Origin of the Inclusions in Production-Scale Electrodes, ESR Ingots, and PESR Ingots in a Martensitic Stainless Steel
Abstract
1. Introduction
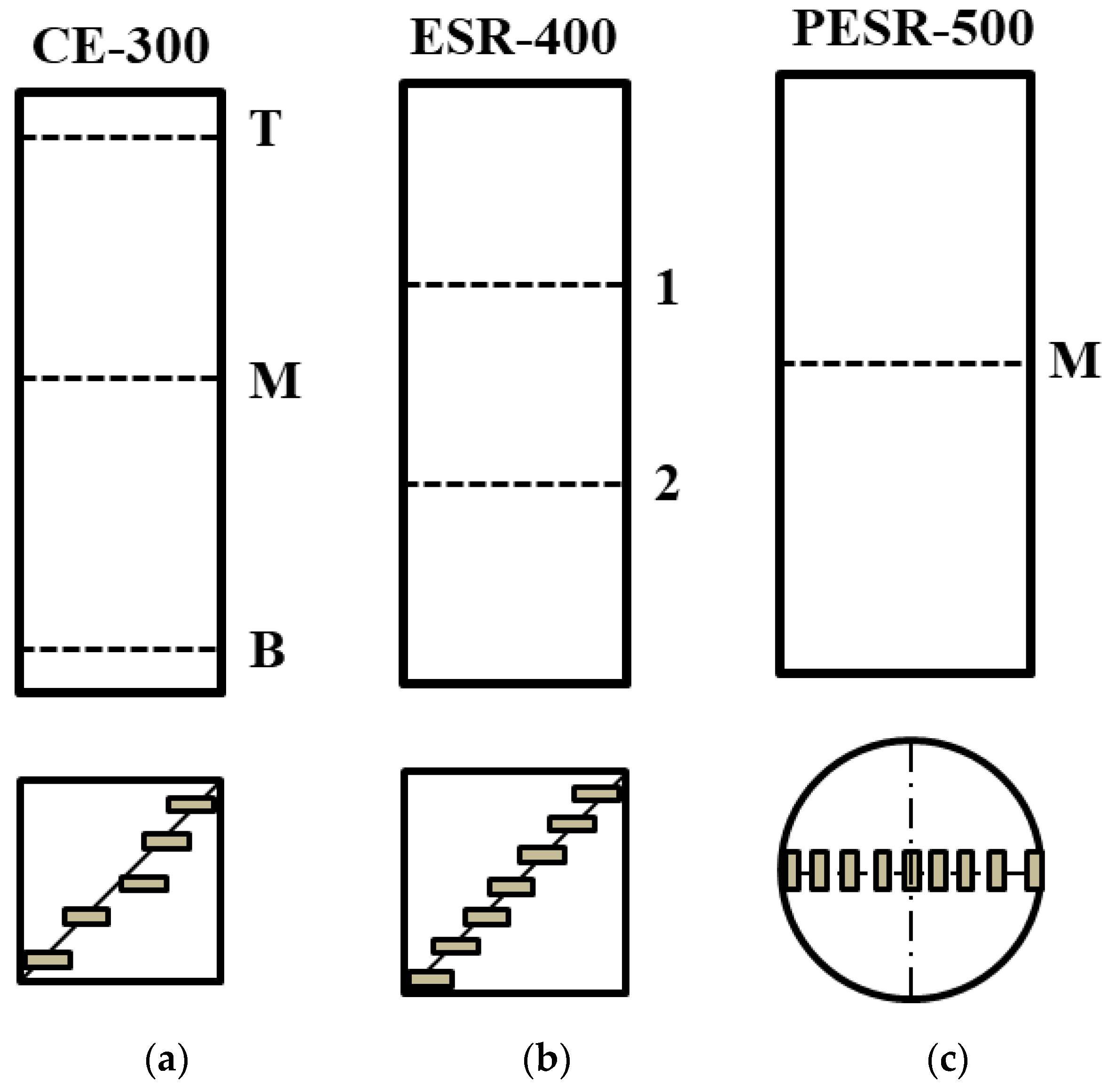
2. Materials and Methods
3. Results
4. Discussion
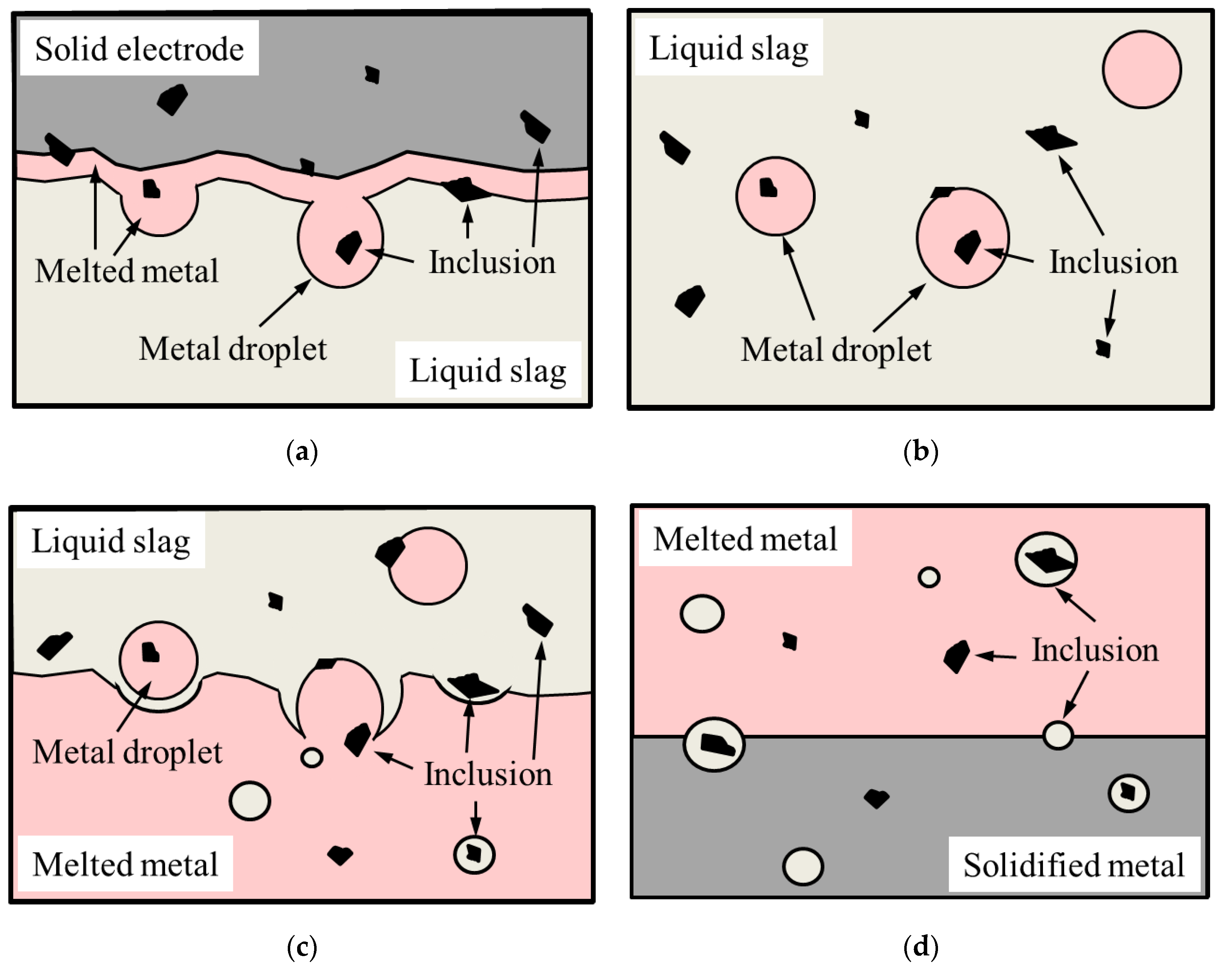
4.1. Contrast between a Conventional Cast Electrode and Remelted Ingots
4.2. Difference between ESR and PESR Remelted Ingots
5. Conclusions
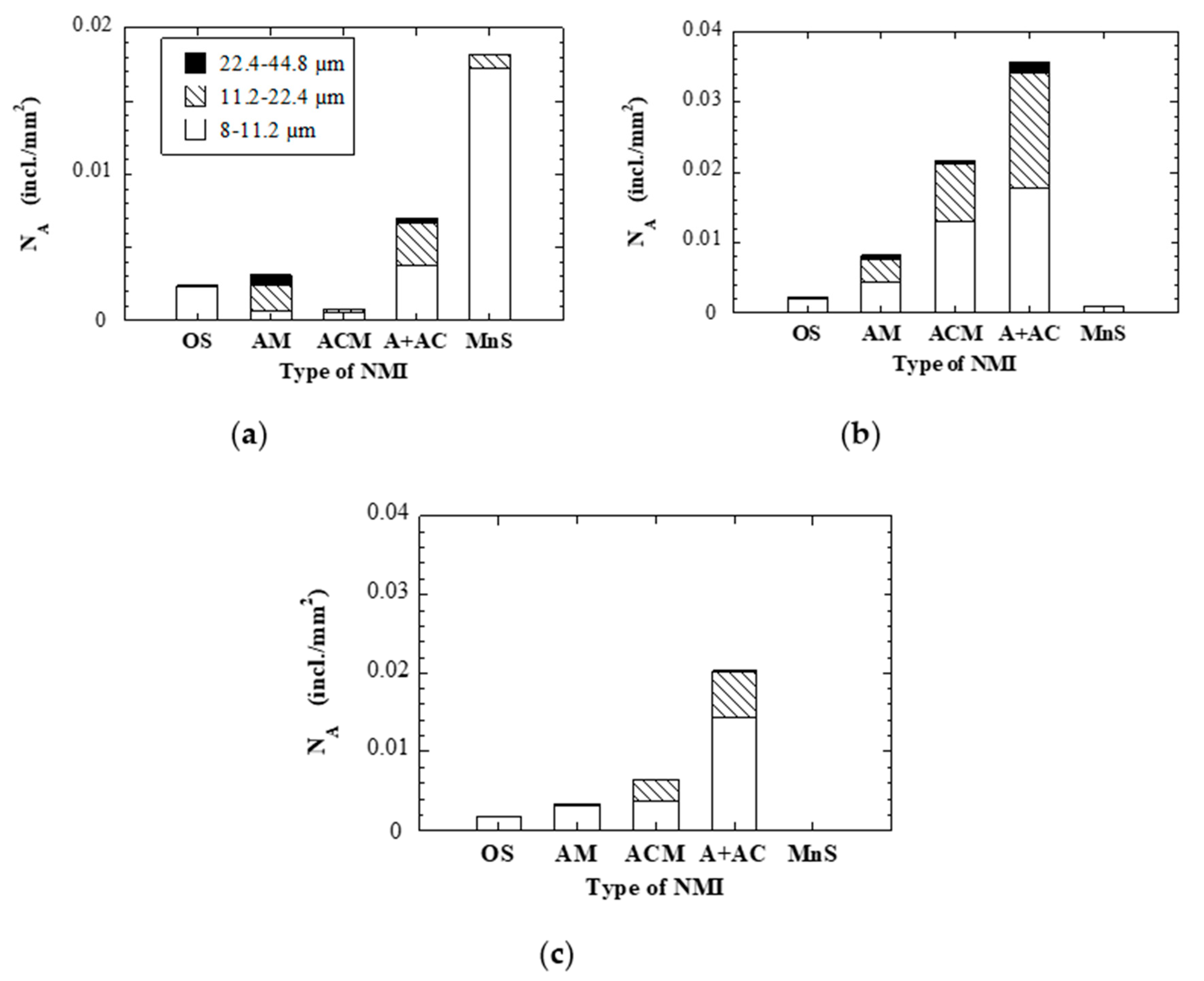
- Both larger inclusions (up to 44.8 µm) and an approximatively two times higher number of oxide inclusions per area were found in the ESR ingot compared to the PESR ingot. This is believed to be caused by the remelting under air atmosphere, the Al deoxidation, the lower filling ratio between the electrode and the ingot, and an unintentional uneven melting during the electrode changes.
- According to the recent classification of inclusions, primary, semi-secondary and secondary inclusions exist in the remelted ingots as explained below.
