semiconductor material with a bandgap energy of 3.2 eV, and thus, it absorbs energy near ultraviolet light with a wavelength of around 380 nm [14].
For the photocatalytic action of TiO2, first, irradiation incidence promotes the shift of electrons (e−) from the valence band to the semiconductor’s conduction band, leaving electron holes (h+) on the valence band. Electron-hole pairs reach the surface of the TiO2 particles and react with the adsorbed oxygen and water from the air [15]. Reactive oxygen species like hydroxyl (OH•), superoxide (O2−) and hydrogen peroxide (H2O2) are formed during the process, and together with oxygen (O2), they may all foster photocatalytic mechanisms, making it possible, finally, to decompose or mineralise pollutants through a reduction-oxidation process [15,16]. Hydroxyl active species can coexist with trapped holes, which are also responsible for oxidant reactions at the photocatalyst surface [17].
Regarding building facades, besides the environmental concern, aesthetic issues also integrate the objectives of applying TiO2 since, when exposed to urban pollution, the coating surfaces may soil due to the deposition of atmospheric particulates [18]. Therefore, the self-cleaning property provided by TiO2 can be especially beneficial. The definitive removal of impurities benefits from high levels of wettability [1]. Thus, the self-cleaning ability is favoured by the photoinduced super hydrophilicity mechanism undergone by the TiO2, which is also based on the production of excited electrons and holes through ultraviolet irradiation [19].
There are mainly two ways of using TiO2 in mortars for building facades: adding it to the mixture proportion or as a surface coating [20]. The efficiency of the photocatalytic activity is affected by the way TiO2 is incorporated into the substrate and by the characteristics of the substrate itself [21]. Adding the photocatalyst into the mixture provides more stability for long-term applications regarding the mortar’s surface-mechanical properties [22]. According to Vulic et al. [22], the resulting photocatalytic activity of mortars with intermixed TiO2 is lower than those with TiO2-based thin layers applied over their surface, although it can still be acceptable; however, it is not always satisfactory [23]. TiO2 additions into mortars’ mixture may not lead to the full benefit from the active material since only the nanoparticles from the outer face of the layer can be activated with UV light; on the other hand, thus, thin TiO2-based coatings might explore the photocatalyst activity more efficiently [20].
The higher photocatalytic activity of mortars coated with thin TiO2-based layers is related to the availability of more active sites on their surfaces, which can lead to greater pollutant oxidation [24]. Krishnan et al. [25] studied the performance of TiO2-based silicate coatings and TiO2-containing mortars and concluded that the coated specimens required 20 times less TiO2 mass for comparable photocatalytic performance. Moreover, applying thin photocatalytic coatings or films on facades is also interesting considering that they can result in minimal changes over original aesthetic features [26] and contribute to the energy performance of the buildings [27] and the reduction of maintenance costs and efforts [28].
However, there are concerns about the durability of thin TiO2-based coatings applied on mortars since surface erosion and environmental agents can affect their stability [20]. It is a challenge to maintain a photocatalytic efficiency associated with an appropriate weathering resistance in photocatalytic building products [29]. Although self-cleaning materials in buildings are receiving increasing interest, their durability still needs to be investigated [30]. The knowledge concerning long-term performance and resilience of building materials is, however, crucial considering not only sustainability but also the expected climate changes and the initiatives for their mitigation [31].
Despite the ability of the TiO2 coatings to degrade atmospheric pollutants and other components, there is also a gap concerning the knowledge of how TiO2 nanoparticles affect the sustainability of cementitious materials [32]. Few studies present Life Cycle Assessments (LCAs) that are broad enough to include not only impacts such as global warming or depletion of fossil resources but also specific critical issues related to nanoparticles, such as leaching ecotoxicity [33]. In addition to investigating whether photocatalytic materials have, in fact, economic benefits, their cost-efficiency can also be determined considering Life Cycle Costing (LCC) [34], and, thus, included in the challenging context of sustainability, positioning their economic performance within the scope of durability and climate change.
The climate change scenario, the search for a more sustainable built environment, as well as the possibility of combining depolluting effects with self-cleaning properties, motivate the study of thin TiO2-based coatings to be applied on the surface of mortars in facades. Therefore, this paper aims to review some main topics regarding photocatalytic coatings with TiO2, involving their efficiency and the challenging issues related to their durability and sustainability. Well-established topics and scientific gaps are discussed, seeking consistency between the construction sector and the protection of the planet.
2. Methods
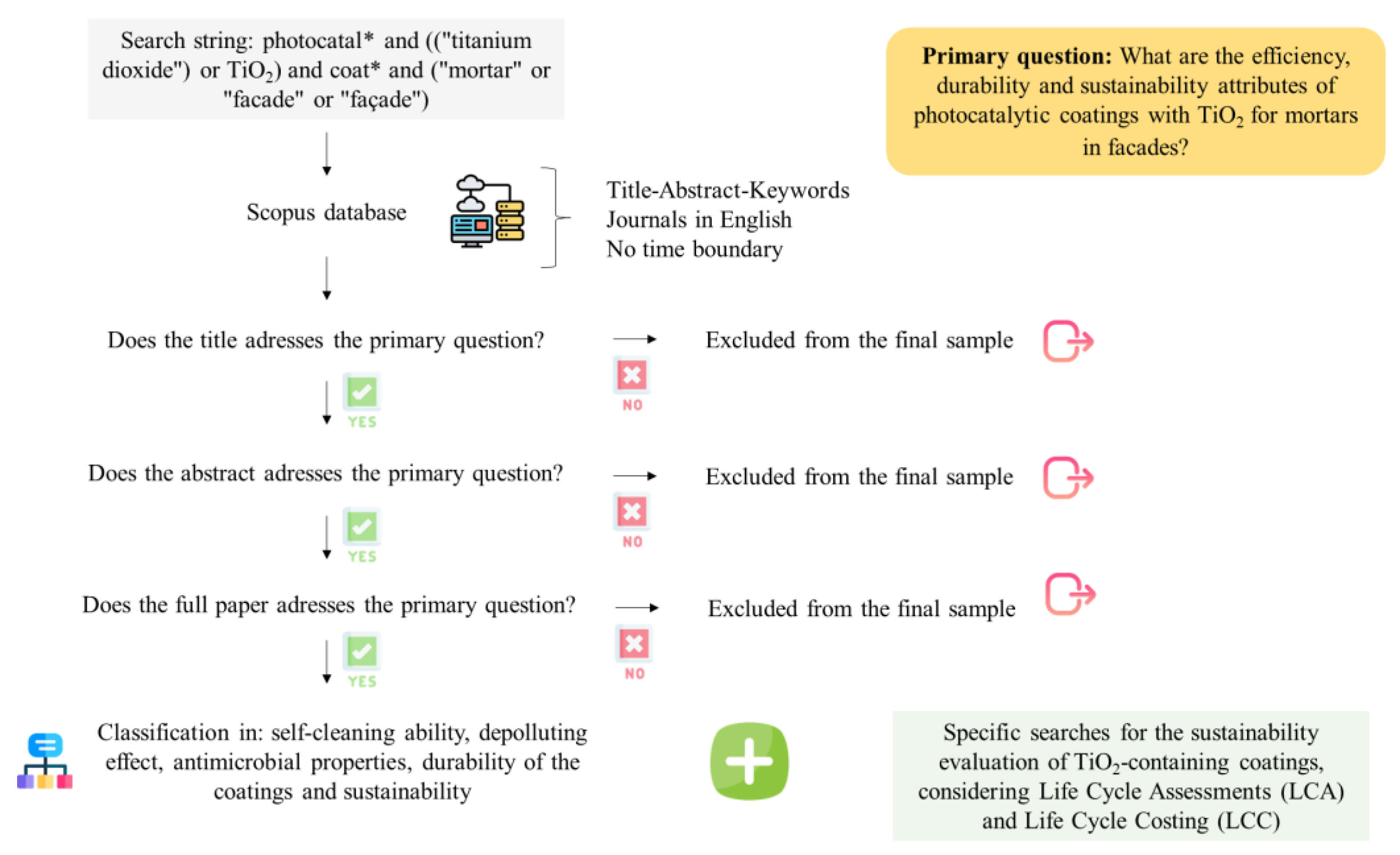
The search for relevant papers explicitly related to the studied subject was carried out through a systematic literature review. First, a search string was used to retrieve the literature from the Scopus database. Afterwards, a qualitative content analysis was carried out to exclude studies unrelated to the review focus [35]. The primary question [36] proposed to be answered by the selected papers was: “What are the efficiency, durability, and sustainability attributes of photocatalytic coatings with TiO2 for mortars in facades?”.
The search was restricted to journals in English, and no time boundary was defined. The keyword string was set as “photocatal* and ((“titanium dioxide”) or TiO2) and coat* and (“mortar” or “facade” or “façade”)”, and the search was carried out on the title, abstract, and keywords of the papers. The studies gathered with the search criteria went through three filtering considerations: a title analysis, an abstract analysis, and, lastly, an in-depth complete document analysis [36].
Through complete in-depth analysis, the sample of the resulting documents was further refined to consider only the TiO2 form of application specified: thin TiO2-based coatings applied on mortar surfaces to be used in facades. Thus, papers addressing, for example, clay bricks [37], TiO2-soaked aggregates [38], pavements [39], application over paints [40], and TiO2-intermixed mortars [41] were left out from the selection. The remaining papers were classified according to the TiO2-related attributes they considered: self-cleaning ability, depolluting effect, antimicrobial properties, the durability of the coatings, and sustainability. Similarly, Gopalan et al. [42] also grouped the significant applications of TiO2-based building materials under environmental pollution remediation, self-cleaning, and self-disinfecting. Other properties and tests presented by the selected papers were disregarded for the classification.
In the present review, sustainability was included to examine, particularly, available LCA and LCC studies related to thin TiO2-based coatings. However, since none of the documents in the final sample used the mentioned tools, a second phase of string searches was performed in the Scopus database to provide at least an overview of the subject. Initially, two searches were conducted on the title, abstract, and keywords, the first with the string “((“titanium dioxide”) or TiO2) and coat* and (“mortar” or “facade” or “façade”) and (“LCA” or “life cycle assess*”),” and the second considering “((“titanium dioxide”) or TiO2) and (coat*) and (“LCA” or “life cycle assess*”).” The second search string was less restrictive than the former due to the initial retrieval of only two papers [33,43]. Again, the search comprised only journals in English, and no time boundary was determined. Titles, abstracts, and full texts were subsequently analysed to filter the relevant documents related to a second proposed question: “What does the LCA indicate about the sustainability of mortars with TiO2 coatings in facades?”. In this case, however, studies addressing coatings for substrates other than mortars were not directly excluded, looking for potential contributions of their LCA within the scope of the present review.
Furthermore, in the same way as for an LCA, to answer a third question, “What do LCC analyses say about the economic sustainability of mortars with TiO2 coatings in facades?”, LCCs related to thin TiO2-based coatings were searched in the Scopus database. The first query string searched was “((“titanium dioxide”) or TiO2) and coat* and (“mortar” or “facade” or “façade”) and (“LCC” or “life cycle cost*”)”; however, it presented no results. Then, a second keyword string considering “((“titanium dioxide”) or TiO2) and (coat*) and (“LCC” or “life cycle cost*”)” was searched in the database.
After the final samples were filtered, classified, and organized, their methodologies and relevant findings were explored and discussed to present state-of-the-art photocatalytic efficiency, durability, and sustainability issues of TiO2-based coatings for mortars and to reveal potential research gaps. Figure 1 depicts the protocol applied to conduct this review.
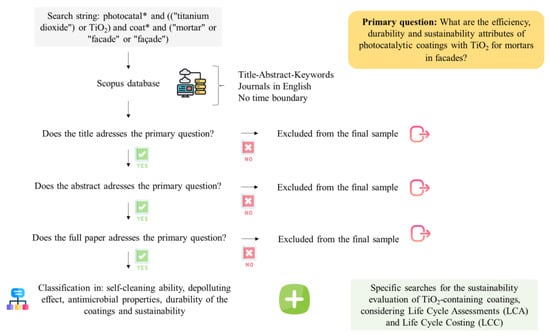
Figure 1.
Protocol applied to conduct the literature review. This flowchart has been designed using resources from Flaticon.com (Eucalyp, Freepik, Icon mania and Vectors Market).
Due to the difficulty in comparing the results of different studies with photocatalytic materials caused by variations in reactors and parameters [44], specifically for the photocatalytic efficiency evaluation focused on the depolluting effect, discussions of the papers were further detailed based on a figure-of-merit called formal quantum efficiency (FQE), presented by Watanabe et al. [45] and Mills and Le Hunte [46]. FQE was calculated regarding Equation 1, in which the rate of reaction designates the number of molecules that are transformed or formed, while the incident light intensity applies to the incident number of photons [46].
FQE = rate of reaction/incident light intensity
3. Overview
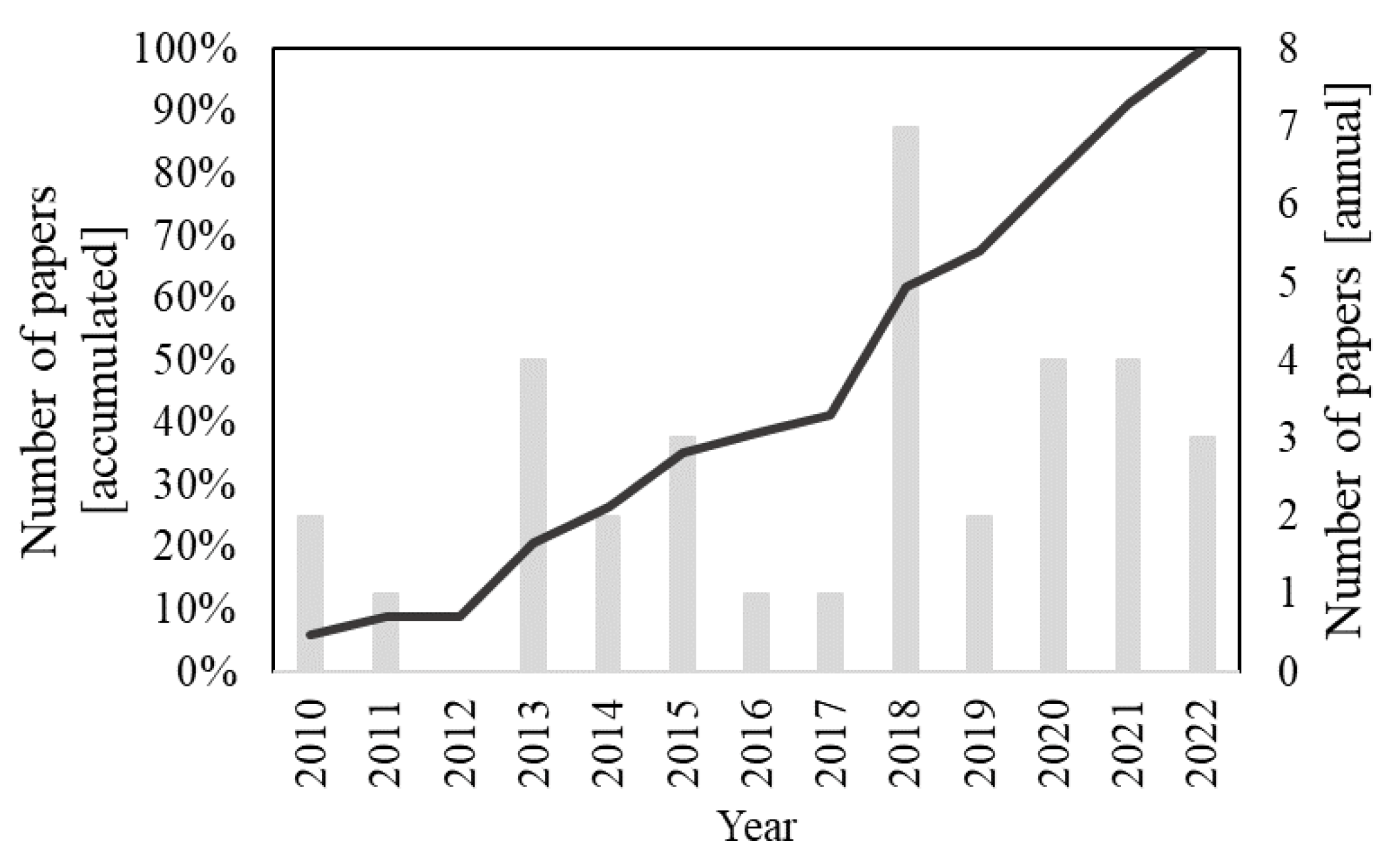
The search of the “photocatal* and ((“titanium dioxide”) or TiO2) and coat* and (“mortar” or “facade” or “façade”)” string in the Scopus database retrieved 77 results. Title screening led to the exclusion of 27 documents and the abstract analysis of 14 other papers. Finally, two additional documents were removed with a full paper in-depth evaluation. The final sample, therefore, was composed of 34 articles. Figure 2 demonstrates the distribution of the selected documents over time; the final sample was entirely composed of papers published after 2010.
