Electrochemical Behavior of the Ni3Al Intermetallic Alloy in Nitrate Salts
Abstract
1. Introduction
2. Materials and Methods
2.1. Ni3Al Alloy
2.2. Corrosive Medium
2.3. Gravimetric Tests
2.4. Electrochemical Tests
3. Results and Discussion
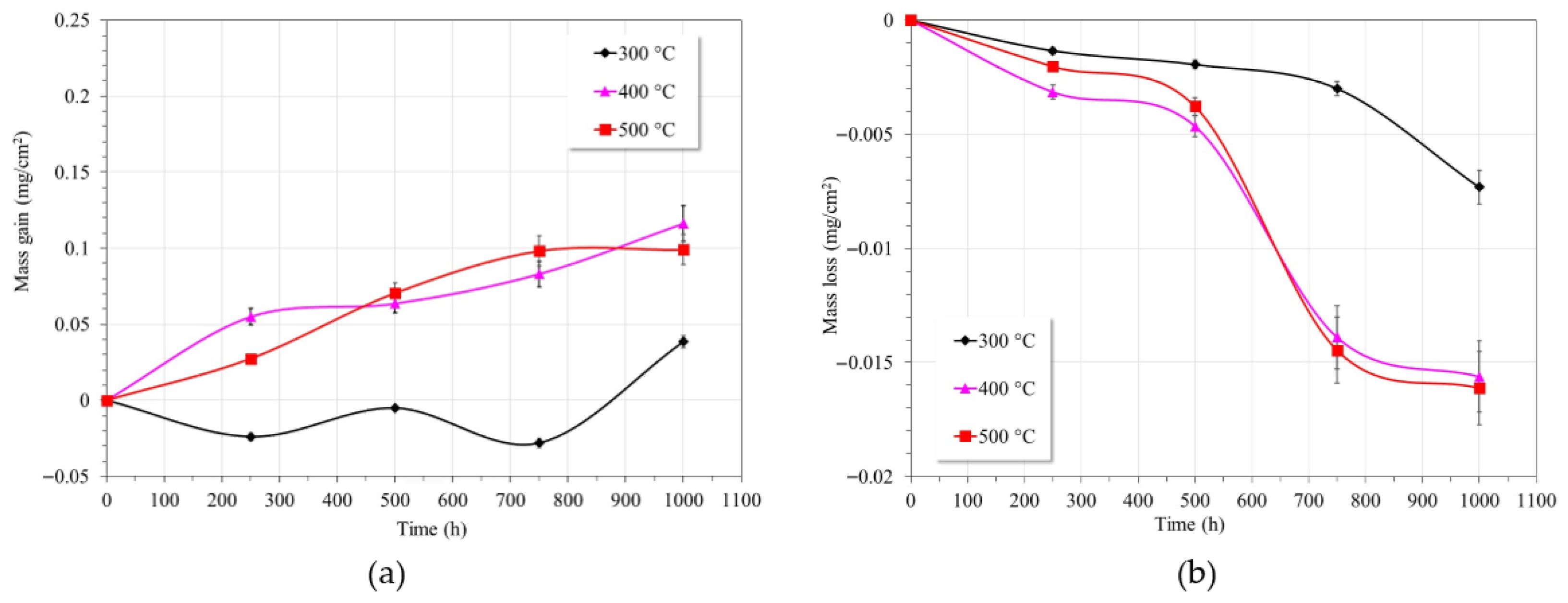
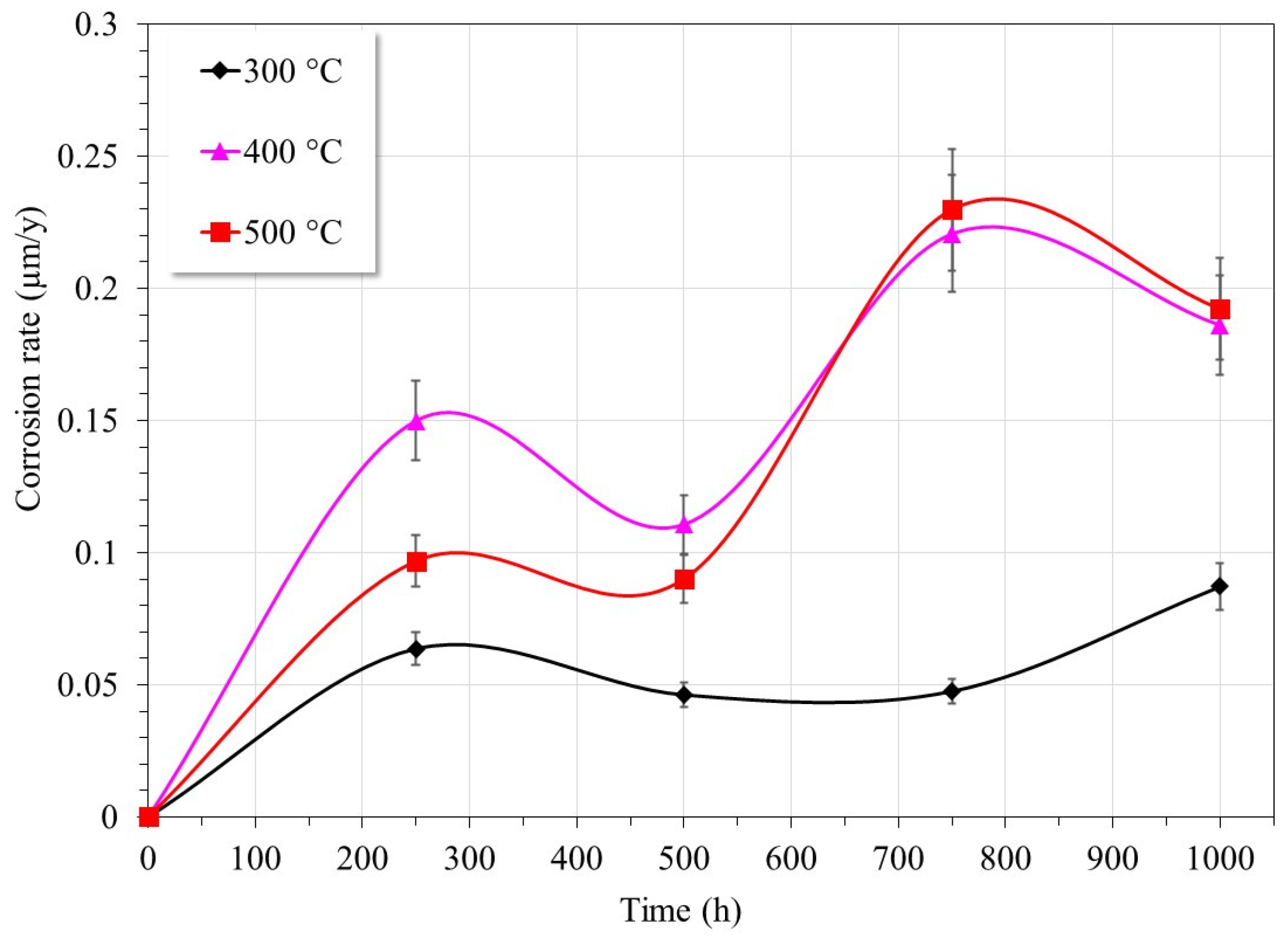
3.1. Gravimetric Tests
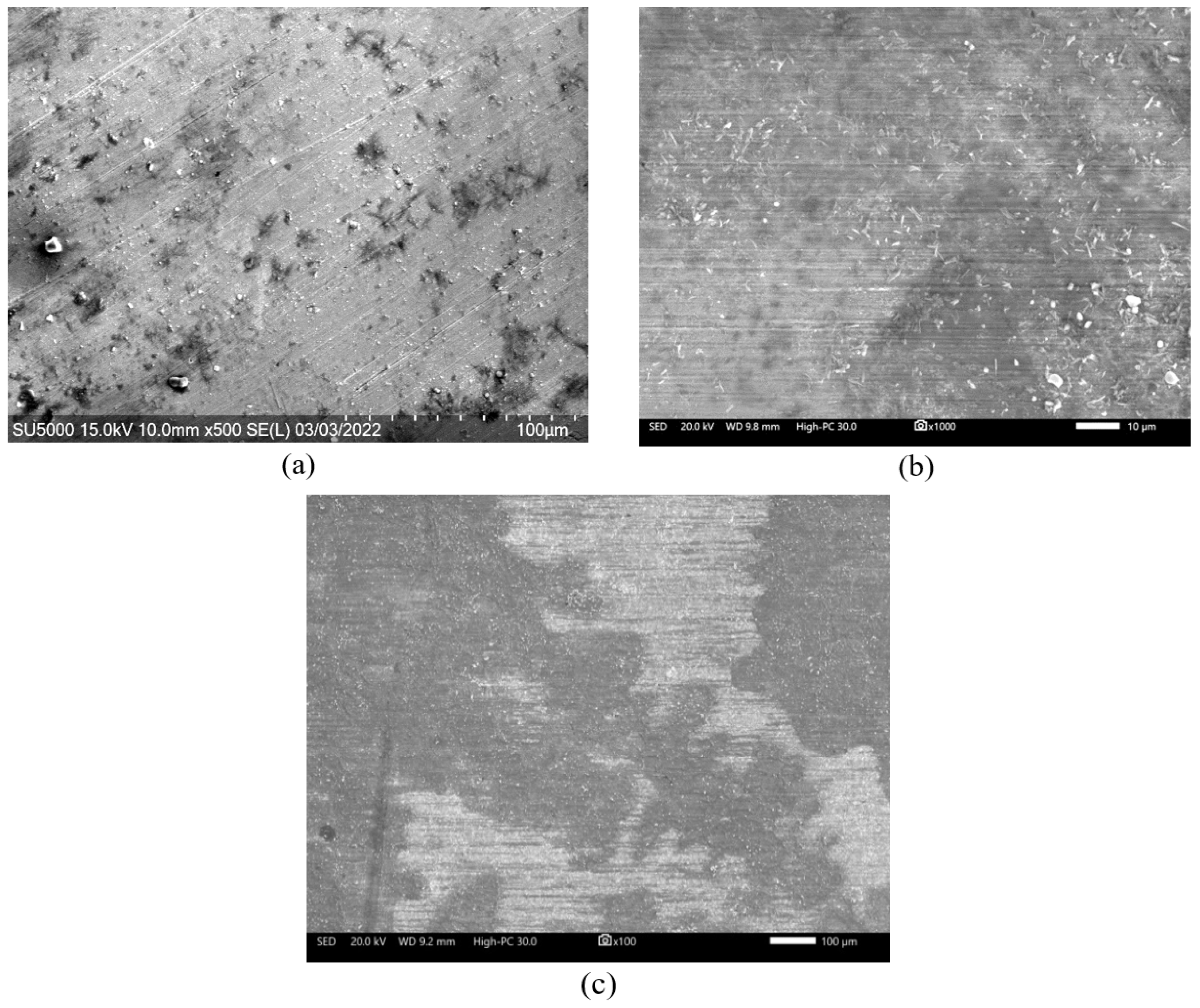
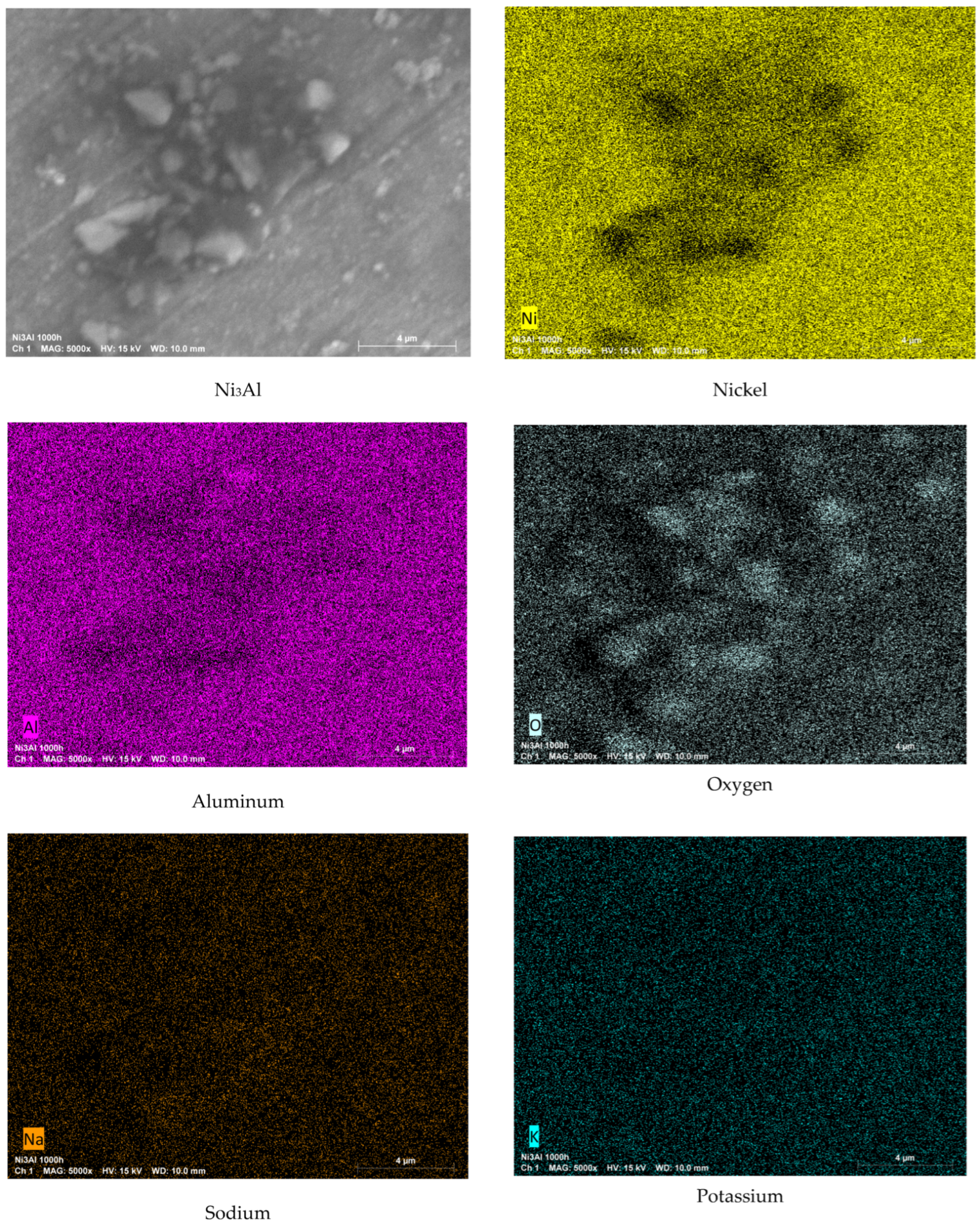
3.2. Surface Analysis by Scanning Electron Microscopy
3.3. Potentiodynamic Polarization Curves
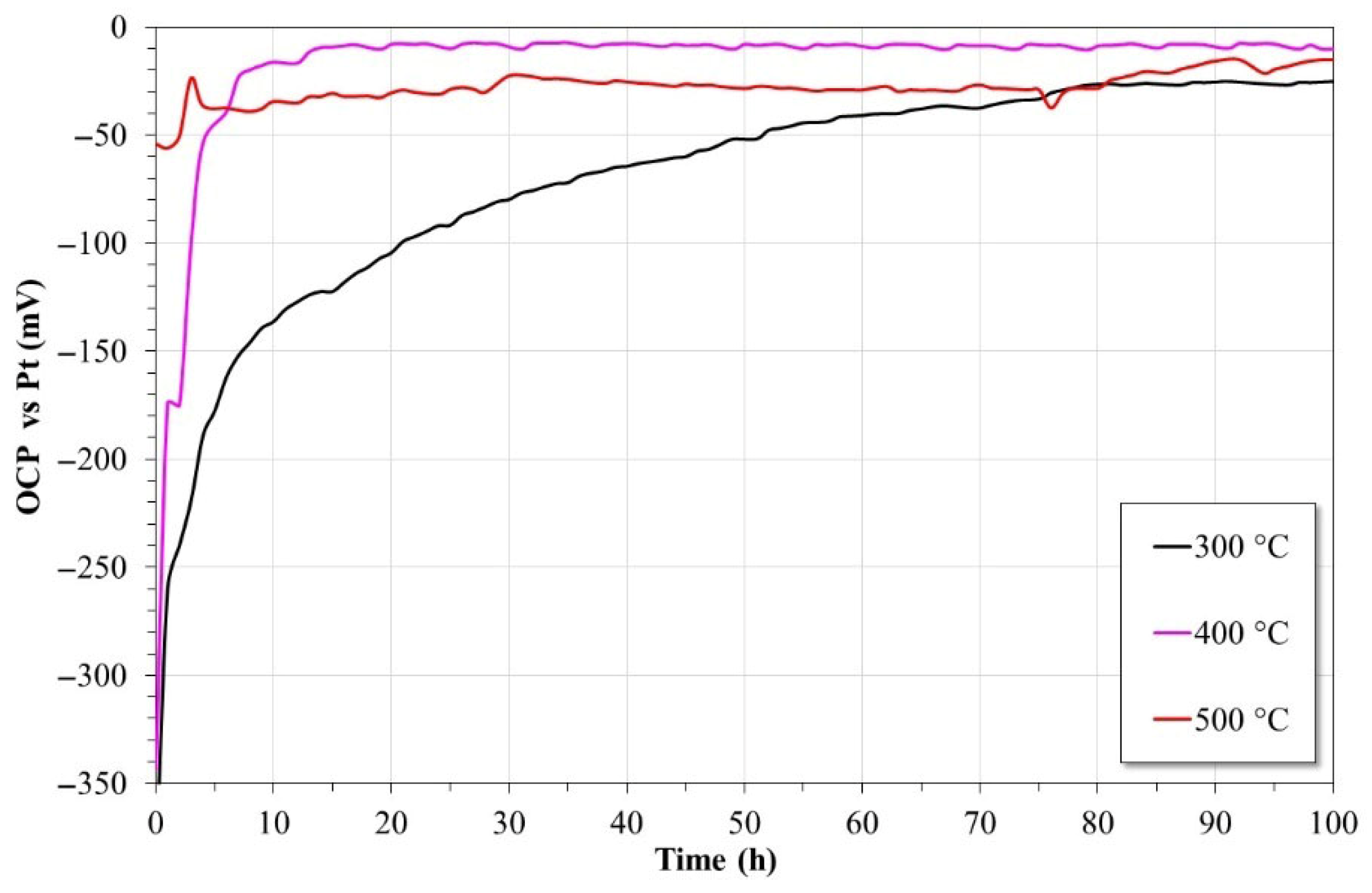
3.4. Open Circuit Potential (OCP)
3.5. Linear Polarization Resistance (LPR)
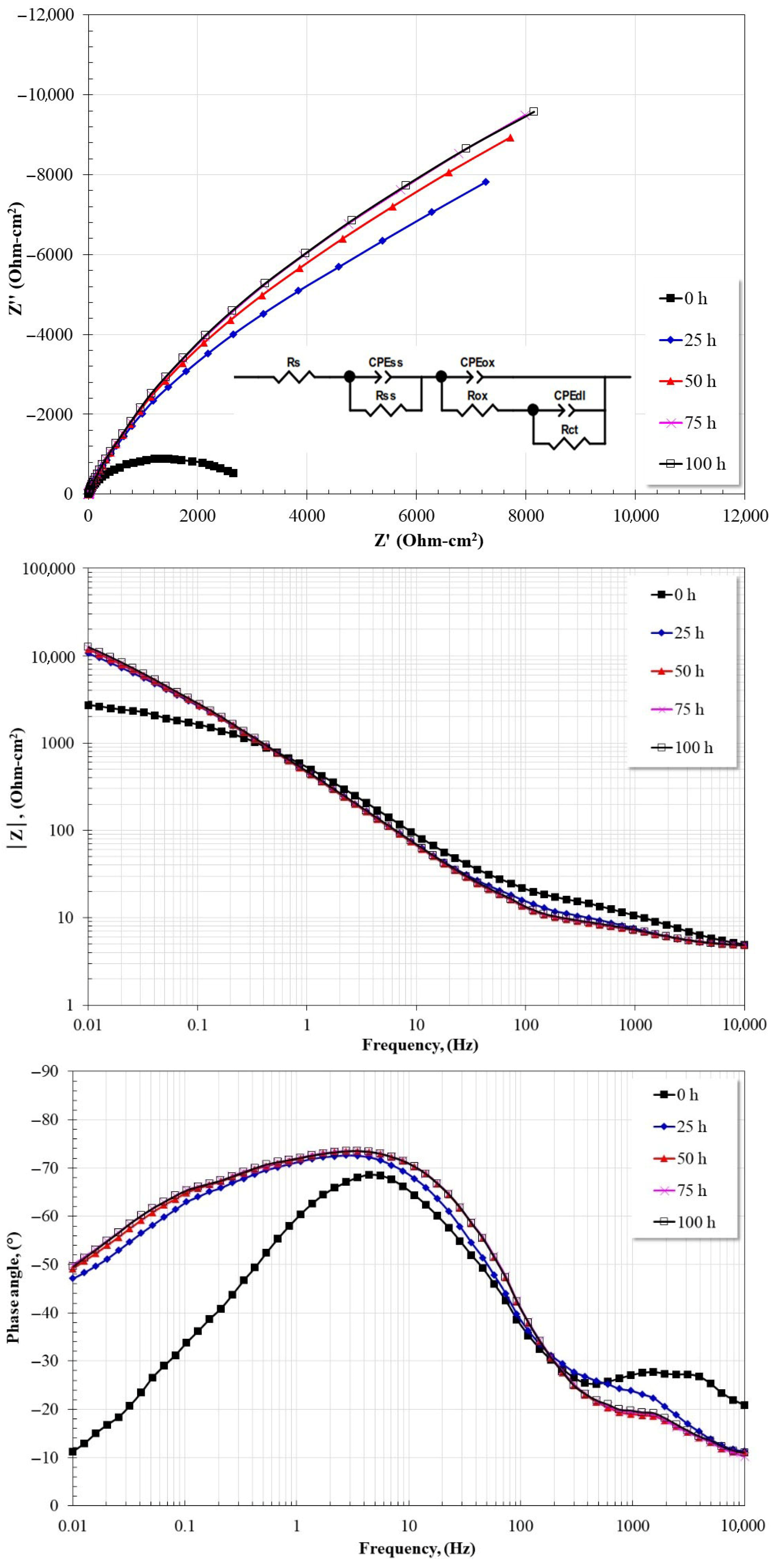
3.6. Electrochemical Impedance Spectroscopy (EIS)
3.7. Reaction Mechanisms
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Encinas-Sánchez, V.; de Miguel, M.T.; Lasanta, M.I.; García-Martín, G.; Pérez, F.J. Electrochemical impedance spectroscopy (EIS): An efficient technique for monitoring corrosion processes in molten salt environments in CSP applications. Sol. Energy Mater. Sol. Cells 2019, 191, 157–163. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, C.; Wu, Y.; Lu, Y. Comparative review of different influence factors on molten salt corrosion characteristics for thermal energy storage. Sol. Energy Mater. Sol. Cells 2022, 235, 111485. [Google Scholar] [CrossRef]
- Zhan, F.; Yu, H.; Yang, P.; Zhu, M.; Bai, Z.; Gao, Y. Review of Molten Salt Corrosion in Stainless Steels and Superalloys. Crystals 2025, 15, 237. [Google Scholar] [CrossRef]
- Mortazavi, A.; Zhao, Y.; Esmaily, M.; Allanore, A.; Vidal, J.; Birbilis, N. High-temperature corrosion of a nickel-based alloy in a molten chloride environment—The effect of thermal and chemical purifications. Sol. Energy Mater. Sol. Cells 2022, 236, 111542. [Google Scholar] [CrossRef]
- Fernández, A.G.; Cabeza, L.F. Corrosion monitoring and mitigation techniques on advanced thermal energy storage materials for CSP plants. Sol. Energy Mater. Sol. Cells 2019, 192, 179–187. [Google Scholar] [CrossRef]
