Effect of Exercise Intensity on Cell-Mediated Immunity
Abstract
1. Introduction
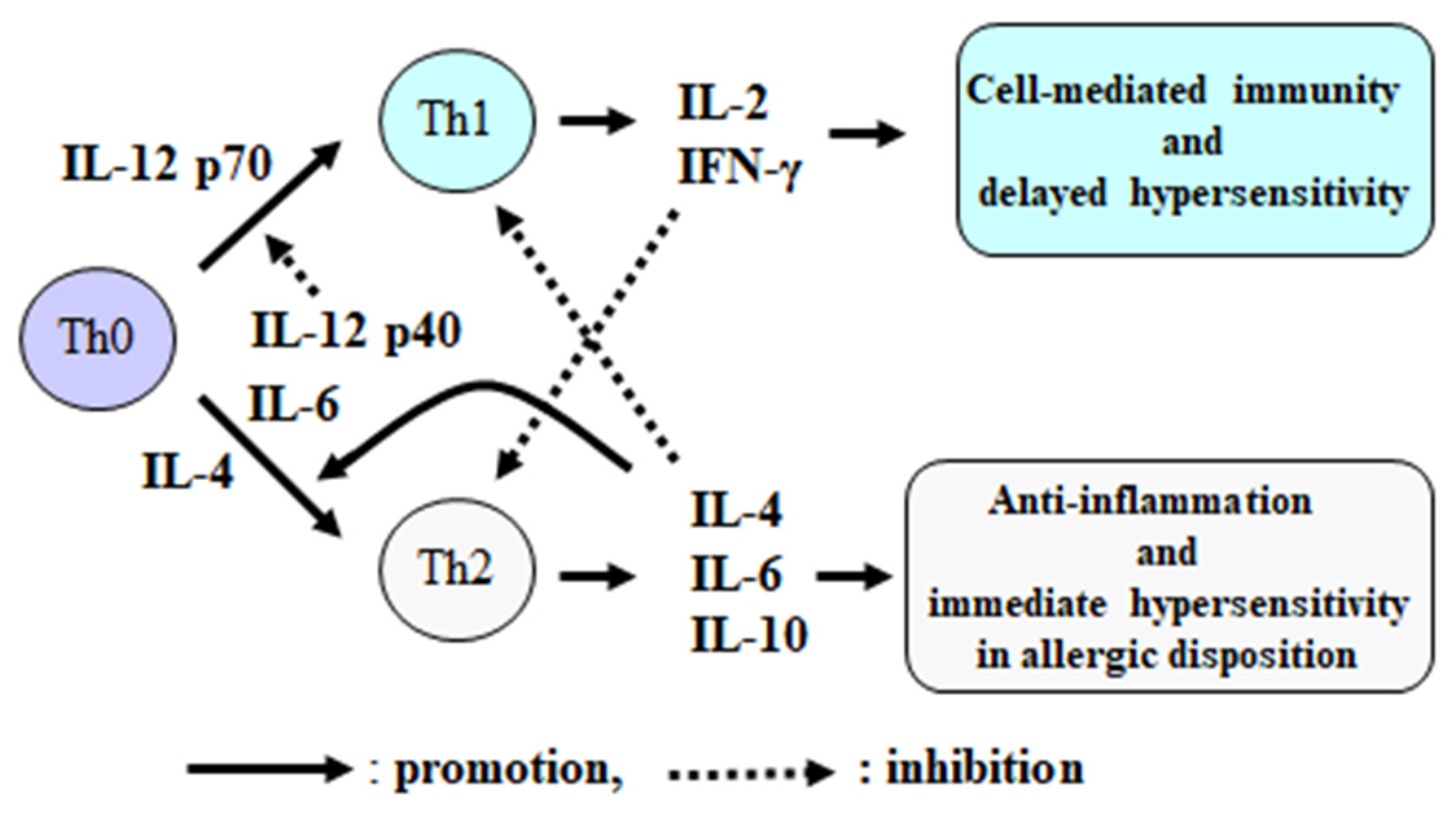
2. Function and Control Mechanism of Cell-Mediated Immunity
3. Effects of High-Intensity and Long Duration Exercise on Immune Variables
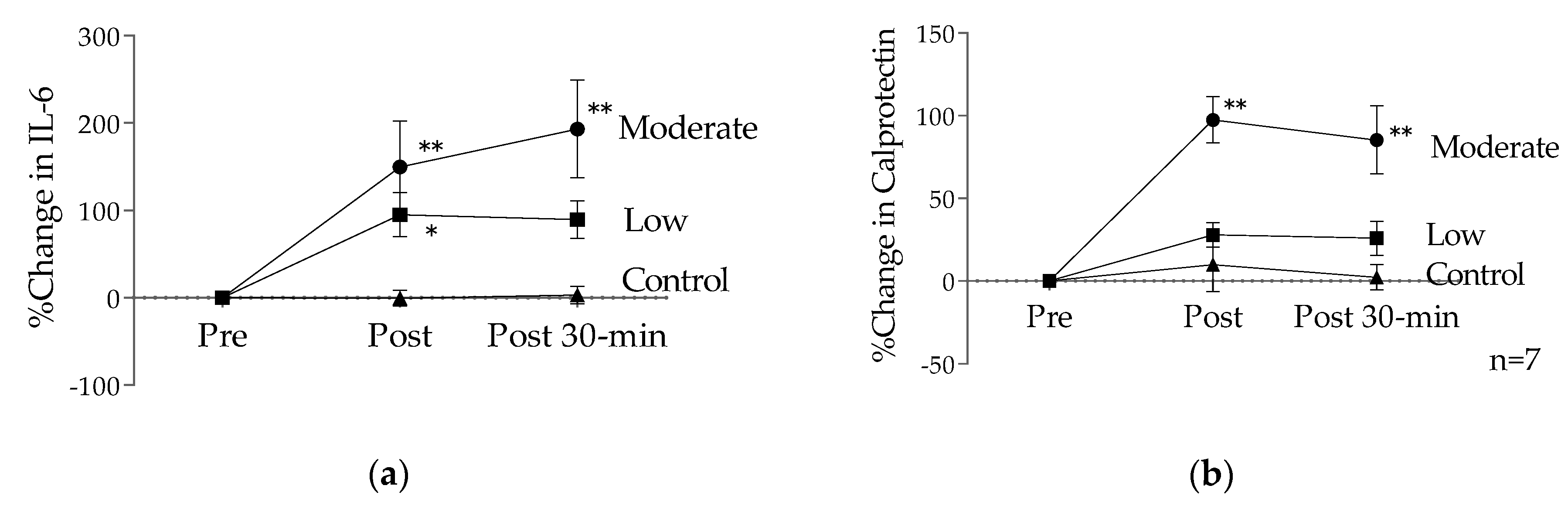
4. Effects of Moderate-Intensity Exercise and Training on Immune Variables
5. Effects of Short-Duration Low-Intensity Exercise and Training on Immune Variables
6. Conclusions and Challenges for the Future
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bobinski, F.; Teixeira, J.M.; Sluka, K.A.; Santos, A.R.S. Interleukin-4 mediates the analgesia produced by low-intensity exercise in mice with neuropathic pain. Pain 2018, 159, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C. Exercise immunology: Practical applications. Int. J. Sports Med. 1997, 18 (Suppl. 1), S91–S100. [Google Scholar] [CrossRef] [PubMed]
- Diment, B.C.; Fortes, M.B.; Edwards, J.P.; Hanstock, H.G.; Ward, M.D.; Dunstall, H.M.; Friedmann, P.S.; Walsh, N.P. Exercise intensity and duration effects on in vivo immunity. Med. Sci. Sports Exerc. 2015, 47, 1390–1398. [Google Scholar] [CrossRef]
- Simpson, R.J.; Campbell, J.P.; Gleeson, M.; Krüger, K.; Nieman, D.C.; Pyne, D.B.; Turner, J.E.; Walsh, N.P. Can exercise affect immune function to increase susceptibility to infection? Exerc. Immunol. Rev. 2020, 26, 8–22. [Google Scholar] [PubMed]
- Cerqueira, É.; Marinho, D.A.; Neiva, H.P.; Lourenço, O. Inflammatory effects of high and moderate intensity exercise—A systematic review. Front. Physiol. 2020, 10, 1550. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Tominaga, T.; Ruhee, R.T.; Ma, S. Characterization and modulation of systemic inflammatory response to exhaustive exercise in relation to oxidative stress. Antioxidants 2020, 9, 401. [Google Scholar] [CrossRef]
- Nieman, D.C.; Henson, D.A.; Austin, M.D.; Brown, V.A. Immune response to a 30-minute walk. Med. Sci. Sports Exerc. 2005, 37, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Terada, O.; Suzuki, K.; Kurihara, Y.; Moriguchi, S. Effects of low-intensity brief exercise and training on cell-mediated immunity. Jpn. J. Complementary Altern. Med. 2007, 4, 71–77. [Google Scholar] [CrossRef][Green Version]
- Field, T. Yoga research review. Complementary Ther. Clin. Pract. 2016, 24, 145–161. [Google Scholar] [CrossRef]
- Falkenberg, R.I.; Eising, C.; Peters, M.L. Yoga and immune system functioning: A systematic review of randomized controlled trials. J. Behav. Med. 2018, 41, 467–482. [Google Scholar] [CrossRef]
