Abstract
Ti2AlNb-based alloys are potential structural materials for high-temperature applications due to their low density and superior specific strength. However, their widespread application is limited by relatively poor oxidation resistance above 700 °C. While Ti2AlNb-based alloys exhibit promising mechanical properties, their oxidation behavior remains inadequately characterized, particularly concerning the role of Nb content. In this study, the high-temperature oxidation behavior of Ti2AlNb-based alloys with different Nb contents was investigated at 800 °C in air. The results revealed a characteristic double-layered oxide structure consisting of an outer TiO2 layer and inner alternating TiO2-rich and AlNbO4-rich sublayers. Thermodynamic calculations confirmed the favorable formation of TiO2, Al2O3, Nb2O5, and AlNbO4 at high temperatures. However, the reaction between Nb2O5 and Al2O3 hinders the formation of a protective Al2O3 layer. Increasing the Nb content was found to replace Ti atoms, reducing the diffusion rate of oxygen and simultaneously decreasing the thickness of porous TiO2 regions. Nevertheless, the inadequate rate of aluminum diffusion inhibited adequate Al2O3 formation, leading to limited overall oxidation protection. These findings elucidate the composition–oxidation relationship in Ti2AlNb-based alloys and provide valuable insights for tailoring Nb and Al contents to achieve a balanced combination of mechanical properties and high-temperature oxidation resistance.
1. Introduction
Ti2AlNb-based alloys, characterized by their orthorhombic O-phase, have attracted significant attention as potential candidates for next-generation lightweight structural materials, particularly in aerospace propulsion systems [1,2]. They offer a favorable combination of high specific strength [3,4], excellent creep resistance [5,6,7], and good thermal stability at elevated temperatures [8,9,10]. In addition, their relatively low density (approximately 5.0–5.2 g/cm3) provides a distinct advantage in reducing structural weight, a critical factor for improving fuel efficiency and payload capacity [11]. These attributes position Ti2AlNb-based alloys as potential alternatives to replace conventional Ni-based superalloys in high-temperature engine components.
The microstructure of Ti2AlNb-based alloys typically consists of a multiphase microstructure including the orthorhombic O-phase (Ti2AlNb), the hexagonal α2-phase (Ti3Al), and the body-centered cubic B2/β-phase [12,13]. The O-phase serves as the primary load-bearing constituent at intermediate temperatures due to its favorable combination of strength and ductility [14,15]. The α2-phase enhances oxidation resistance but reduces room-temperature ductility [16]. The B2/β-phase improves hot workability and facilitates phase transformations, thus enhancing the alloy processability [17,18]. The complex interplay among these three phases allows for precise microstructural tailoring to meet performance requirements under various service conditions. Despite their advantageous high-temperature mechanical properties, the application of Ti2AlNb-based alloys in oxidative environments is constrained by their relatively poor oxidation resistance, especially above 700 °C [19,20,21]. Exposure to high-temperature air can lead to the rapid formation of non-protective oxide scales, internal oxidation, and oxygen embrittlement, which deteriorate structural integrity and service life. Moreover, the oxidation behavior is strongly influenced by compositional parameters—particularly the Nb content, which plays a dual role in both stabilizing the O-phase and modifying oxidation kinetics. Therefore, a comprehensive understanding of how Nb content affects the oxidation resistance and oxide scale evolution is essential for the design of Ti2AlNb-based alloys with improved high-temperature environmental stability.
He et al. [22] reported that high-temperature oxidation of Ti2AlNb-based alloys primarily produces TiO2 and AlNbO4, with Al2O3 and NbO forming under specific conditions depending on alloying elements and temperature. Zhao et al. [23] demonstrated that the addition of Nb inhibits the growth of TiO2 and promotes the selective oxidation of Al, leading to the formation of a dense and protective Al2O3 layer. Moreover, increased Nb content enhances the oxidation resistance of Ti alloys by inhibiting elemental interdiffusion and promoting the formation of a more adherent oxide scale with a reduced spallation tendency [24,25,26]. Li et al. [27] further demonstrated that Nb plays a multifaceted role in enhancing the oxidation resistance of Ti-Al-Nb alloys by suppressing TiO2 formation, promoting Al2O3 scale development, and reducing the diffusion rates of both oxygen and metal ions. However, Kim et al. [28] reported that increasing the Nb content—i.e., decreasing the Al/Nb ratio—markedly reduces the oxidation resistance of Ti2AlNb-based alloys, as exceeding a certain proportion of Nb leads to the formation of more non-protective oxides, and the reduced Al diffusion suppresses the formation of protective Al2O3 at the outermost surface. Overall, while Nb generally acts as a beneficial alloying element by promoting Al2O3 scale formation and suppressing TiO2 growth, excessive Nb additions may lower the Al/Nb ratio, thereby limiting Al activity and impeding the development of a continuous and protective Al2O3 layer.
Although extensive research has been conducted on the high-temperature oxidation behavior of Ti2AlNb-based alloys, most studies have primarily focused on evaluating their oxidation resistance. However, the nature of the oxidation products and the underlying oxidation mechanisms, particularly at 800 °C, remain inadequately elucidated. Ti2AlNb-based alloys have potential for high-temperature aeroengine and aerospace components due to their high strength-to-weight ratio and fatigue resistance. However, at 800 °C, these alloys exhibit extreme oxidation, leading to the formation of non-protective oxide scales and a significant reduction in mechanical strength, which can compromise structural integrity. This study aims to provide insights into the effect of Nb content on oxidation resistance, laying the groundwork for further optimization of alloy composition. Through high-temperature oxidation experiments combined with X-ray diffraction (XRD), scanning electron microscopy (SEM), and electron probe microanalysis (EPMA) characterization, the evolution of oxide scale structures and constituent phases is thoroughly examined. This work aims to establish a correlation between alloy composition, oxidation mechanism, and oxidation resistance, thereby providing guidance for designing Ti2AlNb-based alloys with enhanced environmental stability at elevated temperatures.
2. Materials and Methods
This study employed two rolled Ti2AlNb-based alloys. The first alloy, designated as 23Nb, has a nominal composition of Ti-22Al-23(Nb, Mo, V, Si) (at.%). The second alloy, designated as 14Nb with a nominal composition of Ti-22Al-14(Nb, Mo, Fe, V, Si) (at.%), was developed as a lower-cost variant by substituting a small amount of Nb with Fe. A detailed list of the nominal compositions for both alloys is presented in Table 1. The alloys were prepared by vacuum arc melting, followed by homogenization and multi-pass hot rolling to refine the microstructure and ensure compositional homogeneity. The Ti2AlNb-based alloy specimens were machined into rectangular coupons with dimensions of 10 mm × 10 mm × 2.5 mm. For each oxidation time (2, 6, 12, 24, 48, and 100 hours at 800 °C), three samples were tested to ensure reproducibility. The crucibles separately containing three parallel samples were placed into a large crucible to ensure that no interference occurred between the samples. For consistency, the rolling direction (RD), transverse direction (TD), and normal direction (ND) were defined based on the final rolling pass. High-temperature oxidation tests were conducted at 800 °C in ambient air using a muffle furnace. Figure 1 shows a schematic illustration of the high-temperature oxidation test procedure. The samples were exposed to air for various durations ranging from 1 h to 100 h and subsequently furnace-cooled to room temperature. To ensure that any spalled oxide was included in the mass gain measurements, the experimental setup minimized the effect of oxide spallation by weighing the sample together with the crucible.