- Sutter, F.; Oskay, C.; Galetz, M.C.; Diamantino, T.; Pedrosa, F.; Figueira, I.; Glumm, S.; Bonk, A.; Agüero, A.; Rodríguez, S.; et al. Dynamic corrosion testing of metals in solar salt for concentrated solar power. Sol. Energy Mater. Sol. Cells 2021, 232, 111331. [Google Scholar] [CrossRef]
- Fernandez, A.G.; Rey, A.; Lasanta, I.; Mato, S.; Brady, M.; Perez, F.J. Corrosion of alumina-forming austenitic steel in molten nitrate salts by gravimetric analysis and impedance spectroscopy. Mater. Corros. 2014, 65, 267–275. [Google Scholar] [CrossRef]
- Cheng, W.-J.; Chen, D.-J.; Wang, C.-J. High-temperature corrosion of Cr–Mo steel in molten LiNO3–NaNO3–KNO3 eutectic salt for thermal energy storage. Sol. Energy Mater. Sol. Cells 2015, 132, 563–569. [Google Scholar] [CrossRef]
- Ignatiev, V.; Surenkov, A.; Gnidoy, I. Intergranular tellurium cracking of nickel-based alloys in molten salt mixture. J. Nucl. Mater. 2013, 440, 243–249. [Google Scholar] [CrossRef]
- Kumar, S.; Chattopadhyay, K.; Alam, Z.; Singh, V.; Satyanarayana, D.V.V.; Kumar, V. Corrosion Behavior of Surface Nanostructured IN718 Superalloy at 650 °C. Matls. Perf. Charact. 2022, 11, 157–170. [Google Scholar] [CrossRef]
- Felix-Contreras, R.; de la Vega Olivas, J.; Arrieta-Gonzalez, C.D.; Chacon-Nava, J.G.; Rodriguez-Diaz, R.A.; Gonzalez-Rodriguez, J.G.; Porcayo-Calderon, J. Kinetic and Thermodynamic Aspects of the Degradation of Ferritic Steels Immersed in Solar Salt. Materials 2024, 17, 5776. [Google Scholar] [CrossRef] [PubMed]
- Brady, M.P.; Yamamoto, Y.; Santella, M.L.; Pint, B.A. Effects of minor alloy additions and oxidation temperature on protective alumina scale formation in creep-resistant austenitic stainless steels. Scr. Mater. 2007, 57, 1117–1120. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Brady, M.P.; Santella, M.L.; Bei, H.; Maziasz, P.J.; Pint, B.A. Overview of Strategies for High-Temperature Creep and Oxidation Resistance of Alumina-Forming Austenitic Stainless Steels. Metall. Mater. Trans. A 2011, 42, 922–931. [Google Scholar] [CrossRef]
- Shi, J.; Meng, F.; Huang, G.; Liu, F.; Zhai, L.; Chen, Y.; Zhang, F.; Lin, J.; Wang, L. Improving the oxidation resistance by forming continuous Al2O3 protective layer in alumina-forming austenitic stainless steel. Surf. Coat. Technol. 2024, 493, 131279. [Google Scholar] [CrossRef]
