Fine Morphology of the Mouthparts in Cheilocapsus nigrescens (Hemiptera: Heteroptera: Miridae) Reflects Adaptation for Phytophagous Habits
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Collecting
2.2. Samples for SEM
2.3. Image Processing and Morphometric Measurement
2.4. Terminology
3. Results
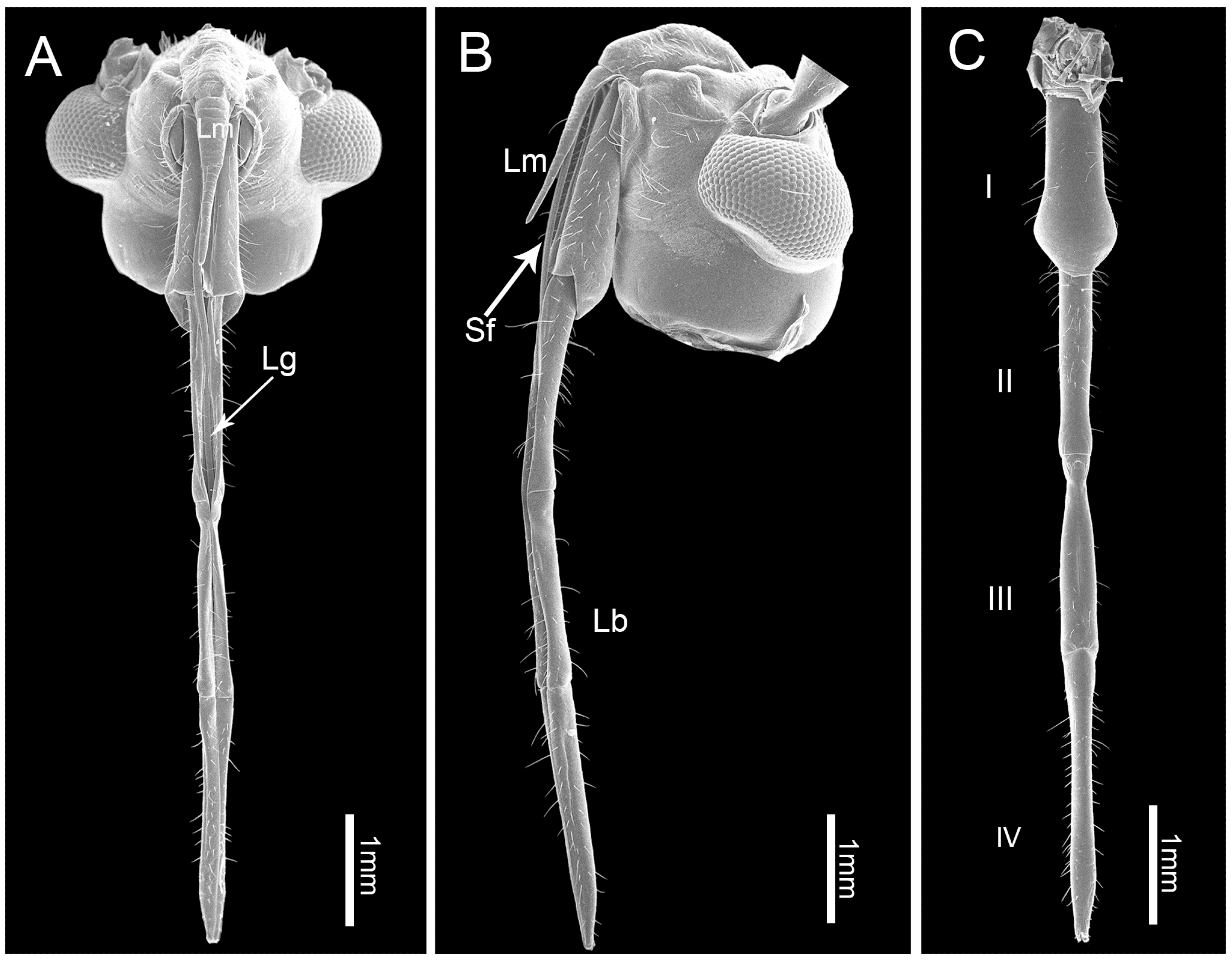
3.1. Gross Morphology of the Mouthparts
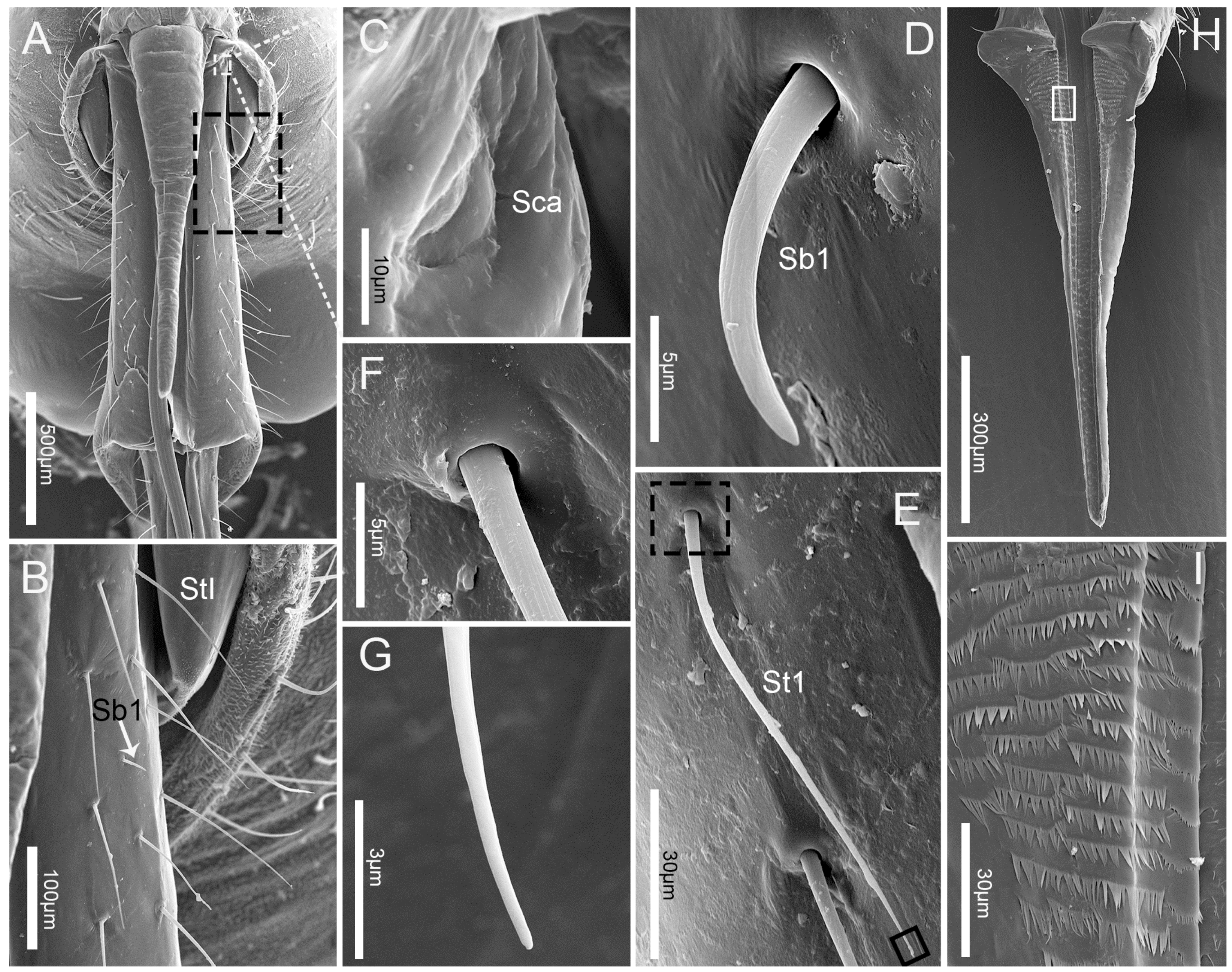
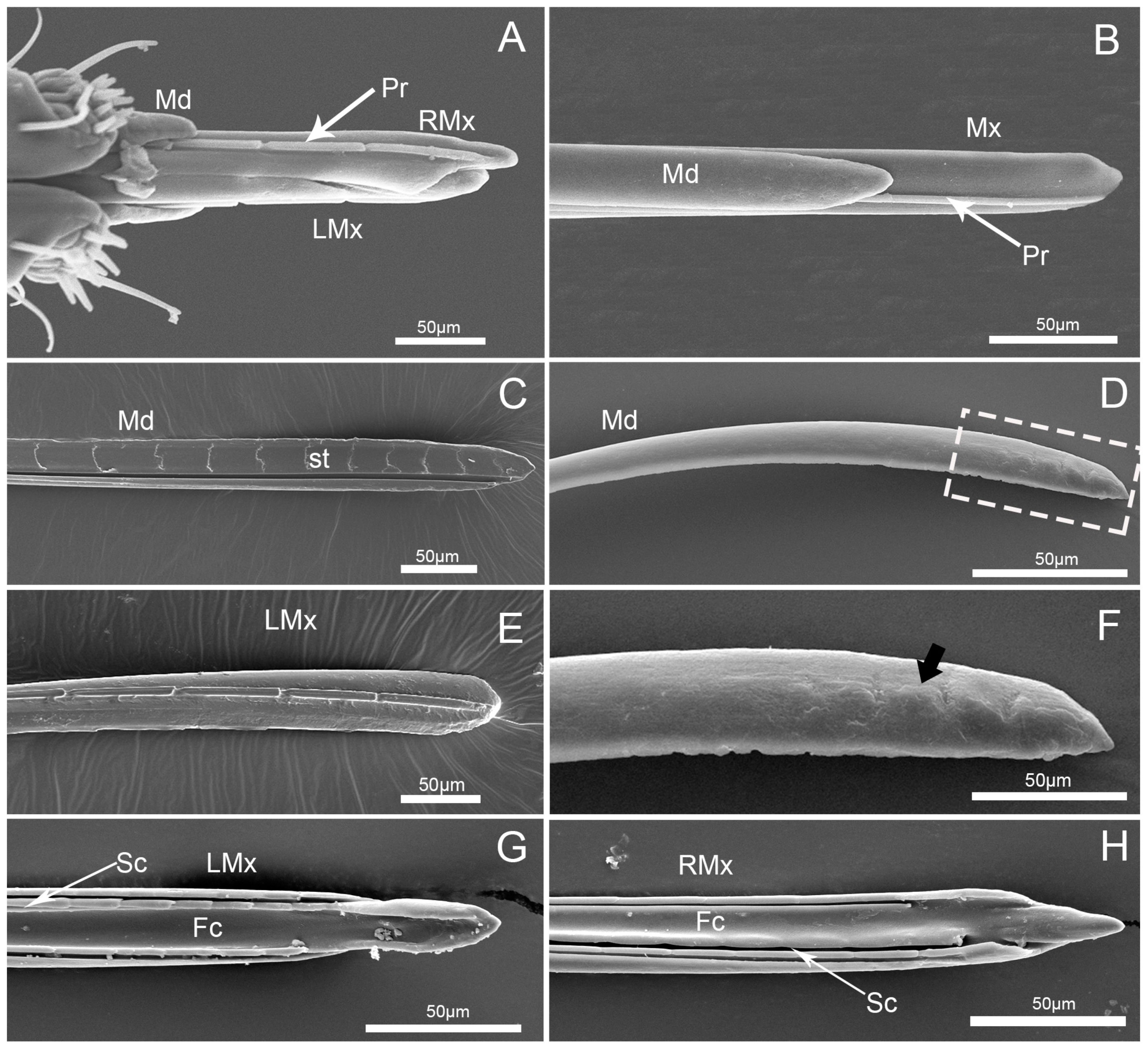
3.2. Labrum
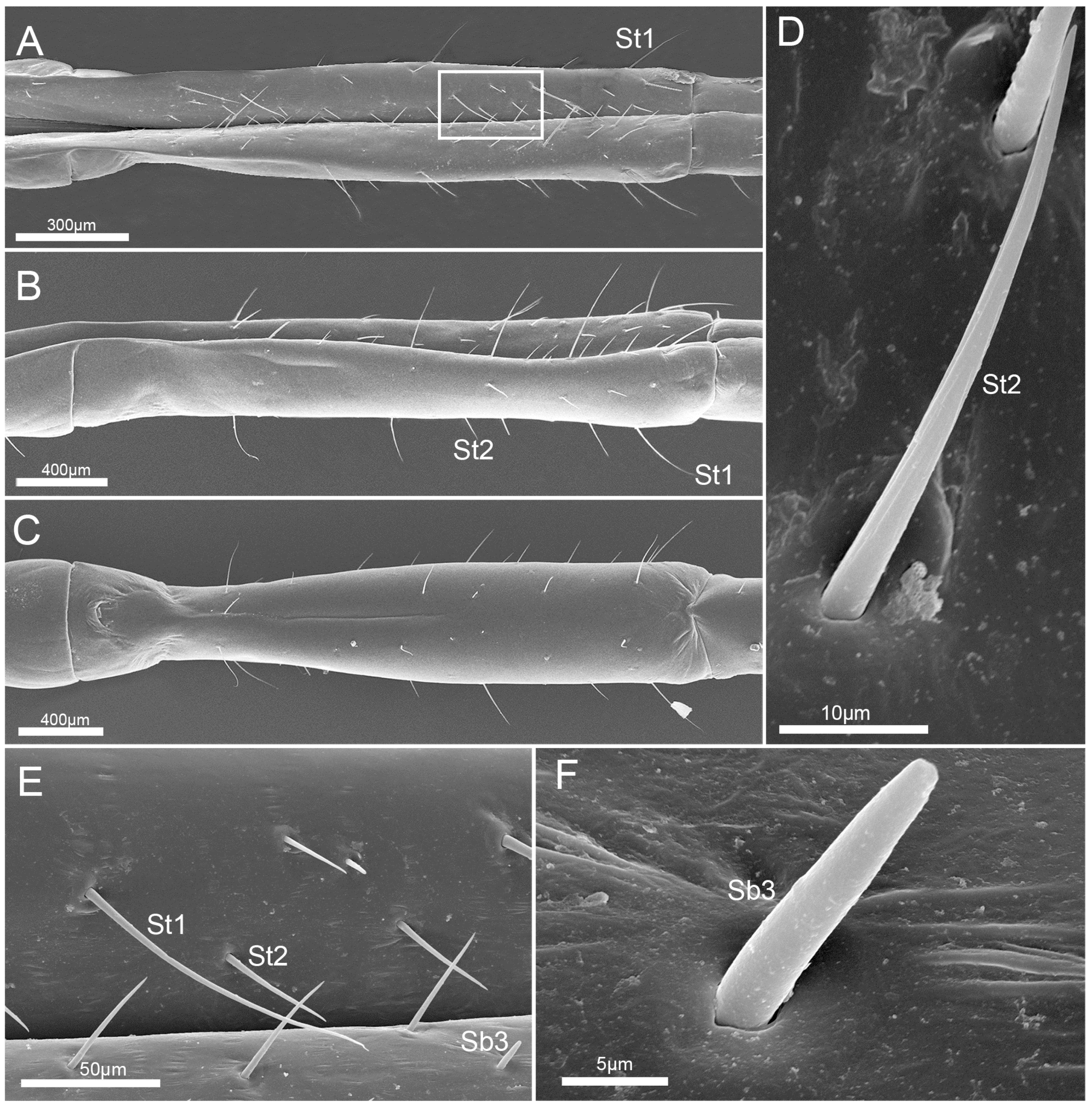
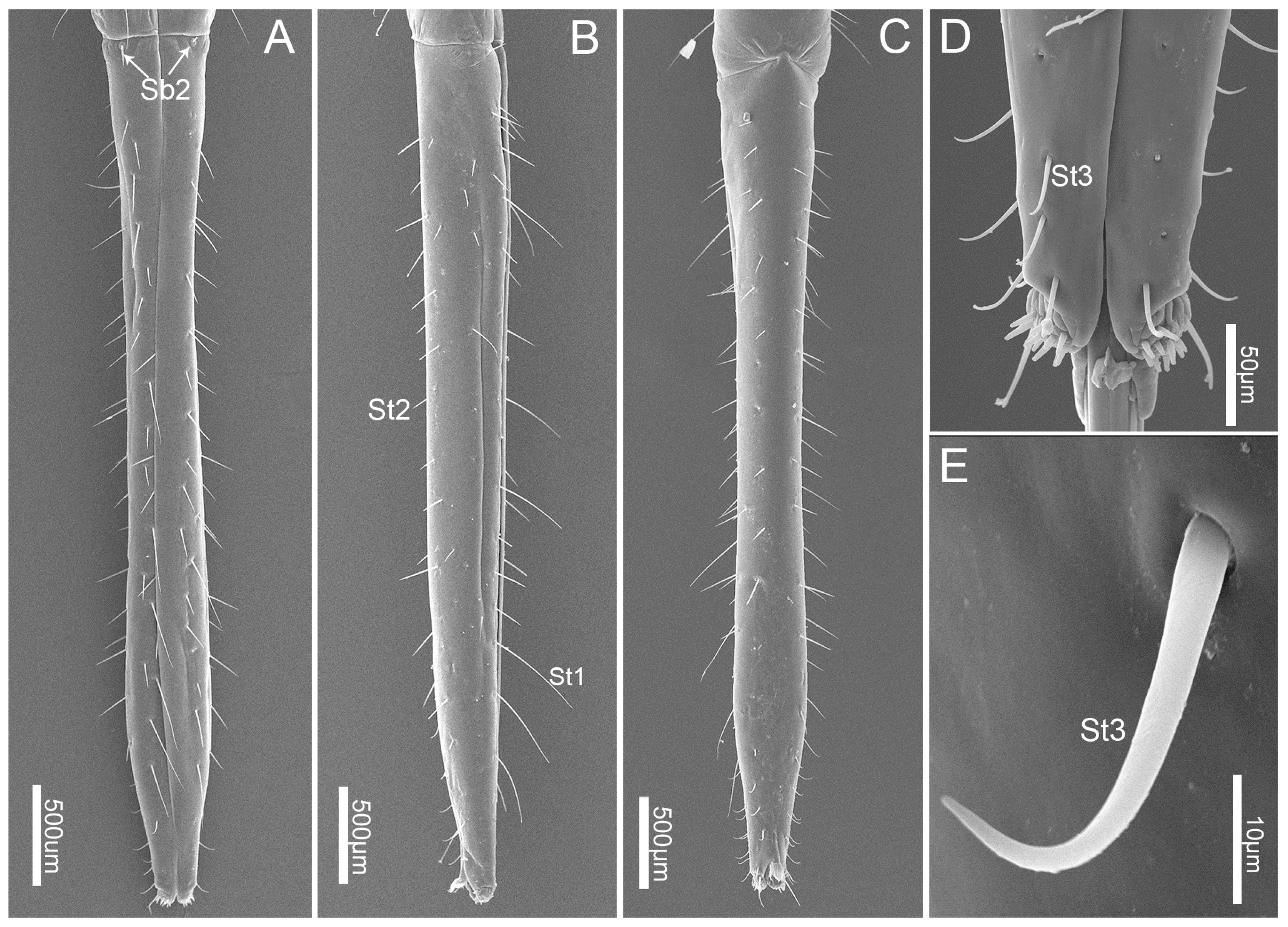
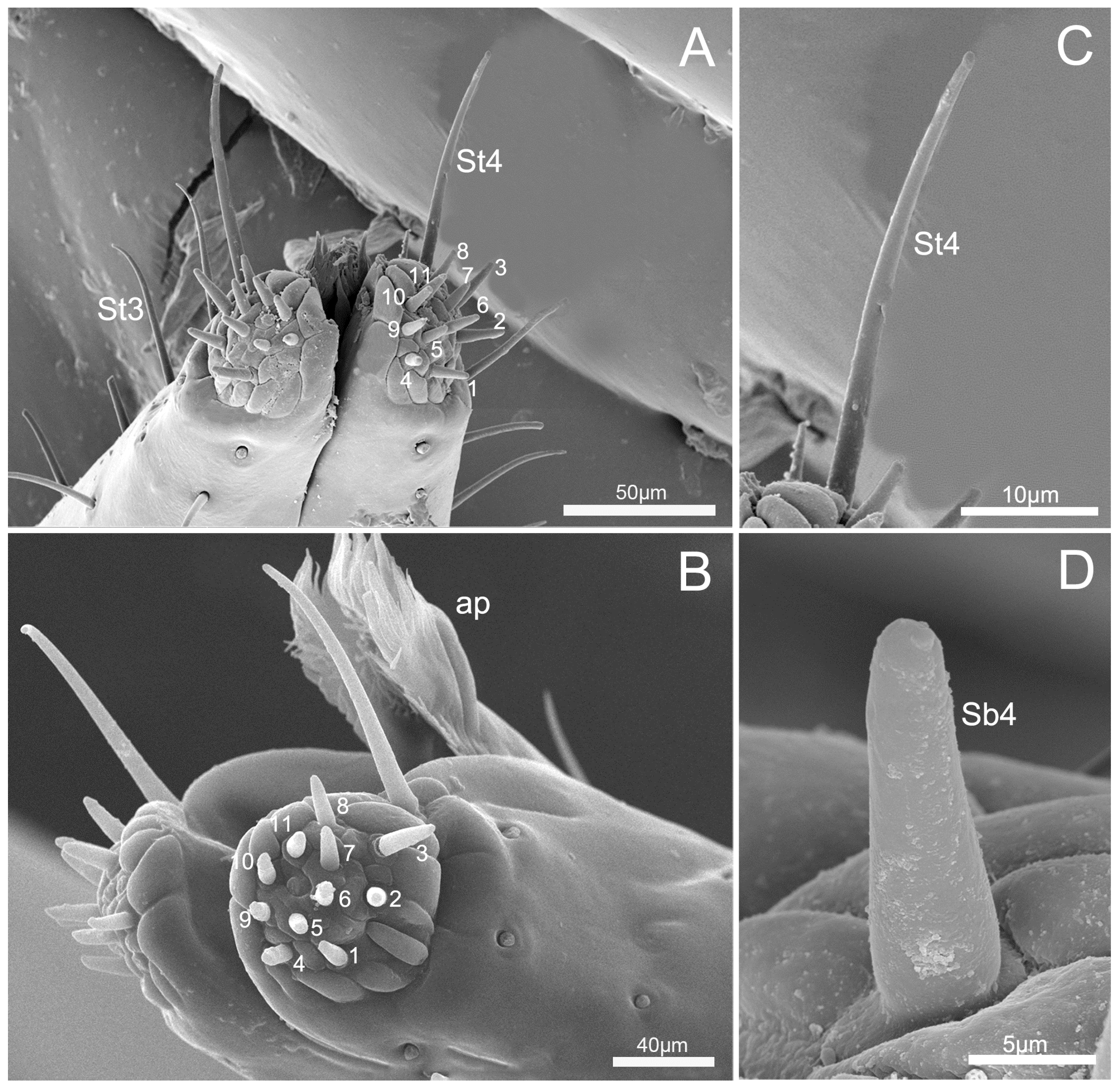
3.3. Labium
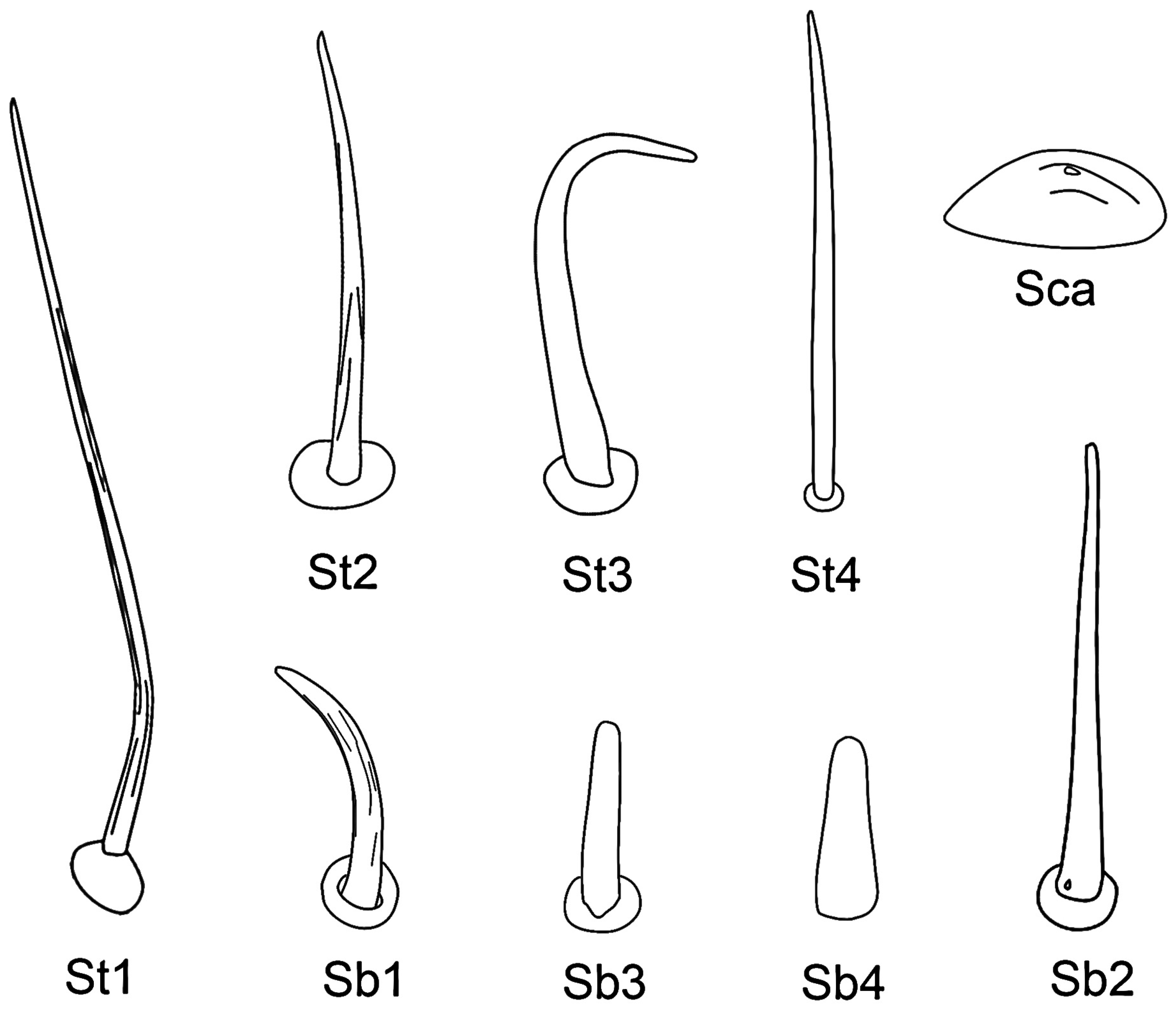
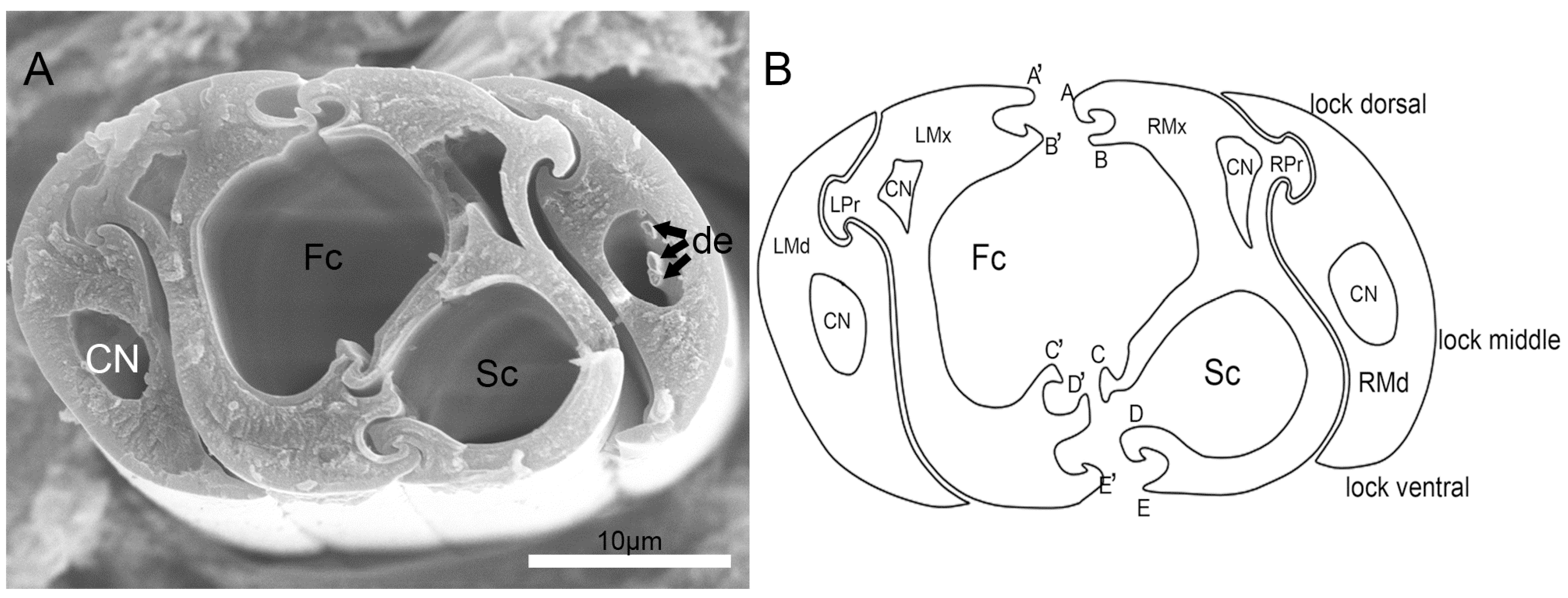
3.4. Stylet Fascicle
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Labandeira, C.C. Insect mouthparts: Ascertaining the paleobiology of insect feeding strategies. Annu. Rev. Ecol. Syst. 1997, 28, 153–193. [Google Scholar] [CrossRef]
- Gullan, P.J.; Cranston, P.S. The Insects: An Outline of Entomology, 5th ed.; Wiley Blackwell: Oxford, UK, 2014; pp. 95–124. [Google Scholar]
- Liu, M.; Ren, D.; Tan, J.J. A brief introduction to insect mouthparts and their evolutionary history. Chin. Bull. Entomol. 2005, 42, 587–592. [Google Scholar]
- Coronado-Gonzalez, P.A.; Vijaysegaran, S.; Robinson, A.S. Functional morphology of the mouthparts of the adult Mediterranean fruit fly, Ceratitis capitata. J. Insect Sci. 2008, 8, 73. [Google Scholar] [CrossRef]
- McShaffrey, D.; McCafferty, W. Feeding behavior and related functional morphology of the mayfly Ephemerella needhami (Ephemeroptera: Ephemerellidae). J. Insect Behav. 1990, 3, 673–688. [Google Scholar] [CrossRef]
- Bigelow, R.S. Morphology of the face in the Hymenoptera. Can. J. Zool. 1954, 32, 378–392. [Google Scholar] [CrossRef][Green Version]
- Cobben, R.H. Evolutionary Trends in Heteroptera. Part II Mouthpart-Structures and Feeding Strategies; Mededelingen Landbouwhogeschool: Wageningen, The Netherlands, 1978; Volume 78, pp. 1–407. [Google Scholar]