- Suzuki, K.; Nakaji, S.; Kurakake, S.; Totsuka, M.; Sato, K.; Kuriyama, T.; Fujimoto, H.; Shibusawa, K.; Machida, K.; Sugawara, K. Exhaustive exercise and type-1/type-2 cytokine balance with special focus on interleukin-12 p40/p70. Exerc. Immunol. Rev. 2003, 9, 48–57. [Google Scholar] [PubMed]
- Hasegawa, H.; Mizoguchi, I.; Chiba, Y.; Ohashi, M.; Xu, M.; Yoshimoto, T. Expanding diversity in molecular structures and functions of the IL-6/IL-12 heterodimeric cytokine family. Front. Immunol. 2016, 7, 479. [Google Scholar] [CrossRef] [PubMed]
- Kaliński, P.; Vieira, P.L.; Schuitemaker, J.H.; de Jong, E.C.; Kapsenberg, M.L. Prostaglandin E(2) is a selective inducer of interleukin-12 p40 (IL-12p40) production and an inhibitor of bioactive IL-12p70 heterodimer. Blood 2001, 97, 3466–3469. [Google Scholar] [CrossRef] [PubMed]
- Sugama, K.; Suzuki, K.; Yoshitani, K.; Shiraishi, K.; Kometani, T. IL-17, neutrophil activation and muscle damage following endurance exercise. Exerc. Immunol. Rev. 2012, 18, 116–127. [Google Scholar] [PubMed]
- Pedersen, B.K.; Rohde, T.; Zacho, M. Immunity in athletes. J. Sports Med. Phys. Fit. 1996, 3, 236–245. [Google Scholar]
- Goh, J.; Lim, C.L.; Suzuki, K. Effects of Endurance-, Strength-, and Concurrent Training on Cytokines and Inflammation. In Concurrent Aerobic and Strength Training; Schumann, M., Rønnestad, B.R., Eds.; Springer: Basel, Switzerland, 2019; pp. 125–138. [Google Scholar] [CrossRef]
- Bruunsgaard, H.; Hartkopp, A.; Mohr, T.; Konradsen, H.; Heron, I.; Mordhorst, C.H.; Pedersen, B.K. In vivo cell-mediated immunity and vaccination response following prolonged, intense exercise. Med. Sci. Sports Exerc. 1997, 29, 1176–1181. [Google Scholar] [CrossRef]
- Walsh, N.P.; Gleeson, M.; Shephard, R.J.; Gleeson, M.; Woods, J.A.; Bishop, N.C.; Fleshner, M.; Green, C.; Pedersen, B.K.; Hoffman-Goetz, L.; et al. Position statement. Part one: Immune function and exercise. Exerc. Immunol. Rev. 2011, 17, 6–63. [Google Scholar]
- Gleeson, M. Immune function in sport and exercise. J. Appl. Physiol. 2007, 103, 693–699. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Ullum, H. NK cell response to physical activity: Possible mechanisms of action. Med. Sci. Sports Exerc. 1994, 26, 140–146. [Google Scholar] [CrossRef]
- Suzuki, K.; Yamada, M.; Kurakake, S.; Okamura, N.; Yamaya, K.; Liu, Q.; Kudoh, S.; Kowatari, K.; Nakaji, S.; Sugawara, K. Circulating cytokines and hormones with immunosuppressive but neutrophil-priming potentials rise after endurance exercise in humans. Eur. J. Appl. Physiol. 2000, 81, 281–287. [Google Scholar] [CrossRef]