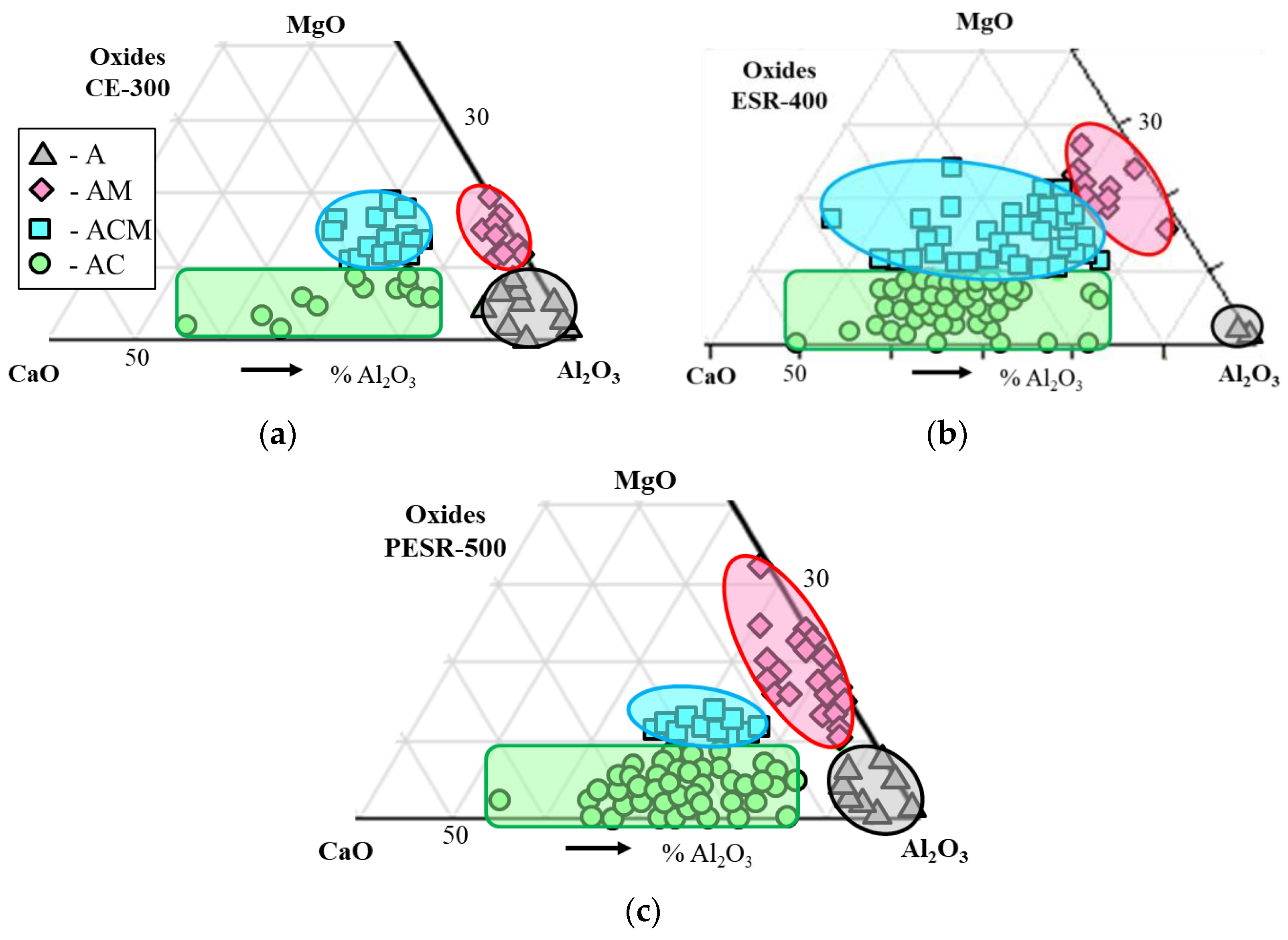
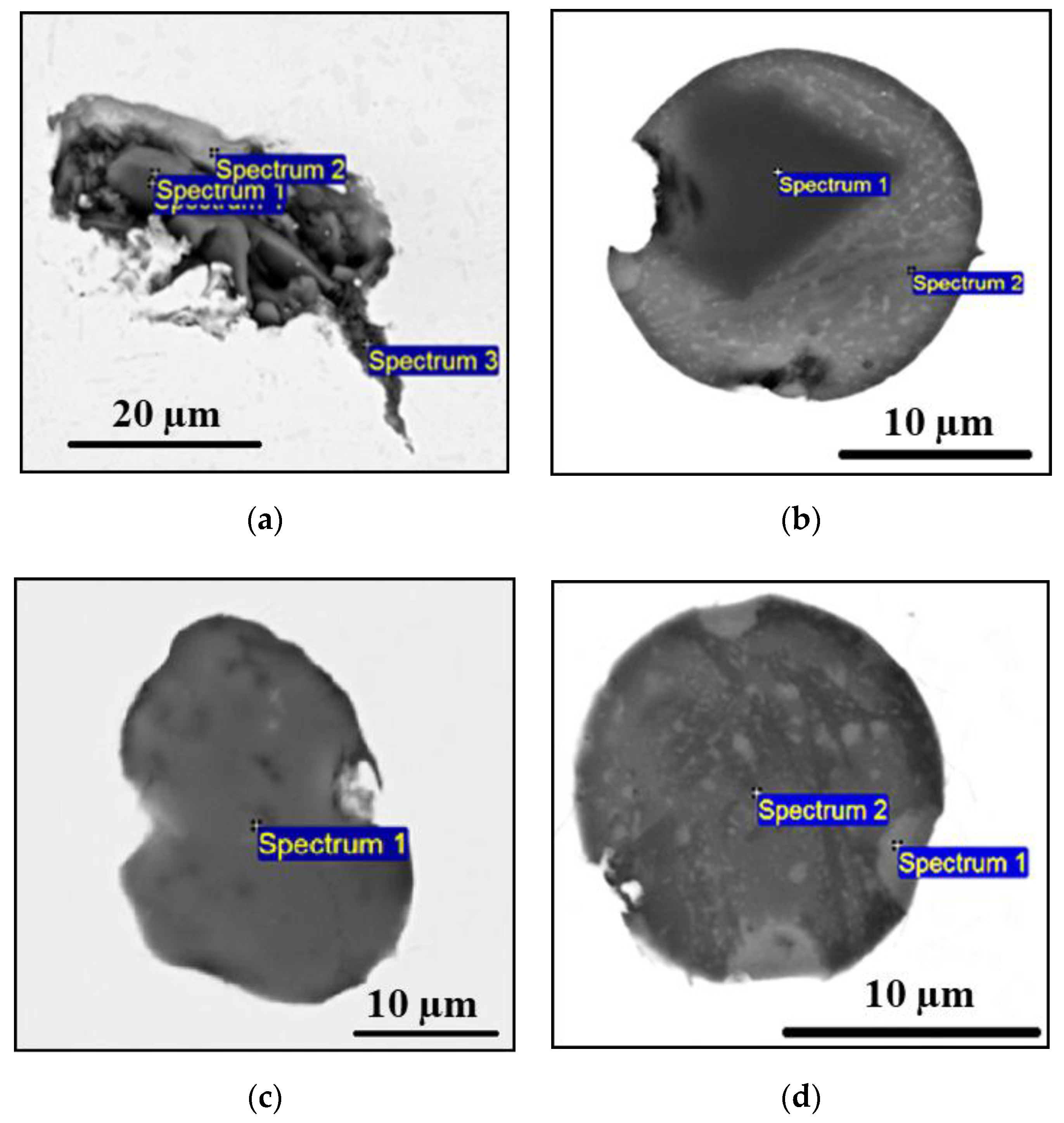
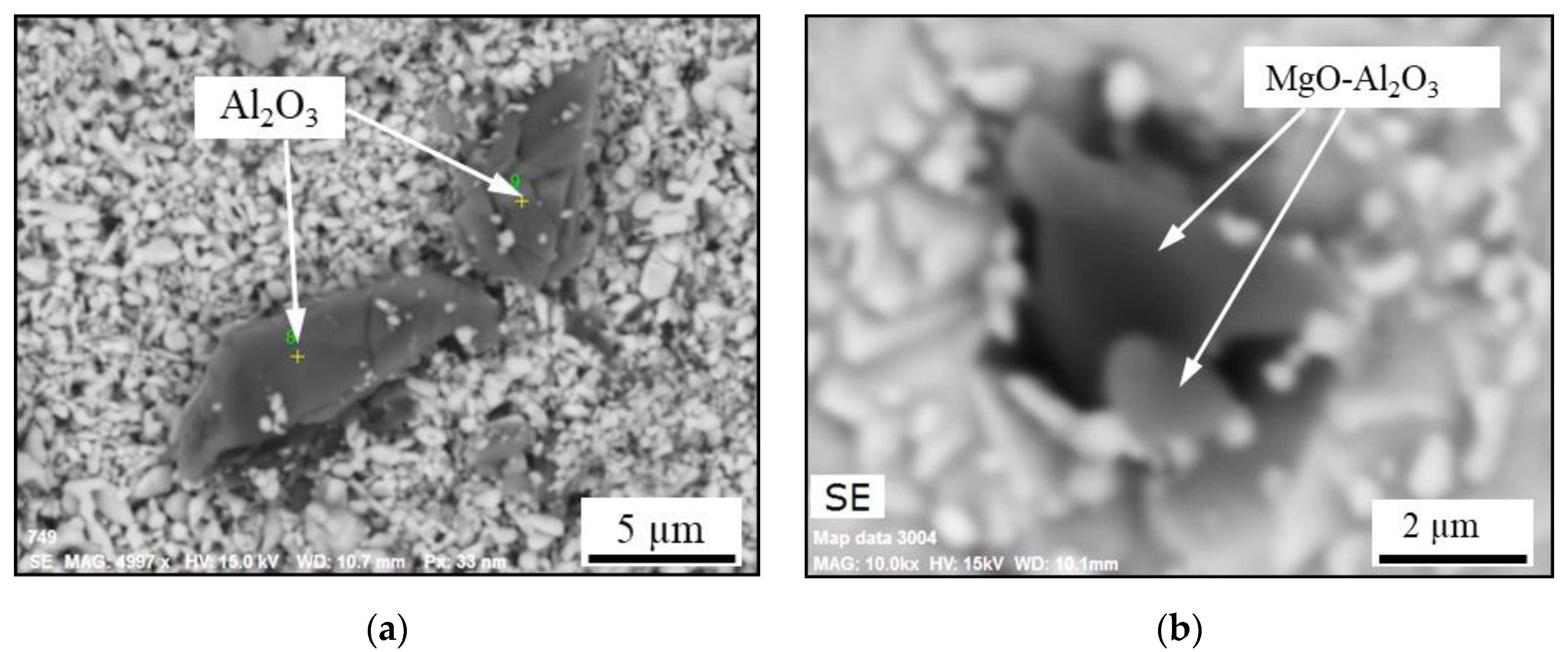
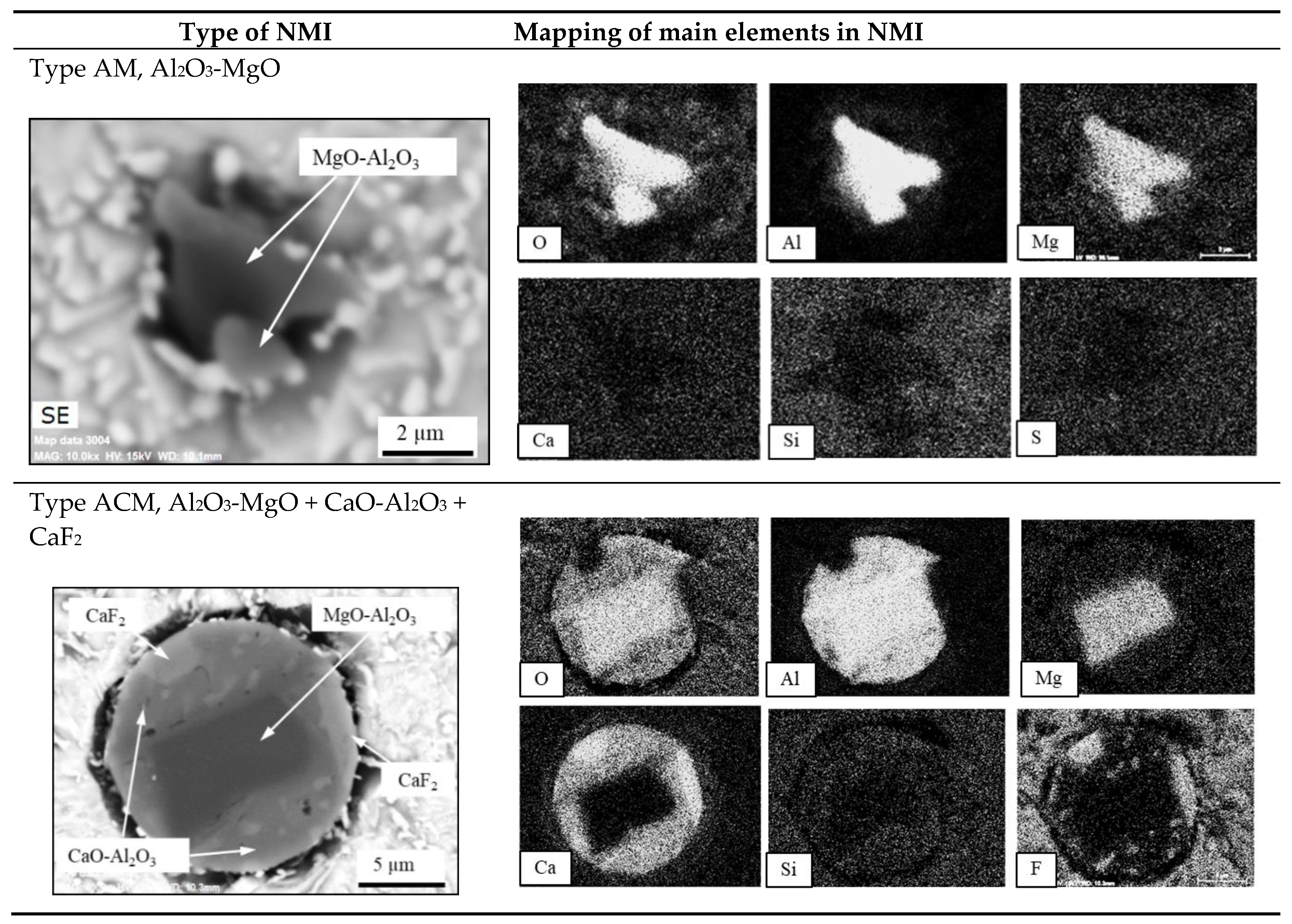
- The primary inclusions (such as Al2O3-MgO oxides, Type AM) are assumed to have survived from the electrode because they were trapped inside a steel drop or a fallen steel fragment, without having contact with the ESR/PESR process slag.
