Corrosion-Induced Damage and Residual Strength of WC-Co,Ni Cemented Carbides: Influence of Microstructure and Corrosion Medium †
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
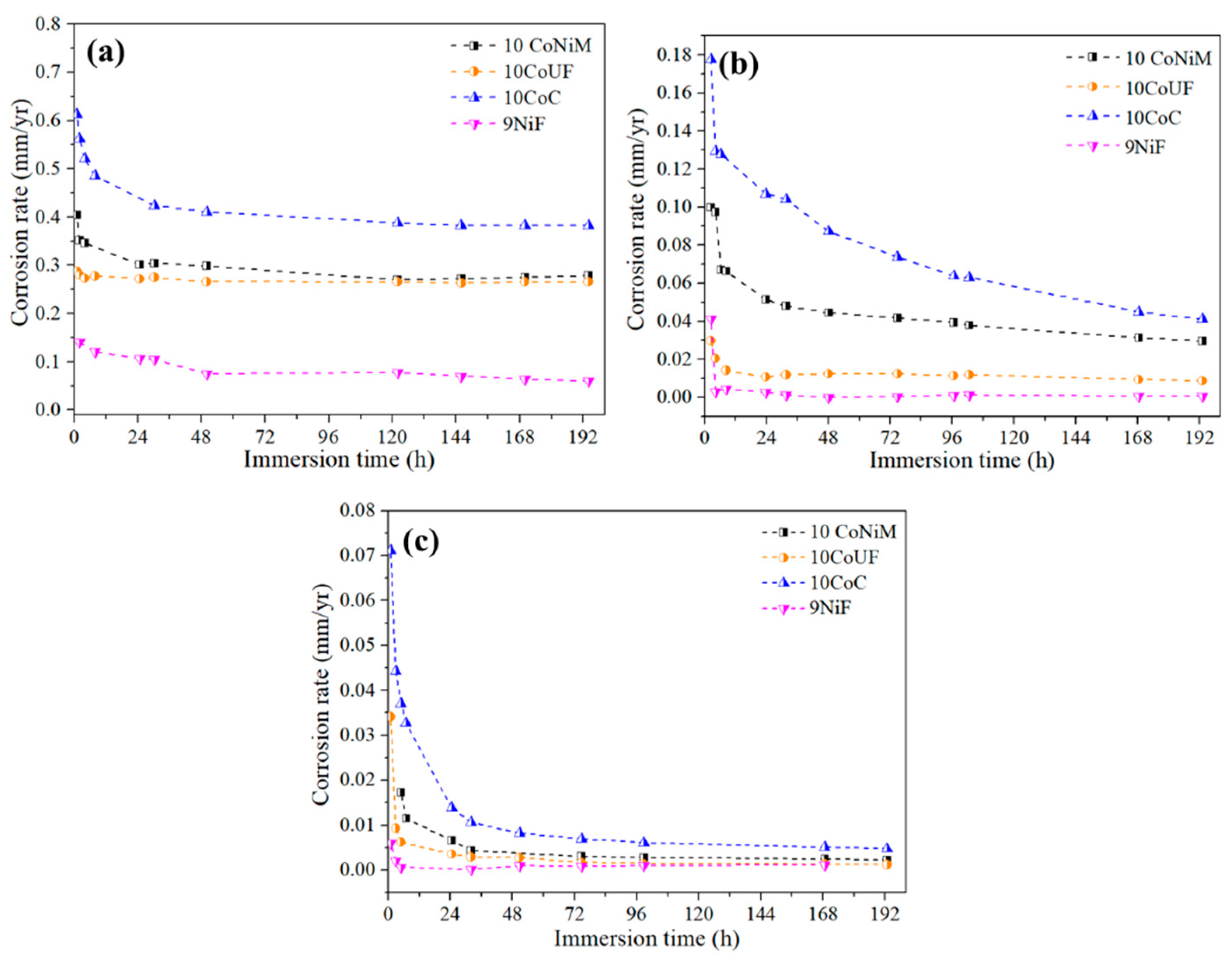
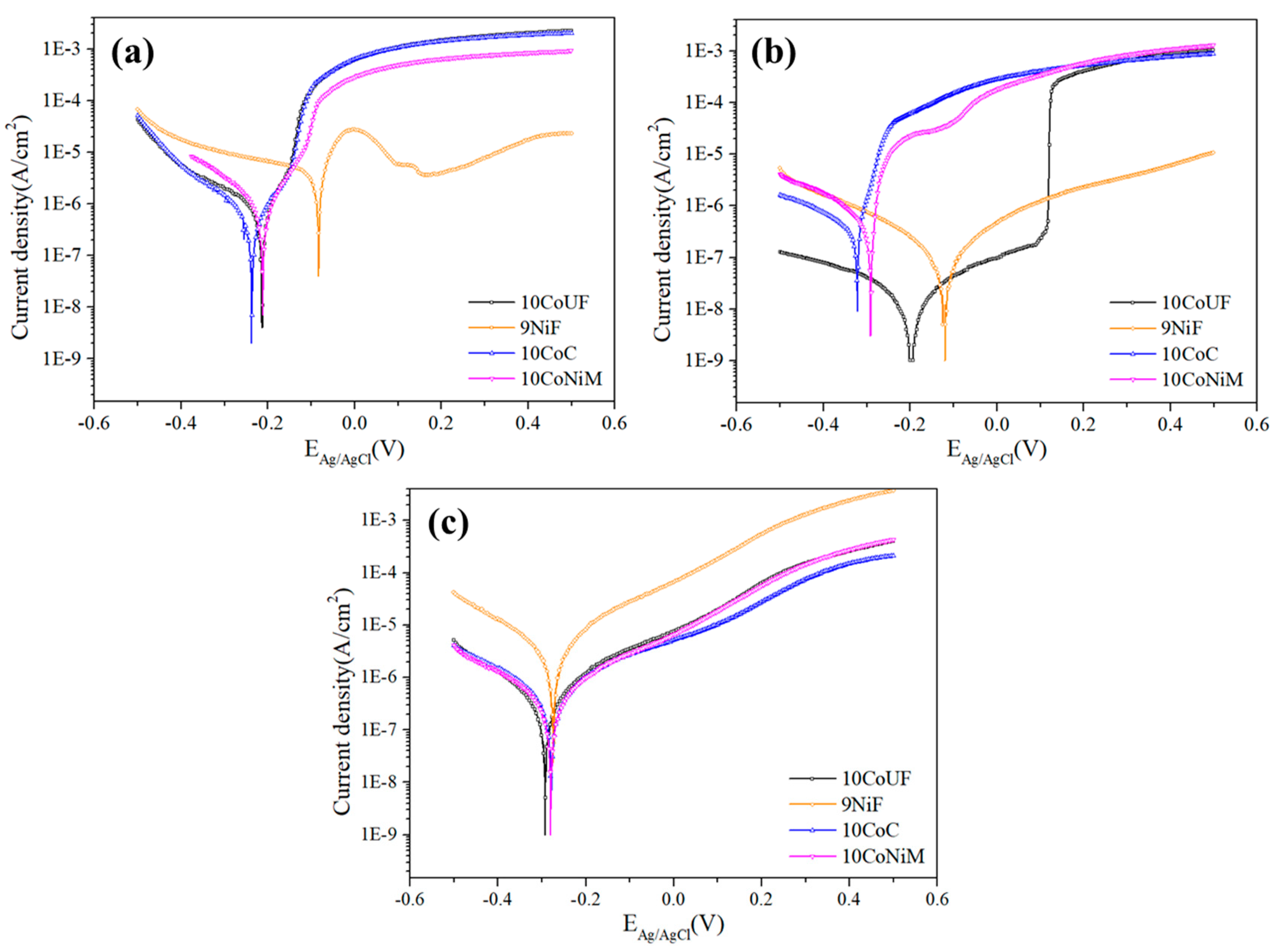
3.1. Corrosion Behavior
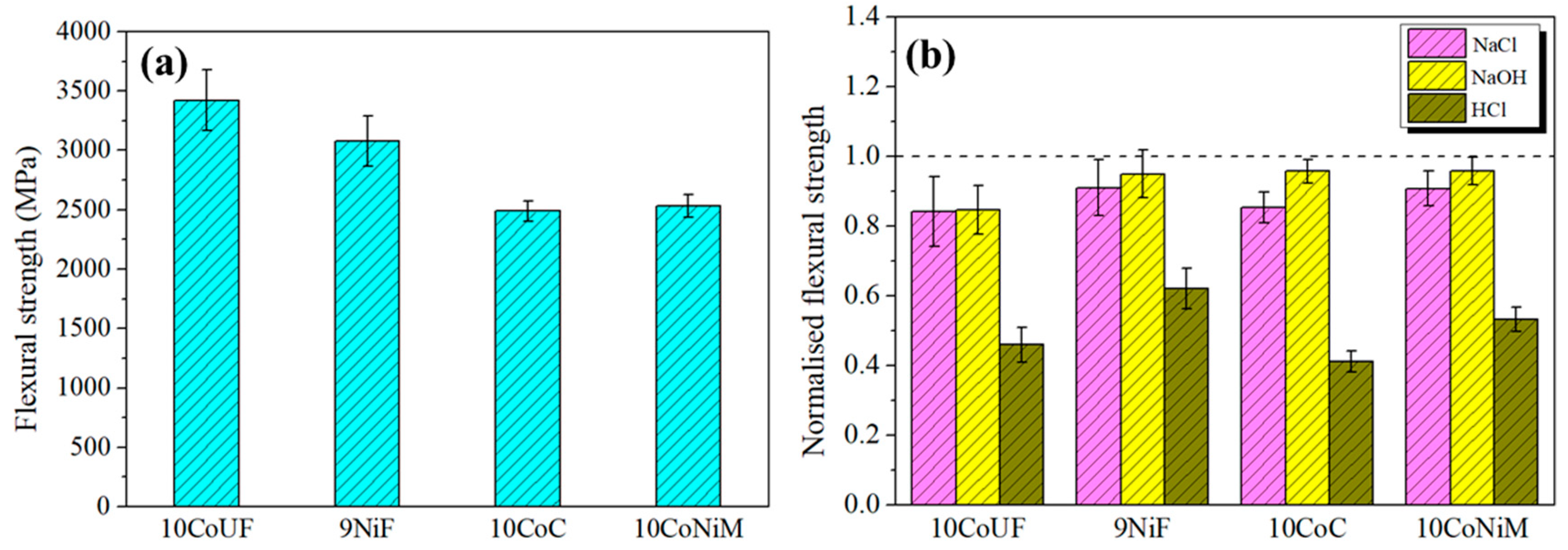
3.2. Residual Strength of Corroded Hardmetals
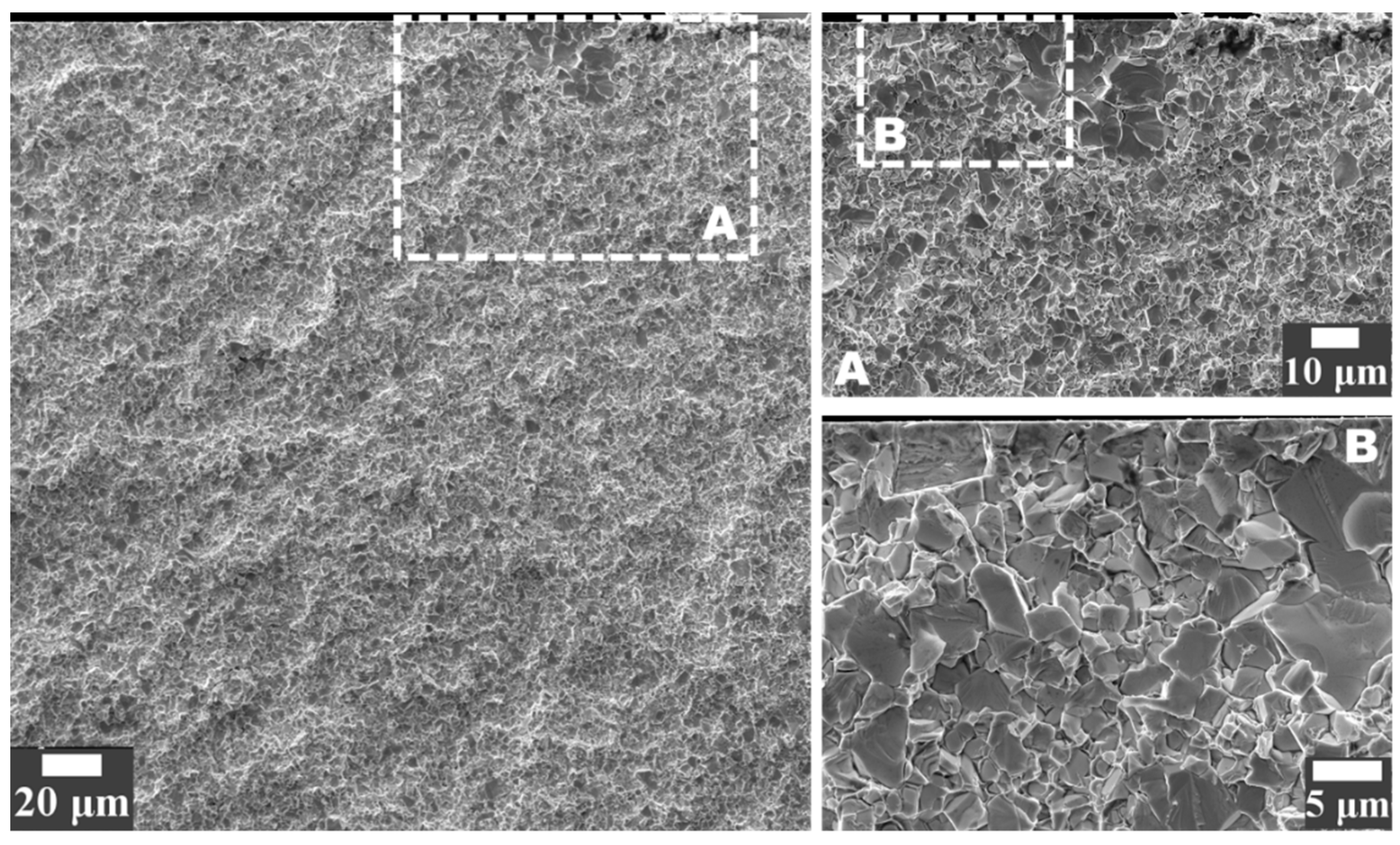
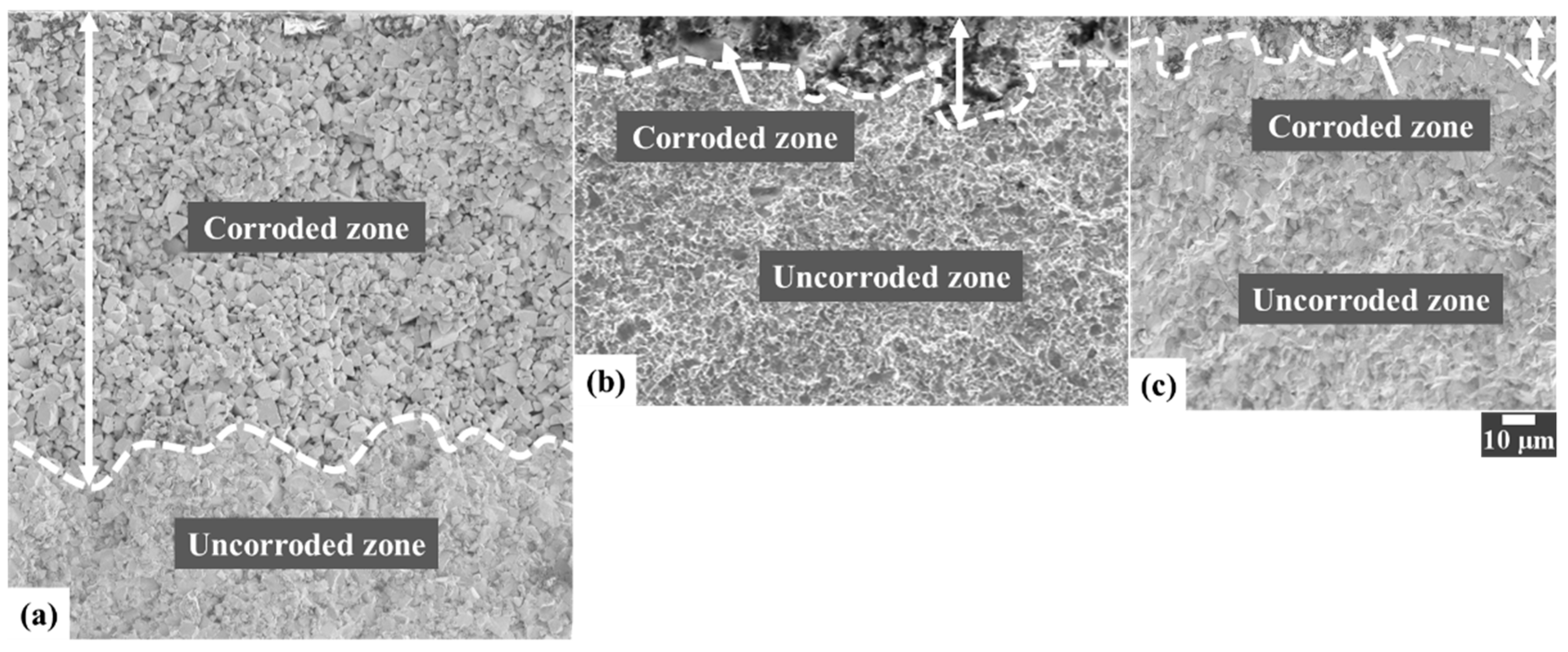
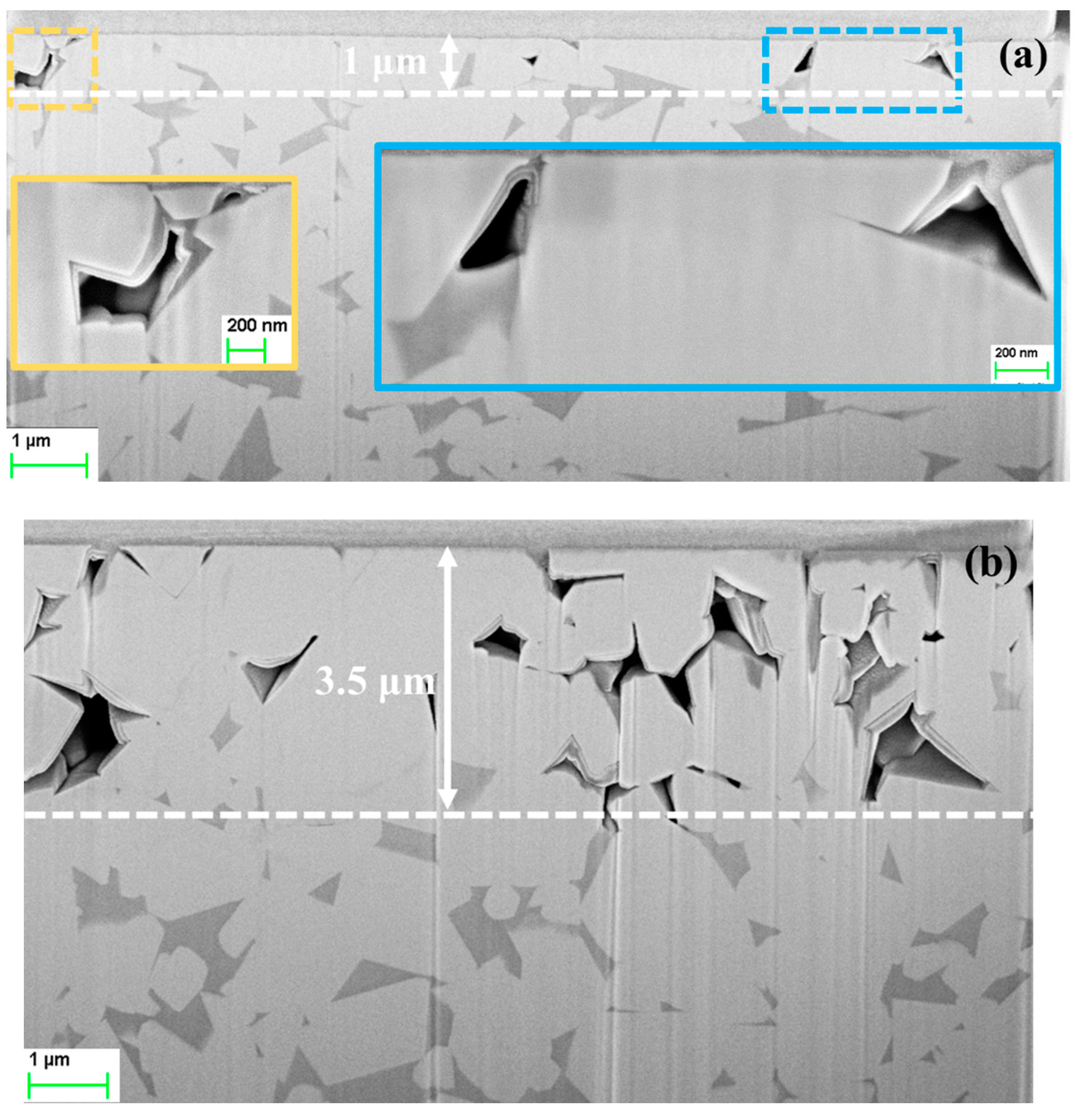
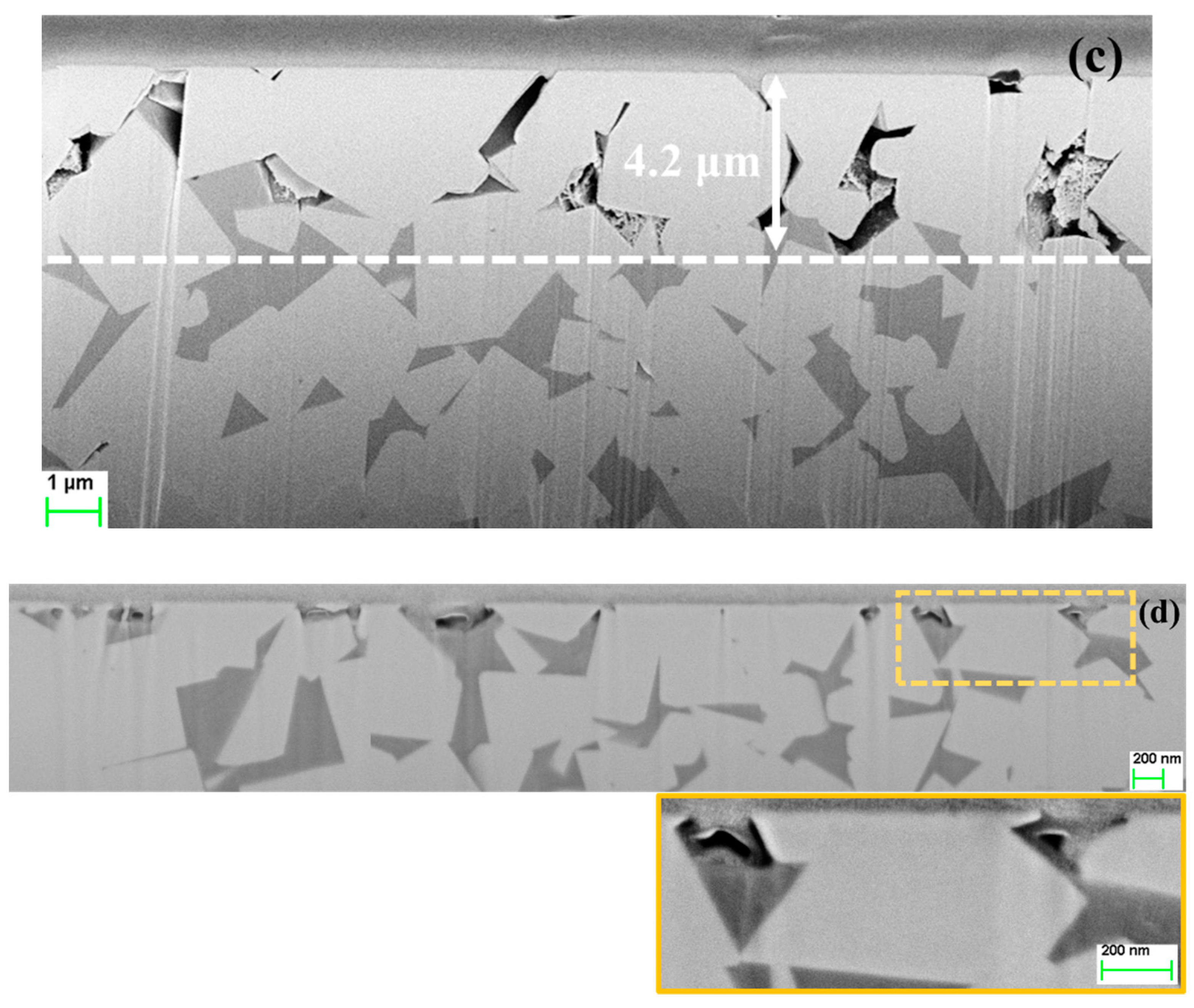
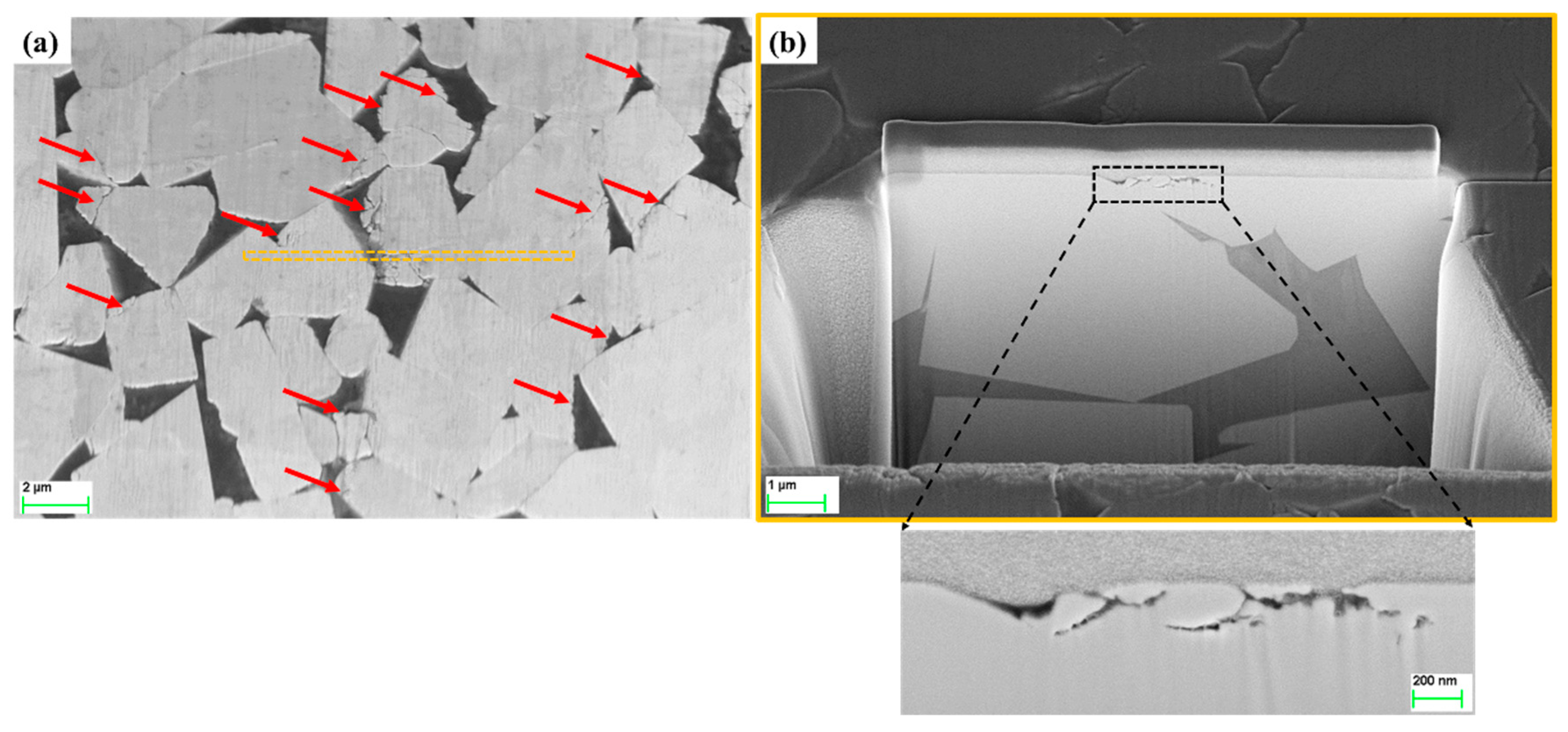
3.3. FIB/FESEM Characterization of Corrosion-Induced Damage
4. Conclusions
- (1)
- Electrochemical and immersion tests revealed that nickel binder displays more noble corrosion potential and critical current density compared to cobalt grades in acidic and neutral solutions containing chlorides. In these conditions, the presence of small amounts of chromium improves more the corrosion resistance of the materials than mixing nickel and cobalt as a binder. No significant differences among studied grades were observed in alkaline solution.
- (2)
- Corrosion damage resulted in strength degradation on the basis of stress rising effects associated with the formation of surface corrosion pits in acidic solution for all studied grades. In neutral and alkaline solutions, corrosion effects on residual strength are less pronounced. Under these conditions, the grade more affected by exposure to corrosion medium is the ultrafine one.