- Wenga, T.; Wu, X.; Yixian, X.; Zeng, S.; Ma, W. High-temperature corrosion mechanisms of a Ni-based alloy in simulated multi-source organic waste co-incineration environments. Fuel 2024, 377, 132784. [Google Scholar] [CrossRef]
- Yang, S.; Gao, S.-Y.; Xue, W.-H.; Wu, B.; Duan, D.L. Oxidation, hot corrosion, and interdiffusion behavior of NiAlTa coating by electro-spark deposition. Rare Met. 2025, 44, 4988–5004. [Google Scholar] [CrossRef]
- Yadav, P.; Abro, M.A.; Lee, D.B.; Yoon, J. High-temperature corrosion of pure Ni3Al and its alloyed (2.99 wt.% Ti) in Ar-0.2% SO2 gas environment. J. Mater. Res. Technol. 2022, 17, 3055–3065. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Rao, A.U.; Kharb, A.S.; Chawla, A.K.; Avasthi, D.K. A review of mechanical and tribological properties of Ni3Al-based coatings-synthesis and high-temperature behavior. Phys. Scr. 2023, 98, 072001. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, Q.; Zhou, Q.; Zhang, C.; Song, B.; Qiao, J.; Fan, D.; He, J.; He, W.; Guo, H.; et al. Research progress in high temperature functional coatings for advanced aeroengines. J. Aeronautical Mater. 2024, 44, 48–69. [Google Scholar] [CrossRef]
- McConohy, G.; Kruizenga, A. Molten nitrate salts at 600 and 680 °C: Thermophysical property changes and corrosion of high-temperature nickel alloys. Sol. Energy 2014, 103, 242–252. [Google Scholar] [CrossRef]
- Dorcheh, A.S.; Durham, R.N.; Galetz, M.C. Corrosion behavior of stainless and low-chromium steels and IN625 in molten nitrate salts at 600 °C. Sol. Energy Mater. Sol. Cells 2016, 144, 109–116. [Google Scholar] [CrossRef]
- Fernández, A.G.; Galleguillos, H.; Fuentealba, E.; Pérez, F.J. Corrosion of stainless steels and low-Cr steel in molten Ca(NO3)2–NaNO3–KNO3 eutectic salt for direct energy storage in CSP plants. Sol. Energy Mater. Sol. Cells 2015, 141, 7–13. [Google Scholar] [CrossRef]
- Bradshaw, R.W. Thermal convection loop study of the corrosion of incoloy 800 in molten NaNO3-KNO3. Corrosion 1987, 43, 173–178. [Google Scholar] [CrossRef]