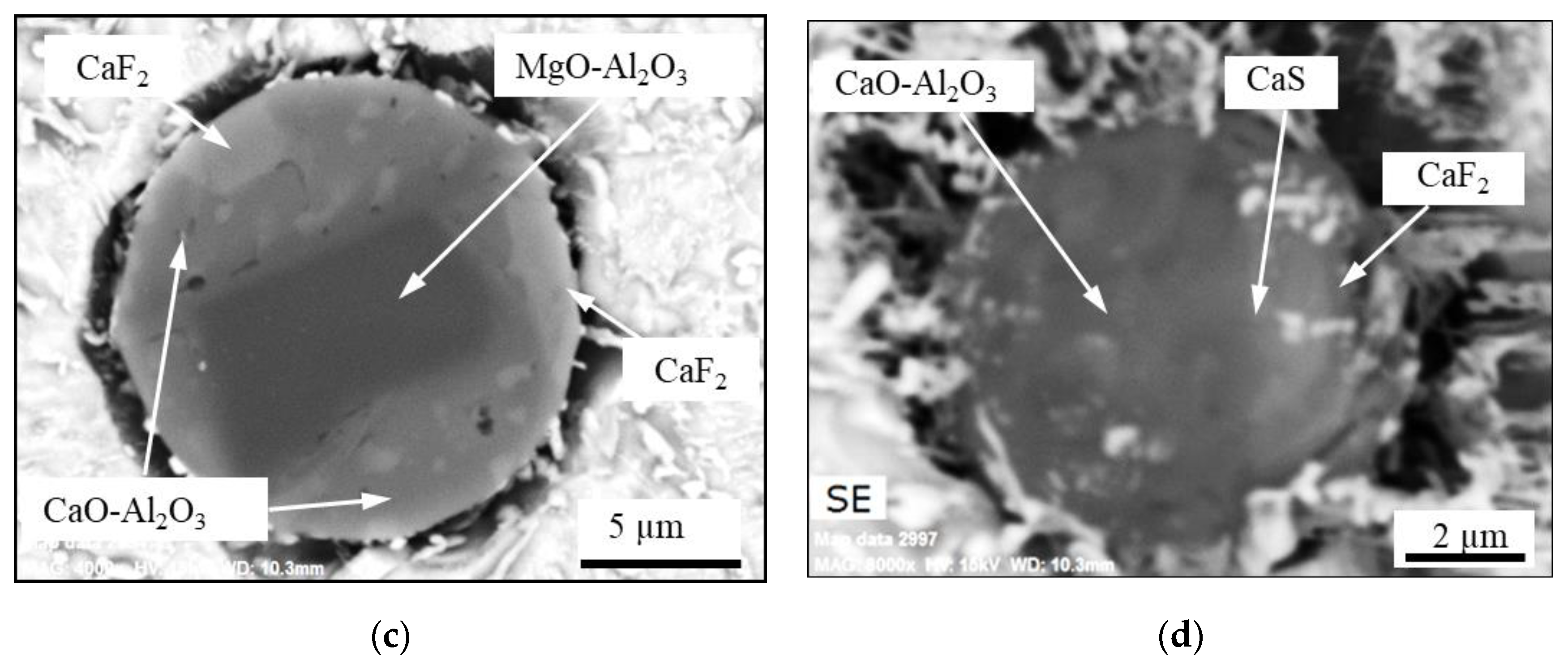
- The most common inclusion type in the remelted ingots has a core of MgO_Al2O3 with an outer layer that corresponds to their corresponding process slag (Type ACM). However, inclusions containing MgO are characterized by having an exogenous origin, where the main sources of MgO are refractories or the furnace or ladle slags. Due to the low solubility of Mg in iron and the high melting point of MgO, it is more likely that at least the majority of the Mg spinel inclusions in the remelted ingots are primary inclusions, which survived from the electrode. This kind of inclusion is denoted as semi-secondary inclusions.
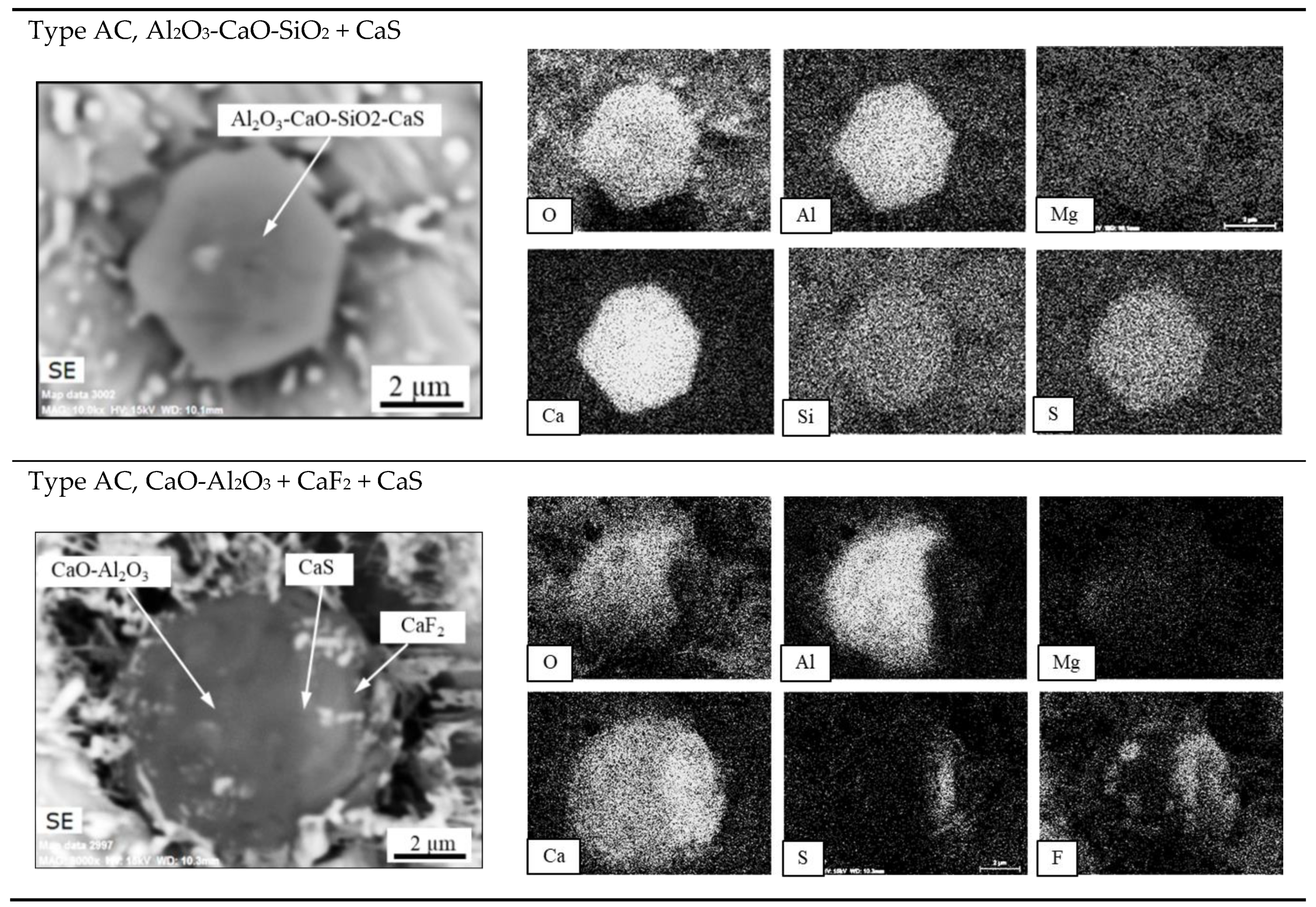
- The secondary inclusions are assumed to be formed in the liquid steel pool as a result of the reactions between alloying elements and the dissolved oxygen.
