First-Principles Calculation for the Influence of C and O on the Mechanical Properties of γ-TiAl Alloy at High Temperature
Abstract
:1. Introduction
2. Computational Details
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kumpfert, J.; Kim, Y.W.; Dimiduk, D.M. Effect of microstructure on fatigue and tensile properties of the gamma TiAl alloy Ti-46.5Al-3.0Nb-2.1Cr-0.2W. Mater. Sci. Eng. A 1995, 192–193, 465–473. [Google Scholar] [CrossRef]
- Mishin, Y.; Herzig, C. Diffusion in the Ti–Al System. Acta Mater. 2000, 48, 589–623. [Google Scholar] [CrossRef]
- Wallgram, W.; Schmölzer, T.; Cha, L.; Das, G.; Güther, V.; Clemens, H. Technology and mechanical properties of advanced γ-TiAl based alloys. Int. J. Mater. Res. 2009, 100, 1021–1030. [Google Scholar] [CrossRef]
- Appel, F.; Clemens, H.; Oehring, M. Recent Advances in Development and Processing of Titanium Aluminide Alloys. Mat. Res. Soc. Symp. Proc. 2001, 646. [Google Scholar] [CrossRef]
- Chen, G.; Peng, Y.; Zheng, G.; Qi, Z.; Wang, M.; Yu, H.; Dong, C.; Liu, C.L. Polysynthetic twinned TiAl single crystals for high-temperature applications. Nat. Mater. 2016, 15, 876. [Google Scholar] [CrossRef] [PubMed]
- Duwez, P.; Taylor, J.L. Crystal Structure of TiAl. JOM 1952, 4, 70–71. [Google Scholar] [CrossRef]
- Hsieh, K.-C.; Austinchang, Y.; Freeman, A.J.; Oguchi, T.; Xu, J.-H. Thermodynamic and structural parameters of the body-center tetragonal TiAl phase. Scr. Metall. 1988, 22, 1267–1272. [Google Scholar] [CrossRef]
- Erdely, P.; Stark, A.; Clemens, H.; Mayer, S. In-situ High-energy X-ray Diffraction on an Intermetallic β-stabilised γ-TiAl Based Alloy. BHM 2015, 160, 221–225. [Google Scholar] [CrossRef]
