Rapid Screening of Liquid Metal Wetting for a Materials Compatibility Library
Abstract
1. Introduction
- (1)
- The wetting material is manufacturable into bulk components via milling, pressing and sintering, casting, or additive manufacturing;
- (2)
- The non-wetting material can be applied as a coating on the outside of the nozzle via spray coating, vapor deposition, or chemical growth methods;
- (3)
- The wettable material, the coating, and their interface are all stable—well above the melting point of the target liquid metal.
2. Materials and Methods
2.1. Sample Preparation
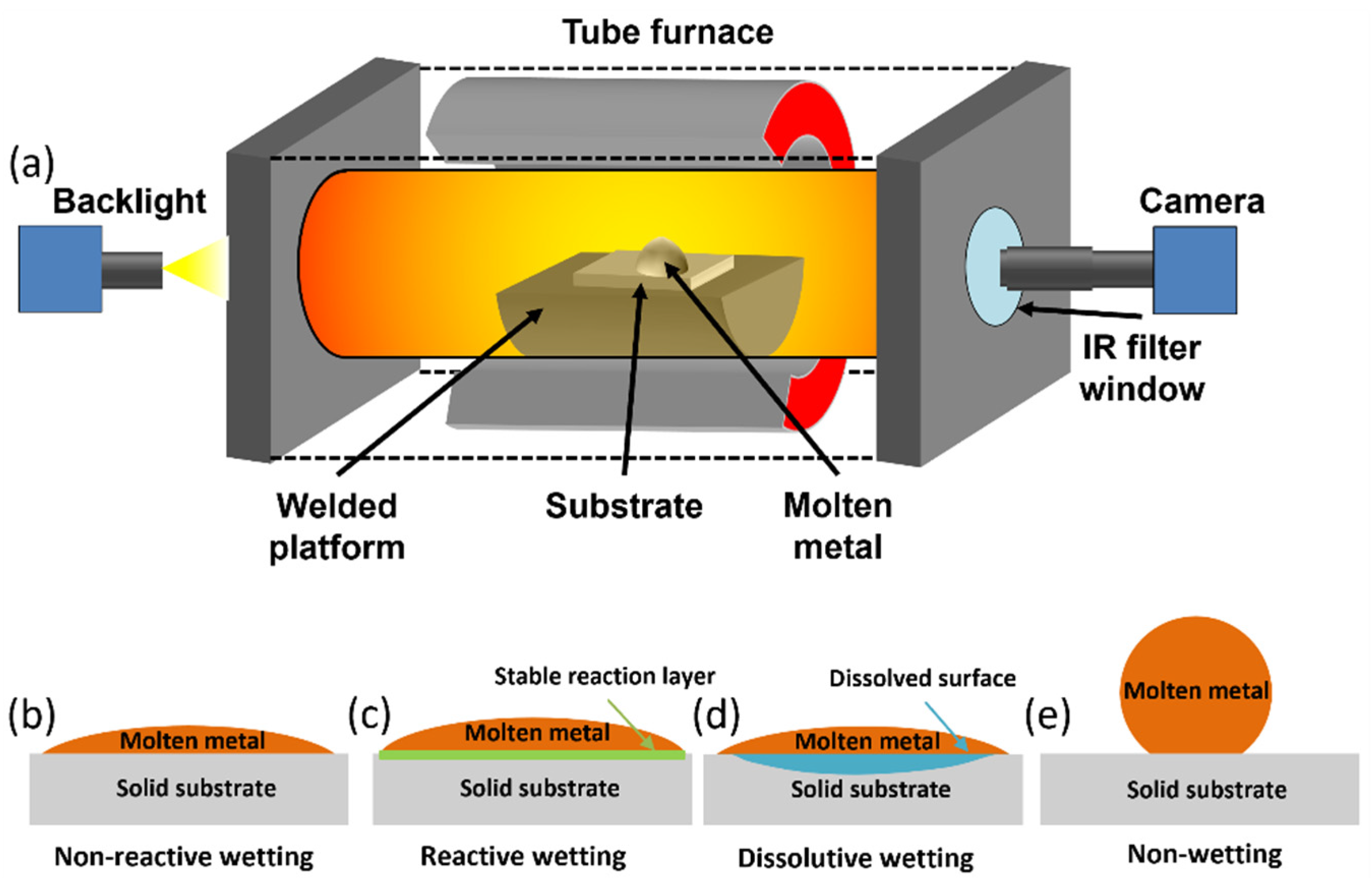
2.2. Wetting Experiment Setup
2.3. Microscopy Preparation and Elemental Mapping
3. Results and Discussion
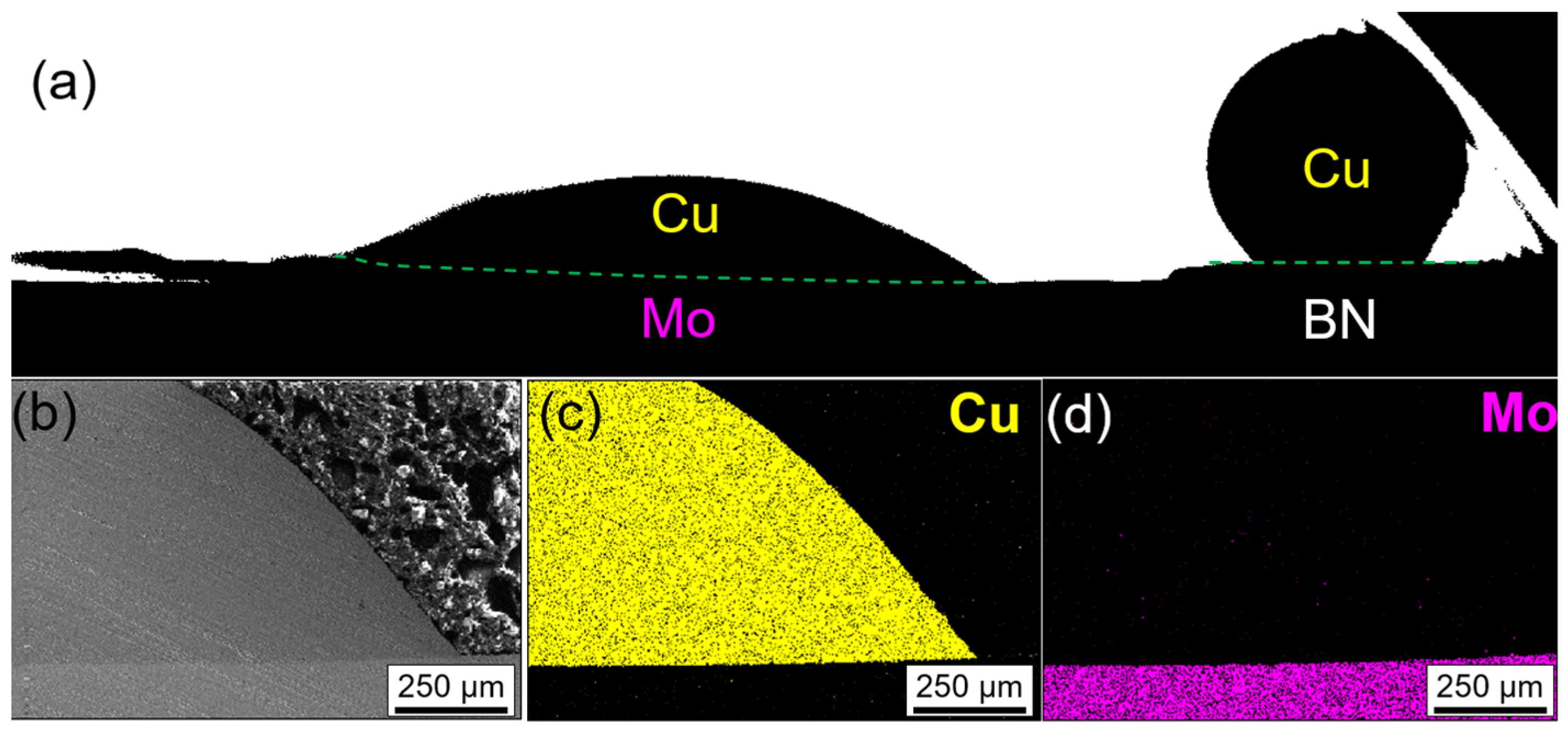
3.1. Wetting of Metals with Negligible Mutual Solubility
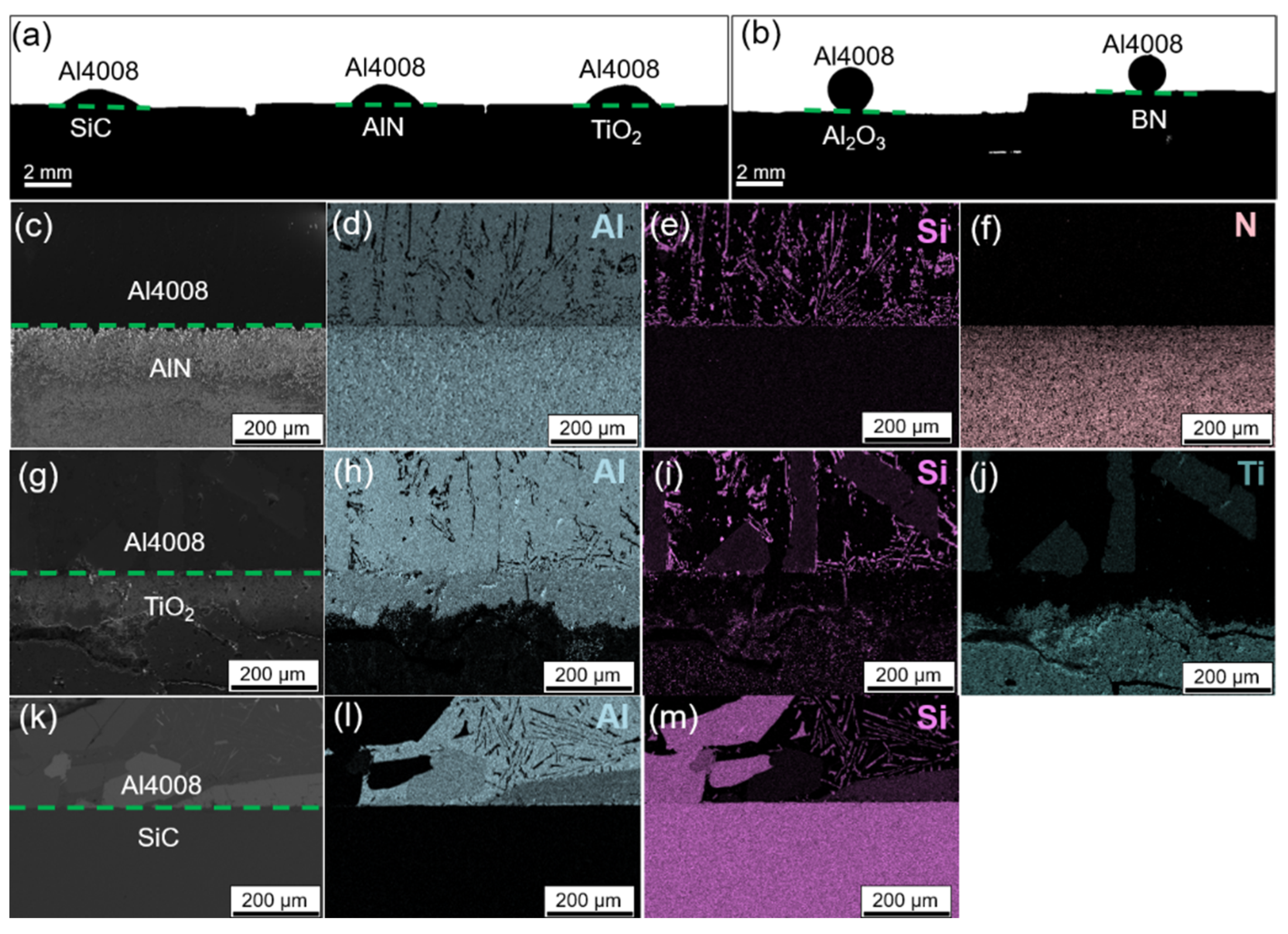
3.2. Wetting of Metals with Ceramics
3.3. Selection of Wetting/Non-Wetting Combinations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eustathopoulos, N. Wetting by Liquid Metals—Application in Materials Processing: The Contribution of the Grenoble Group. Metals 2015, 5, 350–370. [Google Scholar] [CrossRef]
- Hu, X.Y.; Jiang, G.C.; Fan, P.X.; Hu, G.Q.; Xu, G.; Wang, W.; Wang, L.Z.; Zhang, H.J.; Zhong, M.L. 1000 °C High-Temperature Wetting Behaviors of Molten Metals on Laser-Microstructured Metal Surfaces. Langmuir 2023, 39, 17538–17550. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Prabhu, K.N. Review of non-reactive and reactive wetting of liquids on surfaces. Adv. Colloid Interface Sci. 2007, 133, 61–89. [Google Scholar] [CrossRef]