- According to the literature, the inclusions after a calcium treatment modification, (used to modify MgO-Al2O3 to softer inclusions with a lower melting point of the outer layer of inclusions) are very similar to the inclusions found in the ESR/PESR ingots. This observation indicates that the ESR process slag acts in some way like a calcium treatment modification. This hypothesis is supported by the observed appearance of the inclusions described above.
- The results presented above correspond to the results from previous trials, made using a tracer (La2O3) in the ESR process slag, which indicates that the most probable inclusion to survive from the electrode is a Mg-spinel.
- On the whole, we find that the overall cleanliness of the electrode (and also the composition of the inclusions in the electrode) has a direct bearing on the inclusion content of the ESR and PESR ingots.
Author Contributions
Funding
Conflicts of Interest
References
- Kay, D.A.R.; Pomfret, R.J. Removal of inclusions during AC Electroslag remelting. J. Iron Steel Inst. 1971, 209, 962–965. [Google Scholar]
- Li, Z.B.; Zhou, W.H.; Li, Y.D. Mechanism of removal on non-metallic inclusions in the ESR process. Iron Steel 1980, 15, 20–26. [Google Scholar]
- Shi, C.; Chen, X.-C.; Guo, H.-J. Oxygen control and its effect on steel cleanliness during Electroslag remelting of NAK80 die steel. In Proceedings of the Iron and Steel Conference and Exposition AISTech 2012, Atlanta, GA, USA, 7–10 May 2012; pp. 947–957. [Google Scholar]
- Shi, C.; Chen, X.-C.; Guo, H.-J.; Zhu, Z.-J.; Ren, H. Assessment of oxygen control and its effect on inclusion characteristics during ESR remelting of die steel. Steel Res. Int. 2012, 83, 472–486. [Google Scholar] [CrossRef]
- Shi, C.; Chen, X.-C.; Guo, H.-J. Characteristics of inclusions in high-Al steel during electroslag remelting process. Int. J. Miner. Mater. 2012, 19, 295–302. [Google Scholar] [CrossRef]
- Chen, X.C.; Shi, C.B.; Guo, H.J.; Wang, F.; Ren, H.; Feng, D. Investigations of oxide inclusions and primary carbon nitrides in Inconel 718 Superalloy refined through electroslag remelting process. Metall. Mater. Trans. B 2012, 43, 1596–1607. [Google Scholar] [CrossRef]
- Burel, B.C. A Study of Inclusion Behavior during Electroslag Remelting. Ph.D. Thesis, Department of Metallurgy, University of British Columbia, Vancouver, BC, Canada, 1969. [Google Scholar]
- Mitchell, A. Oxide inclusion behavior during consumable electrode remelting. Ironmak. Steelmak. 1974, 1, 172–179. [Google Scholar]
- Chan, J.C.F.; Miller, J.W.G.D.; Cameron, J. The Re-solution of Inclusions in Remelted Stainless Steels. Metall. Mater. Trans. B 1976, 7B, 135–141. [Google Scholar] [CrossRef]
- Shi, C.; Chen, X.-C.; Luo, Y.-W.; Guo, H.-J. Theory analysis of steel cleanliness control during electroslag remelting. In Materials Processing Fundamentals; TMS: Pittsburgh, PA, USA, 2013. [Google Scholar]
- Dong, Y.; Jiang, Z.; Cao, Y.; Fan, J.; Yu, A.; Liu, F. Effect on fluoride containing slag on oxide inclusions in electroslag ingot. In Proceedings of the Liquid Metal Processing & Casting Conference, Austin, TX, USA, 22–25 September 2013. [Google Scholar]
- Dong, Y.-W.; Jiang, Z.-H.; Cao, Y.-L.; Hou, D. Effect of slag on Inclusions during electroslag remelting process of die steel. Metall. Mater. Trans. B 2014, 45B, 1315–1324. [Google Scholar] [CrossRef]
- Shi, C.; Chen, X.-C.; Guo, H.-J.; Sun, X.L.; Zhu, Z.-J. Control of MgO·Al2O3 spinel inclusions during protective gas electroslag remelting of die steel. Metall. Mater. Trans. B 2013, 44B, 378–389. [Google Scholar] [CrossRef]
- Schneider, R.; Schuler, C.; Wurstinger, P.; Reiter, G.; Martinez, C. Einfluss eines höheren SiO2-Gehaltes in der Schlacke beim Umsmschmelzen eines Varmarbeitsstahles auf ESU-prozess. Berg. Und Hüttenmännische 2015, 160, 117–122. (In Germany) [Google Scholar] [CrossRef]
- Shi, C.; Zhu, Q.-t.; Yu, W.-t.; Song, H.-d.; Li, J. Effect on oxide inclusions modification during electroslag remelting on primary carbides and toughness of a high-carbon 17% Cr tool steel. J. Mater. Eng. Perform. 2016, 25, 4785–4795. [Google Scholar] [CrossRef]
- Du, G.; Li, J.; Wang, Z.-B. Effect on operating conditions on inclusion of Die Steel duringElectroslag remelting. ISIJ Int. 2017, 29, 1–10. [Google Scholar]
- Chang, L.Z.; Shi, X.F.; Cong, J.Q. Study on mechanism of oxygen increase and countermeasure to control oxygen content during electroslag remelting process. Ironmak. Steelmak. 2014, 41, 182–186. [Google Scholar] [CrossRef]
- Shi, C.; Park, J.H. Evolution of Oxide inclusions in Si-killed Steel during Protective Atmosphere Electroslag remelting. Metall. Mater. Tans B 2019, 50B, 1139–1147. [Google Scholar] [CrossRef]
- Reiter, G.; Schuetzenhoefer, W.; Tazreiter, A.; Martinez, C.; Wurstinger, P.; Loecker, C. The incluence of different melting and remelting routes in the cleanliness of high alloyed steels. In Proceedings of the Liquid Metal Processing & Casting conference LMPC 2013, Austin, TX, USA, 22–25 September 2013. [Google Scholar]