- Rani, P.U.; Madhavendra, S.S. Morphology and distribution of antennal sense organs and diversity of mouthpart structures in Odontopus nigricornis (Stall) and Nezera viridula L. (Hemiptera). Int. J. Insect Morphol. Embryol. 1995, 24, 119–132. [Google Scholar] [CrossRef]
- Rani, P.U.; Madhavendra, S.S. External morphology of antennal and rostral sensillae in four hemipteran insects and their possible role in host plant selection. Int. J. Trop. Insect Sci. 2005, 25, 198–207. [Google Scholar] [CrossRef]
- Boyd, D.W. Digestive enzymes and stylet morphology of Deraeocoris nigritulus (Uhler) (Hemiptera: Miridae) reflect adaptations for predatory habits. Ann. Entomol. Soc. Am. 2003, 96, 667–671. [Google Scholar] [CrossRef]
- Boyd, D.W.; Cohen, A.C.; Alverson, D.R. Digestive enzymes and stylet morphology of Deraeocoris nebulosus (Hemiptera: Miridae), a predacious plant bug. Ann. Entomol. Soc. Am. 2002, 95, 395–401. [Google Scholar] [CrossRef]
- Anderson, W.G.; Heng-Moss, T.M.; Baxendale, F.P.; Baird, L.M.; Sarath, G.; Higley, L. Chinch bug (Hemiptera: Blissidae) mouthpart morphology, probing frequencies, and locations on resistant and susceptible germplasm. J. Econ. Entomol. 2006, 99, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Brożek, J.; Herczek, A. Internal structure of the mouthparts of true bugs (Hemiptera: Heteroptera). Pol. J. Entomol. 2004, 73, 79–106. [Google Scholar]
- Brożek, J. Morphology and arrangement of the labial sensilla of the water bugs. Bull. Insectol. 2008, 61, 67–168. [Google Scholar]
- Brożek, J. A comparison of external and internal maxilla and mandible morphology of water bugs (Hemiptera: Heteroptera: Nepomorpha). Zootaxa 2013, 3635, 340–378. [Google Scholar] [CrossRef]
- Brożek, J.; Chłond, D. Morphology, arrangement and classification of sensilla on the apical segment of labium in Peiratinae (Hemiptera: Heteroptera: Reduviidae). Zootaxa 2010, 2476, 39–52. [Google Scholar] [CrossRef]