- Suzuki, K.; Nakaji, S.; Yamada, M.; Totsuka, M.; Sato, K.; Sugawara, K. Systemic inflammatory response to exhaustive exercise. Cytokine kinetics. Exerc. Immunol. Rev. 2002, 8, 6–48. [Google Scholar] [PubMed]
- Suzuki, K. Involvement of neutrophils in exercise-induced muscle damage. Gen Intern. Med. Clin. Innov. 2018, 3, 1–8. [Google Scholar] [CrossRef]
- Nieman, D.C. Exercise, immunology and nutrition. World Rev. Nutr. Diet 2001, 90, 89–101. [Google Scholar] [PubMed]
- Suzuki, K. Chronic inflammation as an immunological abnormality and effectiveness of exercise. Biomolecules 2019, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Wentz, L.M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Ann. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Bishop, N.; Oliveira, M.; McCauley, T.; Tauler, P.; Muhamad, A.S. Respiratory infection risk in athletes: Association with antigen-stimulated IL-10 production and salivary IgA secretion. Scand. J. Med. Sci. Sports 2012, 22, 410–417. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C. URI in athletes: Are mucosal immunity and cytokine responses key risk factors? Exerc. Sport Sci. Rev. 2013, 41, 148–153. [Google Scholar] [CrossRef]
- Hayashida, H.; Shimura, M.; Sugama, K.; Kanda, K.; Suzuki, K. Exercise-induced inflammation during different phases of the menstrual cycle. Physiother Rehabil 2016, 1, 121. [Google Scholar] [CrossRef]
- Hayashida, H.; Shimura, M.; Sugama, K.; Kanda, K.; Suzuki, K. Effects of the menstrual cycle and acute aerobic exercise on cytokine levels. J. Sports Med. Doping Stud 2015, 6, 173. [Google Scholar] [CrossRef]
- Rykova, M.P.; Antropova, E.N.; Popov, D.V.; Vinogradova, O.L.; Larina, I.M. The activation processes of the immune system during low intensity exercise without relaxation. Ross. Fiziol. Zhurnal Im. 2008, 94, 212–219. [Google Scholar]
- Tenório, T.R.S.; Balagopal, P.B.; Andersen, L.B.; Ritti-Dias, R.M.; Hill, J.O.; Lofrano-Prado, M.C.; Prado, W.L. Effect of low- versus high-intensity exercise training on biomarkers of inflammation and endothelial dysfunction in adolescents with obesity: A 6-month randomized exercise intervention study. Ped Exerc. Sci. 2018, 30, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Mackinnon, L. Chronic exercise training effects on immune function. Med. Sci. Sports Exerc. 2000, 32, S369–S376. [Google Scholar] [CrossRef] [PubMed]