- Huang, Z.W.; Lin, J.P.; Sun, H.L. Microstructural changes and mechanical behaviour of a near lamellar γ-TiAl alloy during long-term exposure at 700 °C. Intermetallics 2017, 85, 59–68. [Google Scholar] [CrossRef]
- Tanaka, K.; Koiwa, M. Single-crystal elastic constants of intermetallic compounds. Intermetallics 1996, 4, S29–S39. [Google Scholar] [CrossRef]
- Tanaka, K.; Ichitsubo, T.; Inui, H.; Yamaguchi, M.; Koiwa, M. Single-crystal elastic constants of γ-TiAl. Philos. Mag. Lett. 1996, 73, 71–78. [Google Scholar] [CrossRef]
- He, Y.; Schwarz, R.B.; Darling, T.; Hundley, M.; Whang, S.H.; Wang, Z.M. Elastic constants and thermal expansion of single crystal γ-TiAl from 300 to 750 K. Mater. Sci. Eng. A 1997, 239–240, 157–163. [Google Scholar] [CrossRef]
- Zou, J.; Fu, C.L.; Yoo, M.H. Phase stability of intermetallics in the Al-Ti system: A first-principles total-energy investigation. Intermetallics 1995, 3, 265–269. [Google Scholar] [CrossRef]
- Tang, P.-Y.; Huang, G.-H.; Xie, Q.-L.; Li, J.-Y. Ideal shear strength and deformation behaviours of L10 TiAl from first-principles calculations. Bull. Mater. Sci. 2016, 39, 1411–1418. [Google Scholar] [CrossRef]
- Liu, Y.L.; Liu, L.M.; Wang, S.Q.; Ye, H.Q. First-principles study of shear deformation in TiAl and Ti3Al. Intermetallics 2007, 15, 428–435. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, K.; Zhang, J.; Hu, Z.; Lu, G.; Kioussis, N. Electronic effects of oxygen and vanadium impurities in TiAl. J. Phys. Condens. Matter 1997, 9, 9829–9843. [Google Scholar] [CrossRef]
- Zhou, H.-B.; Wei, Y.; Liu, Y.-L.; Zhang, Y.; Lu, G.-H. First-principles investigation of site preference and bonding properties of alloying element in TiAl with O impurity. Modell. Simul. Mater. Sci. Eng. 2010, 18, 015007. [Google Scholar] [CrossRef]
