Abstract
Aging is associated with physiological changes that increase the risk of falls, impacting functional independence and quality of life. Multicomponent exercise training has emerged as an effective strategy for mitigating these risks by enhancing strength, balance, flexibility, and aerobic capacity. This study aimed to evaluate the effects of a 30-week multicomponent training program on functional fitness and fall prevention in older women. A parallel, single-blind randomized controlled trial was conducted with 40 participants (aged ≥ 65 years), divided into an exercise group and a control group. The intervention combined strength, balance, coordination, and aerobic training, following international exercise guidelines for older adults. Functional fitness was assessed using validated tests, including the Timed Up and Go (TUG) test, lower limb strength, flexibility, and aerobic endurance measures. Results demonstrated significant improvements in the intervention group, particularly in TUG performance (p < 0.001), lower limb strength (p < 0.001), and flexibility (p < 0.05), indicating enhanced mobility and reduced fall risk. These findings reinforce the importance of structured, multicomponent training programs for aging populations, particularly women, who experience greater musculoskeletal decline due to menopause-related hormonal changes. Future research should explore long-term retention of benefits and optimize intervention strategies. This study highlights the critical role of tailored exercise programs in promoting active aging, improving functional capacity, and reducing healthcare burdens associated with fall-related injuries.
1. Introduction
Population aging is a global reality that has significantly reshaped demographic, social, and economic structures worldwide [1]. According to United Nations projections, by 2050 the number of people aged 60 and over will exceed 2 billion, representing more than 20% of the global population [2]. In Portugal, this trend is even more pronounced, as the country ranks among the oldest populations in Europe. In 2018, approximately 22% of the population was aged 65 or older [3], with projections indicating an increase to 45% by 2050 [4]. This demographic shift presents major public health challenges, particularly concerning the maintenance of quality of life and functional independence among older adults [5].
Aging is accompanied by various physiological changes that negatively affect individuals’ functional capacity and autonomy [6]. Among the most notable are the progressive loss of muscle mass (sarcopenia) [7], decreased bone density (osteoporosis) [8], and declines in sensory and motor functions [9,10], as well as reduced cardiorespiratory fitness [11]. These changes make older adults more susceptible to multiple health issues, including impaired mobility [12], increased incidence of chronic diseases [13], and, most critically, a heightened risk of falls [14,15].
It is important to highlight, however, that many of these impairments—such as loss of muscle mass, reduced balance, and decreased cardiorespiratory fitness—are not solely caused by the aging process itself but are strongly associated with a sedentary lifestyle and reduced physical activity levels [16]. This distinction is critical, as these disuse-related declines are largely modifiable and can be mitigated through regular, structured exercise interventions [17]. Recognizing inactivity as a key contributing factor reinforces the role of physical activity in preserving function and preventing falls in older adults [16,17].
Falls are among the most serious and prevalent health concerns in the aging population. Their consequences go beyond physical injuries—such as fractures and head trauma—to include psychological effects, such as fear of falling, and social consequences, including loss of independence and the need for institutional care [18,19,20,21]. In Portugal, for instance, between 2000 and 2013, three out of every 100 hospitalizations of individuals over 65 years were due to falls [22]. Moreover, falls are a leading cause of hospitalizations and deaths in older adults, imposing a substantial burden on healthcare and social support systems [23]. Although most falls are non-fatal, they are associated with significant morbidity and greater functional decline. Approximately 0.6% of fall-related hospitalizations in Portugal result in death [24]. In light of this impact, fall prevention has become a priority in promoting the health and well-being of aging populations [14,25].
In response to this pressing issue, non-pharmacological strategies—particularly physical activity and exercise—have gained prominence in fall prevention and the promotion of healthy aging. Regular physical activity is widely recognized as one of the most effective interventions for reducing the risks associated with aging [26], especially in decreasing fall incidence in older adults [27,28]. Various forms of exercise, including aerobic activities, resistance training, and balance exercises, offer specific benefits that contribute to the preservation of functional capacity, independence, and fall risk reduction [29,30,31,32]. Aerobic exercise improves cardiorespiratory capacity and endurance [33,34]; resistance training increases muscle strength and helps combat sarcopenia [35,36]; and balance and coordination exercises enhance postural control and responsiveness, thereby reducing fall risk [37,38].
To address the multifactorial nature of fall risk comprehensively, multicomponent exercise programs have emerged as a particularly effective strategy [39,40]. These programs integrate different exercise modalities—such as strength, balance, flexibility, and endurance—into a unified intervention, allowing older adults to work on multiple aspects of fitness simultaneously [41]. Additionally, these programs can be adapted to individual capacities, making them safe and accessible for people with varying physical abilities [42]. Evidence shows that regular participation in multicomponent programs significantly reduces fall risk by enhancing postural control, motor coordination, and responses to external perturbations, which are essential for stability and mobility in later life [43,44,45,46,47,48].
Despite the proven benefits of multicomponent training in fall prevention, there remains a relative lack of research focused specifically on older women. Many clinical trials include mixed-sex samples or do not report sex-specific outcomes, limiting the generalizability of findings to this population. Some studies suggest that hormonal and musculoskeletal changes related to menopause may influence training responses, underscoring the importance of tailored interventions for postmenopausal women [49,50].
In this regard, strength training enhances muscle mass and functional capacity, reducing lower limb weakness, while balance and agility exercises improve proprioception and reactivity to unexpected events, thus preventing falls [41]. Understanding the benefits and implementation of multicomponent training [51] enables health professionals, physical educators, and public health officials to develop effective, evidence-based interventions tailored to the needs of older adults. Such strategies not only promote healthy and active aging, but also contribute to the sustainability of healthcare systems in an aging society [52].
Aging in women is associated with accelerated losses in muscle mass and bone mineral density, primarily due to the decline in estrogen levels during menopause [53]. These changes increase the risk of falls and fractures, especially in areas such as the hip and spine, highlighting the need for targeted preventive approaches [54]. Moreover, the decline in balance, proprioception, and lower limb strength further compromises postural stability [55]. Therefore, interventions involving structured exercise—particularly multicomponent programs—are essential to mitigate these impacts and support safety and autonomy in older women [49,50,56,57].
Given these challenges, it is essential to investigate evidence-based interventions capable of counteracting the physiological impacts of aging in women. The present study, therefore, aims to evaluate the effects of a multicomponent exercise program on fall prevention and functional fitness in older women, with particular attention to the Portuguese population. By addressing key factors such as balance, strength, and mobility—domains commonly impaired with age—this research seeks to contribute to the development of accessible, non-pharmacological strategies that foster autonomy, safety, and quality of life in this vulnerable group.
2. Materials and Methods
2.1. Study Design
This parallel-group, single-blind randomized controlled trial (RCT), with a total duration of 30 weeks, was approved by the Ethics Committee of the Polytechnic Institute of Bragança (IPB) under protocol number 2067313 and registered at ClinicalTrials.gov (identifier: NCT06843486). The study protocol adhered to the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines [58] and was conducted in accordance with the principles outlined in the Declaration of Helsinki. The reporting followed the Consolidated Standards of Reporting Trials (CONSORT) guidelines [59].
2.2. Sample
The study was initiated following ethical approval from the Ethics Committee of the Polytechnic Institute of Bragança (IPB), under protocol number 2067313. Participant recruitment was conducted based on specific inclusion and exclusion criteria. Eligible participants were community-dwelling women aged 65 years or older, without psychological or physical conditions that could impair their participation in the exercise program or assessments. They were also required to have adequate visual and auditory acuity to perform the activities safely and effectively, and not rely on assistive devices for mobility or demonstrate notable difficulties in performing activities of daily living.