- Gerosa-Neto, J.; Monteiro, P.A.; Inoue, D.S.; Antunes, B.M.; Batatinha, H.; Dorneles, G.P.; Peres, A.; Rosa-Neto, J.C.; Lira, F.S. High- and moderate-intensity training modify LPS-induced ex-vivo interleukin-10 production in obese men in response to an acute exercise bout. Cytokine 2020, 136, 155249. [Google Scholar] [CrossRef] [PubMed]
- Quchan, A.H.S.K.; Kordi, M.R.; Namdari, H.; Shabkhiz, F. Voluntary wheel running stimulates the expression of Nrf-2 and interleukin-10 but suppresses interleukin-17 in experimental autoimmune encephalomyelitis. Neurosci. Let. 2020, 738, 135382. [Google Scholar] [CrossRef] [PubMed]
- Khammassi, M.; Ouerghi, N.; Said, M.; Feki, M.; Khammassi, Y.; Pereira, B.; Thivel, D.; Bouassida, A. continuous moderate-intensity but not high-intensity interval training improves immune function biomarkers in healthy young men. J. Strength Cond. Res. 2020, 34, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Silveira, L.S.; Antunes Bde, M.; Minari, A.L.; Dos Santos, R.V.; Neto, J.C.; Lira, F.S. Macrophage polarization: Implications on metabolic diseases and the role of exercise. Crit. Rev. Eukaryot. Gene Expr. 2016, 26, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef]
- Suzuki, K. Characterization of exercise-induced cytokine release, the impacts on the body, the mechanisms and modulations. Int. J. Sports Exerc. Med. 2019, 5, 1–13. [Google Scholar] [CrossRef]
- Jesus, I.; Vanhee, V.; Deramaudt, T.B.; Bonay, M. Promising effects of exercise on the cardiovascular, metabolic and immune system during COVID-19 period. J. Hum. Hypertens. 2020. [Google Scholar] [CrossRef]
- Morgan, N.; Irwin, M.R.; Chung, M.; Wang, C. The effects of mind-body therapies on the immune system: Meta-analysis. PLoS ONE 2014, 9, e100903. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghava, A.; Doreswamy, V.; Narasipur, O.S.; Kunnavil, R.; Srinivasamurthy, N. Effect of yoga practice on levels of inflammatory markers after moderate and strenuous exercise. J. Clin. Diagn. Res. 2015, 9, CC08–CC12. [Google Scholar] [CrossRef] [PubMed]
- Cahn, B.R.; Goodman, M.S.; Peterson, C.T.; Maturi, R.; Mills, P.J. Yoga, Meditation and mind-body health: Increased BDNF, cortisol awakening response, and altered inflammatory marker expression after a 3-month yoga and meditation retreat. Front. Hum. Neurosci. 2017, 11, 315. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.A.; Cheong, K.J. Regular yoga practice improves antioxidant status, immune function, and stress hormone releases in young healthy people: A randomized, double-blind, controlled pilot study. J. Altern. Complementary Med. 2015, 21, 530–538. [Google Scholar] [CrossRef]
