Recent Advances in Additive Manufacturing of High Entropy Alloys and Their Nuclear and Wear-Resistant Applications
Abstract
:1. Introduction
1.1. The History of High Entropy Alloys
1.2. The Definitions of High Entropy Alloys
2. Manufacturing of HEAs
2.1. Background and Conventional Methods
2.2. Additive Manufacturing of HEAs
3. Applications under Extreme Environments
3.1. Nuclear Applications
3.1.1. Dislocation
3.1.2. Hardness
3.1.3. Phase Stability
3.1.4. Irradiation-Induced Creep (IIC)
3.1.5. Swelling Resistance
3.1.6. Self-Healing
3.1.7. Miscellaneous
3.2. Wear Behavior
3.2.1. Content Variation
3.2.2. Particle Reinforcement
3.2.3. Use of Media and Heat Treatment
3.2.4. Nitriding/Carburizing/Boronizing/Sulfurization
3.2.5. Comparison with Conventional Materials
3.2.6. Higher Temperatures Wear Resistance
4. Summery and Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Radetzki, M. Seven thousand years in the service of humanity—The history of copper, the red metal. Resour. Policy 2009, 34, 176–184. [Google Scholar] [CrossRef]
- Murty, B.; Yeh, J.; Ranganathan, S.; Bhattacharjee, P. High-Entropy Alloys; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Achard, F.C. Recherches sur les Propriétés des Alliages Métalliques; Decker: Berlin, Germany, 1788; Volume IV, p. 37. [Google Scholar]
- Smith, C.S. Four outstanding researches in metallurgical history; American Society for Testing and Materials: West Conshohocken, PA, USA, 1963. [Google Scholar]
- Vincent, A.J.B. A Study of Three Multicomponent Alloys; University of Sussex: Brighton, UK, 1981. [Google Scholar]
- Huang, K.H.; Yeh, J. A Study on the Multicomponent Alloy Systems Containing Equal-Mole Elements; National Tsing Hua University: Hsinchu, Taiwan, 1996. [Google Scholar]
- Ranganathan, S. Alloyed pleasures: Multimetallic cocktails. Curr. Sci. 2003, 85, 1404–1406. [Google Scholar]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Greer, A.L. Confusion by design. Nature 1993, 366, 303–304. [Google Scholar] [CrossRef]
- Gao, M.C.; Liaw, P.K.; Yeh, J.W.; Zhang, Y. High-Entropy Alloys: Fundamentals and Applications; Springer: Cham, Switzerland, 2016; ISBN 9783319270135. [Google Scholar]
- Cantor, B. Multicomponent and high entropy alloys. Entropy 2014, 16, 4749–4768. [Google Scholar] [CrossRef] [Green Version]
- Yeh, J.W. Recent progress in high-entropy alloys. Ann. Chim. Sci. 2006, 31, 633–648. [Google Scholar] [CrossRef]
- Yeh, J.W. Physical Metallurgy of High-Entropy Alloys. JOM 2015, 67, 2254–2261. [Google Scholar] [CrossRef]
- Zhang, W.; Liaw, P.K.; Zhang, Y. Science and technology in high-entropy alloys. Sci. China Mater. 2018, 61, 2–22. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.S.; Yadav, S.; Biswas, K.; Basu, B. High-entropy alloys and metallic nanocomposites: Processing challenges, microstructure development and property enhancement. Mater. Sci. Eng. R Rep. 2018, 131, 1–42. [Google Scholar] [CrossRef]
- Basu, I.; De Hosson, J.T.M. High Entropy Alloys: Ready to Set Sail? Metals 2020, 10, 194. [Google Scholar] [CrossRef] [Green Version]
- Reddy, C.K.; Gopi Krishna, M.; Srikant, P. Brief evolution story and some basic limitations of high entropy alloys (HEAs)—A review. Mater. Today Proc. 2019, 18, 436–439. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, Y.L.; Lin, S.J.; Chen, S.K. High-entropy alloys—A new era of exploitation. Mater. Sci. Forum 2007, 560, 1–9. [Google Scholar] [CrossRef]
- Pickering, E.J.; Jones, N.G. High-entropy alloys: A critical assessment of their founding principles and future prospects. Int. Mater. Rev. 2016, 61, 183–202. [Google Scholar] [CrossRef] [Green Version]
- Tian, F. A review of solid-solution models of high-entropy alloys based on Ab initio calculations. Front. Mater. 2017, 4, 36. [Google Scholar] [CrossRef] [Green Version]
- George, E.P.; Raabe, D.; Ritchie, R.O. High-entropy alloys. Nat. Rev. Mater. 2019, 4, 5151–5534. [Google Scholar] [CrossRef]
- Lu, Z.P.; Wang, H.; Chen, M.W.; Baker, I.; Yeh, J.W.; Liu, C.T.; Nieh, T.G. An assessment on the future development of high-entropy alloys: Summary from a recent workshop. Intermetallics 2015, 66, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Troparevsky, M.C.; Morris, J.R.; Daene, M.; Wang, Y.; Lupini, A.R.; Stocks, G.M. Beyond Atomic Sizes and Hume-Rothery Rules: Understanding and Predicting High-Entropy Alloys. JOM 2015, 67, 2350–2363. [Google Scholar] [CrossRef]
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. High-entropy alloy: Challenges and prospects. Mater. Today 2016, 19, 349–362. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- Miracle, D.B. High-Entropy Alloys: A Current Evaluation of Founding Ideas and Core Effects and Exploring “Nonlinear Alloys”. JOM 2017, 69, 2130–2136. [Google Scholar] [CrossRef] [Green Version]
- Ge, H.; Tian, F. A Review of Ab Initio Calculation on Lattice Distortion in High-Entropy Alloys. JOM 2019, 71, 4225–4237. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Liaw, P.K. Alloy design and properties optimization of high-entropy alloys. JOM 2012, 64, 830–838. [Google Scholar] [CrossRef]
- Yeh, J.W. Alloy design strategies and future trends in high-entropy alloys. JOM 2013, 65, 1759–1771. [Google Scholar] [CrossRef]
- Tsai, M.H. Three strategies for the design of advanced high-entropy alloys. Entropy 2016, 18, 252. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-solution phase formation rules for multi-component alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Guo, S.; Liu, C.T. Phase stability in high entropy alloys: Formation of solid-solution phase or amorphous phase. Prog. Nat. Sci. Mater. Int. 2011, 21, 433–446. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.C.; Zhang, C.; Gao, P.; Zhang, F.; Ouyang, L.Z.; Widom, M.; Hawk, J.A. Thermodynamics of concentrated solid solution alloys. Curr. Opin. Solid State Mater. Sci. 2017, 21, 238–251. [Google Scholar] [CrossRef]
- Kozak, R.; Sologubenko, A.; Steurer, W. Single-phase high-entropy alloys—An overview. Z. Fur Krist. 2015, 230, 55–68. [Google Scholar] [CrossRef] [Green Version]
- Diao, H.; Santodonato, L.J.; Tang, Z.; Egami, T.; Liaw, P.K. Local Structures of High-Entropy Alloys (HEAs) on Atomic Scales: An Overview. JOM 2015, 67, 2321–2325. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Huang, W.; Martin, P.; Zhuang, H.L. Machine-learning phase prediction of high-entropy alloys. Acta Mater. 2019, 169, 225–236. [Google Scholar] [CrossRef]
- Ganesh, U.L.; Raghavendra, H. Review on the transition from conventional to multi-component-based nano-high-entropy alloys—NHEAs. J. Therm. Anal. Calorim. 2020, 139, 207–216. [Google Scholar] [CrossRef]
- Lyu, Z.; Lee, C.; Wang, S.Y.; Fan, X.; Yeh, J.W.; Liaw, P.K. Effects of Constituent Elements and Fabrication Methods on Mechanical Behavior of High-Entropy Alloys: A Review. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2019, 50, 1–28. [Google Scholar] [CrossRef]
- Li, Z.; Raabe, D. Strong and Ductile Non-equiatomic High-Entropy Alloys: Design, Processing, Microstructure, and Mechanical Properties. JOM 2017, 69, 2099–2106. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, S.; Ritchie, R.O.; Meyers, M.A. Mechanical properties of high-entropy alloys with emphasis on face-centered cubic alloys. Prog. Mater. Sci. 2019, 102, 296–345. [Google Scholar] [CrossRef]
- Praveen, S.; Kim, H.S. High-Entropy Alloys: Potential Candidates for High-Temperature Applications—An Overview. Adv. Eng. Mater. 2018, 20, 1700645. [Google Scholar] [CrossRef]
- Cao, B.; Yang, T.; Liu, W.; Liu, C.T. Precipitation-hardened high-entropy alloys for high-temperature applications: A critical review. MRS Bull. 2019, 44, 854–859. [Google Scholar] [CrossRef]
- Huang, E.W.; Hung, G.Y.; Lee, S.Y.; Jain, J.; Chang, K.P.; Chou, J.J.; Yang, W.C.; Liaw, P.K. Mechanical and magnetic properties of the high-entropy alloys for combinatorial approaches. Crystals 2020, 10, 200. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.H. Physical properties of high entropy alloys. Entropy 2013, 15, 5338–5345. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Troparevsky, M.C.; Gao, Y.F.; Morris, J.R.; Stocks, G.M.; Bei, H. Phase stability, physical properties and strengthening mechanisms of concentrated solid solution alloys. Curr. Opin. Solid State Mater. Sci. 2017, 21, 267–284. [Google Scholar] [CrossRef]
- Toda-Caraballo, I.; Wróbel, J.S.; Nguyen-Manh, D.; Pérez, P.; Rivera-Díaz-del-Castillo, P.E.J. Simulation and Modeling in High Entropy Alloys. JOM 2017, 69, 2137–2149. [Google Scholar] [CrossRef]
- Zhao, S.; Weber, W.J.; Zhang, Y. Unique Challenges for Modeling Defect Dynamics in Concentrated Solid-Solution Alloys. JOM 2017, 69, 2084–2091. [Google Scholar] [CrossRef]
- Torralba, J.M.; Alvaredo, P.; García-Junceda, A. High-entropy alloys fabricated via powder metallurgy. A critical review. Powder Metall. 2019, 62, 84–114. [Google Scholar] [CrossRef]
- Chen, S.; Tong, Y.; Liaw, P.K. Additive manufacturing of high-entropy alloys: A review. Entropy 2018, 20, 937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Tang, C.; Rothwell, G.; Li, L.; Wang, Y.C.; Yang, Q.; Ren, X. Welding of high entropy alloys—A review. Entropy 2019, 21, 431. [Google Scholar] [CrossRef] [Green Version]
- Lopes, J.G.; Oliveira, J.P. A short review on welding and joining of high entropy alloys. Metals 2020, 10, 212. [Google Scholar] [CrossRef] [Green Version]
- Filho, F.C.G.; Monteiro, S.N. Welding joints in high entropy alloys: A short-review on recent trends. Materials 2020, 13, 1411. [Google Scholar] [CrossRef] [Green Version]
- Scutelnicu, E.; Simion, G.; Rusu, C.C.; Corneliu Gheonea, M.; Voiculescu, I.; Geanta, V. High Entropy Alloys Behaviour During Welding. Rev. Chim. 2020, 71, 219–233. [Google Scholar] [CrossRef]
- Dong, W.; Zhou, Z.; Zhang, M.; Ma, Y.; Yu, P.; Liaw, P.K.; Li, G. Applications of high-pressure technology for high-entropy alloys: A review. Metals 2019, 9, 876. [Google Scholar] [CrossRef] [Green Version]
- Dąbrowa, J.; Danielewski, M. State-of-the-art diffusion studies in the high entropy alloys. Metals 2020, 10, 347. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Wang, J.; Xia, L.; Wu, Y. Deformation behavior of bulk metallic glasses and high entropy alloys under complex stress fields: A review. Entropy 2019, 21, 54. [Google Scholar] [CrossRef] [Green Version]
- Diao, H.Y.; Feng, R.; Dahmen, K.A.; Liaw, P.K. Fundamental deformation behavior in high-entropy alloys: An overview. Curr. Opin. Solid State Mater. Sci. 2017, 21, 252–266. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, B.; Liaw, P.K. Corrosion-resistant high-entropy alloys: A review. Metals 2017, 7, 43. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.; Thomas, S.; Gibson, M.A.; Fraser, H.L.; Birbilis, N. Corrosion of high entropy alloys. npj Mater. Degrad. 2017, 1, 15. [Google Scholar] [CrossRef]
- Chen, P.Y.; Lee, C.; Wang, S.Y.; Seifi, M.; Lewandowski, J.J.; Dahmen, K.A.; Jia, H.L.; Xie, X.; Chen, B.L.; Yeh, J.W.; et al. Fatigue behavior of high-entropy alloys: A review. Sci. China Technol. Sci. 2018, 61, 168–178. [Google Scholar] [CrossRef]
- Li, W.; Wang, G.; Wu, S.; Liaw, P.K. Creep, fatigue, and fracture behavior of high-entropy alloys. J. Mater. Res. 2018, 33, 3011–3034. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhao, S.; Weber, W.J.; Nordlund, K.; Granberg, F.; Djurabekova, F. Atomic-level heterogeneity and defect dynamics in concentrated solid-solution alloys. Curr. Opin. Solid State Mater. Sci. 2017, 21, 221–237. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Li, C.; Zinkle, S.J.; Zhao, S.; Bei, H.; Zhang, Y. Irradiation responses and defect behavior of single-phase concentrated solid solution alloys. J. Mater. Res. 2018, 33, 3077–3091. [Google Scholar] [CrossRef] [Green Version]
- Jin, K.; Bei, H. Single-phase concentrated solid-solution alloys: Bridging intrinsic transport properties and irradiation resistance. Front. Mater. 2018, 5, 26. [Google Scholar] [CrossRef] [Green Version]
- Senkov, O.N.; Miracle, D.B.; Chaput, K.J.; Couzinie, J.P. Development and exploration of refractory high entropy alloys—A review. J. Mater. Res. 2018, 33, 3092–3128. [Google Scholar] [CrossRef] [Green Version]
- Ram Prabhu, T.; Chodancar, Y.; Arivarasu, M.; Arivazhagan, N.; Mishra, R.K. High Entropy Based Composites—An Overview. Mater. Sci. Forum 2019, 969, 98–113. [Google Scholar] [CrossRef]
- Oses, C.; Toher, C.; Curtarolo, S. High-entropy ceramics. Nat. Rev. Mater. 2020, 5, 295–309. [Google Scholar] [CrossRef]
- Motallebzadeh, A.; Peighambardoust, N.S.; Sheikh, S.; Murakami, H.; Guo, S.; Canadinc, D. Microstructural, mechanical and electrochemical characterization of TiZrTaHfNb and Ti1.5ZrTa0.5Hf0.5Nb0.5 refractory high-entropy alloys for biomedical applications. Intermetallics 2019, 113, 106572. [Google Scholar] [CrossRef]
- Akmal, M.; Hussain, A.; Afzal, M.; Lee, Y.I.; Ryu, H.J. Systematic Study of (MoTa)xNbTiZr Medium- and High-Entropy Alloys for Biomedical Implants- In Vivo Biocompatibility Examination. J. Mater. Sci. Technol. 2021, 78, 183–191. [Google Scholar] [CrossRef]
- Fu, M.; Ma, X.; Zhao, K.; Li, X.; Su, D. High-entropy materials for energy-related applications. Iscience 2021, 24, 102177. [Google Scholar] [CrossRef] [PubMed]
- Canter, N. High-Entropy Alloys; Elsevier: London, UK, 2015; Volume 71, ISBN 9780128160671. [Google Scholar]
- Gopinath, V.M.; Arulvel, S. A review on the steels, alloys/high entropy alloys, composites and coatings used in high temperature wear applications. Mater. Today Proc. 2020, 43, 817–823. [Google Scholar] [CrossRef]
- Voiculescu, I.; Geanta, V.; Stanciu, E.M.; Jianu, D.A.; Postolache, C.; Fugaru, V. Effect of irradiation and temperature on microstructural characteristic of FeCrAl alloys. Acta Phys. Pol. A 2018, 134, 116–118. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiao, J.W.; Liaw, P.K. A Brief Review of High Entropy Alloys and Serration Behavior and Flow Units. J. Iron Steel Res. Int. 2016, 23, 2–6. [Google Scholar] [CrossRef]
- López Ríos, M.; Socorro Perdomo, P.P.; Voiculescu, I.; Geanta, V.; Crăciun, V.; Boerasu, I.; Mirza Rosca, J.C. Effects of nickel content on the microstructure, microhardness and corrosion behavior of high-entropy AlCoCrFeNix alloys. Sci. Rep. 2020, 10, 21119. [Google Scholar] [CrossRef]
- Shi, H.; Fetzer, R.; Jianu, A.; Weisenburger, A.; Heinzel, A.; Lang, F.; Müller, G. Influence of alloying elements (Cu, Ti, Nb) on the microstructure and corrosion behaviour of AlCrFeNi-based high entropy alloys exposed to oxygen-containing molten Pb. Corros. Sci. 2021, 190, 109659. [Google Scholar] [CrossRef]
- Thorhallsson, A.I.; Csáki, I.; Geambazu, L.E.; Magnus, F.; Karlsdottir, S.N. Effect of alloying ratios and Cu-addition on corrosion behaviour of CoCrFeNiMo high-entropy alloys in superheated steam containing CO2, H2S and HCl. Corros. Sci. 2021, 178, 109083. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, H.; Gao, X.; Ren, C.; Gao, J.; Zhang, H.; Zheng, S.; Jin, Q.; Zhao, Y.; Lu, C.; et al. A promising new class of irradiation tolerant materials: Ti2ZrHfV0.5Mo0.2 high-entropy alloy. J. Mater. Sci. Technol. 2019, 35, 369–373. [Google Scholar] [CrossRef]
- Chen, D.; Tong, Y.; Wang, J.; Han, B.; Zhao, Y.L.; He, F.; Kai, J.J. Microstructural response of He+ irradiated FeCoNiCrTi0.2 high-entropy alloy. J. Nucl. Mater. 2018, 510, 187–192. [Google Scholar] [CrossRef]
- Yang, T.; Xia, S.; Liu, S.; Wang, C.; Liu, S.; Fang, Y.; Zhang, Y.; Xue, J.; Yan, S.; Wang, Y. Precipitation behavior of AlxCoCrFeNi high entropy alloys under ion irradiation. Sci. Rep. 2016, 6, 32146. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Gao, M.C.; Yang, T.; Liaw, P.K.; Zhang, Y. Phase stability and microstructures of high entropy alloys ion irradiated to high doses. J. Nucl. Mater. 2016, 480, 100–108. [Google Scholar] [CrossRef] [Green Version]
- Alshataif, Y.A.; Sivasankaran, S.; Al-Mufadi, F.A.; Alaboodi, A.S.; Ammar, H.R. Manufacturing Methods, Microstructural and Mechanical Properties Evolutions of High-Entropy Alloys: A Review. Met. Mater. Int. 2019, 26, 1099–1133. [Google Scholar] [CrossRef]
- He, Q.F.; Tang, P.H.; Chen, H.A.; Lan, S.; Wang, J.G.; Luan, J.H.; Du, M.; Liu, Y.; Liu, C.T.; Pao, C.W.; et al. Understanding chemical short-range ordering/demixing coupled with lattice distortion in solid solution high entropy alloys. Acta Mater. 2021, 216, 117140. [Google Scholar] [CrossRef]
- Stepanov, N.D.; Yurchenko, N.Y.; Zherebtsov, S.V.; Tikhonovsky, M.A.; Salishchev, G.A. Aging behavior of the HfNbTaTiZr high entropy alloy. Mater. Lett. 2018, 211, 87–90. [Google Scholar] [CrossRef]
- Tsai, C.W.; Chen, Y.L.; Tsai, M.H.; Yeh, J.W.; Shun, T.T.; Chen, S.K. Deformation and annealing behaviors of high-entropy alloy Al0.5CoCrCuFeNi. J. Alloys Compd. 2009, 486, 427–435. [Google Scholar] [CrossRef]
- Zhang, K.B.; Fu, Z.Y.; Zhang, J.Y.; Shi, J.; Wang, W.M.; Wang, H.; Wang, Y.C.; Zhang, Q.J. Annealing on the structure and properties evolution of the CoCrFeNiCuAl high-entropy alloy. J. Alloys Compd. 2010, 502, 295–299. [Google Scholar] [CrossRef]
- Bhattacharjee, P.P.; Sathiaraj, G.D.; Zaid, M.; Gatti, J.R.; Lee, C.; Tsai, C.W.; Yeh, J.W. Microstructure and texture evolution during annealing of equiatomic CoCrFeMnNi high-entropy alloy. J. Alloys Compd. 2014, 587, 544–552. [Google Scholar] [CrossRef]
- Stepanov, N.D.; Yurchenko, N.Y.; Tikhonovsky, M.A.; Salishchev, G.A. Effect of carbon content and annealing on structure and hardness of the CoCrFeNiMn-based high entropy alloys. J. Alloys Compd. 2016, 687, 59–71. [Google Scholar] [CrossRef]
- Zhang, K.; Fu, Z. Effects of annealing treatment on phase composition and microstructure of CoCrFeNiTiAlx high-entropy alloys. Intermetallics 2012, 22, 24–32. [Google Scholar] [CrossRef]
- Haase, C.; Barrales-Mora, L.A. Influence of deformation and annealing twinning on the microstructure and texture evolution of face-centered cubic high-entropy alloys. Acta Mater. 2018, 150, 88–103. [Google Scholar] [CrossRef] [Green Version]
- Niu, Z.; Wang, Y.; Geng, C.; Xu, J.; Wang, Y. Microstructural evolution, mechanical and corrosion behaviors of as-annealed CoCrFeNiMox (x = 0, 0.2, 0.5, 0.8, 1) high entropy alloys. J. Alloys Compd. 2020, 820, 153273. [Google Scholar] [CrossRef]
- Abbasi, E.; Dehghani, K. Microstructure and mechanical properties of Co19Cr20Fe20Mn21Ni19 and Co19Cr20Fe20Mn21Ni19Nb0.06C0.8 high-entropy/compositionally-complex alloys after annealing. Mater. Sci. Eng. A 2020, 772, 138812. [Google Scholar] [CrossRef]
- Sathiaraj, G.D.; Pukenas, A.; Skrotzki, W. Texture formation in face-centered cubic high-entropy alloys. J. Alloys Compd. 2020, 826, 154183. [Google Scholar] [CrossRef]
- Munitz, A.; Meshi, L.; Kaufman, M.J. Heat treatments’ effects on the microstructure and mechanical properties of an equiatomic Al-Cr-Fe-Mn-Ni high entropy alloy. Mater. Sci. Eng. A 2017, 689, 384–394. [Google Scholar] [CrossRef] [Green Version]