- (3)
- In acidic solution, the binder was preferentially attacked. The binder dissolution started from the center of binder pools, independent of binder chemical nature, and spreads to the edges until binder phase was completely consumed. In alkaline solution, corrosion process was initially located at the binder/WC interface. As exposure time increased, degradation evolved into microcracks which propagated inside the WC phase, yielding finally a fragmented-like scenario.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Exner, H.E. Physical and chemical nature of cemented carbides. Int. Met. Rev. 1979, 24, 149–173. [Google Scholar] [CrossRef]
- Roebuck, B.; Almond, E.A. Deformation and fracture processes and the physical metallurgy of WC-Co hardmetals. Int. Mater. Rev. 1988, 33, 90–110. [Google Scholar] [CrossRef]
- Upadhyaya, G.S. Materials science of cemented carbides-an overview. Mater. Des. 2001, 22, 483–489. [Google Scholar] [CrossRef]
- Roa, J.J.; Jiménez-Piqué, E.; Verge, C.; Tarragó, J.M.; Mateo, A.; Fair, J.; Llanes, L. Intrinsic hardness of constitutive phases in WC-Co composites: Nanoindentation testing, statistical analysis, WC crystal orientation effects and flow stress for the constrained metallic binder. J. Eur. Ceram. Soc. 2015, 35, 3419–3425. [Google Scholar] [CrossRef]