Table 1.
Nominal chemical compositions of the 14Nb and 23Nb alloys (at.%).

Figure 1.
Schematic illustration of the high-temperature oxidation test procedure.
Phase identification of the oxidized surfaces was performed using XRD (Bruker D8 Discover Plus, Bruker AXS, Karlsruhe, Germany) with Cu Kα radiation (λ = 1.5406 Å), operated at 40 kV and 40 mA. XRD scans were acquired over a 2θ range of 10–100° with a step size of 0.02°/s. Surface and cross-sectional morphologies of the oxide scales were characterized using SEM (ZEISS Gemini 300, Carl Zeiss AG, Oberkochen, Germany) operated at an accelerating voltage of 20 kV. Secondary electron (SE) and backscattered electron (BSE) modes were used for SEM imaging. The SE mode allowed us to distinguish phases based on their distinct topographic features, while the BSE mode was employed to differentiate phases based on their compositional contrast. To reveal the subsurface microstructure, the samples were cold-mounted in epoxy resin, followed by grinding with sandpapers up to 2000 grit. Next, it was polished on a soft cloth using a 0.05 μm SiO2 suspension until a mirror-like surface was obtained. Finally, the sample was etched with Kroll’s reagent. Energy-dispersive X-ray spectroscopy (EDS, Oxford, UK) was employed in conjunction with SEM (ZEISS Gemini 300, Carl Zeiss AG, Oberkochen, Germany) to analyze the elemental distribution near the oxidation layers. Elemental mapping was further conducted using EPMA (JEM-IHP200F, JEOL Ltd., Tokyo, Japan) at a beam current of 1 nA and an accelerating voltage of 20 kV.
Thermodynamic assessments of oxidation reactions were carried out by calculating the Gibbs free energies of relevant oxides using the Dmol3 module in Materials Studio 6.0 (BIOVIA, San Diego, CA, USA). Owing to software limitations, the calculations were performed within a temperature range of 0–1000 K. To obtain the Gibbs free energies at the experimental oxidation temperature of 1073 K (800 °C), the computed data were fitted and extrapolated using Origin software (2025, OriginLab, Northampton, MA, USA). Furthermore, the CASTEP module in Materials Studio was utilized to determine the adsorption energies of O2 molecules on the B2, O, and α2 phase surfaces of the Ti2AlNb-based alloys, to gain further insight into the initial oxidation behavior of different phases.
3. Results and Discussion
3.1. Microstructure
Figure 2 illustrates SEM micrographs and XRD of the 14Nb and 23Nb alloys. The B2, O, and α2 phases were readily distinguished in the secondary electron (SE) micrographs by contrast differences [29,30], as indicated by the yellow arrows in Figure 2a,b,d,e. The phase fractions of the 14Nb and 23Nb alloys were listed in Table 2. Both the 14Nb and 23Nb alloys exhibited a characteristic three-phase microstructure comprising α2, O, and B2 phases, consistent with the XRD pattern shown in Figure 2c,f. The α2 phase appeared as elongated lamellae aligned along the rolling direction, indicative of processing-induced morphological anisotropy [31]. The O phase precipitates from the B2 matrix with a lath-like morphology and coexists with the residual B2 phase, as shown in Figure 2b,e. A higher Nb content is generally known to stabilize the high-temperature B2 phase and promote the B2 to O phase transformation during cooling, owing to the strong partitioning tendency of Nb into the O phase and its effect on shifting the phase equilibrium toward O phase formation [32,33,34]. Nevertheless, as shown in Figure 2b,e, the experimental results reveal only small changes in the volume fractions of the O phase in the 14Nb and 23Nb alloys. This is attributed to the observation that both alloys fall within the α2 + O + B2 three-phase region of the Ti-Al-Nb phase diagram in a given processing condition [11,35]. In such non-equilibrium rolled states, the final phase distribution is primarily governed by the thermomechanical processing history and transformation kinetics, rather than by small variations in nominal Nb content [17].
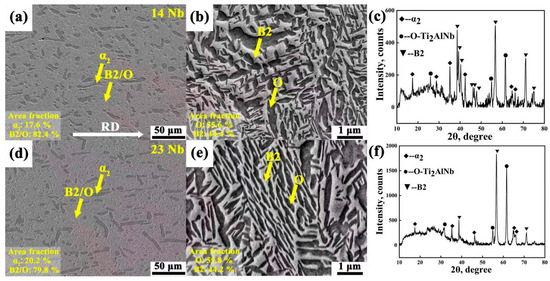
Figure 2.
Microstructure and XRD analysis of the 14Nb and 23Nb alloys: (a,b) SEM micrographs of the 14Nb alloy; (c) XRD pattern of the 14Nb alloy; (d,e) SEM micrographs of the 23Nb alloy; (f) XRD pattern of the 23Nb alloy.

Table 2.
Phase fractions of the 14Nb and 23Nb alloys determined by SEM.
3.2. Oxidation Resistance
As shown in Figure 3, the oxidation weight gain of the 14Nb alloy remained significantly higher than that of the 23Nb alloy throughout the entire exposure duration at 800 °C. Moreover, the weight gain curve for the 14Nb alloy exhibits an approximately linear trend, suggesting that the oxide scale formed on Ti2AlNb-based alloys at this temperature fails to provide effective protection against further oxidation. To quantitatively evaluate the oxidation behavior, the oxidation kinetics were analyzed using the empirical equation [36,37]:
where ΔM is the weight gain per unit area (mg/cm2), kn represents the oxidation kinetics constant, describing the rate of mass gain during oxidation (mgn/(cm2n·h)), t is the oxidation time (h), and n is the kinetic exponent that characterizes the oxidation mechanism. An n value of 1 indicates a linear relationship between weight gain and time, implying that the oxide scale does not effectively inhibit further oxidation, and thus the alloy exhibits insufficient oxidation resistance in a given condition. In contrast, an n value of 2 corresponds to parabolic kinetics, signifying the formation of a dense, protective oxide layer that impedes further oxidation. As summarized in Table 3, the oxidation kinetics fitting results for the rolled Ti2AlNb-based alloys reveal n values close to 1, indicating a linear oxidation behavior. This further confirms that no protective oxide scale was formed at 800 °C in air, and the alloys are highly susceptible to progressive oxidation. Moreover, the kn value of the 14Nb alloy is higher than that of the 23Nb alloy, suggesting that after 100 h of oxidation at 800 °C in air; the 23Nb alloy exhibits better oxidation resistance compared to the 14Nb alloy, as demonstrated by a lower mass gain rate and a more stable oxide layer. The high Nb content in the alloy hinders the formation of a continuous, protective Al2O3 layer and instead promotes the growth of a less-protective AlNbO4 phase [38,39,40].