- Tang, Q.H.; Huang, Y.; Huang, Y.Y.; Liao, X.Z.; Langdon, T.G.; Dai, P.Q. Hardening of an Al0.3CoCrFeNi high entropy alloy via high-pressure torsion and thermal annealing. Mater. Lett. 2015, 151, 126–129. [Google Scholar] [CrossRef] [Green Version]
- Lin, D.; Xu, L.; Jing, H.; Han, Y.; Zhao, L.; Minami, F. Effects of annealing on the structure and mechanical properties of FeCoCrNi high-entropy alloy fabricated via selective laser melting. Addit. Manuf. 2020, 32, 101058. [Google Scholar] [CrossRef]
- Shahmir, H.; He, J.; Lu, Z.; Kawasaki, M.; Langdon, T.G. Effect of annealing on mechanical properties of a nanocrystalline CoCrFeNiMn high-entropy alloy processed by high-pressure torsion. Mater. Sci. Eng. A 2016, 676, 294–303. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Zhang, J.Y.; Wang, Y.Q.; Zhang, P.; Kuang, J.; Liu, G.; Zhang, G.J.; Sun, J. Annealing-dependent microstructure, magnetic and mechanical properties of high-entropy FeCoNiAl0.5 alloy. Mater. Sci. Eng. A 2020, 776, 139003. [Google Scholar] [CrossRef]
- Ma, Y.; Peng, G.J.; Wen, D.H.; Zhang, T.H. Nanoindentation creep behavior in a CoCrFeCuNi high-entropy alloy film with two different structure states. Mater. Sci. Eng. A 2015, 621, 111–117. [Google Scholar] [CrossRef]
- Ma, S.G.; Qiao, J.W.; Wang, Z.H.; Yang, H.J.; Zhang, Y. Microstructural features and tensile behaviors of the Al0.5CrCuFeNi2 high-entropy alloys by cold rolling and subsequent annealing. Mater. Des. 2015, 88, 1057–1062. [Google Scholar] [CrossRef]
- Zhuang, Y.X.; Xue, H.D.; Chen, Z.Y.; Hu, Z.Y.; He, J.C. Effect of annealing treatment on microstructures and mechanical properties of FeCoNiCuAl high entropy alloys. Mater. Sci. Eng. A 2013, 572, 30–35. [Google Scholar] [CrossRef]
- Niu, S.; Kou, H.; Guo, T.; Zhang, Y.; Wang, J.; Li, J. Strengthening of nanoprecipitations in an annealed Al0.5CoCrFeNi high entropy alloy. Mater. Sci. Eng. A 2016, 671, 82–86. [Google Scholar] [CrossRef]
- Gwalani, B.; Gorsse, S.; Choudhuri, D.; Styles, M.; Zheng, Y.; Mishra, R.S.; Banerjee, R. Modifying transformation pathways in high entropy alloys or complex concentrated alloys via thermo-mechanical processing. Acta Mater. 2018, 153, 169–185. [Google Scholar] [CrossRef]
- Wani, I.S.; Bhattacharjee, T.; Sheikh, S.; Bhattacharjee, P.P.; Guo, S.; Tsuji, N. Tailoring nanostructures and mechanical properties of AlCoCrFeNi2.1 eutectic high entropy alloy using thermo-mechanical processing. Mater. Sci. Eng. A 2016, 675, 99–109. [Google Scholar] [CrossRef]
- Wani, I.S.; Sathiaraj, G.D.; Ahmed, M.Z.; Reddy, S.R.; Bhattacharjee, P.P. Evolution of microstructure and texture during thermo-mechanical processing of a two phase Al0.5CoCrFeMnNi high entropy alloy. Mater. Charact. 2016, 118, 417–424. [Google Scholar] [CrossRef]
- Sathiaraj, G.D.; Bhattacharjee, P.P. Effect of starting grain size on the evolution of microstructure and texture during thermo-mechanical processing of CoCrFeMnNi high entropy alloy. J. Alloys Compd. 2015, 647, 82–96. [Google Scholar] [CrossRef]
- Munitz, A.; Salhov, S.; Guttmann, G.; Derimow, N.; Nahmany, M. Heat treatment influence on the microstructure and mechanical properties of AlCrFeNiTi0.5 high entropy alloys. Mater. Sci. Eng. A 2019, 742, 1–14. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, X.; Sun, H.; Shao, Z. Effect of annealing heat treatment on microstructure and mechanical properties of nonequiatomic CoCrFeNiMo medium-entropy alloys prepared by hot isostatic pressing. Nanotechnol. Rev. 2020, 9, 580–595. [Google Scholar] [CrossRef]
- Li, Z.; Fu, L.; Zheng, H.; Yu, R.; Lv, L.; Sun, Y.; Dong, X.; Shan, A. Effect of Annealing Temperature on Microstructure and Mechanical Properties of a Severe Cold-Rolled FeCoCrNiMn High-Entropy Alloy. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2019, 50, 3223–3237. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, X.; Wang, D.; Zhu, W.; Li, J.; Zhao, Y.F. AlCoCuFeNi high-entropy alloy with tailored microstructure and outstanding compressive properties fabricated via selective laser melting with heat treatment. Mater. Sci. Eng. A 2019, 743, 773–784. [Google Scholar] [CrossRef]
- Lin, C.M.; Tsai, H.L.; Bor, H.Y. Effect of aging treatment on microstructure and properties of high-entropy Cu0.5CoCrFeNi alloy. Intermetallics 2010, 18, 1244–1250. [Google Scholar] [CrossRef]
- Wen, L.H.; Kou, H.C.; Li, J.S.; Chang, H.; Xue, X.Y.; Zhou, L. Effect of aging temperature on microstructure and properties of AlCoCrCuFeNi high-entropy alloy. Intermetallics 2009, 17, 266–269. [Google Scholar] [CrossRef]
- Ren, B.; Liu, Z.X.; Cai, B.; Wang, M.X.; Shi, L. Aging behavior of a CuCr2Fe2NiMn high-entropy alloy. Mater. Des. 2012, 33, 121–126. [Google Scholar] [CrossRef]
- Na, T.W.; Park, K.B.; Lee, S.Y.; Yang, S.M.; Kang, J.W.; Lee, T.W.; Park, J.M.; Park, K.; Park, H.K. Preparation of spherical TaNbHfZrTi high-entropy alloy powders by a hydrogenation–dehydrogenation reaction and thermal plasma treatment. J. Alloys Compd. 2020, 817, 152757. [Google Scholar] [CrossRef]
- Zhang, H.; He, Y.; Pan, Y. Enhanced hardness and fracture toughness of the laser-solidified FeCoNiCrCuTiMoAlSiB0.5 high-entropy alloy by martensite strengthening. Scr. Mater. 2013, 69, 342–345. [Google Scholar] [CrossRef]
- Yang, J.; Jo, Y.H.; Kim, D.W.; Choi, W.M.; Kim, H.S.; Lee, B.J.; Sohn, S.S.; Lee, S. Effects of transformation-induced plasticity (TRIP) on tensile property improvement of Fe45Co30Cr10V10Ni5−xMnx high-entropy alloys. Mater. Sci. Eng. A 2020, 772, 138809. [Google Scholar] [CrossRef]
- Lilensten, L.; Couzinié, J.P.; Perrière, L.; Bourgon, J.; Emery, N.; Guillot, I. New structure in refractory high-entropy alloys. Mater. Lett. 2014, 132, 123–125. [Google Scholar] [CrossRef]
- Aryal, A.; Dubenko, I.; Talapatra, S.; Granovsky, A.; Lähderanta, E.; Stadler, S.; Ali, N. Magnetic field dependence of the martensitic transition and magnetocaloric effects in Ni49BiMn35In15. AIP Adv. 2020, 10, 015138. [Google Scholar] [CrossRef]
- Li, R.X.; Liaw, P.K.; Zhang, Y. Synthesis of AlxCoCrFeNi high-entropy alloys by high-gravity combustion from oxides. Mater. Sci. Eng. A 2017, 707, 668–673. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, H.; Hu, S.; Wei, R.; Wang, T.; Cheng, Y.; Zhang, T.; Shi, N.; Li, F.; Guan, S.; et al. Influences of laser surface melting on microstructure, mechanical properties and corrosion resistance of dual-phase Cr–Fe–Co–Ni–Al high entropy alloys. J. Alloys Compd. 2020, 826, 154100. [Google Scholar] [CrossRef]
- He, J.Y.; Wang, H.; Wu, Y.; Liu, X.J.; Mao, H.H.; Nieh, T.G.; Lu, Z.P. Precipitation behavior and its effects on tensile properties of FeCoNiCr high-entropy alloys. Intermetallics 2016, 79, 41–52. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Wen, H.; Zhao, B.; Dong, X.; Zhang, L. Precipitation behavior and its impact on mechanical properties in an aged carbon-containing Al0.3Cu0.5CrFeNi2 high-entropy alloy. Mater. Charact. 2019, 155, 109792. [Google Scholar] [CrossRef]
- Liu, W.H.; Yang, T.; Liu, C.T. Precipitation hardening in CoCrFeNi-based high entropy alloys. Mater. Chem. Phys. 2018, 210, 2–11. [Google Scholar] [CrossRef]
- He, J.Y.; Wang, H.; Huang, H.L.; Xu, X.D.; Chen, M.W.; Wu, Y.; Liu, X.J.; Nieh, T.G.; An, K.; Lu, Z.P. A precipitation-hardened high-entropy alloy with outstanding tensile properties. Acta Mater. 2016, 102, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Park, J.M.; Kang, J.W.; Lee, W.H.; Lee, S.Y.; Min, S.H.; Ha, T.K.; Park, H.K. Preparation of spherical WTaMoNbV refractory high entropy alloy powder by inductively-coupled thermal plasma. Mater. Lett. 2019, 255, 126513. [Google Scholar] [CrossRef]
- Zhang, F.; Lou, H.; Cheng, B.; Zeng, Z.; Zeng, Q. High-Pressure Induced Phase Transitions in High-Entropy Alloys: A Review. Entropy 2019, 21, 239. [Google Scholar] [CrossRef] [Green Version]
- Shahmir, H.; He, J.; Lu, Z.; Kawasaki, M.; Langdon, T.G. Evidence for superplasticity in a CoCrFeNiMn high-entropy alloy processed by high-pressure torsion. Mater. Sci. Eng. A 2017, 685, 342–348. [Google Scholar] [CrossRef] [Green Version]
- Haušild, P.; Čížek, J.; Čech, J.; Zýka, J.; Kim, H.S. Indentation size effect in high pressure torsion processed high entropy alloy. Acta Polytech. CTU Proc. 2020, 27, 141–144. [Google Scholar] [CrossRef]
- Zhang, K.; Peng, S.; Li, N.; Liu, X.; Zhang, M.; Wu, Y.D.; Yang, Y.; Greenberg, E.; Prakapenka, V.B.; Hui, X.; et al. Tuning to more compressible phase in TiZrHfNb high entropy alloy by pressure. Appl. Phys. Lett. 2020, 116, 031901. [Google Scholar] [CrossRef]
- Sonkusare, R.; Biswas, K.; Al-Hamdany, N.; Brokmeier, H.G.; Kalsar, R.; Schell, N.; Gurao, N.P. A critical evaluation of microstructure-texture-mechanical behavior heterogeneity in high pressure torsion processed CoCuFeMnNi high entropy alloy. Mater. Sci. Eng. A 2020, 782, 139187. [Google Scholar] [CrossRef]
- Podolskiy, A.V.; Shapovalov, Y.O.; Tabachnikova, E.D.; Tortika, A.S.; Tikhonovsky, M.A.; Joni, B.; Ódor, E.; Ungar, T.; Maier, S.; Rentenberger, C.; et al. Anomalous Evolution of Strength and Microstructure of High-Entropy Alloy CoCrFeNiMn after High-Pressure Torsion at 300 and 77 K. Adv. Eng. Mater. 2020, 22, 1900752. [Google Scholar] [CrossRef] [Green Version]
- Skrotzki, W.; Pukenas, A.; Odor, E.; Joni, B.; Ungar, T.; Völker, B.; Hohenwarter, A.; Pippan, R.; George, E.P. Microstructure, texture, and strength development during high-pressure torsion of crmnfeconi high-entropy alloy. Crystals 2020, 10, 336. [Google Scholar] [CrossRef]
- Asghari-Rad, P.; Sathiyamoorthi, P.; Thi-Cam Nguyen, N.; Bae, J.W.; Shahmir, H.; Kim, H.S. Fine-tuning of mechanical properties in V10Cr15Mn5Fe35Co10Ni25 high-entropy alloy through high-pressure torsion and annealing. Mater. Sci. Eng. A 2020, 771, 138604. [Google Scholar] [CrossRef]
- Ahmad, A.S.; Su, Y.; Liu, S.Y.; Ståhl, K.; Wu, Y.D.; Hui, X.D.; Ruett, U.; Gutowski, O.; Glazyrin, K.; Liermann, H.P.; et al. Structural stability of high entropy alloys under pressure and temperature. J. Appl. Phys. 2017, 121, 235901. [Google Scholar] [CrossRef] [Green Version]
- LuŽnik, J.; KoŽelj, P.; Vrtnik, S.; Jelen, A.; Jagličić, Z.; Meden, A.; Feuerbacher, M.; Dolinšek, J. Complex magnetism of Ho-Dy-Y-Gd-Tb hexagonal high-entropy alloy. Phys. Rev. B Condens. Matter Mater. Phys. 2015, 92, 224201. [Google Scholar] [CrossRef]
- Feuerbacher, M.; Heidelmann, M.; Thomas, C. Hexagonal High-entropy Alloys. Mater. Res. Lett. 2014, 3, 1–6. [Google Scholar] [CrossRef]
- Heczel, A.; Kawasaki, M.; Lábár, J.L.; Jang, J.I.; Langdon, T.G.; Gubicza, J. Defect structure and hardness in nanocrystalline CoCrFeMnNi High-Entropy Alloy processed by High-Pressure Torsion. J. Alloys Compd. 2017, 711, 143–154. [Google Scholar] [CrossRef] [Green Version]
- Kilmametov, A.; Kulagin, R.; Mazilkin, A.; Seils, S.; Boll, T.; Heilmaier, M.; Hahn, H. High-pressure torsion driven mechanical alloying of CoCrFeMnNi high entropy alloy. Scr. Mater. 2019, 158, 29–33. [Google Scholar] [CrossRef]
- Asghari-Rad, P.; Sathiyamoorthi, P.; Bae, J.W.; Moon, J.; Park, J.M.; Zargaran, A.; Kim, H.S. Effect of grain size on the tensile behavior of V10Cr15Mn5Fe35Co10Ni25 high entropy alloy. Mater. Sci. Eng. A 2019, 744, 610–617. [Google Scholar] [CrossRef]
- Edalati, P.; Floriano, R.; Tang, Y.; Mohammadi, A.; Pereira, K.D.; Luchessi, A.D.; Edalati, K. Ultrahigh hardness and biocompatibility of high-entropy alloy TiAlFeCoNi processed by high-pressure torsion. Mater. Sci. Eng. C 2020, 112, 110908. [Google Scholar] [CrossRef]
- Yu, P.F.; Zhang, L.J.; Cheng, H.; Zhang, H.; Ma, M.Z.; Li, Y.C.; Li, G.; Liaw, P.K.; Liu, R.P. The high-entropy alloys with high hardness and soft magnetic property prepared by mechanical alloying and high-pressure sintering. Intermetallics 2016, 70, 82–87. [Google Scholar] [CrossRef] [Green Version]
- Tracy, C.L.; Park, S.; Rittman, D.R.; Zinkle, S.J.; Bei, H.; Lang, M.; Ewing, R.C.; Mao, W.L. High pressure synthesis of a hexagonal close-packed phase of the high-entropy alloy CrMnFeCoNi. Nat. Commun. 2017, 8, 15634. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.H.; Tong, Y.; Chen, S.W.; Xu, W.W.; Wu, H.H.; Zhao, Y.L.; Yang, T.; Wang, X.L.; Liu, X.; Kai, J.J.; et al. Unveiling the Electronic Origin for Pressure-Induced Phase Transitions in High-Entropy Alloys. Matter 2020, 2, 751–763. [Google Scholar] [CrossRef] [Green Version]
- Yu, P.F.; Zhang, L.J.; Ning, J.L.; Ma, M.Z.; Zhang, X.Y.; Li, Y.C.; Liaw, P.K.; Li, G.; Liu, R.P. Pressure-induced phase transitions in HoDyYGdTb high-entropy alloy. Mater. Lett. 2017, 196, 137–140. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Lou, H.; Chen, S.; Chen, X.; Zeng, Z.; Yan, J.; Zhao, W.; Wu, Y.; Lu, Z.; Zeng, Q. Effects of non-hydrostaticity and grain size on the pressure-induced phase transition of the CoCrFeMnNi high-entropy alloy. J. Appl. Phys. 2018, 124, 115901. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.X.; Zhao, S.; Jin, K.; Bei, H.; Popov, D.; Park, C.; Neuefeind, J.C.; Weber, W.J.; Zhang, Y. Pressure-induced fcc to hcp phase transition in Ni-based high entropy solid solution alloys. Appl. Phys. Lett. 2017, 110, 011902. [Google Scholar] [CrossRef]
- Cheng, B.; Zhang, F.; Lou, H.; Chen, X.; Liaw, P.K.; Yan, J.; Zeng, Z.; Ding, Y.; Zeng, Q. Pressure-induced phase transition in the AlCoCrFeNi high-entropy alloy. Scr. Mater. 2019, 161, 88–92. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Bhandari, U.; Zeng, C.; Ding, H.; Guo, S.; Yan, J.; Yang, S. Carbide formation in refractory Mo15Nb20Re15Ta30W20 alloy under a combined high-pressure and high-temperature condition. Entropy 2020, 22, 718. [Google Scholar] [CrossRef]
- Tillmann, W.; Ulitzka, T.; Wojarski, L.; Manka, M.; Ulitzka, H.; Wagstyl, D. Development of high entropy alloys for brazing applications. Weld. World 2020, 64, 201–208. [Google Scholar] [CrossRef]
- Liu, R.; Wang, Z.; Sparks, T.; Liou, F.; Newkirk, J. Aerospace applications of laser additive manufacturing. In Laser Additive Manufacturing: Materials, Design, Technologies, and Applications; Brandt, M., Ed.; Matthew Deans; Woodhead Publishing: Sawston, England, 2017; pp. 351–371. ISBN 9780081004340. [Google Scholar]
- Zhu, Z.G.; Nguyen, Q.B.; Ng, F.L.; An, X.H.; Liao, X.Z.; Liaw, P.K.; Nai, S.M.L.; Wei, J. Hierarchical microstructure and strengthening mechanisms of a CoCrFeNiMn high entropy alloy additively manufactured by selective laser melting. Scr. Mater. 2018, 154, 20–24. [Google Scholar] [CrossRef]
- Park, J.M.; Choe, J.; Kim, J.G.; Bae, J.W.; Moon, J.; Yang, S.; Kim, K.T.; Yu, J.H.; Kim, H.S. Superior tensile properties of 1%C-CoCrFeMnNi high-entropy alloy additively manufactured by selective laser melting. Mater. Res. Lett. 2020, 8, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Liu, Y.; Zhou, C.; Li, S.; Wu, W.; Song, M.; Liu, B.; Liang, X.; Liaw, P.K. Microstructures and mechanical properties of C-containing FeCoCrNi high-entropy alloy fabricated by selective laser melting. Intermetallics 2018, 94, 165–171. [Google Scholar] [CrossRef]
- Brif, Y.; Thomas, M.; Todd, I. The use of high-entropy alloys in additive manufacturing. Scr. Mater. 2015, 99, 93–96. [Google Scholar] [CrossRef]
- Peyrouzet, F.; Hachet, D.; Soulas, R.; Navone, C.; Godet, S.; Gorsse, S. Selective Laser Melting of Al0.3CoCrFeNi High-Entropy Alloy: Printability, Microstructure, and Mechanical Properties. JOM 2019, 71, 3443–3451. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Peng, W.; Yang, L.; Fang, L. Effect of SLM processing parameters on microstructures and mechanical properties of Al0. 5CoCrFeNi high entropy alloys. Metals 2020, 10, 292. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Niu, S.; Guo, T.; Kou, H.; Li, J. The FCC to BCC phase transformation kinetics in an Al0.5CoCrFeNi high entropy alloy. J. Alloys Compd. 2017, 710, 144–150. [Google Scholar] [CrossRef]
- Zhou, P.F.; Xiao, D.H.; Wu, Z.; Ou, X.Q. Al0.5FeCoCrNi high entropy alloy prepared by selective laser melting with gas-atomized pre-alloy powders. Mater. Sci. Eng. A 2019, 739, 86–89. [Google Scholar] [CrossRef]
- Li, X. Additive Manufacturing of Advanced Multi-Component Alloys: Bulk Metallic Glasses and High Entropy Alloys. Adv. Eng. Mater. 2018, 20, 1490. [Google Scholar] [CrossRef]
- Li, X.P.; Wang, X.J.; Saunders, M.; Suvorova, A.; Zhang, L.C.; Liu, Y.J.; Fang, M.H.; Huang, Z.H.; Sercombe, T.B. A selective laser melting and solution heat treatment refined Al-12Si alloy with a controllable ultrafine eutectic microstructure and 25% tensile ductility. Acta Mater. 2015, 95, 74–82. [Google Scholar] [CrossRef]
- Li, X.P.; Ji, G.; Chen, Z.; Addad, A.; Wu, Y.; Wang, H.W.; Vleugels, J.; Van Humbeeck, J.; Kruth, J.P. Selective laser melting of nano-TiB2 decorated AlSi10Mg alloy with high fracture strength and ductility. Acta Mater. 2017, 129, 183–193. [Google Scholar] [CrossRef]
- Attar, H.; Bönisch, M.; Calin, M.; Zhang, L.C.; Scudino, S.; Eckert, J. Selective laser melting of in situ titanium-titanium boride composites: Processing, microstructure and mechanical properties. Acta Mater. 2014, 76, 13–22. [Google Scholar] [CrossRef]
- Vrancken, B.; Thijs, L.; Kruth, J.P.; Van Humbeeck, J. Microstructure and mechanical properties of a novel β titanium metallic composite by selective laser melting. Acta Mater. 2014, 68, 150–158. [Google Scholar] [CrossRef] [Green Version]
- Li, X.P.; Van Humbeeck, J.; Kruth, J.P. Selective laser melting of weak-textured commercially pure titanium with high strength and ductility: A study from laser power perspective. Mater. Des. 2017, 116, 352–358. [Google Scholar] [CrossRef]
- Wang, Q.; Ren, L.; Li, X.; Zhang, S.; Sercombe, T.B.; Yang, K. Antimicrobial Cu-bearing stainless steel scaffolds. Mater. Sci. Eng. C 2016, 68, 519–522. [Google Scholar] [CrossRef]
- Sercombe, T.B.; Li, X. Selective laser melting of aluminium and aluminium metal matrix composites: Review. Mater. Technol. 2016, 31, 77–85. [Google Scholar] [CrossRef]
- Niu, P.; Li, R.; Zhu, S.; Wang, M.; Chen, C.; Yuan, T. Hot cracking, crystal orientation and compressive strength of an equimolar CoCrFeMnNi high-entropy alloy printed by selective laser melting. Opt. Laser Technol. 2020, 127, 106147. [Google Scholar] [CrossRef]
- Karlsson, D.; Marshal, A.; Johansson, F.; Schuisky, M.; Sahlberg, M.; Schneider, J.M.; Jansson, U. Elemental segregation in an AlCoCrFeNi high-entropy alloy—A comparison between selective laser melting and induction melting. J. Alloys Compd. 2019, 784, 195–203. [Google Scholar] [CrossRef]
- Cui, W.; Zhang, X.; Liou, F. Additive Manufacturing of High-Entropy Alloys—A Review. In Proceedings of the 2017 28th Annual International Solid Freeform Fabrication Symposium—An Additive Manufacturing Conference, Rolla, MO, USA, 7–9 August 2017; pp. 712–724. [Google Scholar]
- Ostovari Moghaddam, A.; Shaburova, N.A.; Samodurova, M.N.; Abdollahzadeh, A.; Trofimov, E.A. Additive manufacturing of high entropy alloys: A practical review. J. Mater. Sci. Technol. 2021, 77, 131–162. [Google Scholar] [CrossRef]
- Yeh, M.C.G.J.; Liaw, P.K.; Zhang, Y. High-Entropy Alloys; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9783319270111. [Google Scholar]