- Jiménez-Piqué, E.; Turon-Vinas, M.; Chen, H.; Trifonov, T.; Fair, J.; Tarrés, E.; Llanes, L. Focused ion beam tomography of WC-Co cemented carbides. Int. J. Refract. Met. Hard Mater. 2017, 67, 9–17. [Google Scholar] [CrossRef]
- Human, A.M.; Exner, H.E. The relationship between electrochemical behaviour and in-service corrosion of WC based cemented carbides. Int. J. Refract. Met. Hard Mater. 1997, 15, 65–71. [Google Scholar] [CrossRef]
- Bozzini, B.; De Gaudenzi, G.P.; Serra, M.; Fanigliulo, A.; Bogani, F. Corrosion behaviour of WC-Co based hardmetal in neutral chloride and acid sulphate media. Mater. Corros. 2002, 53, 328–334. [Google Scholar] [CrossRef]
- Lu, R.; Minarro, L.; Su, Y.Y.; Shemenski, R.M. Failure mechanism of cemented tungsten carbide dies in wet drawing process of steel cord filament. Int. J. Refract. Met. Hard Mater. 2008, 26, 589–600. [Google Scholar] [CrossRef]
- Bozzini, B.; Busson, B.; De Gaudenzi, G.P.; Humbert, C.; Tadjeddine, A. Corrosion of cemented carbide grades in petrochemical slurries. Part I—Electrochemical adsorption of CN¯, SCN¯ and MBT: A study based on in situ SFG. Int. J. Refract. Met. Hard Mater. 2016, 60, 37–51. [Google Scholar] [CrossRef]
- Konadu, D.S.; Van Der Merwe, J.; Potgieter, J.H.; Potgieter-Vermaak, S.; Machio, C.N. The corrosion behaviour of WC-VC-Co hardmetals in acidic media. Corros. Sci. 2001, 52, 3118–3125. [Google Scholar] [CrossRef]
- Kellner, F.J.J.; Hildebrand, H.; Virtanen, S. Effect of WC grain size on the corrosion behaviour of WC-Co based hardmetals in alkaline solutions. Int. J. Refract. Met. Hard Mater. 2009, 27, 806–812. [Google Scholar] [CrossRef]
