Evaluation of the Possibility of Using Non-Conventional Technological Approaches for the Heat Treatment of Hot-Rolled DP Steel
Abstract
1. Introduction
2. Materials and Methods
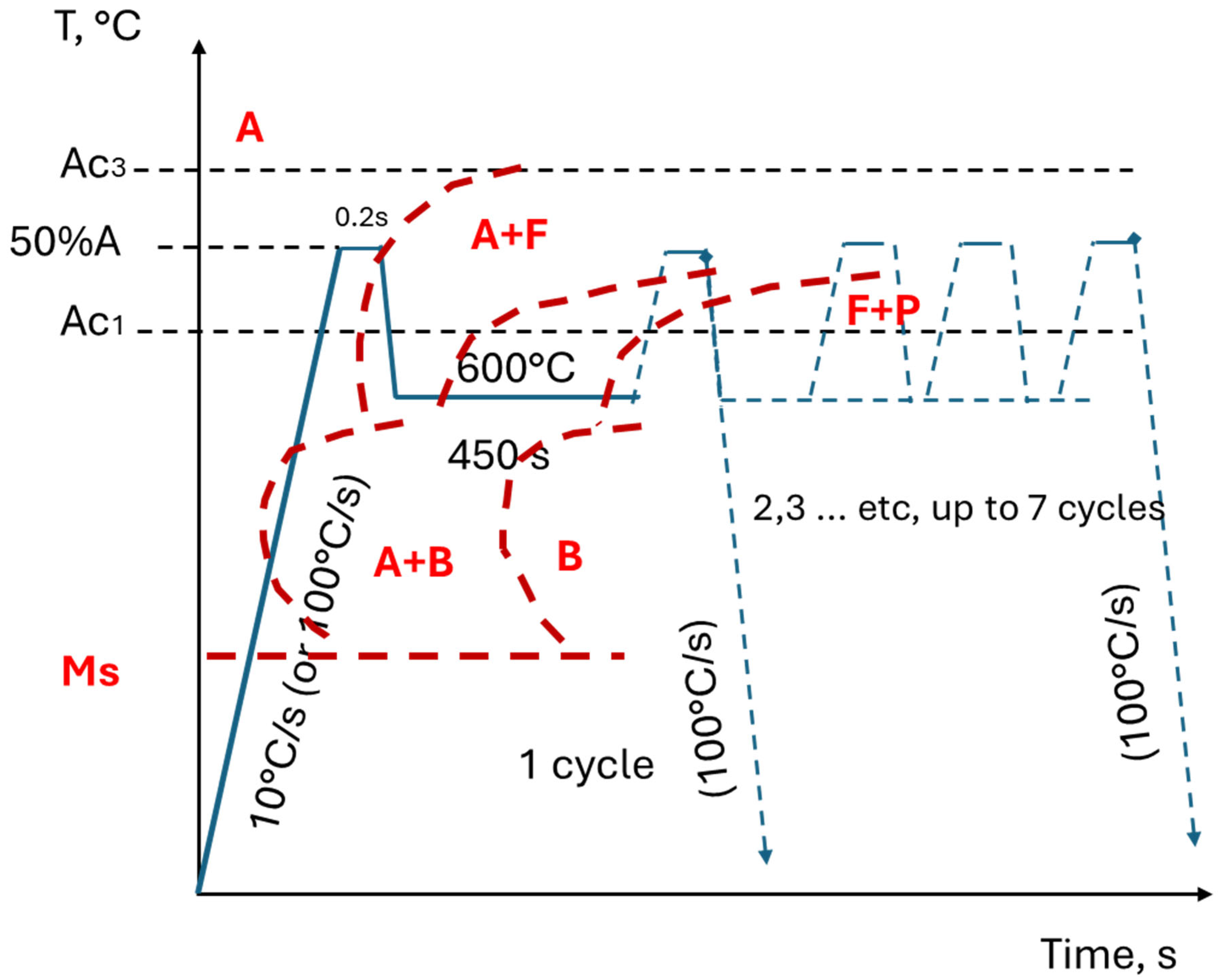
2.1. Material and Heat Treatment
2.2. Sample Preparation and Microstructure Characterization
2.3. Mechanical Properties
3. Results and Discussion
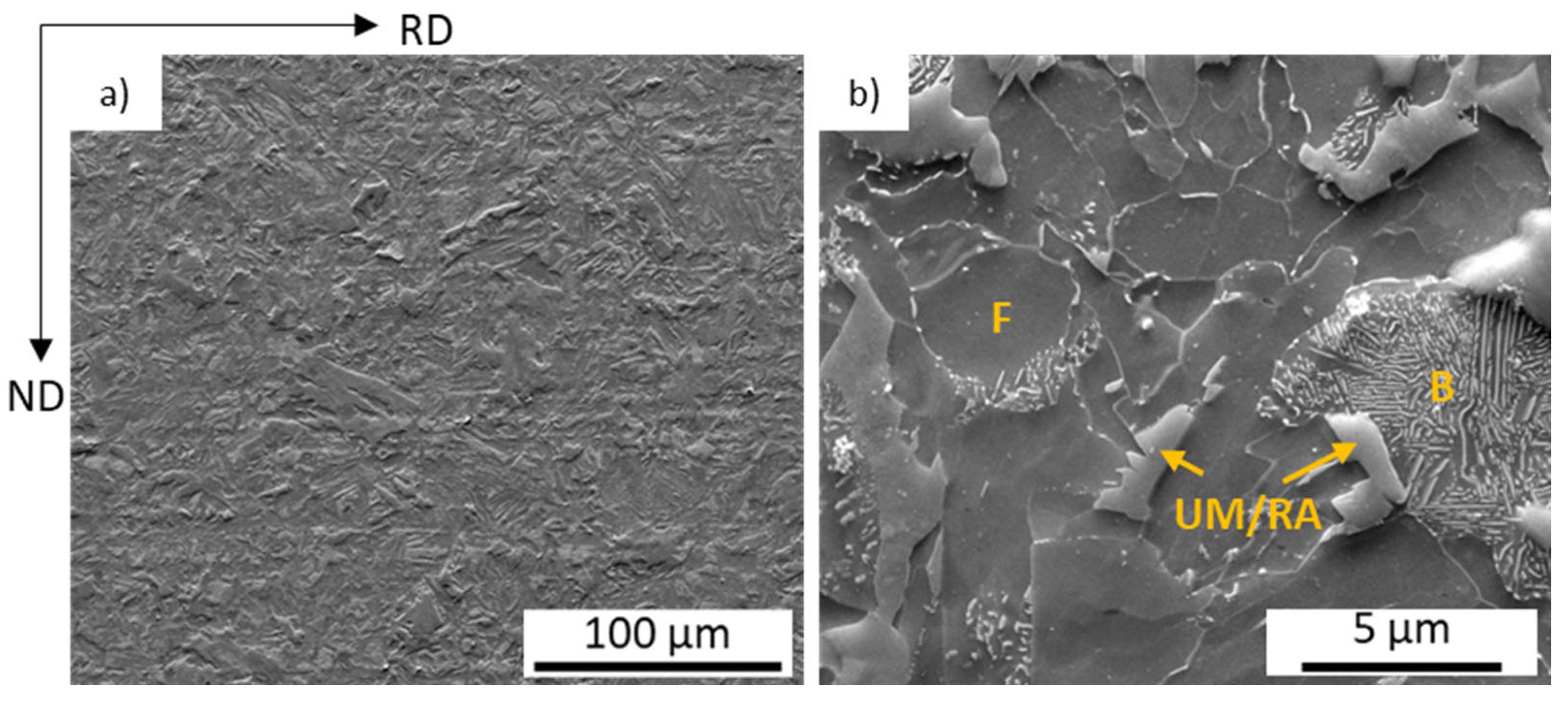
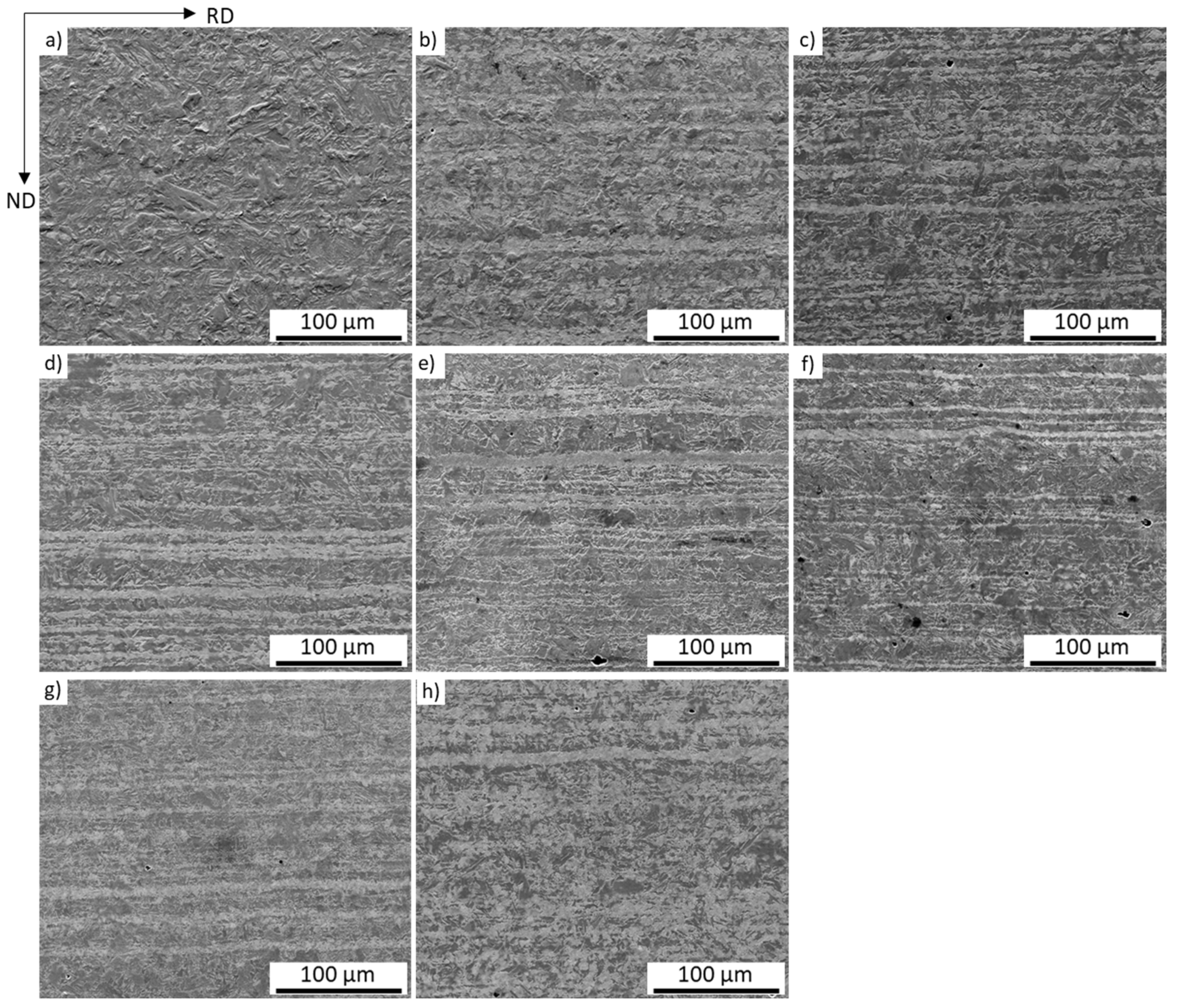
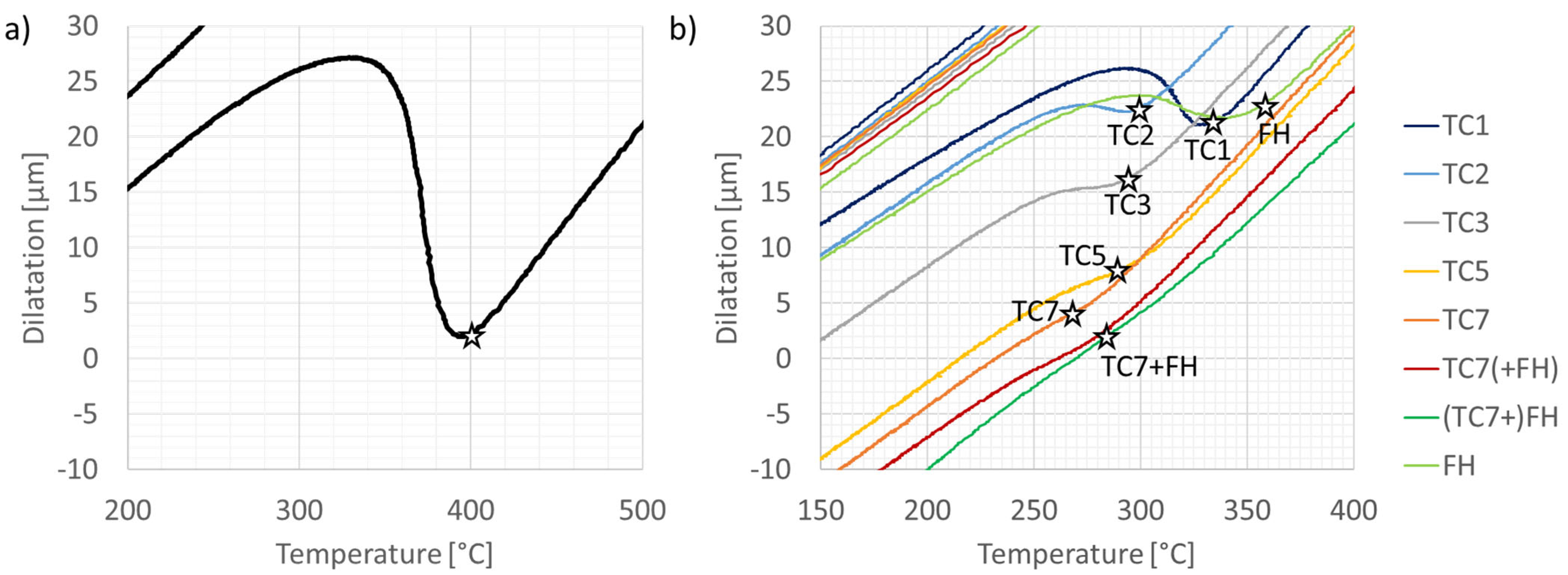
