Electrochemical Corrosion Resistance of Ni and Co Bonded Near-Nano and Nanostructured Cemented Carbides †
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Characteristics of Ni-Bonded and Co-Bonded Near-Nanostructured Cemented Carbides
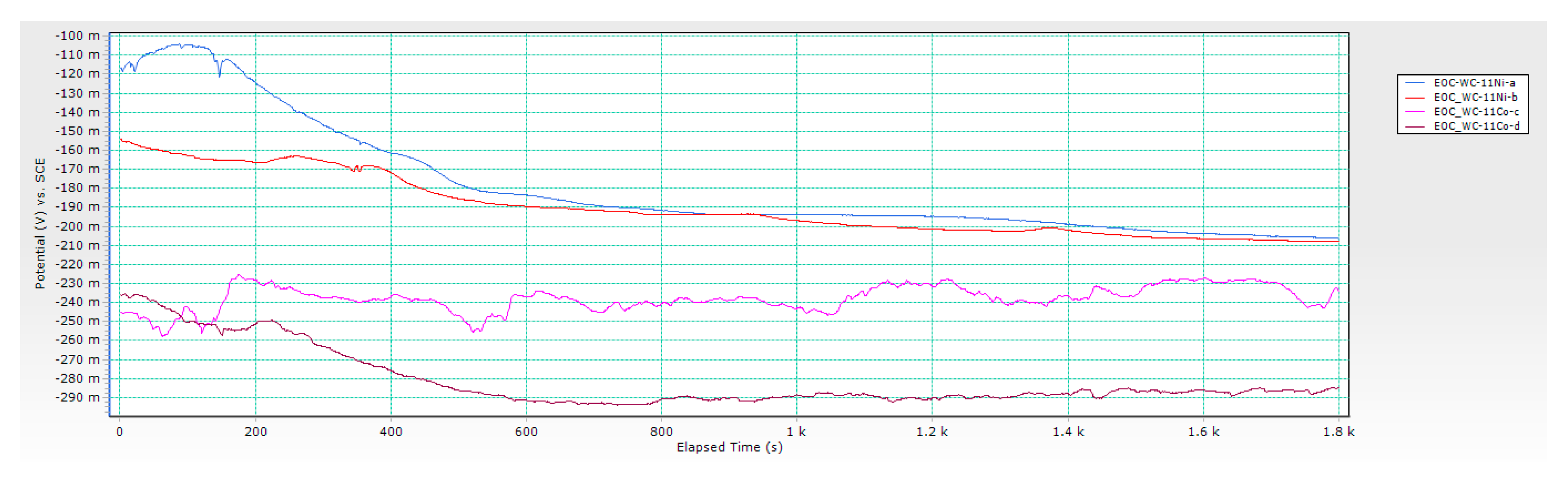
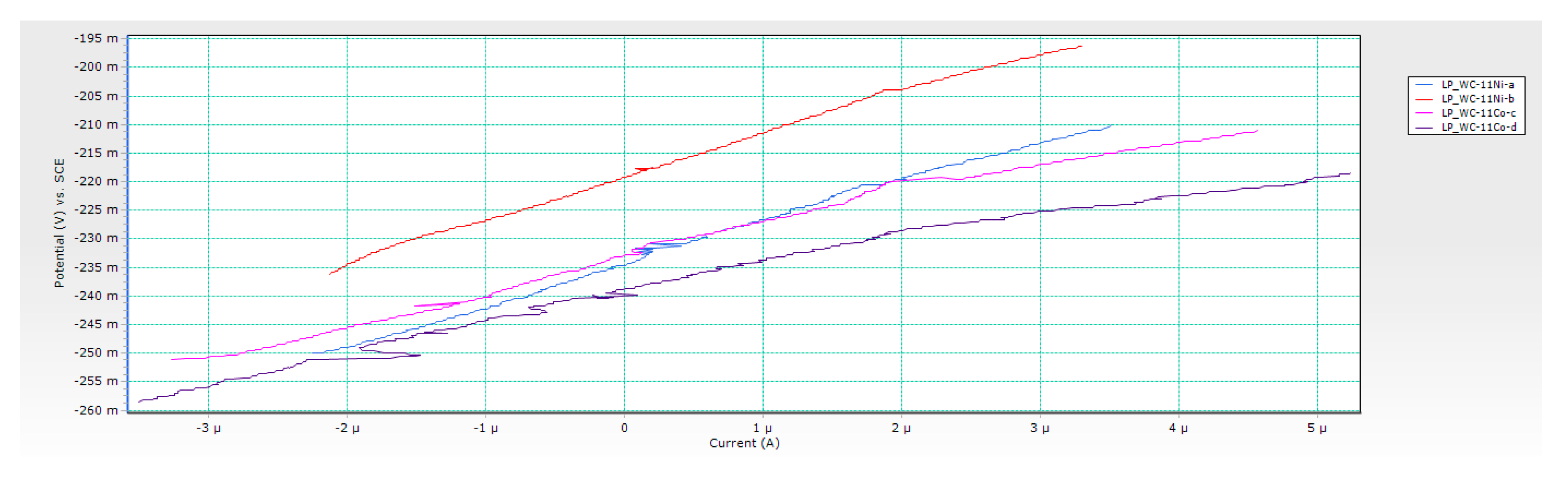
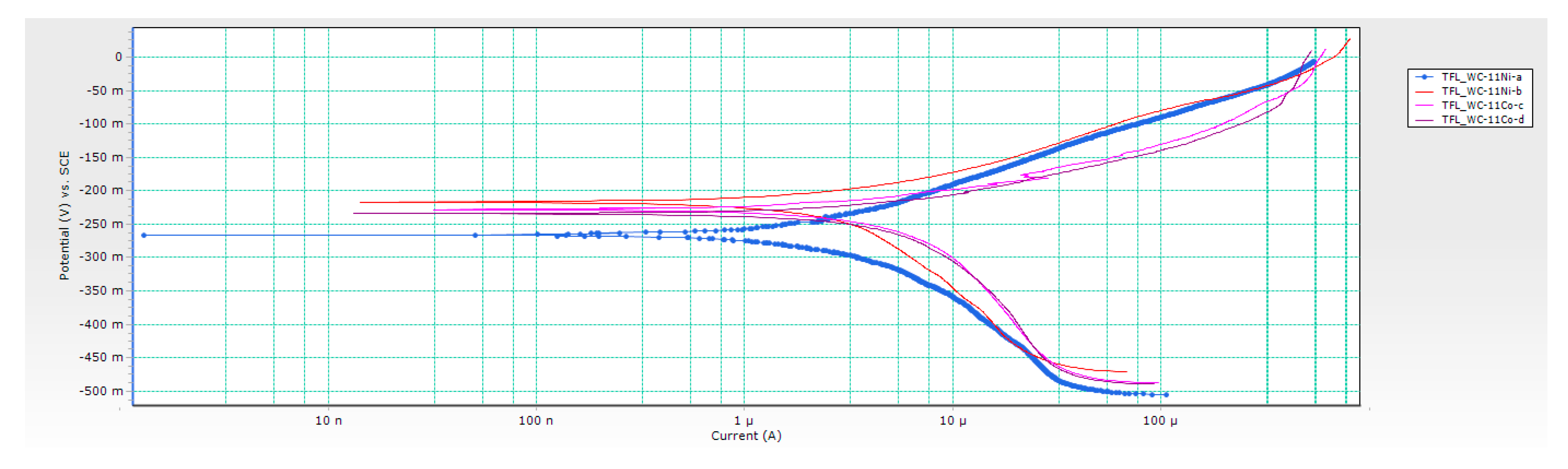
3.2. Results of Electrochemical DC Measurements
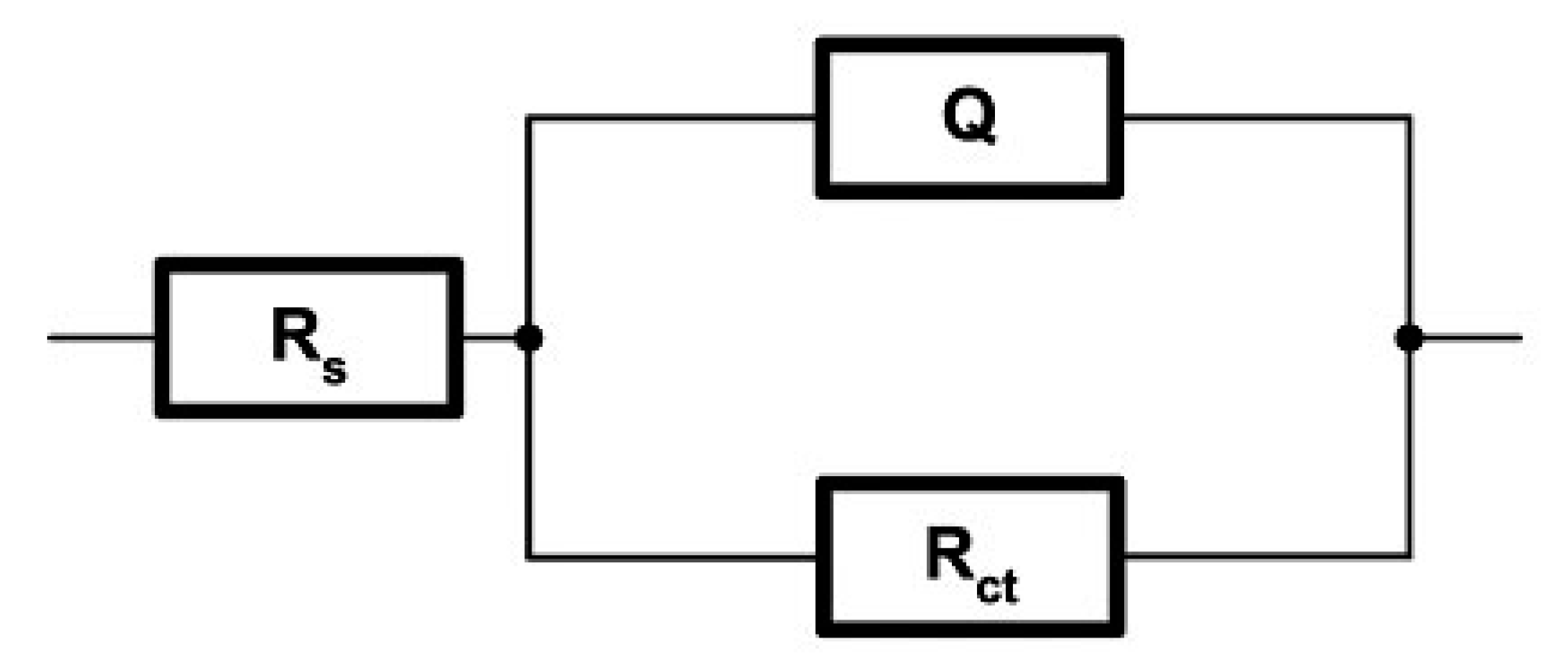
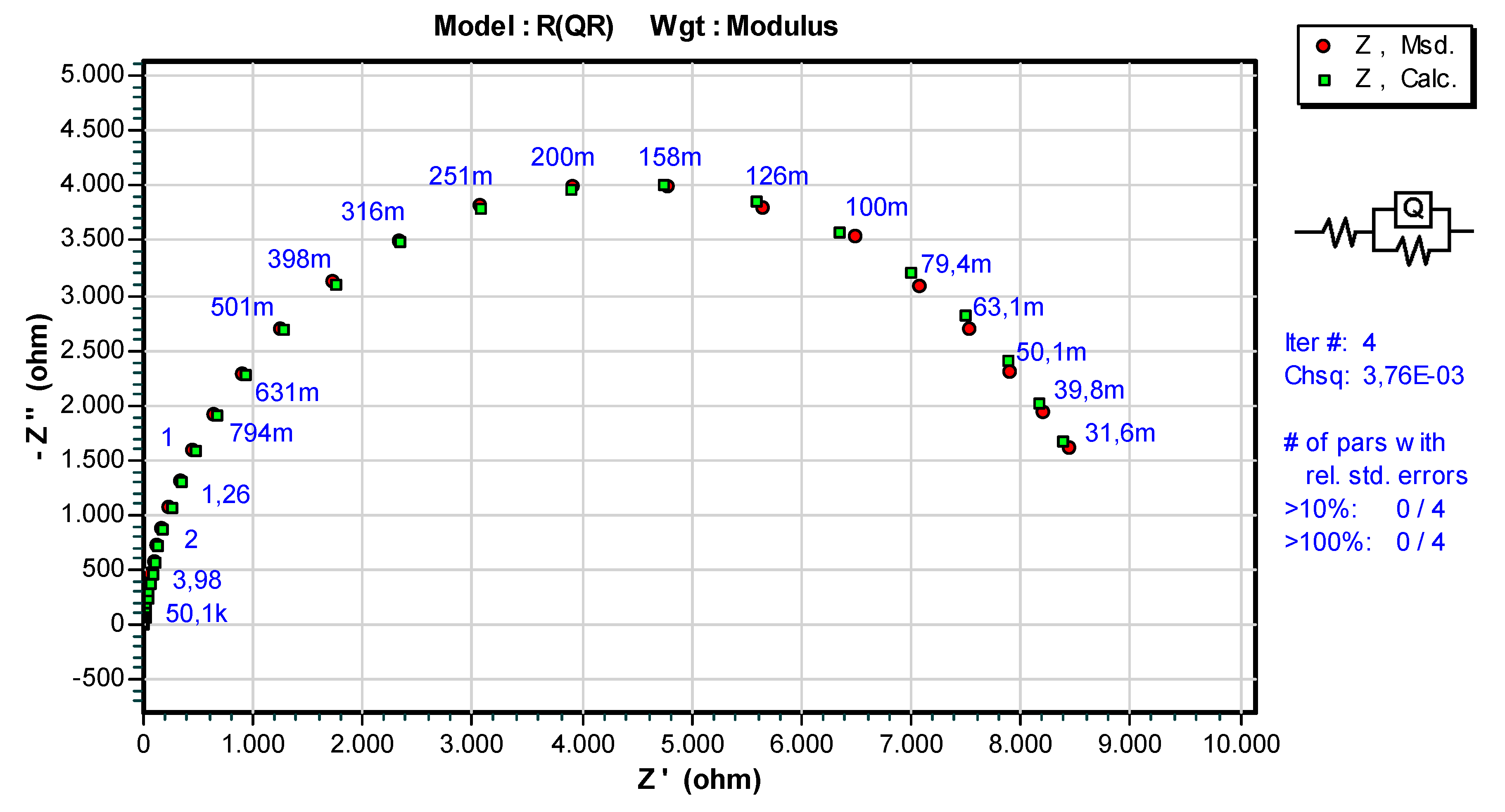
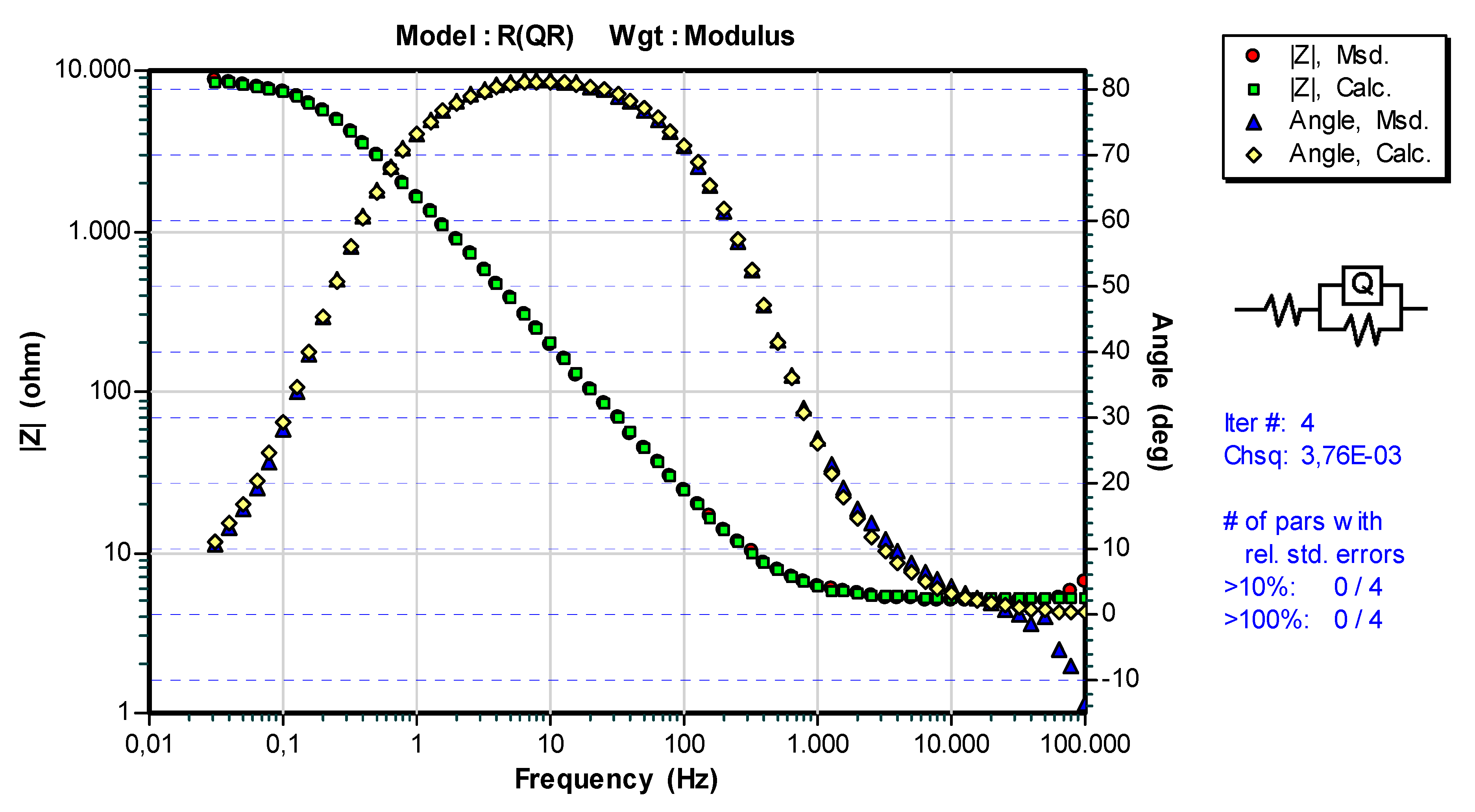