- Fu, H.; Zhao, Z.; Liu, W.; Peng, F.; Gao, T.; Cheng, X. Ab initio calculations of elastic constants and thermodynamic properties of γTiAl under high pressures. Intermetallics 2010, 18, 761–766. [Google Scholar] [CrossRef]
- Zhang, C.; Hou, H.; Zhao, Y.; Yang, X.; Guo, Y. First-principles study on structural, elastic and thermal properties of γ-TiAl and α2-Ti3Al phases in TiAl-based alloy under high pressure. Int. J. Mod. Phys. B 2017, 31, 1750079. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Peng, Y.P.; Mao, Y.L.; Pang, C.J.; Lu, L.Y. Effect of hot-dip aluminizing on the oxidation resistance of Ti-6Al-4V alloy at high temperatures. Corros. Sci. 2012, 55, 187–193. [Google Scholar] [CrossRef]
- Cheng, J.; Li, F.; Zhu, S.; Yu, Y.; Qiao, Z.; Yang, J. Electrochemical corrosion and tribological evaluation of TiAl alloy for marine application. Tribol. Int. 2017, 115, 483–492. [Google Scholar] [CrossRef]
- Du, H.L.; Datta, P.K.; Burnell-gray, J.S.; Lewis, D.B. Effect of Nb coating on the sulphidation/oxidation behaviour of Ti and Ti-6Al-4V alloy. J. Mater. Sci. 1995, 30, 2640–2647. [Google Scholar] [CrossRef]
- Pedersen, A.; Hermansson, M. Inhibition of Metal Corrosion by Bacteria. Biofouling 1991, 3, 1–11. [Google Scholar] [CrossRef]
- Lei, F.; Liu, L.; Yu, Z.; Gao, M.; Li, Y.; Wang, F. Corrosion Behavior of Ti60 Alloy under a Solid NaCl Deposit in a Wet Oxygen Flow at 600 °C. Sci. Rep. 2016, 6, 29019. [Google Scholar]
- Wu, X.; Hu, D.; Loretto, M.H. Alloy and process development of TiAl. J. Mater. Sci. 2004, 39, 3935–3940. [Google Scholar] [CrossRef]