- ISO 17245; Corrosion of Metals and Alloys—Test Method for High Temperature Corrosion Testing of Metallic Materials by Immersing in Molten Salt or Other Liquids Under Static Conditions International Standard, First Edition 2015-04-15. ISO: Geneva, Switzerland, 2015.
- ASTM G31-72; Standard Practice for Laboratory Immersion Corrosion Testing of Metals. ASTM: West Conshohocken, PA, USA, 2004.
- Yang, P.; Deng, Z.; Chen, B.; Wang, Y. High-temperature dynamic corrosion mechanisms of austenitic stainless and carbon steels in nitrates for concentrating solar power. Sol. Energy Mater. Sol. Cells 2024, 266, 112690. [Google Scholar] [CrossRef]
- Gomez-Guzman, N.B.; Lopez-Dominguez, D.; Arrieta-Gonzalez, C.D.; Mayen, J.; Porcayo-Palafox, E.; Chacon-Nava, J.G.; Gonzalez-Rodriguez, J.G.; Porcayo-Calderon, J.; Rodriguez-Diaz, R.A. Behavior of Ni20Cr Alloy in Molten Nitrate Salts. Int. J. Mol. Sci. 2022, 23, 7895. [Google Scholar] [CrossRef] [PubMed]
- Audigié, P.; Encinas-Sánchez, V.; Juez-Lorenzo, M.; Rodríguez, S.; Gutiérrez, M.; Pérez, F.J.; Agüeroa, A. High temperature molten salt corrosion behavior of aluminide and nickel-aluminide coatings for heat storage in concentrated solar power plants. Surf. Coat. Technol. 2018, 349, 1148–1157. [Google Scholar] [CrossRef]
- Sidhu, B.S.; Prakash, S. Evaluation of the corrosion behaviour of plasma-sprayed Ni3Al coatings on steel in oxidation and molten salt environments at 900 °C. Surf. Coat. Technol. 2003, 166, 89–100. [Google Scholar] [CrossRef]
- Ramos-Hernandez, J.J.; Porcayo-Calderon, J.; Gonzalez-Rodriguez, J.G.; Mayén, J.; Lara-Rodriguez, G.; Salinas-Bravo, V.M.; Martinez-Gomez, L. Effect of the Noble Metals Addition on the Oxidation Behavior of Ni3Al. Adv. Mater. Sci. Eng. 2018, 2018, 1616340. [Google Scholar] [CrossRef]
- Ramos-Hernandez, J.J.; Arrieta-Gonzalez, C.D.; Chacon-Nava, J.G.; Porcayo-Palafox, E.; Sanchez-Carrillo, M.; Flores-De los Rios, J.P.; Pedraza-Basulto, G.K.; Diaz-Mendez, S.E.; Porcayo-Calderon, J. Effect of Noble Metal Addition on the Disorder Dynamics of Ni3Al by Means of MonteCarlo Simulation. Materials 2020, 13, 4832. [Google Scholar] [CrossRef]
- Choi, S.C.; Cho, H.J.; Lee, D.B. Effect of Cr, Co, and Ti additions on the high-temperature oxidation behavior of Ni3Al. Oxid. Met. 1996, 46, 109–127. [Google Scholar] [CrossRef]