Exclusion criteria included: women under the age of 65; those with psychological or physical disorders that would limit their participation in training or testing; critical visual and/or auditory impairments that could compromise task execution; use of mobility aids; or evident functional limitations in daily activities. Additionally, women already engaged in structured physical training or taking medications likely to influence outcomes (e.g., hormone replacement therapy or osteoporosis treatments) were excluded.
The participants were divided into two groups: a control group and an exercise group. Randomization was performed using a simple random allocation sequence generated in RStudio software (version 2024.09.0+375) by an independent researcher who was not involved in recruitment or assessments. This ensured allocation concealment and minimized selection bias.
Participant recruitment followed a non-probabilistic approach. All participants were fully informed about the study’s objectives, potential risks, and expected discomforts, and participation was voluntary. Written informed consent was obtained from all participants prior to enrollment.
The sample consisted of 40 participants, with an average age of 68.68 ± 5.74 years and a body mass of 70.47 ± 14.88 kg, all of whom were women. The control group was composed of 20 participants, while the exercise group included 20 participants. All participants were biologically female. The study used “women” to reflect sex as reported by participants.
The volunteers were recruited from community projects and physical exercise programs promoted by the Polytechnic Institute of Bragança (IPB). Furthermore, baseline assessments included self-reported history of falls in the previous year and fear of falling, collected via a brief structured questionnaire. Few participants reported previous falls, and the majority did not report a significant fear of falling. Although these variables were not used as primary outcomes, they contributed to the initial characterization of fall risk in the study sample.
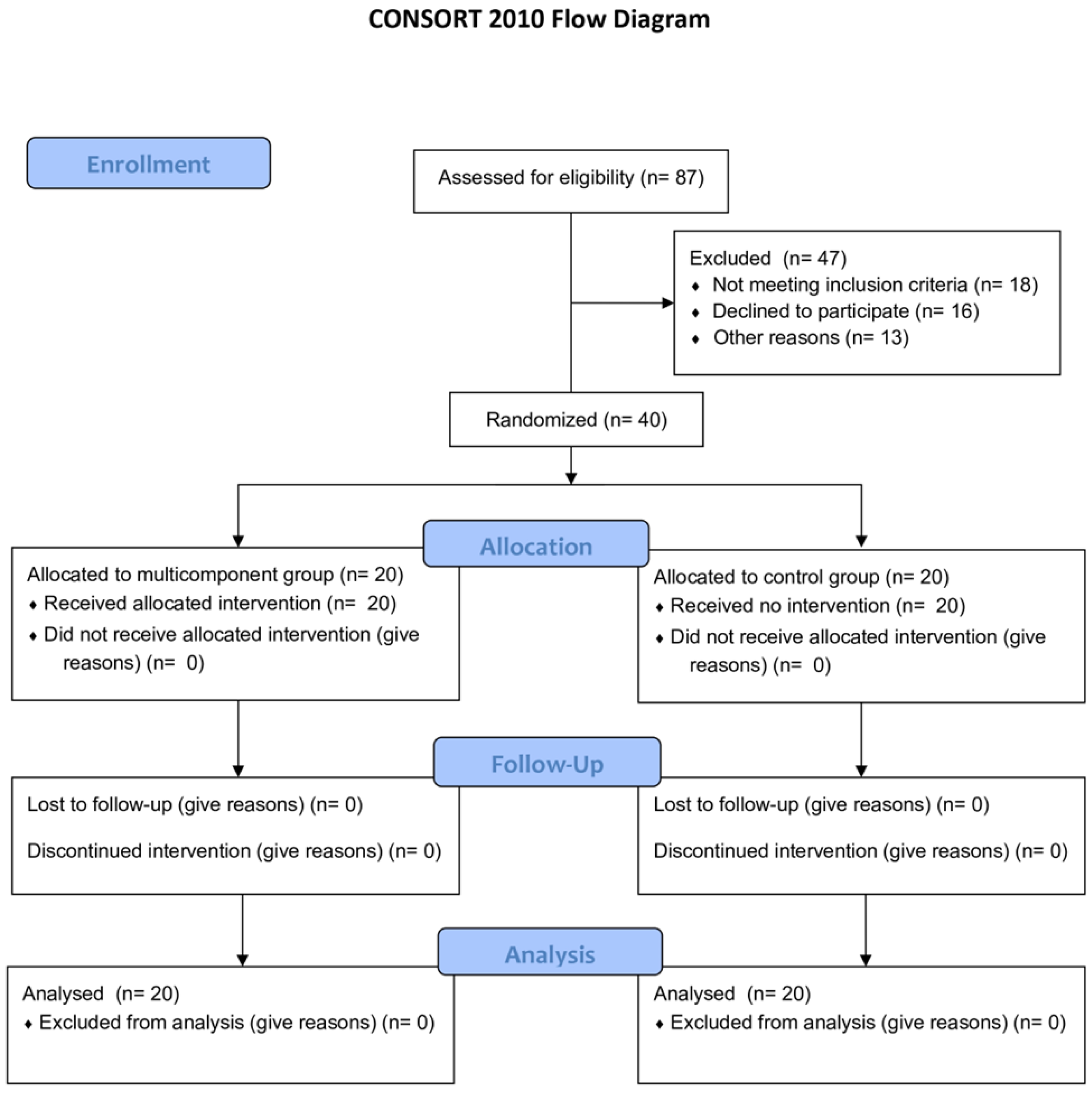
Figure 1 describes the flow diagram of the study.
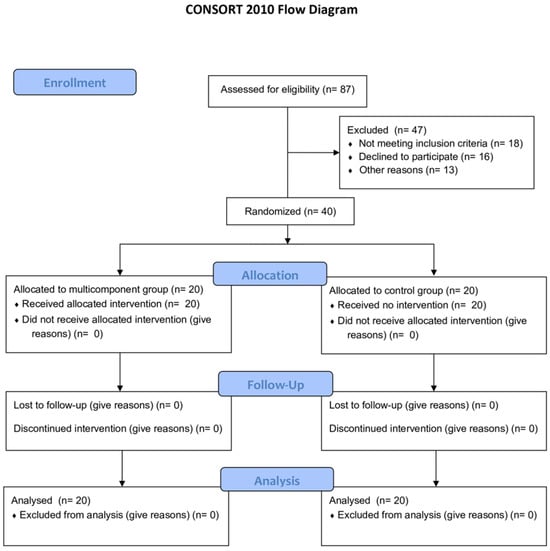
Figure 1.
CONSORT diagram of the study.
2.3. Procedures
2.3.1. Anthropometry and Body Composition
Body weight was measured to the nearest 0.1 kg using a digital scale (SECA®; SECA GmbH & Co. KG, Hamburg, Germany), with participants dressed in light clothing and barefoot. Height was assessed using a stadiometer attached to the scale, measuring the vertical distance from the vertex of the head to the ground reference plane, with an accuracy of 0.1 cm. Body Mass Index (BMI) was calculated using the standard formula [weight (kg)/height2 (m2)] [60], and classified according to the World Health Organization (WHO) criteria (2010): normal weight (18.50–24.99 kg/m2), pre-obesity (25.00–29.99 kg/m2), obesity class I (30.00–34.99 kg/m2), and obesity class II (35.00–39.99 kg/m2) [61].