- Parveen, S.; Ahmad, A.; Brożek, J.; Ramamurthy, V.V. Morphological diversity of the labial sensilla of phytophagous and predatory Pentatomidae (Hemiptera: Heteroptera), with reference to their possible functions. Zootaxa 2015, 4039, 359–372. [Google Scholar] [CrossRef]
- Wheeler, A.G. Economic importance of Heteroptera. In Heteroptera of Economic Importance; Schaefer, C.W., Panizzi, A.R., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 37–83. [Google Scholar]
- Cassis, G.; Schuh, R.T. Systematics, biodiversity, biogeography, and host associations of the Miridae (Insecta: Hemiptera: Heteroptera: Cimicomorpha). Annu. Rev. Entomol. 2012, 57, 377–404. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, A.G. Biology of the Plant Bugs (Hemiptera: Miridae): Pests, Predators, Opportunists; Cornell Univ. Press: Ithaca, NY, USA, 2001. [Google Scholar]
- Cohen, A.C. Feeding adaptations of some predaceous Hemiptera. Ann. Entomol. Soc. Am. 1990, 83, 1215–1223. [Google Scholar] [CrossRef]
- Terra, W.R.; Ferreira, C. Insect digestive enzymes: Properties, compartmentalization and function. Comp. Biochem. Physiol. 1994, 109B, 1–62. [Google Scholar] [CrossRef]
- Avé, D.; Frazier, J.L.; Hatfield, L.D. Contact chemoreception in the tarnished plant bug Lygus lineolaris. Entomol. Exp. Appl. 1978, 24, 217–227. [Google Scholar] [CrossRef]
- Hatfield, L.D.; Frazier, J.L. Ultrastructure of the labial tip sensilla of the tarnished plant bug, Lygus lineolaris (P. De Beauvois) (Hemiptera: Miridae). Int. J. Insect Morphol. Embryol. 1980, 9, 59–66. [Google Scholar] [CrossRef]
- Romani, R.; Salerno, G.; Frati, F.; Conti, E.; Isidoro, N.; Bin, F. Oviposition behaviour in Lygus rugulipennis: A morpho-functional study. Entomol. Exp. Appl. 2005, 115, 17–25. [Google Scholar] [CrossRef]