- Chang, T.T.; Pan, Y.C.; Chuang, T.H. The oxidation behavior of Ni3AlZr alloys with various zirconium contents. J. Alloy Compd. 1996, 243, 126–132. [Google Scholar] [CrossRef]
- Haasch, R.T.; Venezia, A.M.; Loxton, C.M. The relationship between mass transport and oxide chemistry in oxidation of Ni3Al alloys. J. Mater. Res. 1992, 7, 1341–1349. [Google Scholar] [CrossRef]
- Pan, Y.C.; Chuang, T.H.; Yao, Y.D. Long-term oxidation behaviour of Ni3Al alloys with and without chromium additions. J. Mater. Sci. 1991, 26, 6097–6103. [Google Scholar] [CrossRef]
- Singh, H.; Prakash, S.; Puri, D. Some observations on the high temperature oxidation behaviour of plasma sprayed Ni3Al coatings. Mater. Sci. Eng. A 2007, 444, 242–250. [Google Scholar] [CrossRef]
- Meißner, T.M.; Oskay, C.; Bonk, A.; Gregoire, B.; Donchev, A.; Solimani, A.; Galetz, M.C. Improving the corrosion resistance of ferritic-martensitic steels at 600 ◦C in molten solar salt via diffusion coatings. Sol. Energy Mater. Sol. Cells 2021, 227, 111105. [Google Scholar] [CrossRef]
- Zahrani, E.M.; Cuevas-Arteaga, C.; Verhelst, D.; Alfantazi, A. High Temperature Corrosion of 625 Superalloy under Iron-Zinc Oxide/Lead Sulfate Salt Mixture. ECS Meet. Abstr. 2010, MA2010-01, 895. [Google Scholar] [CrossRef]
- Cuevas Arteaga, C. Corrosion study of HK-40m alloy exposed to molten sulfate/vanadate mixtures using the electrochemical noise technique. Corros. Sci. 2008, 50, 650–663. [Google Scholar] [CrossRef]
- Cuevas-Arteaga, C.; Uruchurtu, J.; Gonzalez, J.; Izquierdo-Montalvo, G.; Porcayo Calderon, J.; Cano-Castillo, U. Corrosion evaluation of Alloy 800 in sulfate/vanadate molten salts. Corrosion 2004, 60, 548–560. [Google Scholar] [CrossRef]
- Cuevas-Arteaga, C.; Porcayo-Calderon, J.; Izquierdo, G.; Martinez-Villafañe, A.; Gonzalez-Rodriguez, J.G. Study of hot corrosion of alloy 800 using linear polarisation resistance and weight loss measurement. Mater. Sci. Technol. 2001, 17, 880–885. [Google Scholar] [CrossRef]
- ASTM G102; Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Information. ASTM: West Conshohocken, PA, USA, 2023.