- Zhang, W.; Gao, L.; Li, J.; Yang, B.; Yin, Y. TiAl/B4C marine material-Fabrication, mechanical and corrosion properties. Ceram. Int. 2011, 37, 783–789. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.-K.; Chen, L.-Q. Thermodynamic properties of Al, Ni, NiAl, and Ni3Al from first-principles calculations. Acta Mater. 2004, 52, 2665–2671. [Google Scholar] [CrossRef]
- Guo, Z.-C.; Luo, F.; Zhang, X.-L.; Yuan, C.-Y.; Lin, C.-A.; Cai, L.-C. First-principles calculations of elastic, phonon and thermodynamic properties of W. Mol. Phys. 2016, 114, 3430–3436. [Google Scholar] [CrossRef]
- Fast, F.; Wills, J.M.; Johansson, B.; Eriksson, O. Elastic constants of hexagonal transition metals: Theory. Phys. Rev. B 1995, 51, 17431–17438. [Google Scholar] [CrossRef]
- Voigt, W. Lehrburch der Kristallphys; Vieweg + Teubner Verlag: Leipzig, Germany, 1928. [Google Scholar]
- Wang, J.-H.; Lu, Y.; Zhang, X.-L.; Shao, X.-H. The elastic behaviors and theoretical tensile strength of γ-TiAl alloy from the first principles calculations. Intermetallics 2018, 101, 1–7. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Hammer, B.; Hansen, L.B.; Nørskov, J.K. Improved adsorption energetics within density-functional theory using revised Perdew-Burke-Ernzerhof functionals. Phys. Rev. B 1999, 59, 7413–7421. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Togo, A.; Oba, F.; Tanaka, I. First-principles calculations of the ferroelastic transition between rutile-type and CaCl2-type SiO2 at high pressures. Phys. Rev. B 2008, 78, 134106. [Google Scholar] [CrossRef]
- Zhao, J.; Winey, J.M.; Gupta, Y.M. First-principles calculations of second-and third-order elastic constants for single crystals of arbitrary symmetry. Phys. Rev. B 2007, 75, 094105. [Google Scholar] [CrossRef]
