Liquid Crystals as Lubricants
Abstract
1. Introduction
2. Surface Orientation in Tribology as a Special Case of Mesomorphism
3. Thermotropic Mesogens in Tribology
3.1. Calamitic Mesogens
3.2. Chiral Mesogens
3.3. Mesogens as Additives to Lubricants and Greases
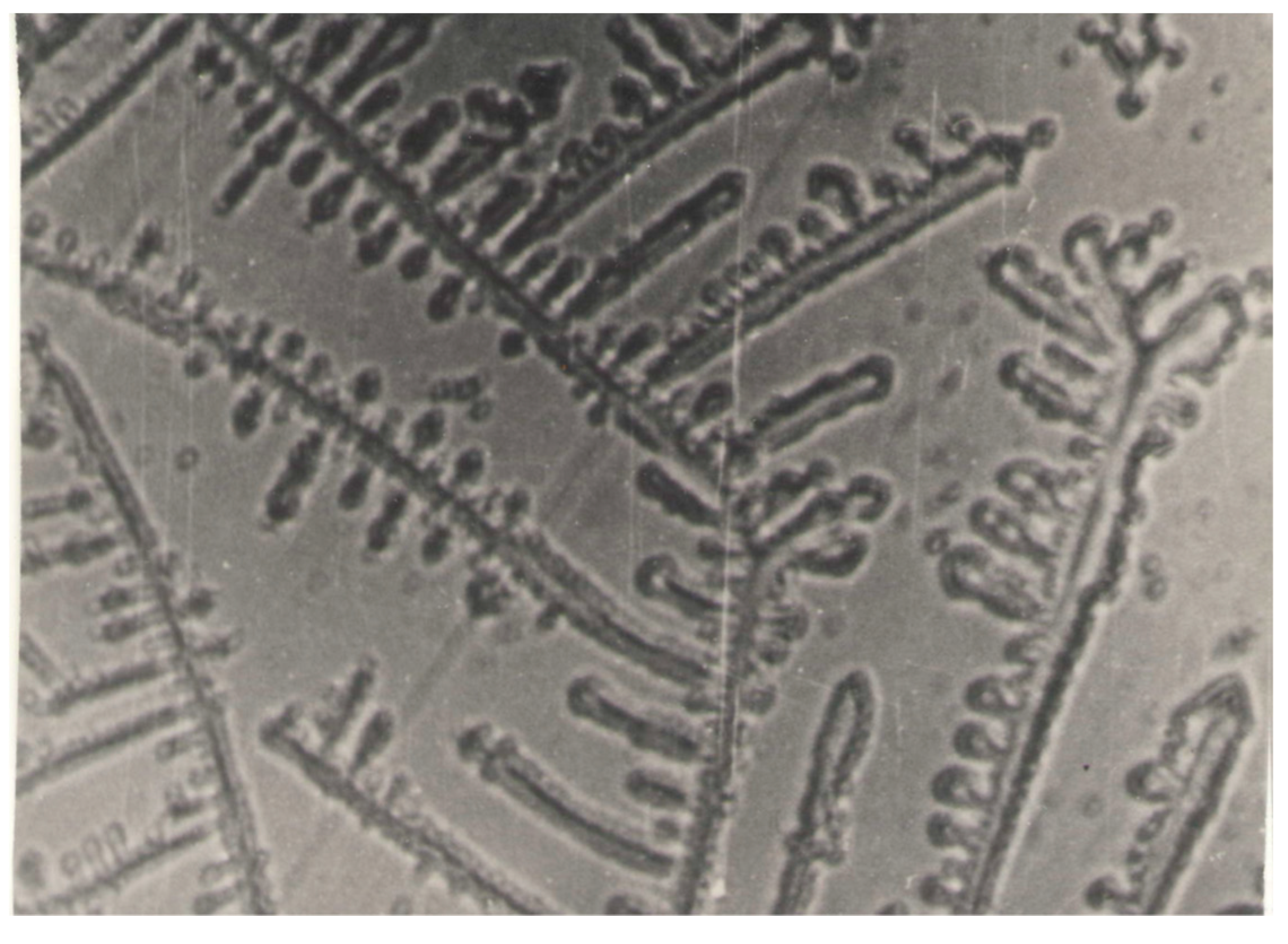
3.4. Discotic Mesogens in Tribology
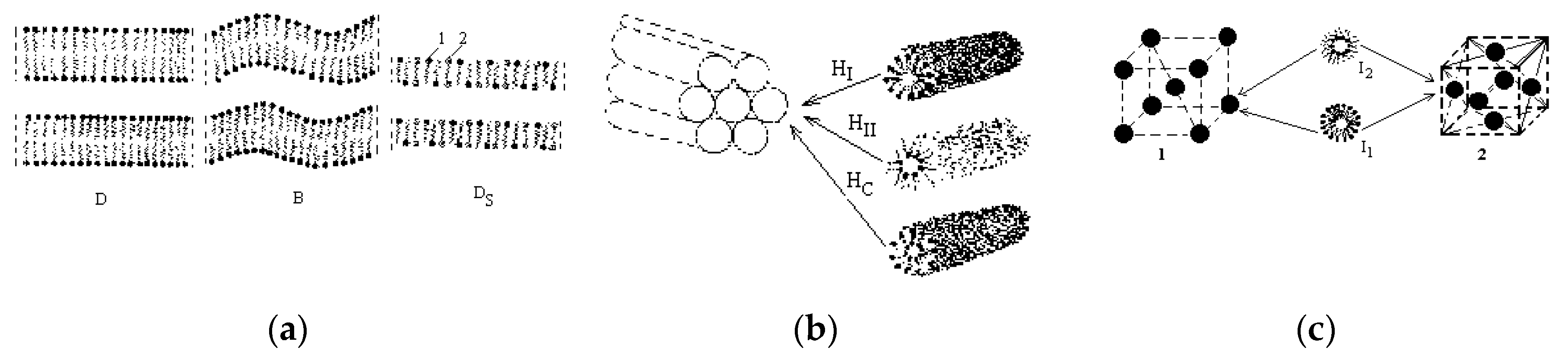
4. Lyotropic Mesogens in Tribology
4.1. Ionogenic Lyotropic Mesogens
4.2. Nonionic Lyotropic Mesogens
4.3. Discotic Heterocyclic Compounds
4.4. Biological Fluids
5. Conclusions
Funding
Conflicts of Interest
References
- Usol’tseva, N.V.; Akopova, O.B. Tribology and Mesomorphism. In Physics, Chemistry and Mechanics of Tribosystems, Interuniversity Collection of Scientific Papers; Latyshev, V.N., Ed.; Ivanovo State University: Ivanovo, Russia, 2011; pp. 14–23. (In Russian) [Google Scholar]
- Myshkin, N.K.; Petrokovec, M.I. Friction, Lubrication, Wear. Physical Fundamentals and Technical Applications of Tribology; FIZMATLIT: Moscow, Russia, 2007; p. 368. ISBN 978-5-9221-0824-9. (In Russian) [Google Scholar]
- Stribeck, R. Die wesentlichen Eigenschaften der Gleit-und Rollenlager. Z. Ver. Dtsch. Ing. 2002, 36, 1341–1348. [Google Scholar]
- Minami, J. Ionic Liquids in Tribology. Molecules 2009, 14, 2286–2305. [Google Scholar] [CrossRef] [PubMed]
- Carrion, F.-J.; Martinez-Nicolas, G.; Iglesias, P.; Sanes, J.; Bermudez, M.-D. Liquid Crystals in Tribology. Int. J. Mol. Sci. 2009, 10, 4102–4115. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Luo, J.; Wen, S.; Yao, J. Nanotribological properties and mechanisms of the liquid crystal as an additive. Chin. Sci. Bull. 2001, 46, 1227–1232. [Google Scholar] [CrossRef]