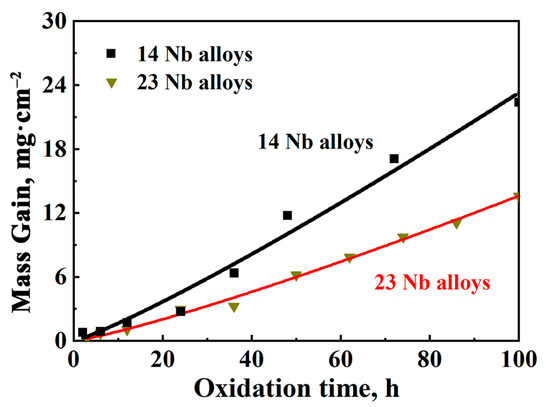
Figure 3.
Oxidation weight gain curves of 14Nb and 23Nb alloys at 800 °C.

Table 3.
Oxidation kinetics parameters of the 14Nb and 23Nb alloys during isothermal oxidation in air at 800 °C.
3.3. Oxidation Mechanism
The linear oxidation kinetics observed for the 14Nb and 23Nb alloys (Figure 3) are indicative of the formation of a non-protective oxide scale, which contrasts with the parabolic kinetics often seen in protective Ti-Al systems. This distinct behavior is attributed to the high concentration of niobium, which fundamentally alters the composition and protective nature of the oxide scale.
As shown in Figure 4, the constitution of this non-protective scale was investigated by XRD. After oxidation at 800 °C for 2 h, the identified main crystal phases were Al2O3, AlNbO4, and TiO2. In addition, XRD analysis did not detect any nitride phases, confirming that the participation of nitrogen in the oxidation of the sample is negligible. However, after continued oxidation for 12 and 100 h, the identified main phases were AlNbO4 and TiO2, with no obvious diffraction peaks corresponding to Al2O3 detected. This suggests that while Al2O3 form initially, it is rapidly consumed through a reaction with niobium oxides to form the less-protective, ternary AlNbO4 phase via the following pathway [41]:
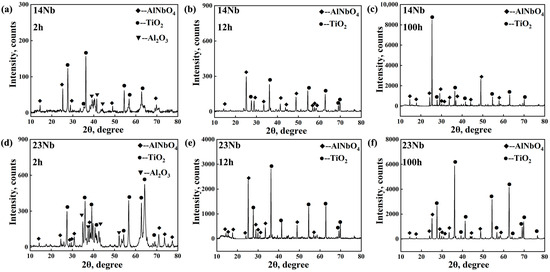
Figure 4.
XRD patterns of the 14Nb and 23Nb alloys; (a–c) 14Nb alloy after oxidation in air at 800 °C for (a) 2 h, (b) 12 h, (c) 100 h; (d–f) 23Nb alloy after oxidation in air at 800 °C for (a) 2 h, (b) 12 h, (c) 100 h, showing the formation and evolution of Al2O3 (PDF#75-1862), AlNbO4 (PDF#41-0347) and TiO2 (PDF#89-4202) phases.
After 12 h, pronounced diffraction peaks for AlNbO4 were observed, while TiO2 peaks remained weak, indicating that AlNbO4 formation is the preferential process during the early stages. As oxidation progressed to 100 h, the significant intensification of TiO2 peaks reflected the continued growth of this non-protective oxide. During the oxidation process, Al2O3 initially underwent a series of phase transitions (amorphous → γ-Al2O3 → δ-Al2O3 → θ-Al2O3 → α-Al2O3) but was subsequently consumed by Nb oxides, leading to its disappearance from the final oxide scale [42,43]. Ultimately, the high Nb content hindered the formation of a continuous, protective Al2O3 layer, instead promoting the growth of a less-protective mixed scale dominated by AlNbO4 and TiO2 [42].
To elucidate the relative thermodynamic stability of oxide formation during high-temperature oxidation, the absolute Gibbs free energies (G) of the Ti2AlNb matrix phases (α2, B2, O) and relevant oxides (TiO2, Al2O3, Nb2O5, AlNbO4) were calculated using the Dmol3 module in Materials Studio 6.0 based on first-principles density functional theory (DFT). Since the computed temperature range was limited to 0–1000 K, extrapolation to the experimental temperature (1073 K) was performed using the fitted G-T relationship (Equation (3)) [44]:
where G is the absolute Gibbs free energy (kcal/mol), T is the temperature (K), and a, b, c, and I are the fitting parameters obtained from regression analysis. The enthalpy term H represents the standard formation enthalpy at 298 K. As shown in Figure 5a, the calculated G values reveal the relative stabilities of the oxides. Throughout the investigated temperature range, Nb2O5 consistently displays the lowest Gibbs free energy (G), followed by Al2O3 and then TiO2, indicating that Nb2O5 is the most thermodynamically favorable oxide. However, this thermodynamic prediction must be considered alongside kinetic factors. Due to the sluggish diffusion of Nb, the formation of Nb2O5 is kinetically hindered and primarily limited to the inner regions of the oxide scale. In contrast, TiO2 and Al2O3 form more readily at the surface owing to the higher diffusivity of Ti and Al [45]. Notably, Al2O3 generated in the early stages reacts with Nb2O5 to form AlNbO4 (Equation (2)), which accounts for the absence of crystalline Al2O3 in the XRD patterns. A comparison of the calculated Gibbs free energies indicates that the reaction between stable, crystalline Al2O3 and Nb2O5 is thermodynamically unfavorable at 1073 K (ΔG > 0). Despite this, AlNbO4 is experimentally observed as a stable product after prolonged oxidation. A plausible explanation for this discrepancy is the high-energy state of the actual reactant oxides; the initially formed Al2O3 and Nb2O5 within the scale are likely amorphous or nanocrystalline, possessing a significantly higher Gibbs free energy than the ideal crystalline structures used in the bulk calculation [46,47]. Consequently, the transformation from these high-energy precursors into the stable, crystalline AlNbO4 phase becomes a thermodynamically favorable pathway.