- Tomlinson, W.J.; Ayerst, N.J. Anodic polarization and corrosion of WC-Co hardmetals containing small amounts of Cr3C2 and/or VC. J. Mater. Sci. 1989, 24, 2348–2354. [Google Scholar] [CrossRef]
- Engqvist, H.; Beste, U.; Axén, N. The influence of pH on sliding wear of WC-based materials. Int. J. Refract. Met. Hard Mater. 2000, 18, 103–109. [Google Scholar] [CrossRef]
- Gant, A.J.; Gee, M.G.; May, A.T. The evaluation of tribo-corrosion synergy for WC-Co hardmetals in low stress abrasion. Wear 2004, 256, 500–516. [Google Scholar] [CrossRef]
- Thakare, M.R.; Wharton, J.A.; Wood, R.J.K.; Menger, C. Exposure effects of alkaline drilling fluid on the microscale abrasion-corrosion of WC-based hardmetals. Wear 2007, 263, 125–136. [Google Scholar] [CrossRef]
- Tomlinson, W.J.; Molyneux, I.D. Corrosion, erosion-corrosion, and the flexural strength of WC-Co hardmetals. J. Mater. Sci. 1991, 26, 1605–1608. [Google Scholar] [CrossRef]
- Pugsley, V.A.; Korn, G.; Luyckx, S.; Sockel, H.G.; Heinrich, W.; Wolf, M.; Feld, H.; Schulte, R. The influence of a corrosive wood-cutting environment on the mechanical properties of hardmetal tools. Int. J. Refract. Met. Hard Mater. 2001, 19, 311–318. [Google Scholar] [CrossRef]
- Pugsley, V.A.; Sockel, H.G. Corrosion fatigue of cemented carbide cutting tool materials. Mater. Sci. Eng. A. 2004, 366, 87–95. [Google Scholar] [CrossRef]