2.3.2. Assessment of Functional Fitness
Functional fitness was assessed using the Rikli and Jones Senior Fitness Test [62], a validated test battery for older adults that evaluates key components such as muscular strength, flexibility, balance, agility, and aerobic capacity. The battery included the following six tests:
- Lower limb strength: 30 s chair stand test, measuring the number of full stands from a seated position.
- Upper limb strength: arm curl test using a 2 kg dumbbell, counting the number of repetitions performed in 30 s.
- Lower body flexibility: chair sit-and-reach test, with the distance (in centimeters) measured between the extended fingertips and the toes.
- Upper body flexibility: back scratch test, measuring the distance (in centimeters) between the middle fingers of each hand.
- Agility: Timed Up and Go (TUG) test, which records the time (in seconds) required to stand up from a chair, walk 3 m, turn around, return, and sit down.
- Aerobic endurance: 2 min step test, counting the number of steps completed while raising the knees to hip level.
This assessment protocol has been used in previous studies to evaluate the functional fitness of older adults [57,63].
2.3.3. Multicomponent Training Program
The multicomponent training program was developed based on international guidelines for exercise in adult and older populations [64]. The intervention lasted 30 weeks and consisted of three 60 min sessions per week (Figure 2), delivered in a group setting. Each session followed a structured format:

Figure 2.
Multicomponent Training program.
- Warm-up (10 min): dynamic stretches, mobility exercises, and light jogging.
- Strength training (20 min): exercises using free weights, dumbbells, and kettlebells, targeting major muscle groups including upper limbs, lower limbs, and core.
- Balance and coordination training (10 min): activities such as single-leg exercises, plyometric movements, and tasks involving unstable surfaces.
- Aerobic training (15 min): dynamic and rhythmic movements like lateral jumps, jumping jacks, running in place, and heel raises.
- Cool-down (5 min): static stretches for upper and lower limbs and trunk, plus guided breathing exercises.
Sessions were supervised by certified physical education professionals and physiotherapists. Exercises were adapted to the individual functional capacity of each participant. Internal load was monitored weekly using the Borg Rating of Perceived Exertion (RPE) scale (6–20), with exercises adjusted to maintain perceived effort between 13 and 15 (moderate to intense).
Group sessions were held in a shared environment with more than 20 participants present, as the intervention coincided with other community-based multicomponent training projects conducted at the same facility. Despite the larger setting, each participant received individualized guidance and supervision from qualified physical education professionals and physiotherapists.
Participants assigned to the control group were instructed to maintain their usual lifestyle and not to engage in any new structured physical activity or exercise program during the 30-week study period. They received no intervention, physical guidance, or supervised sessions.
Monthly follow-up calls were made to ensure continued participation, confirm adherence to the non-intervention condition, and record any changes in physical activity, health status, or adverse events. These participants were included in the final analysis regardless of minor changes in behavior, provided they did not enroll in other exercise programs during the study period.
2.3.4. Pre-Test and Post-Test Evaluations
The initial assessments (pre-test) were conducted before the start of the training program, and the final assessments (post-test) were carried out after the 30 weeks of intervention. The evaluations were conducted at the same times from 8:00 to 11:00 in the morning.
2.3.5. Statistical Analyses
The collected data were analyzed using statistical methods, utilizing RStudio software (version 2024.09.0+375, Posit, PBC). Descriptive analyses, normality tests (Shapiro–Wilk and Levene’s), and comparisons between groups (ANOVA) were performed to evaluate the differences before and after the intervention [65,66,67]. The adopted significance level was p < 0.05 [68].
Initially, the Shapiro–Wilk test was applied to verify the normality of the data. For the variables that showed a normal distribution, a mixed ANOVA was used, which allows for the assessment of main effects (group and time) and the interaction between them (group × time), considering repeated measures within participants. This approach enables a robust analysis of the differences in body composition and functional fitness between the groups over time.
For the variables that did not follow a normal distribution, the non-parametric Brunner–Langer ANOVA was applied, suitable for data with non-normal distributions or variance heterogeneity, common characteristics in studies with specific populations, such as in this study. The analysis was conducted using the nparLD package in R software, generating Wald-type statistics (WTS) and ANOVA-type statistics (ATS), which allow for testing the absence of main effects and interactions without assuming parametric distributions.
3. Results
Table 1 shows the main characteristics and composition of the sample between the experimental group and the control group.

Table 1.
Descriptive statistics for group composition, age, and BMI.
The Brunner–Langer nonparametric analysis for BMI showed that there was no significant group × time interaction (ATS = 1.370, p = 0.241), indicating that the variations over time did not differ significantly between the intervention and control groups. Additionally, no significant main effects were observed for group (ATS = 0.125, p = 0.723) or time (ATS = 1.458, p = 0.227). The effect size (Cohen’s d = 0.06) suggested a negligible and clinically irrelevant effect.
Descriptively, the intervention group exhibited a slight reduction in BMI, from 31.24 ± 5.96 kg/m2 (95% CI: 28.63 to 33.29) at baseline to 30.52 ± 6.13 kg/m2 (95% CI: 27.84 to 33.21) after the intervention, resulting in a Δ of –0.72 kg/m2. Conversely, the control group showed a small increase, from 29.67 ± 5.98 kg/m2 (95% CI: 27.04 to 32.29) to 29.83 ± 5.73 kg/m2 (95% CI: 27.32 to 32.34), corresponding to a Δ of +0.16 kg/m2. These findings indicate that the MTC program did not result in statistically or clinically meaningful changes in BMI among the participants.
The mixed ANOVA for upper limb flexibility, assessed using the Back Scratch Test, revealed a significant group × time interaction (F(1,38) = 19.35, p < 0.001, η2p = 0.951), indicating that the groups responded differently over time. No significant main effects were observed for group (F(1,38) = 0.35, p = 0.556, η2p = 0.261) or time (F(1,38) = 2.37, p = 0.132, η2p = 0.703), suggesting that the observed differences were primarily due to the interaction effect.
Descriptively, the intervention group improved from −11.68 ± 8.40 cm (95% CI: −15.45 to −7.90) at baseline to −7.95 ± 9.14 cm (95% CI: −12.06 to −3.83) post-intervention, resulting in a Δ of +3.73 cm. In contrast, the control group showed a slight decline, from −7.38 ± 10.03 cm (95% CI: −11.88 to −2.87) to −8.60 ± 10.73 cm (95% CI: −13.43 to −3.77), with a Δ of −1.22 cm. These findings suggest a positive effect of the MTC program on upper limb flexibility in postmenopausal women.
The mixed ANOVA for upper limb strength, assessed using the Arm Curl Test, revealed a significant group × time interaction (F(1,38) = 42.59, p < 0.001, η2p = 0.529), indicating that the effects of the intervention differed between the groups over time. Additionally, a significant main effect of time was observed (F(1,38) = 16.47, p < 0.001, η2p = 0.302), suggesting an overall improvement in upper limb strength from pre- to post-intervention. However, the main effect of group was not significant (F(1,38) = 0.63, p = 0.431, η2p = 0.016), indicating that the groups did not differ significantly when analyzed independently of time.
Descriptive results showed that the intervention group improved from 24.60 ± 8.85 repetitions (95% CI: 20.62 to 28.58) at baseline to 29.95 ± 8.78 repetitions (95% CI: 26.00 to 33.90) post-intervention, resulting in a Δ of +5.35 repetitions. In contrast, the control group declined from 31.65 ± 9.94 repetitions (95% CI: 27.18 to 36.12) to 27.45 ± 8.77 repetitions (95% CI: 23.51 to 31.39), corresponding to a Δ of −4.20 repetitions. These findings reinforce the effectiveness of the multicomponent training program in enhancing upper limb strength in postmenopausal women.