- Kullenberg, B. Studien uber die Biologie der Capsiden. Zool. Bidr. Upps. 1946, 23, 1–522. [Google Scholar]
- Roitberg, B.D.; Gillespie, D.R.; Quiring, D.M.J.; Alma, C.R.; Jenner, W.H.; Perry, J.; Peterson, J.H.; Salomon, M.; van Laerhoven, S. The cost of being an omnivore: Mandible wear from plant feeding in a true bug. Naturwissenschaften 2005, 92, 431–434. [Google Scholar] [CrossRef]
- Awati, P.R. The mechanism of suction in the potato capsid bug, Lygus pabulinus Linn. Proc. Zool. Soc. Lond. 1914, 2, 685–733. [Google Scholar] [CrossRef]
- Liu, G.Q.; Wang, H.J. Genus cheilocapsus kirkaldy of Mainland China (Insecta: Hemiptera: Miridae: Mirinae). Reichenbachia 2001, 34, 61–65. [Google Scholar]
- Zheng, L.Y.; Lü, N.; Liu, G.Q.; Xu, B.H. Hemiptera, Miridae, Mirinae; Editorial Committee of Fauna Sinica, Chinese Academy of Sciences, Ed.; Fauna Sinica (Insecta Volume 33); Sciences Press: Beijing, China, 2004; pp. 1–797, (In Chinese, English abstract). [Google Scholar]
- Shields, V.D.C. High resolution ultrastructural investigation of insect sensory organs using field emission scanning electron microscopy. In Microscopy: Science, Technology, Applications and Education; Méndez-Vilas, A., Díaz, J., Eds.; Formatex: Badajoz, Spain, 2010; pp. 321–328. [Google Scholar]
- Altner, H.; Prillinger, L. Ultrastructure of invertebrate chemo-, termo-, and hygroreceptors and its functional significance. Int. Rev. Cytol. 1980, 67, 69–139. [Google Scholar]
- Hartenstein, V. Development of insect sensilla. In Comprehensive Molecular Insect Science; Gilbert, L.I., Ed.; Elsevier: Oxford, UK, 2005; pp. 379–419. [Google Scholar]
- Silva, M.; Jurberg, J.; Galvão, C.; Barbosa, H.S. Comparative study of the stridulatorium sulcus, buccula and rostrum of nymphs of Triatoma kluge Carcavallo et al, Triatoma vandae Carcavallo et al. and Triatoma williami Galvão et al. (Hemiptera: Reduviidae). Neotrop. Entomol. 2010, 39, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Spangenberg, R.; Friedemann, K.; Weirauch, C.H.; Beutel, R.G. The head morphology of the potentially basal Heteropteran lineages Enicocephalomorpha and Dipsocoromorpha (Insecta: Hemiptera: Heteroptera). Arthropod Syst. Phylogeny 2013, 71, 103–136. [Google Scholar]