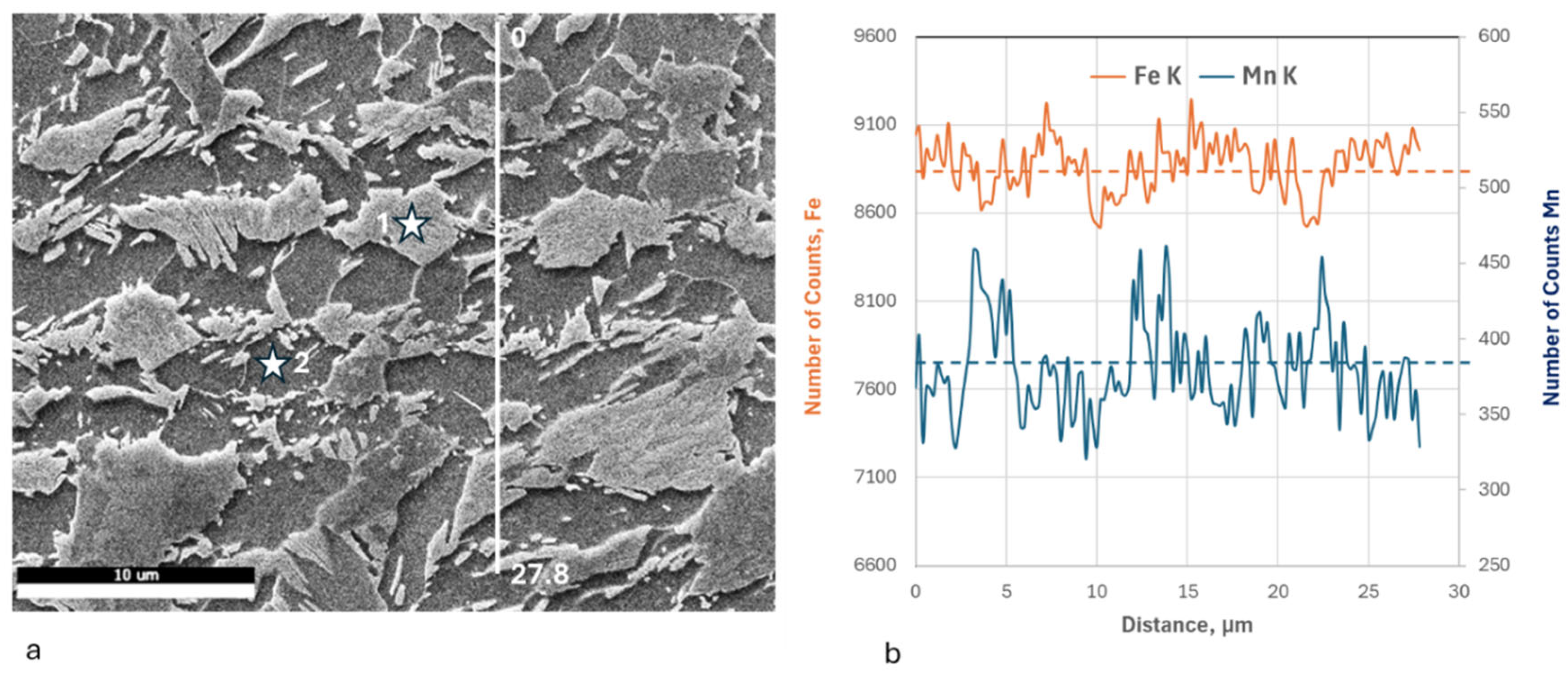
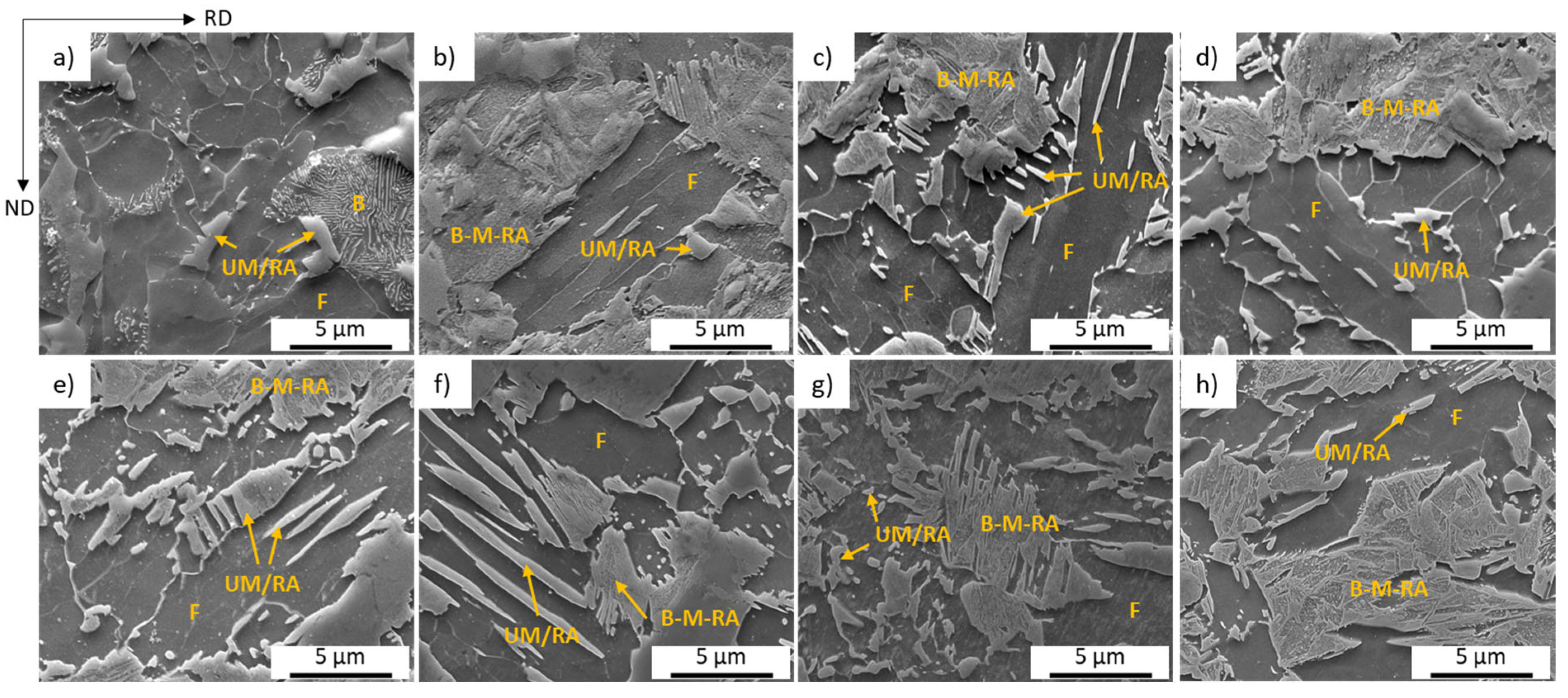
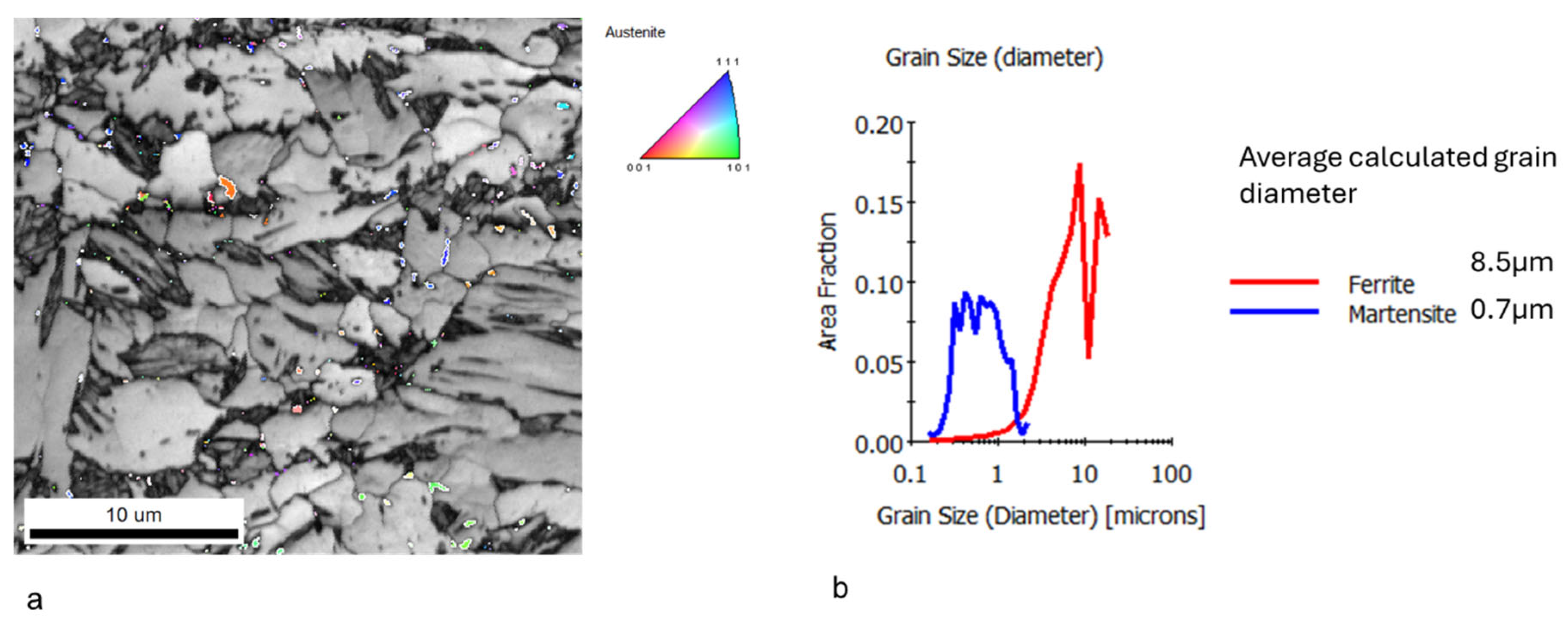
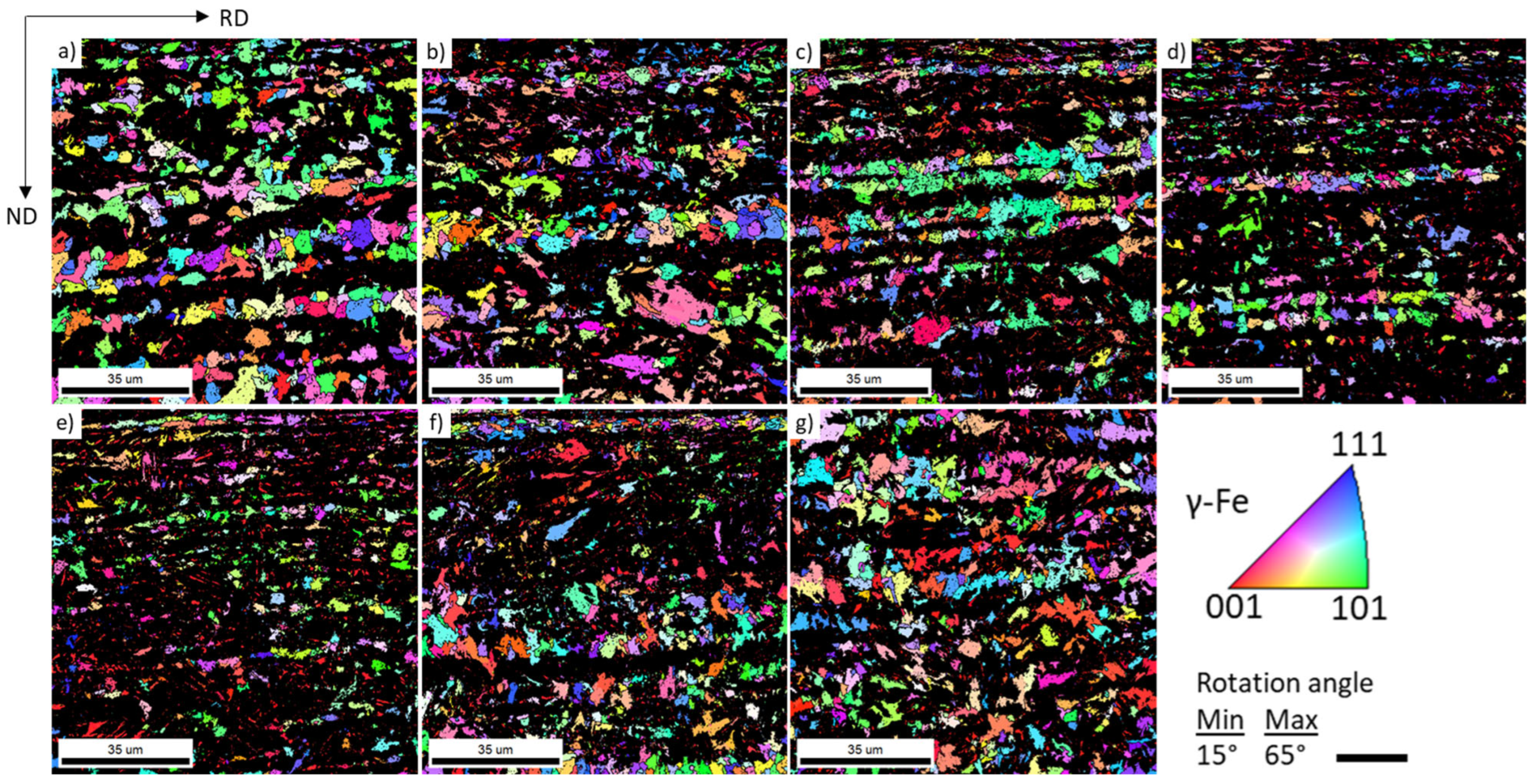
3.1. Microstructure Characterization and Hardness
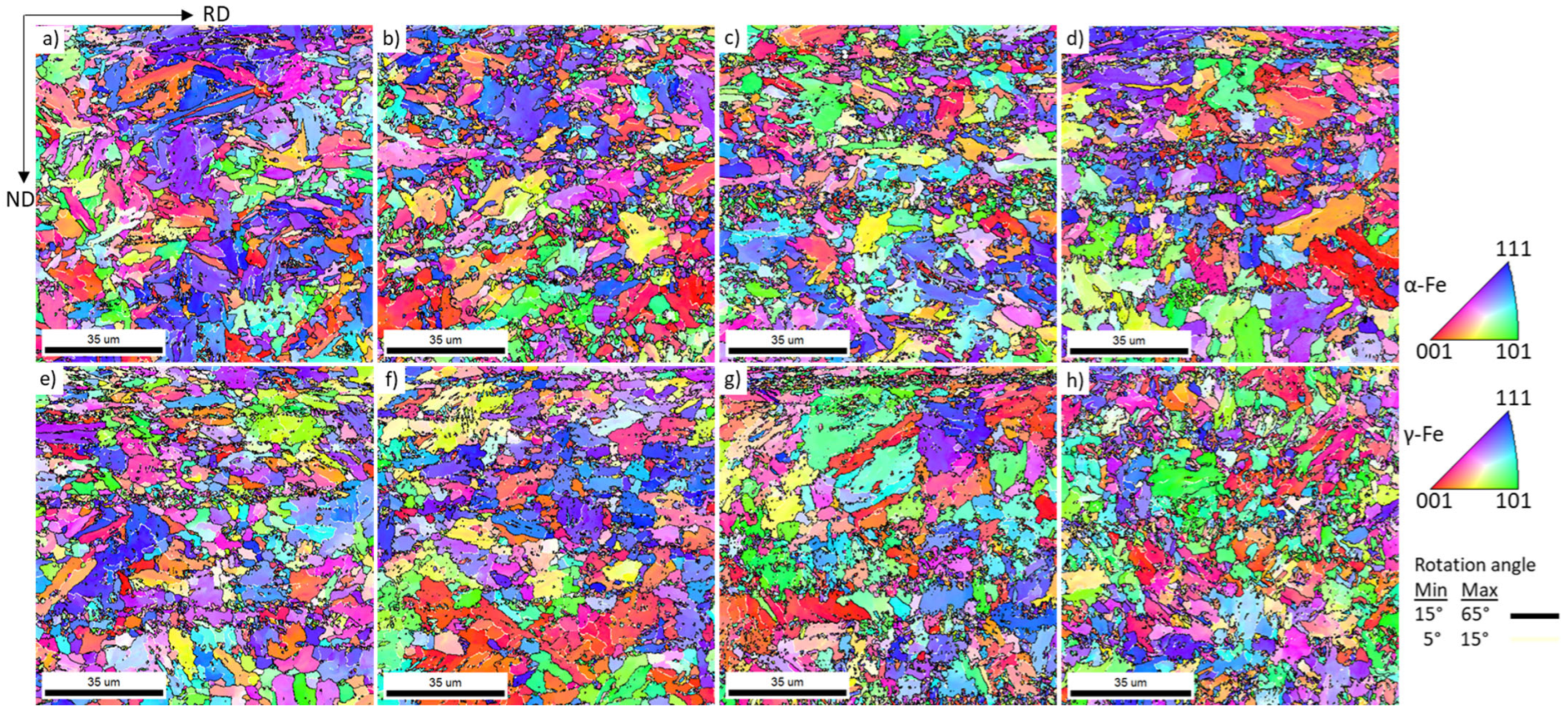
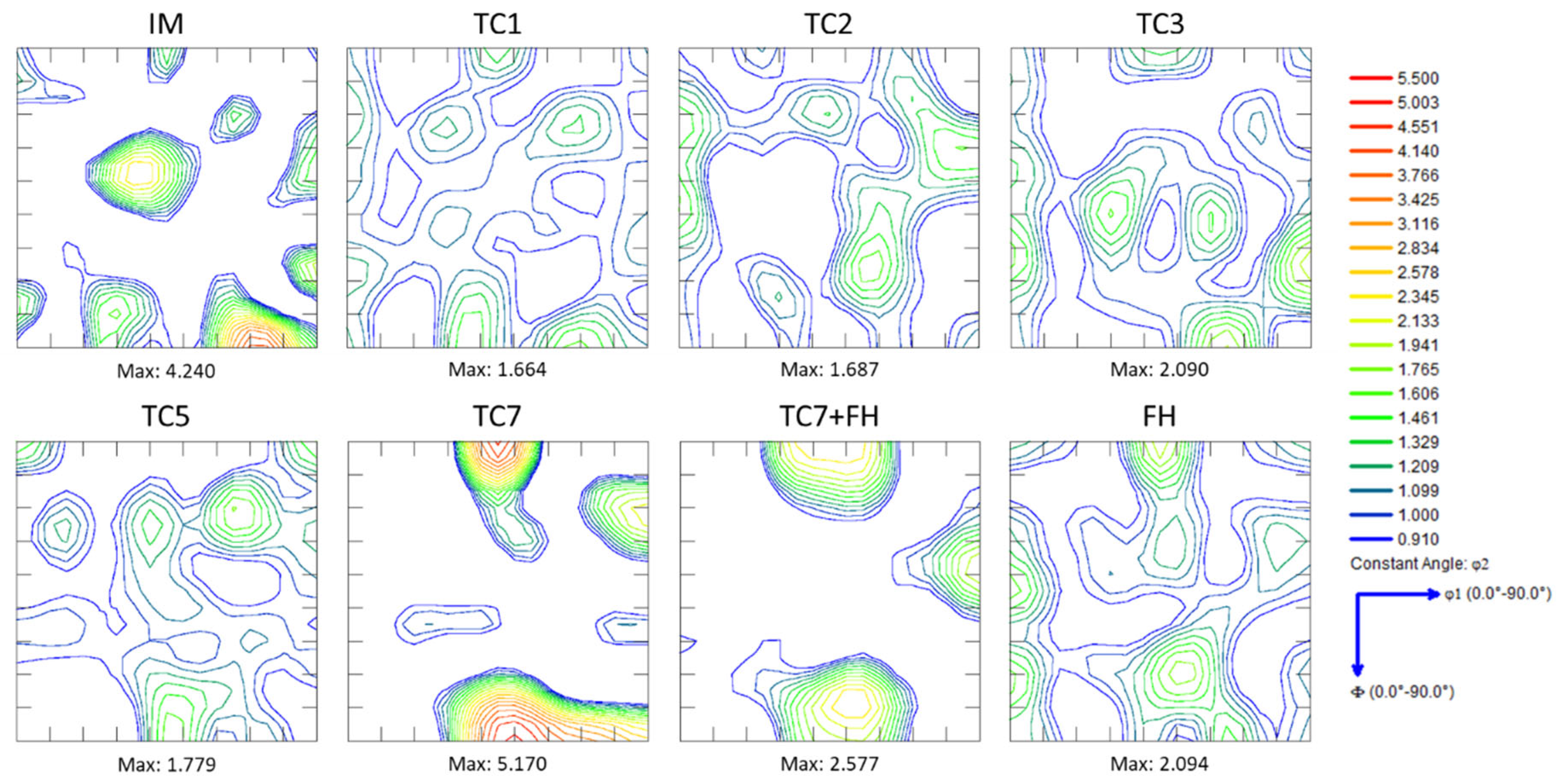
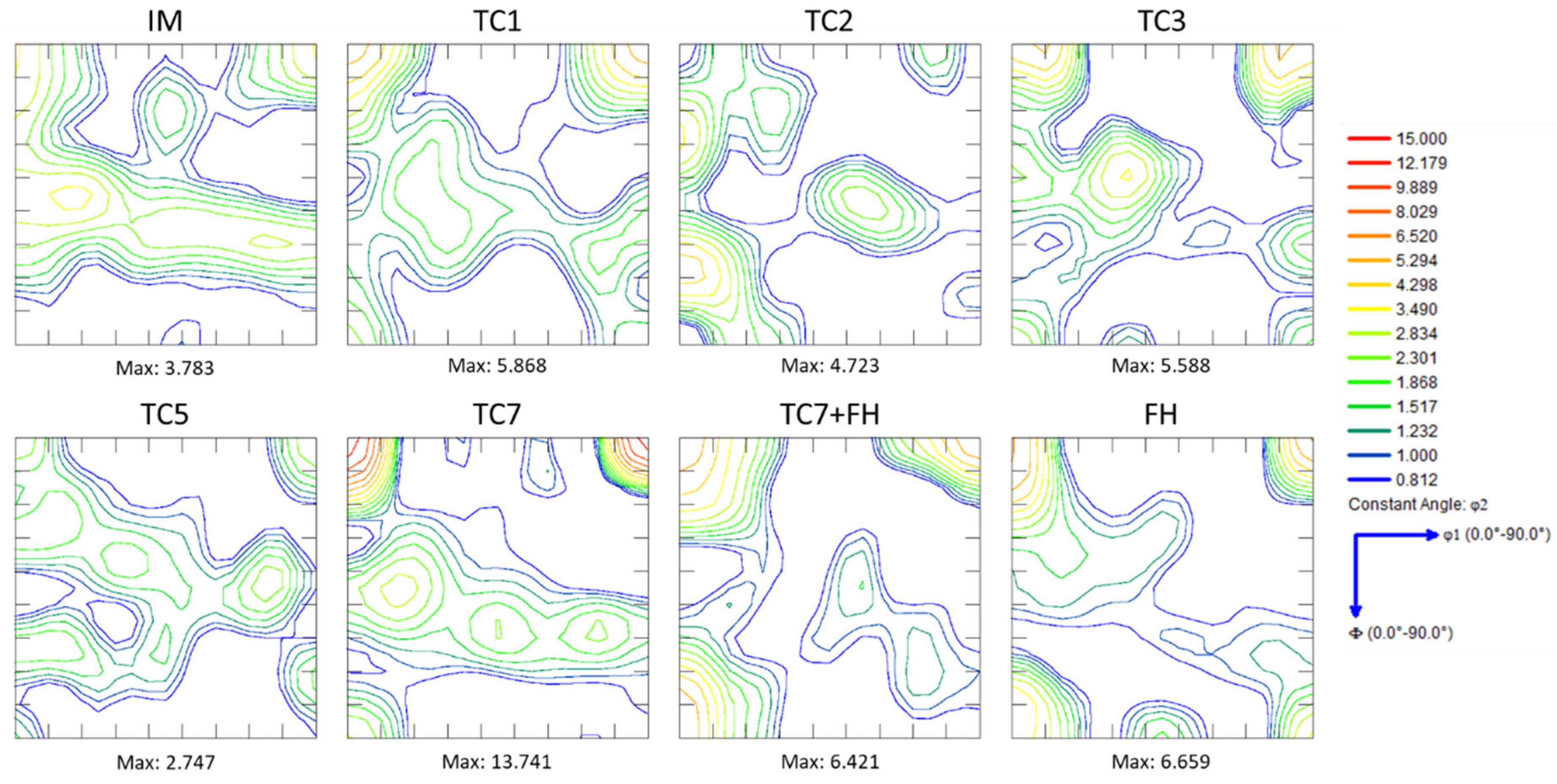
3.2. Crystallographic Texture
4. Summary and Conclusions
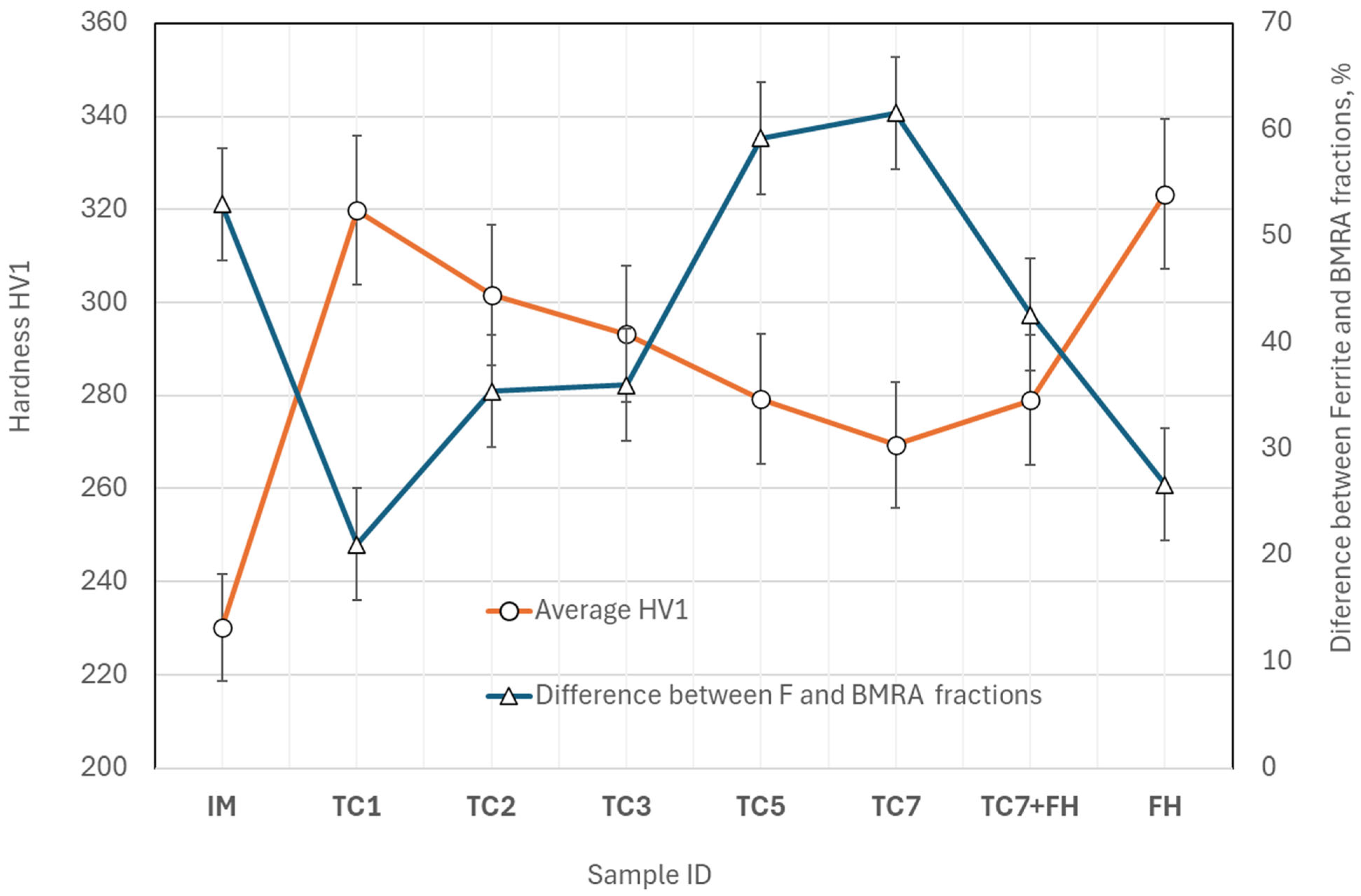