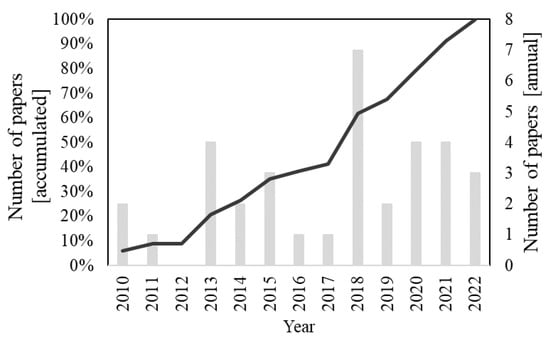
Figure 2.
Distribution of the 34 papers from the final sample (after title, abstract, and full-text screening) related to the search string “photocatal* and ((“titanium dioxide”) or TiO2) and coat* and (“mortar” or “facade” or “façade”)” over time.
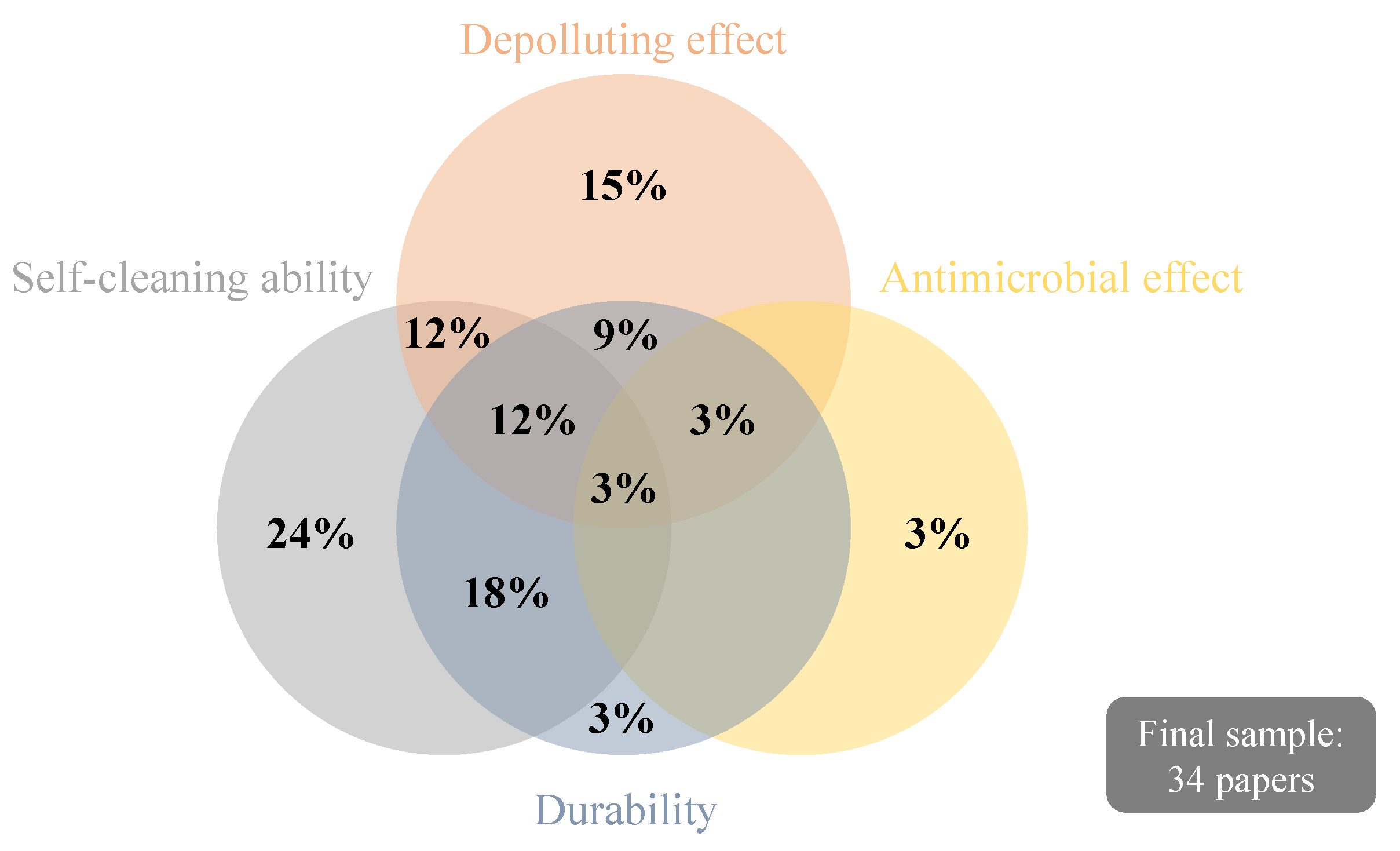
Within the final sample, five papers [1,42,47,48,49] were literature reviews. Among the 34 documents, 23 reported the self-cleaning performance of TiO2-based coatings for mortars to be used in facades, 18 approached their depolluting effect, three studied antimicrobial properties, and 16 presented information regarding the durability of the coatings, as indicated in Table 1. Nevertheless, none of the papers explored the sustainability of TiO2 coatings or focused on the LCA or LCC tools, which are fundamental to achieving efficient and sustainable photocatalysis on building materials/systems [1].

Table 1.
Subjects addressed by the studied papers: self-cleaning ability, depollution effect, antimicrobial effect, and durability.
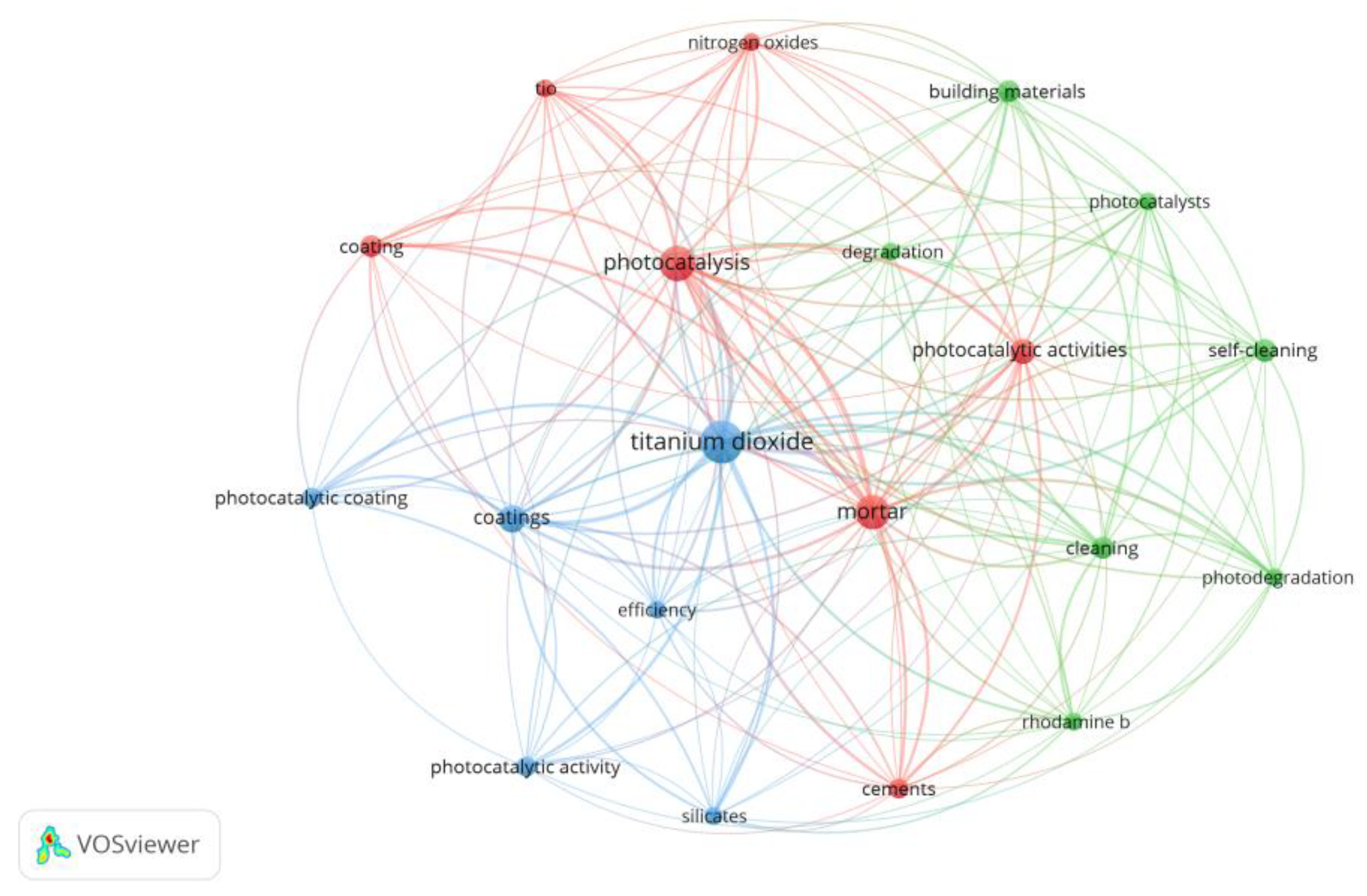
Figure 3 depicts the proportion of papers that studied each one of the subjects of interest presented in Table 1 and the ones encompassing more than one research focus. In Figure 4, a bibliometric network map is shown to evaluate the co-occurrence of keywords found in the 34 papers of the final sample, aiming to understand their relevance and possible knowledge gaps. The minimum number considered for occurrences of a keyword was 5, resulting in the depiction of 20 different words. Nitrogen oxides, self-cleaning, and degradation are among the main clusters identified, following Figure 3; keywords related to the antimicrobial effect cannot be identified in Figure 4 since it was the less recurrent researched topic. Silicates are among the keywords in the cluster related to titanium dioxide, suggesting its recurrent use in the composition of TiO2-based thin films for mortars. Further details and discussions about the studies are given in Section 4 and Section 5.
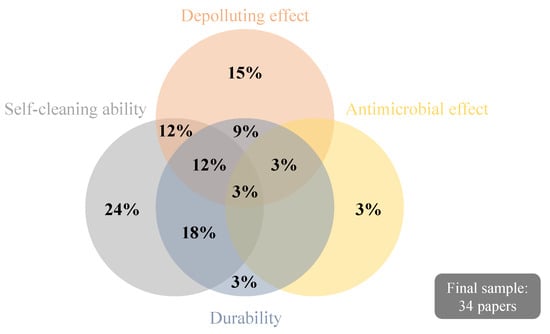
Figure 3.
Proportion of papers addressing the research subjects: self-cleaning ability, depollution effect, antimicrobial effect, and durability.
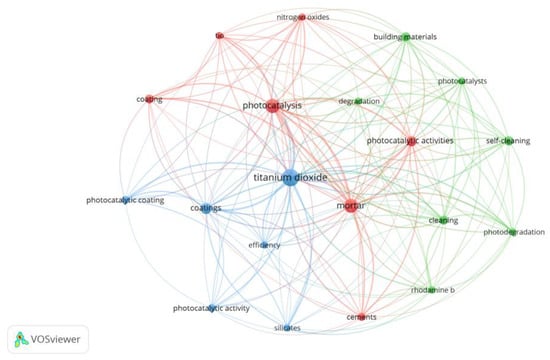
Figure 4.
Bibliometric network map referring to the co-occurrence of 20 keywords from the final sample of 34 papers related to self-cleaning ability, depollution effect, antimicrobial effect, and durability.
Table 2 compiles the core papers studied in this review relating to experimental studies on self-cleaning ability, depolluting effect, antimicrobial effect, and durability. The articles are presented chronologically, starting with the most recently published. The photocatalytic coatings are introduced considering their composition, applying method, doping substance/nanocomposite (if applicable), and evaluated substrate. Dip coating, sol-gel, covering of the surface with an n-TiO2 thin layer, commercial water-based coatings, and spraying are among the principal applying methods [47].
Review articles are not presented in Table 2 since they discuss experimental results provided by several studies. Among the reviews, Castro-Hoyos et al. [1] aimed mainly to present challenges and opportunities related to using TiO2 in cement-based materials, and Singh et al. [47] discussed its synthesis processes, applications, and efficiency. Gopalan et al. [42] studied the usage of modified TiO2 in photocatalytic building materials, while Rosales and Esquivel [48] reviewed the synthesis and applications of SiO2-TiO2 composites with modifiers or dopants. Finally, Khitab et al. [49] worked with synthesis methods, usage, and health concerns related to nano titania.


Table 2.
Core papers presenting experimental results on self-cleaning ability, depolluting effect, antimicrobial effect, or durability. The entry “-“ is for information unavailable or not applicable.
Table 2.
Core papers presenting experimental results on self-cleaning ability, depolluting effect, antimicrobial effect, or durability. The entry “-“ is for information unavailable or not applicable.