- Riva, S.; Brown, S.G.R.; Lavery, N.P.; Tudball, A.; Yusenko, K.V. Spark Plasma Sintering of Materials; Cavaliere, P., Ed.; Springer: Lecce, Italy, 2019; ISBN 978-3-030-05326-0. [Google Scholar]
- Vaidya, M.; Muralikrishna, G.M.; Murty, B.S. High-entropy alloys by mechanical alloying: A review. J. Mater. Res. 2019, 34, 664–686. [Google Scholar] [CrossRef]
- Chen, P.; Li, S.; Zhou, Y.; Yan, M.; Attallah, M.M. Fabricating CoCrFeMnNi high entropy alloy via selective laser melting in-situ alloying. J. Mater. Sci. Technol. 2020, 43, 40–43. [Google Scholar] [CrossRef]
- Li, B.; Zhang, L.; Xu, Y.; Liu, Z.; Qian, B.; Xuan, F. Selective laser melting of CoCrFeNiMn high entropy alloy powder modified with nano-TiN particles for additive manufacturing and strength enhancement: Process, particle behavior and effects. Powder Technol. 2020, 360, 509–521. [Google Scholar] [CrossRef]
- Li, N.; Wu, S.; Ouyang, D.; Zhang, J.; Liu, L. Fe-based metallic glass reinforced FeCoCrNiMn high entropy alloy through selective laser melting. J. Alloys Compd. 2020, 822, 153695. [Google Scholar] [CrossRef]
- Li, B.; Zhang, L.; Yang, B. Grain refinement and localized amorphization of additively manufactured high-entropy alloy matrix composites reinforced by nano ceramic particles via selective-laser-melting/remelting. Compos. Commun. 2020, 19, 56–60. [Google Scholar] [CrossRef]
- Kim, J.G.; Park, J.M.; Seol, J.B.; Choe, J.; Yu, J.H.; Yang, S.; Kim, H.S. Nano-scale solute heterogeneities in the ultrastrong selectively laser melted carbon-doped CoCrFeMnNi alloy. Mater. Sci. Eng. A 2020, 773, 138726. [Google Scholar] [CrossRef]
- Li, B.; Qian, B.; Xu, Y.; Liu, Z.; Xuan, F. Fine-structured CoCrFeNiMn high-entropy alloy matrix composite with 12 wt% TiN particle reinforcements via selective laser melting assisted additive manufacturing. Mater. Lett. 2019, 252, 88–91. [Google Scholar] [CrossRef]
- Piglione, A.; Dovgyy, B.; Liu, C.; Gourlay, C.M.; Hooper, P.A.; Pham, M.S. Printability and microstructure of the CoCrFeMnNi high-entropy alloy fabricated by laser powder bed fusion. Mater. Lett. 2018, 224, 22–25. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, H.; Li, W.; Mao, A.; Wang, L.; Song, G.; He, Y. Microstructure and nanoindentation creep behavior of CoCrFeMnNi high-entropy alloy fabricated by selective laser melting. Addit. Manuf. 2019, 28, 766–771. [Google Scholar] [CrossRef]
- Ren, J.; Mahajan, C.; Liu, L.; Follette, D.; Chen, W.; Mukherjee, S. Corrosion behavior of selectively laser melted CoCrFeMnNi high entropy alloy. Metals 2019, 9, 1029. [Google Scholar] [CrossRef] [Green Version]
- Dovgyy, B.; Pham, M.S. Epitaxial growth in 316L steel and CoCrFeMnNi high entropy alloy made by powder-bed laser melting. AIP Conf. Proc. 2018, 1960, 140008. [Google Scholar] [CrossRef]
- Wu, W.; Zhou, R.; Wei, B.; Ni, S.; Liu, Y.; Song, M. Nanosized precipitates and dislocation networks reinforced C-containing CoCrFeNi high-entropy alloy fabricated by selective laser melting. Mater. Charact. 2018, 144, 605–610. [Google Scholar] [CrossRef]
- Sun, Z.; Tan, X.P.; Descoins, M.; Mangelinck, D.; Tor, S.B.; Lim, C.S. Revealing hot tearing mechanism for an additively manufactured high-entropy alloy via selective laser melting. Scr. Mater. 2019, 168, 129–133. [Google Scholar] [CrossRef]
- Song, M.; Zhou, R.; Gu, J.; Wang, Z.; Ni, S.; Liu, Y. Nitrogen induced heterogeneous structures overcome strength-ductility trade-off in an additively manufactured high-entropy alloy. Appl. Mater. Today 2020, 18, 100498. [Google Scholar] [CrossRef]
- Zhou, R.; Chen, G.; Liu, B.; Wang, J.; Han, L.; Liu, Y. Microstructures and wear behaviour of (FeCoCrNi)1−x(WC)x high entropy alloy composites. Int. J. Refract. Met. Hard Mater. 2018, 75, 56–62. [Google Scholar] [CrossRef]
- Niu, P.D.; Li, R.D.; Yuan, T.C.; Zhu, S.Y.; Chen, C.; Wang, M.B.; Huang, L. Microstructures and properties of an equimolar AlCoCrFeNi high entropy alloy printed by selective laser melting. Intermetallics 2019, 104, 24–32. [Google Scholar] [CrossRef]
- Luo, S.; Gao, P.; Yu, H.; Yang, J.; Wang, Z.; Zeng, X. Selective laser melting of an equiatomic AlCrCuFeNi high-entropy alloy: Processability, non-equilibrium microstructure and mechanical behavior. J. Alloys Compd. 2019, 771, 387–397. [Google Scholar] [CrossRef]
- Luo, S.; Zhao, C.; Su, Y.; Liu, Q.; Wang, Z. Selective laser melting of dual phase AlCrCuFeNix high entropy alloys: Formability, heterogeneous microstructures and deformation mechanisms. Addit. Manuf. 2020, 31, 100925. [Google Scholar] [CrossRef]
- Yao, H.; Tan, Z.; He, D.; Zhou, Z.; Zhou, Z.; Xue, Y.; Cui, L.; Chen, L.; Wang, G.; Yang, Y. High strength and ductility AlCrFeNiV high entropy alloy with hierarchically heterogeneous microstructure prepared by selective laser melting. J. Alloys Compd. 2020, 813, 152196. [Google Scholar] [CrossRef]
- Wang, Y.; Li, R.; Niu, P.; Zhang, Z.; Yuan, T.; Yuan, J.; Li, K. Microstructures and properties of equimolar AlCoCrCuFeNi high-entropy alloy additively manufactured by selective laser melting. Intermetallics 2020, 120, 106746. [Google Scholar] [CrossRef]
- Wang, M.; Li, R.; Yuan, T.; Chen, C.; Zhou, L.; Chen, H.; Zhang, M.; Xie, S. Microstructures and mechanical property of AlMgScZrMn—A comparison between selective laser melting, spark plasma sintering and cast. Mater. Sci. Eng. A 2019, 756, 354–364. [Google Scholar] [CrossRef]
- Sarswat, P.K.; Sarkar, S.; Murali, A.; Huang, W.; Tan, W.; Free, M.L. Additive manufactured new hybrid high entropy alloys derived from the AlCoFeNiSmTiVZr system. Appl. Surf. Sci. 2019, 476, 242–258. [Google Scholar] [CrossRef]
- Agrawal, P.; Thapliyal, S.; Nene, S.S.; Mishra, R.S.; McWilliams, B.A.; Cho, K.C. Excellent strength-ductility synergy in metastable high entropy alloy by laser powder bed additive manufacturing. Addit. Manuf. 2020, 32, 101098. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Huang, S.; Zhu, S.; Wang, F.; Li, D. Manufacturing and analysis of high-performance refractory high-entropy alloy via selective laser melting (SLM). Materials 2019, 12, 720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Xu, W.; Xu, Y.; Lu, Z.; Li, D. The thermal-mechanical behavior of WTaMoNb high-entropy alloy via selective laser melting (SLM): Experiment and simulation. Int. J. Adv. Manuf. Technol. 2018, 96, 461–474. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, Y.; Xi, S.; Chen, Z.; Wei, P.; He, C.; Li, T.; Gao, Y.; Wu, H. Additively manufactured fine grained Ni6Cr4WFe9Ti high entropy alloys with high strength and ductility. Mater. Sci. Eng. A 2019, 767, 138394. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, Y.; Xi, S.; Chen, Z.; Wei, P.; He, C.; Li, T.; Gao, Y.; Wu, H. Grain-anisotropied high-strength Ni6Cr4WFe9Ti high entropy alloys with outstanding tensile ductility. Mater. Sci. Eng. A 2019, 767, 138382. [Google Scholar] [CrossRef]
- Chen, P.; Yang, C.; Li, S.; Attallah, M.M.; Yan, M. In-situ alloyed, oxide-dispersion-strengthened CoCrFeMnNi high entropy alloy fabricated via laser powder bed fusion. Mater. Des. 2020, 194, 108966. [Google Scholar] [CrossRef]
- Litwa, P.; Hernandez-Nava, E.; Guan, D.; Goodall, R.; Wika, K.K. The additive manufacture processing and machinability of CrMnFeCoNi high entropy alloy. Mater. Des. 2021, 198, 109380. [Google Scholar] [CrossRef]
- Zhang, C.; Feng, K.; Kokawa, H.; Han, B.; Li, Z. Cracking mechanism and mechanical properties of selective laser melted CoCrFeMnNi high entropy alloy using different scanning strategies. Mater. Sci. Eng. A 2020, 789, 139672. [Google Scholar] [CrossRef]
- Kim, Y.K.; Suh, J.Y.; Lee, K.A. Effect of gaseous hydrogen embrittlement on the mechanical properties of additively manufactured CrMnFeCoNi high-entropy alloy strengthened by in-situ formed oxide. Mater. Sci. Eng. A 2020, 796, 140039. [Google Scholar] [CrossRef]
- Choi, N.; Kulitckii, V.; Kottke, J.; Tas, B.; Choe, J.; Yu, J.H.; Yang, S.; Park, J.H.; Lee, J.S.; Wilde, G.; et al. Analyzing the ‘non-equilibrium state’ of grain boundaries in additively manufactured high-entropy CoCrFeMnNi alloy using tracer diffusion measurements. J. Alloys Compd. 2020, 844, 155757. [Google Scholar] [CrossRef]
- Su, Y.; Luo, S.; Wang, Z. Microstructure evolution and cracking behaviors of additively manufactured AlxCrCuFeNi2 high entropy alloys via selective laser melting. J. Alloys Compd. 2020, 842, 155823. [Google Scholar] [CrossRef]
- Peng, Y.; Kong, Y.; Zhang, W.; Zhang, M.; Wang, H. Effect of diffusion barrier and interfacial strengthening on the interface behavior between high entropy alloy and diamond. J. Alloys Compd. 2021, 852, 157023. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, Z.G.; Chen, H.; Wang, A.G.; Liu, J.Q.; Liu, H.W.; Zheng, R.K.; Nai, S.M.L.; Primig, S.; Babu, S.S.; et al. Effect of cyclic rapid thermal loadings on the microstructural evolution of a CrMnFeCoNi high-entropy alloy manufactured by selective laser melting. Acta Mater. 2020, 196, 609–625. [Google Scholar] [CrossRef]
- Sun, Z.; Tan, X.; Wang, C.; Descoins, M.; Mangelinck, D.; Tor, S.B.; Jägle, E.A.; Zaefferer, S.; Raabe, D. Reducing hot tearing by grain boundary segregation engineering in additive manufacturing: Example of an AlxCoCrFeNi high-entropy alloy. Acta Mater. 2021, 204, 116505. [Google Scholar] [CrossRef]
- Ishimoto, T.; Ozasa, R.; Nakano, K.; Weinmann, M.; Schnitter, C.; Stenzel, M.; Matsugaki, A.; Nagase, T.; Matsuzaka, T.; Todai, M.; et al. Development of TiNbTaZrMo bio-high entropy alloy (BioHEA) super-solid solution by selective laser melting, and its improved mechanical property and biocompatibility. Scr. Mater. 2021, 194, 113658. [Google Scholar] [CrossRef]
- Park, J.M.; Choe, J.; Park, H.K.; Son, S.; Jung, J.; Kim, T.S.; Yu, J.H.; Kim, J.G.; Kim, H.S. Synergetic strengthening of additively manufactured (CoCrFeMnNi)99C1 high-entropy alloy by heterogeneous anisotropic microstructure. Addit. Manuf. 2020, 35, 101333. [Google Scholar] [CrossRef]
- Lin, D.; Xu, L.; Li, X.; Jing, H.; Qin, G.; Pang, H.; Minami, F. A Si-containing FeCoCrNi high-entropy alloy with high strength and ductility synthesized in situ via selective laser melting. Addit. Manuf. 2020, 35, 101340. [Google Scholar] [CrossRef]
- Kim, Y.K.; Yang, S.; Lee, K.A. Compressive creep behavior of selective laser melted CoCrFeMnNi high-entropy alloy strengthened by in-situ formation of nano-oxides. Addit. Manuf. 2020, 36, 101543. [Google Scholar] [CrossRef]
- Jin, M.; Piglione, A.; Dovgyy, B.; Hosseini, E.; Hooper, P.A.; Holdsworth, S.R.; Pham, M.S. Cyclic plasticity and fatigue damage of CrMnFeCoNi high entropy alloy fabricated by laser powder-bed fusion. Addit. Manuf. 2020, 36, 101584. [Google Scholar] [CrossRef]
- Lin, W.C.; Chang, Y.J.; Hsu, T.H.; Gorsse, S.; Sun, F.; Furuhara, T.; Yeh, A.C. Microstructure and tensile property of a precipitation strengthened high entropy alloy processed by selective laser melting and post heat treatment. Addit. Manuf. 2020, 36, 101601. [Google Scholar] [CrossRef]
- Peng, H.; Lin, Z.; Li, R.; Niu, P.; Zhang, Z. Corrosion Behavior of an Equiatomic CoCrFeMnNi High-Entropy Alloy—A Comparison Between Selective Laser Melting and Cast. Front. Mater. 2020, 7, 244. [Google Scholar] [CrossRef]
- Vogiatzief, D.; Evirgen, A.; Gein, S.; Molina, V.R.; Weisheit, A.; Pedersen, M. Laser Powder Bed Fusion and Heat Treatment of an AlCrFe2Ni2 High Entropy Alloy. Front. Mater. 2020, 7, 248. [Google Scholar] [CrossRef]
- Liao, Y.; Zhu, P.; Li, S. Synthesis of AlFeCrNiV high entropy alloy by gas atomization and selective laser melting. Synthesis (Stuttg) 2020, 7, 11591–11594. [Google Scholar]
- Guo, L.; Gu, J.; Gan, B.; Ni, S.; Bi, Z.; Wang, Z.; Song, M. Effects of elemental segregation and scanning strategy on the mechanical properties and hot cracking of a selective laser melted FeCoCrNiMn-(N,Si) high entropy alloy. J. Alloys Compd. 2021, 865, 158892. [Google Scholar] [CrossRef]
- Kim, Y.K.; Yu, J.H.; Kim, H.S.; Lee, K.A. In-situ carbide-reinforced CoCrFeMnNi high-entropy alloy matrix nanocomposites manufactured by selective laser melting: Carbon content effects on microstructure, mechanical properties, and deformation mechanism. Compos. Part B Eng. 2021, 210, 108638. [Google Scholar] [CrossRef]
- Zhao, W.; Han, J.-K.; Kuzminova, Y.O.; Evlashin, S.A.; Zhilyaev, A.P.; Pesin, A.M.; Jang, J.; Liss, K.-D.; Kawasaki, M. Significance of grain refinement on micro-mechanical properties and structures of additively-manufactured CoCrFeNi high-entropy alloy. Mater. Sci. Eng. A 2021, 807, 140898. [Google Scholar] [CrossRef]
- Gu, Z.; Su, X.; Peng, W.; Guo, W.; Xi, S.; Zhang, X.; Tu, H.; Gao, Y.; Wu, H. An important improvement of strength and ductility on a new type of CoCr2.5FeNi2TiW0.5 high entropy alloys under two different protective gases by selective laser melting. J. Alloys Compd. 2021, 868, 159088. [Google Scholar] [CrossRef]
- Peng, S.; Mooraj, S.; Feng, R.; Liu, L.; Ren, J.; Liu, Y.; Kong, F.; Xiao, Z.; Zhu, C.; Liaw, P.K.; et al. Additive manufacturing of three-dimensional (3D)-architected CoCrFeNiMn high- entropy alloy with great energy absorption. Scr. Mater. 2021, 190, 46–51. [Google Scholar] [CrossRef]
- Wang, P.; Huang, P.; Ng, F.L.; Sin, W.J.; Lu, S.; Nai, M.L.S.; Dong, Z.L.; Wei, J. Additively manufactured CoCrFeNiMn high-entropy alloy via pre-alloyed powder. Mater. Des. 2019, 168, 107576. [Google Scholar] [CrossRef]
- Kuwabara, K.; Shiratori, H.; Fujieda, T.; Yamanaka, K.; Koizumi, Y.; Chiba, A. Mechanical and corrosion properties of AlCoCrFeNi high-entropy alloy fabricated with selective electron beam melting. Addit. Manuf. 2018, 23, 264–271. [Google Scholar] [CrossRef]
- Yang, S.; Liu, Z.; Pi, J. Microstructure and wear behavior of the AlCrFeCoNi high-entropy alloy fabricated by additive manufacturing. Mater. Lett. 2020, 261, 127004. [Google Scholar] [CrossRef]
- Fujieda, T.; Chen, M.; Shiratori, H.; Kuwabara, K.; Yamanaka, K.; Koizumi, Y.; Chiba, A.; Watanabe, S. Mechanical and corrosion properties of CoCrFeNiTi-based high-entropy alloy additive manufactured using selective laser melting. Addit. Manuf. 2019, 25, 412–420. [Google Scholar] [CrossRef]
- Popov, V.V.; Katz-Demyanetz, A.; Koptyug, A.; Bamberger, M. Selective electron beam melting of Al0.5CrMoNbTa0.5 high entropy alloys using elemental powder blend. Heliyon 2019, 5, e01188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, S.; Wan, D.; Solberg, K.; Berto, F.; Welo, T.; Yue, T.M.; Chan, K.C. Additive manufacturing of fine-grained and dislocation-populated CrMnFeCoNi high entropy alloy by laser engineered net shaping. Mater. Sci. Eng. A 2019, 761, 138056. [Google Scholar] [CrossRef]
- Melia, M.A.; Carroll, J.D.; Whetten, S.R.; Esmaeely, S.N.; Locke, J.; White, E.; Anderson, I.; Chandross, M.; Michael, J.R.; Argibay, N.; et al. Mechanical and Corrosion Properties of Additively Manufactured CoCrFeMnNi High Entropy Alloy. Addit. Manuf. 2019, 29, 100833. [Google Scholar] [CrossRef]
- Li, H.G.; Lee, T.L.; Zheng, W.; Lu, Y.Z.; Yin, H.B.C.; Yang, J.X.; Huang, Y.J.; Sun, J.F. Characterization of residual stress in laser melting deposited CoCrFeMnNi high entropy alloy by neutron diffraction. Mater. Lett. 2020, 263, 127247. [Google Scholar] [CrossRef]
- Gao, X.; Lu, Y. Laser 3D printing of CoCrFeMnNi high-entropy alloy. Mater. Lett. 2019, 236, 77–80. [Google Scholar] [CrossRef]
- Xiang, S.; Li, J.; Luan, H.; Amar, A.; Lu, S.; Li, K.; Zhang, L.; Liu, X.; Le, G.; Wang, X.; et al. Effects of process parameters on microstructures and tensile properties of laser melting deposited CrMnFeCoNi high entropy alloys. Mater. Sci. Eng. A 2019, 743, 412–417. [Google Scholar] [CrossRef]
- Xiang, S.; Luan, H.; Wu, J.; Yao, K.F.; Li, J.; Liu, X.; Tian, Y.; Mao, W.; Bai, H.; Le, G.; et al. Microstructures and mechanical properties of CrMnFeCoNi high entropy alloys fabricated using laser metal deposition technique. J. Alloys Compd. 2019, 773, 387–392. [Google Scholar] [CrossRef]
- Chew, Y.; Bi, G.J.; Zhu, Z.G.; Ng, F.L.; Weng, F.; Liu, S.B.; Nai, S.M.L.; Lee, B.Y. Microstructure and enhanced strength of laser aided additive manufactured CoCrFeNiMn high entropy alloy. Mater. Sci. Eng. A 2019, 744, 137–144. [Google Scholar] [CrossRef]
- Qiu, Z.; Yao, C.; Feng, K.; Li, Z.; Chu, P.K. Cryogenic deformation mechanism of CrMnFeCoNi high-entropy alloy fabricated by laser additive manufacturing process. Int. J. Light. Mater. Manuf. 2018, 1, 33–39. [Google Scholar] [CrossRef]
- Li, J.; Xiang, S.; Luan, H.; Amar, A.; Liu, X.; Lu, S.; Zeng, Y.; Le, G.; Wang, X.; Qu, F.; et al. Additive manufacturing of high-strength CrMnFeCoNi high-entropy alloys-based composites with WC addition. J. Mater. Sci. Technol. 2019, 35, 2430–2434. [Google Scholar] [CrossRef]
- Amar, A.; Li, J.; Xiang, S.; Liu, X.; Zhou, Y.; Le, G.; Wang, X.; Qu, F.; Ma, S.; Dong, W.; et al. Additive manufacturing of high-strength CrMnFeCoNi-based High Entropy Alloys with TiC addition. Intermetallics 2019, 109, 162–166. [Google Scholar] [CrossRef]
- Guan, S.; Wan, D.; Solberg, K.; Berto, F.; Welo, T.; Yue, T.M.; Chan, K.C. Additively manufactured CrMnFeCoNi/AlCoCrFeNiTi0.5 laminated high-entropy alloy with enhanced strength-plasticity synergy. Scr. Mater. 2020, 183, 133–138. [Google Scholar] [CrossRef]
- Wang, Q.; Amar, A.; Jiang, C.; Luan, H.; Zhao, S.; Zhang, H.; Le, G.; Liu, X.; Wang, X.; Yang, X.; et al. CoCrFeNiMo0.2 high entropy alloy by laser melting deposition: Prospective material for low temperature and corrosion resistant applications. Intermetallics 2020, 119, 106727. [Google Scholar] [CrossRef]
- Zhou, K.; Li, J.; Wang, L.; Yang, H.; Wang, Z.; Wang, J. Direct laser deposited bulk CoCrFeNiNbx high entropy alloys. Intermetallics 2019, 114, 106592. [Google Scholar] [CrossRef]
- Gwalani, B.; Gangireddy, S.; Shukla, S.; Yannetta, C.J.; Valentin, S.G.; Mishra, R.S.; Banerjee, R. Compositionally graded high entropy alloy with a strong front and ductile back. Mater. Today Commun. 2019, 20, 100602. [Google Scholar] [CrossRef]
- Nartu, M.S.K.K.Y.; Alam, T.; Dasari, S.; Mantri, S.A.; Gorsse, S.; Siller, H.; Dahotre, N.; Banerjee, R. Enhanced tensile yield strength in laser additively manufactured Al0.3CoCrFeNi high entropy alloy. Materialia 2020, 9, 100522. [Google Scholar] [CrossRef]
- Mohanty, A.; Sampreeth, J.K.; Bembalge, O.; Hascoet, J.Y.; Marya, S.; Immanuel, R.J.; Panigrahi, S.K. High temperature oxidation study of direct laser deposited AlXCoCrFeNi (X = 0.3, 0.7) high entropy alloys. Surf. Coat. Technol. 2019, 380, 125028. [Google Scholar] [CrossRef]
- Vikram, R.J.; Murty, B.S.; Fabijanic, D.; Suwas, S. Insights into micro-mechanical response and texture of the additively manufactured eutectic high entropy alloy AlCoCrFeNi2.1. J. Alloys Compd. 2020, 827, 154034. [Google Scholar] [CrossRef]
- Gwalani, B.; Soni, V.; Waseem, O.A.; Mantri, S.A.; Banerjee, R. Laser additive manufacturing of compositionally graded AlCrFeMoVx (x = 0 to 1) high-entropy alloy system. Opt. Laser Technol. 2019, 113, 330–337. [Google Scholar] [CrossRef]