- Shao, T.; Wen, B.; Melnik, R.; Yao, S.; Kawazoe, Y.; Tian, Y. Temperature dependent elastic constants for crystals with arbitrary symmetry: Combined first principles and continuum elasticity theory. J. Appl. Phys. 2012, 111, 083525. [Google Scholar] [CrossRef]
- Mouhat, F.; Coudert, F.-X. Necessary and sufficient elastic stability conditions in various crystal systems. Phys. Rev. B 2014, 90, 224104. [Google Scholar] [CrossRef]
- Wu, Z.-J.; Zhao, E.-J.; Xiang, H.-P.; Hao, X.-F.; Liu, X.-J.; Meng, J. Crystal structures and elastic properties of superhard IrN2 and IrN3 from first principles. Phys. Rev. B 2007, 76, 054115. [Google Scholar] [CrossRef]
- Hill, R. The Elastic Behaviour of a Crystalline Aggregate. Proc. Phys. Soc. A 1952, 65, 349–354. [Google Scholar] [CrossRef]
- Reuss, A. Berechnung der Fließgrenze von Mischkristallen auf Grund der Plastizitätsbedingung für Einkristalle. Z. Angew. Math. Mech. 1929, 9, 49–58. (In German) [Google Scholar] [CrossRef]
- Hu, C.H.; Chen, D.M.; Wang, Y.M.; Yang, K. First-principles investigations of isotope effects in themodynamic properties of TiX2 (X = H, D, and T) system. J. Alloy. Comp. 2008, 450, 369–374. [Google Scholar] [CrossRef]
- Kelkar, T.; Kanhere, D.G.; Pal, S. First principles calculations of thermal equations of state and thermodynamical properties of MgH2 at finite temperatures. Comput. Mater. Sci. 2008, 42, 510–516. [Google Scholar] [CrossRef]
- Fang, H.; Liu, B.; Liu, X.; Huang, S.; Ni, C.; Li, Z.; Wang, R. High-pressure lattice dynamic and thermodynamic properties of Ir by first-principles calculation. Phys. B 2010, 405, 732–737. [Google Scholar] [CrossRef]
- Teter, D.M. Computational Alchemy: The Search for New Superhard Materials. MRS Bull 1998, 23, 22–27. [Google Scholar] [CrossRef]