| Paper | Photocatalytic Coating Composition | Applying Method | Doping Substance/Nanocomposite If Applicable | Substrate Evaluated |
|---|---|---|---|---|
| Khannyra et al. [50] | TiO2/SiO2 and N-TiO2/SiO2 synthesized via sol–gel method. TiO2 proportion of 4% w/v concerning silica oligomer and concentrations of 0, 3.33, 6.66, 8 and 10 M for the nitrogen doping, regarding the synthesis of TiO2/SiO2 and N-TiO2/SiO2. P25 used for comparison. (S0N0T; SN10TiO2; SP25; SN8TiO2; SN3.33TiO2; STiO2; SN6.66TiO2) | Brushed to saturation three times; sols extra removed by paper | Nitrogen | Portland cement mortar |
| Gryparis et al. [51] | TiO2/C-dots composites synthesized using a hydrothermal strategy. TiO2 and several C-dot loadings: TC0, TC25, TC50, TC62.5 and TC75. Each catalyst was added in 4% w/w to a hydrophobic consolidant, synthesized using a sol–gel process. A commercial catalyst Au (1%)/TiO2 (TAu) was also tested under solar conditions | Brushed three times | C-dot loading | Cement mortar |
| Pei et al. [11] | Graphene/TiO2 nanocomposites prepared using a sol-gel assisted electrospray method. Suspensions in methanol of graphene/TiO2 nanocomposites (2.5%) and commercial TiO2 nanoparticles (2.5%) by weight of the cementitious materials used for the cement mortar | Sprayed | Graphene | Portland cement mortar |
| Zahabizadeh et al. [52] | Nano-TiO2 aqueous suspension sprayed over the surface of mortar specimens after 1.5, 5, 9, 24 and 32 h and 7 days after the beginning of the hydration process | 5 mg/cm² to 80 mg/cm² sprayed | - | Cement mortar |
| Zuena et al. [53] | Two distinct sols obtained by mixing TEOS, ethanol, TiO2 nanoparticles, and loaded NC (silica nanocapsules) or MNP (silica mesoporous nanocapsules). Total nanoparticle concentration: 0.1% w/w. Tested coatings: Si-TiO2-NC, Si-TiO2-MNP and Si-Control | Applied using a brush until the surface remained wet for more than 1 min | Silica nanodevices loaded with a commercial biocide (2-mercaptobenzothiazole) | Lime-based mortar |
| Speziale et al. [54] | Two heterostructures of TiO2-ZnO (weight/weight 50/50 and 10/90). Dispersions into plain hydroalcoholic and 3D superhydrophobic medium. Further improvement by addition of superplasticizers (polycarboxylate ether (PCE), melamine sulfonate (MEL), polynaphthalene sulfonate (PNS) and polyacrylate (PA)) in a 1% w/w percentage concerning the weight of photocatalyst. Optimized coatings—Dispersion 1: superhydrophobic coatings (SPHB) 3 w/w% TiO2-ZnO 50/50 + 5% w/w PCE with respect to the nanoparticles. Dispersion 2: SPHB 3 w/w% TiO2-ZnO 10/90 + 5% w/w MEL with respect to the nanoparticles. Dispersion 3: SPHB 1.5 w/w% TiO2-ZnO 50/50 + 2.5% w/w PCE with respect to the nanoparticles. Dispersion 4: SPHB 1.5 w/w% TiO2-ZnO 10/90 + 2.5% w/w MEL with respect to the nanoparticles | 1 mL deposited with a pipette | ZnO | Lime-based mortar |
| Hot et al. [15] | TiO2-based powders obtained from waste due to chemical milling baths of the aeronautical industry. 5 g of TiO2-based powder mechanically dissolved in 100 mL of distilled water solution containing 3.5 g of dispersant and 0.1 g of anti-foaming agent | Applied using a brush: 2–3 layers. 0, 1.4, 1.7, 4.4, 5.4, 5.5, 5.6, 6 g TiO2/m² | - | Cement mortar |
| Kim et al. [55] | Recycled TiO2 nanoparticles produced from Ti-salt flocculated sludge obtained from dye wastewater. Suspension: dispersion of 20, 40, or 60 mg of TiO2 powder in 5 mL of distilled water with 10 mg of mussel adhesive protein (MAP) | 5 mL sprayed | - | Cement mortar |
| Pondelak et al. [56] | Nanocomposites based on layered double hydroxides (LDHs) associated with a photocatalytically active TiO2. Up to 10 wt.% of TiO2 intercalated into the LDH. Preparation of a photocatalytic suspension | Three layers sprayed | ZnAl layered double hydroxide | Lime-based mortar |
| Saeli et al. [24] | Photocatalytic hydroxyapatite (TiHAp). HAp derived from Atlantic codfish bone wastes prepared with 1 wt% TiO2. Suspensions of 1 wt% and 5 wt% TiHAp in distilled water | 1.5 mL deposited with a pipette | - | Natural hydraulic lime-based mortar |
| Wang et al. [57] | Two core-shell nanocomposites with different deposited densities of TiO2 nanoparticles on each SiO2 nanosphere. SiO2@TiO2 photocatalysts (0.025 mg) added into water (2 mL), solution sprayed on one surface (4 cm × 4 cm) of slices. P25 used as reference | 2 mL sprayed | SiO2 | Cement paste and mortar |
| Krishnan et al. [58] | For laboratory studies: TiO2 mixed in a commercial silicate coating in two contents of 1.6% or 2.5% (by volume of the liquid silicate). For field study: silicate containing 0.7% photocatalytic TiO2 | Individual coatings applied in three coats | - | Cement mortar |
| Rosales et al. [59] | Synthesis with sonochemistry: mixing of titanium dioxide sol and silicon dioxide sol. Synthesis without sonochemistry: mixing of titanium dioxide particles and silicon dioxide sol | - | SiO2 | Cement mortar |
| Wu et al. [60] | Photocatalytic top layer prepared by mixing a transparent silicate coating with 1.46% photocatalytic TiO2 by volume. 3 layers: white silicate coating, transparent silicate coating and photocatalytic layer | Each layer applied using a brush | - | Portland cement mortar |
| Pérez-Nicolás et al. [61] | Dispersions with 1 wt% of the photocatalytic additive in water with superplasticizer (three polycarboxylate-based polymers and a commercial polynaphthalene sulfonate (PNS); 1 wt% in relation to the photocatalysts). Average percentage of photocatalytic additive with respect to the binder weight: 0.005% | 28.5 mg sprayed | Bare TiO2, Fe-TiO2 and V-TiO2 | Cement and air lime mortar |
| Hot et al. [62] | TiO2 aqueous dispersions: dilution of a commercial stable aqueous dispersion of ultrafine TiO2 anatase particles. TiO2 dry matter content in solution: 18 wt%, 12 wt%, 6 wt%, 5 wt%, 4 wt%, 3 wt% and 1 wt% | Applied using brush | - | Cement mortar |
| Guo et al. [29] | Commercially available TiO2 containing paint with about 10% TiO2 by weight: TiO2 water suspension prepared from a 0.03 g/L P25 distilled deionized water suspension | Three layers applied using a brush | - | Self-compacting architectural mortar |
| Mendoza et al. [63] | Commercial TiO2 suspensions and homemade titania sol (TEA)—stabilized suspension. SiO2 sol applied on cement surface previously to the TiO2 layer for RhB removal evaluation | SiO2 and TiO2 layer sprayed | - | Cement mortar |
| Rudic et al. [64] | Layered double hydroxides and photocatalytic active TiO2 particles in suspension. 1 wt% of the synthesized nanocomposite (TiO2-LDH) in a stabilized suspension | Three layers sprayed | Zn and Al salts | Lime-based renders |
| Vulic et al. [65] | 3 wt% TiO2 suspensions introduced onto calcined ZnAl-LDH (layered double hydroxides) powder. Nanocomposite powder (1 g) suspended in 100 mL demineralized water. Nanocomposite suspension: 1 wt% of solid phase | Three layers sprayed | ZnAl-LDH | Cement and pozzolanic mortar |
| Bengtsson and Castellote [66] | Two commercial paints (applied directly on the surface). Six TiO2 powders, applied in a solution at a concentration of 8.37 g/L, final TiO2 mass load of 5 g/m2. One of the powders was a homemade S-, N-, and C-doped catalyst (S-TiO2). Samples of the catalysts also submitted to treatments of exposure to water and calcinations (except for the paints and one powder) | 300 g/m² of paint applied with metal roller. 3 mL of dispersions | S, N and C for one of the eight different catalysts tested | White cement mortar |
| Martinez et al. [67] | Acrylic polymer binder, water as solvent, additives (thickeners and wetting agents). Photocatalyst: particle suspension commercialized (40 wt% TiO2 P25). Final photocatalytic coating with 10% (wt) of TiO2 | 40 g/m² (≈5 µm) applied using a brush | - | Cement mortar |
| Krishnan et al. [25] | Commercially available silicate coating material (containing potassium silicate, silica sol, and organic additives, solid content of 13.5%). 5%, 10%, 15%, and 20% of TiO2 by mass of solid silicate mixed with the silicate coating material | Three coats | - | Portland cement mortar |
| Smits et al. [26] | TiO2 P25, P90 and Hombikat dispersed in ethanol; E-UV commercially available in liquid form | One layer deposited with a pipette (24 ± 2 mg, 267 µg/cm²) | - | Cement mortar |
| Guo et al. [21] | Two coating techniques. 1: suspension of 25 g/L P25 ethanol suspension with 25 g/L glycerol; substrate materials dipped into it for 5 min. Coated mortar calcinated at 450 °C for 120 min to burn organic materials and bond the TiO2 film to the substrate. 2: suspension of methanol and P25 (25g/L); mortar dipped for 5 min, and over-dried at 60 °C for 120 min | Dip-coating | - | Self-compacting glass mortars |
| Vulic et al. [22] | Wet impregnation of TiO2 onto Zn–Al layer double hydroxides (LDHs) for the preparation of Ti–Zn–Al LDH nanocomposites. Nanocomposite suspension prepared using sol–gel method with H2O2 solution | Three layers sprayed | Ti–Zn–Al LDH | Cement mortar |
| Martinez et al. [68] | Acrylic binder, water as solvent, additives (thickeners and wetting agents). The photocatalyst was a commercial slurry solution. Different amounts of binders (2.3%, 5.0%, 7.5%, 11.5%, 15.0%) and various concentrations of photocatalyst (0%, 5%, 10%, 15%, 20%) | 40 g/m² applied using a brush | - | Mortar |
| Fonseca et al. [69] | Aqueous solutions of anatase photocatalyst P25 (1% (v/v) in distilled water) | Sprayed | - | Two external walls of the National Palace of Pena |
| Bengtsson and Castellote [70] | Colloidal suspension of TiO2 in deionised water at a concentration of 8.37 g/L, final TiO2 mass load of 5 g/m2 | Layers of around 10 μm | - | White mortar |
Some of the retrieved papers, as presented in Table 2, use TiO2 with doping techniques or its combined use with other substances and nanocomposites, which may be motivated by several reasons. In general, chemical and structural modifications aim to reduce the bandgap, seeking the degradation of pollutants and contaminants with TiO2 under solar or visible light irradiation [42].
Among the doping substances used in the studies, Khannyra et al. [50] applied nitrogen to understand its effect on photocatalytic activity. C-dot loading in TiO2 was chosen because it might increase the photocatalytic activity, benefiting from solar light [51]; graphene/TiO2 nanocomposites, likewise, might enable better exploitation of solar energy [11]. Doped structures such as Fe-TiO2 and V-TiO2 also aim to take advantage of visible light for photocatalytic reactions [61]. The probable more active photocatalyst behaviour under UV and visible radiation also drove the choice for heterostructures of TiO2-ZnO [54]. Layered-structured materials, like ZnAl double hydroxide, may adjust physical and chemical performance attributes during the synthesis of nano-TiO2 [56], contributing, for example, to its compatibility with cement-based mortars [22]. SiO2@TiO2 nanocomposites were researched mainly regarding durability issues due to the commonly weak adhesion between photocatalytic coatings and the underneath substrate; SiO2 may prevent the release of TiO2 nanoparticles from the surface and, thus, improve the adhesion [57].
Specifically, for the sustainability research goal, conducted as a second phase of the review, for the first string searched on an LCA, “((“titanium dioxide”) or TiO2) and coat* and (“mortar” or “facade” or “façade”) and (“LCA” or “life cycle assess*”)”, two papers were retrieved, and, for the second search string, “((“titanium dioxide”) or TiO2) and (coat*) and (“LCA” or “life cycle assess*”),” the Scopus database identified 15 studies. The two papers found for the first string [33,43] were among the 15 identified for the second one; thus, they were considered only once.
Concerning the papers on sustainability using the LCA tool, the title and abstract screening led to the exclusion of three articles each. Full-paper reading resulted in the exclusion of two additional papers. Among the remaining seven articles, one was a literature review [71]; the other six documents from the final sample are presented in Table 3. It is essential to highlight that, as the search string was broadened to sustainability papers because specific studies related to an LCA on TiO2-based thin coatings for mortars on facades had not been retrieved from the search, some of the substrates were not mortars. Further details on the studies are given in Section 6.

Table 3.
Papers with results assessed for sustainability and life cycle assessment (LCA). The entry “-“ is for information unavailable or not applicable.
Finally, regarding sustainability and LCC, although the “((“titanium dioxide”) or TiO2) and coat* and (“mortar” or “facade” or “façade”) and (“LCC” or “life cycle cost*”)” query string did not match any articles, the second, “((“titanium dioxide”) or TiO2) and (coat*) and (“LCC” or “life cycle cost*”)” resulted in two papers published in journals [34,75]. The study of Wu et al. [75] was excluded because it did not refer to life cycle costing; the LCC abbreviation meant lightweight cement composite. Therefore, only the work of Babaizadeh and Hassan [34], already mentioned in Table 3, resulting from the advanced search on sustainability, used the LCC tool.
4. Photocatalytic Efficiency
This section is divided into self-cleaning ability, depolluting, and antimicrobial effects, aiming to cover all the main topics related to the photocatalytic efficiency of thin TiO2-based coatings to be applied on mortars in facades.
4.1. Self-Cleaning Ability
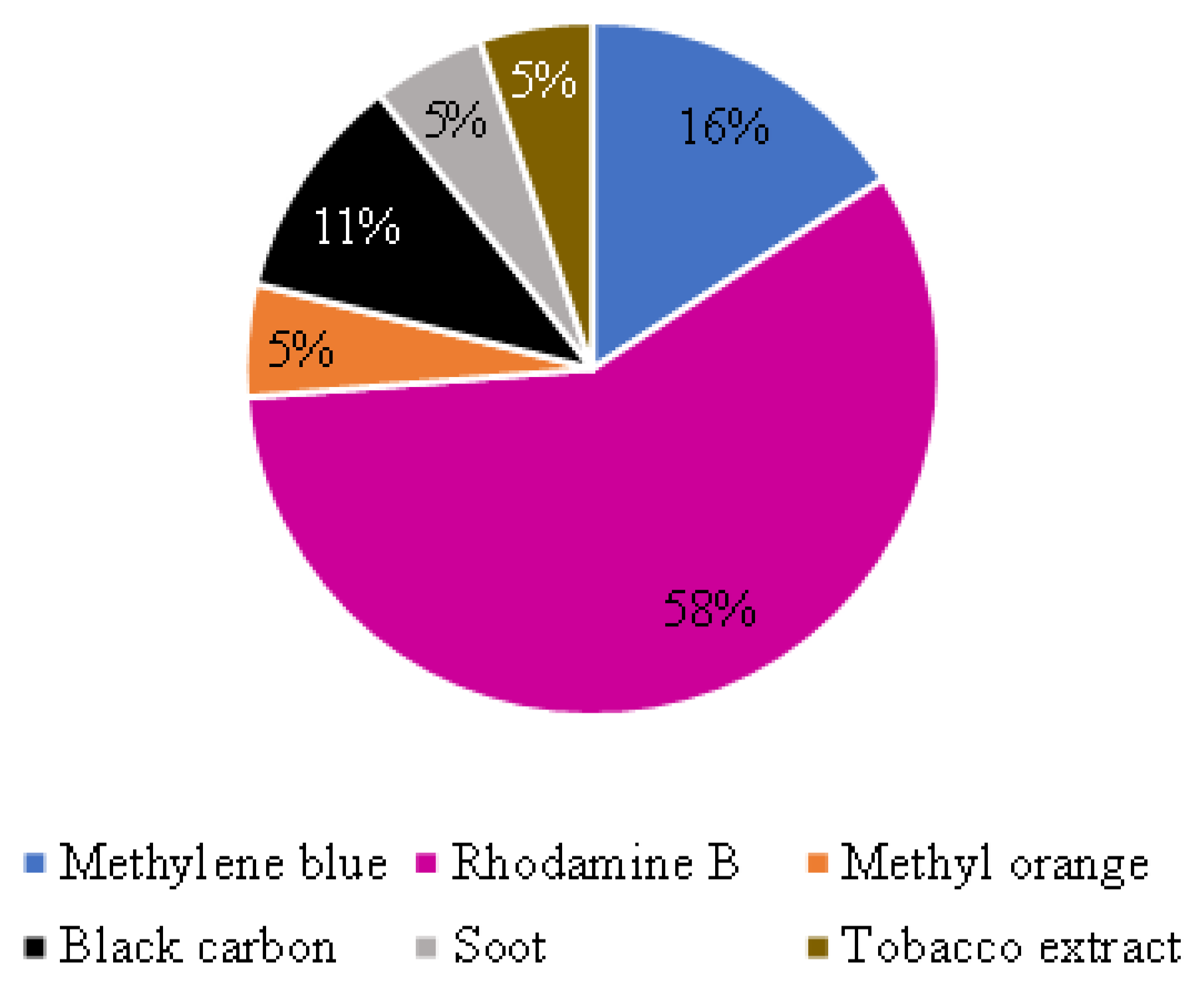
Among the papers retrieved by the review, the self-cleaning ability evaluation of thin TiO2-based coatings for mortars was done with methylene blue (MB) [22,50,51], rhodamine B (RhB) [11,25,29,52,54,56,57,59,63,65,66], methyl orange (MO) [53], black carbon [58,60], soot [26], or tobacco extract [66], as illustrated in Figure 5. Table 4 details the applied dye or contamination agents and the main exposure conditions for the experimental tests.
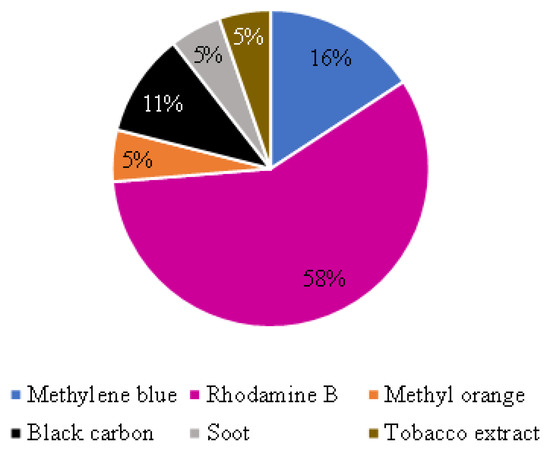
Figure 5.
