Dermacentor reticulatus (Fabricius, 1794) in Southwestern Poland: Changes in Range and Local Scale Updates
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
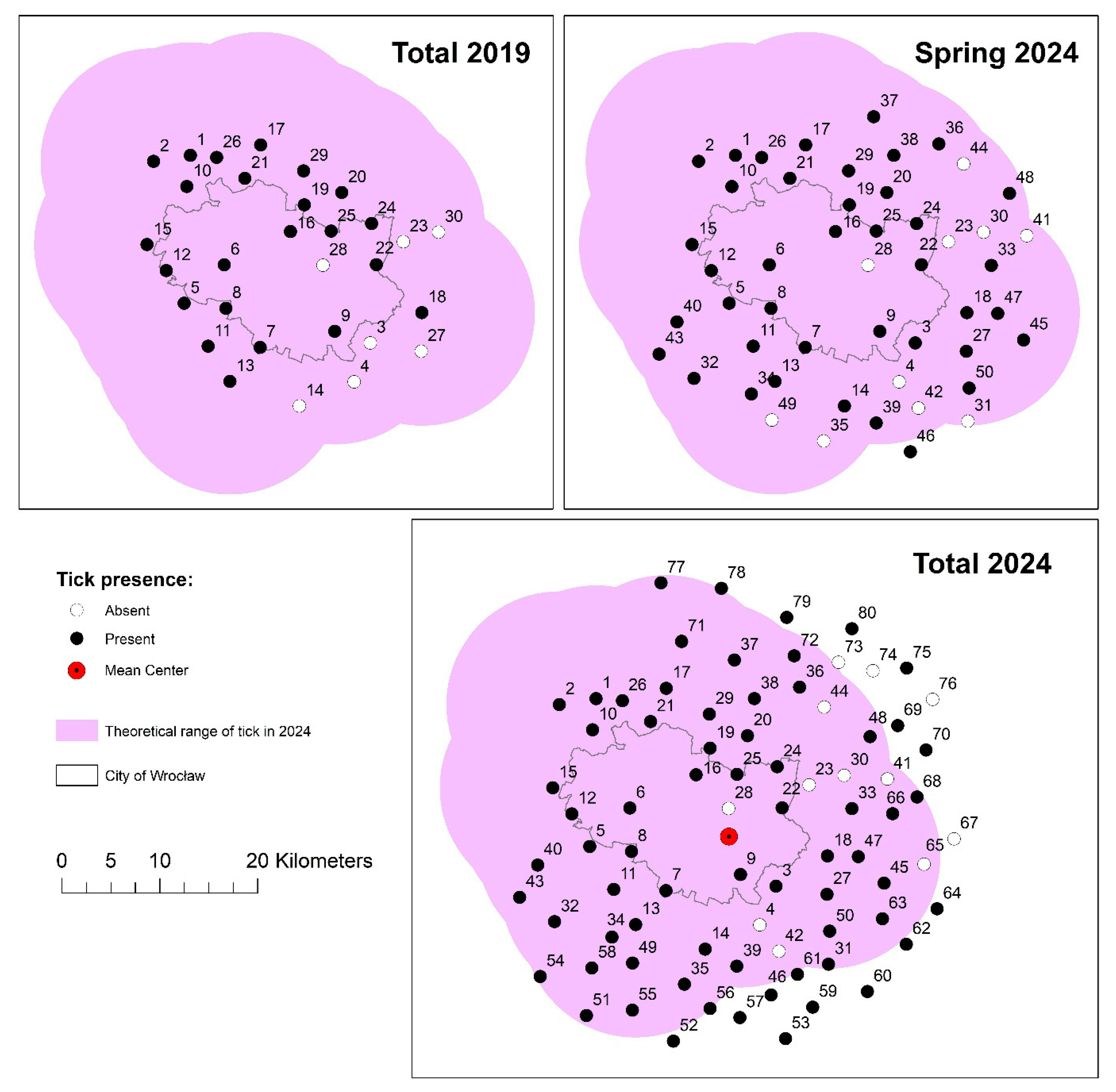
2.1. Tick Collection and Study Area
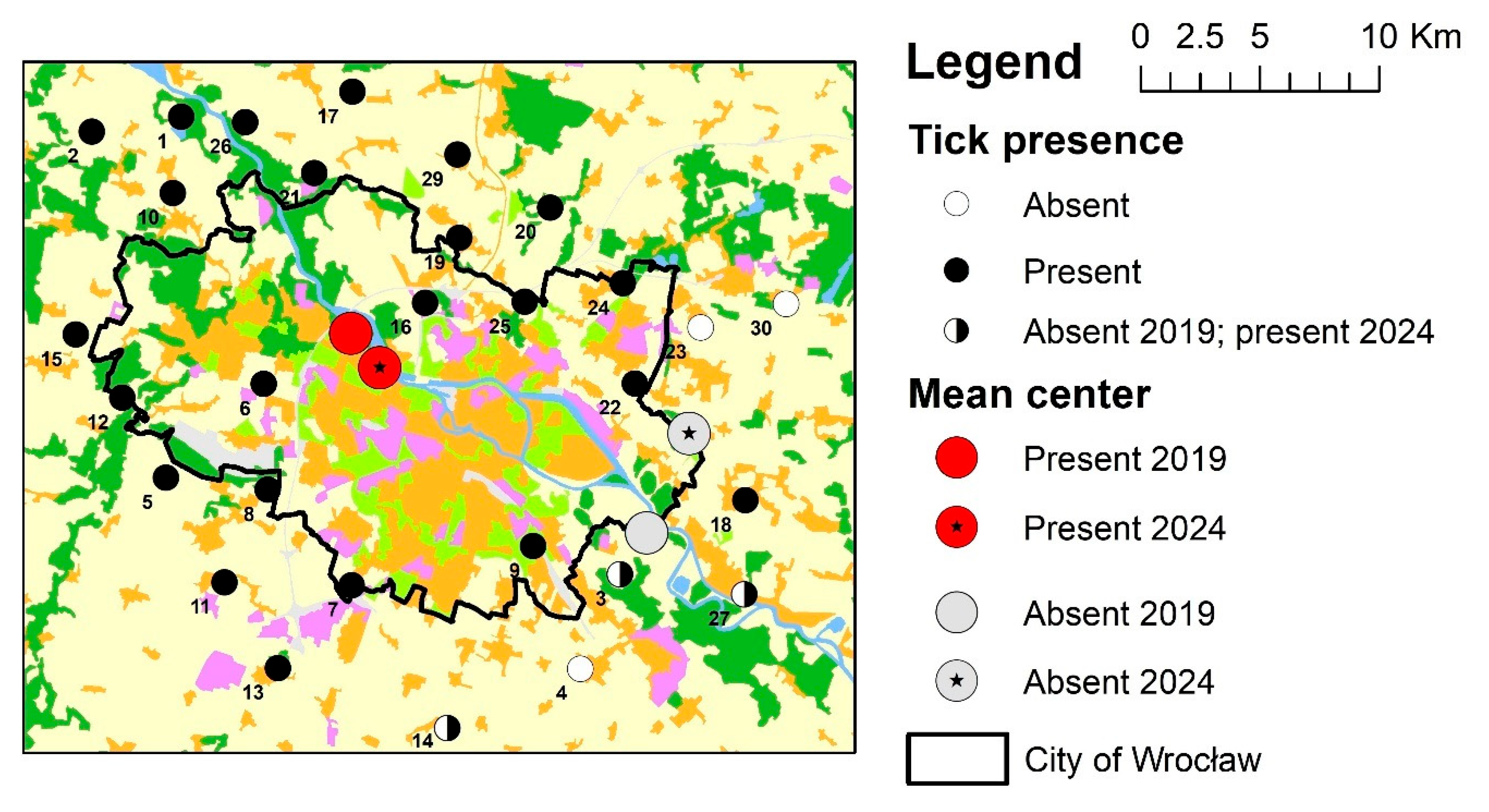
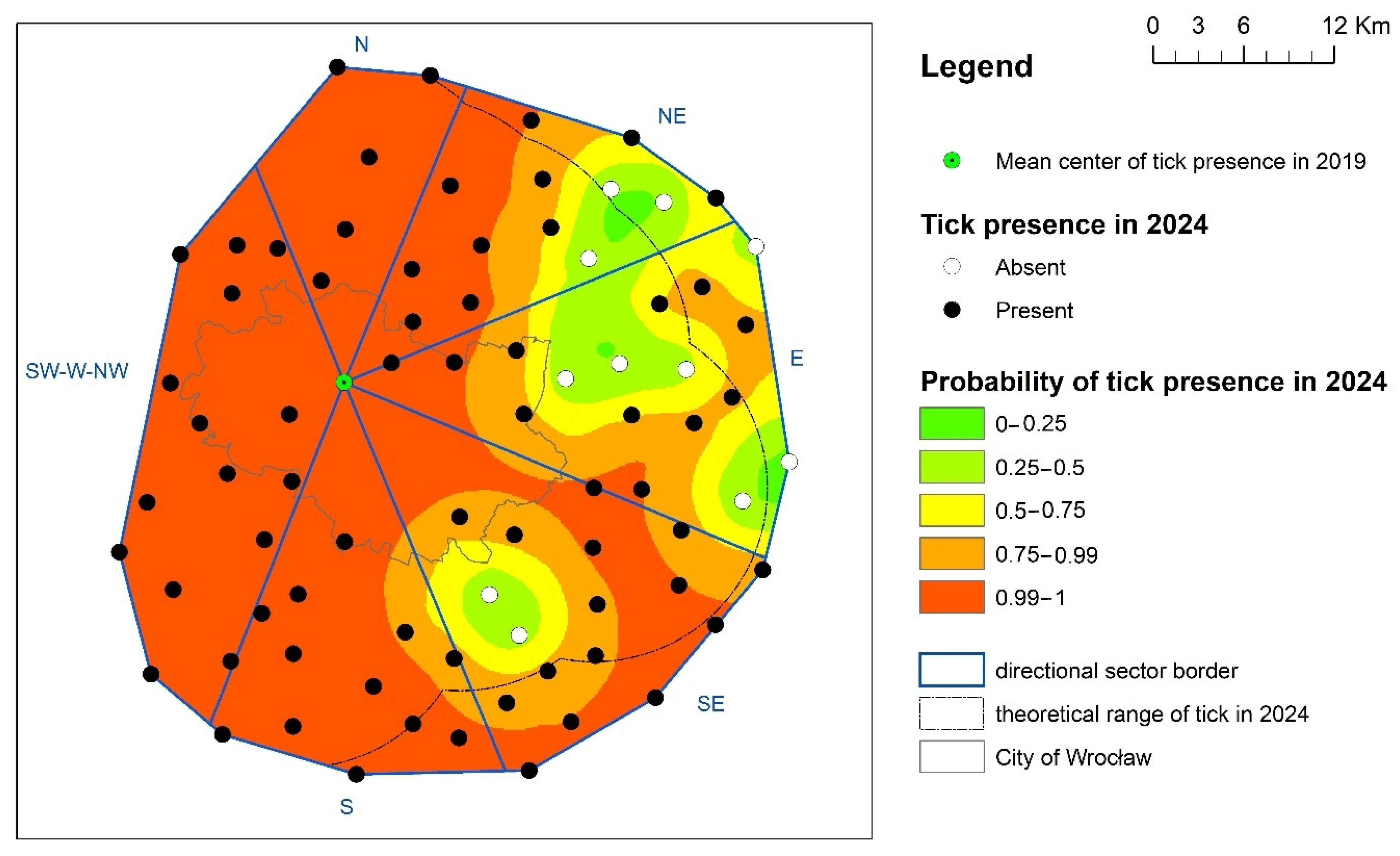
2.2. Analysis of D. reticulatus Tick Distribution and the Rate of Change in Its Range
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Földvári, G.; Široký, P.; Szekeres, S.; Majoros, G.; Sprong, H. Dermacentor reticulatus: A vector on the rise. Parasit Vectors 2016, 9, 314. [Google Scholar] [CrossRef] [PubMed]
- Rubel, F.; Brugger, K.; Belova, O.A.; Kholodilov, I.S.; Didyk, Y.M.; Kurzrock, L.; García-Pérez, A.L.; Kahl, O. Vectors of disease at the northern distribution limit of the genus Dermacentor in Eurasia: D. reticulatus and D. silvarum. Exp. Appl. Acarol. 2020, 82, 95–123. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Available online: https://www.ecdc.europa.eu/en/publications-data/dermacentor-reticulatus-current-known-distribution-october-2023 (accessed on 17 March 2025).