The mixed ANOVA for lower limb strength, assessed using the Chair Stand Test, revealed a significant group × time interaction (F(1,38) = 75.38, p < 0.001, η2p = 0.987), indicating that the changes in performance over time were different between the intervention and control groups. A significant main effect of time was also found (F(1,38) = 21.72, p < 0.001, η2p = 0.956), suggesting an overall improvement in lower limb strength across time points. However, the main effect of group was not significant (F(1,38) = 0.06, p = 0.811, η2p = 0.054), showing that there was no significant difference between groups when time was not considered.
Descriptively, the intervention group improved from 19.75 ± 4.24 repetitions (95% CI: 17.84 to 21.66) at baseline to 24.00 ± 4.31 repetitions (95% CI: 22.06 to 25.94) post-intervention, with a Δ of +4.25 repetitions. In contrast, the control group declined from 22.75 ± 6.60 repetitions (95% CI: 19.78 to 25.72) to 20.15 ± 6.70 repetitions (95% CI: 17.14 to 23.16), corresponding to a Δ of −2.60 repetitions. These results support the effectiveness of the MTC program in promoting significant gains in lower limb strength among postmenopausal women.
The Brunner–Langer nonparametric analysis for aerobic endurance, assessed using the 2 min Step Test, revealed a significant group × time interaction (ATS = 26.42, p < 0.001), indicating that the response to the intervention differed between the groups. A significant main effect of time was also observed (ATS = 5.50, p = 0.019), suggesting changes in aerobic capacity regardless of group assignment. However, the main effect of group was not statistically significant (ATS = 0.605, p = 0.436). The effect size was moderate (Cohen’s d = 0.426), indicating a meaningful impact of the intervention on aerobic endurance.
Descriptively, the intervention group increased its average step count from 114.50 ± 52.21 (95% CI: 91.02 to 137.98) at baseline to 136.60 ± 51.60 (95% CI: 113.40 to 159.80) after the intervention, resulting in a Δ of +22.10 steps. In contrast, the control group showed a decrease from 139.90 ± 40.00 (95% CI: 121.91 to 157.89) to 130.30 ± 35.22 (95% CI: 114.47 to 146.13), with a Δ of −9.60 steps. These results suggest that the multicomponent training program had a positive effect on aerobic endurance in postmenopausal women.
The Brunner–Langer nonparametric analysis for lower limb flexibility, assessed using the Sit-and-Reach Test, revealed a significant group × time interaction (ATS = 34.232, p < 0.001), indicating that the effects of the intervention differed significantly between the groups over time. A significant main effect of time was also found (ATS = 5.078, p = 0.024), suggesting improvements in flexibility regardless of group. However, the main effect of group was not significant (ATS = 0.020, p = 0.885). The effect size was moderate (Cohen’s d = 0.437), indicating a meaningful intervention impact on this variable.
Descriptive analysis showed that the intervention group improved from 4.85 ± 6.75 cm (95% CI: 1.82 to 7.88) at baseline to 7.82 ± 6.85 cm (95% CI: 4.74 to 10.90) after the intervention, resulting in a Δ of +2.97 cm. In contrast, the control group declined from 6.78 ± 7.29 cm (95% CI: 3.49 to 10.06) to 5.10 ± 7.80 cm (95% CI: 1.59 to 8.61), with a Δ of −1.68 cm. These findings suggest that the MTC program contributed to meaningful improvements in lower limb flexibility in postmenopausal women.
The Brunner–Langer nonparametric analysis for agility, assessed using the Timed Up and Go (TUG) test, revealed a significant group × time interaction (ATS = 6.792, p = 0.009), suggesting that the changes in agility over time differed significantly between the intervention and control groups. No significant main effects were observed for group (ATS = 0.768, p = 0.380) or time (ATS = 0.421, p = 0.516). The effect size, expressed as Cohen’s d, was −0.388, indicating a small-to-moderate effect in favor of the intervention group (noting that lower times represent better performance).
Descriptively, the intervention group improved by reducing its average TUG time from 4.87 ± 0.88 s (95% CI: 4.47 to 5.27) at baseline to 4.60 ± 0.44 s (95% CI: 4.40 to 4.80) post-intervention (Δ = −0.27 s). Conversely, the control group showed a decline in performance, increasing from 4.74 ± 0.73 s (95% CI: 4.41 to 5.07) to 4.99 ± 0.78 s (95% CI: 4.63 to 5.34), with a Δ of +0.25 s. These results highlight the effectiveness of the MTC program in enhancing agility among older women.
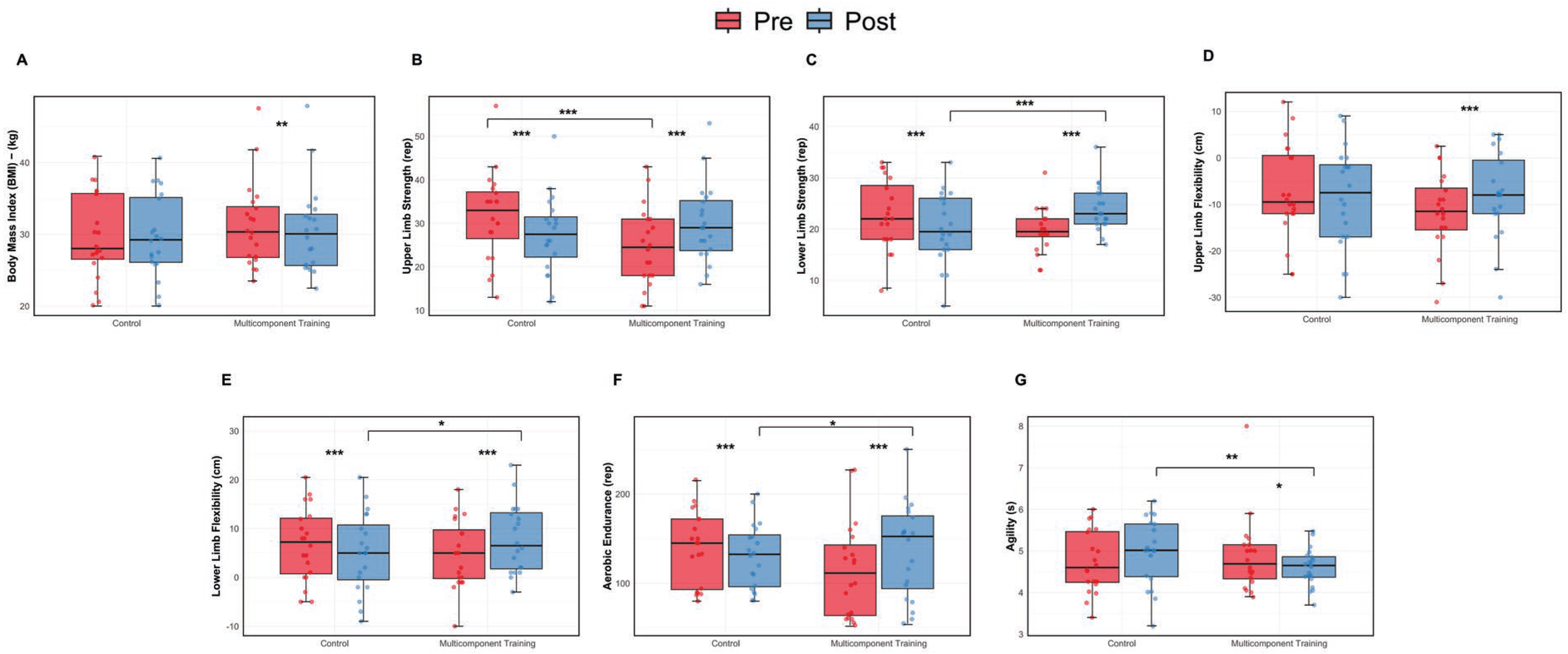
These results highlight the effectiveness of the MTC program in enhancing agility among older women (Figure 3).