Application of different dyes or contamination agents to evaluate self-cleaning ability. Note: one of the papers [66] used tobacco extract and Rhodamine B.

Table 4.
Details on the applied dye or contamination agents and the main exposure conditions for testing the self-cleaning ability.
Even though, in general, the studied papers focused on the search for stable aesthetic appearance in buildings by combining photocatalytic oxidation activity with photo-induced hydrophilicity [42], comparisons between different studies must be made carefully. The thickness and structure of the thin TiO2-based coatings, applying method, nanoparticles’ crystallographic parameters, and the presence of dopants affect the photocatalytic ability [47]. The efficiency of catalysts may also depend on variables such as the type of contaminant or pollutant assessed and the exposure conditions [60,66]. Pondelak et al. [56], for example, reported RhB removal of around 2% in 30 min of testing and almost 16% after 24 h of irradiation. For Vulic et al. [22], however, mortars with nanocomposite coatings enabled 28% MB removal after 3.5 h of irradiation. On the other hand, for Khannyra et al. [50], around 25% and 27% of MB degradation were verified for untreated samples and samples solely with silica (S0N0T), probably due to the dye’s sensitivity to UV–vis light.
The amount of exposed TiO2 and, consequently, of active sites over the mortar surface is probably the main reason for using thin photocatalytic coatings instead of TiO2-intermixing techniques [22,25]. Krishnan et al. [25] observed that a silicate coating containing 15% TiO2 by mass achieved a similar RhB degradation to mortars intermixed with 2% and 4% TiO2 by weight of cement but required at least 20 times less TiO2. In this context, Zahabizadeh et al. [52] suggested that applying a photocatalytic coating on mortars still in a fresh state could improve the immobilization of TiO2 nanoparticles on their surface.
Colour compatibility is an essential criterion to be considered when applying coatings on mortar surfaces. Aiming to preserve their original aesthetic appearance after the application of thin TiO2-based coatings, Pondelak et al. [56], for example, observed minor initial colour differences (ΔE < 3) and no whitening or yellowing since ΔL* was nearly zero, possibly indicating a high level of compatibility with the substrate. Khannyra et al. [50], on the other hand, identified significant total colour changes (ΔE > 5), which points out the importance of individual assessment of proposed photocatalytic coatings.
Although Pei et al. [11] and Mendoza et al. [63] worked with UV–vis diffuse reflectance spectra (DRS) to study the self-cleaning ability, most of the papers [25,29,50,51,54,57,58,59,60,66] used spectrophotometers. Then, mainly CIELab colour space enabled the assessment of colour variation (ΔE), calculated according to Equation 2, and the colourimetric coordinates L*, a*, and b* [57]. The coordinate L* refers to the lightness and luminance and varies from white (100) to black (0), a* expresses values ranging from red (+a*) to green (−a*), and, finally, b* represents values from blue (−b*) to yellow (+b*). In Equation 2, L0*, a0*, and b0* represent the initial colourimetric coordinates, and Lt*, at*, and bt* are the coordinates after a particular irradiation time [57].
∆E = √((Lt* − L0*)2 + (at* − a0*)2 + (bt* − b0*)2)
Some authors [29,59] investigated RhB removal efficiencies after 4 and 26 h of UV-A or visible light irradiation, expressed as R4 and R26. Equation 3 demonstrates the calculation of R4; R26 determination is the same as R4, but the value of a* for 4 h is changed for a* after 26 h [29]. R4 > 20% and R26 > 50% indicate that the material can be considered photocatalytic [76,77]. In Rosales et al. [59], for example, SiO2@TiO2-containing coatings achieved UNI 11259-2016 [77] photocatalytic requirements, with R4 and R26 of 25% and 55%, respectively; sonochemistry further improved the efficiencies of RhB degradation to 30.4% and 70.5%, probably due to a better dispersion of TiO2 on the SiO2 matrix. Coatings solely TiO2-based led to even higher photocatalytic activity, reaching R4 and R26 of 79% and 92%, and layers with SiO2 only led to a significantly lower 0.5% and 8% removal [59], as expected.
R4 (%) = [(a*(0h) − a*(4h))/(a*(0h))] × 100%
More research is needed concerning using SiO2 and TiO2 as a composite, especially regarding their physical and chemical properties, synthesis methods, and applications [48]. RhB degradation provided by coatings with SiO2@TiO2 nanocomposites prepared by Wang et al. [57] was higher than the efficiency promoted with TiO2 P25 up to 1 h of the test, different from that observed by Rosales et al. [59]. After 9 h, however, 63.4%. 66.7%, 68.3%, and 55.1% of RhB removal was verified for coatings with the nanocomposites with higher and lower deposited density of TiO2 on SiO2 nanospheres, P25, and, lastly, for untreated surfaces [57].
Rosales and Esquivel [48] referenced a hydrophobic potential of SiO2-TiO2 composites but indicated that TiO2 decreased these properties because it led to a lower contact angle; the angle can be increased, however, with compounds like siloxanes. Speziale et al. [54] identified a synergistic effect between photocatalysts and hydrophobic components on the coatings, which avoided bonds between the applied dye and the underneath substrate. RhB discolouration, observed through the variation on the colour coordinate a*, was enhanced by 2–16% for lime mortars with active optimized TiO2-ZnO-containing coatings with superplasticizers [54]. In this context, for Zuena et al. [53], Si-TiO2-MNP coating with silica mesoporous nanoparticles and TiO2 nanoparticles led to a worse dye discolouration performance compared to loaded silica nanocapsules due to the probable loss of contact between the dye agent, methyl orange, and the TiO2, caused by an entrapment of the photocatalytic nanoparticles within the MNP. Therefore, it is essential to understand clearly the interaction between SiO2 and TiO2 since the contact between them and other substances may influence the resulting performance.
Furthermore, in the study of Mendoza et al. [63], a SiO2 interlayer between the mortar and the TiO2-coating led to extremely low reaction rates at the initial stages of testing when compared with specimens without the interlayer concerning RhB photodegradation. Wu et al. [60], on the other hand, after 300 h of testing, observed that a white silicate coating incorporating photocatalyst (PSWC) enabled a 99% recovery of solar reflectance and L* values, evidencing the removal of black carbon, different from that observed for transparent silicate coating (TSC) and white silicate coating (WSC). Therefore, even with possibly corresponding components, each study can identify specific photocatalytic behaviour, which refers to the particularities of the researched layers and the applied tests.
In Gryparis et al. [51], testing with different irradiation did not significantly influence the results. Smits et al. [26], however, for daylight irradiation, observed much lower degradation rates than for UV light; more than 150 h of daylight irradiation were necessary to degrade half of the initially deposited soot. However, the soot quantity was higher than in reality, indicating that, under realistic conditions, the TiO2 coating could remove the actual soot particles from the surfaces and provide self-cleaning ability [26].
Still considering different types of irradiation, in Guo et al. [29], for UV-A incidence, both the tested commercial paint containing TiO2 and the water dispersion with TiO2 P25 presented satisfactory photocatalytic performance, reaching, respectively, R4 of 51.4% and 57.2%; R26 was 63.6% for both types of coating. The resulting values were, thus, higher than the recommended targets of 20% and 50%, respectively. For visible light irradiation, similar to Smits et al. [26], the samples of Guo et al. [29] presented lower RhB removal than for UV-A: R4 was 42.0% and 43.2% for the commercial TiO2-containing paint and the aqueous dispersion, respectively, and R26, 61.7% and 51.2%. The dye-sensitized photoreaction undergone using RhB probably contributed to the final high activity identified with visible irradiation [29,78].
Regarding the doping of TiO2 or its use as nanocomposite, Vulic et al. [65] observed similar RhB removal for TiO2/ZnAl layered double hydroxide coated cement mortars and pozzolanic mortars, which achieved 10% and 8% removal, respectively, after 3.5 h of exposure to UV light; the similar behaviour was possibly due to a comparable nature and quantity of active surface sites. For coatings containing TiO2 and/or those which were N-doped, after 60 min of irradiation, Khannyra et al. [50] observed MB degradation efficiencies of 85%, 83%, 79.5%, 79%, 78%, and 72%, respectively, for SN10TiO2 > SP25 > SN8TiO2 > SN3.33TiO2 > STiO2 > SN6.66TiO2. In Pei et al. [11], cement mortar samples coated with TiO2 nanoparticles and graphene/TiO2 nanocomposites led to 20.25% and 69.74% of RhB degradation, respectively, while untreated mortar degraded 3.51% of RhB after 48 h of irradiation.
For Gryparis et al. [51], which studied TiO2/C-dots composites synthesized using a hydrothermal strategy, TC0 (pure TiO2) and TAu had the best performances, reaching ΔE below five (comparing the colour difference of the surfaces after the degradation of the MB stain and before the MB application) within 48 h. A double layer composed of a consolidant layer plus a consolidant with TC25 also led to considerable self-cleaning activity, achieving ΔE of 7.21 and 5.76 under UV-A and visible light exposure, respectively. As expected, untreated surfaces and surfaces applied only with consolidant had an insignificant colour decline [51].
Among the papers that studied the doping of TiO2 or its combined use with other substances, thus, the results indicate that the efficiency of photocatalytic activity may be increased or negatively affected depending on the composition of the adjoining substance. Khannyra et al. [50], for example, verified that nitrogen doping probably extended the absorption of light that enables the photocatalytic reactions to the visible region, improving MB degradation kinetics. Additionally, Pei et al. [11] reported that the low bandgap energy presented using graphene/TiO2 nanocomposites compared to solely TiO2 provided the exploitation of more photons and the consequent generation of more electron-hole pairs with lower recombination rates, contributing to the photocatalytic efficiency of the studied coatings. Gryparis et al. [51], on the other hand, concluded that nanocomposites without C-dot loading into TiO2 led to faster MB degradation.
Considering different TiO2 proportions on coatings, all TiO2-coated and untreated surfaces of Zahabizadeh et al. [52] presented similar photocatalytic efficiency up to 4 h of irradiation. After 8 h, however, increasing coating rates resulted, in general, in higher RhB degradation; a coating rate of 80 mg/cm² led to 21% of efficiency [52]. In the study of Krishnan et al. [58], higher amounts of TiO2 also led to the fastest surface recovery after testing with black carbon; less soot over the surfaces required lower TiO2 content to achieve self-maintenance for a more extended period. For Smits et al. [26], all the tested coatings, with different commercially available TiO2, removed the soot deposited over the samples, although with different performances. The best behaviour, obtained with a coating containing TiO2 P25, led to the complete mineralization of 60% of the soot in 24 h, resulting in its total transformation into CO2 [26].
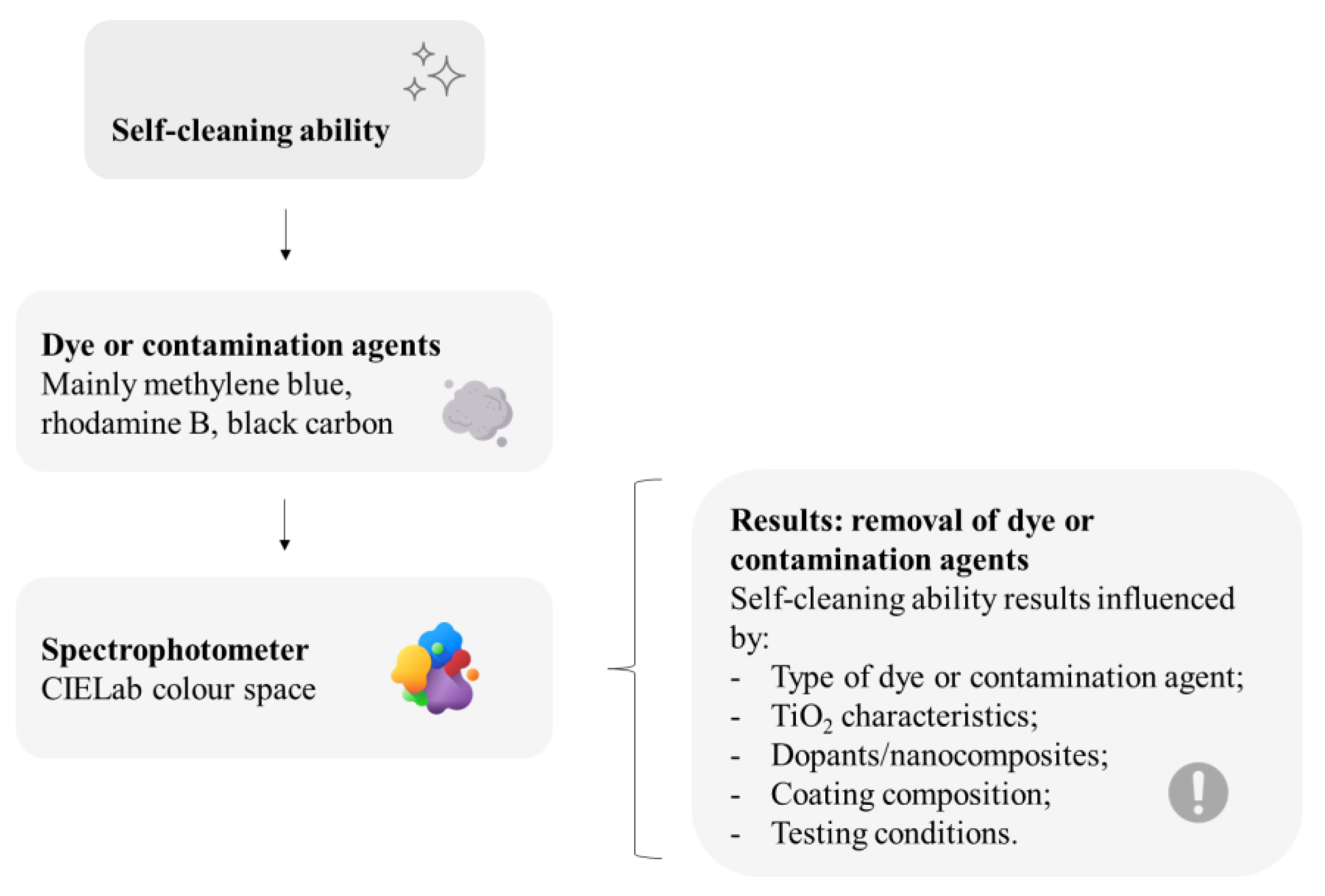
The great exposure of facades to sunlight and rainfall speeds up the implementation of self-cleaning on their surfaces [42,49]. For the spreading and consolidation of TiO2 usage in buildings worldwide, however, higher photocatalytic efficiencies may still be needed, which could be enabled by large-scale or more realistic experimental studies, considering real-life pollution, weather, and lighting, further than dye degradation laboratory tests; architectural designs must also be correlated with the use of TiO2 in facades since construction details might be decisive on the self-cleaning performance [1]. To summarize, Figure 6 depicts some main recurrent issues related to this review on the self-cleaning evaluation of mortar samples with thin TiO2-based coatings.
Figure 6.
Main recurrent issues reviewed on the self-cleaning evaluation of mortar samples with thin TiO2-based coatings. This figure has been designed using resources from Flaticon.com (Creatype, Freepik, Good Ware).
4.2. Depolluting Effect
Different from that verified for the self-cleaning ability, whose evaluation was done with varied dyes and contamination agents, the study of the depolluting effect on TiO2-based coatings was mainly carried out by observing the degradation of nitrogen oxides (NOx). NOx represents two different gases, nitrogen oxide (NO) and nitrogen dioxide (NO2) [42]. Differently, Martinez et al. [67] observed the degradation of a volatile organic compound (VOC) mixture composed of benzene, toluene, ethylbenzene, and xylenes, or BTEX, to represent atmospheric pollution. VOCs are converted into CO2 and H2O under UV irradiation using TiO2 action [42].
Several papers [15,50,54,55,61,62,66,67,68,70] reference ISO 22197-1:2016 [79] or its anterior versions for the method applied (some with adaptations), or, still, UNI 11247:2010 [63,80]. ISO 22197-1:2016 [79] recommends the exposure of the tested samples in a flow-type photoreactor and their activation using UV illumination. NO is the typical air pollutant used for the experiment, during which it is partly converted to NO2. The air-purification performance is obtained through the net removal of NOx, which considers the discount of the NO2 formed from the NO removed [79]. The photocatalytic degradation of NO involves two stages: first, NO is oxidized to NO2 and, afterwards, NO2 is further oxidated into nitrate ions NO3- or nitric acid (HNO3) [42,79]; TiO2 accelerates the chemical reactions without its consumption [49]. Table 5 presents information about the gas flow and exposure conditions used by the studied papers to assess the depolluting effect.