- Zheng, L.Y. Class insect: Order Hemiptera: Suborder Heteroptera (Order Hemiptera s. str.). In Insect Classification; Zheng, L.Y., Gui, H., Eds.; Nanjing Normal University Press: Nanjing, China, 1999; Volume 1, pp. 442–520. (In Chinese) [Google Scholar]
- Faucheux, M.M. Relations entre l’ultrastructure des stylets manibulaires et maxillaires et la prise de nourriture chez les insects Hemipteres. CR Acad. Sci. Paris (Ser. D) 1975, 281, 41–44. [Google Scholar]
- Gaffal, K.P. Terminal sensilla on the labium of Dysdercus intermedius distant (Heteroptera: Pyrrhocoridae). Int. J. Insect Morphol. Embryol. 1981, 10, 1–6. [Google Scholar] [CrossRef]
- Depieri, R.A.; Panizzi, A.R. Rostrum length, mandible serration, and food and salivary canals areas of selected species of stink bugs (Heteroptera, Pentatomidae). Rev. Bras. Entomol. 2010, 54, 584–587. [Google Scholar] [CrossRef][Green Version]
- Depieri, R.A.; Siqueira, F.; Panizzi, A.R. Aging and food source effects on mandibular stylets teeth wear of Phytophagous stink bug (Heteroptera: Pentatomidae). Neotrop. Entomol. 2010, 39, 952–956. [Google Scholar] [CrossRef]
- Cohen, A.C. Feeding fitness and quality of domesticated and feral predators: Effects of long-term rearing on artificial diet. Biol. Control 2000, 17, 50–54. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, W. Fine structure of mouthparts and feeding performance of Pyrrhocoris sibiricus Kuschakevich with remarks on the specialization of sensilla and stylets for seed feeding. PLoS ONE 2017, 12, e0177209. [Google Scholar] [CrossRef]