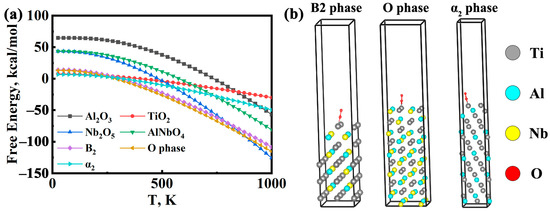
Figure 5.
(a) temperature dependence of Gibbs free energy for the α2, β/B2, and O phases in the Ti2AlNb-based alloys and their corresponding oxides; (b) Adsorption of O2 molecules on Ti2AlNb-based alloy phase and oxide surface, B2 phase, O phase, and α2 phase.
To further elucidate the oxidation behavior at the atomic scale, DFT simulations based on the CASTEP module in Materials Studio were employed to evaluate the adsorption behavior of O2 on the B2, O, and α2 phase surfaces of the Ti2AlNb-based alloy. The adsorption energies indicate the relative affinity of oxygen for α2, B2 and O phases, which reflects the initial oxide formation tendencies. As shown in Figure 5b, surface models were constructed for each phase, and the adsorption energy (Eabs) was calculated as:
where Esystem is the total energy of the surface with adsorbed O2, Esurface is the energy of the clean surface, and EO2 is the energy of a free O2 molecule. As listed in Table 4, all three phases exhibit negative Eabs values, indicating spontaneous adsorption. Among them, the O phase displays the lowest adsorption energy, indicating the strongest affinity with O2 and reflecting its high surface reactivity. The α2 phase shows intermediate adsorption energy, suggesting moderate oxygen affinity, while the B2 phase exhibits the weakest oxygen adsorption capacity. These findings are consistent with the in situ XRD results reported by Cheng et al. [48], in which the O phase preferentially forms during the initial oxidation stage and acts as the primary oxygen-absorbing phase, followed by the formation of α2 to accommodate further oxygen ingress. Overall, the B2 phase exhibits the highest oxidation resistance, whereas the α2 and O phases exhibit a poor resistance to oxidation [49].

Table 4.
Calculation of adsorption energy of O2 molecule on Ti2AlNb-based alloy phase and oxide surface.
As shown in Figure 6, after oxidation at 800 °C for 100 h, the substrate of the 14Nb alloy consists of B2 and α2 phases, while the 23Nb alloy retains a three-phase microstructure composed of B2, O, and α2 phases. The disappearance of the O phase in the 14Nb alloy is attributed to its rapid consumption during the early stage of oxidation, as the O phase possesses higher surface activity and reacts preferentially with oxygen [50]. In addition, the lower Nb content in the 14Nb alloy reduces the thermodynamic stability of the O phase, further promoting its depletion [51]. First-principles calculations reveal that the O phase exhibits the most negative O2 adsorption energy among the three phases, confirming its susceptibility to oxidation. From the perspective of phase constitution alone, the 23Nb alloy, which contains more oxidation-prone O phase, would be expected to exhibit an inferior oxidation resistance. However, the experimental results show the opposite trend: the 23Nb alloy demonstrates significantly improved oxidation resistance. This apparent inconsistency indicates that the enhancement in oxidation resistance with increasing Nb content cannot be simply explained by phase constitution, underscoring the necessity of considering other Nb-related factors such as oxide scale adhesion, density, and diffusion kinetics.
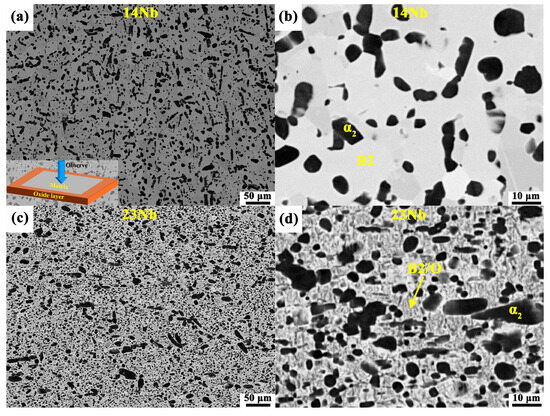
Figure 6.
BSE images of the substrate microstructure after oxidation at 800 °C for 100 h. (a,b) 14Nb alloy at lower and higher magnifications; (c,d) 23Nb alloy at lower and higher magnifications.
Figure 7 illustrates the surface morphologies of the oxide scales formed on the 14Nb alloy after oxidation at 800 °C for various durations. The oxide products exhibit a well-defined cuboidal morphology, which can be attributed to the crystalline growth of TiO2 as the dominant oxide phase, in agreement with the XRD results. At the early oxidation stage of 2 h (Figure 7d), the oxides appear as uniformly distributed fine cuboids. With increasing oxidation time to 12 and 100 h (Figure 7e,f), the TiO2 particles grow progressively larger, indicating continuous growth and coarsening under prolonged exposure. This time-dependent growth behavior suggests that the oxidation is governed by the outward diffusion of Ti and inward diffusion of oxygen, facilitating the preferential formation and accumulation of TiO2 at the oxide-gas interface [52,53]. Moreover, the increased particle size and surface roughness over time reflect the limited protective capability of the oxide scale, allowing sustained oxygen ingress and further oxidation.
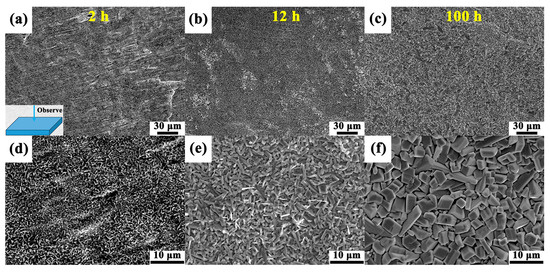
Figure 7.
Surface morphologies of the oxide scales formed on the 14Nb alloy after oxidation in air at 800 °C for (a) 2 h, (b) 12 h, and (c) 100 h; (d–f) the corresponding high-magnification images illustrating the size evolution and morphological changes in the oxide products.