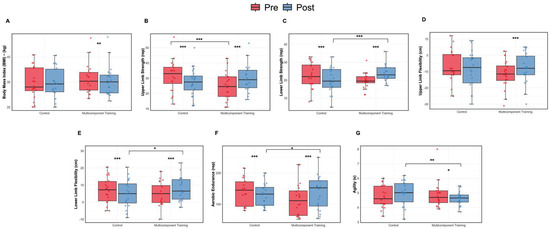
Figure 3.
Body composition and functional fitness assessment: pre and post intervention comparisons. (A) Body Mass Index (BMI). (B) Upper limb strength. (C) Lower limb strength. (D) Upper limb flexibility. (E) Lower limb flexibility. (F) Aerobic endurance. (G) Agility. Data are presented as boxplots showing median and individual values. * p < 0.05, ** p < 0.01; *** p < 0.001.
4. Discussion
The objective of this study was to evaluate the role of multicomponent exercise in fall prevention and the improvement of functional fitness in older women, with a particular focus on the Portuguese population. One of the main functional outcomes assessed was mobility, evaluated through the Timed Up and Go (TUG) test. This test is a widely used and validated tool to assess functional mobility and dynamic balance in community-dwelling older adults [69]. Although it is commonly included in fall risk assessments, its ability to predict falls is limited in individuals with preserved mobility [70].
However, clinical cut-off points have been proposed in the literature. For instance, individuals who take longer than 13.5 s to complete the TUG test are considered at increased risk of falls, with both sensitivity and specificity reported at 87% [71]. Therefore, the improvements observed in TUG performance in this study should be interpreted primarily as gains in functional mobility and balance, rather than conclusive evidence of reduced fall risk.
The results demonstrated that participants who engaged in the multicomponent training program showed a significant improvement in TUG performance, indicating gains in agility and motor control. These findings suggests that the exercise program was effective in enhancing the functional capacity of the participants, making them more capable of performing daily activities with a lower risk of falls. Previous studies have already demonstrated that interventions based on physical exercises, especially those that include strength, balance, and coordination training, are essential in fall prevention among older adults [49,50,56,57].
In addition to the TUG, the results also indicated improvements in lower limb strength, flexibility, and aerobic endurance. The significant interaction between group and time for these outcomes reinforces the idea that a well-structured exercise program can not only maintain but also reverse age-related functional decline [72]. The improvement in lower limb strength is particularly relevant, as muscle weakness is directly associated with the inability to respond appropriately to postural disturbances, increasing the risk of falls [73]. These results are consistent with previous findings that highlight the role of strength gains in enhancing postural stability and reducing fall risk in older adults [74,75,76].
The literature indicates that multicomponent training programs are more effective in fall prevention [77,78], when compared to interventions focused on just one component, such as aerobic or strength training in isolation [29,79]. In the meta-analysis conducted by Sadaqa et al., the combination of strength, balance, and agility exercises proved to be an effective strategy to improve functionality and reduce risk factors for falls, reinforcing the need to promote this type of intervention in aging populations [29].
It is worth noting that several functional fitness tests used in this study have established cut-off values that are associated with an increased risk of falling. For instance, in the Timed Up and Go (TUG) test, values above 13.5 s have been linked to a higher fall risk in older adults [71]. Similarly, reduced performance in lower limb strength and flexibility tests has been associated with impaired balance and mobility [29]. Therefore, the significant improvements observed in TUG performance and other functional parameters in the intervention group may reflect not only statistical but also clinically meaningful reductions in fall risk.
Despite the relevant findings, this study presents some limitations. The sample size, while sufficient to detect differences, limits generalizability. Moreover, the exclusive inclusion of women prevents assessment of possible sex-related differences in training response, which should be explored in future research. External factors such as physical activity outside the program, diet, and session adherence were not fully controlled and may have influenced the outcomes.
In interpreting these findings, several methodological limitations should also be considered. Although the study followed a single-blind design with blinded outcome assessors, participants and instructors were aware of group allocation, which may have introduced performance or expectancy bias. Additionally, the control group did not receive a placebo or structured intervention, and incidental physical activity was not systematically monitored. While exercise intensity was guided by the Borg Rating of Perceived Exertion and adjusted individually, objective measures of intensity (e.g., heart rate, workload) were not recorded. The sample consisted solely of community-dwelling women, further limiting generalizability. Finally, although improvements were seen in functional outcomes associated with fall risk, the study did not directly assess fall incidence. These factors must be taken into account when interpreting the results and drawing conclusions about the broader applicability of the intervention.
Future studies could include larger, more diverse samples and adopt a longitudinal design to assess the long-term effects of multicomponent training. Exploring its application in different settings—such as community centers, clinics, or institutions—and among populations with reduced mobility or prior falls may enhance the understanding of its effectiveness. The integration of technologies, like motion sensors or mobile apps, may also support monitoring and adherence.
Finally, combining multicomponent training with other interventions, such as nutritional or cognitive strategies, may further enhance its benefits. This multifaceted approach can be key to promoting healthier, more independent aging and contributing to the sustainability of healthcare systems.
5. Conclusions
This study demonstrated that multicomponent exercise is an effective strategy to improve functional fitness in older women. The observed gains in mobility, strength, balance, and endurance—particularly the improvement in TUG performance—highlight the positive impact of this approach on physical function. These findings support the use of structured multicomponent training as a practical and beneficial intervention to enhance functional capacity and promote autonomy during aging. While improvements were seen in parameters associated with fall risk, further research is needed to determine whether such interventions lead to a direct reduction in fall incidence.
Author Contributions
Conceptualization, A.S. and L.B.L.; data curation, A.S.; formal analysis, A.S. and L.B.L.; investigation, A.S. and J.T.; methodology, A.S. and L.B.L.; project administration, A.M.M.; resources, A.M.M.; supervision, T.M.B. and A.M.M.; writing—original draft, A.S.; writing–review and editing, L.B.L., J.T., P.F., T.M.B. and A.M.M. All authors will be updated at each stage of manuscript processing, including submission, revision, and revision reminder, via emails from our system or the assigned Assistant Editor. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee at the Polytechnic Institute of Bragança (IPB) (2067313) on 5 March 2024.
Informed Consent Statement
Informed consent was obtained from all participants included in the study.
Data Availability Statement
The data supporting the findings of this study are not publicly available due to privacy or ethical restrictions but can be provided by the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ismail, Z.; Ahmad, W.I.W.; Hamjah, S.H.; Astina, I.K. The Impact of Population Ageing: A Review. Iran. J. Public Health 2021, 50, 2451–2460. [Google Scholar] [CrossRef] [PubMed]
- Ageing and Health [Internet]. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 20 December 2024).
- Bárrios, M.J.; Marques, R.; Fernandes, A.A. Aging with health: Aging in place strategies of a Portuguese population aged 65 years or older. Rev. Saude Publica 2020, 54, 129. [Google Scholar] [CrossRef]
- Ageing Europe—Statistics on Population Developments [Internet]. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Ageing_Europe_-_statistics_on_population_developments (accessed on 14 March 2025).