- The hardness of dual-phase (DP) steel is primarily governed by the balance between ferrite and the transformation products—bainite, martensite, and retained austenite (B-M-RA)—which can be effectively tailored through thermal cycling. The ferrite grain size has only a secondary influence on hardness.
- Thermal cycling produces a pronounced grain-refining effect in both the prior austenite grains (PAGs) and the B-M-RA structures, with refinement intensifying as the number of cycles increases. However, excessive cycling or the addition of a fast-heating (FH) step after multiple cycles leads to grain coarsening. Ferrite grain size shows no consistent trend but tends to increase after several cycles, while FH samples consistently exhibit the smallest overall grain sizes.
- Repeated thermal cycling enhances carbon and manganese diffusion toward transformed regions, resulting in the formation of banded microstructures and a reduction in the martensite start (Ms) temperature due to solute enrichment of intercritical austenite. Crystallographic texture analysis further indicates recrystallization of both BCC and FCC constituents, even at rapid heating rates of 100 °C/s.
- Mechanical properties, particularly hardness and yield strength, can be improved by applying up to two thermal cycles or by employing fast heating without isothermal soaking. Increasing the number of cycles beyond this point reduces the B-M-RA fraction due to higher Ac1 temperatures and enhanced ferrite formation during cooling.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Basoeki, P.D. Improving lightweight materials processing for automotive by using intercritical annealing. MATEC Web Conf. 2018, 175, 02023. [Google Scholar] [CrossRef]
- Arcelor Mittal North America. Dual Phase Steels. Available online: https://northamerica.arcelormittal.com/media/vtmdpdq2/arcelormittal_data-sheet_dual-phase-steels_2024_x1a.pdf (accessed on 5 September 2025).