- Persson, E.; Mitchell, A.; Fredriksson, H. The behavior of Inclusiond during ESR remelting. In Proceedings of the 2nd Ingot Casting Rolling Forging Conference ICRF 2014, Milano, Italy, 7–9 May 2014. [Google Scholar]
- Persson, E.S.; Fredriksson, H.; Mitchell, A. Differences in inclusion morphology between ESR remelted and ingot casted common martensitic steel. In Proceedings of the 5th International Conference on Process Development in Iron and Steelmaking Scanmet-V 2016, Luleå, Sweden, 12–15 June 2016. [Google Scholar]
- Wang, H.; Li, J.; Shi, C.B.; Li, J. Evolution of Al2O3 inclusions by magnesium treatment in H13 hot work die steel. Ironmak. Steelmak. 2017, 44, 128–133. [Google Scholar] [CrossRef]
- Persson, E.S.; Karasev, A.; Jönsson, P. Studies of three dimension inclusions from ESR remelted and conventional cast steel. In Proceedings of the Liquid Metal Processing & Casting Conference LMPC, Philadelphia, PA, USA, 10–13 September 2017. [Google Scholar]
- Persson, E.S.; Mitchell, A. Differences in inclusion morphology between ESR remelted steel with and without tracer in the slag. In Proceedings of the Liquid Metal Processing & Casting Conference LMPC, Philadelphia, PA, USA, 10–13 September 2017. [Google Scholar]
- Persson, E.S.; Mitchell, A. The importance of Electrode Cleaness in the ESR/PESR Processes. In Proceedings of the 3rd Ingot Casting Rolling Forging conference ICRF, Stockholm, Sweden, 16–19 October 2018. [Google Scholar]
- Fredriksson, H.; Åkerlind, U. Materials Processing During Casting; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 27, 185, 207, 219, 265. [Google Scholar]
- Hoyle, G. Electroslag Processes, Principles and Practice; Applied Science Publisher Ltd.: New York, NY, USA, 1983; p. 29. [Google Scholar]
- Mitchell, A. Solidification in remelting processes. Mater. Sci. Eng. A 2005, 413–414, 10–18. [Google Scholar] [CrossRef]
- Mitchell, A.; Burel, B. The solution of aliumina in CaF2-Al2O3slags. Metall. Trans. B 1970, 1, 2253–2256. [Google Scholar] [CrossRef]
- Li, J.L.; Shu, Q.F.; Liu, Y.A.; Chou, K.C. Dissolution rate of Al2O3into molten CaO-Al2O3-CaF2flux. Ironmak. Steelmak. 2014, 41, 732–737. [Google Scholar] [CrossRef]
- Du, G.; Li, J.; Wang, Z.B. Effect of initial large sized inclusion content on removal in ESR. Ironmak. Steelmak. 2018, 45, 919–923. [Google Scholar] [CrossRef]
- Paton, B.E. Investigation Methods Removal of Non-Metallic Inclusions during ESR. In Proceedings of the 5th International Symposium on ESR, Pittsburgh, PA, USA, 16–18 October 1974; Bhat, E.G.K., Simcovitch, A., Eds.; Mellon Institute: Pittsburgh, PA, USA, 1974; pp. 433–448. [Google Scholar]
- Zhang, L.; Rietow, B.; Thomas, B.G.; Eakin, K. Large inclusions in plain carbon steel ingots cast by bottom teeming. JISI 2006, 46, 670–679. [Google Scholar] [CrossRef]
- Xuan, C.; Persson, E.S.; Sevastopolev, R.; Nzotta, M. Motion and Detachment Behaviors of Liquid Inclusion at Molten Steel-Slag Interfaces. Metall. Mater. Trans. B 2019, 4, 1957–1973. [Google Scholar] [CrossRef]
- Voronov, V.A. Kinetics of dissolution of MgO in CaF2 slags. Izv. Akad. Nauk. SSSR Met. 1975, 3, 62–65. [Google Scholar]
- Shi, C.; Guo, H.J.; Chen, X.C. Kinetic study on Oxygen during Protective Gas Electroslag Remelting Process. Special Steel 2012, in press. [Google Scholar]
- Medina, S.F.; Cores, A. Thermodynamic Aspects in the Manufacturing of Microalloyed Steels by the Electroslag Remelting Process. ISIJ Int. 1993, 33, 1244–1251. [Google Scholar] [CrossRef]
- Wang, C.S.; Liu, S.G.; Xu, M.D. Reducing oxygen content in Electro-slag Remelted Bearing Steel GCr15. Special Steel 1997, 18, 31–35. [Google Scholar]
- Chang, L.Z.; Yang, H.S.; Li, Z.B. Study on Oxygen Behavior Steelmaking during Electroslag Remelting. Steelmaking 2010, 26, 46–50. [Google Scholar]
- Wang, F.; Chen, X.C.; Guo, H.J. Aluminum Deoxidization of H13 Hot Die Steel through Inert Gas Protection Electroslag Remelting. In Proceedings of the Iron and Steel Conference and Exposition ATS Tech 2012, Atlanta, GA, USA, 7–10 May 2012; pp. 1005–1015. [Google Scholar]
- Mitchell, A. The chemistry of ESR slag. Canadian Metall. Q. 1981, 20, 101–112. [Google Scholar] [CrossRef]
- Frazer, M.E.; Mitchell, A. Mass transfer in the electro slag process, Part 2. Mass-transfer model. Ironmak. Steelmak 1976, 3, 288. [Google Scholar]
- Cao, Y.; Dong, Y.; Jiang, Z.-h.; Cao, H.-b.; Feng, Q.-l. Research on droplet formation and dripping behavior during the electroslag remelting process. Metall. Mater. 2016, 23, 399–407. [Google Scholar] [CrossRef]
- Xuan, C.; Persson, E.S.; Jensen, J.; Sevastopolev, R.; Nzotta, M. A novel evolution mechanism of Mg-Al-oxides in liquid steel. Integration of chemical reaction and coalescence-collision. J. Alloys Compd. 2019, 812. [Google Scholar] [CrossRef]
- Shin, J.H.; Park, J.H. Optimization of slag-metal reaction model for prediction of inclusion composition. In Proceedings of the 5th International conference on Process Development in Iron and Steelmaking Scanmet-V 2016, Luleå, Sweden, 12–15 June 2016. [Google Scholar]
- Kiessling, R.; Lange, N. Non-Metallic Inclusions in Steel; Part 2; The Institute of Materials: London, UK, 1977; pp. 1–6. [Google Scholar]