Table 5.
Details on the gas flow and exposure conditions used for the depolluting effect assessment by the studied papers.
The characteristics of the mortar substrate may influence the depolluting of thin TiO2-based coatings. Saeli et al. [24], for example, reported that a layer with 5 wt% of TiHAp provided photocatalytic activity four times greater than with 1 wt% and even more significant than for the pure TiHAp powder (18.7% of NOx reduction after 45 min of the test), which might indicate a synergistic effect due to the high porosity of hydraulic lime mortars, enables a good spreading of the powder contained using the coating and, thus, an increased contact area with the NOx gas. According to Kim et al. [55], the porous surface of cementitious materials can contribute to the mass transfer of NO to the TiO2 surface, enhancing the removal rates. On the other hand, in lime mortars studied by Speziale et al. [54], coated with hydroalcoholic and superhydrophobic dispersions with 50/50 and 10/90 TiO2-ZnO nano-heterostructures, although the surface roughness should lead to increased activity, its high porosity possibly caused the absorption of active particles and, therefore, reduced the exposure of active photocatalysts to NO molecules and irradiation; optimized coatings also did not always increase the photocatalytic activity, probably due to the porosity of the substrate.
Depolluting reactions may also be influenced by the test conditions to which the samples are exposed. For Bengtsson and Castellote [70], catalyst load and light intensity were the most influential parameters on the reaction, compared with relative humidity, temperature, and contaminant concentration. Martinez et al. [68] observed that with initial NO concentrations of 400 ppb and 1000 ppb, the humidity did not significantly influence the degradation rates; however, degradation rates decreased with lower humidity values for NO concentrations of 1500 ppb and 2000 ppb. Water on the active sites at high humidity values probably did not limit the reactions; on the other hand, in low humidity conditions, a lack of oxygen-reactive species possibly affected the efficiency of NO degradation at higher pollutant concentrations [68].
Martinez et al. [67] investigated the influence of different humidity conditions on the catalytic oxidation of BTEX. Regarding an initial VOC concentration of 260 ppb, BTEX conversion reduced with increases in humidity, possibly because of competition between water vapour and pollutants at the adsorption sites of the photocatalysts. For an initial VOC concentration of 2600 ppb, the optimum humidity level was probably lower than 45%; however, oxidation rates increased with humidity below the optimum level, possibly due to the hydroxyl radical creation by dissociating the additional water under irradiation incidence [67]. Therefore, testing parameters may influence the results, and combinations among different proposed conditions, such as humidity and pollutant concentration, may also affect the observed final depolluting efficiency.
Still, regarding the NO degradation test concerning the dynamic of the experiment, Khannyra et al. [50] identified no photocatalytic activity in the absence of UV light since the photocatalytic sites were not activated. With UV–vis light irradiation, NO concentration decreased abruptly and, afterwards, remained stable through the 5 h of testing due to the complete activation of the photocatalytic sites. During this period, NO2 concentration was increased because of the reaction between NO and O2. Even so, the total NOx was removed stably [50]. The importance of proper definition and communication of the test conditions must be, thus, highlighted.
Concerning irradiance, Hot et al. [15], as expected, observed that NO degradation was higher under 20 W/m² of UV lighting intensity than under 5 W/m² due to the more extensive formation of electron-hole pairs. Considering the TiO2 obtained from waste resulting from chemical milling baths of the aeronautical industry, the maximum NO degradation was around 7%, lower than enabled using commercial products; some probable reasons are the micrometric size of the synthesized particles, resulting in fewer active sites, and the fact that more than 70% of the compounds in the powders obtained from waste were not TiO2, but other unwanted phases [15].
Regarding the composition of the coatings, Bengtsson and Castellote [66] observed that the tested TiO2-containing white paints and the S-TiO2 solution were not photoactive. However, for the other studied catalysts, NO concentration after 120 min of irradiation was reduced from 1000 ppb to 39-175 ppb [66]. For Mendoza et al. [63], there were no significant differences in total NOx conversion obtained with different TiO2-based coatings applied on mortar samples; more than 53% of NOx photodegradation was verified during the continuous flow test.
TiO2 content is an important aspect influencing the results. Hot et al. [62] identified, among others, no relevant influence of TiO2 dry matter content superior to 8 g/m² on coatings applied on mortars, considering UV light exposure; NO degradation was considered efficient between 46% and 50%, suggesting an optimal content, above which the degradation did not improve significantly due to the absence of direct incidence of light over the photocatalyst and of contact with the pollutants. Under visible light, the same trend was observed by Hot et al. [62] but with a higher potential optimal content, 20 g/m². For Kim et al. [55], NO removal increased with higher TiO2 particle content, substantial up to a TiO2/MAP weight ratio of 4:1.
The dispersion of the photocatalyst over the mortar surfaces is fundamental to the efficiency of depolluting reactions. Agglomeration may negatively affect the TiO2 photocatalytic efficiency by reducing available active sites [47]. Pérez-Nicolás et al. [61] proved the effectiveness of superplasticizers, especially polycarboxylate-based, to disperse photocatalysts and, thus, obtain high NO abatement percentages under UV light, even close to 50% (solar and visible light led to lower NO removal than UV). The polycarboxylate superplasticizers were responsible for an increase in NO abatement under UV light, on average, by 15%. Regarding solar irradiation, polycarboxylate superplasticizers led to an average of 76% higher values, especially considering air lime mortars and Fe-doped TiO2; NO removal under visible irradiation was also improved. Polynaphthalene sulfonate superplasticizer, on the other hand, caused agglomeration, leading to smaller NOx degradation values than the control coating [61]. In Speziale et al. [54], the addition of superplasticizers to the coatings also positively affected the photocatalytic activity expressed through NOx removal, in the majority of cases, except for samples with polyacrylate, which caused agglomeration; coatings with TiO2-ZnO 10/90 and superplasticizers led to the best-observed behaviour.
Doping of the TiO2 may also influence depolluting efficiency. In Pérez-Nicolás et al. [61], doping enhanced the NO removal results. Under UV light, Fe-TiO2 was, in general, more effective than V-TiO2; under visible light, bare TiO2 still had a positive outcome because of the UV light fraction, but Fe-TiO2 also led to more considerable NO degradation. In Khannyra et al. [50], the synthesized photocatalysts with nitrogen doping were less efficient than solely TiO2 P25, which showed almost 39% NO conversion, compared to around 24% of SN10TiO2; up to 8 M (SN8TiO2) nitrogen doping did not cause a significant difference on the results. The lower photoactivity of the synthesized photocatalysts is possibly related to aggregation, affecting their distribution on the substrate’s surface; in addition, their silica gel covering possibly prevented contact with light and the adsorption of NO on the photocatalytic surfaces [50].
For cement mortars without coatings containing TiO2 nanoparticles or graphene/TiO2 nanocomposites, Pei et al. [11] verified almost no change in the total concentrations of NO and NO2 throughout 12 h of visible light illumination. For 420 nm irradiation, a subtle reduction in the NO concentration and a low increase in the NO2 concentration were verified for the TiO2-based coating, probably due to a portion of illumination belonging to the UV region; after almost 4 h, the NO concentration stabilized with nearly 5% of removal, being the photocatalyst deactivation probably due to the occupation of active sites with products like nitrite and nitrate. Mortars with graphene/TiO2 nanocomposites coatings presented the most efficient NO removal rate, reaching 54.2%, with much later photocatalyst deactivation; thus, graphene incorporation enhanced photocatalytic activity with superior adsorption capacity [11]. Therefore, different dopant elements and synthesis parameters are decisive for depollutant reactions.
Among the studied papers, photocatalyst deactivation was observed or not in different situations. In Guo et al. [21], no catalyst deactivation was observed, different from Pei et al. [11]; TiO2 dip-coated self-compacting glass mortars removed about 14 mg h−1m−2 of NO and the two different dip-coating methods tested did not have a significant effect on the removal [21]. Martinez et al. [68] also did not identify photocatalyst deactivation since, although nitrate ions were formed over the mortar substrate, its large adsorption capacity limited or retarded the deactivation.
Relating to self-cleaning ability, it must be highlighted that, to preserve the desirable appearance of mortars in facades, the photocatalytic coatings must be able to remove pollutants faster than their deposition rate [58]; additional strategies may be needed to obtain truly efficient air-purifying infrastructures, beyond the promising laboratory and pilot projects results [1]. Facades coated with white photocatalytic materials may, furthermore, assist in urban temperature control and even contribute to lower ozone formation during summer, considering the correlation between higher temperatures and smog [42]. Thin TiO2-based coatings for facades could act, in this context, as instruments in the pursuit of climate change control and global warming mitigation.
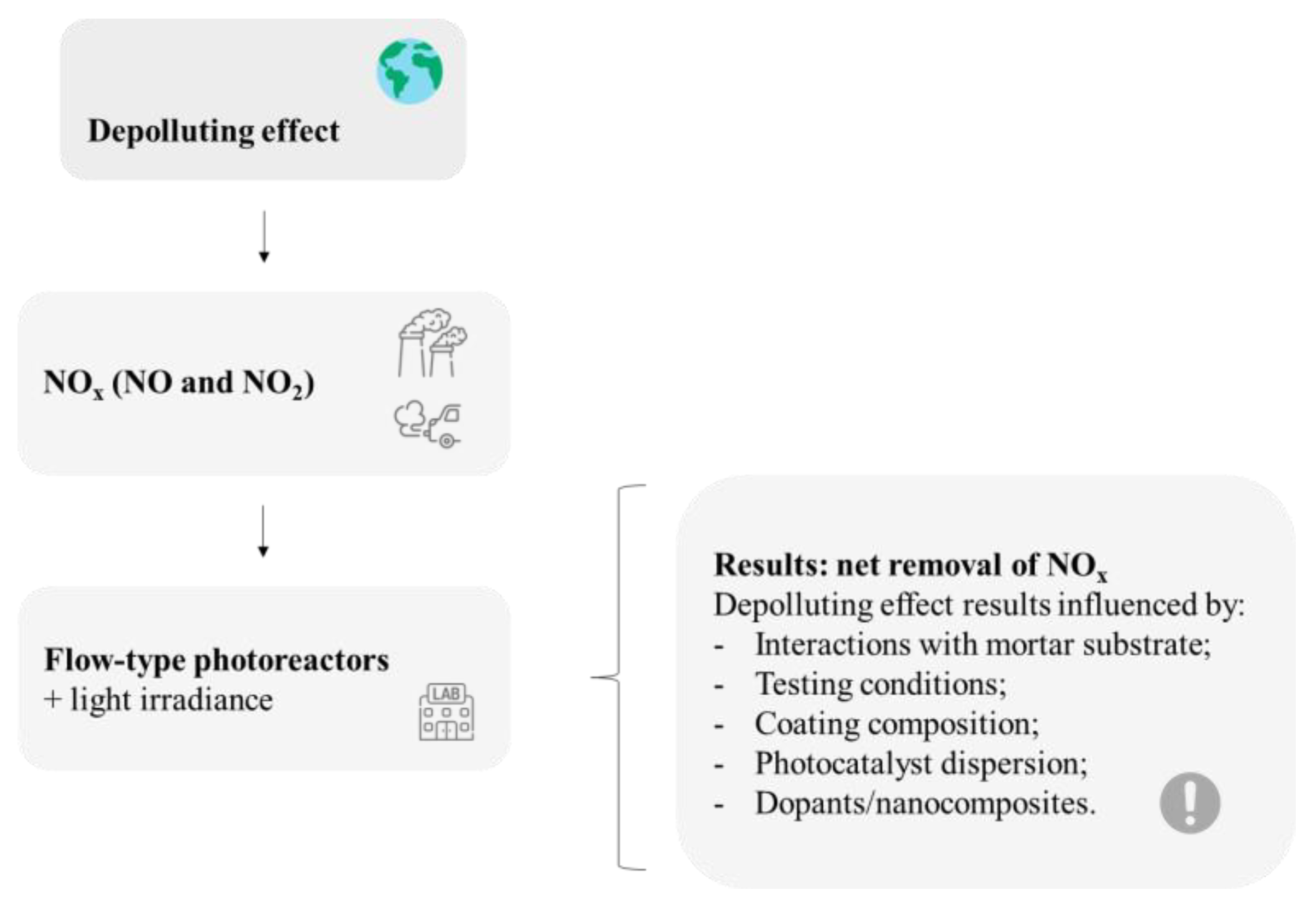
In general, the retrieved papers point to a coherent air-purification effect. Results on the depolluting effect provided using thin TiO2-based coatings applied on mortars depend on several features, as summarized in Figure 7. The influencing parameters are related to each particular study regarding surface characteristics, coating composition, and exposure conditions.
Figure 7.
Main recurrent issues reviewed on the depollution effect of mortar samples with thin TiO2-based coatings. This figure has been designed using resources from Flaticon.com (Freepik, Tempo_doloe, turkkub).
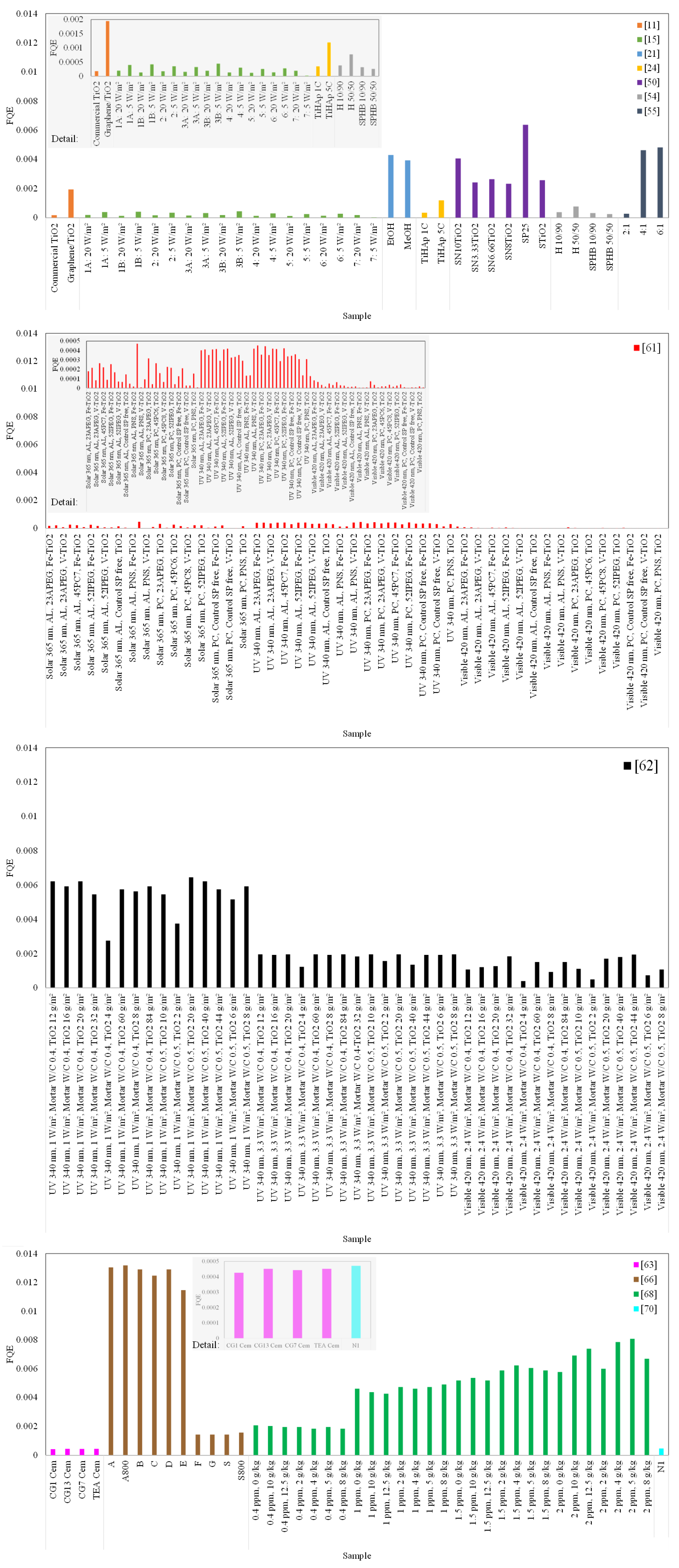
To mitigate comparison incompatibilities among the studied papers resulting from the main influencing parameters, the FQE was calculated relating to depolluting effect. Available information on the articles related to irradiation [W/m²], flow rates [L/min], NO concentration [ppm], NO removal [%], and samples’ exposure area [m²] was used. The number of photons was estimated based on the wavelength of peak emission from the different lamps applied in the studies. When data were not available, assumptions were made based on the literature, namely for peak emission wavelengths [15,24,50,54,61,62,66,68], which were considered 340 nm for UV light [81], 365 nm for solar light [63], and 420 nm for visible light [11], and flow rates [15,50,66], which were assumed as 3 L/min [79,82]. Mean values were considered when the results for individual samples of the same composition were presented.