Cross-sectional observations revealed distinct multilayered oxide scale structures in both the 14Nb and 23Nb alloys after oxidation at 800 °C, as shown in Figure 8. Both alloys exhibited a stratified morphology, with loosely bonded and porous interfaces between layers, offering limited protection against further oxidation. Notably, the oxide layer thickness of the 14Nb and 23Nb alloys showed no significant difference in the early stages of oxidation. However, after 100 h of oxidation, the 14Nb alloy developed a significantly thicker oxide scale (~945 μm) compared to the 23Nb alloy (~103 μm), indicating that 14Nb alloy had inferior oxidation resistance. The oxide layers formed on both alloys after 100 h of oxidation were unstable. EPMA elemental mapping of the oxidized 14Nb alloy, as illustrated in Figure 9a–i, revealed a complex layered architecture. The outermost layer consists predominantly of TiO2 with negligible Al and Nb content, indicating that Ti diffused outward and oxidized at the gas-oxide interface. Beneath this layer, alternating Ti-rich and Al/Nb-rich sublayers were observed. Oxygen enrichment was observed at the interface between the oxide layer and the alloy substrate, which indicates that oxygen inward diffusion is one of the driving forces for the ongoing oxidation process. As shown in Figure 10, the cross-sectional morphology and EDS mapping of the oxide scale in the 23Nb alloy reveal a layered structure similar to that of the 14Nb alloy. The outermost layer consists predominantly of TiO2, while the inner layer features alternating Nb- and Al-enriched regions and Ti-rich regions. This periodic structure reflects the complex interplay between differential elemental diffusion and local phase transformations during oxidation, and is characteristic of the oxidation behavior of Ti2AlNb-based alloys. The Al/Nb-rich layers are notably thinner, which can be attributed to the lower Nb content in the 14Nb alloy. Nb plays a crucial role in enhancing oxidation resistance by stabilizing complex oxides such as AlNbO4 and suppressing Ti outward diffusion [54,55]. Therefore, the reduced Nb content in the 14Nb alloy limits the formation of these protective phases, resulting in inferior oxidation resistance.
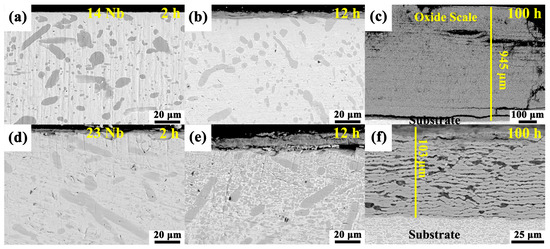
Figure 8.
(a–c) BSE image of the 14Nb alloy after oxidation in air at 800 °C for (a) 2 h, (b) 12 h, and (c) 100 h; (d–f) BSE image of the 23Nb alloy oxidized in air at 800 °C for (d) 2 h, (e) 12 h, and (f) 100 h.
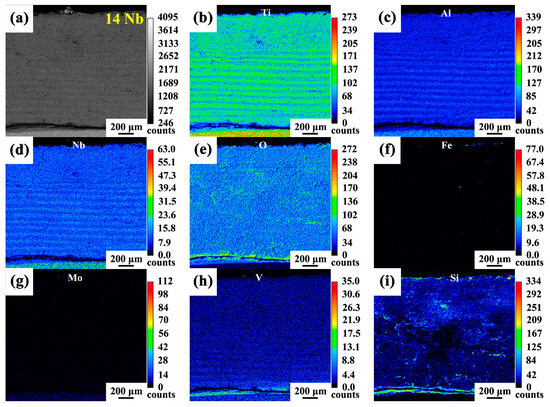
Figure 9.
(a) selected EPMA mapping region of the 14Nb alloy; (b–i) the corresponding elemental distribution maps of Ti, Al, Nb, O, Fe, Mo, V and Si, respectively.
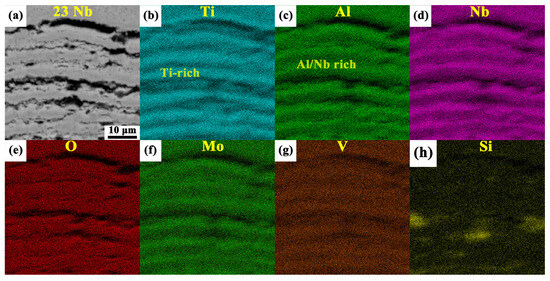
Figure 10.
(a) Cross-sectional morphology of the oxide scale of the 23Nb alloy after oxidation at 800 °C, obtained by BSE imaging; (b–h) EDS elemental distribution maps of Ti, Al, Nb, O, Mo, V, and Si corresponding to the region shown in (a).
As illustrated in Figure 11, the oxidation of Ti2AlNb-based alloys at 800 °C in air produces a bilayered oxide scale consisting of an outer TiO2 layer and an inner complex sublayer composed of alternating TiO2-rich and AlNbO4-rich regions. The thickness of the outer TiO2 layer is greater than that of each individual inner sublayer, which can be attributed to the higher diffusivity of Ti. The effect of Nb content on the oxidation resistance of Ti2AlNb-based alloys can be rationalized by several interrelated mechanisms. First, due to the relatively low Al content and high Nb concentration in Ti2AlNb-based alloys, a continuous and dense Al2O3 layer—a hallmark of oxidation-resistant Ti-Al systems—fails to form [56,57]. Although Nb facilitates the selective oxidation of Al, the final product is typically AlNbO4 rather than Al2O3 [58]. While AlNbO4 exhibits inferior protective capabilities compared to Al2O3, it is more adherent to the substrate and denser than TiO2, thereby offering better protection than TiO2 alone [59]. When TiO2 forms, the Ti content on the metal surface decreases, and the relative activity of Al increases, promoting the formation of AlNbO4. Conversely, when AlNbO4 forms, the activity of Al beneath the oxide layer decreases, while the activity of Ti increases, favoring the growth of TiO2. This reciprocal process gives rise to the alternating oxide layers observed experimentally [60]. In addition, the high Nb content modifies the oxidation pathway by promoting the formation of discontinuous Nb-containing oxides (e.g., Nb2O5, AlNbO4), which act as effective diffusion barriers and suppress both inward oxygen diffusion and outward metal transport [61]. From a thermodynamic perspective, Nb5+ can substitute for Ti4+ in TiO2, reducing oxygen vacancy concentration and thus suppressing oxygen diffusion and slowing the oxidation rate [62,63]. Collectively, these factors highlight the complex but beneficial role of Nb in regulating the oxidation behavior of Ti2AlNb-based alloys.
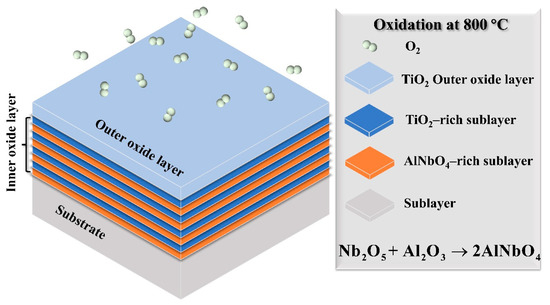
Figure 11.
Schematic illustration of the oxidation mechanism of the rolled Ti2AlNb-based alloy after exposure to air at 800 °C.