- Suski, C.A.; Filho, J.F.S.; Cortez, A.; Gonçalves, F.E.; Ferreira, R.R. The environmental impact of lightweight body in white structures in vehicle manufacturing. J. Clean. Prod. 2024, 449, 141833. [Google Scholar] [CrossRef]
- Calcagnotto, M.; Ponge, D.; Raabe, D. Effect of grain refinement to 1μm on strength and toughness of dual-phase steels. Mater. Sci. Eng. A 2010, 527, 7832–7840. [Google Scholar] [CrossRef]
- Asadi, M.; De Cooman, B.C.; Palkowski, H. Influence of martensite volume fraction and cooling rate on the properties of thermomechanically processed dual phase steel. Mater. Sci. Eng. A 2012, 538, 42–52. [Google Scholar] [CrossRef]
- Movahed, P.; Kolahgar, S.; Marashi, S.P.H.; Pouranvari, M.; Parvin, N. The effect of intercritical heat treatment temperature on the tensile properties and work hardening behavior of ferrite–martensite dual phase steel sheets. Mater. Sci. Eng. A 2009, 518, 1–6. [Google Scholar] [CrossRef]
- Lu, J.; Yu, H.; Yang, S. Mechanical behavior of multi-stage heat-treated HSLA steel based on examinations of microstructural evolution. Mater. Sci. Eng. A 2021, 803, 140493. [Google Scholar] [CrossRef]
- Hernandez-Duran, E.I.; Bliznuk, V.; Ros-Yanez, T.; Iquilio-Abarzua, R.; Castro-Cerda, F.M.; Petrov, R.H. Improvement of the strength-ductility balance in ultrafast heated steels by combining high-temperature annealing and quenching and partitioning process. Mater. Sci. Eng. A 2021, 827, 142045. [Google Scholar] [CrossRef]
- Gaggiotti, M.; Albini, L.; Di Nunzio, P.; Di Schino, A.; Stornelli, G.; Tiracorrendo, G. Ultrafast Heating Heat Treatment Effect on the Microstructure and Properties of Steels. Metals 2022, 12, 1313. [Google Scholar] [CrossRef]
- Cerda, F.M.C. Third Generation Advanced High Strength Steels via Ultrafast Heating. Ph.D. Thesis, Ghent University, Gent, Belgium, 2017. [Google Scholar]
- Banis, A.; Bouzouni, M.; Petrov, R.H.; Papaefthymiou, S. Simulation and characterisation of the microstructure of ultra-fast heated dual-phase steel. Mater. Sci. Technol. 2020, 36, 1282–1291. [Google Scholar] [CrossRef]
- Bouzouni, M.; Papaefthymiou, S. Preliminary Study of Carbide Dissolution during an Ultra-Fast Heat Treatment in Chromium Molybdenum Steel. Int. J. Metall. Met. Phys. 2017, 2, 1–7. [Google Scholar] [CrossRef]
- Carreno-Saavedra, J.I.; Ros-Yanez, T.; García, C.I.; Hernández-Durán, E.I.; Iquilio, R.A.; Cerda, F.M.C. Application of ultrafast heating and tempering to plate steel. Mater. Sci. Technol. 2023, 39, 2417–2432. [Google Scholar] [CrossRef]
- Cryderman, R.L.; Arterbury, E.; Bamrud, F. Effects of Initial Microstructure and Induction Heating Cycle on the Microstructure and Torsional Fatigue Performance of a Steel Containing 0.6 wt pct Carbon. J. Mater. Eng. Perform. 2024, 33, 2680–2693. [Google Scholar] [CrossRef]
- Matlock, D.K.; Kang, S.; De Moor, E.; Speer, J.G. Applications of rapid thermal processing to advanced high strength sheet steel developments. Mater. Charact. 2020, 166, 110397. [Google Scholar] [CrossRef]
- Pedraza, J.P.; Landa-Mejia, R.; García-Rincon, O.; Garcia, C.I. The Effect of Rapid Heating and Fast Cooling on the Transformation Behavior and Mechanical Properties of an Advanced High Strength Steel (AHSS). Metals 2019, 9, 545. [Google Scholar] [CrossRef]
- Grange, R.A. The rapid heat treatment of steel. Metall. Trans. 1971, 2, 65–78. [Google Scholar] [CrossRef]
- Konopleva, E.V.; Éntin, R.I.; Bayazitov, V.M.; Kogan, L.I.; Spasskii, M.N. Thermal cycling treatment of low-carbon martensitic-bainitic steels. Met. Sci. Heat. Treat. 1987, 29, 489–492. [Google Scholar] [CrossRef]
- Hidalgo, J.; Santofimia, M.J. Effect of Prior Austenite Grain Size Refinement by Thermal Cycling on the Microstructural Features of As-Quenched Lath Martensite. Metall. Mater. Trans. A 2016, 47, 5288–5301. [Google Scholar] [CrossRef]
- Hernandez-Duran, E.; Corallo, L.; Ros-Yanez, T.; Castro-Cerda, F.; Petrov, R.H. The Effect of Different Annealing Strategies on the Microstructure Development and Mechanical Response of Austempered Steels. Metals 2021, 11, 1041. [Google Scholar] [CrossRef]
- Koo, J.Y.; Thomas, G. Thermal cycling treatments and microstructures for improved properties of Fe-0.12% C-0.5% Mn steels. Mater. Sci. Eng. 1976, 24, 187–198. [Google Scholar] [CrossRef]
- Ghaemifar, S.; Mirzadeh, H. Refinement of Banded Structure via Thermal Cycling and Its Effects on Mechanical Properties of Dual Phase Steel. Steel Res. Int. 2018, 89, 1700531. [Google Scholar] [CrossRef]
- Ghaemifar, S.; Mirzadeh, H. Enhanced mechanical properties of dual-phase steel by repetitive intercritical annealing. Can. Metall. Q. 2017, 56, 459–463. [Google Scholar] [CrossRef]
- Ghaemifar, S.; Mirzadeh, H.; Khorrami, M.S.; Soltani, R.; Nasiri, Z. Improved properties of dual-phase steel via pre-intercritical annealing treatment and thermal cycling. Mater. Sci. Technol. 2020, 36, 1663–1670. [Google Scholar] [CrossRef]
- Nasiri, Z.; Ghaemifar, S.; Naghizadeh, M.; Mirzadeh, H. Thermal Mechanisms of Grain Refinement in Steels: A Review. Met. Mater. Int. 2021, 27, 2078–2094. [Google Scholar] [CrossRef]
- Ashrafi, H.; Shamanian, M.; Emadi, R.; Saeidi, N. A novel and simple technique for development of dual phase steels with excellent ductility. Mater. Sci. Eng. A 2017, 680, 197–202. [Google Scholar] [CrossRef]
- Xie, Z.J.; Han, G.; Zhou, W.H.; Wang, X.L.; Shang, C.J.; Misra, R.D.K. A novel multi-step intercritical heat treatment induces multi-phase microstructure with ultra-low yield ratio and high ductility in advanced high-strength steel. Scr. Mater. 2018, 155, 164–168. [Google Scholar] [CrossRef]
- Craeynest, A. Experimental Study & Simulations of the Transformation Behavior of Dual-Phase Steels. Master’s Thesis, Ghent University, Gent, Belgium, 2022. [Google Scholar]
- Fujita, M.; Kuki, K. An Evaluation of Mechanical Properties with the Hardness of Building Steel Structural Members for Reuse by NDT. Metals 2016, 6, 247. [Google Scholar] [CrossRef]
- Xu, X.; van der Zwaag, S.; Xu, W. The effect of martensite volume fraction on the scratch and abrasion resistance of a ferrite–martensite dual phase steel. Wear 2016, 348–349, 80–88. [Google Scholar] [CrossRef]
- Li, P.; Li, J.; Meng, Q.; Hu, W.; Xu, D. Effect of heating rate on ferrite recrystallization and austenite formation of cold-roll dual phase steel. J. Alloys Compd. 2013, 578, 320–327. [Google Scholar] [CrossRef]
- HajyAkbary, F.; Sietsma, J.; Petrov, R.H.; Miyamoto, G.; Furuhara, T.; Santofimia, M.J. A quantitative investigation of the effect of Mn segregation on microstructural properties of quenching and partitioning steels. Scr. Mater. 2017, 137, 27–30. [Google Scholar] [CrossRef]
- Zhang, D.; Li, H.; Song, Y.; Sun, H.; Zheng, Y.; Ma, C.; She, Y.; Wang, B.; Yang, S. Toughness and plasticity mechanism of medium manganese steel in cyclic phase transformation strengthening. J. Mater. Sci. 2025, 60, 4897–4914. [Google Scholar] [CrossRef]
- Celotto, S.; Ghadbeigi, H.; Pinna, C.; Shollock, B.A.; Efthymiadis, P. Deformation-Induced Microstructural Banding in TRIP Steels. Metall. Mater. Trans. A 2018, 49, 2893–2906. [Google Scholar] [CrossRef]
- Petrov, R.; Kestens, L.; Houbaert, Y. Recrystallization of a Cold Rolled Trip-assisted Steel during Reheating for Intercritical Annealling. ISIJ Int. 2001, 41, 883–890. [Google Scholar] [CrossRef]
- Thomas, L.S.; Matlock, D.K. Formation of Banded Microstructures with Rapid Intercritical Annealing of Cold-Rolled Sheet Steel. Metall. Mater. Trans. A 2018, 49, 4456–4473. [Google Scholar] [CrossRef]
- Gulyaev, A. Physical Metallurgy; Mir Publishers: Moscow, Russia, 1980; Volume 1, p. 173. Available online: https://books.google.gr/books?id=fYNkwAEACAAJ (accessed on 8 October 2025).