- Guan, S.; Solberg, K.; Wan, D.; Berto, F.; Welo, T.; Yue, T.M.; Chan, K.C. Formation of fully equiaxed grain microstructure in additively manufactured AlCoCrFeNiTi0.5 high entropy alloy. Mater. Des. 2019, 184, 108202. [Google Scholar] [CrossRef]
- Malatji, N.; Popoola, A.P.I.; Lengopeng, T.; Pityana, S. Tribological and corrosion properties of laser additive manufactured AlCrFeNiCu high entropy alloy. Mater. Today Proc. 2020, 28, 944–948. [Google Scholar] [CrossRef]
- Dada, M.; Patricia, P.; Mathe, N.; Pityana, S.; Adeosun, S.; Lengopeng, T. Fabrication and Hardness Behaviour of High Entropy Alloys. In Proceedings of the TMS 2020 149th Annual Meeting & Exhibition Supplemental Proceedings, San Diego, CA, USA, 23–27 February 2020; pp. 1581–1591. [Google Scholar]
- Dada, M.; Popoola, P.; Mathe, N.; Pityana, S.; Adeosun, S. Effect of laser parameters on the properties of high entropy alloys: A preliminary study. Mater. Today Proc. 2020, 38, 756–761. [Google Scholar] [CrossRef]
- Moorehead, M.; Bertsch, K.; Niezgoda, M.; Parkin, C.; Elbakhshwan, M.; Sridharan, K.; Zhang, C.; Thoma, D.; Couet, A. High-throughput synthesis of Mo-Nb-Ta-W high-entropy alloys via additive manufacturing. Mater. Des. 2020, 187, 108358. [Google Scholar] [CrossRef]
- Kunce, I.; Polanski, M.; Bystrzycki, J. Microstructure and hydrogen storage properties of a TiZrNbMoV high entropy alloy synthesized using Laser Engineered Net Shaping (LENS). Int. J. Hydrogen Energy 2014, 39, 9904–9910. [Google Scholar] [CrossRef]
- Dobbelstein, H.; Gurevich, E.L.; George, E.P.; Ostendorf, A.; Laplanche, G. Laser metal deposition of a refractory TiZrNbHfTa high-entropy alloy. Addit. Manuf. 2018, 24, 386–390. [Google Scholar] [CrossRef]
- Pegues, J.W.; Melia, M.A.; Puckett, R.; Whetten, S.R.; Argibay, N.; Kustas, A.B. Exploring additive manufacturing as a high-throughput screening tool for multiphase high entropy alloys. Addit. Manuf. 2021, 37, 101598. [Google Scholar] [CrossRef]
- Li, H.; Huang, Y.; Jiang, S.; Lu, Y.; Gao, X.; Lu, X.; Ning, Z.; Sun, J. Columnar to equiaxed transition in additively manufactured CoCrFeMnNi high entropy alloy. Mater. Des. 2021, 197, 109262. [Google Scholar] [CrossRef]
- Tong, Z.; Liu, H.; Jiao, J.; Zhou, W.; Yang, Y.; Ren, X. Improving the strength and ductility of laser directed energy deposited CrMnFeCoNi high-entropy alloy by laser shock peening. Addit. Manuf. 2020, 35, 101417. [Google Scholar] [CrossRef]
- Shen, Q.; Kong, X.; Chen, X.; Yao, X.; Deev, V.B.; Prusov, E.S. Powder plasma arc additive manufactured CoCrFeNi(SiC)x high-entropy alloys: Microstructure and mechanical properties. Mater. Lett. 2021, 282, 128736. [Google Scholar] [CrossRef]
- Cai, Y.; Zhu, L.; Cui, Y.; Han, J. Manufacturing of FeCoCrNi + FeCoCrNiAl laminated high-entropy alloy by laser melting deposition (LMD). Mater. Lett. 2021, 289, 129445. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Cai, J.; Ji, S.; Geng, J.; Sun, X.; Li, D. High-strength NbMoTaX refractory high-entropy alloy with low stacking fault energy eutectic phase via laser additive manufacturing. Mater. Des. 2021, 201, 109462. [Google Scholar] [CrossRef]
- Peng, H.; Xie, S.; Niu, P.; Zhang, Z.; Yuan, T.; Ren, Z.; Wang, X.; Zhao, Y.; Li, R. Additive manufacturing of Al0.3CoCrFeNi high-entropy alloy by powder feeding laser melting deposition. J. Alloys Compd. 2021, 863, 158286. [Google Scholar] [CrossRef]
- Kuzminova, Y.O.; Firsov, D.G.; Dagesyan, S.A.; Konev, S.D.; Sergeev, S.N.; Zhilyaev, A.P.; Kawasaki, M.; Akhatov, I.S.; Evlashin, S.A. Fatigue behavior of additive manufactured CrFeCoNi medium-entropy alloy. J. Alloys Compd. 2021, 863, 158609. [Google Scholar] [CrossRef]
- Malatji, N.; Lengopeng, T.; Pityana, S.; Popoola, A.P.I. Effect of heat treatment on the microstructure, microhardness, and wear characteristics of AlCrFeCuNi high-entropy alloy. Int. J. Adv. Manuf. Technol. 2020, 111, 2021–2029. [Google Scholar] [CrossRef]
- Dong, B.; Wang, Z.; Pan, Z.; Muránsky, O.; Shen, C.; Reid, M.; Wu, B.; Chen, X.; Li, H. On the development of pseudo-eutectic AlCoCrFeNi2.1 high entropy alloy using Powder-bed Arc Additive Manufacturing (PAAM) process. Mater. Sci. Eng. A 2021, 802, 140639. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, Z.; He, F.; Liu, S.; Li, J.; Kai, J.J.; Wang, J. A precipitation-strengthened high-entropy alloy for additive manufacturing. Addit. Manuf. 2020, 35, 101410. [Google Scholar] [CrossRef]
- Zheng, M.; Li, C.; Zhang, X.; Ye, Z.; Yang, X.; Gu, J. The influence of columnar to equiaxed transition on deformation behavior of FeCoCrNiMn high entropy alloy fabricated by laser-based directed energy deposition. Addit. Manuf. 2020, 37, 101660. [Google Scholar] [CrossRef]
- Luo, S.; Wang, Z.; Zeng, X. Study on the formability, microstructures and mechanical properties of AlCrCuFeNi high-entropy alloys prepared by selective laser melting. In Proceedings of the Solid Freeform Fabrication 2019: Proceedings of the 30th Annual International Solid Freeform Fabrication Symposium—An Additive Manufacturing Conference, Wuhan, China, 12–14 August 2019; pp. 625–635. [Google Scholar]
- Jiang, F.; Zhao, C.; Liang, D.; Zhu, W.; Zhang, Y.; Pan, S.; Ren, F. In-situ formed heterogeneous grain structure in spark-plasma-sintered CoCrFeMnNi high-entropy alloy overcomes the strength-ductility trade-off. Mater. Sci. Eng. A 2020, 771, 138625. [Google Scholar] [CrossRef]
- Rogal, Ł.; Kalita, D.; Tarasek, A.; Bobrowski, P.; Czerwinski, F. Effect of SiC nano-particles on microstructure and mechanical properties of the CoCrFeMnNi high entropy alloy. J. Alloys Compd. 2017, 708, 344–352. [Google Scholar] [CrossRef]
- Yim, D.; Sathiyamoorthi, P.; Hong, S.J.; Kim, H.S. Fabrication and mechanical properties of TiC reinforced CoCrFeMnNi high-entropy alloy composite by water atomization and spark plasma sintering. J. Alloys Compd. 2019, 781, 389–396. [Google Scholar] [CrossRef]
- Rogal, Ł.; Kalita, D.; Litynska-Dobrzynska, L. CoCrFeMnNi high entropy alloy matrix nanocomposite with addition of Al2O3. Intermetallics 2017, 86, 104–109. [Google Scholar] [CrossRef]
- Li, X.; Feng, Y.; Liu, B.; Yi, D.; Yang, X.; Zhang, W.; Chen, G.; Liu, Y.; Bai, P. Influence of NbC particles on microstructure and mechanical properties of AlCoCrFeNi high-entropy alloy coatings prepared by laser cladding. J. Alloys Compd. 2019, 788, 485–494. [Google Scholar] [CrossRef]
- Chen, S.; Chen, X.; Wang, L.; Liang, J.; Liu, C. Laser cladding FeCrCoNiTiAl high entropy alloy coatings reinforced with self-generated TiC particles. J. Laser Appl. 2017, 29, 012004. [Google Scholar] [CrossRef]
- Fan, Q.C.; Li, B.S.; Zhang, Y. The microstructure and properties of (FeCrNiCo)AlxCuy high-entropy alloys and their TiC-reinforced composites. Mater. Sci. Eng. A 2014, 598, 244–250. [Google Scholar] [CrossRef]
- Jiang, L.; Jiang, H.; Lu, Y.; Wang, T.; Cao, Z.; Li, T. Mechanical properties improvement of AlCrFeNi2Ti0.5 high entropy alloy through annealing design and its relationship with its particle-reinforced microstructures. J. Mater. Sci. Technol. 2015, 31, 397–402. [Google Scholar] [CrossRef]
- Guo, L.; Ou, X.; Ni, S.; Liu, Y.; Song, M. Effects of carbon on the microstructures and mechanical properties of FeCoCrNiMn high entropy alloys. Mater. Sci. Eng. A 2019, 746, 356–362. [Google Scholar] [CrossRef]
- Zhu, S.; Yu, Y.; Zhang, B.; Zhang, Z.; Yan, X.; Wang, Z. Microstructure and wear behaviour of in-situ TiN-Al2O3 reinforced CoCrFeNiMn high-entropy alloys composite coatings fabricated by plasma cladding. Mater. Lett. 2020, 272, 127870. [Google Scholar] [CrossRef]
- Lim, K.R.; Kwon, H.J.; Kang, J.H.; Won, J.W.; Na, Y.S. A novel ultra-high-strength duplex Al–Co–Cr–Fe–Ni high-entropy alloy reinforced with body-centered-cubic ordered-phase particles. Mater. Sci. Eng. A 2020, 771, 138638. [Google Scholar] [CrossRef]
- Fu, A.; Guo, W.; Liu, B.; Cao, Y.; Xu, L.; Fang, Q.; Yang, H.; Liu, Y. A particle reinforced NbTaTiV refractory high entropy alloy based composite with attractive mechanical properties. J. Alloys Compd. 2020, 815, 152466. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Tang, Y.C.; Luo, L.; Luo, L.S.; Su, Y.Q.; Guo, J.J.; Fu, H.Z. Microstructure and mechanical properties of CoCrFeNiWx high entropy alloys reinforced by μ phase particles. J. Alloys Compd. 2020, 843, 155997. [Google Scholar] [CrossRef]
- Jinhong, P.; Ye, P.; Hui, Z.; Lu, Z. Microstructure and properties of AlCrFeCuNi x (0.6 ≤ x ≤ 1.4) high-entropy alloys. Mater. Sci. Eng. A 2012, 543, 228–233. [Google Scholar] [CrossRef]
- He, J.Y.; Liu, W.H.; Wang, H.; Wu, Y.; Liu, X.J.; Nieh, T.G.; Lu, Z.P. Effects of Al addition on structural evolution and tensile properties of the FeCoNiCrMn high-entropy alloy system. Acta Mater. 2014, 62, 105–113. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Miracle, D.B.; Chuang, C.P.; Liaw, P.K. Refractory high-entropy alloys. Intermetallics 2010, 18, 1758–1765. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Mechanical properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 refractory high entropy alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef]
- Yvon, P.; Carré, F. Structural materials challenges for advanced reactor systems. J. Nucl. Mater. 2009, 385, 217–222. [Google Scholar] [CrossRef]
- Allen, T.; Busby, J.; Meyer, M.; Petti, D. Materials challenges for nuclear systems. Mater. Today 2010, 13, 14–23. [Google Scholar] [CrossRef]
- Murty, K.L.; Charit, I. Structural materials for Gen-IV nuclear reactors: Challenges and opportunities. J. Nucl. Mater. 2008, 383, 189–195. [Google Scholar] [CrossRef]
- Zinkle, S.J.; Boutard, J.L.; Hoelzer, D.T.; Kimura, A.; Lindau, R.; Odette, G.R.; Rieth, M.; Tan, L.; Tanigawa, H. Development of next generation tempered and ODS reduced activation ferritic/martensitic steels for fusion energy applications. Nucl. Fusion 2017, 57, 092005. [Google Scholar] [CrossRef]
- Zinkle, S.J.; Busby, J.T. Structural materials for fission & fusion energy. Mater. Today 2009, 12, 12–19. [Google Scholar] [CrossRef]
- Li, C. Characterization of Radiation Effects and Ab Initio Modeling of Defects in a High Entropy Alloy for Nuclear Power Application; The University of Tennessee: Knoxville, TN, USA, 2018. [Google Scholar]
- Hoffman, A.K. Development and characterization of nanostructured steels and high entropy alloys for nuclear applications. J. Mater. Res. 2019, 33, 3077–3091. [Google Scholar]
- King, D.J.M. Investigation of High-Entropy Alloys for Use in Advanced Nuclear Applications; University of Technology Sydney: Sydney, Australia, 2016. [Google Scholar]
- Yeh, J.W.; Lin, S.J. Breakthrough applications of high-entropy materials. J. Mater. Res. 2018, 33, 3129–3137. [Google Scholar] [CrossRef]
- Xia, S.Q.; Wang, Z.; Yang, T.F.; Zhang, Y. Irradiation Behavior in High Entropy Alloys. J. Iron Steel Res. Int. 2015, 22, 879–884. [Google Scholar] [CrossRef]
- Barron, P.J.; Carruthers, A.W.; Fellowes, J.W.; Jones, N.G.; Dawson, H.; Pickering, E.J. Towards V-based high-entropy alloys for nuclear fusion applications. Scr. Mater. 2020, 176, 12–16. [Google Scholar] [CrossRef]
- Xiang, C.; Fu, H.M.; Zhang, Z.M.; Han, E.H.; Zhang, H.F.; Wang, J.Q.; Hu, G.D. Effect of Cr content on microstructure and properties of Mo0.5VNbTiCrx high-entropy alloys. J. Alloys Compd. 2020, 818, 153352. [Google Scholar] [CrossRef]
- Yang, T.; Guo, W.; Poplawsky, J.D.; Li, D.; Wang, L.; Li, Y.; Hu, W.; Crespillo, M.L.; Yan, Z.; Zhang, Y.; et al. Structural damage and phase stability of Al0.3CoCrFeNi high entropy alloy under high temperature ion irradiation. Acta Mater. 2020, 188, 1–15. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, M.; Wang, L.; Liu, C.H.; Chang, H.; Yang, J.J.; Liao, J.L.; Yang, Y.Y.; Liu, N. Interface stability, mechanical and corrosion properties of AlCrMoNbZr/(AlCrMoNbZr)N high-entropy alloy multilayer coatings under helium ion irradiation. Appl. Surf. Sci. 2019, 485, 108–118. [Google Scholar] [CrossRef]
- Xiang, C.; Han, E.H.; Zhang, Z.M.; Fu, H.M.; Wang, J.Q.; Zhang, H.F.; Hu, G.D. Design of single-phase high-entropy alloys composed of low thermal neutron absorption cross-section elements for nuclear power plant application. Intermetallics 2019, 104, 143–153. [Google Scholar] [CrossRef]
- Jawaharram, G.S.; Barr, C.M.; Monterrosa, A.M.; Hattar, K.; Averback, R.S.; Dillon, S.J. Irradiation induced creep in nanocrystalline high entropy alloys. Acta Mater. 2020, 182, 68–76. [Google Scholar] [CrossRef]
- Lu, C.; Yang, T.; Jin, K.; Gao, N.; Xiu, P.; Zhang, Y.; Gao, F.; Bei, H.; Weber, W.J.; Sun, K.; et al. Radiation-induced segregation on defect clusters in single-phase concentrated solid-solution alloys. Acta Mater. 2017, 127, 98–107. [Google Scholar] [CrossRef]
- Barr, C.M.; Nathaniel, J.E.; Unocic, K.A.; Liu, J.; Zhang, Y.; Wang, Y.; Taheri, M.L. Exploring radiation induced segregation mechanisms at grain boundaries in equiatomic CoCrFeNiMn high entropy alloy under heavy ion irradiation. Scr. Mater. 2018, 156, 80–84. [Google Scholar] [CrossRef]
- Lu, C.; Niu, L.; Chen, N.; Jin, K.; Yang, T.; Xiu, P.; Zhang, Y.; Gao, F.; Bei, H.; Shi, S.; et al. Enhancing radiation tolerance by controlling defect mobility and migration pathways in multicomponent single-phase alloys. Nat. Commun. 2016, 7, 13564. [Google Scholar] [CrossRef]
- Tong, Y.; Velisa, G.; Zhao, S.; Guo, W.; Yang, T.; Jin, K.; Lu, C.; Bei, H.; Ko, J.Y.P.; Pagan, D.C.; et al. Evolution of local lattice distortion under irradiation in medium- and high-entropy alloys. Materialia 2018, 2, 73–81. [Google Scholar] [CrossRef]
- Jin, K.; Lu, C.; Wang, L.M.; Qu, J.; Weber, W.J.; Zhang, Y.; Bei, H. Effects of compositional complexity on the ion-irradiation induced swelling and hardening in Ni-containing equiatomic alloys. Scr. Mater. 2016, 119, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.Y.; Liu, X.; Chen, Y.; Yeh, J.W.; Tseng, K.K.; Natesan, K. Irradiation effects in high entropy alloys and 316H stainless steel at 300 °C. J. Nucl. Mater. 2018, 510, 421–430. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, K.; Feng, Y.; Li, Y.; Tang, W.; Wei, B. Evaluation of radiation response in CoCrFeCuNi high-entropy alloys. Entropy 2018, 20, 835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, M.R.; Wang, S.; Shi, S.; Jin, K.; Bei, H.; Yasuda, K.; Matsumura, S.; Higashida, K.; Robertson, I.M. Mechanisms of radiation-induced segregation in CrFeCoNi-based single-phase concentrated solid solution alloys. Acta Mater. 2017, 126, 182–193. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.N.; Lu, C.; Velisa, G.; Jin, K.; Xiu, P.; Zhang, Y.; Bei, H.; Wang, L. Influence of irradiation temperature on void swelling in NiCoFeCrMn and NiCoFeCrPd. Scr. Mater. 2019, 158, 57–61. [Google Scholar] [CrossRef]
- Yang, L.; Ge, H.; Zhang, J.; Xiong, T.; Jin, Q.; Zhou, Y.; Shao, X.; Zhang, B.; Zhu, Z.; Zheng, S.; et al. High He-ion irradiation resistance of CrMnFeCoNi high-entropy alloy revealed by comparison study with Ni and 304SS. J. Mater. Sci. Technol. 2019, 35, 300–305. [Google Scholar] [CrossRef]
- Hashimoto, N.; Ono, Y. Mobility of point defects in CoCrFeNi-base high entropy alloys. Intermetallics 2021, 133, 107182. [Google Scholar] [CrossRef]
- Zhang, Y.; Tunes, M.A.; Crespillo, M.L.; Zhang, F.; Boldman, W.L.; Rack, P.D.; Jiang, L.; Xu, C.; Greaves, G.; Donnelly, S.E.; et al. Thermal stability and irradiation response of nanocrystalline CoCrCuFeNi high-entropy alloy. Nanotechnology 2019, 30, 294004. [Google Scholar] [CrossRef]
- Yang, T.N.; Lu, C.; Jin, K.; Crespillo, M.L.; Zhang, Y.; Bei, H.; Wang, L. The effect of injected interstitials on void formation in self-ion irradiated nickel containing concentrated solid solution alloys. J. Nucl. Mater. 2017, 488, 328–337. [Google Scholar] [CrossRef] [Green Version]
- Abhaya, S.; Rajaraman, R.; Kalavathi, S.; David, C.; Panigrahi, B.K.; Amarendra, G. Effect of dose and post irradiation annealing in Ni implanted high entropy alloy FeCrCoNi using slow positron beam. J. Alloys Compd. 2016, 669, 117–122. [Google Scholar] [CrossRef]
- Sellami, N.; Debelle, A.; Ullah, M.W.; Christen, H.M.; Keum, J.K.; Bei, H.; Xue, H.; Weber, W.J.; Zhang, Y. Effect of electronic energy dissipation on strain relaxation in irradiated concentrated solid solution alloys. Curr. Opin. Solid State Mater. Sci. 2019, 23, 107–115. [Google Scholar] [CrossRef]
- Chen, D.; Tong, Y.; Li, H.; Wang, J.; Zhao, Y.L.; Hu, A.; Kai, J.J. Helium accumulation and bubble formation in FeCoNiCr alloy under high fluence He+ implantation. J. Nucl. Mater. 2018, 501, 208–216. [Google Scholar] [CrossRef]
- Kombaiah, B.; Jin, K.; Bei, H.; Edmondson, P.D.; Zhang, Y. Phase stability of single phase Al0.12CrNiFeCo high entropy alloy upon irradiation. Mater. Des. 2018, 160, 1208–1216. [Google Scholar] [CrossRef]
- Lu, C.; Yang, T.; Jin, K.; Velisa, G.; Xiu, P.; Song, M.; Peng, Q.; Gao, F.; Zhang, Y.; Bei, H.; et al. Enhanced void swelling in NiCoFeCrPd high-entropy alloy by indentation-induced dislocations. Mater. Res. Lett. 2018, 6, 584–591. [Google Scholar] [CrossRef] [Green Version]
- Tunes, M.A.; Edmondson, P.D.; Vishnyakov, V.M.; Donnelly, S.E. Displacement damage and self-healing in high-entropy alloys: A TEM with in situ ion irradiation study. In Fusion Materials Research at Oak Ridge National Laboratory in Fiscal Year 2017; Oak Ridge National Lab. (ORNL): Oak Ridge, TN, USA, 2017; Volume 1, pp. 62–64. [Google Scholar] [CrossRef]
- AlTabbaa, O.; Ankrah, S. Social capital to facilitate ‘engineered’ university–industry collaboration for technology transfer: A dynamic perspective. Technol. Forecast. Soc. Chang. 2016, 104, 1–15. [Google Scholar]
- Fan, Z.; Zhong, W.; Jin, K.; Bei, H.; Osetsky, Y.N.; Zhang, Y. Diffusion-mediated chemical concentration variation and void evolution in ion-irradiated NiCoFeCr high-entropy alloy. J. Mater. Res. 2021, 36, 298–310. [Google Scholar] [CrossRef]
- Lyu, P.; Peng, T.; Miao, Y.; Liu, Z.; Gao, Q.; Zhang, C.; Jin, Y.; Qingfeng Guan, J.C. Microstructure and properties of CoCrFeNiMo0.2 high-entropy alloy enhanced by high-current pulsed electron beam. Surf. Coat. Technol. 2021, 410, 126911. [Google Scholar] [CrossRef]
- Xu, Q.; Zhu, T.; Zhong, Z.H.; Cao, X.Z.; Tsuchida, H. Investigation of irradiation resistance characteristics of precipitation strengthened high-entropy alloy (CoCrFeNi)95Ti1Nb1Al3 using slow positron beam. J. Alloys Compd. 2021, 888, 161518. [Google Scholar] [CrossRef]
- Cao, P.P.; Wang, H.; He, J.Y.; Xuc, C.; Jiang, S.H.; Du, J.L.; Cao, X.Z.; Fu, E.G.; Lu, Z.P. Effects of nanosized precipitates on irradiation behavior of CoCrFeNi high entropy alloys. J. Alloys Compd. 2021, 859, 158291. [Google Scholar] [CrossRef]
- Tolstolutskaya, G.D.; Rostova, G.Y.; Voyevodin, V.N.; Velikodnyi, A.N.; Tikhonovsky, M.A.; Tolmachova, G.N.; Kalchenko, A.S.; Vasilenko, R.L.; Kopanets, I.E. Section 2 thermal and fast reactor materials hardening of Cr-Fe-Ni-Mn high-entropy alloys caused by the irradiation with argon ions. Probl. At. Sci. Technol. 2017, 5, 40–47. Available online: http://dspace.nbuv.gov.ua/handle/123456789/136159 (accessed on 30 November 2021).