- Peng, F.; Chen, D.; Yang, X. First-principles calculations on elasticity of OsN2 under pressure. Solid State Commun. 2009, 149, 2135–2138. [Google Scholar] [CrossRef]
- Brazhkin, V.V.; Lyapin, A.G.; Popova, S.V.; Antonov, Y.V.; Kluev, Y.A.; Naletov, A.M. Mechanical Properties of the Superhard Polymeric and Disordered Phases Prepared From C60, C70, and C2N under High Pressure. Rev. High Pressure Sci. Technol. 1998, 7, 989–991. [Google Scholar] [CrossRef]
- Pugh, S.F. XCII. Relations between the elastic moduli and the plastic properties of polycrystalline pure metals. Philos. Mag. 1954, 45, 823–843. [Google Scholar] [CrossRef]
- Morinaga, M.; Saito, J.; Yukawa, N.; Adachi, H. Electronic effect on the ductility of alloyed TiAl compound. Acta Metall. Mater. 1990, 38, 25–29. [Google Scholar] [CrossRef]
- Song, Y.; Xu, D.S.; Yang, R.; Li, D.; Hu, Z.Q. Theoretical investigation of ductilizing effects of alloying elements on TiAl. Intermetallics 1998, 6, 157–165. [Google Scholar] [CrossRef]
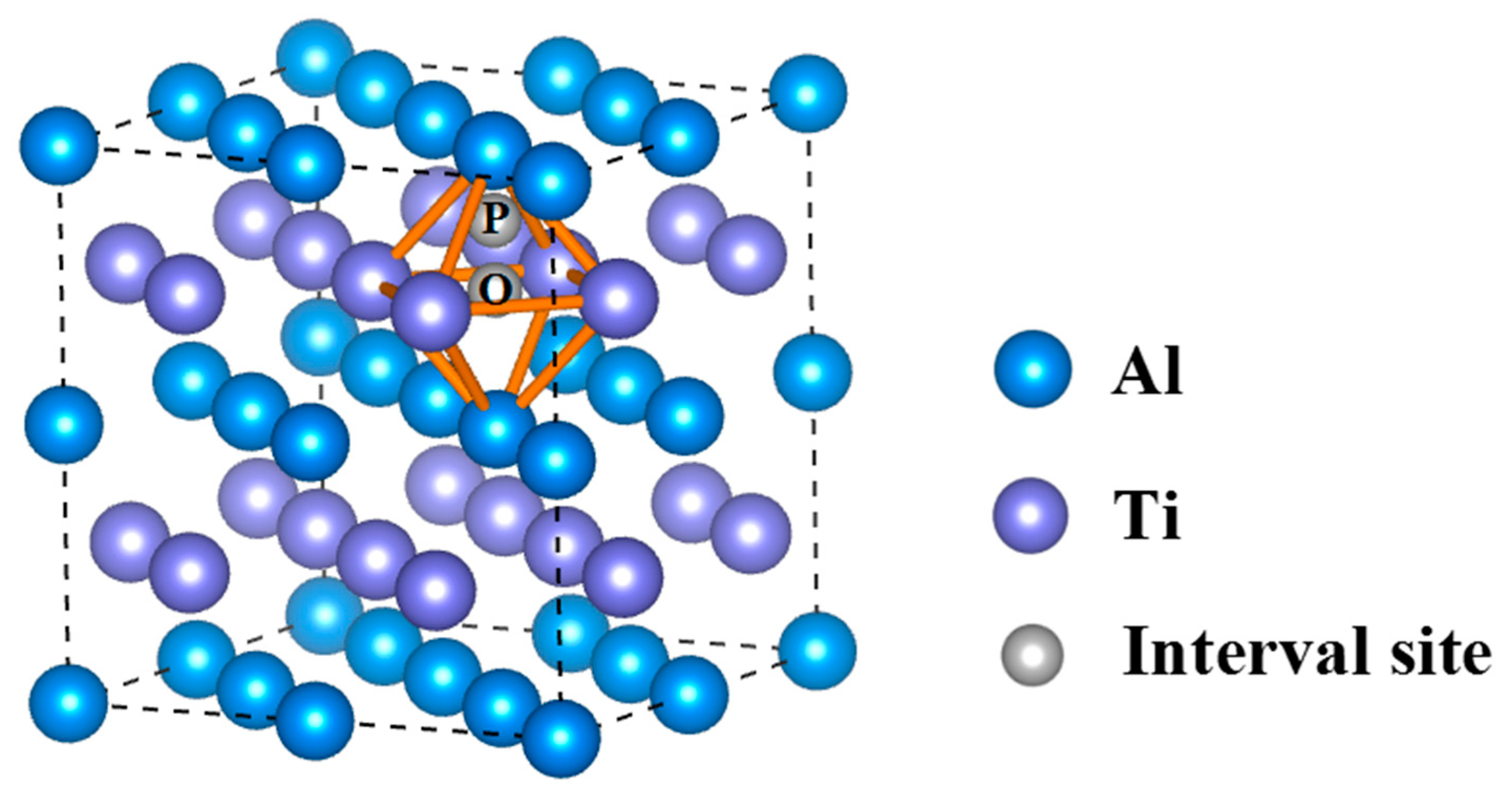
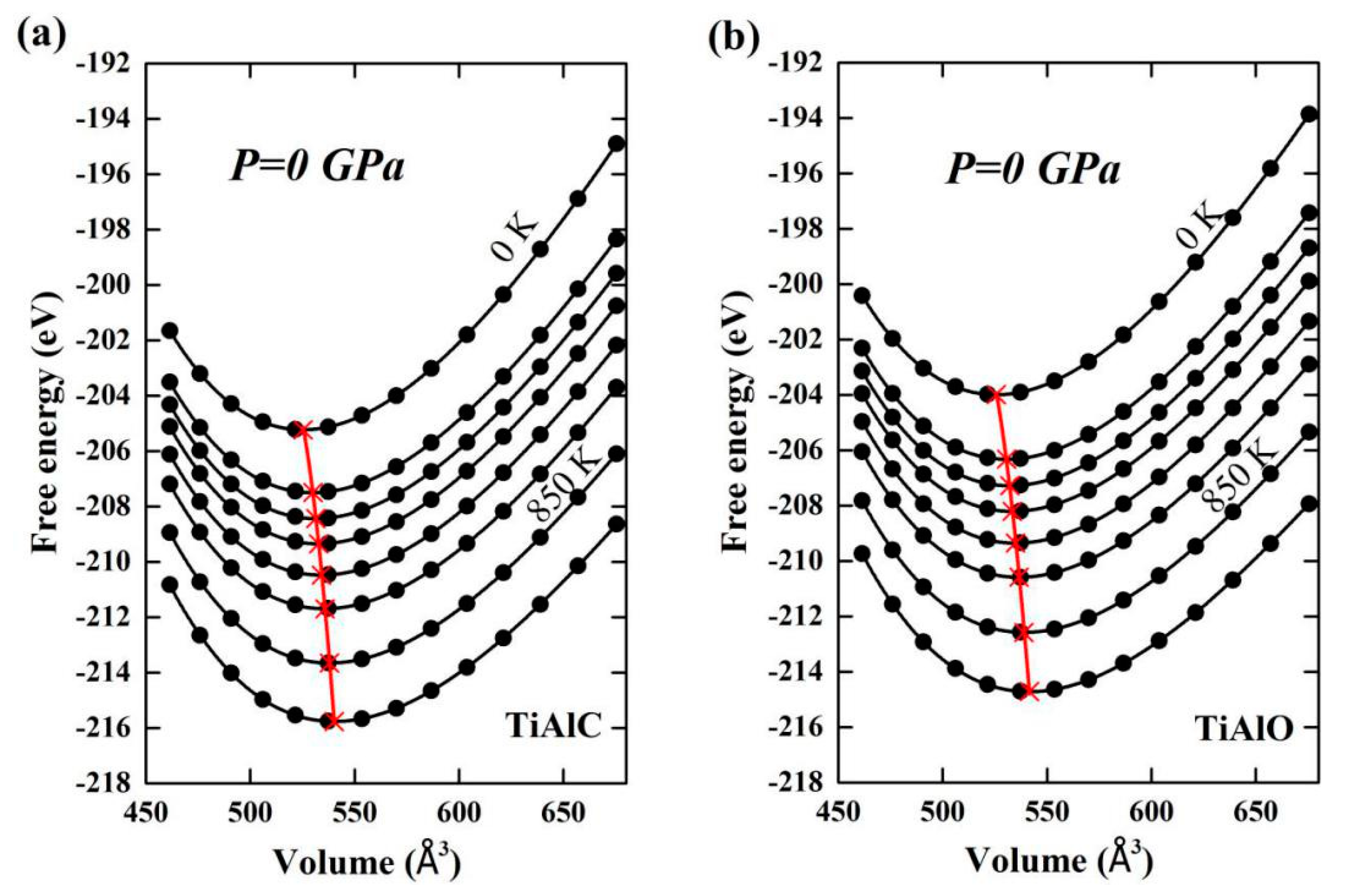
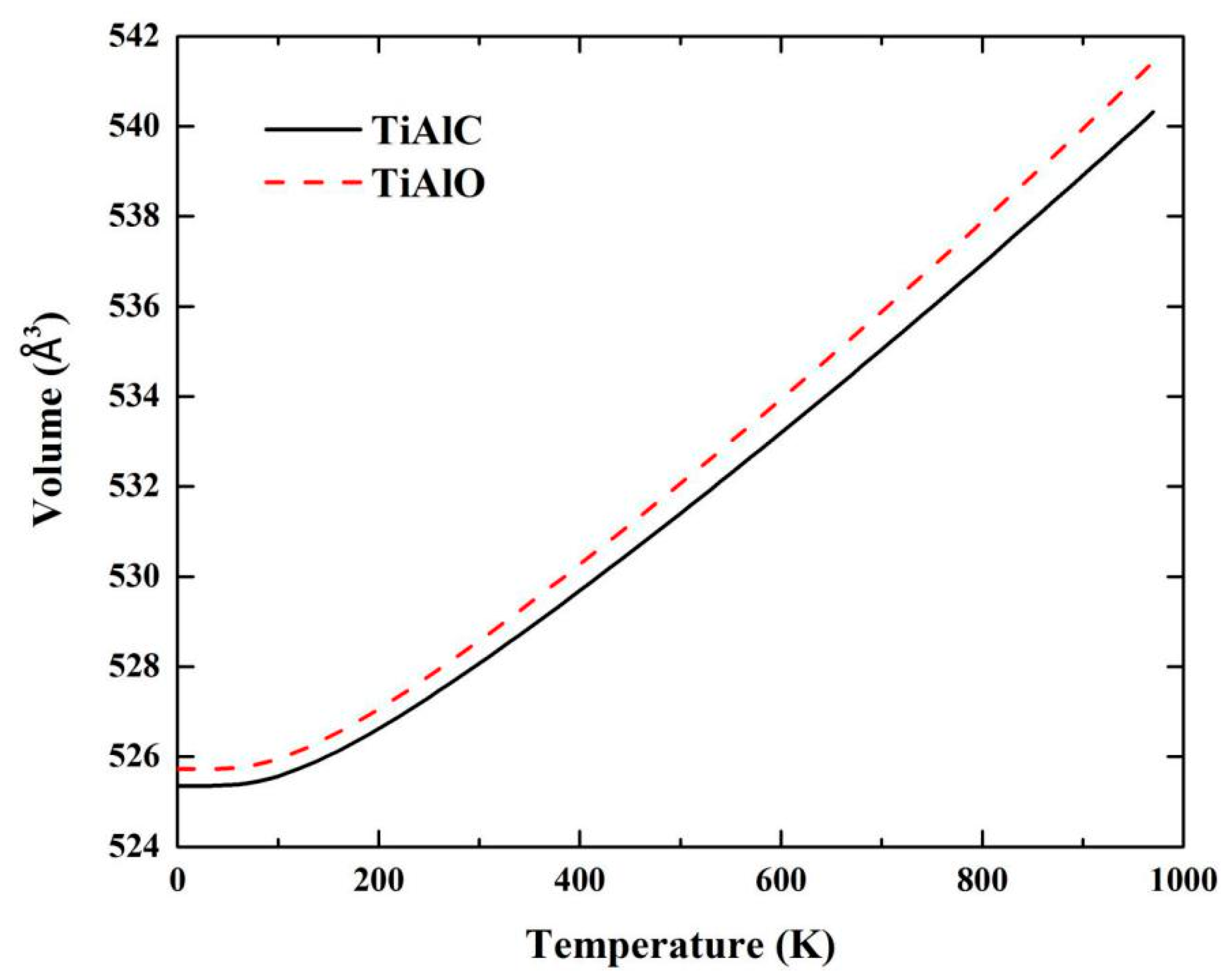
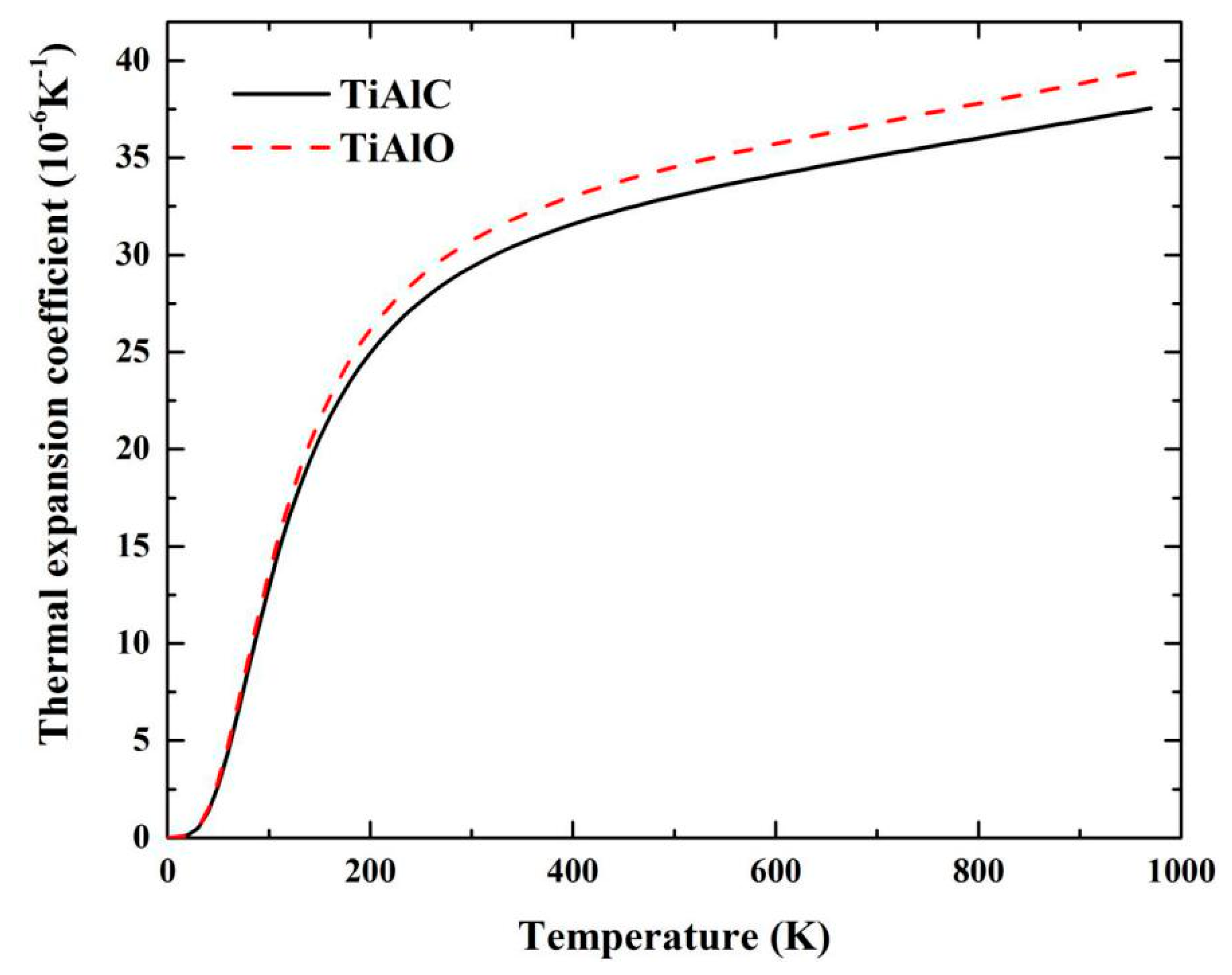
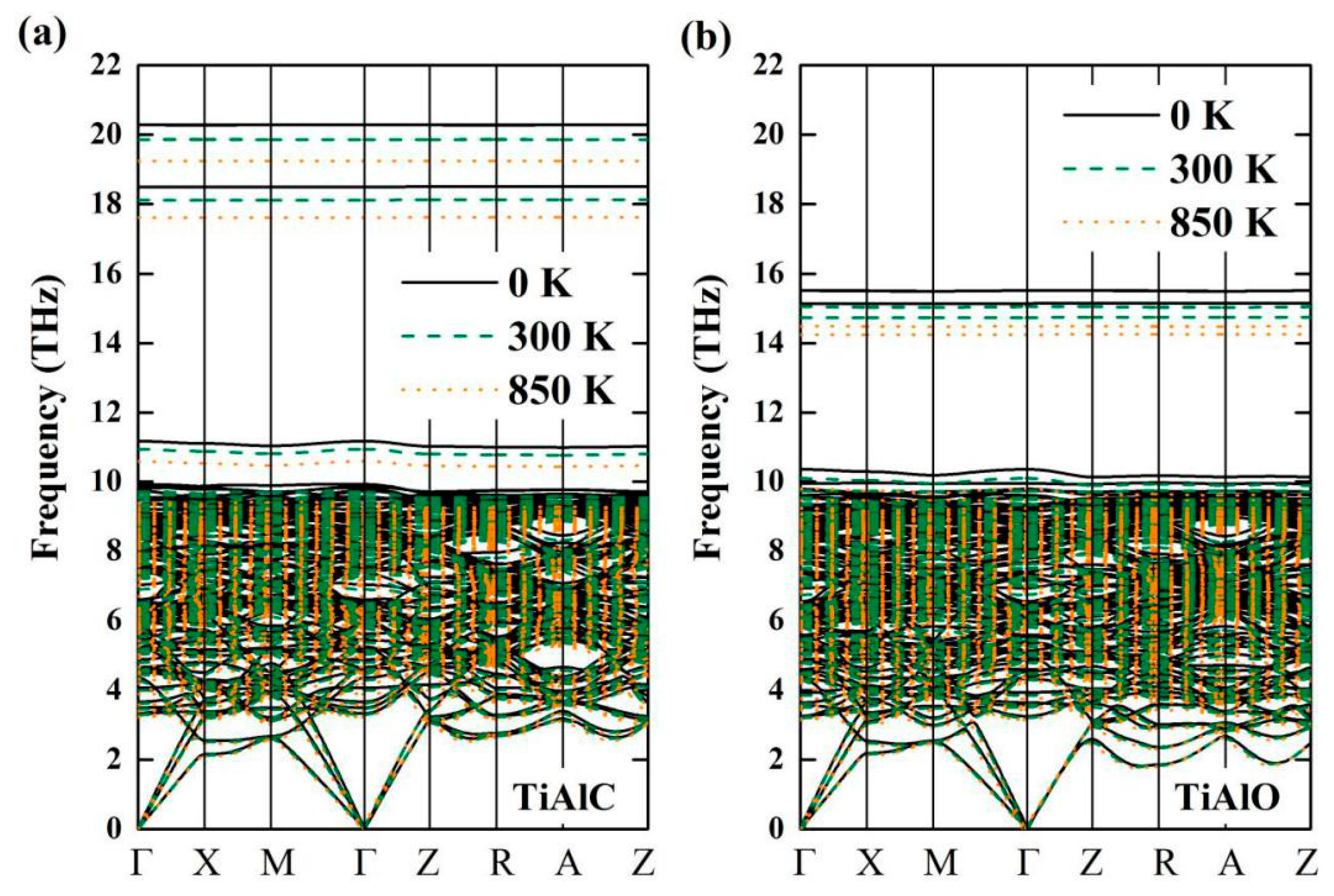
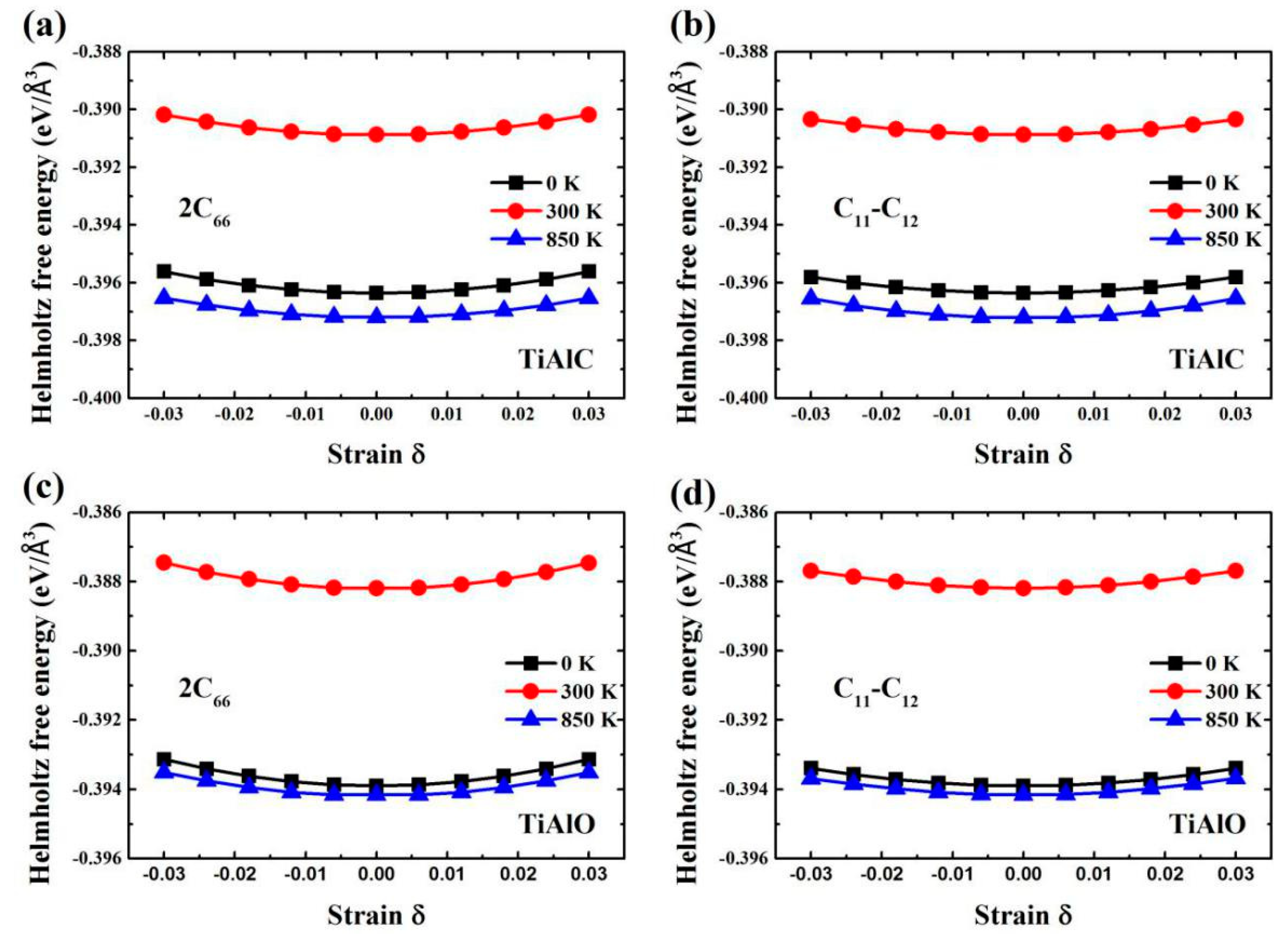
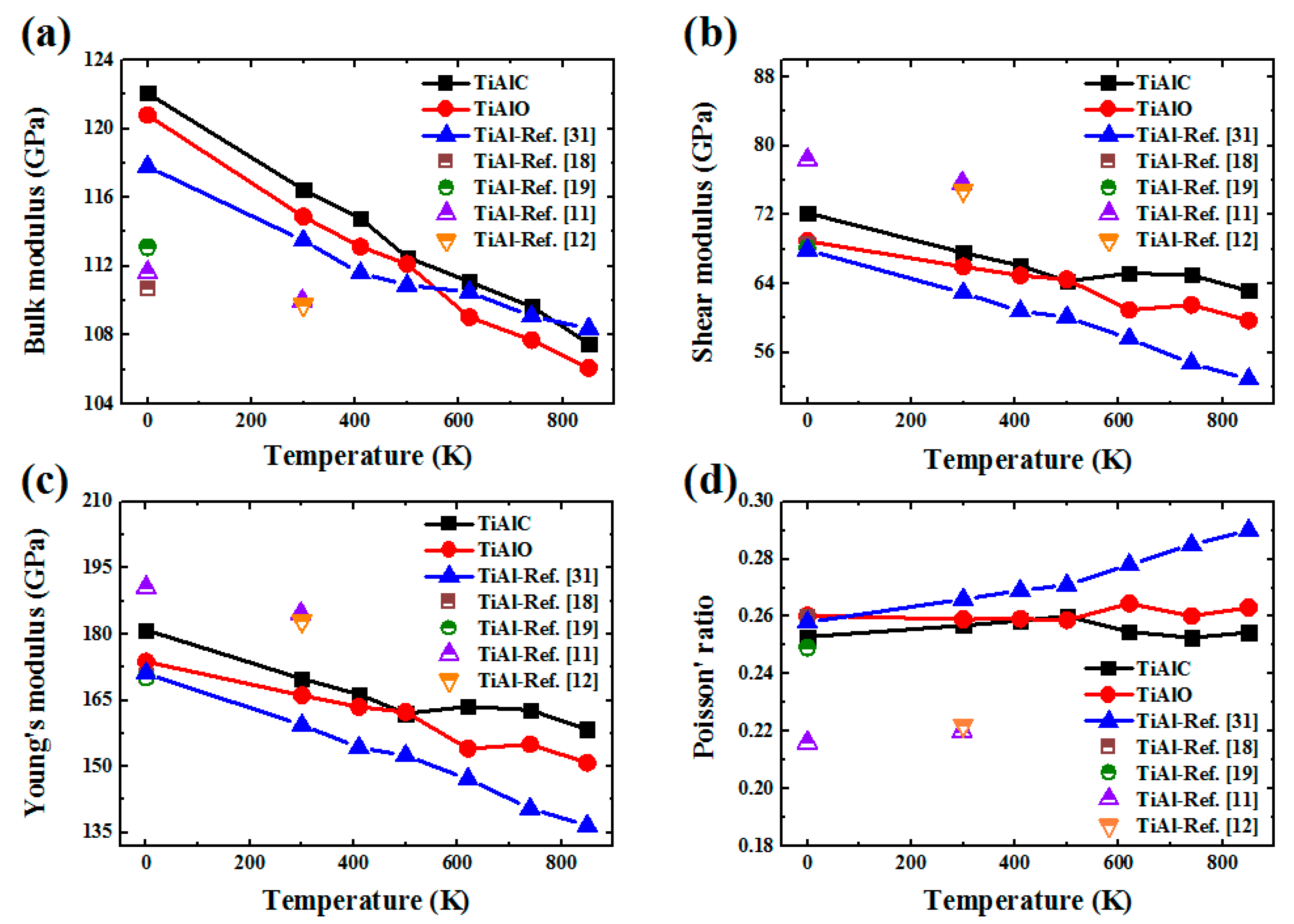
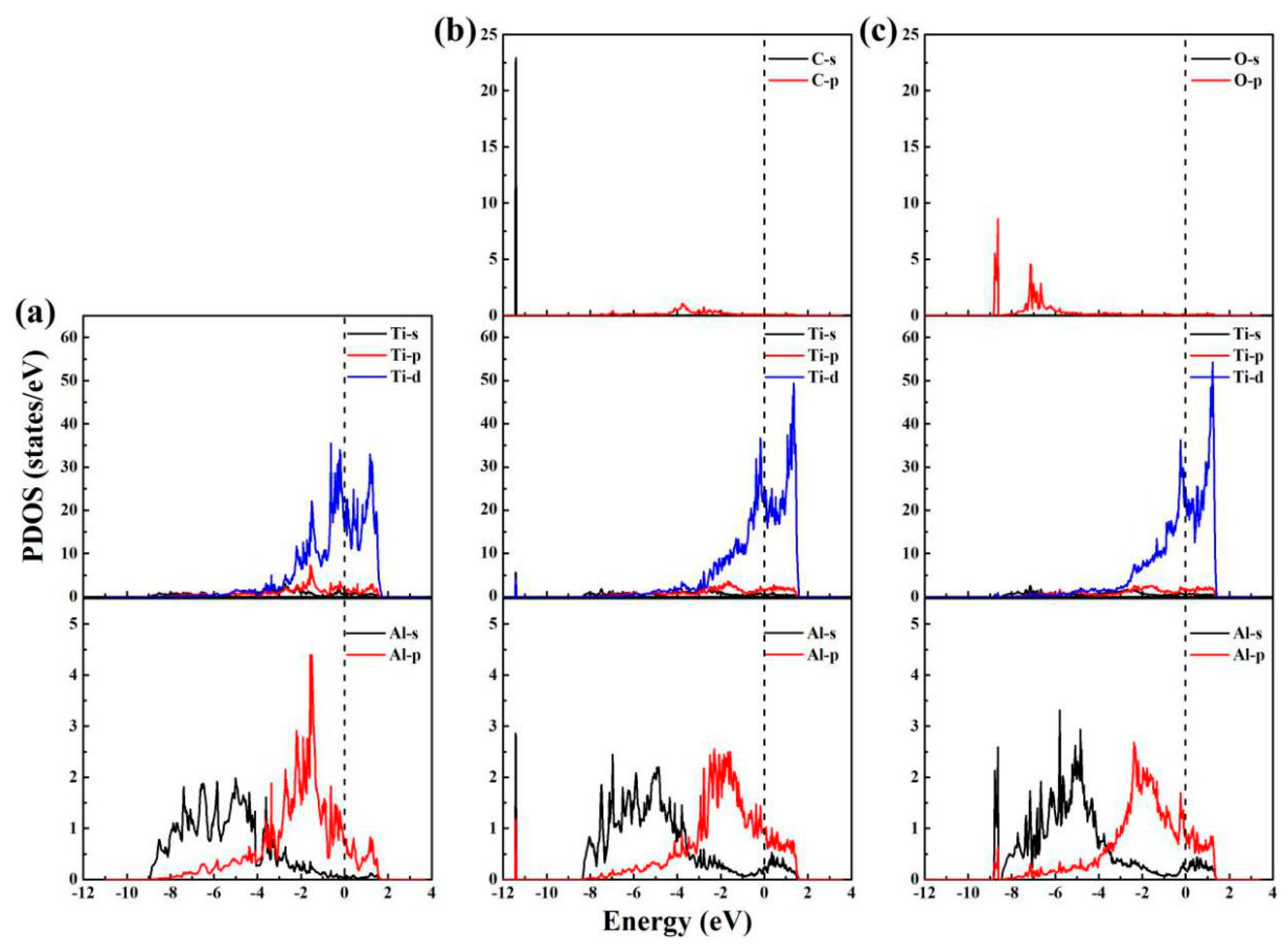
| Phase | Temperature (K) | C11 (GPa) | C12 (GPa) | C13 (GPa) | C33 (GPa) | C44 (GPa) | C66 (GPa) |
|---|---|---|---|---|---|---|---|
| TiAl | 0 a | 171.6 | 89.7 | 91.3 | 172.2 | 114.5 | 65.4 |
| 0 b | 164 | 85.5 | 81.04 | 178.57 | 109.6 | 72.6 | |
| 0 c | 167.4 | 87.7 | 86.1 | 164.2 | 111.1 | 73.7 | |
| 0 d | 187 | 74.8 | 74.8 | 182 | 109 | 81.2 | |
| 298 d | 183 | 74.1 | 74.4 | 178 | 105 | 78.4 | |
| 300 e | 186 | 72 | 74 | 176 | 101 | 77 | |