Figure 8 depicts the results obtained for the FQE. The highest photocatalytic efficiencies observed through NO degradation were obtained using commercial photocatalysts tested by Bengtsson and Castellote [66], followed by the samples studied by Martinez et al. [68], with increasing humidity contents. Samples tested by Hot et al. [62] under UV radiation with 1 W/m² also had high values among the studied papers, comparable to the majority of the samples studied by Guo et al. [21], Saeli et al. [24], and Kim et al. [55]. The lowest values were observed for the samples studied by Hot et al. [15] and Pérez-Nicolás et al. [61]. In Cortes et al. [44], FQEs for CO, CH4, and CO2 are presented, calculated by dividing the moles of electrons passed to form products by the moles of photons; the results have the same order of magnitude as several obtained in the present review.
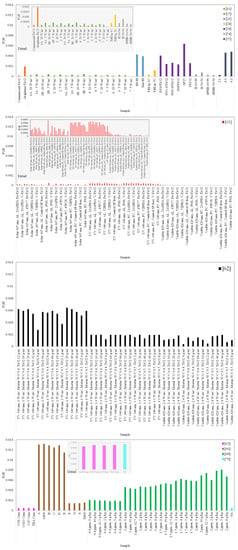
Figure 8.
Formal quantum efficiency (FQE) determined for the samples of the papers discussed on depollution effects of thin TiO2-based coatings applied on mortars [11,15,21,24,50,54,55,61,62,63,66,68,70].
The FQE reinforces the caution that must be taken when comparing different studies with photocatalytic coatings and highlights, at the same time, the relevance of making parallels between research studies. A standard form of TiO2 or a measuring parameter should be available so repeatability can be achieved [46].
4.3. Antimicrobial Properties
The antimicrobial properties provided by TiO2 within thin coatings applied on mortars may enable the reduction of the development of algae, fungi, and bacteria [1]. Table 6 presents the two experimental studies retrieved with the query string.

Table 6.
Details on the microorganisms and methods used to investigate the antimicrobial properties provided by thin TiO2-based coatings in the studied papers.
The results observed by Guo et al. [21] indicated that within 60 min of UV irradiation, E. coli was wholly inactivated on all the studied samples, regardless of the dip-coating method. At the beginning of the test, the photocatalytic inactivation rate was slower, and later, it was faster, resulting in two different stages: in the first step, a longer time was needed until the oxidative damage could destroy the cell wall and membrane, but afterwards, intracellular contents could flow out of the cells [21]. In Fonseca et al. [69], total colour variation indicated that the anatase suspension was the most efficient agent, with higher ΔE* than both biocides, especially Anios. The parameter L*, regarding luminosity and shining of the surface, had the most significant increase for anatase if compared to the biocides due to the removal of dark organic matter; a* also increased after applying anatase because of the elimination of photosynthetic microorganisms. After two weeks, almost all biological growth was removed from the walls [69]. Both studies, thus, suggest the efficiency of TiO2 in suppressing microbial activity under appropriate exposure conditions.
Guo et al. [21] furthermore indicated that with the dip-coating method, photo-generated electrons and holes could react with oxygen and water and form highly reactive species much more efficiently than observed for TiO2 intermixing techniques. It reinforces some advantages that may be obtained by using TiO2 in thin coatings applied over the mortar surface. Additionally, the photoinduced hydrophilicity resulting from the coating possibly affects the bacteria inactivation [21].
To conclude, nanotechnology and preventive conservation potentially prevent the biodeterioration of building materials [69]. Some major topics to consolidate TiO2 as an antimicrobial agent are improving action towards all fouling microorganisms and demonstrating its adequate durability [1]. Further studies are, therefore, needed to disseminate the photocatalyst use concerning its antimicrobial effect.
5. Durability of Thin TiO2-Based Coatings
Table 7 summarizes the properties evaluated using the experimental retrieved papers concerning durability issues for TiO2-based coatings applied on mortars, as well as an overview of the method used for ageing or assessing the results. Regarding the possible negative effects of coatings when exposed to adverse weathering conditions or aggressive environments [1], mainly adhesion and photocatalytic efficiency after ageing or deactivation were evaluated.

Table 7.
Properties evaluated and method for ageing or assessing the results used by the papers to study durability-related issues for TiO2-based coatings.
Concerning adhesion properties, Khannyra et al. [50] reported that the application of surface treatments on mortars led to almost no removal of material from the substrate with a peeling test, while untreated samples lost 6.57 × 10−5 g/cm² of material, suggesting that the studied photocatalytic coatings could be durable for outdoor applications. Similarly, Rudic et al. [64] tested surface treatments which could preserve historical material’s original properties since their application caused minor changes in the adhesion strength of the investigated porous render. Further than providing self-cleaning ability, depolluting, and antimicrobial effects, therefore, the usage of thin TiO2-based coatings with adequate compositions and durability might protect the underneath substrates.
Still, regarding the bonding strength, Kim et al. [55] identified 6.7 N ± 2.1 N for the studied coating applied over a cement-base substrate; it was lower than the adhesion for glass and stainless-steel substrates. However, no change in adhesion was identified after NO degradation tests, indicating that MAP could thus bind the TiO2 to the substrate without deteriorating its photocatalytic performance. In Rosales et al. [59], 10% detachment was observed for SiO2@TiO2 coated mortars; considering solely TiO2-based coatings, 40% detachment was observed, while for SiO2-containing only, the test indicated 5% detachment. In addition, after the adherence test, TiO2-based coating reduced its photocatalytic activity from 79% to 44% after 4 h of the test (R4) and from 92% to 70% after 26 h (R26). On the other hand, no difference was observed for SiO2@TiO2 coatings, possibly reinforcing the SiO2@TiO2 coatings’ potential for air-purifying applications [59]. With the retrieved studies, it can be seen, thus, that improvements in the composition of the thin TiO2-based coatings may enhance their durability and long-term photocatalytic efficiency, indicating the importance of further studies to fill in the related knowledge gaps.
Weathering tests are essential to understanding the durability of TiO2-based coatings to be applied on mortars in facades since they will be exposed to climatic agents and, probably, to climate change. After weathering, Speziale et al. [54], for example, generally verified a significant loss of integrity in coatings applied over lime mortar, which caused a relevant decrease in the samples’ hydrophobicity and affected NOx abatement results. On the other hand, also after ageing, Saeli et al. [24] verified, using XRD, that the photocatalytic active layer with 5 wt% TiHAp in water was still present on the surface of the studied specimens since the same species found before the ageing were identified; the photocatalytic coating could be, thus, resistant to washing and climatic conditions. Running three turns of the NOx abatement test over the TiHAp-coated mortars without any treatment for regeneration, Saeli et al. [24] observed a slight decrease after the first cycle and an almost 20% reduction for the second cycle, which then stabilized. Different TiO2-based coatings, thus, can result in different durability conditions when subjected to ageing tests, possibly requiring specific studies.
In this context, in Guo et al. [29], regarding UV-A irradiation for RhB degradation, after weathering, the studied TiO2-based paint maintained a high efficiency, resulting in R4 and R26 of, respectively, 50.2% and 52.6%. The TiO2-P25 suspension, on the other hand, after one week of weathering, decreased from R4 and R26 of, respectively, 57.2% and 63.6% to 30.7% and 41.2%; after two weeks of weathering, the RhB removal capacity was further reduced to 25.5% and 41.7% because TiO2 particles were removed from the coating. Considering the removal of RhB assessed through visible light exposure, TiO2-P25 did not have good photocatalytic activity, while the paint led to 40% and 51% removal after 4 and 26 h of testing [29].
Pérez-Nicolás et al. [61] observed that weathering worsened the NO degradation ability of the coated mortars due to washing with rain and UV light in a climatic chamber. However, durability was still considered good since moderate values of NO removal were obtained: 35% for cement mortars and 16% for air lime mortars. On average, cement mortars and air lime mortars without superplasticizers on the coatings had 33.8% and 35.2% of removal efficiency reduction, respectively; with superplasticizers, the decline was 35.2% and 16.4% [61]. Vulic et al. [65] also verified that rain rinsing caused some washing of the photocatalytic coatings over cement and pozzolanic mortars. However, at the beginning of UV irradiation for cement mortars, photocatalytic activity was higher than before the weathering, probably due to the presence of water; despite that, after some time, the washing-out effect became predominant and reduced the photocatalytic efficiency. The photoactivity of cement mortar was decreased by 10%, and of pozzolanic mortar, by 12.5%, which was considered a low decrease; both mortars showed an increase in the contact angle with water due to the washing out of the coating, which was coherent with the decrease in the photocatalytic activity [65]. It can be suggested, therefore, that even with some losses in photocatalytic efficiency after ageing, the coatings might still perform satisfactorily, depending on what is required for their long-term activity.
Moreover, Pondelak et al. [56], for example, did not identify reductions in photocatalytic degradation of RhB after rinsing, which suggests that the studied suspension may be suitable for application on mineral building materials. Moreover, a small and positive effect was found for the photocatalytic activity with freezing-thawing cycles, possibly related to a TiO2/LDH structure opening [56]. For Krishnan et al. [25], regarding coatings with silicate and TiO2, after three repeated RhB degradation cycles, the removal rate also increased, but, in this case, probably due to the degradation of organic additives from the silicate coating; after 2500 h of UV irradiation, the silicate coatings still had degradation of 95% at 26 h.
After the washing of the samples, Guo et al. [21] observed a reduction in the NO removal ability from 14.33 mg.h−1.m−2 to 12.14 mg.h−1.m−2 for dip-coated mortars with calcination and from 13.06 mg.h−1.m−2 to 11.11 mg.h−1.m−2 for dip-coated mortars with oven-drying; a similar effect was observed for bactericidal ability, with 90 min required to total inactivation. With abrasion, NO removal resulted in 8.75 mg.h−1.m−2 for the mortar with coating calcination and 6.97 mg.h−1.m−2 for the mortar with coating oven-drying, but no inactivation of E. coli was observed. The mortar porosity and rough texture were possibly positive to retain the TiO2 coatings over the surface; however, the TiO2 particles mainly responsible for bacteria inactivation were probably on the outer surface and removed during abrasion. TiO2 particles in the pores of the mortars could still act as a bactericide but with lower efficiency [21]. Durability, thus, should be evaluated carefully, according to the activity expected from the coatings, since weathering could affect the depolluting effect differently from the antimicrobial performance, for example.
Concerning photocatalyst deactivation, Pei et al. [11] studied the regeneration of deactivated graphene/TiO2 nanocomposites and found NO removal rates reaching 62.5%, 68.8%, 73.1%, 83.7%, 86.3%, and 89.7% of the initial rate after 6 h, 12h, 24 h, 36 h, and 48 h of UV irradiance, respectively, corresponding to 33.8%, 37.3%, 39.6%, 45.4%, 46.8%, and 48.6% of NO removal. Repeated cycles of 12-h UV treatments increased NO removal rates until three cycles, after which they became stable at around 41%. For commercial TiO2, the regeneration was less efficient than the graphene/TiO2 nanocomposites, reaching 18.86%, 24.5%, and 30.2% of the initial removal rates [11]. Studies on the regeneration of photocatalyst activity may represent an opportunity for the long-term efficiency of TiO2-based coatings.
In field exposure, Krishnan et al. [58] did not observe significant appearance changes in panels without TiO2-based coatings after nine months; however, after 25 months, there was a relevant decrease in L* from 72 to 67 and SR from 0.46 to 0.38, and after 3.5 years, 4 µg/cm² of black carbon equivalent was observed over the surfaces. A panel coated with silicate and 0.7% TiO2 presented bleaching in the first month and, after 3.5 years, achieved satisfactory self-cleaning and depolluting results, removing around 16 g/m² of black carbon equivalent [58]. After two years of natural ageing, Saeli et al. [24] also observed a minor degradation of the depolluting activity of coated lime mortars, still resulting in an 18% NOx reduction. Monitoring samples exposed in the field might be considered of the utmost importance to confirm or, eventually, oppose conclusions from laboratory experiments and contribute to the extensive usage of thin TiO2-based coatings in facades to preserve their adequate performance.
Unfortunately, none of the articles retrieved for the durability of thin TiO2-based coatings for mortars on facades referred directly to the influence of climate change and global warming over their long-term performance. However, since effects on the longevity and resilience of building products are expected due to climate change [31], and weathering influences durability, studies on TiO2 coatings considering prospects in the climate scenario represent an urgent gap to be fulfilled. To reinforce the consistency of this gap, an additional search was conducted in the Scopus database with the query string “photocatal* and ((“titanium dioxide”) or TiO2) and durab* and ((“clim* chang*”) or (“global warming”))”, regarding title, abstract, and keywords of journal papers in English. Only 1 document [85] was retrieved; however, it is not related to the construction sector.
Appropriate coatings should ensure stability and durability, further than protecting the underneath substrate and providing photocatalytic properties [42]. Durability investigations are fundamental, considering that, among others, to enable the large-scale application of photocatalytic technology, the deactivation of the photocatalysts [11] must be fully understood. Adherence and photocatalytic efficiency conditions may be improved through the coating composition, including doping or combined substances. Although many studies were found concerning the long-term performance of TiO2-based coatings, further research will be needed, mainly including field exposures and the climate change scenario.
6. Sustainability Potentials and Concerns
Since no specific results were found concerning sustainability for the primary question proposed for this review, potentials and concerns related to the life cycle of TiO2-coated products were raised in this section considering the broader search applied. In LCA studies related to sustainability for nanotechnology, there are several challenges in the obtainment of good results, including the definition of an adequate functional unit, the selection of inventory data, and the impact assessing factors, indicating that the problem may begin within the initial phases of the studies, in the goal and scope definition [33]. Among the retrieved papers, Fufa et al. [74], Babaizadeh and Hassan [34] and Hischier et al. [43] were published before the establishment of the Sustainable Development Goals by the member countries of the United Nations [6].
There needs to be more understanding of the long-term applications of nanoparticles regarding their benefits or, on the other hand, their toxic effects [71]. The availability of accurate information on nanoparticles’ released amount is fundamental, as well as established rules determining how to consider this information on the inventory modelling and characterization factors for taking it into account effectively within the life cycle impact assessment, especially regarding toxicity potentials; however, the consideration of nanoparticles released through the life cycle, and their ecological relevance is often lacking in LCA studies [33]. For the experimental works assessed, Table 8 presents some main parameters regarding the LCA scopes, including impact assessment methods and the environmental impacts or damage categories considered; concerning data sources, only the databases used were referred to. Although all of the papers presented in Table 8 address at least one toxicity aspect, Tichá et al. [73] suggest a lack of internationally accepted characterization factors concerning, for example, human toxicity of nano-TiO2 releases.

Table 8.
Parameters regarding LCA scopes of the studied papers for sustainability evaluation.
In Hischier et al. [33], one of the scenarios evaluated was the application of nano-TiO2 in a separate coating over a traditional paint without modifications to the paint itself; after the end of the coating’s life, only the outer layer should be replaced, and not the paint underneath. The functionality of the studied products must be accurately defined, indicating, for example, the amount of paint or coating saved [33]. This may be an interesting point to be considered when studying the application of TiO2-based coatings in facades.