- Rony, M.K.K.; Parvin, M.R.; Wahiduzzaman, M.d.; Akter, K.; Ullah, M. Challenges and Advancements in the Health-Related Quality of Life of Older People. Adv. Public Health 2024, 2024, 8839631. [Google Scholar] [CrossRef]
- Dharmarajan, T.S. Physiology of Aging. In Geriatr Gastroenterol; Pitchumoni, C.S., Dharmarajan, T.S., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–54. Available online: https://doi.org/10.1007/978-3-319-90761-1_5-1 (accessed on 14 March 2025).
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef] [PubMed]
- Mi, B.; Xiong, Y.; Knoedler, S.; Alfertshofer, M.; Panayi, A.C.; Wang, H.; Lin, S.; Li, G.; Liu, G. Ageing-related bone and immunity changes: Insights into the complex interplay between the skeleton and the immune system. Bone Res. 2024, 12, 42. [Google Scholar] [CrossRef]
- Völter, C.; Thomas, J.P.; Maetzler, W.; Guthoff, R.; Grunwald, M.; Hummel, T. Sensory Dysfunction in Old Age. Dtsch. Ärzteblatt Int. 2021, 118, 512–520. [Google Scholar] [CrossRef]
- Seidler, R.D.; Bernard, J.A.; Burutolu, T.B.; Fling, B.W.; Gordon, M.T.; Gwin, J.T.; Kwak, Y.; Lipps, D.B. Motor Control and Aging: Links to Age-Related Brain Structural, Functional, and Biochemical Effects. Neurosci. Biobehav. Rev. 2010, 34, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Kohler, R.; Rorato, P.; Braga, A.L.F.; Velho, R.B.; Krause, M.P. Effects of Aging and Exercise on the Cardiorespiratory Fitness of Older Women. Rev. Bras. Geriatr. Gerontol. 2016, 19, 603–612. [Google Scholar] [CrossRef]
- Ferrucci, L.; Cooper, R.; Shardell, M.; Simonsick, E.M.; Schrack, J.A.; Kuh, D. Age-Related Change in Mobility: Perspectives from Life Course Epidemiology and Geroscience. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2016, 71, 1184–1194. [Google Scholar] [CrossRef]
- Oduro, J.K.; Okyere, J.; Nyador, J.K.M.T. Risky health behaviours and chronic conditions among aged persons: Analysis of SAGE selected countries. BMC Geriatr. 2023, 23, 145. [Google Scholar] [CrossRef]
- Montero-Odasso, M.; Van Der Velde, N.; Martin, F.C.; Petrovic, M.; Tan, M.P.; Ryg, J.; Aguilar-Navarro, S.; Alexander, N.B.; Becker, C.; Blain, H.; et al. World guidelines for falls prevention and management for older adults: A global initiative. Age Ageing 2022, 51, afac205. [Google Scholar] [CrossRef] [PubMed]
- Dionyssiotis, Y. Analyzing the problem of falls among older people. Int. J. Gen. Med. 2012, 5, 805–813. [Google Scholar] [CrossRef]
- Cunningham, C.; O’Sullivan, R.; Caserotti, P.; Tully, M.A. Consequences of physical inactivity in older adults: A systematic review of reviews and meta-analyses. Scand. J. Med. Sci. Sports 2020, 30, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Langhammer, B.; Bergland, A.; Rydwik, E. The Importance of Physical Activity Exercise Among Older People. BioMed Res. Int. 2018, 2018, 7856823. [Google Scholar] [CrossRef] [PubMed]
- Vaishya, R.; Vaish, A. Falls in Older Adults are Serious. Indian J. Orthop. 2020, 54, 69–74. [Google Scholar] [CrossRef]
- Berg, R.L.; Cassells, J.S. Falls in Older Persons: Risk Factors and Prevention. In The Second Fifty Years: Promoting Health and Preventing Disability; National Academies Press (US): Washington, DC, USA, 1992. Available online: https://www.ncbi.nlm.nih.gov/books/NBK235613/ (accessed on 14 March 2025).
- Giovannini, S.; Brau, F.; Galluzzo, V.; Santagada, D.A.; Loreti, C.; Biscotti, L.; Laudisio, A.; Zuccala, G.; Bernabei, R. Falls Among Older Adults: Screening, Identification, Rehabilitation, and Management. Appl. Sci. 2022, 12, 7934. [Google Scholar] [CrossRef]
- Lee, D.; Tak, S.H. A concept analysis of fear of falling in older adults: Insights from qualitative research studies. BMC Geriatr. 2023, 23, 651. [Google Scholar] [CrossRef]
- Ferreira, C.R.; Mascarenhas-Melo, F.; Rodrigues, A.R.; Lima, M.J.R.; Pinheiro, J.P.; Chaves, C.; Teixeira-Lemos, E.; Bell, V. Characterisation of institutionalised Portuguese older adult fallers: Is there a place for pharmacist intervention? A preliminary study. Pharm. Pract. 2022, 20, 2717. [Google Scholar] [CrossRef]
- Fuller, G.F. Falls in the elderly. Am. Fam. Physician 2000, 61, 2159–2168. [Google Scholar]
- Tropeções, Quedas e Trambolhões [Internet]. Available online: https://www.sns.gov.pt/noticias/2017/12/19/tropecoes-quedas-e-trambolhoes/ (accessed on 14 March 2025).
- Chidume, T. Promoting older adult fall prevention education and awareness in a community setting: A nurse-led intervention. Appl. Nurs. Res. 2021, 57, 151392. [Google Scholar] [CrossRef]
- Garatachea, N.; Pareja-Galeano, H.; Sanchis-Gomar, F.; Santos-Lozano, A.; Fiuza-Luces, C.; Morán, M.; Emanuele, E.; Joyner, M.J.; Lucia, A. Exercise attenuates the major hallmarks of aging. Rejuvenation Res. 2015, 18, 57–89. [Google Scholar] [CrossRef]
- Sun, M.; Min, L.; Xu, N.; Huang, L.; Li, X. The Effect of Exercise Intervention on Reducing the Fall Risk in Older Adults: A Meta-Analysis of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2021, 18, 12562. [Google Scholar] [CrossRef]
- Sherrington, C.; Fairhall, N.; Kwok, W.; Wallbank, G.; Tiedemann, A.; Michaleff, Z.A.; Ng, C.A.; Bauman, A. Evidence on physical activity and falls prevention for people aged 65+ years: Systematic review to inform the WHO guidelines on physical activity and sedentary behaviour. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 144. [Google Scholar] [CrossRef]
- Sadaqa, M.; Németh, Z.; Makai, A.; Prémusz, V.; Hock, M. Effectiveness of exercise interventions on fall prevention in ambulatory community-dwelling older adults: A systematic review with narrative synthesis. Front. Public Health 2023, 11, 1209319. [Google Scholar] [CrossRef]
- Sherrington, C.; Michaleff, Z.A.; Fairhall, N.; Paul, S.S.; Tiedemann, A.; Whitney, J.; Cumming, R.G.; Herbert, R.D.; Close, J.C.; Lord, S.R. Exercise to Prevent Falls in Older Adults: An Updated Systematic Review and Meta-Analysis. 2017. Available online: https://bjsm.bmj.com/content/51/24/1750 (accessed on 14 March 2025).