| 300 a | 161.2 | 88.2 | 91.3 | 157.8 | 109.6 | 65.7 | |
| 410 a | 157.7 | 87.2 | 91.4 | 149.3 | 108.4 | 65.3 | |
| 500 a | 158.8 | 81.0 | 93.2 | 145.4 | 107.2 | 65.1 | |
| 620 a | 152.9 | 80.8 | 95.9 | 144.1 | 104.7 | 68.9 | |
| 740 a | 151.1 | 79.0 | 96.1 | 137.0 | 103.3 | 63.0 | |
| 850 a | 148.0 | 82.4 | 95.4 | 133.3 | 101.7 | 62.5 | |
| TiAlC | 0 | 191.0 | 92.4 | 87.6 | 181.9 | 109.5 | 66.0 |
| 300 | 181.9 | 85.9 | 85.8 | 169.9 | 102.7 | 62.3 | |
| 410 | 178.8 | 84.2 | 85.4 | 166.1 | 100.7 | 61.2 | |
| 500 | 175.1 | 81.9 | 84.5 | 161.4 | 98.2 | 59.7 | |
| 620 | 178.1 | 78.9 | 83.1 | 155.3 | 98.3 | 62.2 | |
| 740 | 175.4 | 76.8 | 80.7 | 160.6 | 95.5 | 61.2 | |
| 850 | 171.9 | 74.8 | 79.7 | 156.1 | 93.0 | 59.2 | |
| TiAlO | 0 | 181.4 | 91.5 | 92.3 | 172.4 | 109.7 | 68.2 |
| 300 | 173.8 | 84.4 | 88.2 | 164.9 | 103.0 | 66.3 | |
| 410 | 171.2 | 82.5 | 87.1 | 162.8 | 101.1 | 65.3 | |
| 500 | 164.4 | 71.6 | 94.4 | 161.8 | 106.9 | 69.3 | |
| 620 | 162.5 | 78.2 | 86.0 | 156.0 | 96.6 | 60.4 | |
| 740 | 161.4 | 76.8 | 83.9 | 157.2 | 95.9 | 61.8 | |
| 850 | 158.9 | 74.4 | 84.3 | 150.9 | 94.3 | 60.7 |
| Phase | Temperature (K) | B (GPa) | G (GPa) | E (GPa) | ν | G/B |
|---|---|---|---|---|---|---|
| TiAl | 0 a | 117.8 | 67.9 | 171.1 | 0.258 | 0.576 |
| 0 b | 110.69 | 68.57 | 170.50 | 0.26 | 0.619 | |
| 0 c | 113.1 | 68.1 | 170.2 | 0.249 | 0.602 | |
| 0 d | 111.64 | 78.36 | 190.51 | 0.216 | 0.702 | |
| 298 d | 109.97 | 75.68 | 184.67 | 0.220 | 0.688 | |
| 300 e | 109.76 | 74.83 | 182.92 | 0.222 | 0.682 | |
| 300 a | 113.5 | 62.9 | 159.4 | 0.266 | 0.554 | |
| 410 a | 111.6 | 60.8 | 154.3 | 0.269 | 0.545 | |
| 500 a | 110.9 | 60.1 | 152.5 | 0.271 | 0.541 | |
| 620 a | 110.5 | 57.6 | 147.2 | 0.278 | 0.521 | |