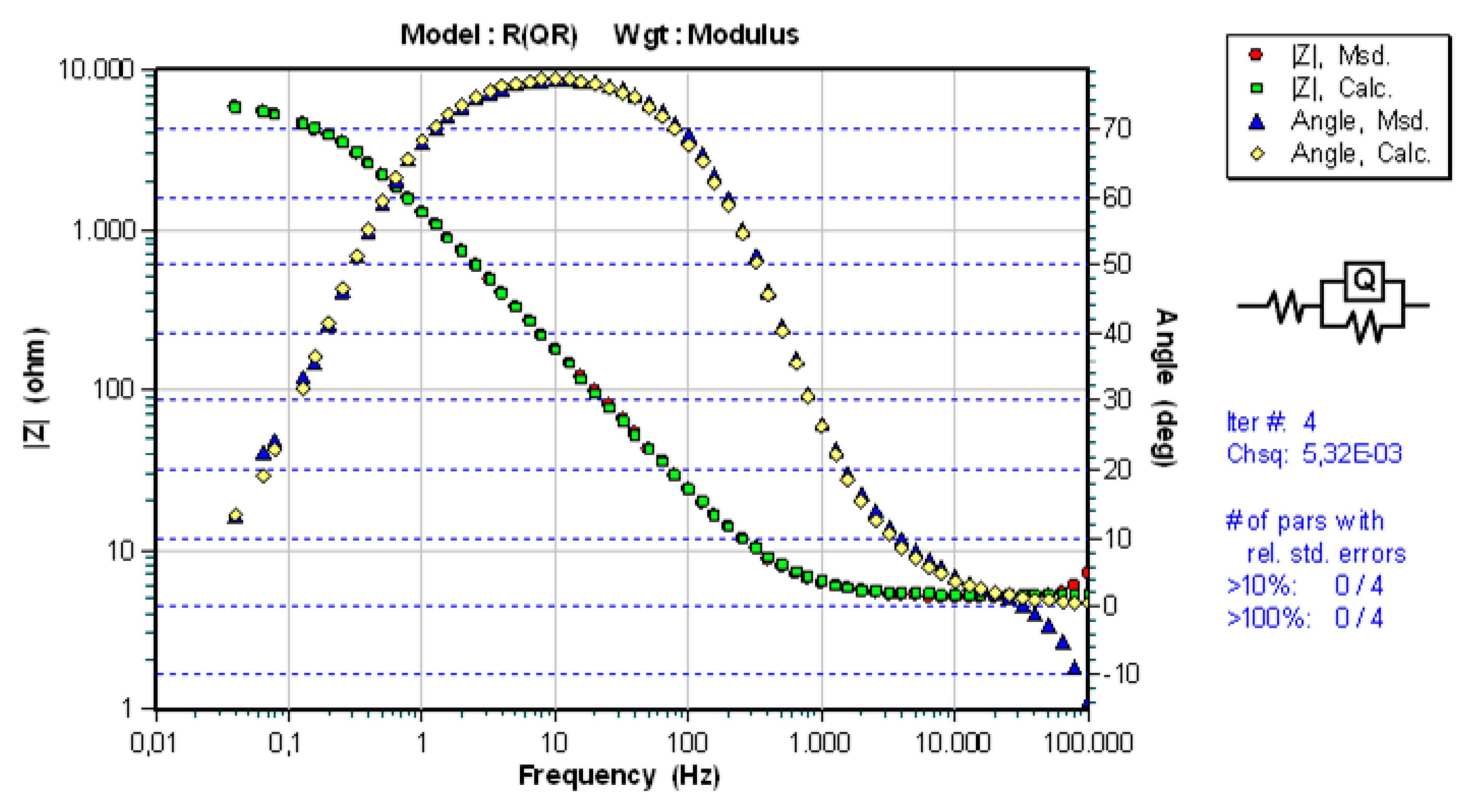
3.3. Results of Electrochemical Impedance Spectroscopy (EIS)
4. Discussion
5. Conclusions
- i.
- The samples of Ni and Co-bonded near-nanostructured and nanostructured cemented carbides have been successfully consolidated. A very fine homogeneous microstructure with relatively uniform grain-size distribution, and without microstructural defects in the form of carbide agglomerates and abnormal grain growth, was achieved for both Ni and Co-bonded samples. Achieved mechanical properties, Vickers hardness, and