- Kumar, N.A.P.K.; Li, C.; Leonard, K.J.; Bei, H.; Zinkle, S.J. Microstructural stability and mechanical behavior of FeNiMnCr high entropy alloy under ion irradiation. Acta Mater. 2016, 113, 230–244. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Hu, X.; Yang, T.; Kumar, N.K.; Wirth, B.D.; Zinkle, S.J. Neutron irradiation response of a Co-free high entropy alloy. J. Nucl. Mater. 2019, 527, 151838. [Google Scholar] [CrossRef]
- Voyevodin, V.N.; Karpov, S.A.; Tolstolutskaya, G.D.; Tikhonovsky, M.A.; Velikodnyi, A.N.; Kopanets, I.E.; Tolmachova, G.N.; Kalchenko, A.S.; Vasilenko, R.L.; Kolodiy, I.V. Effect of irradiation on microstructure and hardening of Cr–Fe–Ni–Mn high-entropy alloy and its strengthened version. Philos. Mag. 2020, 100, 822–836. [Google Scholar] [CrossRef]
- Dias, M.; Antão, F.; Catarino, N.; Galatanu, A.; Galatanu, M.; Ferreira, P.; Correia, J.B.; da Silva, R.C.; Gonçalves, A.P.; Alves, E. Sintering and irradiation of copper-based high entropy alloys for nuclear fusion. Fusion Eng. Des. 2020, 146, 1824–1828. [Google Scholar] [CrossRef]
- Gromov, V.; Ivanov, Y.; Konovalov, S.; Osintsev, K.; Semin, A.; Rubannikova, Y. Modification of high-entropy alloy AlCoCrFeNi by electron beam treatment. J. Mater. Sci. Technol. 2021, 13, 787–797. [Google Scholar] [CrossRef]
- Yang, T.; Xia, S.; Guo, W.; Hu, R.; Poplawsky, J.D.; Sha, G.; Fang, Y.; Yan, Z.; Wang, C.; Li, C.; et al. Effects of temperature on the irradiation responses of Al0.1CoCrFeNi high entropy alloy. Scr. Mater. 2018, 144, 31–35. [Google Scholar] [CrossRef]
- Zhou, J.; Islam, M.I.; Guo, S.; Zhang, Y.; Lu, F. Radiation-induced grain growth of nanocrystalline alxcocrfeni high-entropy alloys. J. Phys. Chem. C 2021, 125, 3509–3516. [Google Scholar] [CrossRef]
- Zhou, J. Radiation Effects in Apatite and High Entropy Alloy under Energetic Ions and Electrons; Louisiana State University and Agricultural and Mechanical College: Baton Rouge, LA, USA, 2020. [Google Scholar]
- Zhou, J.; Kirk, M.; Baldo, P.; Guo, S.; Lu, F. Phase stability of novel HfNbTaTiVZr refractory high entropy alloy under ion irradiation. Mater. Lett. 2021, 305, 130789. [Google Scholar] [CrossRef]
- Moschetti, M.; Xu, A.; Schuh, B.; Hohenwarter, A.; Couzinié, J.P.; Kruzic, J.J.; Bhattacharyya, D.; Gludovatz, B. On the Room-Temperature Mechanical Properties of an Ion-Irradiated TiZrNbHfTa Refractory High Entropy Alloy. JOM 2020, 72, 130–138. [Google Scholar] [CrossRef]
- Sadeghilaridjani, M.; Ayyagari, A.; Muskeri, S.; Hasannaeimi, V.; Salloom, R.; Chen, W.Y.; Mukherjee, S. Ion irradiation response and mechanical behavior of reduced activity high entropy alloy. J. Nucl. Mater. 2020, 529, 151955. [Google Scholar] [CrossRef]
- Li, D.; Jia, N.; Huang, H.; Chen, S.; Dou, Y.; He, X.; Yang, W.; Xue, Y.; Hua, Z.; Zhang, F.; et al. Helium ion irradiation enhanced precipitation and the impact on cavity formation in a HfNbZrTi refractory high entropy alloy. J. Nucl. Mater. 2021, 552, 153023. [Google Scholar] [CrossRef]
- Kareer, A.; Waite, J.C.; Li, B.; Couet, A.; Armstrong, D.E.J.; Wilkinson, A.J. Short communication: ‘Low activation, refractory, high entropy alloys for nuclear applications’. J. Nucl. Mater. 2019, 526, 151744. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, K.; Feng, Y.; Li, Y.; Tang, W.; Zhang, Y.; Wei, B.; Hu, Z. Excellent irradiation tolerance and mechanical behaviors in high-entropy metallic glasses. J. Nucl. Mater. 2019, 527, 151785. [Google Scholar] [CrossRef]
- El-Atwani, O.; Li, N.; Li, M.; Devaraj, A.; Baldwin, J.K.S.; Schneider, M.M.; Sobieraj, D.; Wróbel, J.S.; Nguyen-Manh, D.; Maloy, S.A.; et al. Outstanding radiation resistance of tungsten-based high-entropy alloys. Sci. Adv. 2019, 5, eaav2002. [Google Scholar] [CrossRef] [Green Version]
- Komarov, F.F.; Konstantinov, S.V.; Pogrebnyak, A.D. Effect of high-fluence ion irradiation on the structure and mechanical properties of coatings based on nanostructured nitrides of high-entropy alloys (Ti, Hf, Zr, V, Nb). Dokl. Natsional’noj Akad. Nauk Belarusi 2015, 48, 24–30. [Google Scholar] [CrossRef]
- Gandy, A.S.; Jim, B.; Coe, G.; Patel, D.; Hardwick, L.; Akhmadaliev, S.; Reeves-McLaren, N.; Goodall, R. High temperature and ion implantation-induced phase transformations in novel reduced activation si-fe-v-cr (-mo) high entropy alloys. Front. Mater. 2019, 6, 146. [Google Scholar] [CrossRef]
- Patel, D.; Richardson, M.D.; Jim, B.; Akhmadaliev, S.; Goodall, R.; Gandy, A.S. Radiation damage tolerance of a novel metastable refractory high entropy alloy V2.5Cr1.2WMoCo0.04. J. Nucl. Mater. 2020, 531, 152005. [Google Scholar] [CrossRef]
- Zhang, Z.; Han, E.H.; Xiang, C. Irradiation behaviors of two novel single-phase bcc-structure high-entropy alloys for accident-tolerant fuel cladding. J. Mater. Sci. Technol. 2021, 84, 230–238. [Google Scholar] [CrossRef]
- Zhang, Z.; Han, E.H.; Xiang, C. Effect of helium ion irradiation on short-time corrosion behavior of two novel high-entropy alloys in simulated PWR primary water. Corros. Sci. 2021, 191, 109742. [Google Scholar] [CrossRef]
- El-Atwani, O.; Alvarado, A.; Unal, K.; Fensin, S.; Hinks, J.A.; Greaves, G.; Baldwin, J.K.S.; Maloy, S.A.; Martinez, E. Helium implantation damage resistance in nanocrystalline W-Ta-V-Cr high entropy alloys. Mater. Today Energy 2021, 19, 100599. [Google Scholar] [CrossRef]
- Garner, F.A.; Toloczko, M.B.; Sencer, B.H. Comparison of swelling and irradiation creep behavior of fcc-austenitic and bcc-ferritic/martensitic alloys at high neutron exposure. J. Nucl. Mater. 2000, 276, 123–142. [Google Scholar] [CrossRef]
- Zinkle, S.J.; Was, G.S. Materials challenges in nuclear energy. Acta Mater. 2013, 61, 735–758. [Google Scholar] [CrossRef]
- Egami, T.; Guo, W.; Rack, P.D.; Nagase, T. Irradiation resistance of multicomponent alloys. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2014, 45, 180–183. [Google Scholar] [CrossRef]
- Tsai, M.H.; Yeh, J.W. High-entropy alloys: A critical review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Kasar, A.K.; Scalaro, K.; Menezes, P.L. Tribological properties of high-entropy alloys under dry conditions for a wide temperature range—a review. Materials 2021, 14, 5814. [Google Scholar] [CrossRef]
- Menghani, J.; Vyas, A.; Patel, P.; Natu, H.; More, S. Wear, erosion and corrosion behavior of laser cladded high entropy alloy coatings—A review. Mater. Today Proc. 2020, 38, 2824–2829. [Google Scholar] [CrossRef]
- Ayyagari, A.; Hasannaeimi, V.; Grewal, H.S.; Arora, H.; Mukherjee, S. Corrosion, erosion andwear behavior of complex concentrated alloys: A review. Metals 2018, 8, 603. [Google Scholar] [CrossRef] [Green Version]
- Joseph, J.; Haghdadi, N.; Annasamy, M.; Kada, S.; Hodgson, P.D.; Barnett, M.R.; Fabijanic, D.M. On the enhanced wear resistance of CoCrFeMnNi high entropy alloy at intermediate temperature. Scr. Mater. 2020, 186, 230–235. [Google Scholar] [CrossRef]
- Wang, H.; Ren, K.; Xie, J.; Zhang, C.; Tang, W. Friction and wear behavior of single-phase high-entropy alloyFeCoNiCrMn under MoS2-oil lubrication. Ind. Lubr. Tribol. 2019, 2019, 2–9. [Google Scholar] [CrossRef]
- Xiao, J.K.; Tan, H.; Wu, Y.Q.; Chen, J.; Zhang, C. Microstructure and wear behavior of FeCoNiCrMn high entropy alloy coating deposited by plasma spraying. Surf. Coat. Technol. 2020, 385, 125430. [Google Scholar] [CrossRef]
- Jones, M.R.; Nation, B.L.; Wellington-Johnson, J.A.; Curry, J.F.; Kustas, A.B.; Lu, P.; Chandross, M.; Argibay, N. Evidence of Inverse Hall-Petch Behavior and Low Friction and Wear in High Entropy Alloys. Sci. Rep. 2020, 10, 14336. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, B.; Tao, X.; Yu, Y.; Zhang, Z.; Wang, Z.; Lu, B. Microstructure and tribology performance of plasma clad intermetallics reinforced CoCrFeMnNi-based high-entropy alloy composite coatings. Tribol. Trans. 2020, 64, 264–274. [Google Scholar] [CrossRef]
- Deng, G.; Tieu, A.K.; Su, L.; Wang, P.; Wang, L.; Lan, X.; Cui, S.; Zhu, H. Investigation into reciprocating dry sliding friction and wear properties of bulk CoCrFeNiMo high entropy alloys fabricated by spark plasma sintering and subsequent cold rolling processes: Role of Mo element concentration. Wear 2020, 460–461, 203440. [Google Scholar] [CrossRef]
- Lindner, T.; Löbel, M.; Saborowski, E.; Rymer, L.M.; Lampke, T. Wear and corrosion behaviour of supersaturated surface layers in the high-entropy alloy systems CrMnFeCoNi and CrFeCoNi. Crystals 2020, 10, 110. [Google Scholar] [CrossRef] [Green Version]
- Sha, C.; Zhou, Z.; Xie, Z.; Munroe, P. FeMnNiCoCr-based high entropy alloy coatings: Effect of nitrogen additions on microstructural development, mechanical properties and tribological performance. Appl. Surf. Sci. 2020, 507, 145101. [Google Scholar] [CrossRef]
- Xiao, J.K.; Tan, H.; Chen, J.; Martini, A.; Zhang, C. Effect of carbon content on microstructure, hardness and wear resistance of CoCrFeMnNiCx high-entropy alloys. J. Alloys Compd. 2020, 847, 156533. [Google Scholar] [CrossRef]
- Cheng, H.; Fang, Y.; Xu, J.; Zhu, C.; Dai, P.; Xue, S. Tribological properties of nano/ultrafine-grained FeCoCrNiMnAlx high-entropy alloys over a wide range of temperatures. J. Alloys Compd. 2020, 817, 153305. [Google Scholar] [CrossRef]
- Joseph, J.; Haghdadi, N.; Shamlaye, K.; Hodgson, P.; Barnett, M.; Fabijanic, D. The sliding wear behaviour of CoCrFeMnNi and AlxCoCrFeNi high entropy alloys at elevated temperatures. Wear 2019, 428–429, 32–44. [Google Scholar] [CrossRef]
- Liu, X.; Yin, H.; Xu, Y. Microstructure, mechanical and tribological properties of Oxide Dispersion Strengthened high-entropy alloys. Materials 2017, 10, 1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Zhang, B.; Yu, Y.; Zhang, Z.; Zhu, S.; Lou, X.; Wang, Z. Study of high temperature friction and wear performance of (CoCrFeMnNi)85Ti15 high-entropy alloy coating prepared by plasma cladding. Surf. Coat. Technol. 2020, 384, 125337. [Google Scholar] [CrossRef]
- Zhang, A.; Han, J.; Su, B.; Meng, J. A novel CoCrFeNi high entropy alloy matrix self-lubricating composite. J. Alloys Compd. 2017, 725, 700–710. [Google Scholar] [CrossRef]
- Geng, Y.; Chen, J.; Tan, H.; Cheng, J.; Yang, J.; Liu, W. Vacuum tribological behaviors of CoCrFeNi high entropy alloy at elevated temperatures. Wear 2020, 456, 203368. [Google Scholar] [CrossRef]
- Zhang, A.; Han, J.; Su, B.; Li, P.; Meng, J. Microstructure, mechanical properties and tribological performance of CoCrFeNi high entropy alloy matrix self-lubricating composite. Mater. Des. 2017, 114, 253–263. [Google Scholar] [CrossRef]
- Brownlie, F.; Hodgkiess, T.; Fanicchia, F. Erosion-corrosion behaviour of CoCrFeNiMo0.85 and Al0.5CoCrFeNi complex concentrated alloys produced by laser metal deposition. Surf. Coatings Technol. 2021, 423, 127634. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, W.; Liu, Y.; Liu, B.; Wang, J. FeCoCrNiMo high-entropy alloys prepared by powder metallurgy processing for diamond tool applications. Powder Metall. 2018, 61, 123–130. [Google Scholar] [CrossRef]
- Huang, L.; Wang, X.; Jia, F.; Zhao, X.; Huang, B.; Ma, J.; Wang, C. Effect of Si element on phase transformation and mechanical properties for FeCoCrNiSix high entropy alloys. Mater. Lett. 2021, 282, 128809. [Google Scholar] [CrossRef]
- Cui, G.; Han, B.; Yang, Y.; Wang, Y.; Chunyang, H. Microstructure and tribological property of CoCrFeMoNi High entropy alloy treated by ion sulfurization. J. Mater. Res. Technol. 2020, 9, 2598–2609. [Google Scholar] [CrossRef]
- Li, T.; Liu, Y.; Liu, B.; Guo, W.; Xu, L. Microstructure and wear behavior of FeCoCrNiMo0.2 high entropy coatings prepared by air plasma spray and the high velocity oxy-fuel spray processes. Coatings 2017, 7, 151. [Google Scholar] [CrossRef] [Green Version]
- Ji, X.; Zhao, J.; Wang, H.; Luo, C. Sliding wear of spark plasma sintered CrFeCoNiCu high entropy alloy coatings with MoS2 and WC additions. Int. J. Adv. Manuf. Technol. 2018, 96, 1685–1691. [Google Scholar] [CrossRef]
- Verma, A.; Tarate, P.; Abhyankar, A.C.; Mohape, M.R.; Gowtam, D.S.; Deshmukh, V.P.; Shanmugasundaram, T. High temperature wear in CoCrFeNiCux high entropy alloys: The role of Cu. Scr. Mater. 2019, 161, 28–31. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, J.; Li, Y.; Zhu, W.; Lin, L. Effects of boron content on microstructure and wear properties of FeCoCrNiBx high-entropy alloy coating by laser cladding. Appl. Sci. 2020, 10, 49. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Jiang, L.; Qiao, D.; Lu, Y.; Wang, T.; Cao, Z.; Li, T. Effect of Niobium on Microstructure and Properties of the CoCrFeNbxNi High Entropy Alloys. J. Mater. Sci. Technol. 2017, 33, 712–717. [Google Scholar] [CrossRef]
- Yu, Y.; He, F.; Qiao, Z.; Wang, Z.; Liu, W.; Yang, J. Effects of temperature and microstructure on the triblogical properties of CoCrFeNiNbx eutectic high entropy alloys. J. Alloys Compd. 2019, 775, 1376–1385. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, S.; Xu, Y. Microstructure and tribological performance of Fe50Mn30Co10Cr10 high-entropy alloy based self-lubricating composites. Mater. Lett. 2018, 233, 142–145. [Google Scholar] [CrossRef]
- Wang, J.; Yang, H.; Liu, Z.; Li, R.; Ruan, J.; Ji, S. Synergistic effects of WC nanoparticles and MC nanoprecipitates on the mechanical and tribological properties of Fe40Mn40Cr10Co10 medium-entropy alloy. J. Mater. Res. Technol. 2019, 8, 3550–3564. [Google Scholar] [CrossRef]
- Derimow, N.; MacDonald, B.E.; Lavernia, E.J.; Abbaschian, R. Duplex phase hexagonal-cubic multi-principal element alloys with high hardness. Mater. Today Commun. 2019, 21, 100658. [Google Scholar] [CrossRef]
- Guo, Y.; Li, C.; Zeng, M.; Wang, J.; Deng, P.; Wang, Y. In-situ TiC reinforced CoCrCuFeNiSi0.2 high-entropy alloy coatings designed for enhanced wear performance by laser cladding. Mater. Chem. Phys. 2020, 242, 122522. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, T.; Xiao, M.; Shen, Y. Tribological behavior of diamond reinforced FeNiCoCrTi0.5 carbonized high-entropy alloy coating. Surf. Coat. Technol. 2020, 401, 126233. [Google Scholar] [CrossRef]
- Erdoğan, A.; Gök, M.S.; Zeytin, S. Analysis of the high-temperature dry sliding behavior of CoCrFeNiTi0.5Alx high-entropy alloys. Friction 2020, 8, 198–207. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Xie, Y.; Cui, S.; Yi, Y.; Xing, X.; Wang, X.; Li, W. Effect of mo element on the mechanical properties and tribological responses of cocrfenimox high-entropy alloys. Metals 2021, 11, 486. [Google Scholar] [CrossRef]
- Moazzen, P.; Toroghinejad, M.R.; Cavaliere, P. Effect of Iron content on the microstructure evolution, mechanical properties and wear resistance of FeXCoCrNi high-entropy alloy system produced via MA-SPS. J. Alloys Compd. 2021, 870, 159410. [Google Scholar] [CrossRef]
- Yang, Y.; Ren, Y.; Tian, Y.; Li, K.; Zhang, W.; Shan, Q.; Tian, Y.; Huang, Q.; Wu, H. Microstructure and properties of FeCoCrNiMoSix high-entropy alloys fabricated by spark plasma sintering. J. Alloys Compd. 2021, 884, 161070. [Google Scholar] [CrossRef]
- Li, Y.; Liang, H.; Nie, Q.; Qi, Z.; Deng, D.; Jiang, H.; Cao, Z. Microstructures and Wear Resistance of CoCrFeNi2V0.5Tix High-Entropy Alloy Coatings Prepared by Laser Cladding. Crystals 2020, 10, 352. [Google Scholar] [CrossRef]
- Islak, S.; Eski, Ö.; Koç, V.; Özorak, C. Wear properties and synthesis of crfenimoti high entropy alloy coatings produced by TIG process. Indian J. Eng. Mater. Sci. 2020, 27, 659–664. [Google Scholar]
- Wen, X.; Cai, Z.; Yin, B.; Cui, X.; Zhang, X.; Jin, G. Tribological and Corrosion Properties of Ni-Cr-Co-Ti-V Multi-Principal Element Alloy Prepared by Vacuum Hot-Pressing Sintering. Adv. Eng. Mater. 2019, 21, 1801239. [Google Scholar] [CrossRef]
- Wang, X.R.; Wang, Z.Q.; He, P.; Lin, T.S.; Shi, Y. Microstructure and wear properties of CuNiSiTiZr high-entropy alloy coatings on TC11 titanium alloy produced by electrospark—computer numerical control deposition process. Surf. Coat. Technol. 2015, 283, 156–161. [Google Scholar] [CrossRef]
- Cheng, J.; Sun, B.; Ge, Y.; Hu, X.; Zhang, L.; Liang, X.; Zhang, X. Nb doping in laser-cladded Fe25Co25Ni25(B0.7Si0.3)25 high entropy alloy coatings: Microstructure evolution and wear behavior. Surf. Coat. Technol. 2020, 402, 126321. [Google Scholar] [CrossRef]
- Yadav, S.; Sarkar, S.; Aggarwal, A.; Kumar, A.; Biswas, K. Wear and mechanical properties of novel (CuCrFeTiZn)100−xPbx high entropy alloy composite via mechanical alloying and spark plasma sintering. Wear 2018, 410–411, 93–109. [Google Scholar] [CrossRef]
- Gou, Q.; Xiong, J.; Guo, Z.; Liu, J.; Yang, L.; Li, X. Influence of NbC additions on microstructure and wear resistance of Ti(C,N)-based cermets bonded by CoCrFeNi high-entropy alloy. Int. J. Refract. Met. Hard Mater. 2020, 94, 105375. [Google Scholar] [CrossRef]
- Yadav, S.; Kumar, A.; Biswas, K. Wear behavior of high entropy alloys containing soft dispersoids (Pb, Bi). Mater. Chem. Phys. 2018, 210, 222–232. [Google Scholar] [CrossRef]
- Cui, Y.; Shen, J.; Manladan, S.M.; Geng, K.; Hu, S. Wear resistance of FeCoCrNiMnAlx high-entropy alloy coatings at high temperature. Appl. Surf. Sci. 2020, 512, 145736. [Google Scholar] [CrossRef]