- Yang, S.F.; Wang, Q.Q.; Zhang, L.; Li, J.; Peaslee, K. Formation of MgO·Al2O3-based inclusions in alloy steels. Metall. Mater. Trans. B 2012, 43, 731–750. [Google Scholar] [CrossRef]
- Mitchell, A.; Szekely, J.; Elliott, F.J. Electroslag Refining; The Iron and Steel Institute: London, UK, 1973; pp. 3–15. [Google Scholar]
- Reyes-Carmona, F.; Mitchell, A. Deoxidation of ESR Slags. ISIJ Int. 1992, 32, 529–537. [Google Scholar] [CrossRef]


| Year | Author | Steel | Scale | Diameter/Width | Inclusions Size | Ref. |
|---|---|---|---|---|---|---|
| 1971 | D.A.R. Kay et al. | N.A. | N.A. | N.A. | N.A. | [1] |
| 1980 | Z.B. Li et al. | N.A. | N.A. | N.A. | N.A. | [2] |
| 2012 | C-B. SHI et al. | NAK80 die steel | N.A. | N.A. | N.A. | [3] |
| 2012 | C-B. SHI et al. | Die steel | Laboratory | N.A. | N.A. | [4] |
| 2012 | C-B. SHI et al. | High-al steel | N.A. | N.A. | <5µm | [5] |
| 2012 | X.C. Chen et al. | Inconel 718 | N.A. | N.A. | CN 5–15 µm | [6] |
| 1969 | B.C. Burel | steel, iron | Laboratory | Mould 77 mm | <15 µm | [7] |
| 1974 | A. Mitchell et al. | oxygen containing iron, iron-OFHC copper | Laboratory | Mould 76.2 mm | 1–5 µm | [8] |
| 1976 | J.C.F. Chan et al. | Stainless steel | Laboratory | Electrode 35 mm | N.A. | [9] |
| 2013 | C-B. SHI et al. | Die steel, superallloys …. | Laboratory | N.A. | N.A. | [10] |
| 2013 | Y. Dong et al. | Cold rolls steel MC5 | Laboratory 800 gr | N.A. | N.A. | [11] |
| 2014 | Y-W. Dong et al. | Die steel CR-5A | Laboratory 800 gr | N.A. | N.A. | [12] |
| 2013 | C-B. SHI et al. | H13 die steel | Laboratory, 50 kg | Electrcode 90 mm | abt 2 µm | [13] |
| 2015 | R. Scheinder et al. | Hot work steel abt H11 | Pilot | Electrode 101.5 mm | N.A. | [14] |
| 2016 | C-B. SHI et al. | High-Carbon 17% Cr Tool Steel | Pilot | PESR mould 170 mm | abt 5 µm | [15] |
| 2017 | G. Du et al. | H13 die steel | Pilot | ESR mould 300 mm | 0–15 µm, >15 µm | [16] |
| 2014 | L.Z. Cang et al. | N.A. | Pilot 15 kg | Mould abt 105 mm | [17] | |
| 2019 | C-B SHI et al. | Si-Mn killed steel ≈ H13 | Pilot | PESR 95 mm | 1–3 µm, few > 3µm | [18] |
| 2013 | G. Reiter et al. | abt H11, H13 die steel, martensitic Cr-Ni steel | Industrial | N.A. | ASTM E45 Heavy | [19] |
| 2014 | E.S. Persson et al. | Martensitic stainless steel | Industrial | Moulds 400–1050 mm | 8–30 µm | [20] |
| 2016 | E.S. Persson et al. | Martensitic stainless steel | Industrial | Moulds 400–1050 mm | N.A. | [21] |
| 2017 | H. Wang et al. | H13 die steel | Laboratory | Electrode 25 mm | abt 1–2 µm | [22] |
| 2017 | E.S. Persson et al. | Martensitic stainless steel | Industrial | Moulds 400–500 mm | 8–45 µm | [23] |
| 2017 | E.S. Persson et al. | Martensitic stainless steel | Industrial | ESR mould 300 mm | 8–20 µm | [24] |
| 2018 | E.S. Persson et al. | Martensitic stainless steel | Industrial | ESR mould 300 mm | 8–20 µm | [25] |
| Inclusion | Primary Inclusions (PI) | Semi-Secondary Inclusions (SSI) | Secondary Inclusions (SI) |
|---|---|---|---|
| Schematic illustration |  | 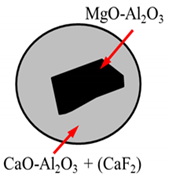 |  |
| Type of NMI | AM | ACM, A and AC | A, AC |
| Size on NMI | Large, see below | Medium (normally ≈ < 30 µm) | Small (<10 µm) |
| Source or formation mechanism | From the electrode, the size depends on the size of the inclusions in the electrode and the size of the steel droplets. In the ingot the spinels are often found in inclusion clusters with complex shapes, often together with other inclusions and elements (C, Cr, Si, Mn, S, Ca). | Primary AM oxides covered by process slag, sometimes with CaF2 attached. If CaF2 is found, it is proof that the inclusion has been in contact with the ESR/PESR process slag. | Precipitated in the ingot during solidification of the liquid steel |
| Type of Inclusion | Melting Point |
|---|---|
| MgO-Al2O3 spinels | 2135 °C |
| CaO-Al2O3 calcium aluminates | 1455–1850 °C |
| CaO-SiO2 calcium silicates | 1475–2070 °C |
| MnS manganese sulfide | 1610 °C |
| CaS calcium sulfide | about 2500 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sjöqvist Persson, E.; Karasev, A.; Mitchell, A.; Jönsson, P.G. Origin of the Inclusions in Production-Scale Electrodes, ESR Ingots, and PESR Ingots in a Martensitic Stainless Steel. Metals 2020, 10, 1620. https://doi.org/10.3390/met10121620
Sjöqvist Persson E, Karasev A, Mitchell A, Jönsson PG. Origin of the Inclusions in Production-Scale Electrodes, ESR Ingots, and PESR Ingots in a Martensitic Stainless Steel. Metals. 2020; 10(12):1620. https://doi.org/10.3390/met10121620
Chicago/Turabian StyleSjöqvist Persson, Ewa, Andrey Karasev, Alec Mitchell, and Pär G. Jönsson. 2020. "Origin of the Inclusions in Production-Scale Electrodes, ESR Ingots, and PESR Ingots in a Martensitic Stainless Steel" Metals 10, no. 12: 1620. https://doi.org/10.3390/met10121620
APA StyleSjöqvist Persson, E., Karasev, A., Mitchell, A., & Jönsson, P. G. (2020). Origin of the Inclusions in Production-Scale Electrodes, ESR Ingots, and PESR Ingots in a Martensitic Stainless Steel. Metals, 10(12), 1620. https://doi.org/10.3390/met10121620