Palmqvist toughness of Ni-bonded near-nanostructured cemented carbides are slightly lower but still comparable with Co-bonded nanostructured cemented carbides. Reduction of the WC grain of the starting powder improved the mechanical properties of Ni-bonded cemented carbides.
- ii.
- Chemical composition of the binder significantly influenced the electrochemical corrosion resistance. Better corrosion resistance was observed for Ni-bonded samples compared to Co-bonded samples. Higher values of polarisation resistance Rp and lower values of corrosion current densities icorr were measured for WC-Ni samples. The corrosion rate of Ni-bonded cemented carbides is approximately four times lower compared to Co-bonded cemented carbides.
- iii.
- Chemical composition of the binder and microstructural characteristics might have a more significant influence than the weight content of the binder phase.
Author Contributions
Funding
Conflicts of Interest
References
- Mohammadpour, M.; Abachi, P.; Parvin, N.; Pourazrang, K. Study of cemented carbonitrides with nickel as binder: Experimental investigations and computer calculations. Int. J. Refract. Met. Hard Mater. 2012, 31, 164–170. [Google Scholar] [CrossRef]
- Tracy, V.A. Nickel in hardmetal. Int. J. Refract. Met. Hard Mater. 1992, 11, 137–149. [Google Scholar] [CrossRef]
- García, J.; Collado Ciprés, V.; Blomqvist, A.; Kaplan, B. Cemented carbide microstructures: A review. Int. J. Refract. Met. Hard Mater. 2018, 80, 40–68. [Google Scholar] [CrossRef]
- Aleksandrov Fabijanić, T.; Pötschke, J.; Mayer, M.; Škrinjarić, I.; Kurtela, M. Comparison of nickel and cobalt bonded nanoscaled hardmetals. In Proceedings of the EURO PM 2019, Maastricht, The Netherlands, 13–16 October 2019. [Google Scholar]
- Heinrichs, J.; Norgren, S.; Jacobson, S.; Yvell, K.; Olsson, M. Influence of cemented carbide binder type on wear initiation in rock drilling—Investigated in sliding wear against magnetite rock. Int. J. Refract. Met. Hard Mater. 2019, 85, 105035. [Google Scholar] [CrossRef]
- Nickel in Hardmetals. Available online: https://www.ipen.br/biblioteca/cd/ptech/2003/PDF/11_07.pdf (accessed on 14 January 2019).