- Noll, M.; Wall, R.; Makepeace, B.L.; Vineer, H.R. Distribution of ticks in the Western Palearctic: An updated systematic review (2015–2021). Parasit Vectors 2023, 16, 141. [Google Scholar] [CrossRef] [PubMed]
- Heile, C.; Heydorn, A.O.; Schein, E. Dermacentor reticulatus (Fabricius, 1794) -distribution, biology and vector for Babesia canis in Germany. Berl. Munch Tierarztl Wochenschr. 2006, 119, 330–334. [Google Scholar] [PubMed]
- Dautel, H.; Dippel, C.; Oehme, R.; Hartelt, K.; Schettler, E. Evidence for an increased geographical distribution of Dermacentor reticulatus in Germany and detection of Rickettsia sp. RpA4. Int. J. Med. Microbiol. 2006, 296 (Suppl. 40), 149–156. [Google Scholar] [CrossRef] [PubMed]
- Mierzejewska, E.J.; Estrada-Peña, A.; Bajer, A. Spread of Dermacentor reticulatus is associated with the loss of forest area. Exp. Appl. Acarol. 2017, 72, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Karbowiak, G. Changes in the occurrence range of hosts cause the expansion of the ornate dog tick Dermacentor reticulatus (Fabricius, 1794) in Poland. Biologia 2022, 77, 1513–1522. [Google Scholar] [CrossRef]
- Asman, M.; Bartosik, K.; Jakubas-Zawalska, J.; Świętek, A.; Witecka, J. A new endemic locality of Dermacentor reticulatus in central–southern Poland and its potential epidemiological implications. Insects 2024, 15, 580. [Google Scholar] [CrossRef] [PubMed]
- Karbowiak, G.; Kiewra, D. New locations of Dermacentor reticulatus ticks in Western Poland: The first evidence of the merge in D. reticulatus occurrence areas? Wiad Parazytol. 2010, 56, 33333–33336. [Google Scholar]
- Kiewra, D.; Czułowska, A. Evidence for an increased distribution range of Dermacentor reticulatus in south-west Poland. Exp. Appl. Acarol. 2013, 59, 501–506. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kiewra, D.; Szymanowski, M.; Czułowska, A.; Kolanek, A. The local-scale expansion of Dermacentor reticulatus ticks in Lower Silesia, SW Poland. Ticks Tick Borne Dis. 2021, 12, 101599. [Google Scholar] [CrossRef] [PubMed]
- Bullová, E.; Lukán, M.; Stanko, M.; Petko, B. Spatial distribution of Dermacentor reticulatus tick in Slovakia in the beginning of the 21st century. Vet. Parasitol. 2009, 165, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Kiewra, D.; Szymanowski, M.; Czułowska, A.; Dyczko, D.; Jawień, P.; Plewa-Tutaj, K. Update on the occurrence of Dermacentor reticulatus in the Wrocław Agglomeration, SW Poland. Ann. Parasitol. 2024, 70 (Suppl. 1), 52. [Google Scholar]
- Kiewra, D.; Szymanowski, M.; Lonc, E.; Czułowska, A. Dermacentor reticulatus (Fabr.) in Wrocław area—Planning field measurements and analyzing spatial distribution of ticks using GIS tools. In Stawonogi We Współczesnym Świecie; Buczek, A., Błaszak, C., Eds.; Wydawnictwo Koliber: Lublin, Poland, 2015; pp. 79–85. [Google Scholar]
- Copernicus Land Monitoring Service. Available online: https://land.copernicus.eu/en/products/corine-land-cover/clc2018 (accessed on 17 March 2025).