- Sherrington, C.; Fairhall, N.J.; Wallbank, G.K.; Tiedemann, A.; Michaleff, Z.A.; Howard, K.; Clemson, L.; Hopewell, S.; Lamb, S.E. Exercise for preventing falls in older people living in the community. Cochrane Database Syst. Rev. 2019, 1, CD012424. [Google Scholar] [CrossRef]
- Dyer, S.M.; Suen, J.; Kwok, W.S.; Dawson, R.; McLennan, C.; Cameron, I.D.; Hill, K.D.; Sherrington, C. Exercise for falls prevention in aged care: Systematic review and trial endpoint meta-analyses. Age Ageing 2023, 52, afad217. [Google Scholar] [CrossRef]
- Murray, K.O.; Mahoney, S.A.; Venkatasubramanian, R.; Seals, D.R.; Clayton, Z.S. Aging, aerobic exercise, and cardiovascular health: Barriers, alternative strategies and future directions. Exp. Gerontol. 2023, 173, 112105. [Google Scholar] [CrossRef]
- Chapman, S.B.; Aslan, S.; Spence, J.S.; DeFina, L.F.; Keebler, M.W.; Didehbani, N.; Lu, H. Shorter term aerobic exercise improves brain, cognition, and cardiovascular fitness in aging. Front. Aging Neurosci. 2013, 5, 75. [Google Scholar] [CrossRef]
- Lavin, K.M.; Roberts, B.M.; Fry, C.S.; Moro, T.; Rasmussen, B.B.; Bamman, M.M. The Importance of Resistance Exercise Training to Combat Neuromuscular Aging. Physiology 2019, 34, 112–122. [Google Scholar] [CrossRef]
- Cannataro, R.; Cione, E.; Bonilla, D.A.; Cerullo, G.; Angelini, F.; D’Antona, G. Strength training in elderly: An useful tool against sarcopenia. Front. Sports Act. Living 2022, 4, 950949. [Google Scholar] [CrossRef]
- Dunsky, A. The Effect of Balance and Coordination Exercises on Quality of Life in Older Adults: A Mini-Review. Front. Aging Neurosci. 2019, 11, 318. [Google Scholar] [CrossRef] [PubMed]
- Dunsky, A.; Unger, L.; Carasso, R.; Fox, O. The Effect of a Single Session of Balance and Coordination Training on Cognitive Function in Older Adults. Appl. Sci. 2023, 13, 3598. [Google Scholar] [CrossRef]
- Labata-Lezaun, N.; Gonzalez-Rueda, V.; Llurda-Almuzara, L.; Lopez-de-Celis, C.; Rodriguez-Sanz, J.; Bosch, J.; Vicente-Rodriguez, G.; Gorczakowska, D.; Araluze-Arizti, P.; Perez-Bellmunt, A. Effectiveness of multicomponent training on physical performance in older adults: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2023, 104, 104838. [Google Scholar] [CrossRef]
- Rodrigues, F.; Matos, R.; Jacinto, M.; Antunes, R.; Monteiro, D.; Amaro, N. A Comparison of Multicomponent and Concurrent Exercise Protocols on Muscle Strength in Older Adults. Motricidade 2024, 20. [Google Scholar] [CrossRef]
- Casas-Herrero, Á.; Saez de Asteasu, M.L.; Antón-Rodrigo, I.; Sánchez-Sánchez, J.L.; Montero-Odasso, M.; Marín-Epelde, I.; Ramón-Espinoza, F.; Zambom-Ferraresi, F.; Petidier-Torregrosa, R.; Elexpuru-Estomba, J.; et al. Effects of Vivifrail multicomponent intervention on functional capacity: A multicentre, randomized controlled trial. J. Cachexia Sarcopenia Muscle 2022, 13, 884–893. [Google Scholar] [CrossRef]
- Kruse, A.; Cordes, T.; Schulz, S.; Wollesen, B. Feasibility of Multicomponent Training for People with Moderate to Severe Dementia Living in a Long-Term Care Home: A Social Ethical Approach. Int. J. Environ. Res. Public Health 2021, 18, 7631. [Google Scholar] [CrossRef]
- Philippe, A.G.; Goncalves, A.; Martinez, C.; Deshayes, M.; Charbonnier, E. Can an Eight-Session Multicomponent Physical Exercise Program Reduce Fall Risk and Fear of Falling among the Elderly? Int. J. Environ. Res. Public Health 2022, 19, 8262. [Google Scholar] [CrossRef]
- Sadaqa, M.; Debes, W.A.; Németh, Z.; Bera-Baka, Z.; Vachtler-Szepesi, M.; Nácziné Földes, L.; Prémusz, V.; Hock, M. Multicomponent Exercise Intervention for Preventing Falls and Improving Physical Functioning in Older Nursing Home Residents: A Single-Blinded Pilot Randomised Controlled Trial. J. Clin. Med. 2024, 13, 1577. [Google Scholar] [CrossRef]
- Dolenc, M.; Knific, T.; Tomažin, K. A multicomponent exercise programme to prevent falls and frailty in community-dwelling older adults. Eur. J. Public Health 2023, 33, ckad133.174. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, J.L.; Udina, C.; Medina-Rincón, A.; Esbrí-Victor, M.; Bartolomé-Martín, I.; Moral-Cuesta, D.; Marín-Epelde, I.; Ramon-Espinoza, F.; Latorre, M.S.; Idoate, F.; et al. Effect of a multicomponent exercise program and cognitive stimulation (VIVIFRAIL-COGN) on falls in frail community older persons with high risk of falls: Study protocol for a randomized multicenter control trial. BMC Geriatr. 2022, 22, 612. [Google Scholar] [CrossRef]
- Osuka, Y.; Nofuji, Y.; Seino, S.; Maruo, K.; Oka, H.; Shinkai, S.; Fujiwara, Y.; Sasai, H. The effect of a multicomponent intervention on occupational fall-related factors in older workers: A pilot randomized controlled trial. J. Occup. Health 2022, 64, e12374. [Google Scholar] [CrossRef] [PubMed]
- Sedaghati, P.; Goudarzian, M.; Ahmadabadi, S.; Tabatabai-Asl, S. The impact of a multicomponent-functional training with postural correction on functional balance in the elderly with a history of falling. J. Exp. Orthop. 2022, 9, 23. [Google Scholar] [CrossRef]
- Linhares, D.G.; Borba-Pinheiro, C.J.; de Castro, J.B.P.; dos Santos, A.O.B.; dos Santos, L.L.; Cordeiro, L.D.S.; Drigo, A.J.; Nunes, R.D.A.M.; Vale, R.G.D.S. Effects of Multicomponent Exercise Training on the Health of Older Women with Osteoporosis: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 14195. [Google Scholar] [CrossRef]
- Rodrigues, K.; Prado, L.; de Almeida, M.; Yamada, A.; Finzeto, L.; Bueno Junior, C. Effects of Combined Versus Multicomponent Training in Physically Active Women Aged 50–75 Years. Res. Q. Exerc. Sport 2022, 93, 710–717. [Google Scholar] [CrossRef]
- Cancela Carral, J.; Rodrigues, L.; Bezerra, P. The Long-Term Benefits of a Multicomponent Physical Activity Program to Body Composition, Muscle Strength, Cardiorespiratory Capacity, and Bone Mineral Density in a Group of Nonagenarians. Rejuvenation Res. 2019, 23, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Healthy Ageing and Functional Ability [Internet]. Available online: https://www.who.int/news-room/questions-and-answers/item/healthy-ageing-and-functional-ability (accessed on 14 March 2025).