4. Conclusions
The high-temperature oxidation behavior of Ti2AlNb-based alloys with varying Nb contents at 800 °C was investigated. It was found that (i) the oxide scale exhibited a multilayer structure with an outer TiO2 layer and alternating TiO2-rich and AlNbO4-rich sublayers, where the thicker outer TiO2 layer is attributed to the higher diffusion rate of Ti. (ii) From the perspective of weight gain due to oxidation and oxide layer thickness, the oxidation resistance of the 23Nb alloy is superior to that of the 14Nb alloy, rendering it more suitable for service at 800 °C. This is because, on the one hand, Nb ions substitute for Ti ions, reducing the diffusion rate of oxygen within the oxide layer; on the other hand, an appropriate amount of Nb can enhance the oxidation activity of Al, thereby improving the alloy’s oxidation resistance. (iii) Thermodynamic analysis confirmed the favorable formation of AlNbO4 under the test conditions, explaining the absence of crystalline Al2O3, and overall oxidation kinetics followed an approximately linear trend, indicating a non-protective oxide scale.
Author Contributions
Conceptualization, Y.G., J.L., and S.Q.; methodology, Y.G., J.L., and G.C.; software, J.L., H.W., and Y.G.; validation, S.Q., G.C., and H.W.; formal analysis, Y.G., J.L., and A.F.; investigation, Y.G., J.L., and H.W.; resources, S.Q., D.C., and A.F.; data curation, Y.G. and J.L.; writing—original draft, Y.G. and J.L.; writing—review & editing, S.Q., A.F., and D.C.; visualization, Y.G. and J.L.; supervision, S.Q., A.F., and D.C.; project administration, S.Q. and A.F.; funding acquisition, S.Q. and D.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (NSFC) (Grant 52271012, 52571022, 51871168) and Science and Technology Major Project of Liaoning Province (2024JH1/11700028). DLC would like to thank the Natural Sciences and Engineering Research Council of Canada (NSERC) for the financial support.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
The authors also acknowledge the Advanced Materials Research Institute, and the Yangtze Delta Analytical Characterization Platform.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| XRD | X-ray diffraction |
| SEM | Scanning electron microscopy |
| EPMA | Electron probe microanalysis |
| EDS | Energy-dispersive X-ray spectroscopy |
| BSE | Backscattered electron |
| RD | Rolling direction |
| TD | Transverse direction |
| ND | Normal direction |
| ΔM | Weight gain per unit area |
| kn | Oxidation rate constant |
| n | kinetic exponent |
| t | Oxidation time |
| G | Gibbs free energies |
| H | Standard formation enthalpy |
| T | Temperature |
| DFT | Density functional theory |
| Eabs | The adsorption energy |
| Esystem | The total energy of the surface |
| Esurface | The energy of the clean surface |
| EO2 | The energy of a free O2 molecule |
References
- Banerjee, D.; Gogia, A.; Nandi, T.; Joshi, V. A new ordered orthorhombic phase in a Ti3Al-Nb alloy. Acta Metall. 1988, 36, 871–882. [Google Scholar] [CrossRef]
- Feng, A.; Chen, Q.; Wang, J.; Wang, H.; Qu, S.; Chen, D. Thermal stability of microstructures in low-density Ti2AlNb-based alloy hot rolled plate. Acta Metall. Sin. 2022, 59, 777–786. [Google Scholar]
- Man, J.; Huang, L.; He, J.; Yang, H.; Lin, X. Effect of heat treatment on microstructure and properties of Ti2AlNb alloy formed by selective laser melting. J. Mater. Res. Technol. 2024, 33, 2549–2559. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Li, W.P.; Wang, D.W.; Zhang, L.; Ohara, K.; Shen, J.; Ebel, T.; Yan, M. Selective laser melting enabled additive manufacturing of Ti–22Al–25Nb intermetallic: Excellent combination of strength and ductility, and unique microstructural features associated. Acta Mater. 2019, 173, 117–129. [Google Scholar] [CrossRef]
- Liu, Y.; Shan, Z.; Yang, X.; Liu, Q. Investigation on creep behavior and microstructural features of an additively manufactured Ti2AlNb alloy upon isothermal deformation at elevated temperature. Vacuum 2024, 223, 113112. [Google Scholar] [CrossRef]
- Yang, S.J.; Nam, S.W.; Hagiwara, M. Investigation of creep deformation mechanisms and environmental effects on creep resistance in a Ti2AlNb based intermetallic alloy. Intermetallics 2004, 12, 261–274. [Google Scholar] [CrossRef]
- Wei, W.; Weidong, Z.; Chen, X.; Xiaobo, L.; Jianwei, Z. Designed bimodal size lamellar O microstructures in Ti2AlNb based alloy: Microstructural evolution, tensile and creep properties. Mater. Sci. Eng. A 2014, 618, 288–294. [Google Scholar] [CrossRef]
- Esin, V.A.; Mallick, R.; Dadé, M.; Denand, B.; Delfosse, J.; Sallot, P. Combined synchrotron X-ray diffraction, dilatometry and electrical resistivity in situ study of phase transformations in a Ti2AlNb alloy. Mater. Charact. 2020, 169, 110654. [Google Scholar] [CrossRef]
- Zhang, K.; Lei, Z.; Chen, Y.; Yang, K.; Bao, Y. Heat treatment of laser-additive welded Ti2AlNb joints: Microstructure and tensile properties. Mater. Sci. Eng. A 2019, 744, 436–444. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, B.; Bai, Q.; Xie, G. Correlation of microstructure and mechanical properties of Ti2AlNb manufactured by SLM and heat treatment. Intermetallics 2021, 139, 107367. [Google Scholar] [CrossRef]
- Shen, J.; Feng, A. Recent advances on microstructural controlling and hot forming of Ti2AlNb-based alloys. Acta Metall. Sin. 2013, 49, 1286–1294. [Google Scholar] [CrossRef]