- Tarragó, J.M.; Fargas, G.; Jimenez-Piqué, E.; Felip, A.; Isern, L.; Coureaux, D.; Roa, J.J.; Al-Dawery, I.; Fair, J.; Llanes, L. Corrosion damage in WC-Co cemented carbides: Residual strength assessment and 3D FIB-FESEM tomography characterization. Powder Metall. 2014, 57, 324–330. [Google Scholar] [CrossRef]
- Tarragó, J.M.; Fargas, G.; Isern, L.; Dorvlo, S.; Llanes, L. Microstructural influence on tolerance to corrosion-induced damage in hardmetals. Mater. Des. 2016, 111, 36–43. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, L.; Chen, Y.; Zhang, H.; Zhou, L. Corrosion and strength degradation behaviors of binderless WC material and WC-Co hardmetal in alkaline solution: A comparative investigation. Int. J. Refract. Met. Hard Mater. 2017, 68, 1–8. [Google Scholar] [CrossRef]
- Tarragó, J.M.; Coureaux, D.; Torres, Y.; Yu, F.; Al-Dawery, I.; Llanes, L. Implementation of an effective time saving two-stage methodology for microstructural characterization of cemented carbides. Int. J. Refract. Met. Hard Mater. 2016, 55, 80–86. [Google Scholar] [CrossRef]
- Tarragó, J.M.; Dorvlo, S.; Esteve, J.; Llanes, L. Influence of the microstructure on the thermal shock behavior of cemented carbides. Ceram. Int. 2016, 42, 12701–12708. [Google Scholar]
- Tarragó, J.M.; Roa, J.J.; Valle, V.; Marshall, J.M.; Llanes, L. Fracture and fatigue behavior of WC-Co and WC-CoNi cemented carbides. Int. J. Refract. Met. Hard Mater. 2015, 49, 184–191. [Google Scholar] [CrossRef]