- Boyd, D.W. Deraeocoris nebulosus (Uhler) (Hemiptera: Miridae): A Potential Biological Control Agent. Ph.D. Thesis, Clemson University, Clemson, SC, USA, 2001. [Google Scholar]
- Backus, E.A. Sensory systems and behaviours which mediate hemipteran plant-feeding: A taxonomic overview. J. Insect Physiol. 1988, 34, 151–165. [Google Scholar] [CrossRef]
- Liang, X.M.; Zhang, C.N.; Li, Z.L.; Xu, L.F.; Dai, W. Fine structure and sensory apparatus of the mouthparts of the pear psyllid, Cacopsylla chinensis (Yang et Li) (Hemiptera: Psyllidae). Arthropod Struct. Dev. 2013, 42, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.N.; Dietrich, C.H.; Dai, W. Structure and sensilla of the mouthparts of the spotted lanternfly Lycorma delicatula (Hemiptera: Fulgoromorpha: Fulgoridae). PLoS ONE 2016, 11, e0156640. [Google Scholar] [CrossRef] [PubMed]
- Ge, F.R.; Dietrich, C.D.; Dai, W. Mouthpart structure in the woolly apple aphid Eriosoma lanigerum (Hausmann) (Hemiptera: Aphidoidea: Pemphigidae). Arthropod Struct. Dev. 2016, 45, 230–241. [Google Scholar] [CrossRef]
- Rani, P.U. Sensillary morphology on the rostral apex and their possible role in prey location behaviour of the carnivorous stinkbug, Eocanthecona furcellata (Wolff) (Heteroptera: Pentatomidae). Acta Zool. 2009, 90, 246–256. [Google Scholar] [CrossRef]
- Baker, G.T.; Chen, X.; Ma, P.W.K. Labial tip sensilla of Blissus leucopterus leucopterus (Hemiptera: Blissidae): Ultrastructure and behavior. Insect Sci. 2008, 15, 271–275. [Google Scholar] [CrossRef]
- Schoonhoven, L.M.; Henstra, S. Morphology of some rostrum receptors in Dysdercus spp. Neth. J. Zool. 1972, 22, 343–346. [Google Scholar] [CrossRef]
- Peregrine, D.J. Fine structures of sensilla basiconica on the labium of the cotton stainer, Dysdercus fasciatus (Signoret) (Heteroptera: Pyrrhocoridae). Int. J. Insect Morphol. Embryol. 1972, 1, 241–251. [Google Scholar] [CrossRef]

| Gender | Position | Length (μm) | Width (μm) | Height (μm) | N |
|---|---|---|---|---|---|