- Nowak-Chmura, M. Fauna kleszczy (Ixodida) Europy Środkowej; Wydawnictwo Naukowe Uniwersytetu Pedagogicznego: Kraków, Poland, 2013; p. 404. [Google Scholar]
- Estrada-Peña, A.; Mihalca, A.D.; Petney, N.T. Ticks of Europe and North Africa: A Guide to Species Identification; Springer: Cham Switzerland, 2017; pp. XXI, 404. [Google Scholar]
- Mitchell, A. The ESRI Guide to GIS Analysis: Spatial Measurements & Statistics; ESRI Press: Redlands, CA, USA, 2005; p. 240. [Google Scholar]
- Cressie, N. Statistics for Spatial Data (Revised Edition); John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1993; p. 928. [Google Scholar] [CrossRef]
- Široký, P.; Kubelová, M.; Bednář, M.; Modrý, D.; Hubálek, Z.; Tkadlec, E. The distribution and spreading pattern of Dermacentor reticulatus over its threshold area in the Czech Republic—How much is range of this vector expanding? Vet. Parasitol. 2011, 183, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Orłowski, G.; Nowak, L. Road mortality of hedgehogs in agricultural landscape. Pol. J. Ecol. 2004, 52, 377–382. [Google Scholar]
- Orłowski, G.; Nowak, L. Factors influencing mammal roadkills in the agricultural landscape of South-Western Poland. Pol. J. Ecol. 2006, 54, 283–294. [Google Scholar]
- Nordt, A.; Klenke, R. Sleepless in Town—Drivers of the Temporal Shift in Dawn Song in Urban European Blackbirds. PLoS ONE 2013, 8, e71476. [Google Scholar] [CrossRef] [PubMed]
- Ciach, M.; Fröhlich, A. Ungulates in the city: Light pollution and open habitats predict the probability of roe deer occurring in an urban environment. Urban Ecosyst. 2019, 22, 513–523. [Google Scholar] [CrossRef]
- Skorb, K. Wpływ ekologicznego zanieczyszczenia sztucznym światłem na ptaki na przykładzie dużego kompleksu szklarni w siechnicach. [The impact of ecological pollution from artificial light on birds as exemplified by a large greenhouse complex in siechnice]. In Proceedings of the 6. Ogólnopolska Konferencja na Temat Zanieczyszczenia Światłem, Łódź, Poland, 24–25 September 2021; p. 12. Available online: https://cbkpan.pl/wp-content/uploads/6okzs_streszczenia.pdf (accessed on 25 June 2025).
- Zaręba, A.D.; Próchnicka, P. Korytarze ekologiczne a prawo i polityka ekologiczna. Korytarz ekologiczny Doliny Odry jako podstawowy element systemu przyrodniczego Wrocławia. [Ecological corridors and environmental law and policy. The Odra Valley Ecological Corridor as a fundamental element of Wrocław’s natural system]. Gosp. Prak. Teor. 2015, 40, 93–109. [Google Scholar] [CrossRef]
- Zając, Z.; Sędzikowska, A.; Maślanko, W.; Woźniak, A.; Kulisz, J. Occurrence and abundance of Dermacentor reticulatus in the habitats of the ecological corridor of the Wieprz River, Eastern Poland. Insects 2021, 12, 96. [Google Scholar] [CrossRef] [PubMed]

| 0.99–1 | 0.75–0.99 | 0.5–0.75 | 0.25–0.5 | 0–0.25 | |
| N | 100 | 0 | 0 | 0 | 0 |
| NE | 45.3 | 20.4 | 14.2 | 16.8 | 3.4 |
| E | 10.9 | 34.4 | 29.7 | 23.2 | 1.8 |
| SE | 44.7 | 36.9 | 12.2 | 6.2 | 0 |
| S | 87.7 | 11.6 | 0.7 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiewra, D.; Ojrzyńska, H.; Czułowska, A.; Dyczko, D.; Jawień, P.; Plewa-Tutaj, K. Dermacentor reticulatus (Fabricius, 1794) in Southwestern Poland: Changes in Range and Local Scale Updates. Insects 2025, 16, 935. https://doi.org/10.3390/insects16090935
Kiewra D, Ojrzyńska H, Czułowska A, Dyczko D, Jawień P, Plewa-Tutaj K. Dermacentor reticulatus (Fabricius, 1794) in Southwestern Poland: Changes in Range and Local Scale Updates. Insects. 2025; 16(9):935. https://doi.org/10.3390/insects16090935
Chicago/Turabian StyleKiewra, Dorota, Hanna Ojrzyńska, Aleksandra Czułowska, Dagmara Dyczko, Piotr Jawień, and Kinga Plewa-Tutaj. 2025. "Dermacentor reticulatus (Fabricius, 1794) in Southwestern Poland: Changes in Range and Local Scale Updates" Insects 16, no. 9: 935. https://doi.org/10.3390/insects16090935
APA StyleKiewra, D., Ojrzyńska, H., Czułowska, A., Dyczko, D., Jawień, P., & Plewa-Tutaj, K. (2025). Dermacentor reticulatus (Fabricius, 1794) in Southwestern Poland: Changes in Range and Local Scale Updates. Insects, 16(9), 935. https://doi.org/10.3390/insects16090935