For the self-cleaning glass studied by Pini et al. [72], a mild positive effect was observed regarding the reduction of airborne pollutants (toluene and NOx) in the use phase due to the nano-TiO2 film. However, nano-TiO2 adverse effects were primarily related to the release of particles, which may affect several impact categories. In the use phase, when particles are released into the air, they may affect human toxicity (cancer and non-cancer, indoor); when inhaled by humans in the end-of-life phase, they may impact carcinogens inhaled and human toxicity (cancer and non-cancer) [72]. In the work of Hischier et al. [33], most of the studied scenarios led to lower impacts for nano-paints or nano-coatings compared to traditional paints, except for those considered low lifetimes for the nanomaterial. However, among all the scenarios, ecotoxicity potential was identified as the most significant environmental impact. Human toxicity potential led to much lower variability than ecotoxicity; the release patterns of TiO2 probably explain the difference since ecotoxicity potential considered emissions into water, while human toxicity regarded emissions into the air, which were from 3 to 4 times less significant [33]. The studies reinforce, thus, that toxicity aspects are critical when studying the sustainability of TiO2-based coatings.
According to Pini et al. [72], however, the positive effects of reducing emissions using the studied self-cleaning glass may offset the damages caused by nanoparticle releases if adequate eco-design choices are considered. Babaizadeh and Hassan [34] similarly investigated residential window manufacture and in-service life cycle phases when applied with a nano-TiO2-based coating. Positive effects were identified concerning the impact categories’ acidification potential, eutrophication potential, air pollutants, and smog formation potential; however, the coating negatively affected global warming, fossil fuel depletion, water intake, human health, and ecological toxicity. The final result could be positive if an overall normalized performance were considered for the environment and air purification [34].
In Hischier et al. [43], nano-TiO2 replaced part of pigment-grade TiO2 in a paint system. The production of nano-TiO2 was responsible for less than 10% of the total life cycle impacts, and the application of the paint in the facade and its end-of-life were even less significant; production of the pigment-grade TiO2 and other substances were the main responsible for the total environmental impacts. Regarding ecotoxicity potential, the production of nano-TiO2 or pigment-grade TiO2 led to similar results. The presence of nano-TiO2 in the paint allowed the repainting of the facade once less during the reference period of 80 years. There was high variability related to the knowledge of nanoparticle release during its lifetime, but this release could be even more relevant than the prolonged lifetime [43].
Tichá et al. [73] compared TiO2-based coatings with an air purifier, considering their ability to degrade microorganisms and pollutants from indoor spaces, and concluded, despite some standardization difficulties, that the photocatalytic option led to the best results. Similarly to Hischier et al. [43], Tichá et al. [73] suggested that the production method of the photocatalyst may have minimal influence when the whole life cycle of a TiO2-based coating application is considered.
From the studied papers, it can be observed, through the LCA tool, that there may be trade-offs between the positive effects of TiO2-based coatings and the environmental impacts related to the coatings materials and techniques, which can result, inclusively, in an overall negative impact due to the photocatalysts’ usage. There is possibly a correspondence between nanoparticle release and longer lifetimes of TiO2-based surface coatings, which must be carefully considered to understand the offsets and final life cycle impacts. Fufa et al. [74], for example, suggested that if the main objective in using TiO2-based coatings is not the reduction of the period between maintenance interventions, nano-TiO2 coatings may lead to higher final environmental impacts.
There are some differences between the conclusions among the papers, which reinforce the need for LCA studies directed specifically to TiO2-based coatings to be applied on mortars in facades. Knowledge related to the toxicity assessment of nanomaterials is still missing, suggesting the need for a database including information from toxicological tests and nanoparticle emissions [72]. Scientific gaps were pointed out concerning long-term emissions and impacts of the material when disposed of in landfills [43], research on by-products’ effects, the long-term effectiveness of the technology and unknown harmful emissions during the life cycle [34]. Uncertainty considerations in LCA studies are also of fundamental importance [74]. Furthermore, although it does not refer directly to LCA, the work of Khitab et al. [49] concerns health and ecosystem problems that may arise from the usage of nano-TiO2; great care is recommended for its widespread application before the availability of reliable risk assessments.
Finally, regarding the economic performance of thin TiO2-based coatings, Babaizadeh and Hassan [34] applied the ASTM E917-05 method [86] for LCC and, for the interpretation, environmental and economic performance was grouped with Multi-Attribute Decision Analysis. For the functional unit, the prices were $107.52 for the float glass and $10.76 for 5.38 mL of nano-sized TiO2; a monthly average for 2011 was considered for the discount rate obtained from the U.S. Bureau of Labor Statistics [34]. Considering scenarios of different weights between environmental and economic performance, TiO2-coated glass presented a superior overall performance in most cases if compared with the standard float glass [34].
Since only one paper was found for the studied materials that included an economic performance evaluation, an evident gap can be highlighted. Further research needs to be published to enable comparisons and consolidate the knowledge on the cost-efficiency of TiO2 coatings. The innovative character of building materials should be assessed, considering their life cycle costs. Moreover, as Babaizadeh and Hassan [34] suggested, with an increase in the incorporation of TiO2 in construction, this photocatalyst may become more competitive in the market.
7. Conclusions
This paper presents a literature review about thin TiO2-based coatings to be applied on mortars in facades concerning their photocatalytic efficiency, represented by the self-cleaning ability, depolluting effect, and antimicrobial properties, their durability, and an overview of life cycle sustainability. Even with no time boundary imposed for the search, all the studied papers were published after 2010.
Although the efficiency of photocatalytic TiO2-based coatings is generally reinforced, especially if compared to intermixing techniques, the studied papers suggest that an individual analysis is essential for each formulated composition. The investigation must also be particular to the specific properties of interest since the response of the same coating composition may differ under various contaminant, pollutant or microbial agents or even different exposure conditions. Doping agents, substrate properties, TiO2 characteristics, and their dispersion in the coating also influence the results. Therefore, measuring parameters that allow comparison between different research on the same calculation basis may be essential. The discussion about NO degradation, based on the figure-of-merit formal quantum efficiency (FQE), might contribute to this purpose.
Among the research scopes, within the 34 papers answering the primary question proposed, “What are the efficiency, durability, and sustainability attributes of photocatalytic coatings with TiO2 for mortars in facades?” self-cleaning performance was the most investigated topic in the literature, followed by depolluting effect and durability. The thin TiO2-based coatings were mainly applied by brushing, spraying, with a pipette, or by dip-coating.
Different dyes and contamination agents were used to study the self-cleaning behaviour of coated mortars, mainly methylene blue, rhodamine B, and black carbon; the results were usually assessed with CIELab colour space. The depollution effect was mainly investigated through NOx degradation in flow-type photoreactors with light irradiance; however, different exposure conditions were observed among the studied papers. Antimicrobial properties were the least characteristic tested on experimental works concerning coatings applied on mortars in facades; even so, TiO2 was considered adequate for degrading bacterium and other organisms. For durability, the conclusions should be further compared and analysed and focus primarily on the resilience of TiO2-based coatings against climate changes and extreme events. Adhesion and photocatalytic efficiency after ageing or deactivation were the main focus of research related to long-term performance.
Regarding sustainability, since no results using Life Cycle Assessment (LCA) and Life Cycle Costing (LCC) tools were found for the first query string from this review, additional broad searches were required to identify coherent papers; however, the results were not necessarily related to facade mortars. For LCC, only one paper was retrieved. Different parameters were adopted within the scope of the LCA studies discussed; however, all the papers generally addressed at least one toxicity environmental impact. A clear gap was identified in this subject, which is reinforced considering the trade-offs between the advantages and disadvantages of TiO2-based coatings when environmental impacts are considered, pointing out the need for additional work in order to validate the innovative character of TiO2 to be incorporated into building materials.
Further research is also especially needed to fully understand the performance over time of thin TiO2-based coatings for facade mortars and to increase its widespread application by the construction sector with more resilience under a changing climate. The potential of photocatalytic coatings is well documented, but realistic field studies and implementations would contribute to its maturity in the market; especially antimicrobial properties should be more thoroughly investigated. Regarding the current challenges related to climate change, human health, and environmental requirements, durability and sustainability studies are urgent to determine the photocatalytic activity’s long-term performance and its impacts on the coatings’ life cycle. Nanoparticle releases and emissions, toxicological effects, and end-of-life concerns should be addressed, aiming to obtain coatings with positive final impacts, and mitigate the drawbacks of these innovative solutions.
Author Contributions
Conceptualization, J.D.B. and I.F.-C.; methodology, J.D.B., I.F.-C., A.B.M. and D.C.C.D.M.; investigation, J.D.B.; resources, A.B.M., D.C.C.D.M. and I.F.-C.; writing—original draft preparation, J.D.B.; writing—review and editing, J.D.B., I.F.-C., A.B.M. and D.C.C.D.M.; visualization, J.D.B.; supervision, I.F.-C., A.B.M. and D.C.C.D.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CAPES (COORDENAÇÃO DE APERFEIÇOAMENTO DE PESSOAL DE NÍVEL SUPERIOR), grant number 88887.702359/2022-00, and CNPq (CONSELHO NACIONAL DE DESENVOLVIMENTO CIENTÍFICO E TECNOLÓGICO). The second author acknowledges the Civil Engineering Research and Innovation for Sustainability (CERIS) research unit (UIDB/04625/2020) from IST funded by Fundação para a Ciência e a Tecnologia (FCT).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors appreciate the support of LAMTAC (Laboratório de Materiais e Tecnologia do Ambiente Construído), PPGCI (Programa de Pós-Graduação em Engenharia Civil: Construção e Infraestrutura), UFRGS (Universidade Federal do Rio Grande do Sul), CERIS (Civil Engineering Research and Innovation for Sustainability), FCT (Fundação para a Ciência e Tecnologia) and IST (Instituto Superior Técnico).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Castro-Hoyos, A.M.; Manzano, M.A.R.; Maury-Ramírez, A. Challenges and Opportunities of Using Titanium Dioxide Photocatalysis on Cement-Based Materials. Coatings 2022, 12, 968. [Google Scholar] [CrossRef]
- Li, Z.; Ming, T.; Liu, S.; Peng, C.; de Richter, R.; Li, W.; Zhang, H.; Wen, C.Y. Review on pollutant dispersion in urban areas-part A: Effects of mechanical factors and urban morphology. Build. Environ. 2021, 190, 107534. [Google Scholar] [CrossRef]
- Baral, A.; Sen, S.; Roesler, J.R. Use phase assessment of photocatalytic cool pavements. J. Clean. Prod. 2018, 190, 722–728. [Google Scholar] [CrossRef]
- De la Rosa, J.M.; Miller, A.Z.; Pozo-Antonio, J.S.; González-Pérez, J.A.; Jiménez-Morillo, N.T.; Dionisio, A. Assessing the effects of UVA photocatalysis on soot-coated TiO2-containing mortars. Sci. Total Environ. 2017, 605–606, 147–157. [Google Scholar] [CrossRef]
- Abolhassani, S.S.; Joybari, M.M.; Hosseini, M.; Parsaee, M.; Eicker, U. A systematic methodological framework to study climate change impacts on heating and cooling demands of buildings. J. Build. Eng. 2023, 63, 105428. [Google Scholar] [CrossRef]
- United Nations. Available online: https://sdgs.un.org/2030agenda (accessed on 9 October 2022).
- Luo, X.J.; Oyedele, L.O. Life cycle optimisation of building retrofitting considering climate change effects. Energy Build. 2022, 258, 111830. [Google Scholar] [CrossRef]
- Farahani, A.V.; Jokisalo, J.; Korhonen, N.; Jylhä, K.; Kosonen, R.; Lestinen, S. Performance assessment of ventilative and radiant cooling systems in office buildings during extreme weather conditions under a changing climate. J. Build. Eng. 2022, 57, 104951. [Google Scholar] [CrossRef]
- Kharbouch, Y. Effectiveness of phase change material in improving the summer thermal performance of an office building under future climate conditions: An investigation study for the Moroccan Mediterranean climate zone. J. Energy Storage 2022, 54, 105253. [Google Scholar] [CrossRef]
- De Richter, R.; Caillol, S. Fighting global warming: The potential of photocatalysis against CO2, CH4, N2O, CFCs, tropospheric O3, BC and other major contributors to climate change. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 1–19. [Google Scholar] [CrossRef]
- Pei, C.; Zhu, J.H.; Xing, F. Photocatalytic property of cement mortars coated with graphene/TiO2 nanocomposites synthesized via sol-gel assisted electrospray method. J. Clean. Prod. 2021, 279, 123590. [Google Scholar] [CrossRef]
- Saeli, M.; Tobaldi, D.M.; Rozman, N.; Škapin, A.S.; Labrincha, J.A.; Pullar, R.C. Photocatalytic nano-composite architectural lime mortar for degradation of urban pollutants under solar and visible (interior) light. Constr. Build. Mater. 2017, 152, 206–213. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Nath, R.K.; Zain, M.F.M.; Jamil, M. An environment-friendly solution for indoor air purification by using renewable photocatalysts in concrete: A review. Renew. Sustain. Energy Rev. 2016, 62, 1184–1194. [Google Scholar] [CrossRef]
- Hot, J.; Dasque, A.; Topalov, J.; Mazars, V.; Ringot, E. Titanium valorization: From chemical milling baths to air depollution applications. J. Clean. Prod. 2020, 249, 119344. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Jimenez-Relinque, E.; Castellote, M. Quantification of hydroxyl radicals on cementitious materials by fluorescence spectrophotometry as a method to assess the photocatalytic activity. Cem. Concr. Res. 2015, 74, 108–115. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Ormellese, M.; Pedeferri, M.P. Characterization of photocatalytic and superhydrophilic properties of mortars containing titanium dioxide. Cem. Concr. Res. 2008, 38, 1349–1353. [Google Scholar] [CrossRef]