- ASTM C1161-13. Standard Test Method for Flexural Strength of Advanced Ceramics at Ambient Temperature; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Sutthiruangwong, S.; Mori, G.; Kösters, R. Passivity and pseudopassivity of cemented carbides. Int. J. Refrac. Met. Hard Mater. 2005, 23, 129–136. [Google Scholar] [CrossRef]
- Sutthiruangwong, S.; Mori, G. Corrosion properties of Co-based cemented carbides in acidic solutions. Int. J. Refrac. Met. Hard Mater. 2003, 21, 135–145. [Google Scholar] [CrossRef]
- Schnyder, B.; Stössel-Sittig, C.; Kötz, R.; Hochstrasser-Kurz, S.; Virtanen, S.; Jaeggi, C. Investigation of the electrochemical behaviour of WC-Co hardmetal with electrochemical and surface analytical methods. Surf. Sci. 2004, 566, 1240–1245. [Google Scholar] [CrossRef]
- Henjered, A.; Hellsing, M.; Andrén, H.O.; Nordén, H. Quantitative microanalysis of carbide/carbide interfaces in WC-Co-base cemented carbides. Mater. Sci. Technol. 1986, 8, 847–855. [Google Scholar] [CrossRef]
- Krawitz, A.D.; Drake, E.F.; Clausen, B. The role of residual stress in the tension and compression response of WC-Ni. Mater. Sci. Eng. A. 2010, 527, 3595–3601. [Google Scholar] [CrossRef]
- Krawitz, A.; Drake, E. Residual stresses in cemented carbides—An overview. Int. J. Refract. Met. Hard Mater. 2015, 49, 27–35. [Google Scholar] [CrossRef]