| Female | Lb | 3798.4 ± 37.8 | - | - | 3 |
| Male | Lm | 705.6 ± 48.9 | 230.0 ± 3.5 | - | 5 |
| Lb-sg1 | 1012.6 ± 30.3 | 224.8 ± 17.1 | 128.7 ± 22.4 | 12 | |
| Lb-sg2 | 886.9 ± 38.3 | 154.3 ± 7.5 | 121.5 ± 5.8 | 12 | |
| Lb-sg3 | 796.6 ± 52.8 | 121.8 ± 8.5 | 126.0 ± 7.8 | 12 | |
| Lb-sg4 | 1004.3 ± 50.4 | 136.4 ± 8.2 | 104.3 ± 5.6 | 12 | |
| Md | 3929.5 ± 193.2 | 16.2 ± 0.5 | - | 11 | |
| Mx | 4123.4 ± 107.8 | 18.0 ± 0.6 | - | 11 |
| Sensilla Type | Distribution | Length (μm) | Basal Diameter (μm) | N |
|---|---|---|---|---|
| St1 | Lm, Lb-sg1, 2, 3, 4 | 107.1 ± 14.1 | 2.8 ± 0.3 | 20 |
| St2 | Lb-sg1, 2, 3, 4 | 39.2 ± 11.2 | 2.5 ± 0.4 | 20 |
| St3 | Lb-sg4 | 22.9 ± 4.5 | 1.8 ± 0.3 | 20 |
| St4 | Lb-sg4 | 33.2 ± 2.8 | 2.4 ± 0.2 | 15 |
| Sb1 | Lb-sg1, 2 | 13.4 ± 3.0 | 1.9 ± 0.2 | 15 |
| Sb2 | Lb-sg2, 4 | 41.2 ± 8.9 | 3.1 ± 0.4 | 15 |
| Sb3 | Lb-sg3 | 8.9 ± 1.20 | 1.9 ± 0.4 | 15 |
| Sb4 | SF | 7.1 ± 1.3 | 2.1 ± 0.2 | 15 |
| Sca | Lb-sg1, 2 | - | 9.8 ± 1.1 | 15 |
| Species Name | Labrum | Labium | The Number of Basiconica Sensilla of Labium tip | The Number of Sensilla Type | Apical Plate | The Number of Barbs on the Right Maxillary Stylet Tips | Squamous Texture on Mandibular Stylet | The Number of Teech on the Distal Mandibular Stylet | References |
|---|---|---|---|---|---|---|---|---|---|
| Lygus lineolari | unreported | unreported | 11 pairs | unreported | √ | unreported | unreported | unreported | Hatfield and Frazier 1980 [24]; Avé et al. 1978 [23] |
| Lygus pabulinus | tapers to a point, the base being broader | 4-segment | unreported | unreported | √ | (right stylet being more roughly armoured than the left one) | √ | 7–9 recurved hooks | Cobben 1978 [7] * Awati 1914 [28] |
| Lygus rugulipennis | unreported | 4-segment | 11-12 pairs | unreported | √ | unreported | unreported | (cuticular teeth) | Romani et al. 2005 [25] |