- Nunes, V.M.B.; Queirós, C.S.; Lourenço, M.J.V.; Santos, F.J.V.; Nieto de Castro, C.A. Molten salts as engineering fluids—A review Part I. Molten alkali nitrates. Appl. Energy 2016, 183, 603–611. [Google Scholar] [CrossRef]
- Bradshaw, R.W.; Meeker, D.E. High-temperature stability of ternary nitrate molten salts for solar thermal energy systems. Sol. Energy Mater. 1990, 21, 51–60. [Google Scholar] [CrossRef]
- Fernández, A.G.; Ushak, S.; Galleguillos, H.; Pérez, F.J. Development of new molten salts with LiNO3 and Ca(NO3)2 for energy storage in CSP plants. Appl. Energy 2014, 119, 131–140. [Google Scholar] [CrossRef]
- Carranza, R.M.; Alvarez, M.G. The effect of temperature on the passive film properties and pitting behaviour of a Fe-Cr-Ni alloy. Corros. Sci. 1996, 38, 909–925. [Google Scholar] [CrossRef]
- Guilemany, J.M.; Fernandez, J.; Espallargas, N.; Suegama, P.H.; Benedetti, A.V. Influence of spraying parameters on the electrochemical behaviour of HVOF thermally sprayed stainless steel coatings in 3.4% NaCl. Surf. Coat. Technol. 2006, 200, 3064–3072. [Google Scholar] [CrossRef]
- Romo, L.; Gonzalez-Rodriguez, J.G.; Porcayo-Calderon, J.; Guardian, R.; Salinas-Bravo, V.M. A study on the effect of Co, Cr and Ti on the corrosion of FE40AL intermetallic in molten NaCl–KCl mixture. Intermetallics 2015, 67, 156–165. [Google Scholar] [CrossRef]
- Szczygieł, B.; Kołodziej, M. Composite Ni/Al2O3 coatings and their corrosion resistance. Electrochim. Acta 2005, 50, 4188–4195. [Google Scholar] [CrossRef]
- Fattah-alhosseini, A.; Soltani, F.; Shirsalimi, F.; Ezadi, B.; Attarzadeh, N. The semiconducting properties of passive films formed on AISI 316 L and AISI 321 stainless steels: A test of the point defect model (PDM). Corros. Sci. 2011, 53, 3186–3192. [Google Scholar] [CrossRef]
- Hsu, C.H.; Mansfeld, F. Technical Note: Concerning the Conversion of the Constant Phase Element Parameter Y0 into a Capacitance. Corrosion 2001, 57, 747–748. [Google Scholar] [CrossRef]
- Brug, G.J.; van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. Electroanal. Chem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Bell, S.; Steinberg, T.; Will, G. Corrosion mechanisms in molten salt thermal energy storage for concentrating solar power. Renew. Sustain. Energy Rev. 2019, 114, 109328. [Google Scholar] [CrossRef]
- Macdonald, J.R. Comparison and application of two methods for the least squares analysis of immittance data. Solid State Ion. 1992, 58, 97–107. [Google Scholar] [CrossRef]
- Sadkowski, A. CNLS fits and Kramers–Kronig validation of resonant EIS data. J. Electroanal. Chem. 2004, 573, 241–253. [Google Scholar] [CrossRef]