- Yin, L.; Chauhan, A.; Singler, T.J. Reactive wetting in metal/metal systems: Dissolutive versus compound-forming systems. Mater. Sci. Eng. A 2008, 495, 80–89. [Google Scholar] [CrossRef]
- Protsenko, P.; Kozova, O.; Voytovych, R.; Eustathopoulos, N. Dissolutive wetting of si by molten Cu. J. Mater. Sci. 2008, 43, 5669–5671. [Google Scholar] [CrossRef]
- Eustathopoulos, N.; Drevet, B.; Nicholas, M.G. Wettability at High Temperatures; Elsevier: Amsterdam, The Netherlands, 1999; Volume 3. [Google Scholar]
- Iwamoto, C.; Tanaka, S.-i. Atomic morphology and chemical reactions of the reactive wetting front. Acta Mater. 2002, 50, 749–755. [Google Scholar] [CrossRef]
- Shen, P.; Fujii, H.; Nogi, K. Effect of temperature and surface roughness on the wettability of boron nitride by molten Al. J. Mater. Sci. 2007, 42, 3564–3568. [Google Scholar] [CrossRef]
- Uday, M.B.; Ahmad-Fauzi, M.N.; Noor, A.M.; Rajoo, S. Current Issues and Problems in the Joining of Ceramic to Metal. In Joining Technologies; IntechOpen: London, UK, 2016. [Google Scholar]
- Wu, Z.Y.; Zhao, X.; Liu, Y.; Cai, Y.; Li, J.Y.; Chen, H.; Wan, Q.; Yang, D.; Tan, J.; Liu, H.D.; et al. Lead-bismuth eutectic (LBE) corrosion behavior of AlTiN coatings at 550 and 600 °C. J. Nucl. Mater. 2020, 539, 152280. [Google Scholar] [CrossRef]
- Liu, H.; Lavernia, E.J.; Rangel, R.H. An analysis of freeze-up phenomena during gas atomization of metals. Int. J. Heat Mass Transf. 1995, 38, 2183–2193. [Google Scholar] [CrossRef]
- Hsiao, W.; Chun, J.; Saka, N. The Effects of Wetting and Surface Roughness on Liquid Metal Droplet Bouncing. J. Manuf. Sci. Eng. 2009, 131, 021010. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Hao, H.; Basu, S.; Feng, X.; Xu, Y.; Boscoboinik, J.A.; Nanda, J.; Watt, J.; Mitlin, D. Stable Potassium Metal Anodes with an All-Aluminum Current Collector through Improved Electrolyte Wetting. Adv. Mater. 2020, 32, e2002908. [Google Scholar] [CrossRef]