- Ayadh, W.; Denand, B.; Halkoum, A.; Boulet, P.; Sennour, M.; Delfosse, J.; Sallot, P.; Esin, V.A. Effect of prior α2 phase on precipitation kinetics of O-phase in advanced Ti2AlNb alloy. Acta Mater. 2023, 252, 118930. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Feng, A.H.; Qu, S.J.; Shen, J.; Chen, D.L. Microstructure and low cycle fatigue of a Ti2AlNb-based lightweight alloy. J. Mater. Sci. Technol. 2020, 44, 140–147. [Google Scholar] [CrossRef]
- Shao, B.; Tang, W.; Guo, S.; Zong, Y.; Shan, D.; Guo, B. Investigation of the O phase in the Ti–22Al–25Nb alloy during deformation at elevated temperatures: Plastic deformation mechanism and effect on B2 grain boundary embrittlement. Acta Mater. 2023, 242, 118467. [Google Scholar] [CrossRef]
- Shao, B.; Shan, D.; Guo, B.; Zong, Y. Plastic deformation mechanism and interaction of B2, α2, and O phases in Ti-22Al-25Nb alloy at room temperature. Int. J. Plast. 2019, 113, 18–34. [Google Scholar] [CrossRef]
- Koike, J.; Shimoyama, Y.; Ohnuma, I.; Okamura, T.; Kainuma, R.; Ishida, K.; Maruyama, K. Stress-induced phase transformation during superplastic deformation in two-phase Ti–Al–Fe alloy. Acta Mater. 2000, 48, 2059–2069. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, N.; Liang, H.; Liu, Y. Phase transformation and microstructure control of Ti2AlNb-based alloys: A review. J. Mater. Sci. Technol. 2021, 80, 203–216. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, Y.; Zhang, J.; Liu, Y. Synergistic effect of B2 phase and O phase in hot-rolled Ti2AlNb alloy and its influences on mechanical properties. J. Mater. Res. Technol. 2024, 30, 2004–2017. [Google Scholar] [CrossRef]
- Dadé, M.; Esin, V.A.; Nazé, L.; Sallot, P. Short- and long-term oxidation behaviour of an advanced Ti2AlNb alloy. Corros. Sci. 2019, 148, 379–387. [Google Scholar] [CrossRef]
- Leyens, C. Oxidation of orthorhombic titanium aluminide Tl-22AL-25NB in air between 650 and 1000 °C. J. Mater. Eng. Perform. 2001, 10, 225–230. [Google Scholar] [CrossRef]
- Koo, C.H.; Evans, J.W.; Song, K.Y.; Yu, T.H. High-temperature oxidation of Ti3Al−Nb alloys. Oxid. Met. 1994, 42, 529–544. [Google Scholar] [CrossRef]
- He, Y.-S.; Hu, R.; Luo, W.-Z.; He, T.; Liu, X.-H. Oxidation behavior of a novel multi-element alloyed Ti2AlNb-based alloy in temperature range of 650–850 °C. Rare Met. 2018, 37, 838–845. [Google Scholar] [CrossRef]
- Zhao, P.-x.; Li, X.-b.; Xing, W.-w.; Chen, B.; Ma, Y.-c.; Liu, K. Cyclic oxidation behavior of Nb/Mn/Si alloying beta-gamma TiAl alloys. Trans. Nonferrous Met. Soc. China 2023, 33, 128–140. [Google Scholar] [CrossRef]
- Malecka, J. Resistance to High-Temperature Oxidation of Ti-Al-Nb Alloys. Materials 2022, 15, 2137. [Google Scholar] [CrossRef]
- Xiang, L.L.; Zhao, L.L.; Wang, Y.L.; Zhang, L.Q.; Lin, J.P. Synergistic effect of Y and Nb on the high temperature oxidation resistance of high Nb containing TiAl alloys. Intermetallics 2012, 27, 6–13. [Google Scholar] [CrossRef]
- Vojtěch, D.; Popela, T.; Kubásek, J.; Maixner, J.; Novák, P. Comparison of Nb- and Ta-effectiveness for improvement of the cyclic oxidation resistance of TiAl-based intermetallics. Intermetallics 2011, 19, 493–501. [Google Scholar] [CrossRef]
- Li, Y.; Dai, J.; Song, Y. Research Progress of First Principles Studies on Oxidation Behaviors of Ti-Al Alloys and Alloying Influence. Metals 2021, 11, 985. [Google Scholar] [CrossRef]
- Kim, J.; Emura, S.; Lee, Y. High Temperature Oxidation Behavior of Ti2AlNb Intermetallic Alloys. Int. J. Metall. Mater. Eng. 2015, 2015, 112. [Google Scholar] [CrossRef][Green Version]
- Wang, S.; Xu, W.; Shao, B.; Yang, G.; Zong, Y.; Sun, W.; Yang, Z.; Shan, D. Process design and microstructure-property evolution during shear spinning of Ti2AlNb-based alloy. J. Mater. Sci. Technol. 2022, 101, 1–17. [Google Scholar] [CrossRef]
- Jing, C.; Qu, S.; Feng, A.; Wang, H.; Chen, D. Influence of Microstructure and Texture on Tensile Properties of an As-Rolled Ti2AlNb-Based Alloy. Metals 2025, 15, 631. [Google Scholar] [CrossRef]
- Zhao, P.; Cao, G.; Feng, A.; Qu, S.; Wang, H.; Chen, D. Novel insights into anisotropy in a rolled Ti2AlNb-based alloy: The role of α2, O and B2 phases. Mater. Lett. 2025, 400, 139188. [Google Scholar] [CrossRef]
- Sadi, F.A.; Servant, C. On the B2→O phase transformation in Ti–Al–Nb alloys. Mater. Sci. Eng. A 2003, 346, 19–28. [Google Scholar] [CrossRef]
- Li, D.; Zeng, W.; Zhang, F.; Xu, J.; Ma, X.; Liang, X. Precipitation Behavior of O Phase during Continuous Cooling of Ti-22Al-25Nb Alloy. Metals 2022, 12, 291. [Google Scholar] [CrossRef]
- Dai, C.-r.; Yang, Z.-b.; Sun, J.; Lu, S.; Vitos, L. Composition and temperature dependence of α2 phase decomposition in high Nb-containing lamellar γ-TiAl alloys: Experiments and first-principles calculations. Acta Mater. 2021, 221, 117419. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Wang, D.W.; Song, L.J.; Mukhtar, A.; Huang, D.N.; Yang, C.; Yan, M. Effect of heat treatments on the microstructure and mechanical properties of Ti2AlNb intermetallic fabricated by selective laser melting. Mater. Sci. Eng. A 2021, 817, 141352. [Google Scholar] [CrossRef]
- Zheng, J.; Hou, X.; Wang, X.; Meng, Y.; Zheng, X.; Zheng, L. Isothermal oxidation mechanism of Nb–Ti–V–Al–Zr alloy at 700–1200 °C: Diffusion and interface reaction. Corros. Sci. 2015, 96, 186–195. [Google Scholar] [CrossRef]
- Estupinán-López, F.; Orquiz-Muela, C.; Gaona-Tiburcio, C.; Cabral-Miramontes, J.; Bautista-Margulis, R.G.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Almeraya-Calderón, F.; Lopes, A.J. Oxidation Kinetics of Ti-6Al-4V Alloys by Conventional and Electron Beam Additive Manufacturing. Materials 2023, 16, 1187. [Google Scholar] [CrossRef]