- Gwalani, B.; Torgerson, T.; Dasari, S.; Jagetia, A.; Nartu, M.S.K.K.Y.; Gangireddy, S.; Pole, M.; Wang, T.; Scharf, T.W.; Banerjee, R. Influence of fine-scale B2 precipitation on dynamic compression and wear properties in hypo-eutectic Al0.5CoCrFeNi high-entropy alloy. J. Alloys Compd. 2021, 853, 157126. [Google Scholar] [CrossRef]
- Chen, M.; Lan, L.; Shi, X.; Yang, H.; Zhang, M.; Qiao, J. The tribological properties of Al0.6CoCrFeNi high-entropy alloy with the σ phase precipitation at elevated temperature. J. Alloys Compd. 2019, 777, 180–189. [Google Scholar] [CrossRef]
- Du, L.M.; Lan, L.W.; Zhu, S.; Yang, H.J.; Shi, X.H.; Liaw, P.K.; Qiao, J.W. Effects of temperature on the tribological behavior of Al0.25CoCrFeNi high-entropy alloy. J. Mater. Sci. Technol. 2019, 35, 917–925. [Google Scholar] [CrossRef]
- Chen, M.; Shi, X.H.; Yang, H.J.; Liaw, P.K.; Gao, M.C.; Hawk, J.A. Wear behavior of Al0.6CoCrFeNi high-entropy alloy: Effect of environments. J. Mater. Res. 2018, 33, 3310–3320. [Google Scholar] [CrossRef]
- Ji, X.; Duan, H.; Zhang, H.; Ma, J. Slurry Erosion Resistance of Laser Clad NiCoCrFeAl3 High-Entropy Alloy Coatings. Tribol. Trans. 2015, 58, 1119–1123. [Google Scholar] [CrossRef]
- Haghdadi, N.; Guo, T.; Ghaderi, A.; Hodgson, P.D.; Barnett, M.R.; Fabijanic, D.M. The scratch behaviour of AlXCoCrFeNi (x = 0.3 and 1.0) high entropy alloys. Wear 2019, 428–429, 293–301. [Google Scholar] [CrossRef]
- Fang, Y.; Chen, N.; Du, G.; Zhang, M.; Zhao, X.; Cheng, H.; Wu, J. High-temperature oxidation resistance, mechanical and wear resistance properties of Ti(C,N)-based cermets with Al0.3CoCrFeNi high-entropy alloy as a metal binder. J. Alloys Compd. 2020, 815, 152486. [Google Scholar] [CrossRef]
- Wu, Y.H.; Yang, H.J.; Guo, R.P.; Wang, X.J.; Shi, X.H.; Liaw, P.K.; Qiao, J.W. Tribological behavior of boronized Al0.1CoCrFeNi high-entropy alloys under dry and lubricated conditions. Wear 2020, 460–461, 203452. [Google Scholar] [CrossRef]
- Nair, R.B.; Arora, H.S.; Boyana, A.V.; Saiteja, P.; Grewal, H.S. Tribological behavior of microwave synthesized high entropy alloy claddings. Wear 2019, 436–437, 203028. [Google Scholar] [CrossRef]
- Kumar, S.; Rani, P.; Patnaik, A.; Pradhan, A.K.; Kumar, V. Effect of cobalt content on wear behaviour of Al0.4FeCrNiCox (x = 0, 0.25, 0.5, 1.0 mol) high entropy alloys tested under demineralised water with and without 3.5% NaCl solution. Mater. Res. Express 2019, 6, 0865b3. [Google Scholar] [CrossRef]
- Mu, Y.; Zhang, L.; Xu, L.; Prashanth, K.; Zhang, N.; Ma, X.; Jia, Y.; Xu, Y.; Jia, Y.; Wang, G. Frictional wear and corrosion behavior of AlCoCrFeNi high-entropy alloy coatings synthesized by atmospheric plasma spraying. Entropy 2020, 22, 740. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, K.; Xu, Z.; Li, D.Y. Effect of Ti addition on the sliding wear behavior of AlCrFeCoNi high-entropy alloy. Wear 2020, 462–463, 203493. [Google Scholar] [CrossRef]
- Zhao, D.; Yamaguchi, T.; Wang, W. Fabrication and wear performance of Al0.8FeCrCoNi high entropy alloy coating on magnesium alloy by resistance seam welding. Mater. Lett. 2020, 265, 127250. [Google Scholar] [CrossRef]
- Kumar, S.; Patnaik, A.; Pradhan, A.K.; Kumar, V. Room temperature wear study of Al0.4FeCrNiCox (x = 0, 0.25, 0.5, 1.0 mol) high-entropy alloys under oil lubricating conditions. J. Mater. Res. 2019, 34, 841–853. [Google Scholar] [CrossRef]
- Li, Y.; Shi, Y. Phase assemblage and wear resistance of laser-cladding Al0.8FeCoNiCrCu0.5Six high-entropy alloys on aluminum. Mater. Res. Express 2020, 7, 086504. [Google Scholar] [CrossRef]
- Kafexhiu, F.; Podgornik, B.; Feizpour, D. Tribological behavior of as-cast and aged AlCoCrFeNi2.1 CCA. Metals 2020, 10, 208. [Google Scholar] [CrossRef] [Green Version]
- Miao, J.; Liang, H.; Zhang, A.; He, J.; Meng, J.; Lu, Y. Tribological behavior of an AlCoCrFeNi2.1 eutectic high entropy alloy sliding against different counterfaces. Tribol. Int. 2021, 153, 106599. [Google Scholar] [CrossRef]
- Ye, F.; Yang, Y.; Lou, Z.; Feng, L.; Guo, L.; Yu, J. Microstructure and wear resistance of TiC reinforced AlCoCrFeNi2.1 eutectic high entropy alloy layer fabricated by micro-plasma cladding. Mater. Lett. 2021, 284, 128859. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Yang, H.; Zhang, M.; Ma, S.; Qiao, J. Microstructure and wear properties of nitrided AlCoCrFeNi high-entropy alloy. Mater. Chem. Phys. 2018, 210, 233–239. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, S.; Gao, M.C.; Zhang, C.; Zhang, T.; Yang, H.; Wang, Z.; Qiao, J. Tribological Properties of AlCrCuFeNi2 High-Entropy Alloy in Different Conditions. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2016, 47, 3312–3321. [Google Scholar] [CrossRef]
- Kong, D.; Guo, J.; Cui, X.; Zhang, X. Effect of superheating on microstructure and wear resistance of high-entropy Al1.8CrCuFeNi2 alloy. Mater. Lett. 2020, 274, 128021. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Yang, H.; Zhang, M.; Qiao, J. Effect of nitriding on the tribological properties of Al1.3CoCuFeNi2 high-entropy alloy. J. Alloys Compd. 2017, 725, 365–372. [Google Scholar] [CrossRef]
- Xiao, J.K.; Wu, Y.Q.; Chen, J.; Zhang, C. Microstructure and tribological properties of plasma sprayed FeCoNiCrSiAlx high entropy alloy coatings. Wear 2020, 448–449, 203209. [Google Scholar] [CrossRef]
- Liu, H.; Sun, S.; Zhang, T.; Zhang, G.; Yang, H.; Hao, J. Effect of Si addition on microstructure and wear behavior of AlCoCrFeNi high-entropy alloy coatings prepared by laser cladding. Surf. Coat. Technol. 2020, 405, 126522. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Yeh, J.W.; Chen, S.K.; Shun, T.T. Wear resistance and high-temperature compression strength of Fcc CuCoNiCrAl0.5Fe alloy with boron addition. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2004, 35A, 1465–1469. [Google Scholar] [CrossRef]
- Chen, M.R.; Lin, S.J.; Yeh, J.W.; Chen, S.K.; Huang, Y.S.; Tu, C.P. Microstructure and properties of Al0.5CoCrCuFeNiTix (x = 0–2.0) high-entropy alloys. Mater. Trans. 2006, 47, 1395–1401. [Google Scholar] [CrossRef] [Green Version]
- Löbel, M.; Lindner, T.; Mehner, T.; Lampke, T. Microstructure and wear resistance of AlCoCrFeNiTi high-entropy alloy coatings produced by HVOF. Coatings 2017, 7, 144. [Google Scholar] [CrossRef] [Green Version]
- Kane, S.N.; Mishra, A.; Dutta, A.K. Preface: International Conference on Recent Trends in Physics (ICRTP 2016). J. Phys. Conf. Ser. 2016, 755, 011001. [Google Scholar] [CrossRef]
- Wu, C.L.; Zhang, S.; Zhang, C.H.; Zhang, H.; Dong, S.Y. Phase evolution and cavitation erosion-corrosion behavior of FeCoCrAlNiTix high entropy alloy coatings on 304 stainless steel by laser surface alloying. J. Alloys Compd. 2017, 698, 761–770. [Google Scholar] [CrossRef]
- Erdogan, A.; Döleker, K.M.; Zeytin, S. Effect of laser re-melting on electric current assistive sintered CoCrFeNiAlxTiy high entropy alloys: Formation, micro-hardness and wear behaviors. Surf. Coat. Technol. 2020, 399, 126179. [Google Scholar] [CrossRef]
- Xin, B.; Zhang, A.; Han, J.; Su, B.; Meng, J. Tuning composition and microstructure by doping Ti and C for enhancing mechanical property and wear resistance of Al0.2Co1.5CrFeNi1.5Ti0.5 high entropy alloy matrix composites. J. Alloys Compd. 2020, 836, 155273. [Google Scholar] [CrossRef]
- Moravcikova-Gouvea, L.; Moravcik, I.; Omasta, M.; Veselý, J.; Cizek, J.; Minárik, P.; Cupera, J.; Záděra, A.; Jan, V.; Dlouhy, I. High-strength Al0.2Co1.5CrFeNi1.5Ti high-entropy alloy produced by powder metallurgy and casting: A comparison of microstructures, mechanical and tribological properties. Mater. Charact. 2020, 159, 110046. [Google Scholar] [CrossRef]
- Chuang, M.H.; Tsai, M.H.; Wang, W.R.; Lin, S.J.; Yeh, J.W. Microstructure and wear behavior of AlxCo 1.5CrFeNi1.5Tiy high-entropy alloys. Acta Mater. 2011, 59, 6308–6317. [Google Scholar] [CrossRef]
- Liu, H.; Liu, J.; Li, X.; Chen, P.; Yang, H.; Hao, J. Effect of heat treatment on phase stability and wear behavior of laser clad AlCoCrFeNiTi0.8 high-entropy alloy coatings. Surf. Coat. Technol. 2020, 392, 125758. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, W.M.; Zhang, T.B.; Li, J.S.; Wang, J.; Kou, H.C.; Li, J. Microstructure and tribological properties of AlCoCrFeNiTi0.5 high-entropy alloy in hydrogen peroxide solution. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2014, 45, 201–207. [Google Scholar] [CrossRef]
- Löbel, M.; Lindner, T.; Lampke, T. High-temperature wear behaviour of AlCoCrFeNiTi0.5 coatings produced by HVOF. Surf. Coatings Technol. 2020, 403, 126379. [Google Scholar] [CrossRef]
- Chen, L.; Bobzin, K.; Zhou, Z.; Zhao, L.; Öte, M.; Königstein, T.; Tan, Z.; He, D. Wear behavior of HVOF-sprayed Al0.6TiCrFeCoNi high entropy alloy coatings at different temperatures. Surf. Coat. Technol. 2019, 358, 215–222. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, J.; Yang, J.; Qiao, Z.; Duan, H.; Li, J.; Li, J.; Liu, W. Corrosive and tribological behaviors of AlCoCrFeNi-M high entropy alloys under 90 wt. % H2O2 solution. Tribol. Int. 2019, 131, 24–32. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, J.; Li, J.; Kou, H.; Duan, H.; Li, J.; Liu, W. Tribological behavior of AlCoCrCuFeNi and AlCoCrFeNiTi0.5 high entropy alloys under hydrogen peroxide solution against different counterparts. Tribol. Int. 2015, 92, 203–210. [Google Scholar] [CrossRef]
- Jin, G.; Cai, Z.; Guan, Y.; Cui, X.; Liu, Z.; Li, Y.; Dong, M.; Zhang, D. High temperature wear performance of laser-cladded FeNiCoAlCu high-entropy alloy coating. Appl. Surf. Sci. 2018, 445, 113–122. [Google Scholar] [CrossRef]
- Zhu, T.; Wu, H.; Zhou, R.; Zhang, N.; Yin, Y.; Liang, L.; Liu, Y.; Li, J.; Shan, Q.; Li, Q.; et al. Microstructures and Tribological Properties of TiC Reinforced FeCoNiCuAl High-Entropy Alloy at Normal and Elevated Temperature. Metals 2020, 10, 387. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.M.; Lin, S.J.; Yeh, J.W.; Chen, S.K.; Huang, Y.S.; Chen, H.C. Adhesive wear behavior of AlxCoCrCuFeNi high-entropy alloys as a function of aluminum content. Wear 2006, 261, 513–519. [Google Scholar] [CrossRef] [Green Version]
- Yan, G.; Zheng, M.; Ye, Z.; Gu, J.; Li, C.; Wu, C.; Wang, B. In-situ Ti(C, N) reinforced AlCoCrFeNiSi-based high entropy alloy coating with functional gradient double-layer structure fabricated by laser cladding. J. Alloys Compd. 2021, 886, 161252. [Google Scholar] [CrossRef]
- Li, Z.; Fu, P.; Hong, C.; Chang, F.; Dai, P. Tribological behavior of Ti(C, N)-TiB2 composite cermets using FeCoCrNiAl high entropy alloys as binder over a wide range of temperatures. Mater. Today Commun. 2021, 26, 102095. [Google Scholar] [CrossRef]
- Kumar, A.; Chandrakar, R.; Chandraker, S.; Rao, K.R.; Chopkar, M. Microstructural and mechanical properties of AlCoCrCuFeNiSix (x = 0.3 and 0.6) high entropy alloys synthesized by spark plasma sintering. J. Alloys Compd. 2021, 184, 158193. [Google Scholar] [CrossRef]
- Xin, B.; Zhang, A.; Han, J.; Meng, J. Improving mechanical properties and tribological performance of Al0.2Co1.5CrFeNi1.5Ti0.5 high entropy alloys via doping Si. J. Alloys Compd. 2021, 869, 159122. [Google Scholar] [CrossRef]
- Karakaş, M.S.; Günen, A.; Çarboğa, C.; Karaca, Y.; Demir, M.; Altınay, Y.; Erdoğan, A. Microstructure, some mechanical properties and tribocorrosion wear behavior of boronized Al0.07Co1.26Cr1.80Fe1.42Mn1.35Ni1.10 high entropy alloy. J. Alloys Compd. 2021, 886, 161222. [Google Scholar] [CrossRef]
- Xin, B.; Zhang, A.; Han, J.; Meng, J. The tribological properties of carbon doped Al0.2Co1.5CrFeNi1.5Ti0.5 high entropy alloys. Wear 2021, 484, 204045. [Google Scholar] [CrossRef]
- Zhao, P.; Li, J.; Lei, R.; Yuan, B.; Xia, M.; Li, X.; Zhang, Y. Investigation into microstructure, wear resistance in air and nacl solution of alcrconifectax high-entropy alloy coatings fabricated by laser cladding. Coatings 2021, 11, 358. [Google Scholar] [CrossRef]
- Ghanbariha, M.; Farvizi, M.; Ebadzadeh, T.; Alizadeh Samiyan, A. Effect of ZrO2 particles on the nanomechanical properties and wear behavior of AlCoCrFeNi–ZrO2 high entropy alloy composites. Wear 2021, 484–485, 204032. [Google Scholar] [CrossRef]
- Li, Y.; Shi, Y. Microhardness, wear resistance, and corrosion resistance of AlxCrFeCoNiCu high-entropy alloy coatings on aluminum by laser cladding. Opt. Laser Technol. 2021, 134, 106632. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, Z.; Hong, Y.; Lu, B.; Liu, J.; Li, Y.; Pu, J. Improved tribological behavior of plasma-nitrided AlCrTiV and AlCrTiVSi high-entropy alloy films. Tribol. Int. 2021, 163, 107195. [Google Scholar] [CrossRef]
- Chandrakar, R.; Kumar, A.; Chandraker, S.; Rao, K.R.; Chopkar, M. Microstructural and mechanical properties of AlCoCrCuFeNiSix (x = 0 and 0.9) high entropy alloys. Vacuum 2021, 184, 109943. [Google Scholar] [CrossRef]
- Erdogan, A.; Sunbul, S.E.; Icin, K.; Doleker, K.M. Microstructure, wear and oxidation behavior of AlCrFeNiX (X = Cu, Si, Co) high entropy alloys produced by powder metallurgy. Vacuum 2021, 187, 110143. [Google Scholar] [CrossRef]
- Duan, H.; Wu, Y.; Hua, M.; Yuan, C.; Wang, D.; Tu, J.; Kou, H.; Li, J. Tribological properties of AlCoCrFeNiCu high-entropy alloy in hydrogen peroxide solution and in oil lubricant. Wear 2013, 297, 1045–1051. [Google Scholar] [CrossRef]
- Chen, M.R.; Lin, S.J.; Yeh, J.W.; Chen, S.K.; Huang, Y.S.; Chuang, M.H. Effect of vanadium addition on the microstructure, hardness, and wear resistance of Al0.5CoCrCuFeNi high-entropy alloy. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2006, 37, 1363–1369. [Google Scholar] [CrossRef]
- Gu, Z.; Xi, S.; Mao, P.; Wang, C. Microstructure and wear behavior of mechanically alloyed powder AlxMo0.5NbFeTiMn2 high entropy alloy coating formed by laser cladding. Surf. Coat. Technol. 2020, 401, 126244. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Sheu, T.S.; Yeh, J.W.; Chen, S.K. Effect of iron content on wear behavior of AlCoCrFexMo0.5Ni high-entropy alloys. Wear 2010, 268, 653–659. [Google Scholar] [CrossRef]
- Liang, H.; Miao, J.; Gao, B.; Deng, D.; Wang, T.; Lu, Y.; Cao, Z.; Jiang, H.; Li, T.; Kang, H. Microstructure and tribological properties of AlCrFe2Ni2W0.2Mo0.75 high-entropy alloy coating prepared by laser cladding in seawater, NaCl solution and deionized water. Surf. Coat. Technol. 2020, 400, 126214. [Google Scholar] [CrossRef]
- Qiu, X.W.; Liu, C.G. Microstructure and properties of Al2CrFeCoCuTiNix high-entropy alloys prepared by laser cladding. J. Alloys Compd. 2013, 553, 216–220. [Google Scholar] [CrossRef]
- Kanyane, L.R.; Popoola, A.P.; Malatji, N. Influence of Sintering Temperature on Microhardness and Tribological Properties of Equi-Atomic Ti-Al-Mo-Si-W Multicomponent Alloy. IOP Conf. Ser. Mater. Sci. Eng. 2019, 538, 012009. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, Y.; Vilar, R.; Shen, J. Dry sliding wear behavior of laser clad TiVCrAlSi high entropy alloy coatings on Ti-6Al-4V substrate. Mater. Des. 2012, 41, 338–343. [Google Scholar] [CrossRef]
- Zhang, H.X.; Dai, J.J.; Sun, C.X.; Li, S.Y. Microstructure and wear resistance of TiAlNiSiV high-entropy laser cladding coating on Ti-6Al-4V. J. Mater. Process. Technol. 2020, 282, 116671. [Google Scholar] [CrossRef]
- Lin, Y.C.; Cho, Y.H. Elucidating the microstructure and wear behavior for multicomponent alloy clad layers by in situ synthesis. Surf. Coat. Technol. 2008, 202, 4666–4672. [Google Scholar] [CrossRef]
- Yadav, S.; Aggrawal, A.; Kumar, A.; Biswas, K. Effect of TiB2 addition on wear behavior of (AlCrFeMnV)90Bi10 high entropy alloy composite. Tribol. Int. 2019, 132, 62–74. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Zhou, Q.; Zhang, F.; Han, W.; Du, Y.; Hua, K.; Wang, H. Effect of Al addition on the microstructure, mechanical and wear properties of TiZrNbHf refractory high entropy alloys. Tribol. Int. 2021, 160, 107031. [Google Scholar] [CrossRef]
- Zhao, P.; Li, J.; Zhang, Y.; Li, X.; Xia, M.M.; Yuan, B.G. Wear and high-temperature oxidation resistances of AlNbTaZrx high-entropy alloys coatings fabricated on Ti6Al4V by laser cladding. J. Alloys Compd. 2021, 862, 158405. [Google Scholar] [CrossRef]
- Tüten, N.; Canadinc, D.; Motallebzadeh, A.; Bal, B. Microstructure and tribological properties of TiTaHfNbZr high entropy alloy coatings deposited on Ti–6Al–4V substrates. Intermetallics 2019, 105, 99–106. [Google Scholar] [CrossRef]
- Pole, M.; Sadeghilaridjani, M.; Shittu, J.; Ayyagari, A.; Mukherjee, S. High temperature wear behavior of refractory high entropy alloys based on 4-5-6 elemental palette. J. Alloys Compd. 2020, 843, 156004. [Google Scholar] [CrossRef]
- Ye, Y.X.; Liu, C.Z.; Wang, H.; Nieh, T.G. Friction and wear behavior of a single-phase equiatomic TiZrHfNb high-entropy alloy studied using a nanoscratch technique. Acta Mater. 2018, 147, 78–89. [Google Scholar] [CrossRef]
- Pogrebnjak, A.D.; Yakushchenko, I.V.; Abadias, G.; Chartier, P.; Bondar, O.V.; Beresnev, V.M.; Takeda, Y.; Sobol’, O.V.; Oyoshi, K.; Andreyev, A.A.; et al. The effect of the deposition parameters of nitrides of high-entropy alloys (TiZrHfVNb)N on their structure, composition, mechanical and tribological properties. J. Superhard Mater. 2013, 35, 356–368. [Google Scholar] [CrossRef]