- Watkins, N.N.; Traxel, K.D.; Wilson-Heid, A.E.; Reeve, T.C.; Silva, C.M.; Jeffries, J.R.; Pascall, A.J. Process-structure-property relationships for droplet-on-demand liquid-metal-jetted parts. Addit. Manuf. 2023, 73, 103709. [Google Scholar] [CrossRef]
- Gilani, N.; Aboulkhair, N.T.; Simonelli, M.; East, M.; Hague, R.J.M. Drop-on-demand metal jetting of pure copper: On the interaction of molten metal with ceramic and metallic substrates. Mater. Des. 2024, 240, 112834. [Google Scholar] [CrossRef]
- Seo, J.; Somarakis, C.; Korneev, S.; Behandish, M.; Lew, A.J. Physics-based nozzle design rules for high-frequency liquid metal jetting. Phys. Fluids 2022, 34, 102113. [Google Scholar] [CrossRef]
- Meda, M.; Mehta, P.; Mahajan, C.; Kahn, B.; Cormier, D. Magnetohydrodynamic liquid metal droplet jetting of highly conductive electronic traces. Flex. Print. Electron. 2021, 6, 035002. [Google Scholar] [CrossRef]
- Kirchebner, B.; Traxel, K.D.; Wilson-Heid, A.E.; Elton, E.S.; Pascall, A.J.; Jeffries, J.R. Molten metal jetting for repairing aluminum components. Addit. Manuf. Lett. 2024, 11, 100259. [Google Scholar] [CrossRef]
- Elliott, A.; Shyam, A.; Rios, O.; Muth, T. Advancing Liquid Metal Jet Printing; ORNL/TM-2019/1349; NFE-18-07178; CRADA/NFE-18-07178; Oak Ridge National Laboratory (ORNL): Oak Ridge, TN, USA, 2019. [Google Scholar] [CrossRef]
- Sun, Q.; Qi, H.; Wang, X.; Zuo, D. Liquid Tin Droplet Generator in Laser-Produced Plasma Extreme Ultraviolet Source: A Review. IEEE Trans. Instrum. Meas. 2024, 73, 1–14. [Google Scholar] [CrossRef]