- Hochstrasser-Kurz, S.; Reiss, D.; Suter, T.; Latkoczy, C.; Günther, D.; Virtanen, S.; Schmutz, P. ICP-MS, SKPFM, XPS, and microcapillary investigation of the local corrosion mechanisms of WC-Co hardmetal. J. Electrochem. Soc. 2008, 155, C415–C426. [Google Scholar] [CrossRef]
- Lin, N.; He, Y.; Wu, C.; Liu, S.; Xiao, X.; Jiang, Y. Influence of TiC additions on the corrosion behavior of WC-Co hardmetals in alkaline solution. Int. J. Refract. Met. Hard Mater. 2014, 46, 52–57. [Google Scholar] [CrossRef]
| Specimen Code | %wt. Co | %wt. Ni | dWC (µm) | CWC | λbinder (µm) | HV30 (GPa) | KIc (MPa) |
|---|---|---|---|---|---|---|---|
| 10CoUF | 10 | - | 0.39 ± 0.19 | 0.46 ± 0.06 | 0.16 ± 0.06 | 15.7 ± 0.6 | 10.4 ± 0.3 |
| 10CoC | 10 | - | 2.33 ± 1.38 | 0.31 ± 0.11 | 0.68 ± 0.48 | 11.4 ± 0.2 | 15.8 ± 0.3 |
| 10CoNiM | 8 | 2 | 1.44 ± 0.86 | 0.38 ± 0.08 | 0.47 ± 0.30 | 11.6 ± 0.1 | 15.3 ± 0.3 |
| 9NiF | - | 9 | 0.83 ± 0.49 | 0.44 ± 0.08 | 0.29 ± 0.18 | 13.2 ± 0.2 | 11.5 ± 0.2 |
| Specimen Code | Corrosion Rate (mm/y) | ||
|---|---|---|---|
| 0.1M HCl | 0.1M NaCl | 0.1M NaOH | |
| 10CoNiM | 2.8 × 10−1 | 2.96 × 10−2 | 2.23 × 10−3 |
| 10CoUF | 2.7 × 10−1 | 8.67 × 10−3 | 1.19 × 10−3 |
| 10CoC | 3.8 × 10−1 | 4.12 × 10−2 | 4.77 × 10−3 |
| 9NiF | 5.97 × 10−2 | 4.76 × 10−4 | 1.09 × 10−3 |
| Corrosive Media | Specimen Code | Ecorr (V) | icorr (A/cm2) | ic (A/cm2) |
|---|---|---|---|---|
| 0.1M HCl | 10CoUF | −0.213 | 1.05 × 10−6 | 1.90 × 10−3 |
| 10CoC | −0.237 | 9.27 × 10−7 | 1.90 × 10−3 | |
| 10CoNiM | −0.212 | 1.30 × 10−6 | 8.92 × 10−4 | |
| 9NiF | −0.084 | 1.54 × 10−5 | 2.11 × 10−4 | |