- Qu, S.J.; Tang, S.Q.; Feng, A.H.; Feng, C.; Shen, J.; Chen, D.L. Microstructural evolution and high-temperature oxidation mechanisms of a titanium aluminide based alloy. Acta Mater. 2018, 148, 300–310. [Google Scholar] [CrossRef]
- Gao, Z.; Hu, R.; Zou, H.; Zhou, M.; Luo, X. Insight into the Ta alloying effects on the oxidation behavior and mechanism of cast TiAl alloy. Mater. Des. 2024, 241, 112941. [Google Scholar] [CrossRef]
- Shiwei, T.; Anrui, H.; Jianhua, L.; Yefei, Z.; Yonggang, Y.; Yun, Z.; Haitao, J. Oxidation resistance of TiAl alloy improved by hot-pack rolling and cyclic heat treatment. Mater. Charact. 2021, 178, 111196. [Google Scholar] [CrossRef]
- Ralison, A.; Dettenwanger, F.; Schütze, M. Oxidation of orthorhombic Ti2AlNb alloys in the temperature range 550–1000 °C in air. Mater. High Temp. 2003, 20, 607–629. [Google Scholar] [CrossRef]
- Xiang, J.M.; Mi, G.B.; Qu, S.J.; Huang, X.; Chen, Z.; Feng, A.H.; Shen, J.; Chen, D.L. Thermodynamic and microstructural study of Ti2AlNb oxides at 800 °C. Sci. Rep. 2018, 8, 12761. [Google Scholar] [CrossRef]
- Wu, G.D.; Cui, G.R.; Qu, S.J.; Feng, A.H.; Cao, G.J.; Ge, B.H.; Xiang, H.P.; Shen, J.; Chen, D.L. High-temperature oxidation mechanisms of nano-/submicro-scale lamellar structures in an intermetallic alloy. Scr. Mater. 2019, 171, 102–107. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Q.; Qu, S.J.; Xiang, H.P.; Wang, C.; Gao, J.B.; Feng, A.H.; Chen, D.L. Oxidation mechanisms of an intermetallic alloy at high temperatures. Scr. Mater. 2021, 199, 113852. [Google Scholar] [CrossRef]
- Tripathi, A.; Middleton, S.; Lavernia, E.J.; Sachdev, A.K.; Kulkarni, K.N. Ternary Interdiffusion in β (BCC) Phase of the Ti-Al-Nb System. J. Phase Equilib. Diffus. 2018, 39, 841–852. [Google Scholar] [CrossRef]
- Haanappel, V.A.C.; Clemens, H.; Stroosnijder, M.F. The High Temperature Oxidation Behaviour of High and Low Alloyed TiAl-Based Intermetallics. Intermetallics 2002, 10, 293–305. [Google Scholar] [CrossRef]
- Lu, W.; Chen, C.; Xi, Y.; Wang, F.; He, L. The oxidation behavior of Ti–46.5Al–5Nb at 900 °C. Intermetallics 2007, 15, 989–998. [Google Scholar] [CrossRef]
- Cheng, J.W.; Li, J.F.; Rao, Q.L. Time-resolved in-situ XRD study on oxidation evolution of Ti2AlNb-based alloys. Mater. Today Commun. 2023, 36, 106660. [Google Scholar] [CrossRef]
- Chen, X.; Xie, F.; Ma, T.; Li, W.; Wu, X. Oxidation Behavior of Three Different Zones of Linear Friction Welded Ti2AlNb Alloy. Adv. Eng. Mater. 2016, 18, 1944–1951. [Google Scholar] [CrossRef]
- Li, Y.; Dai, J.; Song, Y.; Yang, R. Adsorption properties of oxygen atom on the surface of Ti2AlNb by first principles calculations. Comput. Mater. Sci. 2017, 139, 412–418. [Google Scholar] [CrossRef]
- Zong, Y.; Wang, J.; Shao, B.; Tang, W.; Shan, D. Mechanism and morphology evolution of the O phase transformation in Ti-22Al-25Nb alloy. J. Mater. Sci. Technol. 2021, 89, 97–106. [Google Scholar] [CrossRef]
- Chicardi, E.; Córdoba, J.M.; Gotor, F.J. Kinetics of high-temperature oxidation of (Ti,Ta)(C,N)-based cermets. Corros. Sci. 2016, 102, 168–177. [Google Scholar] [CrossRef]
- Zhao, Z.; Yu, X.; Wang, C.; Yao, S.; Qi, Q.; Wang, L. Oxidation mechanism of in-situ TiC/Ni composites at 1073 K. Corros. Sci. 2022, 194, 109958. [Google Scholar] [CrossRef]
- Valenza, T.C.; Weber, P.K.; Marquis, E.A. Role of niobium in the high-temperature oxidation of titanium. Corros. Sci. 2023, 225, 111603. [Google Scholar] [CrossRef]
- Jiang, H.; Hirohasi, M.; Lu, Y.; Imanari, H. Effect of Nb on the high temperature oxidation of Ti–(0–50 at.%)Al. Scr. Mater. 2002, 46, 639–643. [Google Scholar] [CrossRef]
- Pflumm, R.; Friedle, S.; Schütze, M. Oxidation protection of γ-TiAl-based alloys–A review. Intermetallics 2015, 56, 1–14. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, J.; Wang, Y.; Ma, T.; Zhu, D.; Xing, Q.; Fang, H.; Chen, R. High-temperature oxidation behavior of ceramic particles-reinforced TiAl composites with multilayered structure. Ceram. Int. 2024, 50, 2233–2241. [Google Scholar] [CrossRef]
- Feng, L.; Li, B.; Li, Q.; Gao, Y.; Pei, Z.; Liang, C. Enhancement of mechanical properties and oxidation resistance of TiAl alloy with addition of Nb and Mo alloying elements. Mater. Chem. Phys. 2024, 316, 129148. [Google Scholar] [CrossRef]
- Sun, T.; Guo, Z.; Cao, J.; Liang, Y.; Lin, J. Isothermal oxidation behavior of high-Nb-containing TiAl alloys doped with W, B, Y, and C/Si. Corros. Sci. 2023, 213, 110980. [Google Scholar] [CrossRef]
- Liu, X.; Sun, H.; Jiang, X.; Liu, R.; Yan, W.; Chen, S.; Wang, L. Isothermal oxidation behaviour of TiAl alloys prepared by spark plasma sintering with the addition of Gd under water vapour at 900 °C. Intermetallics 2023, 153, 107796. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Z.; Wu, J.; Li, Q.; Wu, C.; Li, Y. Effects of Nb on the elements diffusion and mechanical properties of laminated Ti/Al2O3 composites. Mater. Sci. Eng. A 2015, 636, 263–268. [Google Scholar] [CrossRef]
- Lin, J.P.; Zhao, L.L.; Li, G.Y.; Zhang, L.Q.; Song, X.P.; Ye, F.; Chen, G.L. Effect of Nb on oxidation behavior of high Nb containing TiAl alloys. Intermetallics 2011, 19, 131–136. [Google Scholar] [CrossRef]
- Liang, Z.; Xiao, S.; Yue, H.; Li, X.; Li, Q.; Zheng, Y.; Xu, L.; Xue, X.; Tian, J.; Chen, Y. Tailoring microstructure and improving oxidation resistance of an additively manufactured high Nb containing TiAl alloy via heat treatment. Corros. Sci. 2023, 220, 111287. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).