| 740 a | 109.1 | 54.7 | 140.5 | 0.285 | 0.501 | |
| 850 a | 108.4 | 52.9 | 136.6 | 0.290 | 0.488 | |
| TiAlC | 0 | 122.0 | 72.2 | 180.9 | 0.253 | 0.592 |
| 300 | 116.5 | 67.6 | 169.9 | 0.257 | 0.580 | |
| 410 | 114.8 | 66.1 | 166.3 | 0.259 | 0.576 | |
| 500 | 112.5 | 64.3 | 162.0 | 0.260 | 0.571 | |
| 620 | 111.1 | 65.2 | 163.6 | 0.253 | 0.587 | |
| 740 | 109.7 | 65.0 | 162.8 | 0.253 | 0.593 | |
| 850 | 107.5 | 63.1 | 158.4 | 0.254 | 0.587 | |
| TiAlO | 0 | 120.8 | 68.9 | 173.7 | 0.260 | 0.571 |
| 300 | 114.9 | 66.0 | 166.1 | 0.259 | 0.574 | |
| 410 | 113.1 | 64.9 | 163.5 | 0.259 | 0.574 | |
| 500 | 112.1 | 64.5 | 162.3 | 0.259 | 0.575 | |
| 620 | 109.0 | 60.9 | 154.1 | 0.265 | 0.559 | |
| 740 | 107.7 | 61.5 | 155.0 | 0.260 | 0.571 | |
| 850 | 106.1 | 59.7 | 150.8 | 0.263 | 0.563 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Lu, Y.; Shao, X. First-Principles Calculation for the Influence of C and O on the Mechanical Properties of γ-TiAl Alloy at High Temperature. Metals 2019, 9, 262. https://doi.org/10.3390/met9020262
Wang J, Lu Y, Shao X. First-Principles Calculation for the Influence of C and O on the Mechanical Properties of γ-TiAl Alloy at High Temperature. Metals. 2019; 9(2):262. https://doi.org/10.3390/met9020262
Chicago/Turabian StyleWang, Jiahua, Yong Lu, and Xiaohong Shao. 2019. "First-Principles Calculation for the Influence of C and O on the Mechanical Properties of γ-TiAl Alloy at High Temperature" Metals 9, no. 2: 262. https://doi.org/10.3390/met9020262
APA StyleWang, J., Lu, Y., & Shao, X. (2019). First-Principles Calculation for the Influence of C and O on the Mechanical Properties of γ-TiAl Alloy at High Temperature. Metals, 9(2), 262. https://doi.org/10.3390/met9020262