- Gopal, A.; Mondal, S.; Gandhi, A.; Arora, S.; Bhattacharjee, J. Effect of integrated yoga practices on immune responses in examination stress—A preliminary study. Int. J. Yoga 2011, 4, 26–32. [Google Scholar]
- Rajbhoj, P.H.; Shete, S.U.; Verma, A.; Bhogal, R.S. Effect of yoga module on pro-inflammatory and anti-inflammatory cytokines in industrial workers of lonavla: A randomized controlled trial. J. Clin. Diagn. Res. 2015, 9, CC01-5. [Google Scholar] [CrossRef]
- Lim, C.L.; Suzuki, K. Systemic inflammation mediates the effects of endotoxemia in the mechanisms of heat stroke. Biol. Med. 2017, 9, 1000376. [Google Scholar] [CrossRef]
- Suzuki, K.; Hashimoto, H.; Oh, T.; Ishijima, T.; Mitsuda, H.; Peake, J.M.; Sakamoto, S.; Muraoka, I.; Higuchi, M. The effects of sports drink osmolality on fluid intake and immunoendocrine responses to cycling in hot conditions. J. Nutr. Sci. Vitaminol. 2013, 59, 206–212. [Google Scholar] [CrossRef][Green Version]
- Suzuki, K. Cytokine response to exercise and its modulation. Antioxidants 2018, 7, 17. [Google Scholar] [CrossRef]
- Eda, N.; Ito, H.; Shimizu, K.; Suzuki, S.; Lee, E.; Akama, T. Yoga stretching for improving salivary immune function and mental stress in middle-aged and older adults. J. Women Aging 2018, 30, 227–241. [Google Scholar] [CrossRef]
- Chen, P.J.; Yang, L.; Chou, C.C.; Li, C.C.; Chang, Y.C.; Liaw, J.J. Effects of prenatal yoga on women’s stress and immune function across pregnancy: A randomized controlled trial. Complement. Ther. Med. 2017, 31, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Nakaji, S.; Yamada, M.; Liu, Q.; Kurakake, S.; Okamura, N.; Kumae, T.; Umeda, T.; Sugawara, K. Impact of a competitive marathon race on systemic cytokine and neutrophil responses. Med. Sci. Sports Exerc. 2003, 35, 348–355. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, K.; Hayashida, H. Effect of Exercise Intensity on Cell-Mediated Immunity. Sports 2021, 9, 8. https://doi.org/10.3390/sports9010008
Suzuki K, Hayashida H. Effect of Exercise Intensity on Cell-Mediated Immunity. Sports. 2021; 9(1):8. https://doi.org/10.3390/sports9010008
Chicago/Turabian StyleSuzuki, Katsuhiko, and Harumi Hayashida. 2021. "Effect of Exercise Intensity on Cell-Mediated Immunity" Sports 9, no. 1: 8. https://doi.org/10.3390/sports9010008
APA StyleSuzuki, K., & Hayashida, H. (2021). Effect of Exercise Intensity on Cell-Mediated Immunity. Sports, 9(1), 8. https://doi.org/10.3390/sports9010008