- Munafò, P.; Goffredo, G.B.; Quagliarini, E. TiO2-based nanocoatings for preserving architectural stone surfaces: An overview. Constr. Build. Mater. 2015, 84, 201–218. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Del Curto, B.; Ormellese, M.; Pedeferri, M.P. Photocatalytic and self-cleaning activity of colored mortars containing TiO2. Constr. Build. Mater. 2013, 46, 167–174. [Google Scholar] [CrossRef]
- Guo, M.Z.; Ling, T.C.; Poon, C.S. Nano-TiO2-based architectural mortar for NO removal and bacteria inactivation: Influence of coating and weathering conditions. Cem. Concr. Compos. 2013, 36, 101–108. [Google Scholar] [CrossRef]
- Vulic, T.; Hadnadjev-Kostic, M.; Rudic, O.; Radeka, M.; Marinkovic-Neducin, R.; Ranogajec, J. Improvement of cement-based mortars by application of photocatalytic active Ti–Zn–Al nanocomposites. Cem. Concr. Compos. 2013, 36, 121–127. [Google Scholar] [CrossRef]
- Guo, M.Z.; Maury-Ramirez, A.; Poon, C.S. Versatile photocatalytic functions of self-compacting architectural glass mortars and their inter-relationship. Mater. Des. 2015, 88, 1260–1268. [Google Scholar] [CrossRef]
- Saeli, M.; Piccirillo, C.; Tobaldi, D.M.; Binions, R.; Castro, P.M.L.; Pullar, R.C. A sustainable replacement for TiO2 in photocatalyst construction materials: Hydroxyapatite-based photocatalytic additives, made from the valorisation of food wastes of marine origin. J. Clean. Prod. 2018, 193, 115–127. [Google Scholar] [CrossRef]
- Krishnan, P.; Zhang, M.H.; Yu, L.; Feng, H. Photocatalytic degradation of particulate pollutants and self-cleaning performance of TiO2-containing silicate coating and mortar. Constr. Build. Mater. 2013, 44, 309–316. [Google Scholar] [CrossRef]
- Smits, M.; Chan, C.K.; Tytgat, T.; Craeye, B.; Costarramone, N.; Lacombe, S.; Lenaerts, S. Photocatalytic degradation of soot deposition: Self-cleaning effect on titanium dioxide coated cementitious materials. Chem. Eng. J. 2013, 222, 411–418. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Paolini, R.; Rossini, M.; Aslan, A.B.; Zinzi, M.; Poli, T.; Pedeferri, M.P. Long term self-cleaning and photocatalytic performance of anatase added mortars exposed to the urban environment. Constr. Build. Mater. 2015, 96, 270–278. [Google Scholar] [CrossRef]
- Fernandes, C.N.; Ferreira, R.L.S.; Bernardo, R.D.S.; Avelino, F.; Bertini, A.A. Using TiO2 nanoparticles as a SO2 catalyst in cement mortars. Constr. Build. Mater. 2020, 257, 119542. [Google Scholar] [CrossRef]
- Guo, M.Z.; Maury-Ramirez, A.; Poon, C.S. Self-cleaning ability of titanium dioxide clear paint coated architectural mortar and its potential in field application. J. Clean. Prod. 2016, 112, 3583–3588. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Luongo, N.; Massari, S.; Spagnolo, S.L.; Daniotti, B.; Pedeferri, M.P. Durability of self-cleaning cement-based materials. Constr. Build. Mater. 2021, 280, 122442. [Google Scholar] [CrossRef]
- Lacasse, M.A.; Gaur, A.; Moore, T.V. Durability and Climate Change—Implications for Service Life Prediction and the Maintainability of Buildings. Buildings 2020, 10, 53. [Google Scholar] [CrossRef]
- Moro, C.; Francioso, V.; Schrager, M.; Velay-Lizancos, M. TiO2 nanoparticles influence on the environmental performance of natural and recycled mortars: A life cycle assessment. Environ. Impact Assess. Rev. 2020, 84, 106430. [Google Scholar] [CrossRef]
- Hischier, R.; Salieri, B.; Pini, M. Most important factors of variability and uncertainty in an LCA study of nanomaterials—Findings from a case study with nano-Titanium dioxide. NanoImpact 2017, 7, 17–26. [Google Scholar] [CrossRef]
- Babaizadeh, H.; Hassan, M. Life cycle assessment of nano-sized titanium dioxide coating on residential windows. Constr. Build. Mater. 2013, 40, 314–321. [Google Scholar] [CrossRef]
- Jäger-Roschko, M.; Petersen, M. Advancing the circular economy through information sharing: A systematic literature review. J. Clean. Prod. 2022, 369, 133210. [Google Scholar] [CrossRef]
- Saade, M.R.M.; Guest, G.; Amor, B. Comparative whole building LCAs: How far are our expectations from the documented evidence? Build. Environ. 2020, 167, 106449. [Google Scholar] [CrossRef]
- Graziani, L.; Quagliarini, E.; Osimani, A.; Aquilanti, L.; Clementi, F.; D’Orazio, M. The influence of clay brick substratum on the inhibitory efficiency of TiO2 nanocoating against biofouling. Build. Environ. 2014, 82, 128–134. [Google Scholar] [CrossRef]
- Xu, Y.; Jin, R.; Hu, L.; Li, B.; Chen, W.; Shen, J.; Wu, P.; Fang, J. Studying the mix design and investigating the photocatalytic performance of pervious concrete containing TiO2-Soaked recycled aggregates. J. Clean. Prod. 2020, 248, 119281. [Google Scholar] [CrossRef]
- Wang, D.; Leng, Z.; Hüben, M.; Oeser, M.; Steinauer, B. Photocatalytic pavements with epoxy-bonded TiO2-containing spreading material. Constr. Build. Mater. 2016, 107, 44–51. [Google Scholar] [CrossRef]
- Vučetić, S.; Rudić, O.L.; Markov, S.L.; Bera, O.J.; Vidaković, A.M.; Skapin, A.S.S.; Ranogajec, J.G. Antifungal efficiency assessment of the TiO2 coating on façade paints. Environ. Sci. Pollut. Res. 2014, 21, 11228–11237. [Google Scholar] [CrossRef]
- Guo, M.Z.; Maury-Ramirez, A.; Poon, C.S. Photocatalytic activities of titanium dioxide incorporated architectural mortars: Effects of weathering and activation light. Build. Environ. 2015, 94, 395–402. [Google Scholar] [CrossRef]
- Gopalan, A.I.; Lee, J.C.; Saianand, G.; Lee, K.P.; Sonar, P.; Dharmarajan, R.; Hou, Y.L.; Ann, K.Y.; Kannan, V.; Kim, W.J. Recent Progress in the Abatement of Hazardous Pollutants Using Photocatalytic TiO2-Based Building Materials. Nanomaterials 2020, 10, 1854. [Google Scholar] [CrossRef] [PubMed]
- Hischier, R.; Nowack, B.; Gottschalk, F.; Hincapie, I.; Steinfeldt, M.; Som, C. Life cycle assessment of façade coating systems containing manufactured nanomaterials. J. Nanopart. Res. 2015, 17, 68. [Google Scholar] [CrossRef]
- Cortes, M.A.L.R.M.; Hamilton, J.W.J.; Sharma, P.K.; Brown, A.; Nolan, M.; Gray, K.A.; Byrne, J.A. Formal quantum efficiencies for the photocatalytic reduction of CO2 in a gas phase batch reactor. Catal. Today 2019, 326, 75–81. [Google Scholar] [CrossRef]
- Watanabe, T.; Takizawa, T.; Honda, K. Photocatalysis through excitation of adsorbates. 1. Highly efficient N-deethylation of rhodamine B adsorbed to cadmium sulfide. J. Phys. Chem. 1977, 81, 1845–1851. [Google Scholar] [CrossRef]
- Mills, A.; Le Hunte, S. An overview of semiconductor photocatalysis. J. Photochem. Photobiol. 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Singh, L.P.; Dhaka, R.K.; Ali, D.; Tyagi, I.; Sharma, U.; Banavath, S.N. Remediation of noxious pollutants using nano-titania-based photocatalytic construction materials: A review. Environ. Sci. Pollut. Res. 2021, 28, 34087–34107. [Google Scholar] [CrossRef]
- Rosales, A.; Esquivel, K. SiO2@TiO2 Composite Synthesis and Its Hydrophobic Applications: A Review. Catalysts 2020, 11, 171. [Google Scholar] [CrossRef]
- Khitab, A.; Ahmad, S.; Munir, M.J.; Kazmi, S.M.S.; Arshad, T.; Khushnood, R.A. Synthesis and applications of nano-Titania particles: A review. Rev. Adv. Mater. Sci. 2018, 53, 90–105. [Google Scholar] [CrossRef]
- Khannyra, S.; Gil, M.L.A.; Addou, M.; Mosquera, M.J. Dye decomposition and air de-pollution performance of TiO2/SiO2 and N-TiO2/SiO2 photocatalysts coated on Portland cement mortar substates. Environ. Sci. Pollut. Res. 2022, 29, 63112–63125. [Google Scholar] [CrossRef]
- Gryparis, C.; Krasoudaki, T.; Maravelaki, P.N. Self-Cleaning Coatings for the Protection of Cementitious Materials: The Effect of Carbon Dot Content on the Enhancement of Catalytic Activity of TiO2. Coatings 2022, 12, 587. [Google Scholar] [CrossRef]
- Zahabizadeh, B.; Rocha Segundo, I.; Pereira, J.; Freitas, E.; Camões, A.; Tavares, C.J.; Teixeira, V.; Cunha, V.M.C.F.; Costa, M.F.M.; Carneira, J.O. Development of Photocatalytic 3D-Printed Cementitious Mortars: Influence of the Curing, Spraying Time Gaps and TiO2 Coating Rates. Buildings 2021, 11, 381. [Google Scholar] [CrossRef]
- Zuena, M.; Ruggiero, L.; Della Ventura, G.; Bemporad, E.; Ricci, M.A.; Sodo, A. Effectiveness and Compatibility of Nanoparticle Based Multifunctional Coatings on Natural and Man-Made Stones. Coatings 2021, 11, 480. [Google Scholar] [CrossRef]
- Speziale, A.; González-Sánchez, J.F.; Taşcı, B.; Pastor, A.; Sánchez, L.; Fernández-Acevedo, C.; Oroz-Mateo, T.; Salazar, C.; Navarro-Blasco, I.; Fernández, J.M.; et al. Development of Multifunctional Coatings for Protecting Stones and Lime Mortars of the Architectural Heritage. Int. J. Archit. Herit. 2020, 14, 1008–1029. [Google Scholar] [CrossRef]
- Kim, J.H.; Han, J.H.; Jung, Y.C.; Kim, Y.A. Mussel adhesive protein-coated titanium oxide nanoparticles for effective NO removal from versatile substrates. Chem. Eng. J. 2019, 378, 122164. [Google Scholar] [CrossRef]
- Pondelak, A.; Kramar, S.; Ranogajec, J.; Škrlep, L.; Vucetic, S.; Ducman, V.; Škapin, A.S. Efficiency of Novel Photocatalytic Coating and Consolidants for Protection of Valuable Mineral Substrates. Materials 2019, 12, 521. [Google Scholar] [CrossRef]
- Wang, D.; Hou, P.; Zhang, L.; Xie, N.; Yang, P.; Cheng, X. Photocatalytic activities and chemically-bonded mechanism of SiO2@TiO2 nanocomposites coated cement-based materials. Mater. Res. Bull. 2018, 102, 262–268. [Google Scholar] [CrossRef]
- Krishnan, P.; Zhang, M.H.; Yu, L.E. Removal of black carbon using photocatalytic silicate-based coating: Laboratory and field studies. J. Clean. Prod. 2018, 183, 436–448. [Google Scholar] [CrossRef]
- Rosales, A.; Maury-Ramírez, A.; Mejía-De Gutiérrez, R.; Guzmán, C.; Esquivel, K. SiO2@TiO2 Coating: Synthesis, Physical Characterization and Photocatalytic Evaluation. Coatings 2018, 8, 120. [Google Scholar] [CrossRef]
- Wu, Y.; Krishnan, P.; Zhang, M.H.; Yu, L.E. Using photocatalytic coating to maintain solar reflectance and lower cooling energy consumption of buildings. Energy Build. 2018, 164, 176–186. [Google Scholar] [CrossRef]
- Pérez-Nicolás, M.; Plank, J.; Ruiz-Izuriaga, D.; Navarro-Blasco, I.; Fernández, J.M.; Alvarez, J.I. Photocatalytically active coatings for cement and air lime mortars: Enhancement of the activity by incorporation of superplasticizers. Constr. Build. Mater. 2018, 162, 628–648. [Google Scholar] [CrossRef]
- Hot, J.; Topalov, J.; Ringot, E.; Bertron, A. Investigation on Parameters Affecting the Effectiveness of Photocatalytic Functional Coatings to Degrade NO: TiO2 Amount on Surface, Illumination, and Substrate Roughness. Int. J. Photoenergy 2017, 2017, 6241615. [Google Scholar] [CrossRef]
- Mendoza, C.; Valle, A.; Castellote, M.; Bahamonde, A.; Faraldos, M. TiO2 and TiO2–SiO2 coated cement: Comparison of mechanic and photocatalytic properties. Appl. Catal. B Environ. 2015, 178, 155–164. [Google Scholar] [CrossRef]
- Rudic, O.; Rajnovic, D.; Cjepa, D.; Vucetic, S.; Ranogajec, J. Investigation of the durability of porous mineral substrates with newly designed TiO2-LDH coating. Ceram. Int. 2015, 41, 9779–9792. [Google Scholar] [CrossRef]
- Vulic, T.; Rudic, O.; Vucetic, S.; Lazar, D.; Ranogajec, J. Photocatalytic activity and stability of TiO2/ZnAl layered double hydroxide based coatings on mortar substrates. Cem. Concr. Compos. 2015, 58, 50–58. [Google Scholar] [CrossRef]
- Bengtsson, N.; Castellote, M. Heterogeneous photocatalysis on construction materials: Effect of catalyst properties on the efficiency for degrading NOx and self-cleaning. Mater. Construcción 2014, 64, e013. [Google Scholar] [CrossRef]
- Martinez, T.; Bertron, A.; Escadeillas, G.; Ringot, E.; Simon, V. BTEX abatement by photocatalytic TiO2-bearing coatings applied to cement mortars. Build. Environ. 2014, 71, 186–192. [Google Scholar] [CrossRef]
- Martinez, T.; Bertron, A.; Ringot, E.; Escadeillas, G. Degradation of NO using photocatalytic coatings applied to different substrates. Build. Environ. 2011, 46, 1808–1816. [Google Scholar] [CrossRef]
- Fonseca, A.J.; Pina, F.; Macedo, M.F.; Leal, N.; Romanowska-Deskins, A.; Laiz, L.; Gómez-Bolea, A.; Saiz-Jimenez, C. Anatase as an alternative application for preventing biodeterioration of mortars: Evaluation and comparison with other biocides. Int. Biodeterior. Biodegrad. 2010, 64, 388–396. [Google Scholar] [CrossRef]
- Bengtsson, N.; Castellote, M. Photocatalytic Activity for NO Degradation by Construction Materials: Parametric Study and Multivariable Correlations. J. Adv. Oxid. Technol. 2010, 13, 341–349. [Google Scholar] [CrossRef]
- Borm, P.J.A.; Berube, D. A tale of opportunities, uncertainities, and risks. Nanotoday 2008, 3, 56–59. [Google Scholar] [CrossRef]
- Pini, M.; González, E.I.C.; Neri, P.; Siligardi, C.; Ferrari, A.M. Assessment of Environmental Performance of TiO2 Nanoparticles Coated Self-Cleaning Float Glass. Coatings 2017, 7, 8. [Google Scholar] [CrossRef]
- Tichá, M.; Zilka, M.; Stieberová, B.; Freiberg, F. Life Cycle Assessment Comparison of Photocatalytic Coating and Air Purifier. Integr. Environ. Assess. Manag. 2016, 12, 478–485. [Google Scholar] [CrossRef]
- Fufa, S.M.; Jelle, B.P.; Hovde, P.J. Durability, reaction to fire properties, and environmental impact of treated and untreated wooden claddings. Wood Mater. Sci. Eng. 2013, 8, 175–187. [Google Scholar] [CrossRef]
- Wu, Y.; Krishnan, P.; Yu, L.E.; Zhang, M.H. Using lightweight cement composite and photocatalytic coating to reduce cooling energy consumption of buildings. Constr. Build. Mater. 2017, 145, 555–564. [Google Scholar] [CrossRef]
- Cassar, L.; Beeldens, A.; Pimpinelli, N.; Guerrini, G.L. Photocatalysis of cementitious materials. In Proceedings of the International Rilem Symposium on Photocatalysis, Florence, Italy, 8–9 October 2007. [Google Scholar]
- UNI 11259:2016; Photocatalysis—Determination of the Photocatalytic Activity of Hidraulic Binders—Rodammina Test Method. UNI (Ente Italiano di Normazione): Milan, Italy, 2016.
- Folli, A.; Pade, C.; Hansen, T.B.; De Marco, T.; Macphee, D.E. TiO2 photocatalysis in cementitious systems: Insights into self-cleaning and depollution chemistry. Cem. Concr. Res. 2012, 42, 539–548. [Google Scholar] [CrossRef]
- ISO 22197-1:2016; Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Test Method for Air-Purification Performance of Semiconducting Photocatalytic Materials—Part 1: Removal of Nitric Oxide. ISO (International Organization for Standardization): Geneva, Switzerland, 2016.
- UNI 11247:2010; Determination of the Degradation of Nitrogen Oxides in the Air by Inorganic Photocatalytic Materials: Continuous Flow Test Method. UNI (Ente Italiano di Normazione): Milan, Italy, 2010.
- Weathering Testing Guidebook; ATLAS Material Testing Solutions: Mount Prospect, IL, USA, 2001.
- Mills, A.; Burns, L.; O’Rourke, C.; Elouali, S. Kinetics of the photocatalysed oxidation of NO in the ISO 22197 reactor. J. Photochem. Photobiol. A Chem. 2016, 321, 137–142. [Google Scholar] [CrossRef]
- ISO 4624:2002; Paints and Varnishes—Pull-Off Test for Adhesion. ISO (International Organization for Standardization): Geneva, Switzerland, 2002.
- ISO 2409:2013; Paints and Varnishes—Cross-Cut Test. ISO (International Organization for Standardization): Geneva, Switzerland, 2013.
- Liu, Z.; Lu, Z.; Bosman, M.; Li, N.; Frankcombe, T.J.; Kia, G.; Tricoli, A.; Liu, Y.; Du, Y.; Yin, Z. Photoactivity and Stability Co-Enhancement: When Localized Plasmons Meet Oxygen Vacancies in MgO. Small 2018, 14, 1803233. [Google Scholar] [CrossRef]
- ASTM E917-05; Standard Practice for Measuring Life-Cycle Costs of Buildings and Building Systems. ASTM (American Society for Testing and Materials): West Conshohocken, PA, USA, 2005.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).