- Scarpioni, F.; Khalid, S.; Chukwu, R.; Pianta, N.; La Mantia, F.; Ruffo, R. Electrochemical Impedance Spectroscopy for Electrode Process Evaluation: Lithium Titanium Phosphate in Concentrated Aqueous Electrolyte. ChemElectroChem 2023, 10, e202201133. [Google Scholar] [CrossRef]
- Tortorelli, P.F.; Bishop, P.S.; DiStefano, J.R. Selection of Corrosion-Resistant Materials for Use in Molten Nitrate Salts; No. ORNL/TM-11162; Oak Ridge National Lab. (ORNL): Oak Ridge, TN, USA, 1989. [Google Scholar] [CrossRef]
- Kim, W.-B.; Choi, W.-S.; Lim, K.-S.; Cho, S.-H.; Lee, J.-H. High-Temperature Corrosion Behavior of Al-Coated Ni-Base Alloys in Lithium Molten Salt for Electroreduction. Coatings 2021, 11, 328. [Google Scholar] [CrossRef]
- Pettersson, C.; Pettersson, J.; Asteman, H. KCl-induced high temperature corrosion of the austenitic Fe-Cr-Ni alloys 304L and Sanicro 28 at 600 °C. Corros. Sci. 2006, 48, 1368–1378. [Google Scholar] [CrossRef]
- Barin, I.; Platzki, G. Thermochemical Data of Pure Substances, 3rd ed.; VCH: New York, NY, USA, 1995; ISBN 3-527-28745-0. [Google Scholar]
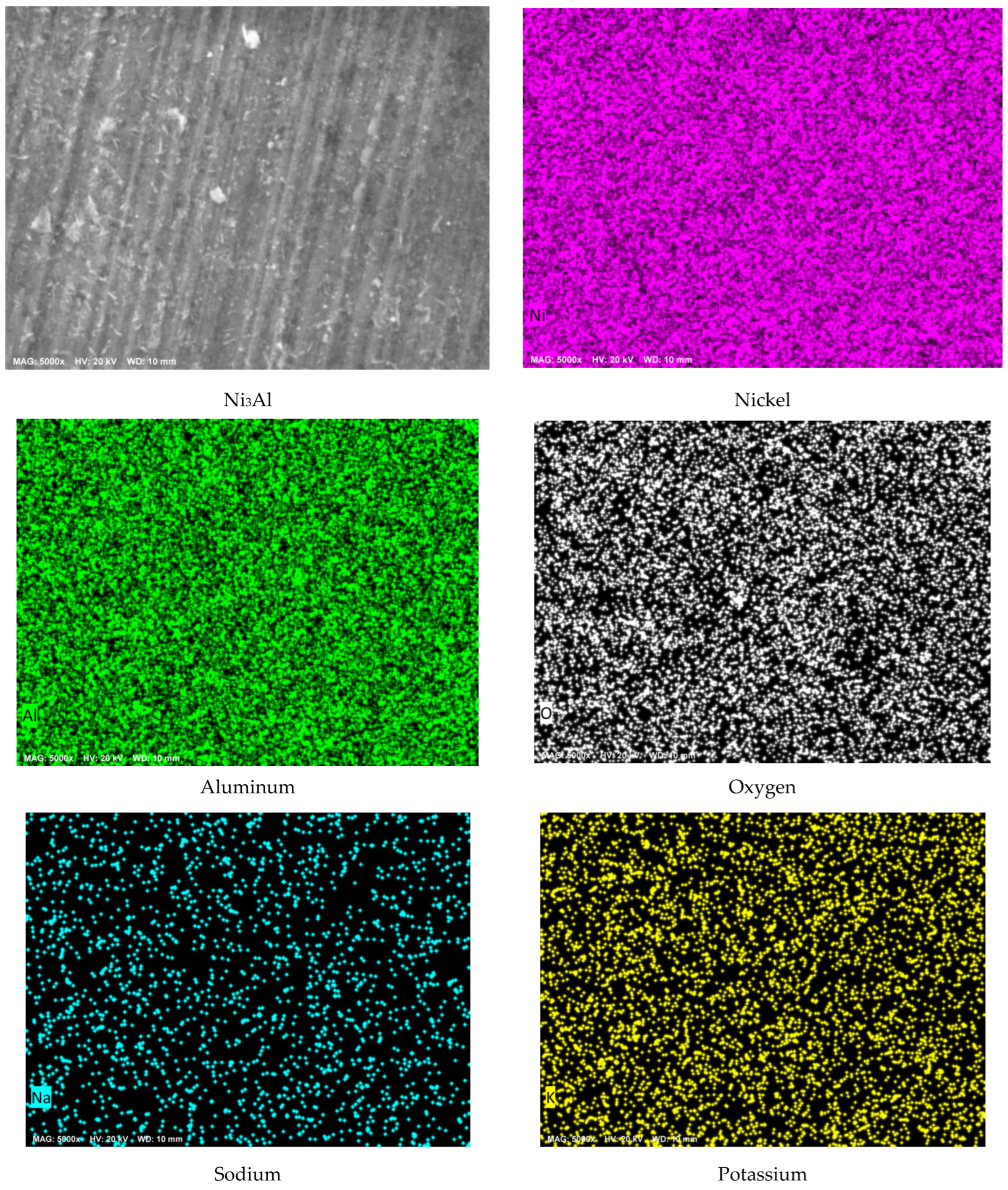

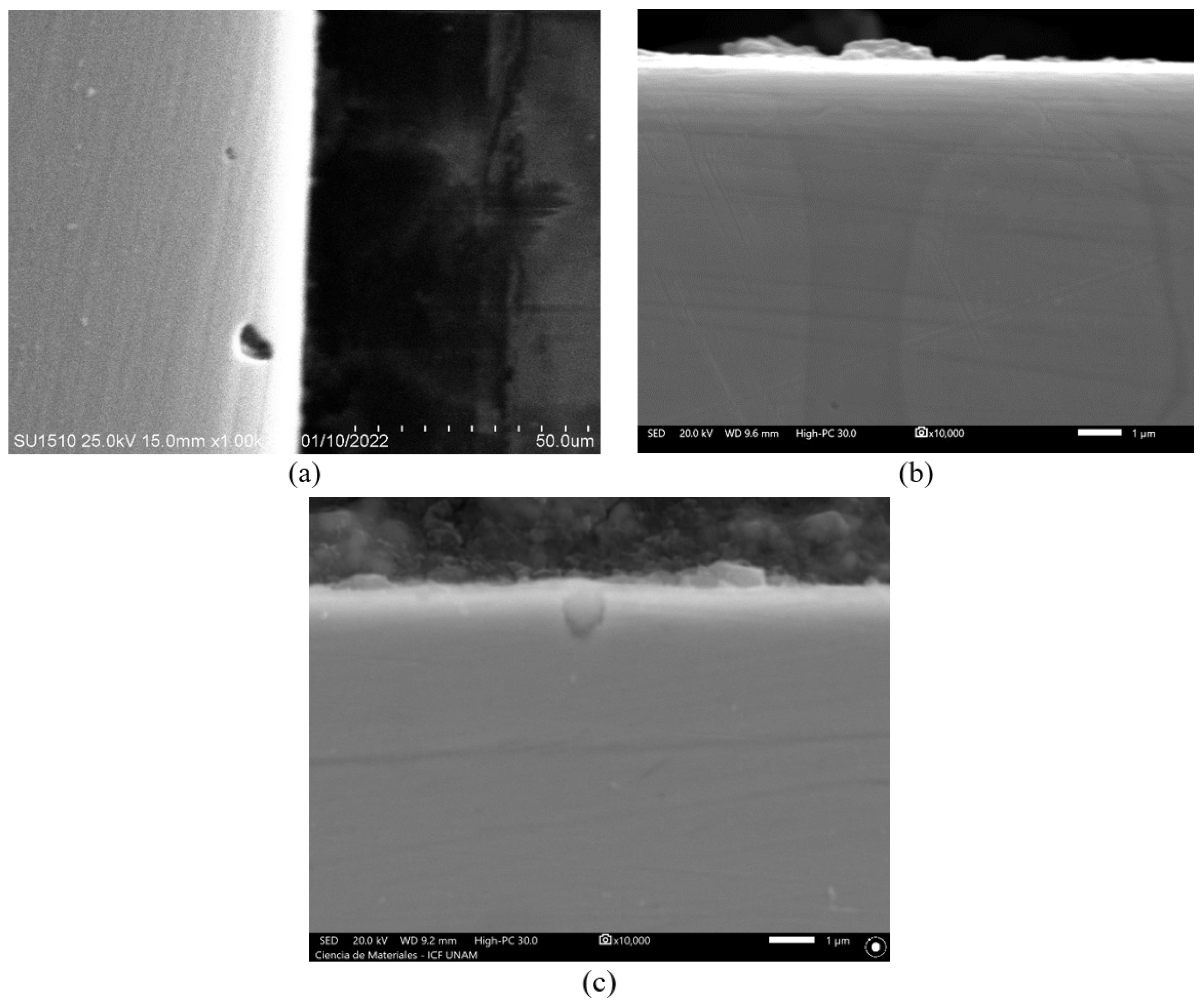







| Temperature | Ecorr (mV) | icorr (mA/cm2) | βa (mV/Dec) | βc (mV/Dec) | CR (μm/Year) |
|---|---|---|---|---|---|
| 300 °C | 29 | 0.00140 | 213 | 203 | 0.0105 |
| 400 °C | 68 | 0.00354 | 306 | 286 | 0.0355 |
| 500 °C | 97 | 0.01417 | 485 | 355 | 0.1428 |
| Temperature | NiO | Al2O3 | NaAlO2 | NaNO3 |
|---|---|---|---|---|
| 300 °C | −186.342 | −1495.763 | −1008.431 | −274.889 |
| 400 °C | −177.582 | −1464.543 | −986.191 | −245.049 |
| 500 °C | −168.822 | −1433.323 | −963.951 | −216.810 |
| Temperature 300 °C | ||||||||||||
| Time (h) | Rs (Ω·cm2) | Rss (Ω·cm2) | Yss (Ω−1·cm−2·sn) | nss | Rox (Ω·cm2) | Yox (Ω−1·cm−2·sn) | nox | Rct (Ω·cm2) | Ydl (Ω−1·cm−2·sn) | ndl | Cox (F·cm−2) | Cdl (F·cm−2) |
| 0 | 2.15 | 11.23 | 0.00050141 | 0.60 | 13370 | 0.000481 | 0.92 | 6056 | 0.00052324 | 0.88 | 0.000567681 | 0.000612356 |
| 25 | 3.39 | 7.096 | 0.00022206 | 0.79 | 9301 | 0.000414 | 0.92 | 46612 | 0.00018629 | 0.73 | 0.000468424 | 0.000423254 |
| 50 | 3.37 | 6.623 | 0.00029233 | 0.77 | 8047 | 0.000417 | 0.92 | 51589 | 0.00021794 | 0.81 | 0.000465492 | 0.000381354 |
| 75 | 3.51 | 5.927 | 0.00026114 | 0.79 | 7112 | 0.000405 | 0.92 | 50629 | 0.00023031 | 0.81 | 0.000444341 | 0.000406408 |
| 100 | 2.15 | 6.012 | 0.00031622 | 0.77 | 8256 | 0.000413 | 0.92 | 51188 | 0.00024975 | 0.88 | 0.00046159 | 0.000353521 |
| Temperature 400 °C | ||||||||||||
| Time (h) | Rs (Ω·cm2) | Rss (Ω·cm2) | Yss (Ω−1·cm−2·sn) | nss | Rox (Ω·cm2) | Yox (Ω−1·cm−2·sn) | nox | Rct (Ω·cm2) | Ydl (Ω−1·cm−2·sn) | ndl | Cox (F·cm−2) | Cdl (F·cm−2) |