- Mohammadpour, M.; Abachi, P.; Pourazarang, K. Effect of cobalt replacement by nickel on functionally graded cemented carbonitrides. Int. J. Refract. Met. Hard Mater. 2012, 30, 42–47. [Google Scholar] [CrossRef]
- Tomlinson, W.J.; Linzell, C.R. Anodic polarization and corrosion of cemented carbides with cobalt and nickel binders. J. Mater. Sci. 1988, 23, 914–918. [Google Scholar] [CrossRef]
- Liu, C. Alternative Binder Phases for WC Cemented Carbides. Master’s Thesis, KTH Royal Institute of Technology, Stockholm, Sweden, 29 May 2015. [Google Scholar]
- Castberg, T.S.; Johnsen, R.; Berget, J. Corrosion and subsequent erosion of hardmetals: Dependence of WC grain size and distribution, and binder composition. Mater. Corros. 2015, 66, 899–906. [Google Scholar] [CrossRef]
- Wittmann, B.; Schubert, W.D.; Lux, B. WC grain growth and grain growth inhibition in nickel and iron binder hardmetals. Int. J. Refract. Met. Hard Mater. 2002, 20, 51–60. [Google Scholar] [CrossRef]
- Rocha, A.M.F.; Bastos, A.C.; Cardoso, J.P.; Rodrigues, F.; Fernandes, C.M.; Soares, E.; Sacramento, J.; Senos, A.M.R.; Ferreira, M.G.S. Corrosion behaviour of WC hardmetals with nickel-based binders. Corros. Sci. 2019, 147, 384–393. [Google Scholar] [CrossRef]
- Richter, J.; Michalik, R. Corrosion resistance of WC–Co and WC–Ni type sintered carbides in acetic acid water solution. Mater. Corros. 2018, 70, 128–134. [Google Scholar] [CrossRef]
- Ekemar, S.; Lindholm, L.; Hartzell, T. Nickel as binder in WC-based cemented carbides. Int. J. Refract. Met. Hard Mater. 1982, 1, 37–40. [Google Scholar]
- Andrews, N.; Giourntas, L.; Galloway, A.M.; Pearson, A. Erosion–corrosion behaviour of zirconia, WC–6Co, WC–6Ni and UNS S31600. Int. J. Refract. Met. Hard Mater. 2015, 48, 229–237. [Google Scholar] [CrossRef]
- Richter, V.; Poetschke, J.; Holke, R.; Michaelis, A. Nanoscaled Hardmetals—Friction or Reality? In Proceedings of the 18th International Plansee Seminar 2013, Reutte, Austria, 3–7 June 2003; pp. 795–804. [Google Scholar]
- Aleksandrov Fabijanić, T.; Alar, Ž.; Pötschke, J. Potentials of nanostructured WC-Co hardmetal as reference material for Vickers hardness. Int. J. Refract. Met. Hard Mater. 2015, 50, 126–132. [Google Scholar] [CrossRef]
- Eisen, W.B.; Ferguson, B.L.; German, R.M.; Iacocca, R.; Lee, P.W.; Madan, D.; Moyer, K.; Sanderow, H.; Trudel, Y. (Eds.) Powder Metal Technologies and Applications, 2nd ed.; ASM International: Cleveland, OH, USA, 1998; Volume 7. [Google Scholar]
- ISO 4499-1:2008. Hardmetals—Metallographic Determination of Microstructure—Part 1: Photomicrographs and Description; International Organization for Standardization: Geneva, Switzerland, 2008. [Google Scholar]
- ISO 17475:2005. Corrosion of Metals and Alloys—Electrochemical Test Methods—Guidelines for Conducting Potentiostatic and Potentiodynamic Polarization Measurements; International Organization for Standardization: Geneva, Switzerland, 2005. [Google Scholar]
- Kelly, R.G.; Scully, J.R.; Shoesmith, D.; Buchheit, R.G. Electrochemical Techniques in Corrosion Science and Engineering; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Application Note CORR-4. Electrochemistry and Corrosion: Overview and Technigues; Princeton Applied Research: Oak Ridge, TN, USA.