| Isometopus intrusus | unreported | unreported | unreported | unreported | √ | (recurved barbs) | √ | (lateral notches) | Cobben 1978 [7] * |
| Dicyphus hesperus | unreported | unreported | unreported | unreported | unreported | unreported | unreported | (Serrations on the lateral margins) | Roitberg et al. 2005 [27] |
| Deraeocoris oliveceus | unreported | unreported | unreported | unreported | √ | (two rows strongly teeth) | √ | (lateral notches) | Cobben 1978 [7] * |
| Cheilocapsus nigrescens | elongated conical | 4-segment | 11 pairs | 10 | √ | no | √ | about 6–8 obscure lateral notches | This study |
| Deraeocoris nebulosus | unreported | unreported | unreported | unreported | unreported | two rows of at least six recurved barbs | unreported | unreported | Boyd et al. 2002 [11] |
| Deraeocoris nigritulus | unreported | unreported | unreported | unreported | unreported | two rows of at least seven strongly recurved teeth | unreported | unreported | Boyd 2003 [10] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, L.; Dai, W. Fine Morphology of the Mouthparts in Cheilocapsus nigrescens (Hemiptera: Heteroptera: Miridae) Reflects Adaptation for Phytophagous Habits. Insects 2019, 10, 143. https://doi.org/10.3390/insects10050143
Wang Y, Li L, Dai W. Fine Morphology of the Mouthparts in Cheilocapsus nigrescens (Hemiptera: Heteroptera: Miridae) Reflects Adaptation for Phytophagous Habits. Insects. 2019; 10(5):143. https://doi.org/10.3390/insects10050143
Chicago/Turabian StyleWang, Yan, Lingfei Li, and Wu Dai. 2019. "Fine Morphology of the Mouthparts in Cheilocapsus nigrescens (Hemiptera: Heteroptera: Miridae) Reflects Adaptation for Phytophagous Habits" Insects 10, no. 5: 143. https://doi.org/10.3390/insects10050143
APA StyleWang, Y., Li, L., & Dai, W. (2019). Fine Morphology of the Mouthparts in Cheilocapsus nigrescens (Hemiptera: Heteroptera: Miridae) Reflects Adaptation for Phytophagous Habits. Insects, 10(5), 143. https://doi.org/10.3390/insects10050143