- Talaulikar, V. Menopause transition: Physiology and symptoms. Best Pract. Res. Clin. Obstet. Gynaecol. 2022, 81, 3–7. [Google Scholar] [CrossRef]
- Gale, C.R.; Cooper, C.; Aihie Sayer, A. Prevalence and risk factors for falls in older men and women: The English Longitudinal Study of Ageing. Age Ageing. 2016, 45, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Fu, H. Relationship between proprioception and balance control among Chinese senior older adults. Front. Physiol. 2022, 13, 1078087. [Google Scholar] [CrossRef]
- Sobrinho, A.; Almeida, M.; Rodrigues, G.; Bertani, R.; Lima, J.; Bueno Junior, C. Stretching and Multicomponent Training to Functional Capacities of Older Women: A Randomized Study. Int. J. Environ. Res. Public Health 2021, 19, 27. [Google Scholar] [CrossRef]
- Monteiro, A.; Rodrigues, S.; Matos, S.; Teixeira, J.; Barbosa, T.; Forte, P. The Effects of 32 Weeks of Multicomponent Training with Different Exercises Order in Elderly Women’s Functional Fitness and Body Composition. Medicina 2022, 58, 628. [Google Scholar] [CrossRef]
- Chan, A.-W.; Tetzlaff, J.M.; Altman, D.G.; Laupacis, A.; Gøtzsche, P.C.; Krleža-Jerić, K.; Hróbjartsson, A.; Mann, H.; Dickersin, K.; Berlin, J.A.; et al. SPIRIT 2013 statement: Defining standard protocol items for clinical trials. Ann. Intern. Med. 2013, 158, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Eldridge, S.M.; Chan, C.L.; Campbell, M.J.; Bond, C.M.; Hopewell, S.; Thabane, L.; Lancaster, G.A. CONSORT 2010 statement: Extension to randomised pilot and feasibility trials. BMJ 2016, 355, i5239. [Google Scholar] [CrossRef] [PubMed]
- Body Mass Index: Obesity, BMI, and Health: A Critical Review—PMC. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC4890841/ (accessed on 14 March 2025).
- A Healthy Lifestyle—WHO Recommendations [Internet]. Available online: https://www.who.int/europe/news-room/fact-sheets/item/a-healthy-lifestyle---who-recommendations (accessed on 14 March 2025).
- Rikli, R.E.; Jones, C.J. Development and Validation of a Functional Fitness Test for Community-Residing Older Adults. J. Aging Phys. Act. 1999, 7, 129–161. [Google Scholar] [CrossRef]
- Carvalho, M.; Marques, E.; Mota, J. Training and detraining effects on functional fitness after a multicomponent training in older women. Gerontology 2009, 55, 41–48. [Google Scholar] [CrossRef]
- Izquierdo, M.; Merchant, R.A.; Morley, J.E.; Anker, S.D.; Aprahamian, I.; Arai, H.; Aubertin-Leheudre, M.; Bernabei, R.; Cadore, E.L.; Cesari, M.; et al. International Exercise Recommendations in Older Adults (ICFSR): Expert Consensus Guidelines. J. Nutr. Health Aging 2021, 25, 824–853. [Google Scholar] [CrossRef]
- Hanusz, Z.; Tarasinska, J.; Zieliński, W. Shapiro–Wilk Test with Known Mean. REVSTAT-Stat. J. 2016, 14, 89–100. [Google Scholar]
- Gastwirth, J.; Gel, Y.; Miao, W. The Impact of Levene’s Test of Equality of Variances on Statistical Theory and Practice. Stat. Sci. 2010, 24, 343–360. [Google Scholar] [CrossRef]
- Sawyer, S. Analysis of Variance: The Fundamental Concepts. J. Man. Manip. Ther. 2009, 17, 27E–38E. [Google Scholar] [CrossRef]
- Andrade, C. The P Value and Statistical Significance: Misunderstandings, Explanations, Challenges, and Alternatives. Indian J. Psychol. Med. 2019, 41, 210–215. [Google Scholar] [CrossRef]
- Rodrigues, F.; Teixeira, J.E.; Forte, P. The Reliability of the Timed Up and Go Test Among Portuguese Elderly. Healthcare 2023, 11, 928. [Google Scholar] [CrossRef]
- Barry, E.; Galvin, R.; Keogh, C.; Horgan, F.; Fahey, T. Is the Timed Up and Go Test a Useful Predictor of Risk of Falls in Community Dwelling Older Adults: A Systematic Review and Meta- Analysis. BMC Geriatr. 2014, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Timed Up and Go Test—An Overview|ScienceDirect Topics [Internet]. Available online: https://www.sciencedirect.com/topics/medicine-and-dentistry/timed-up-and-go-test (accessed on 14 May 2025).
- Halma, M.; Marik, P.; Varon, J.; Tuszynski, J. Reversing Decline in Aging Muscles: Expected Trends, Impacts and Remedies. J. Funct. Morphol. Kinesiol. 2025, 10, 29. [Google Scholar] [CrossRef]
- Cho, K.H.; Bok, S.K.; Kim, Y.-J.; Hwang, S.L. Effect of Lower Limb Strength on Falls and Balance of the Elderly. Ann. Rehabil. Med. 2012, 36, 386–393. [Google Scholar] [CrossRef]
- de Almeida Nagata, C.; Hamu, T.C.D.D.S.; Pelicioni, P.H.S.; Durigan, J.L.Q.; Garcia, P.A. Influence of lower limb isokinetic muscle strength and power on the occurrence of falls in community-dwelling older adults: A longitudinal study. PLoS ONE 2024, 19, e0300818. [Google Scholar] [CrossRef]
- Porto, J.M.; Cangussu-Oliveira, L.M.; Freire Junior, R.C.; Vieira, F.T.; Capato, L.L.; de Oliveira, B.G.M.; de Abreu, D.C.C. Relationship Between Lower Limb Muscle Strength and Future Falls Among Community-Dwelling Older Adults with No History of Falls: A Prospective 1-Year Study. J. Appl. Gerontol. 2021, 40, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.T.L.; Zuo, J.J.J.; Li, K.J.; Lam, F.M.H.; Wong, A.Y.L.; Yang, L.; Bai, X.; Wong, M.S.; Kwok, T.; Zheng, Y.P.; et al. Association of lower-limb strength with different fall histories or prospective falls in community-dwelling older people: A systematic review and meta-analysis. BMC Geriatr. 2025, 25, 83. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, H.; Rezola-Pardo, C.; Gil, S.M.; Virgala, J.; Iturburu, M.; Antón, I.; González-Templado, V.; Irazusta, J.; Rodriguez-Larrad, A. Effects of Multicomponent Exercise on Frailty in Long-Term Nursing Homes: A Randomized Controlled Trial. J. Am. Geriatr. Soc. 2019, 67, 1145–1151. [Google Scholar] [CrossRef]
- de Souto Barreto, P.; Cesari, M.; Denormandie, P.; Armaingaud, D.; Vellas, B.; Rolland, Y. Exercise or Social Intervention for Nursing Home Residents with Dementia: A Pilot Randomized, Controlled Trial. J. Am. Geriatr. Soc. 2017, 65, E123–E129. [Google Scholar] [CrossRef]
- Sousa, N.; Mendes, R.; Silva, A.; Oliveira, J. Combined exercise is more effective than aerobic exercise in the improvement of fall risk factors: A randomized controlled trial in community-dwelling older men. Clin. Rehabil. 2017, 31, 478–486. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).