| 0 | 3.83 | 10.43 | 0.00012099 | 0.77 | 1739 | 0.000361 | 0.85 | 1318 | 0.0023484 | 0.70 | 0.000331862 | 0.003811244 |
| 25 | 4.40 | 5.451 | 0.00023016 | 0.78 | 6423 | 0.000448 | 0.85 | 27384 | 0.0004071 | 0.68 | 0.000537762 | 0.00129236 |
| 50 | 4.45 | 3.23 | 0.00011466 | 0.86 | 6627 | 0.00044 | 0.86 | 27048 | 0.00033749 | 0.72 | 0.000524032 | 0.000793804 |
| 75 | 4.53 | 3.193 | 0.0000995 | 0.88 | 7093 | 0.000432 | 0.86 | 27150 | 0.00032223 | 0.75 | 0.000519241 | 0.000669377 |
| 100 | 4.47 | 3.346 | 0.0001041 | 0.87 | 7301 | 0.000431 | 0.86 | 24914 | 0.00032732 | 0.77 | 0.000520225 | 0.000607913 |
| Temperature 500 °C | ||||||||||||
| Time (h) | Rs (Ω·cm2) | Rss (Ω·cm2) | Yss (Ω−1·cm−2·sn) | nss | Rox (Ω·cm2) | Yox (Ω−1·cm−2·sn) | nox | Rct (Ω·cm2) | Ydl (Ω−1·cm−2·sn) | ndl | Cox (F·cm−2) | Cdl (F·cm−2) |
| 0 | 2.09 | -- | -- | -- | 2.20 | 0.0010226 | 0.61 | 3705 | 0.000296 | 0.93 | 0.000393304 | 0.000298051 |
| 25 | 2.85 | -- | -- | -- | 1655 | 0.0013096 | 0.79 | 2324 | 0.004046 | 0.60 | 0.001607568 | 0.017880809 |
| 50 | 2.82 | -- | -- | -- | 1875 | 0.0015673 | 0.78 | 1631 | 0.011129 | 0.60 | 0.002105584 | 0.076127497 |
| 75 | 2.82 | -- | -- | -- | 1920 | 0.001571 | 0.78 | 831.6 | 0.017393 | 0.78 | 0.002127587 | 0.037563319 |
| 100 | 2.74 | -- | -- | -- | 568.6 | 0.0022598 | 0.84 | 947.1 | 0.031691 | 0.78 | 0.002371678 | 0.081726827 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez-Dominguez, D.; Gomez-Guzman, N.B.; Arrieta-Gonzalez, C.D.; de la Vega Olivas, J.; Gonzalez-Rodriguez, J.G.; Porcayo-Calderon, J.; Chacon-Nava, J.G. Electrochemical Behavior of the Ni3Al Intermetallic Alloy in Nitrate Salts. Metals 2025, 15, 764. https://doi.org/10.3390/met15070764
Lopez-Dominguez D, Gomez-Guzman NB, Arrieta-Gonzalez CD, de la Vega Olivas J, Gonzalez-Rodriguez JG, Porcayo-Calderon J, Chacon-Nava JG. Electrochemical Behavior of the Ni3Al Intermetallic Alloy in Nitrate Salts. Metals. 2025; 15(7):764. https://doi.org/10.3390/met15070764
Chicago/Turabian StyleLopez-Dominguez, Daniel, Nestor Belisario Gomez-Guzman, Cinthya Dinorah Arrieta-Gonzalez, Jonathan de la Vega Olivas, Jose Gonzalo Gonzalez-Rodriguez, Jesus Porcayo-Calderon, and Jose Guadalupe Chacon-Nava. 2025. "Electrochemical Behavior of the Ni3Al Intermetallic Alloy in Nitrate Salts" Metals 15, no. 7: 764. https://doi.org/10.3390/met15070764
APA StyleLopez-Dominguez, D., Gomez-Guzman, N. B., Arrieta-Gonzalez, C. D., de la Vega Olivas, J., Gonzalez-Rodriguez, J. G., Porcayo-Calderon, J., & Chacon-Nava, J. G. (2025). Electrochemical Behavior of the Ni3Al Intermetallic Alloy in Nitrate Salts. Metals, 15(7), 764. https://doi.org/10.3390/met15070764