| 0.1M NaCl | 10CoUF | −0.196 | 7.38 × 10−8 | 2.21 × 10−7 |
| 10CoC | −0.322 | 5.05 × 10−6 | 3.67 × 10−4 | |
| 10CoNiM | −0.291 | 5.22 × 10−6 | 2.07 × 10−4 | |
| 9NiF | −0.124 | 5.20 × 10−7 | - | |
| 0.1M NaOH | 10CoUF | −0.292 | 1.19 × 10−6 | - |
| 10CoC | −0.278 | 1.12 × 10−6 | - | |
| 10CoNiM | −0.279 | 1.02 × 10−6 | - | |
| 9NiF | −0.274 | 1.20 × 10−5 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Fargas, G.; Armelin, E.; Lavigne, O.; Llanes, L. Corrosion-Induced Damage and Residual Strength of WC-Co,Ni Cemented Carbides: Influence of Microstructure and Corrosion Medium. Metals 2019, 9, 1018. https://doi.org/10.3390/met9091018
Zheng Y, Fargas G, Armelin E, Lavigne O, Llanes L. Corrosion-Induced Damage and Residual Strength of WC-Co,Ni Cemented Carbides: Influence of Microstructure and Corrosion Medium. Metals. 2019; 9(9):1018. https://doi.org/10.3390/met9091018
Chicago/Turabian StyleZheng, Yafeng, Gemma Fargas, Elaine Armelin, Olivier Lavigne, and Luis Llanes. 2019. "Corrosion-Induced Damage and Residual Strength of WC-Co,Ni Cemented Carbides: Influence of Microstructure and Corrosion Medium" Metals 9, no. 9: 1018. https://doi.org/10.3390/met9091018
APA StyleZheng, Y., Fargas, G., Armelin, E., Lavigne, O., & Llanes, L. (2019). Corrosion-Induced Damage and Residual Strength of WC-Co,Ni Cemented Carbides: Influence of Microstructure and Corrosion Medium. Metals, 9(9), 1018. https://doi.org/10.3390/met9091018