- Alar, Ž.; Alar, V.; Aleksandrov Fabijanić, T. Electrochemical corrosion behavior of near-nano and nanostructured WC-Co cemented carbides. Metals 2017, 7, 69. [Google Scholar] [CrossRef]
- Aleksandrov Fabijanić, T.; Pötschke, J.; Alar, V.; Alar, Ž. Influence of C content on electrochemical corrosion resistance of nanostructured hardmetals. In Proceedings of the EURO PM 2017, Milano, Italy, 1–5 October 2017. [Google Scholar]



| Mixture | Sample | Starting Powders | Grain Size dBET, nm | Specific Surface, m2/g | Content, wt.% |
|---|---|---|---|---|---|
| WC-11Ni | WC-11Ni-a WC-11Ni-b | WC DN 2.5 (H. C. Starck) | 150 | 2.6 | remaining |
| Ni 2800B (Eurotungsten) | 720 | 0.9 | 11.0 | ||
| Cr3C2 160 (H. C. Starck) | 450 | 2.0 | 0.935 | ||
| VC 160 (H. C. Starck) | 350 | 3.0 | 0.605 | ||
| WC-11Co | WC-11Co-c WC-11Co-d | WC DN 2.5 (H. C. Starck) | 150 | 2.6 | remaining |
| HMP Co (Umicore) | 240 | 2.8 | 11 | ||
| Cr3C2 160 (H. C. Starck) | 450 | 2.0 | 0.935 | ||
| VC 160 (H. C. Starck) | 350 | 3.0 | 0.605 |
| Sample | Ts [°C] | Ecorr vs. SCE [mV] | Rp [kΩcm2] | βa [mV/dec] | βc [mV/dec] | icorr [μA/cm2] | vcorr [mm/y] |
|---|---|---|---|---|---|---|---|
| WC-11Ni-a | 20 ± 2 | −206 | 7.623 | 88.86 | 88.86 | 0.467 | 0.0127 |
| WC-11Ni-b | 20 ± 2 | −208 | 7.603 | 80.13 | 212.75 | 2.743 | 0.0238 |
| WC-11Co-c | 20 ± 2 | −234 | 5.820 | 80.39 | 305.63 | 5.991 | 0.0540 |
| WC-11Co-d | 20 ± 2 | −284 | 4.954 | 82.80 | 290.08 | 6.149 | 0.0554 |
| Sample | Ts [°C] | Rs [Ωcm2] | Q | n1 | Rct [Ωcm2] |
|---|---|---|---|---|---|
| WC-11Ni-a | 20 ± 2 | 5.201 | 1.047·× 10−4 | 0.935 | 8.888·× 103 |
| WC-11Co-c | 20 ± 2 | 5.188 | 1.402·× 10−4 | 0.896 | 6.134·× 103 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleksandrov Fabijanić, T.; Kurtela, M.; Škrinjarić, I.; Pötschke, J.; Mayer, M. Electrochemical Corrosion Resistance of Ni and Co Bonded Near-Nano and Nanostructured Cemented Carbides. Metals 2020, 10, 224. https://doi.org/10.3390/met10020224
Aleksandrov Fabijanić T, Kurtela M, Škrinjarić I, Pötschke J, Mayer M. Electrochemical Corrosion Resistance of Ni and Co Bonded Near-Nano and Nanostructured Cemented Carbides. Metals. 2020; 10(2):224. https://doi.org/10.3390/met10020224
Chicago/Turabian StyleAleksandrov Fabijanić, Tamara, Marin Kurtela, Irbas Škrinjarić, Johannes Pötschke, and Markus Mayer. 2020. "Electrochemical Corrosion Resistance of Ni and Co Bonded Near-Nano and Nanostructured Cemented Carbides" Metals 10, no. 2: 224. https://doi.org/10.3390/met10020224
APA StyleAleksandrov Fabijanić, T., Kurtela, M., Škrinjarić, I., Pötschke, J., & Mayer, M. (2020). Electrochemical Corrosion Resistance of Ni and Co Bonded Near-Nano and Nanostructured Cemented Carbides. Metals, 10(2), 224. https://doi.org/10.3390/met10020224