- Eustathopoulos, N.; Sobczak, N.; Passerone, A.; Nogi, K. Measurement of contact angle and work of adhesion at high temperature. J. Mater. Sci. 2005, 40, 2271–2280. [Google Scholar] [CrossRef]
- Joshipura, I.D.; Persson, K.A.; Truong, V.K.; Oh, J.H.; Kong, M.; Vong, M.H.; Ni, C.J.; Alsafatwi, M.; Parekh, D.P.; Zhao, H.; et al. Are Contact Angle Measurements Useful for Oxide-Coated Liquid Metals? Langmuir 2021, 37, 10914–10923. [Google Scholar] [CrossRef] [PubMed]
- Daniel, D.; Vuckovac, M.; Backholm, M.; Latikka, M.; Karyappa, R.; Koh, X.Q.; Timonen, J.V.I.; Tomczak, N.; Ras, R.H.A. Probing surface wetting across multiple force, length and time scales. Commun. Phys. 2023, 6, 152. [Google Scholar] [CrossRef]
- Uryupina, D.S.; Ivanov, K.A.; Brantov, A.V.; Savel’ev, A.B.; Bychenkov, V.Y.; Povarnitsyn, M.E.; Volkov, R.V.; Tikhonchuk, V.T. Femtosecond laser-plasma interaction with prepulse-generated liquid metal microjets. Phys. Plasmas 2012, 19, 013104. [Google Scholar] [CrossRef]
- Versolato, O.O. Physics of laser-driven tin plasma sources of EUV radiation for nanolithography. Plasma Sources Sci. Technol. 2019, 28, 083001. [Google Scholar] [CrossRef]
- Saiz, E.; Tomsia, A.P.; Rauch, N.; Scheu, C.; Ruehle, M.; Benhassine, M.; Seveno, D.; de Coninck, J.; Lopez-Esteban, S. Nonreactive spreading at high temperature: Molten metals and oxides on molybdenum. Phys. Rev. E Stat. Nonlin Soft Matter Phys. 2007, 76, 041602. [Google Scholar] [CrossRef]
- Toy, C.; Scott, W.D. Wetting and spreading of molten aluminium against AlN surfaces. J. Mater. Sci. 1997, 32, 3243–3248. [Google Scholar] [CrossRef]