- Gong, P.; Li, F.; Deng, L.; Wang, X.; Jin, J. Research on nano-scratching behavior of TiZrHfBeCu(Ni) high entropy bulk metallic glasses. J. Alloys Compd. 2020, 817, 153240. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Ye, Y.X.; Liu, C.Z.; Feng, R.; Yao, K.F.; Nieh, T.G. Tribological behavior of an amorphous Zr20Ti20Cu20Ni20Be20 high-entropy alloy studied using a nanoscratch technique. Intermetallics 2019, 113, 1065601. [Google Scholar] [CrossRef]
- Jhong, Y.S.; Huang, C.W.; Lin, S.J. Effects of CH4 flow ratio on the structure and properties of reactively sputtered (CrNbSiTiZr)Cx coatings. Mater. Chem. Phys. 2018, 210, 348–352. [Google Scholar] [CrossRef]
- Mathiou, C.; Poulia, A.; Georgatis, E.; Karantzalis, A.E. Microstructural features and dry—Sliding wear response of MoTaNbZrTi high entropy alloy. Mater. Chem. Phys. 2018, 210, 126–135. [Google Scholar] [CrossRef]
- Petroglou, D.; Poulia, A.; Mathiou, C.; Georgatis, E.; Karantzalis, A.E. A further examination of MoTaxNbVTi (x = 0.25, 0.50, 0.75 and 1.00 at.%) high-entropy alloy system: Microstructure, mechanical behavior and surface degradation phenomena. Appl. Phys. A Mater. Sci. Process. 2020, 126, 364. [Google Scholar] [CrossRef]
- Poulia, A.; Georgatis, E.; Lekatou, A.; Karantzalis, A.E. Microstructure and wear behavior of a refractory high entropy alloy. Int. J. Refract. Met. Hard Mater. 2016, 57, 50–63. [Google Scholar] [CrossRef]
- Poulia, A.; Georgatis, E.; Lekatou, A.; Karantzalis, A. Dry-Sliding Wear Response of MoTaWNbV High Entropy Alloy. Adv. Eng. Mater. 2017, 19, 1600535. [Google Scholar] [CrossRef]
- Poulia, A.; Georgatis, E.; Karantzalis, A. Evaluation of the Microstructural Aspects, Mechanical Properties and Dry Sliding Wear Response of MoTaNbVTi Refractory High Entropy Alloy. Met. Mater. Int. 2019, 25, 1529–1540. [Google Scholar] [CrossRef]
- Alvi, S.; Akhtar, F. High temperature tribology of CuMoTaWV high entropy alloy. Wear 2019, 426–427, 412–419. [Google Scholar] [CrossRef]
- Hua, N.; Wang, W.; Wang, Q.; Ye, Y.; Lin, S.; Zhang, L.; Guo, Q.; Brechtl, J.; Liaw, P.K. Mechanical, corrosion, and wear properties of biomedical Ti–Zr–Nb–Ta–Mo high entropy alloys. J. Alloys Compd. 2021, 861, 157997. [Google Scholar] [CrossRef]
- Gu, Z.; Peng, W.; Guo, W.; Zhang, Y.; Hou, J.; He, Q.; Zhao, K.; Xi, S. Design and characterization on microstructure evolution and properties of laser-cladding Ni1.5CrFeTi2B0.5Mox high-entropy alloy coatings. Surf. Coat. Technol. 2021, 408, 126793. [Google Scholar] [CrossRef]
- Xin, B.; Yu, Y.; Zhou, J.; Wang, L.; Ren, S. Effect of copper molybdate on the lubricating properties of NiCrAlY laser clad coating at elevated temperatures. Surf. Coat. Technol. 2017, 313, 328–336. [Google Scholar] [CrossRef]
- Archard, J.F. Contact and Rubbing of Flat Surfaces. J. Appl. Phys. 1953, 24, 981–988. [Google Scholar] [CrossRef]
- Mohan, S.; Agarwala, V.; Ray, S. Friction characteristics of stir-cast Al-Pb alloys. Wear 1992, 157, 9–17. [Google Scholar] [CrossRef]
- Ding, C.H.; Li, P.L.; Ran, G.; Tian, Y.W.; Zhou, J.N. Tribological property of self-lubricating PM304 composite. Wear 2007, 262, 575–581. [Google Scholar] [CrossRef]
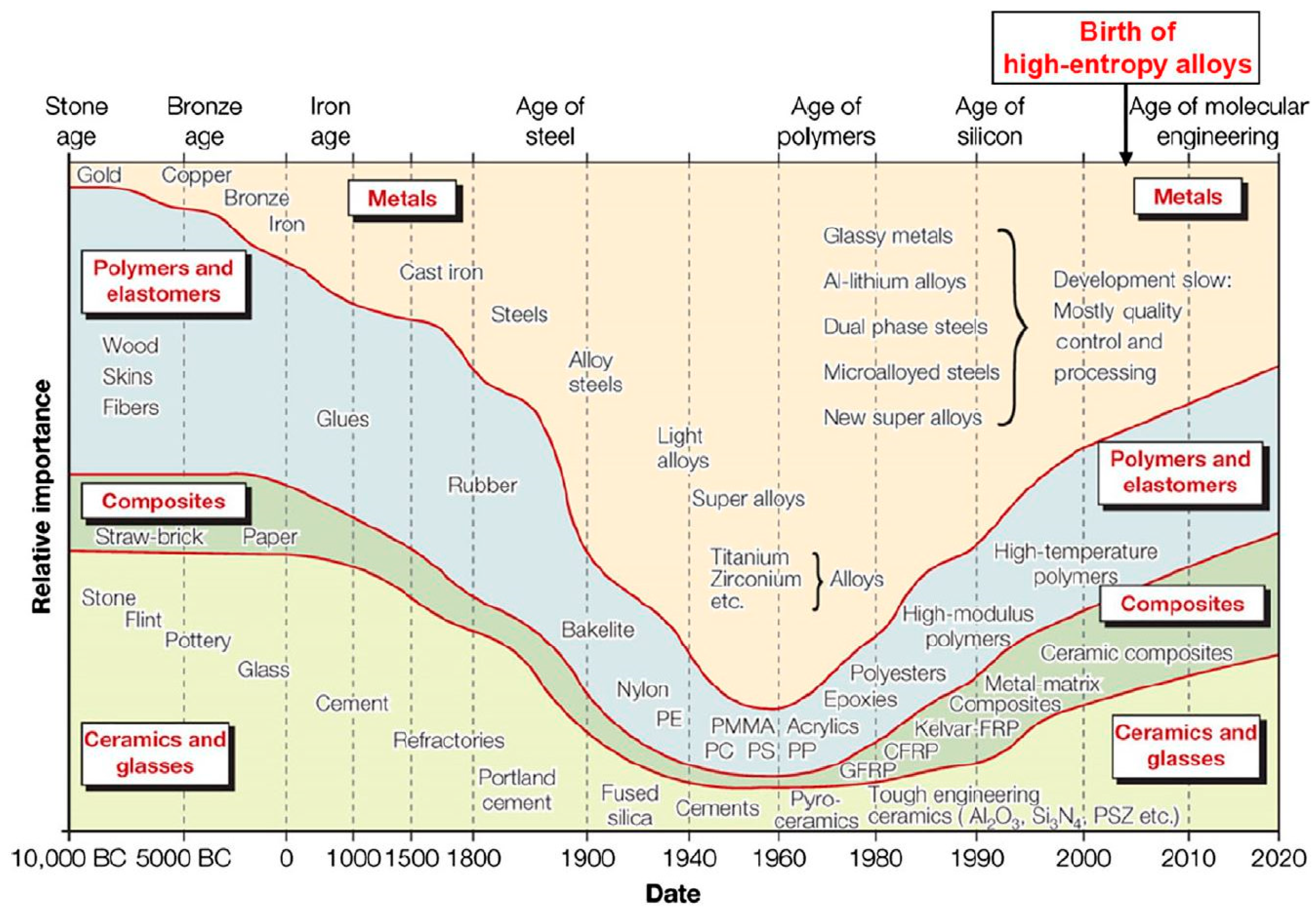
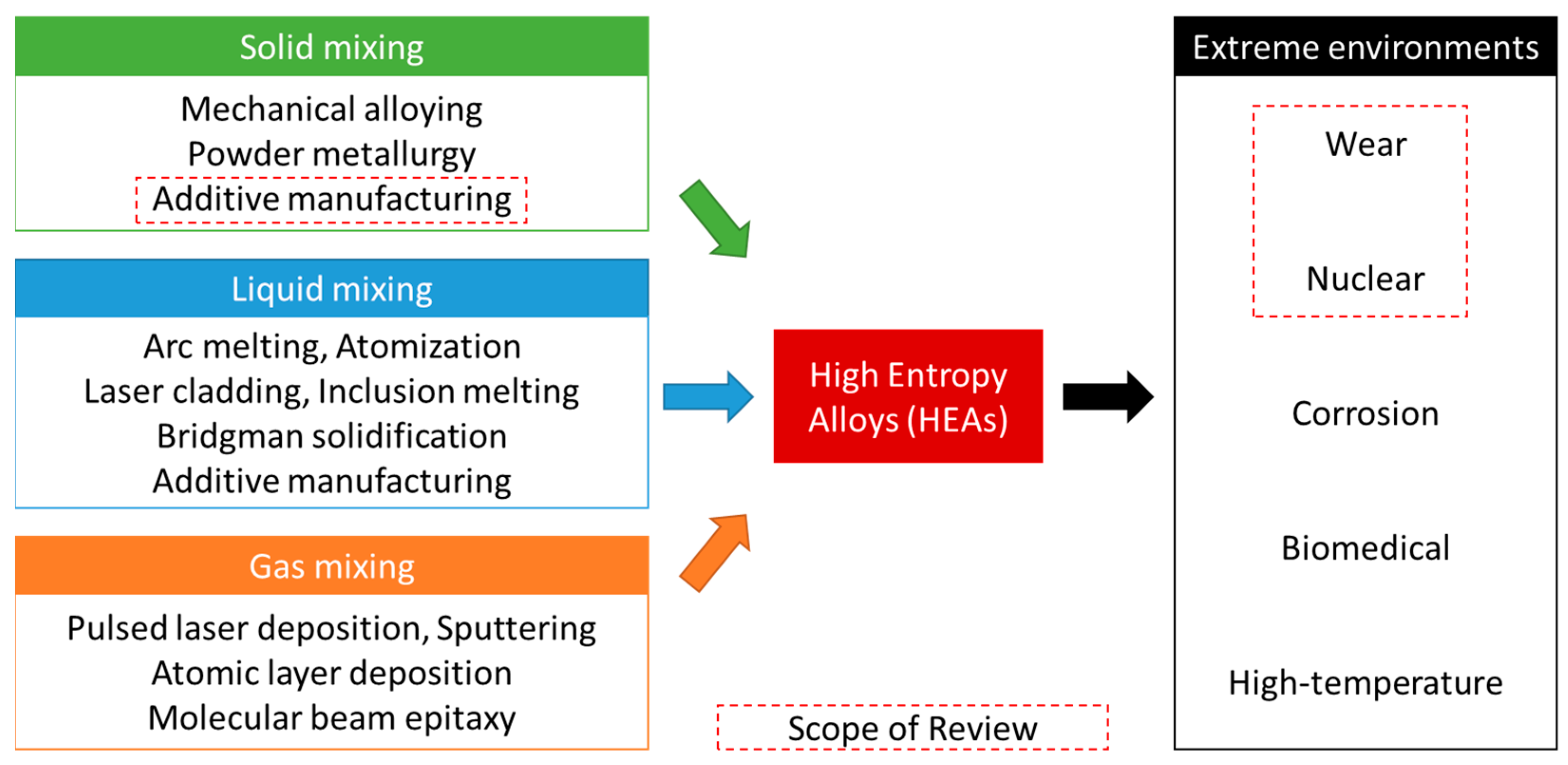
| Source | Alloy Composition | Microstructure (Grain Size) | Result UTS (MPa), YS (MPa), BS (MPa), δb (mm), ε (%), H, CS (MPa), C (%) |
|---|---|---|---|
| Chen et al. [176] | CoCrFeMnNi | FCC (53.1 µm) | UTS = 281 ± 18, YS = 12.5 ± 0.5, H = 261 ± 7 HV |
| Niu et al. [169] | CoCrFeMnNi | FCC (<5 µm) | CS = 2447.7 |
| Li et al. [177] | CoCrFeMnNi + TiNp nanoparticles | FCC | UTS = 601–1036, ε = 12–30 |
| Li et al. [178] | CoCrFeMnNi + Fe based metallic glass | FCC | UTS = 916–1517 |
| Li et al. [179] | CoCrFeMnNi + TiN nanoparticles | FCC | - |
| Kim et al. [180] | (CoCrFeMnNi)C | FCC (180–330 nm) | YS = 800–900, ε = 25–30 |
| Li et al. [181] | CoCrFeMnNi + 12 wt% nano-TiNp | FCC (<2 µm) | UTS = 1100 |
| Piglione et al. [182] | CoCrFeMnNi | FCC (0.52–0.64 µm) | H = 212 HV |
| Zhu et al. [153] | CoCrFeMnNi | FCC | - |
| Xu et al. [183] | CoCrFeMnNi | FCC (1–2 µm) | H = 2.84 ± 0.13 GPa |
| Park et al. [154] | CoCrFeMnNi +1 at%C | FCC (20–35 µm) | UTS = 829–989, YS = 741, ε = 24.3 |
| Ren et al. [184] | CoCrFeMnNi | - | - |
| Dovgyy et al. [185] | CoCrFeMnNi | FCC & cubic (0.2–0.8 µm) | - |
| Zhou et al. [155] | CoCrFeNi + 0.5 at%C | FCC (40–50 µm) | UTS = 776–797, YS = 630–656, ε = 7.7–13.5 |
| Wu et al. [186] | CoCrFeNi + 0.5 at%C | FCC (40–50 µm) | UTS = 795, YS = 638 |
| Lin et al. [98] | CoCrFeNi | FCC | |
| Sun et al. [187] | CoCrFeNi | -, ~3 mm in length and ~200 μm in width | UTS = 676.7–691, YS = 556.7–572, ε = 12.4–17.9 |
| Song et al. [188] | CoCrFeNi + N (1.8%) | FCC | UTS = 600–853, YS = 520–650, ε = 27 |
| Zhou et al. [189] | (CoCrFeNi)1−x (WC)x | FCC | H = 603–768 HV |
| Brif et al. [156] | CoCrFeNi | FCC | UTS = 480–745, YS = 402–600, ε = 8–32, H = 205–238 |
| Niu et al. [190] | AlCoCrFeNi | Disordered (A2) + Ordered (B2) BCC | H = 632.8 HV |
| Karlsson et al. [170] | AlCoCrFeNi | FCC & BCC (<20 µm) | - |
| Peyrouzet et al. [157] | Al0.3CoCrFeNi | FCC (width~13 and length~70–120 µm) | UTS = 896, YS = 730, ε = 29 |
| Sun et al. [158] | Al0.5CoCrFeNi | FCC & BCC (1 µm) | UTS = 878, YS = 609, H = 270HV |
| Zhou et al. [160] | Al0.5CoCrFeNi | FCC | UTS = 721, YS = 579, ε = 22 |
| Luo et al. [191] | AlCrCuFeNi | BCC (avg. width~4 µm) | CS = 1655.2–2052.8, C = 6.5–6.8 |
| Luo et al. [192] | AlCrCuFeNix (2 ≤ x ≤ 3) | FCC (thickness~490 nm) & BCC (~140 nm) Avg. thickness of both ~ 650 nm | UTS = 957, ε = 14.3 |
| Li et al. [112] | AlCoCuFeNi | BCC | YS = 744, ε = 13.1, CS = 1600 |
| Yao et al. [193] | AlCrFeNiV | FCC (width~15 µm, length~75–200 µm) | UTS = 1057.47, ε = 30.3 |
| Wang et al. [194] | AlCoCrCuFeNi | FCC & BCC | H = 710.4 HV |
| Wang et al. [195] | AlMgScZrMn | Al3 (Sc, Zr) (1–10 nm + 7 µm) | UTS = 394, ε = 10.5 |
| Sarawat et al. [196] | AlCoFeNiV0.9Sm0.1 AlCoFeNiSm0.1TiV0.9 AlCoFeNiSm0.05TiV0.95Zr, AlCoFeNiTiVZr | FCC | H~42.8–86.7 HV |
| Agrawal et al. [197] | Fe40Mn20Co20Cr15Si5 | HCP | UTS = 1100, YS = 530, ε = 30 |
| Zhang et al. [198,199] | NbMoTaW | BCC (13.4 μm) | H = 826 HV |
| Yang et al. [200,201] | Ni6Cr4WFe9Ti | FCC (300–1000 nm) + unknown phase | UTS = 972, YS = 742, ε = 12.2 |
| Chen et al. [202] | CoCrFeNiMn | FCC + Mn2O3 particles | YS = 620, UTS = 730, ε~12 |
| Litwa et al. [203] | CoCrFeNiMn | FCC | H~320 HV |
| Zhang et al. [204] | CoCrFeNiMn | FCC | YS~729.6 |
| Kim et al. [205] | CoCrFeNiMn | FCC | YS = 752.6 |
| Choi et al. [206] | CoCrFeNiMn | FCC | |
| Su et al. [207] | CrCuFeNi2 Al0.5CrCuFeNi2 Al0.75CrCuFeNi2 AlCrCuFeNi2 | FCC FCC FCC + BCC/B2 FCC + BCC/B2 | |
| Peng et al. [208] | CoCrFeNi + Ti coated diamond CoCrFeNi + diamond | FCC + diamond particles FCC + Cr7C3 + diamond particles | H = 622 HV, BS = 530, δb = 0.64 H = 615 HV, BS = 925, δb = 0.48 |
| Wang et al. [209] | CoCrFeNiMn | FCC | H = 164–370 HV |
| Sun et al. [210] | Al0.1CrCuFeNi Al0.5CrCuFeNi AlCrCuFeNi | FCC FCC FCC + BCC/B2 (NiAl) | |
| Ishimoto et al. [211] | Ti1.4Nb0.6Ta0.6Zr1.4Mo0.6 | BCC | YS = 1690, |
| Park et al. [212] | (CoCrFeMnNi)99C1 | FCC | YS~741, UTS~874 |
| Lin et al. [213] | CoCrFeNi | FCC | YS = 701 ± 14, UTS = 907 ± 25 |
| Kim et al. [214] | CoCrFeNiMn | FCC | - |
| Jin et al. [215] | CoCrFeNiMn | FCC | YS = 520 ± 10, UTS = 770 ± 10, ε~25 |
| Lin et al. [216] | Al0.2Co1.5CrFeNi1.5Ti0.3 | FCC + σ + L12 | YS = 1235, UTS = 1550 |
| Peng et al. [217] | CoCrFeNiMn | FCC | - |
| Vogiatzief et al. [218] | AlCrFe2Ni2 Heat treatment (750–950 °C, 3 h & 6 h) | FCC + BCC | H = 276–483 HV |
| Liao et al. [219] | Al0.5FeCrNi2.5V0.2 | FCC | H = 220–240 HV |
| Guo et al. [220] | CoCrFeNiMn | FCC | YS = 622, UTS = 763, ε~16 |
| Kim et al. [221] | (CoCrFeNiMn)100−xCx | FCC (15–22 µm) | YS = 653–753, UTS = 766–911 |
| Zhao et al. [222] | CoCrFeNi | FCC | H = 238–525 HV |
| Gu et al. [223] | CoCr2.5FeNi2TiW0.5 | FCC | YS = 449–581, CS = 823–893, ε = 4.4–9.9, H = 436.7–499.2 HV |
| Source | Alloy Composition | Microstructure (Grain Size) | Result UTS (MPa), YS (MPa), ε (%), H, CS (MPa), C (%) |
|---|---|---|---|
| Peng et al. [224] | CoCrFeNiMn | FCC | YS = 196 |
| Wang et al. [225] | CoCrFeMnNi | FCC (65) | UTS = 497, 205, H = 157.1HV |
| Kuwabara et al. [226] | AlCoCrFeNi | BCC & FCC | UTS = 1073, YS = 769, ε = 0–1.2 YS = 944–1015, CS = 1447–1668, C = 14.5–26.4 |
| Wang et al. [227] | AlCoCrFeNi | BCC | - |
| Fujieda et al. [228] | CoCrFeNiTi | FCC + Cubic | UTS = 1178, YS = 773, ε = 25.8 |
| Popov et al. [229] | Al0.5CrMoNbTa0.5 | BCC |
| Scheme | Alloy Composition | Microstructure (Grain Size) | Result UTS (MPa), YS (MPa), ε (%), H, CS (MPa), C (%) |
|---|---|---|---|
| Guan et al. [230] | CoCrFeMnNi | FCC (13 μm) | YS = 517, ε = 26 |
| Melia et al. [231] | CoCrFeMnNi | FCC (~4 μm) | UTS = 647–651, YS = 232–424 |
| Li et al. [232] | CoCrFeMnNi | FCC | |
| Gao et al. [233] | CoCrFeMnNi | FCC (30–150 μm) + BCC | UTS = 620, YS = 448 |
| Xiang et al. [234,235] | CoCrFeNiMn | FCC | UTS = 400–600 |
| Chew et al. [236] | CoCrFeNiMn | FCC (3.68 ± 0.85 μm) | UTS = 660, YS = 518 |
| Qiu et al. [237] | CoCrFeMnNi | FCC | UTS = 891, YS = 564 |
| Li et al. [238] | CoCrFeMnNi + WC (0–10 wt%) | FCC | UTS = 550–845, YS = 300–675, ε = 9 |
| Amar et al. [239] | CoCrFeMnNi + TiC (0–5 wt%) | FCC | UTS = 550–723, YS = 300–385, ε = 32 |
| Guan et al. [240] | CoCrFeMnNi AlCoCrFeNiTi0.5 | FCC (24 µm) BCC (7 µm) + FCC | YS = 888–1100, H = 197–657 HV |
| Wang et al. [241] | CoCrFeNiMo0.2 | FCC | UTS = 532–928, ε = 37 |
| Zhou et al. [242] | CoCrFeNiNbx (x = 0–0.2) | FCC | UTS = 400–820, YS = 220–750 |
| Gwalani et al. [243] | AlxCoCrFeNi (x = 0.3–0.7) | FCC | |
| Nartu et al. [244] | Al0.3CoCrFeNi | FCC | YS = 410–630, ε = 18–28 |
| Mohanty et al. [245] | AlxCoCrFeNi (x = 0.3–0.7) | FCC + BCC | H = 170–380 HV |
| Vikram et al. [246] | AlCoCrFeNi2.1 | FCC & BCC | YS = 309–711, H = 278 ± 11–316 ± 14 HV |
| Gwalani et al. [247] | AlCrFeMoVx (x = 0–1) | BCC (68–165 μm) | H = 485–581 HV |
| Guan et al. [248] | AlCoCrFeNiTi0.5 | BCC (12 μm) | - |
| Malatji et al. [249] | AlCrCuFeNi | BCC & FCC | H = 350 HV, |
| Dada et al. [250,251] | AlCoCrFeNiCu AlTiCrFeCoNi | H = 600 HV, H = 850 HV | |
| Moorehead et al. [252] | NbMoTaW | BCC | - |
| Kunce et al. [253] | TiZrNbMoV | BCC | - |
| Dobbelstein et al. [254] | TiZrNbHfTa | BCC | H = 509 HV0.2 |
| Pegues et al. [255] | CoCrFeNiMn | FCC | - |
| Li et al. [256] | CoCrFeNiMn | FCC | - |
| Tong et al. [257] | CoCrFeNiMn Vacuum arc melting 1 impact Laser shock peening 3 impact Laser shock peening 5 impact Laser shock peening | FCC | YS = 320.7, UTS = 531.7 YS = 427.4, UTS = 570.7 YS~435, UTS~600 YS = 489.9, UTS = 639.9 |
| Shen et al. [258] | CoCrFeNi (SiC)x | FCC + Cr7C3 (1 µm) | UTS = 2155–2499, YS = 142–713, H = 139–310 |
| Cai et al. [259] | CoCrFeNi AlCoCrFeNi | BCC (102.27 µm) BCC (18.75 µm) | YS = 318, UTS = 440, ε = 8.56 YS = 383, UTS = 533, ε = 10.6 |
| Zhang et al. [260] | NbMoTa NbMoTaTi NbMoTaNi NbMoTaTi0.5Ni0.5 | BCC BCC + α-Ti BCC BCC + Ni3Ta + β-Ti | YS = 1252, CS = 1282, ε = 15 YS = 1200, CS = 1350, ε = 23 YS = 1350, CS = 1380, ε = 11 YS = 1750, CS = 2277.79, ε = 15 CS of NbMoTaTi0.5Ni0.5 at 600, 800 and 1000 °C is 1699.75 MPa, 1033.63 MPa and 651.36 MPa |
| Peng et al. [261] | Al0.3CoCrFeNi | FCC + B2 | YS = 373–476, CS = 473–508, ε = 0.6–2.96, H = 208–221 HV |
| Kuzminova et al. [262] | CoCrFeNi | FCC | YS = 456–551, UTS = 637–658, H = 209–259 HV |
| Malatji et al. [263] | AlCuCrFeNi Heat treated (800–1100 °C) | FCC + BCC | H = 310–381 HV |
| Dong et al. [264] | AlCoCrFeNi2.1 | FCC + BCC | YS = 388, UTS = 719, ε~27, H = 221–228 |
| Zhou et al. [265] | CoCrFeNb0.2Ni2.1 Solution treatment (2 h,1250 °C) 96 h aged (650 °C) | FCC + HCP (Laves C14) + Nb rich carbide | YS~340, UTS~735 YS~239, UTS~607 YS~896, UTS~1127, ε~17 |
| Zheng et al. [266] | CoCrFeNiMn | FCC | YS = 330, UTS = 630 |
| Reactor System | Core Outlet Temperature (°C) | Coolant |
|---|---|---|
| Super critical water-cooled reactor | 350–620 | Water |
| Sodium-cooled fast reactor | ~550 | Na liquid metal |
| Lead-cooled reactor | 550–800 | Pb, Pb-Bi liquid Metals |
| Molten salt reactor | 700–800 | Fluoride salts |
| Gas-cooled fast reactor | ~850 | Helium gas |
| Very high temperature reactor | >900 | Helium gas |
| Source | Material (Fabrication) | Phase | Irradiation Conditions (Energy, Ion, Fluence, Temperature) |
|---|---|---|---|
| Jawaharram et al. [301] | CoCrFeNiMn | FCC | 2.6 MeV, Ag3+, 1.5 × 10−3 & 1.9 × 10−3 dpa−1 s−1, 23–500 °C |
| Lu et al. [302] | NiCoFeCr, CoCrFeNiMn | FCC | 3 MeV, Ni2+, 5 × 1016 ions·cm−2, 500 °C |
| Barr et al. [303] | CoCrFeNiMn | FCC | 3 MeV, Ni2+, 3 × 1015 ions·cm−2, 500 °C |
| Lu et al. [304] | CoCrFeNi, CoCrFeNiMn | FCC | 1.5 MeV, Ni+, 4 × 1014 & 3 × 1015 ions·cm−2 (peak dose~4 dpa), 500 °C 3 MeV, Ni+, 5 × 1016 ions·cm−2 (peak dose~60 dpa), 500 °C |
| Tong et al. [305] | CoCrFeNiMn CoCrFeNi CoCrFeNiPd | FCC | 16 MeV, Ni5+, 8 MeV Ni3+, 4 MeV Ni1+& 2 MeV Ni1+, 0.1–1 dpa, 420 °C |
| Jin et al. [306] | CoCrFeNi, CoCrFeNiMn | FCC | 3 MeV, Ni2+, 5 × 1016 ions·cm−2 (peak dose~53 dpa), 500 °C |
| Chen et al. [307] | CoCrFeMnNi Al0.3CoCrFeNi | FCC FCC | 1 MeV, Kr ions, 6.3 × 1015 ions·cm−2, 300 °C |
| Wang et al. [308] | CoCrFeNiCu | FCC | 100 keV, He+, 2.5 × 1017, 5 × 1017 & 1 × 1018 ions·cm−2, RT |
| He et al. [309] | CoCrFeNi, CoCrFeNiMn, CoCrFeNiPd | FCC | electrons, 5 × 1018 e·cm−2·s−1, 400 °C |
| Yang et al. [310] | CoCrFeNiMn, CoCrFeNiPd | FCC | 3MeV, Ni2+, 5 × 1016 ions·cm−2, 420, 500 & 580 °C |
| Yang et al. [311] | CoCrFeNiMn | FCC | -, He ion, -, RT & 450 °C |
| Hashimoto et al. [312] | CoCrFeNiMn, CoCrFeNiAl0.3 | FCC | 1250 keV, 1.5 dpa, 300–400 °C |
| Zhang et al. [313] | CoCrFeNiCu | FCC | 3 MeV Ni2+, 1014 ions·cm−2, RT |
| Yang et al. [314] | CoNi, FeNi, CoCrFeNi | - FCC | 3 MeV, Ni2+, 1.5 × 1016 (peak dose~17 dpa) & 5.0 × 1016 (peak dose~53 dpa) ions·cm−2, 500 °C |
| Abhaya et al. [315] | CrCoFeNi | FCC | 1.5 MeV, Ni2+, 1 × 1015 (peak dose~2 dpa) & 5 × 1016 (peak dose~96 dpa) ions·cm−2, RT |
| Sellami et al. [316] | CoCrFeNi | 1.5 MeV, Ni2+, 1 × 1013–1 × 1014 ions·cm−2 21 MeV, Ni2+, 2 × 1013 & 1 × 1014 ions·cm−2, RT | |
| Chen et al. [317] | CoCrFeNi | FCC | 275 keV, He+, 5.14 × 1020 ions·m−2, 250, 300, 400 °C |
| Kombaiah et al. [318] | CoCrFeNi, Al0.12CoCrFeNi | FCC | 3 MeV, Ni2+, 1 × 1017 ions·cm−2 (peak dose~100 dpa), 500 °C |
| Lu et al. [319] | CoCrFeNiPd | FCC | 3 MeV, Ni2+, 5 × 1016 ions·cm−2, 580 °C |
| Tunes et al. [320] | CrFeNiMn | FCC | 30 keV, Xe+, 2.6 × 1016 ions·cm−2, 500 °C |
| Edmondson et al. [321] | CrFeNiMn | BCC | 30 keV, Xe+, 9.3×1016 ions·cm−2 6 keV He+, 6.4 × 1016 ions·cm−2, RT |
| Fan et al. [322] | CoCrFeNi | FCC | 3 MeV, Ni ions, 5 × 1016–8 × 1016 ions/cm−2, 580 °C |
| Chen et al. [81] | CoCrFeNiTi0.2 | FCC | 275 keV, He2+, 5.14 × 1020 ions·m−2, 400 °C |
| Lyu et al. [323] | CoCrFeNiMo0.2 | FCC | 27 keV, electrons, -, RT |
| Xu et al. [324] | (CoCrFeNi)95Ti1Nb1Al3 | FCC | 2.5 MeV, Fe ions, 1.5 × 1019 ions·m−2, RT-500 °C |
| Cao et al. [325] | (CoCrFeNi)94Ti2Al4 | FCC | 4 MeV, Au ions, 10–49 dpa, RT |
| Tolstolutskaya et al. [326] | Cr0.18Fe0.4Mn0.28Ni0.14 Cr0.18Fe0.28Mn0.27Ni0.28 Cr0.2Fe0.4Mn0.2Ni0.2 | FCC | 1.4 MeV, Ar ions, 0, 0.3, 1 & 5 dpa, RT |
| Kumar et al. [327] | Fe0.27Ni0.28Mn0.27Cr0.18 | FCC | 3 MeV, Ni2+, 4.2 × 1013, 4.2 × 1014 & 4.2 × 1015 ions·cm−2, RT & 500 °C 3 MeV, Ni2+, 2.43 × 1015 & 2.43 × 1016 ions·cm−2, 400–700 °C |
| Li et al. [328] | Cr0.18Fe0.27Ni0.28Mn0.27 | FCC | Neutron, 8.9 × 1014 n·cm−2.s, 60 °C |
| Voyevodin et al. [329] | Cr0.2Fe0.4Mn0.2Ni0.2+ Y2O3 + ZrO2 | FCC | 1.4 MeV, Ar ions, 2.2 × 1015 ions·cm−2, RT |
| Dias et al. [330] | CuxCrFeTiV (x = 0.21–1.7) | BCC + FCC | 300 keV, Ar+, 3 × 1020 at·m−2, RT |
| Yang et al. [298] | Al0.3CoCrFeNi | FCC | 3 MeV, Au ions, 6 × 1015 ion·cm−2 (peak dose ~31 dpa), 250–650 °C |
| Gromov et al. [331] | AlCoCrFeNi | - | 18 keV, electrons, -, RT |
| Zhang et al. [299] | AlCrMoNbZr, (AlCrMoNbZr)N | FCC | 400 keV, He+, 8 × 1015 & 8 × 1016 ion·cm−2, RT |
| Yang et al. [82] | Al0.1CoCrFeNi, Al0.75CoCrFeNi, Al1.5CoCrFeNi, | FCC FCC + B2 A2 + B2 | 3 MeV, Au ions, 1 × 1014–1 × 1016 ions·cm−2, RT |
| Xia et al. [83] | Al0.1CoCrFeNi, Al0.75CoCrFeNi, Al1.5CoCrFeNi | FCC FCC + B2 B2 + A2 | 3 MeV, Au ions, 1 × 1014–1 × 1016 ions·cm−2, RT |
| Yang et al. [332] | Al0.1CoCrFeNi | FCC | 3 MeV, Au ions, 6 × 1015 ions·cm−2, 250–650 °C |
| Zhou et al. [333] | AlxCoCrFeNi (x = 0–2) | FCC + BCC | 1 MeV, Kr2+, -, RT |
| Zhou et al. [334] | AlxCoCrFeNi, HfNbTaTiZrV | FCC Amorphous | MeV Kr & 200 KeV, electrons, 2 dpa, RT & 150 °C |
| Zhou et al. [335] | HfNbTaTiZrV | BCC | 1 MeV Kr2+, -, RT-150 °C |
| Moschetti et al. [336] | HfNbTaTiZr | BCC | 5 MeV, He2+, 1.6 × 1012–4.4 × 1017 ions·cm−2s, 50 °C |
| Sadeghilaridjani et al. [337] | HfTaTiZrV | BCC | 4.4 MeV, Ni2+, 1.08 × 1017 ion·cm−2, RT |
| Li et al. [338] | HfNbTiZr | BCC | 1.5 MeV, He ions, 5 × 1015–1 × 1017 ions·m−2, 700 °C |
| Kareer et al. [339] | TaTiVZr, TaTiVCr, TaTiVNb | BCC BCC BCC | 2 MeV, V+, 2.26 × 1015 ions·cm−2, 500 °C |
| Wang et al. [340] | ZrTiHfCuBe, ZrTiHfCuBeNi, ZrTiHfCuNi | Amorphous | 100 keV, He ions, 5.0 × 1017, 1.0 × 1018 & 2.0 × 1018 ions·cm−2, RT |
| Lu et al. [80] | Ti2ZrHfV0.5Mo0.2 | BCC | 3 MeV, He+, 5 × 1015, 1 × 1016 & 3 × 1016 ions·cm−2, 600 °C |
| Atwani et al. [341] | W0.38Ta0.36Cr0.15V0.11 | BCC | 1 MeV, Kr+2, 0.0006–8 dpa·s−1, 800 °C |
| Komarov et al. [342] | (TiHfZrVNb)N | - | 500 KeV He2+, 5 × 1016–3 × 1017 ions·cm−2, 500 °C |