- Gulyaev, A. Physical Metallurgy; Mir Publishers: Moscow, Russia, 1980; Volume 2, p. 23. Available online: https://books.google.gr/books?id=Vm6NzwEACAAJ (accessed on 8 October 2025).
- Sohrab, S.H.; Catrakis, H.J.; Kobasko, N. Recent Advances in Heat Transfer, Thermal Engineering and Environment; WSEAS Press: Stevens Point, WI, USA, 2009. [Google Scholar]
- Skowronek, A.; Radwański, K.; Sozańska-Jędrasik, L.; Matus, K.; Grajcar, A. Continuous annealing of medium-Mn steels: Sensitivity of mechanical performance to minor changes in time-temperature heat treatment parameters. Mater. Sci. Eng. A 2025, 940, 148568. [Google Scholar] [CrossRef]
- Skowronek, A.; Grajcar, A.; Kozłowska, A.; Janik, A.; Morawiec, M.; Petrov, R.H. Temperature-Dependent Microstructural Evolution of Al-Rich Medium-Mn Steel During Intercritical Annealing. Metall. Mater. Trans. A 2022, 53, 3012–3021. [Google Scholar] [CrossRef]
- Colás, R.; Totten, G.E. (Eds.) Encyclopedia of IRON, Steel, and Their Alloys; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]
- Davut, K.; Zaefferer, S. Statistical Reliability of Phase Fraction Determination Based on Electron Backscatter Diffraction (EBSD) Investigations on the Example of an Al-TRIP Steel. Metall. Mater. Trans. A 2010, 41, 2187–2196. [Google Scholar] [CrossRef]
- Zaefferer, S.; Ohlert, J.; Bleck, W. A study of microstructure, transformation mechanisms and correlation between microstructure and mechanical properties of a low alloyed TRIP steel. Acta Mater. 2004, 52, 2765–2778. [Google Scholar] [CrossRef]
- Farshchi, Y.K.; Mirzadeh, H.; Tavakoli, M.; Zamani, M. Microstructure tailoring for property improvements of DP steel via cyclic intercritical annealing. Mater. Res. Express 2019, 6, 126513. [Google Scholar] [CrossRef]
- Jonas, J.J. Transformation Textures Associated with Steel Processing; Haldar, A., Suwas, S., Bhattacharjee, D., Eds.; Microstructure and Texture in Steels; Springer: London, UK, 2009; pp. 3–17. [Google Scholar] [CrossRef]

| C | Mn | Si | Cr | Al | Ti | V |
|---|---|---|---|---|---|---|
| 0.10–0.15 | 1.7–2.0 | 0.15–0.20 | 0.15–0.20 | 0.02–0.04 | 0.02–0.04 | <0.005 |
| Sn | Nb | N | P | Ni | S | Cu |
| <0.003 | <0.002 | - | <0.05 | - | <0.05 | - |
| Heating Rate | Ac1 (°C) | Ac3 (°C) | T50%austenite (°C) |
|---|---|---|---|
| 10 °C/s | 744 | 824 | 788 |
| 100 °C/s | 757 | 840 | 807 |
| Sample Reference | Heating Rate (°C/s) | Tpeak (°C) | tsoak (s) | Cooling Rate (°C/s) | Thold (°C) | thold (s) | Quenching Rate (°C/s) |
|---|---|---|---|---|---|---|---|
| TC(i) For i = 1–7 | 10 | 788 | 0.2 | 20 | 600 | 450 | >100 |
| FH | 100 | 807 | 0.2 | - | - | - | 100 |
| Sample ID | IM | TC1 | TC2 | TC3 | TC5 | TC7 | TC7 + FH | FH |
|---|---|---|---|---|---|---|---|---|
| B-M-RA, [%] | 24 | 40 | 32 | 32 | 20 | 19 | 29 | 37 |
| Ferrite, [%] | 77 | 61 | 68 | 68 | 80 | 81 | 71 | 63 |
| AvgGS_ F, [µm] | 148 | 97 | 128 | 101 | 104 | 281 | 193 | 72 |
| AvgGS B-M-RA, [µm] | 11 | 5 | 3 | 3 | 2 | 2 | 3 | 5 |
| AvgGS PAGs, [µm] | 19 | 21 | 19 | 8 | 5 | 11 | 20 | |
| Avg hardness, HV1 | 230 ± 1 | 320 ± 2 | 302 ± 4 | 293 ± 5 | 279 ± 3 | 269 ± 3 | 279 ± 6 | 323 ± 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banis, A.; Arijs, J.F.; Petrov, R.H. Evaluation of the Possibility of Using Non-Conventional Technological Approaches for the Heat Treatment of Hot-Rolled DP Steel. Metals 2025, 15, 1230. https://doi.org/10.3390/met15111230
Banis A, Arijs JF, Petrov RH. Evaluation of the Possibility of Using Non-Conventional Technological Approaches for the Heat Treatment of Hot-Rolled DP Steel. Metals. 2025; 15(11):1230. https://doi.org/10.3390/met15111230
Chicago/Turabian StyleBanis, Alexandros, Jasmien Flore Arijs, and Roumen H. Petrov. 2025. "Evaluation of the Possibility of Using Non-Conventional Technological Approaches for the Heat Treatment of Hot-Rolled DP Steel" Metals 15, no. 11: 1230. https://doi.org/10.3390/met15111230
APA StyleBanis, A., Arijs, J. F., & Petrov, R. H. (2025). Evaluation of the Possibility of Using Non-Conventional Technological Approaches for the Heat Treatment of Hot-Rolled DP Steel. Metals, 15(11), 1230. https://doi.org/10.3390/met15111230