- Taranets, N.Y.; Naidich, Y.V. Wettability of aluminium nitride by molten metals. Powder Metall. Met. Ceram. 1996, 35, 282–285. [Google Scholar] [CrossRef]
- Cao, J.; Liu, Y.; Ning, X.S. Influence of AlN(0001) Surface Reconstructions on the Wettability of an Al/AlN System: A First-Principle Study. Materials 2018, 11, 775. [Google Scholar] [CrossRef]
- Metzger, F.; Rienzi, V.; Mascetti, C.; Nguyen, T.; Pimputkar, S. Properties of Titanium Zirconium Molybdenum Alloy after Exposure to Indium at Elevated Temperatures. Materials 2022, 15, 5270. [Google Scholar] [CrossRef] [PubMed]
- Delannay, F.; Froyen, L.; Deruyttere, A. The wetting of solids by molten metals and its relation to the preparation of metal-matrix composites. J. Mater. Sci. 1987, 22, 1–16. [Google Scholar] [CrossRef]
- Sui, R.; Tong, J.; Zhang, Z.; Lin, Q. Wetting of laser-textured tungsten substrate by molten tin. Surf. Interfaces 2024, 46, 104115. [Google Scholar] [CrossRef]
- Bao, S.; Tang, K.; Kvithyld, A.; Engh, T.; Tangstad, M. Wetting of pure aluminium on graphite, SiC and Al2O3 in aluminium filtration. Trans. Nonferrous Met. Soc. 2012, 22, 1930–1938. [Google Scholar] [CrossRef]
- Stull, D.R.; Prophet, H. Janaf Thermochemical Tables; National Institute of Standards and Technology: Gaithersburg, MD, USA, 1971. [Google Scholar]
- Reed, T.B. Free Energy of Formation of Binary Compounds; MIT Press: Cambridge, MA, USA, 1971. [Google Scholar]
- Xue, X.M.; Wang, J.T.; Quan, M.X. Wettability and Spreading Kinetics of Liquid Aluminium on Boron-Nitride. J. Mater. Sci. 1991, 26, 6391–6395. [Google Scholar] [CrossRef]