| Gandy et al. [343] | SiFeVCrMo SiFeVCr | sigma BCC+ sigma | 5 MeV, Au2+, 5 × 1015 ions·cm−2, RT |
| Patel et al. [344] | V2.5Cr1.2WMoCo0.04 | BCC | 5 MeV, Au+, 5 × 1015 ion·cm−2 (peak dose~42 dpa), RT |
| Zhang et al. [345] | Mo0.5NbTiVCr0.25, Mo0.5NbTiV0.5Zr0.25 | BCC | 400 He2+, 1 × 1017–5 × 1017 ions·m−2, 350 °C |
| Zhang et al. [346] | Mo0.5NbTiVCr0.25, Mo0.5NbTiV0.5Zr0.25 | BCC | 400 keV, He2+, peak dose~10.5 dpa, 350 °C |
| Atwani et al. [347] | WtaCrV | BCC | 2 keV, He+, 1.65 × 1017 ions·cm−2, 950 °C |
| Source | Composition | Microstructure | Method, Medium, Antagonist Material, Temperature, Wear Rate |
|---|---|---|---|
| Joseph et al. [355] | CoCrFeNiMn | FCC | Pin-on-disc, dry, Al2O3, 600–800 °C, RT, 0.5 × 10−4–3.8 × 10−4 mm3·N−1·m−1 |
| Wang et al. [356] | CoCrFeNiMn | FCC | Ball-on-disc, MoS2-oil lubrication, GCr15, RT-140 °C |
| Xiao et al. [357] | CoCrFeNiMn | FCC | Ball-on-flat, dry, WC-Co, RT, 0.5 × 10−4–5.4 × 10−4 mm3·N−1·m−1 |
| Jones et al. [358] | CoCrFeNiMn | FCC | Rotary tribometer, -, -, ~0.5 × 10−6 mm3·N−1·m−1 |
| Zhu et al. [359] | CoCrFeNiMn CoCrFeNiMnV CoCrFeNiMnNb CoCrFeNiMnNbV | FCC + HCP (Laves) + σ | Ball-on-disc, dry, Si3N4, RT, 1.85 × 10−5–6.39 × 10−5 mm3·N−1·m−1 |
| Deng et al. [360] | CoCrFeNiMox (x = 0–0.3) | FCC | Ball-on-disc, dry, GCr15, RT, 0.33 × 10−3–0.53 × 10−3 mm3·N−1·m−1 |
| Lindner et al. [361] | CoCrFeNiMn CoCrFeNi | FCC FCC | Ball-on-disc, dry, Al2O3, RT |
| Sha et al. [362] | (CoCrFeNiMn)N | FCC + BCC | Ball-on-disc, dry, ruby, RT, 1 × 10−7–1.4 × 10−6 mm3·N−1·m−1 |
| Xiao et al. [363] | CoCrFeNiMnCx (x = 0–1.2) | FCC | Ball-on-disk, dry, Si3N4, RT, 0.47 × 10−5–6.5 × 10−5 mm3·N−1·m−1 |
| Zhu et al. [277] | CoCrFeNiMn + TiN-Al2O3 | FCC + TiN | Ball-on-disc, dry, 440C steel, RT |
| Cheng et al. [364] | CoCrFeNiMn Al0.5CoCrFeNiMn AlCoCrFeNiMn | FCC FCC + BCC FCC + BCC | Ball-on-disc, dry, Si3N4, RT-800 °C, 0.5 × 10−4–3.8 × 10−4 mm3·N−1·m−1 |
| Joseph et al. [365] | CoCrFeNiMn Al0.3CoCrFeNi Al0.6CoCrFeNi AlCoCrFeNi | FCC FCC FCC + BCC BCC | Pin-on-disc, dry, Al2O3, 25 & 900 °C |
| Liu et al. [366] | CoCrFeNiMn + Y2O3 | FCC + Y2O3 (particles) | Ball-on-disc, dry, GCr15, RT |
| Wang et al. [367] | (CoCrFeMnNi)85Ti15 | FCC + BCC | Ball-on-disc, dry, Si3N4, RT-800 °C, 4 × 10−6–2.23 × 10−5 mm3·N−1·m−1 |
| Zhang et al. [368] | CoCrFeNi + (Ag or BaF2/CaF2) | FCC | Ball-on-disk, dry, Inconel-718, RT, ~4 × 10−5–40 × 10−5 mm3·N−1·m−1 |
| Geng et al. [369] | CoCrFeNi | FCC | Pin-on-disc, vacuum (4 Pa) & air, Inconel 718, RT, 0.6 × 10−4–8 × 10−4 mm3·N−1·m−1 |
| Zhang et al. [370] | CoCrFeNi + (graphite or MoS2) | FCC | Ball-on-disk, dry, Si3N4, RT-800 °C, ~1 × 10−5–23 × 10−5 mm3·N−1·m−1 |
| Zhou et al. [371] | CoCrFeNiMo0.85 Al0.5CoCrFeNi | FCC FCC | Slurry jet test-rig, HCl+NaCl, -, 40 °C, - |
| Zhang et al. [372] | CoCrFeNiMo | FCC | Ball-on-disc, dry, -, RT |
| Huang et al. [373] | FeCoCrNiSix | FCC + BCC | Ball-on-disk, dry, GCr15, RT |
| Cui et al. [374] | CoCrFeNiMo Sulfurized at 260 °C for 2 h | FCC + FeS/MoS2 film | Pin-on-disk, dry, GCr15, RT |
| Li et al. [375] | CoCrFeNiMo0.2 | FCC | Ball on disc, dry, GCr15, RT, 3.9 × 10−4–5.4 × 10−4 mm3·N−1·m−1 |
| Ji et al. [376] | CoCrFeNiCu + 2% MoS2 CoCrFeNiCu + 5% MoS2 CoCrFeNiCu + 20% WC CoCrFeNiCu + 50% WC CoCrFeNiCu + 80% WC | FCC + MoS2 (particles) FCC + MoS2 (particles) FCC + WC (particles) FCC + WC (particles) FCC + WC (particles) | Ball-on-disk, dry, Si3N4, RT |
| Verma et al. [377] | CoCrFeNiCux (x = 0–1) | FCC | Pin-on-disk, dry, -, RT & 600 °C, ~1.3 × 10−5–2.5 × 10−5 mm3·N−1·m−1 |
| Liu et al. [378] | CoCrFeNiBx (x = 0.5–1.5) | FCC + Borides | Roller friction wear tester, dry, W18Cr5V, RT |
| Jiang et al. [379] | CoCrFeNiNbx (x = 0–1.2) | FCC + HCP (Laves) HCP (Co2Nb) | Ball-on-disc, dry, BN, RT |
| Yu et al. [380] | CoCrFeNiNbx (x = 0.5–0.8) | FCC + HCP (Laves) | Pin-on-disk, dry, Si3N4, RT-800 °C, ~1.8 × 10−4–9 × 10−4 mm3·N−1·m−1 |
| Liu et al. [381] | Co10Cr10Fe50Mn30 + graphene nanoplatelets (0.2–0.8 wt%) | FCC | Ball-on-plate, dry, GCr15, RT |
| Wang et al. [382] | Co10Cr10Fe40Mn40 + WC (10 wt%) | FCC+ WC + M23C6 | Ball-on-disc, dry, Si3N4, RT |
| Derimow et al. [383] | (CoCrCuTi)100−xMnx (x = 5–10) (CoCrCuTi)100−xMnx (x = 10–20) | FCC + BCC FCC + HCP (Laves) | Ball-on-disc, dry, GCr15, RT |
| Guo et al. [384] | CoCrFeNiCuSi0.2 (Ti or C)x (x = 0–1.5) | FCC + TiC | Brooks sliding friction & wear tester, dry, RT |
| Zhang et al. [385] | (CoCrFeNiTi0.5)Cx (x = 3–12 wt%) | BCC + Cr23C6 + TiC | ML-100 friction and wear tester, -, -, RT |
| Erdoğan et al. [386] | CoCrFeNiTi0.5 CoCrFeNiTi0.5Al0.5 CoCrFeNiTi0.5Al | FCC BCC BCC | Ball-on-disc, dry, WC, RT |
| Liu et al. [387] | CoCrFeNiMo CoCrFeNiMox (x ≥ 0.3) CoCrFeNiMox (x ≥ 1) | FCC FCC + σ FCC + σ + µ | Pin-on-disk, dry, YG6, RT, 1 × 10−5–8.5 × 10−5 mm3·N−1·m−1 |
| Moazzen et al. [388] | CoCrFexNi (x = 1–1.6) | FCC + BCC | Pin-on-disk, dry, AISI52100 steel, 20–30 °C, - |
| Yang et al. [389] | CoCrFeNiMoSix (x = 0.5–1.5) | FCC | Pin-on-disk, dry, Si3N4, RT, 0.292 × 10−4–0.892 × 10−4 mm3·N−1·m−1 |
| Li et al. [390] | CoCrFeNi2V0.5Tix (x = 0.5–1.25) | BCC + (Co,Ni)Ti2 | Ball-on-disc, dry, Si3N4, RT, 4.4 × 10−5- 37.5 × 10−5 mm3·N−1·m−1 |
| Islak et al. [391] | CrFeNiMoTi | FCC | Ball-on-flat, dry, 100Cr6, RT, 2.7 × 10−3–9.4 × 10−3 mm3·N−1·m−1 |
| Wen et al. [392] | CrCoNiTiV | FCC + BCC + TiO | HT-1000 tribometer, -, WC, RT & 600 °C |
| Wang et al. [393] | CuNiSiTiZr | BCC | CJS111A wear tester, dry, -, RT |
| Cheng et al. [394] | (Fe25Co25Ni25 (B0.7Si0.3)25)100−xNbx (x = 0–4 wt%) | BCC + HCP (Laves) + FCC | Ball-on-disc, dry, GCr15, RT, ~1.5 × 10−6–3.6 × 10−6 mm3·N−1·m−1 |
| Yadav et al. [395] | (CuCrFeTiZn)1−xPbx (x = 0.05–0.2) | FCC + BCC + Pb (particles) | Ball-on-disk, dry, -, SAE 52100, RT, 1.17 × 10−5–50 × 10−5 mm3·N−1·m−1 |
| Gou et al. [396] | CoCrFeNi + WC + Mo2C + NbC | FCC | Ball-on-disc, dry, GCr15, 700 °C |
| Yadav et al. [397] | (CuCrFeTiZn)100−xPbx (x = 0–10) (CuCrFeTiZn)100−xBix (x = 0–10) | FCC + BCC BCC | Ball-on-disk, dry, steel, RT |
| Cui et al. [398] | AlxCoCrFeNiMn (x = 0–0.75) | FCC + BCC | MDW- 02 abrasive wear tester, RT |
| Gwalani et al. [399] | Al0.5CoCrFeNi | FCC + B2 | Pin-on-disc, dry, Si3N4, RT, 1.8 × 10−5–11 × 10−5 mm3·N−1·m−1 |
| Chen et al. [400] | Al0.6CoCrFeNi | FCC + BCC | Ball-on-plate, dry, Si3N4, RT-600 °C, ~0.5 × 10−4–5 × 10−4 mm3·N−1·m−1 |
| Du et al. [401] | Al0.25CoCrFeNi | FCC | Universal wear testing machine, dry, Si3N4 20–600 °C, ~1.5 × 10−4–3.5 × 10−4 mm3·N−1·m−1 |
| Chen et al. [402] | Al0.6CoCrFeNi | FCC + BCC | Ball-on-block, deionized water & acid rain (pH = 2), seawater, GCr15, RT, 1.58 × 10−4–6.52 × 10−4 mm3·N−1·m−1 |
| Ji et al. [403] | Al3CoCrFeNi | Jet erosion testing machine, water and 15 wt% SiO2 particles (350–600 mm), RT | |
| Haghdadi et al. [404] | Al0.3CoCrFeNi AlCoCrFeNi | FCC BCC | Scratch testing, dry, -, RT |
| Fang et al. [405] | Al0.3CoCrFeNi | FCC | Pin-on-disc, dry, -, 900 °C |
| Wu et al. [406] | Al0.1CoCrFeNi | FCC | Ball-on-block, dry and deionized water, Si3N4, RT, ~0.2 × 10−4–1.86 × 10−4 mm3·N−1·m−1 |
| Nair et al. [407] | Al0.1CoCrFeNi AlCoCrFeNi Al3CoCrFeNi | FCC FCC + BCC (B2) BCC (B2) + A2 + σ | Ball-on-disc, dry, WC, RT |
| Kumar et al. [408] | Al0.4CoxCrFeNi (x = 0–1) | - | Pin-on-disc, demineralized water & (demineralized water + 3.5 wt% NaCl), EN-31, RT, 0.81 × 10−4–1.86 × 10−4 mm3·N−1·m−1 |
| Mu et al. [409] | AlCoCrFeNi | BCC + FCC | Ball-on disc, dry, Si3N4, RT |
| Wu et al. [410] | AlCoCrFeNi AlCoCrFeNiTi0.5 | BCC | Pin-on-disc, dry, Si3N4, RT |
| Zhao et al. [411] | Al0.8CoCrFeNi | FCC + BCC | Ball-on-disk, dry, deionized water + 0.5 wt% NaCl, RT, ~2 × 10−5–7.5 × 10−5 mm3·N−1·m−1 |
| Kumar et al. [412] | Al0.4CoxCrFeNi (x = 0–0.5) Al0.4CoxCrFeNi (x = 1) | FCC + BCC FCC | Pin-on-disk, engine oil (SAE Grade:20W-40), EN-31 steel, RT, 2.1 × 10−5–11 × 10−5 mm3·N−1·m−1 |
| Li et al. [413] | Al0.8CoCrFeNiCu0.5Six (x = 0–0.5) | FCC + BCC1 + BCC2 | -, -, CGr15, RT, 0.9 × 10−6–1.19 × 10−6 mm3·N−1·m−1 |
| Li et al. [272] | (AlCoCrFeNi)100-x (NbC)x (x = 0–30 wt%) | FCC + BCC | Reciprocating tester, dry, N4Si3, RT |
| Kafexhiu et al. [414] | AlCoCrFeNi2.1 | BCC + FCC | Ball-on-plate, dry, 100Cr6 steel, RT, 7 × 10−5–11 × 10−5 mm3·N−1·m−1 |
| Miao et al. [415] | AlCoCrFeNi2.1 | FCC (L12) + BCC (B2) | Ball-on-disk, dry, Al2O3/Si3N4/SiC/GCr15, RT-900 °C, ~1 × 10−4–4.2 × 10−4 mm3·N−1·m−1 |
| Ye et al. [416] | AlCoCrFeNi2.1 + TiC (0–15 wt%) | FCC + B2 + TiC | MM-200 wear testing machine, dry, -, RT |
| Wang et al. [417] | (AlCoCrFeNi)N | BCC + nitrides (AlN,CrN,Fe4N) | Ball-on block, dry, deionized water & acid rain (pH = 2), Si3N4, RT, 2.8 × 10−5–7 × 10−5 mm3·N−1·m−1 |
| Liu et al. [418] | AlCrCuFeNi2 | Ball-on-block, dry, simulated rainwater & deionized water, Si3N4, RT, 2.163 × 10−3–0.23 × 10−3 mm3·N−1·m−1 | |
| Kong et al. [419] | Al1.8CrCuFeNi2 | BCC | MMS-2A roller friction wear tester, dry, -, RT |
| Malatji et al. [263] | AlCrCuFeNi | FCC + BCC | Ball-on-disk, dry, SiC, RT |
| Wang et al. [420] | Al1.3CoCuFeNi2 | FCC + BCC | Ball-on block, dry, deionized water & acid rain (pH = 2), Si3N4, RT, 1 × 10−4–12 × 10−4 mm3·N−1·m−1 |
| Xiao et al. [421] | AlxCoCrFeNiSi (x = 0.5–1.5) | FCC + BCC | Ball-on-flat, distilled water, WC-12Co, RT, 6.7 × 10−6–5.5 × 10−5 mm3·N−1·m−1 |
| Liu et al. [422] | AlCoCrFeNiSix (0–0.5) | BCC | Pin-on-disk, dry, ZrO2, RT, 1.3 × 10−4–5.1 × 10−4 mm3·N−1·m−1 |
| Hsu et al. [423] | Al0.5CoCrFeNiCuBx (x = 0–1) | FCC + boride precipitates | Pin-on-disk, dry, Al2O3, RT |
| Chen et al. [424] | Al0.5CoCrFeNiCuTix (x = 0–0.2) Al0.5CoCrFeNiCuTix (x = 0.4–1) Al0.5CoCrFeNiCuTix (x = 1.2–2) | FCC FCC + BCC FCC + BCC + Ti2N | Pin-on-disk, dry, Al2O3, RT |
| Lobel et al. [425] | AlCoCrFeNiTi | BCC | Ball-on-disc, dry, Al2O3, RT |
| Lobel et al. [426] | AlCoCrFeNiTi | BCC | Ball-on-plate, dry, 100Cr6 Steel, RT |
| Wu et al. [427] | AlCoCrFeNiTix (x = 0.5–1) AlCoCrFeNiTix (x = 1.5) AlCoCrFeNiTix (x = 2) | FCC + BCC FCC + BCC + Ti2Ni FCC + BCC + Ti2Ni + ordered BCC | Cavitation erosion tests, Distilled water+ 3.5 wt% NaCl, RT |
| Erdogan et al. [428] | AlxCoCrFeNiTiy (x = 0–0.5, y = 0–0.5) | FCC + BCC | Ball-on-disc, dry, WC, RT, 0.25 × 10−4–1.78 × 10−4 mm3·N−1·m−1, 0.25 × 10−4–1.78 × 10−4 mm3·N−1·m−1 |
| Xin et al. [429] | Al0.2Co1.5CrFeNi1.5Ti0.5 + TiC | FCC | Ball-on-disc, dry, Si3N4, RT, 0.3 × 10−5–12.6 × 10−5 mm3·N−1·m−1 |
| Gouvea et al. [430] | Al0.2Co1.5CrFeNi1.5Ti | FCC | Ball-on-plate, dry, AISI 52,100 steel, RT, 1.6 × 10−8–7.5 × 10−5 mm2·N−1 |
| Chuang et al. [431] | AlxCo1.5CrFeNi1.5Tiy (x = 0–0.2, y = 0.5–1) | FCC | Pin-on-disk, dry, SKH51 steel, RT, ~4 × 10−4–1.8 × 10−4 mm3·N−1·m−1 |
| Liu et al. [432] | AlCoCrFeNiTi0.8 | BCC + B2 | Ball-on-disc, dry, Si3N4, RT, 1.36 × 10−6–6.96 × 10−6 mm3·N−1·m−1, 0.7 × 10−4–6 × 10−4 mm3·N−1·m−1 |
| Yu et al. [433] | AlCoCrFeNiTi0.5 | BCC1 + BCC2 | Pin-on-disk, H2O2, SiC & ZrO2, RT |
| Lobel et al. [434] | AlCoCrFeNiTi0.5 | BCC (A2 + B2) | SRV-Tribometer, dry, Al2O3, 22–900 °C |
| Chen et al. [435] | Al0.6CoCrFeNiTi | BCC | Pin-on-disc, Dry, Al2O3 RT-500 °C |
| Yu et al. [436] | AlCoCrFeNiTi0.5 AlCoCrFeNiCu | Pin-on-disc, dry, Si3N4 | |
| Yu et al. [437] | AlCoCrFeNiCu AlCoCrFeNiTi0.5 | FCC + BCC1 BCC1 + BCC2 | Pin-on-disk, H2O2, 1Cr18Ni9Ti steel & ZrO2/SiC ceramic, RT |
| Jin et al. [438] | AlCoFeNiCu | FCC + BCC | Ball-on-disk, dry, WC, 200–800 °C |
| Zhu et al. [439] | AlCoFeNiCu + TiC (10–30 wt%) | FCC + BCC | Ball-on-disk, dry, Si3N420–600 °C, ~0.1 × 10−5–6.5 × 10−5 mm3·N−1·m−1 |
| Wu et al. [440] | Al0.5CoCrFeNiCu Al1.0CoCrFeNiCu Al2.0CoCrFeNiCu | FCC FCC + BCC BCC | Pin-on-disk, dry, SKH-51 steel, RT |
| Yan et al. [441] | AlCoCrFeNiSi + Ti (C, N) | BCC + FCC | Ball-on-disc, dry, GCr15, RT, - |
| Li et al. [442] | AlCoCrFeNi + Ti (C,N) + TiB2 | FCC | Ball-on-disc, dry, WC-6Co, 200–800 °C, 2.69 × 10−5–8.66 × 0−5 mm3·N−1·m−1 |
| Kumar et al. [443] | AlCoCrCuFeNiSi0.3 AlCoCrCuFeNiSi0.6 | FCC + BCC FCC + BCC + σ | Pin-on-disk, dry, -, RT, - |
| Xin et al. [444] | Al0.2Co1.5CrFeNi1.5Ti0.5 | FCC | Pin-on-disk, dry, Si3N4, 25–800 °C, 1.21 × 10−5–6.7 × 10−5 mm3·N−1·m−1 |
| Karakaş et al. [445] | Al0.07Co1.26Cr1.80Fe1.42Mn1.35Ni1.1 | FCC | Ball-on-disc, 3.5%NaCl & 5%H2SO4, -, RT, 16.26 × 10−9–77.84 × 10−8 mm3·N−1·m−1 |
| Xin et al. [446] | Al0.2Co1.5CrFeNi1.5Ti (0.5+x) + Cx (x = 0) | FCC | Pin-on-disk, dry, Si3N4, 25–800 °C, 3.12 × 10−6–12.59 × 10−5 mm3·N−1·m−1 |
| Zhao et al. [447] | AlCrCoFeNiCTax (x = 0–1) | BCC | Pin-on-disk, 3.5%NaCl & air, Si3N4, RT, 1.67 × 10−6–2.22 × 10−5 mm3·N−1·m−1 |
| Ghanbariha et al. [448] | AlCoCrFeNi + ZrO2 | FCC + BCC | Pin-on-disk, dry, WC, RT, 1.11 × 10−3–2.52 × 10−3 mm3·N−1·m−1 |
| Li et al. [449] | AlxCrFeCoNiCu (x = 0–0.5) AlxCrFeCoNiCu (x = 0.5–2) | FCC FCC + BCC | -, dry, GCr15, RT, 6.64 × 10−7–2.26 × 10−4 mm3·N−1·m−1 |
| Cai et al. [450] | AlCrTiV, AlCrTiVSi | BCC | Nanoindenter G200, dry, CGr15 &Al2O3, RT, - |
| Chandrakar et al. [451] | AlCoCrCuFeNiSix (x = 0–0.9) | BCC | Pin-on-disk, dry, -, RT, - |
| Erdogan et al. [452] | AlCrFeNiSi AlCrFeNix (x = Cu,Co) | BCC BCC + FCC | Ball-on-disc, dry, WC, RT, - |
| Duan et al. [453] | AlCoCrFeNiCu | - | Pin-on-disc, H2O2, Si3N4, RT |
| Chen et al. [454] | Al0.5CoCrFeNiCuVx (x = 0–0.2) Al0.5CoCrFeNiCuVx (x = 0.4–0.8) Al0.5CoCrFeNiCuVx (x = 1–2) | FCC FCC + BCC BCC | Pin-on-disk, dry, Al2O3, RT, 1 × 10−4–2.7 × 10−4 mm3·N−1·m−1 |
| Gu et al. [455] | AlxMo0.5NbFeTiMn2 (x = 1–2) | BCC | Pin-on-disk, dry, Al2O3, RT |
| Hsu et al. [456] | AlCoCrFexNiMo0.5 (x = 0.6–2) | BCC + σ | Pin-on-disk, dry, SKH51 steel, RT |
| Liang et al. [457] | AlCrFe2Ni2W0.2Mo0.75 | BCC | Ball-on-disc, deionized water, Al2O3, RT, ~5 × 10−6–22 × 10−6 mm3·N−1·m−1 |
| Qui et al. [458] | Al2CoCrFeCuTiNix (x = 0–2) | FCC + BCC | Tribometer, -, -, RT |
| Kanyane et al. [459] | AlTiSiMoW | BCC + TiSi2 (ordered FCC) | Ball-on-disc, dry, stainless steel, RT |
| Huang et al. [460] | AlTiSiVCr | BCC+ (Ti,V)5Si3 precipitates | Ball-on-disc, dry, GCr15 steel, RT, 2 × 10−5–2.5 × 10−5 mm3·N−1·m−1 |
| Zhang et al. [461] | AlTiSiVNi | B2 (NiAl) + (Ti,V)5Si3 + TiN | Ball-on-disc, dry, Si3N4, RT & 800 °C |
| Lin et al. [462] | AlCoCrNiW AlCoCrNiSi | W + AlNi + Cr15.58Fe7.42C6 BCC | Pin-on-disc, dry, AISI 52100, RT |
| Yadav et al. [463] | AlCrFeMnV (AlCrFeMnV)90Bi10 (AlCrFeMnV)90Bi10 + 10 wt% TiB2 (AlCrFeMnV)90Bi10 + 15 wt% TiB2 | BCC BCC + AlV3 + Bi BCC + AlV3 + Bi + TiB2 BCC + AlV3 + Bi + TiB2 | Ball-on-disk, dry, SAE 52,100 steel, RT, 1.02 × 10−5–7.02 × 10−5 mm3·N−1·m−1 |
| Bhardwaj et al. [464] | AlTiZrNbHf | BCC | Pin-on-disk, dry, CGr15, RT, - |
| Zhao et al. [465] | AlNbTaZrx (x = 0.2–1) | BCC + HCP | Ball-on-disc, dry, Si3N4, RT, 1.85 × 10−4–2.41 × 10−4 mm3·N−1·m−1 |
| Tuten et al. [466] | TiZrHfNbTa | Amorphous | Ball-on-disc, dry, Al2O3, RT |
| Pole et al. [467] | TiZrHfTaV, TiZrTaVW | BCC | Ball-on-disk, dry, Si3N4, RT-500 °C, ~1 × 10−4–8 × 10−4 mm3·N−1·m−1 |
| Ye et al. [468] | TiZrHfNb | BCC | Nano-scratch, dry, diamond indenter, RT |
| Pogrebnjak et al. [469] | (TiZrHfNbV)N | FCC | Ball-on-disc, dry, Al2O3, 20 °C |
| Gong et al. [470] | TiZrHfBeCu TiZrHfBeNi Ti20Zr20Hf20Be20Cu10Ni10 Ti13.8Zr41.2Ni10Be22.5Cu12.5 | Amorphous | Nano-scratch, dry, diamond indenter, RT |
| Zhao et al. [471] | TiZrNiBeCu | Amorphous | Nano-scratch, dry, diamond indenter, RT |
| Jhong et al. [472] | (TiZrNbCrSi)Cx (x = 36.7–87.8 at.%) | FCC | Ball-on-disc, dry, 100Cr6 steel, RT, 0.2 × 10–3.3 × 10−6 mm3·N−1·m−1 |
| Mathiou et al. [473] | TiZrNbMoTa | BCC + HCP | Ball-on disc, dry, 100Cr6 steel, Al2O3, RT, 0.154 × 10−1–0.199 × 10−1 mm3·N−1·m−1 |
| Petroglou et al. [474] | MoTaxNbVTi (x = 0.25–1) | BCC | Ball-on-disk, dry, 100Cr6 steel, RT, 0.19 × 10−6–0.38 × 10−6 g·N−1·m−1 |
| Poulia et al. [475] | MoTaNbVW | BCC | Ball-on-disc, dry, 100Cr6 steel & Al2O3, RT |
| Poulia et al. [476] | MoTaNbVW | BCC | Ball-on-disc, dry, 100Cr6 steel & Al2O3, RT, 1.05 × 10−4–4.89 × 10−4 mm3·N−1·m−1 |
| Poulia et al. [477] | MoTaNbVTi | BCC + hexagonal C14 Laves + cubic C15 laves | Ball-on disc, dry, 100Cr6 steel, Al2O3, RT |
| Alvi et al. [478] | MoTaWVCu | BCC | Ball-on-disc, dry, E52100 steel & Si3N4, RT-600 °C, 2.3 × 10−2–5 × 10−2 mm3·N−1·m−1 |
| Hua et al. [479] | TixZrNbTaMo (x = 0.5–2) | BCC | HSR-2M tester, dry, Si3N4, RT, 2.22 × 10−7–2.42 × 10−7 mm3·N−1·m−1 |
| Gu et al. [480] | Ni1.5CrFeTi2.0.5Mox (x = 0–0.25) Ni1.5CrFeTi2.0.5Mox (x = 0.5–0.25) | BCC BCC + FCC | Ball-on-disc, dry, Al2O3, RT, 7.99 × 107–2.7 × 107 µm3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonal, S.; Lee, J. Recent Advances in Additive Manufacturing of High Entropy Alloys and Their Nuclear and Wear-Resistant Applications. Metals 2021, 11, 1980. https://doi.org/10.3390/met11121980
Sonal S, Lee J. Recent Advances in Additive Manufacturing of High Entropy Alloys and Their Nuclear and Wear-Resistant Applications. Metals. 2021; 11(12):1980. https://doi.org/10.3390/met11121980
Chicago/Turabian StyleSonal, Sonal, and Jonghyun Lee. 2021. "Recent Advances in Additive Manufacturing of High Entropy Alloys and Their Nuclear and Wear-Resistant Applications" Metals 11, no. 12: 1980. https://doi.org/10.3390/met11121980
APA StyleSonal, S., & Lee, J. (2021). Recent Advances in Additive Manufacturing of High Entropy Alloys and Their Nuclear and Wear-Resistant Applications. Metals, 11(12), 1980. https://doi.org/10.3390/met11121980