- Caskey, C.M.; Richards, R.M.; Ginley, D.S.; Zakutayev, A. Thin film synthesis and properties of copper nitride, a metastable semiconductor. Mater. Horiz. 2014, 1, 424–430. [Google Scholar] [CrossRef]
- Ansari, M.Z.; Janicek, P.; Nandi, D.K.; Palka, K.; Slang, S.; Kim, D.H.; Cheon, T.; Kim, S.-H. Influence of post-annealing on structural, optical and electrical properties of tin nitride thin films prepared by atomic layer deposition. Appl. Surf. Sci. 2021, 538, 147920. [Google Scholar] [CrossRef]

| Compound System | Estimated Free Energy of Formation at or Around 1000 °C (kJ/mol) |
|---|---|
| Cu3N | Decomposes above 500 °C [37] |
| SnNx | Decomposes above 500 °C [38] |
| AlN | −343.1 [35] |
| hBN | −272.0 [35] |
| CeN | −376.6 [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mooraj, S.; Baker, A.; Rietema, C.J.; Ahlquist, J.; Henderson, H.; Sukhotskiy, V. Rapid Screening of Liquid Metal Wetting for a Materials Compatibility Library. Metals 2025, 15, 1121. https://doi.org/10.3390/met15101121
Mooraj S, Baker A, Rietema CJ, Ahlquist J, Henderson H, Sukhotskiy V. Rapid Screening of Liquid Metal Wetting for a Materials Compatibility Library. Metals. 2025; 15(10):1121. https://doi.org/10.3390/met15101121
Chicago/Turabian StyleMooraj, Shahryar, Alexander Baker, Connor J. Rietema, Jesse Ahlquist, Hunter Henderson, and Viktor Sukhotskiy. 2025. "Rapid Screening of Liquid Metal Wetting for a Materials Compatibility Library" Metals 15, no. 10: 1121. https://doi.org/10.3390/met15101121
APA StyleMooraj, S., Baker, A., Rietema, C. J., Ahlquist, J., Henderson, H., & Sukhotskiy, V. (2025). Rapid Screening of Liquid Metal Wetting for a Materials Compatibility Library. Metals, 15(10), 1121. https://doi.org/10.3390/met15101121