- Berezina, E.V. Phthalocyanine Derivatives as Additives to Lubricating Compositions; Ivanovo State University: Ivanovo, Russia, 2007; p. 240. ISBN 5-7807-0614-X. (In Russian) [Google Scholar]
- Gao, Y.; Jiang, Y.; Hu, W.; Jiang, H.; Li, J. Cholesteric liquid crystals as oil based lubricant additives: Effect of mesogenic phases and structures on tribological characteristics. Langmuir 2019, 35, 6981–6992. [Google Scholar] [CrossRef]
- Deryagin, B.V. Research in Surface Forces: Surface Forces in Thin Films and Disperse Systems; Springer: Moscow, Russia, 1972; p. 326. (In Russian) [Google Scholar]
- Sonin, A.S.; Frenkel, V.Y. Vsevolod Konstantinovich Frederiksz: 1885–1944; FIZMATLIT: Moscow, Russia, 1995; p. 176. ISBN 5-02-015179-3. (In Russian) [Google Scholar]
- Comstock, M.J. Tribology and the Liquid Crystalline State; ACS Symposium Series; Biresaw, G., Ed.; American Chemical Society: Washington, DC, USA, 1990; Volume 441, p. 133. [Google Scholar]
- Latyshev, V.N.; Karabanov, V.B.; Chaikovsky, V.M.; Chistyakov, I. Cutting Fluid for Machining of Metals. SU No. 601304, 27 April 1978. (In Russian). [Google Scholar]
- Latyshev, V.N.; Korotkov, V.B.; Godlevskiy, V.A.; Volkov, V.F.; Usol’tseva, N.V. Antifriction Additive for Oils. SU No. 1086009, 15 April 1984. (In Russian). [Google Scholar]
- Latyshev, V.N.; Korotkov, V.B.; Godlevskiy, V.A.; Alexandrov, A.I.; Usol’tseva, N.V.; Volkov, V.F. Cutting Fluid for Machining of Metals. SU No. 1149622, 22 December 1983. (In Russian). [Google Scholar]
- Latyshev, V.N.; Lazuk Yu, N.; Usol’tseva, N.V. Liquid Crystals and Their Practical Application. In Proceedings of the Republican Conference, Baku, Azerbaijan, 20 January 1990; pp. 37–38. (In Russian). [Google Scholar]
- Goodby, J.W.; Gray, G.W. Guide to the Nomenclature and Classification of Liquid Crystals. In Handbook of Liquid Crystals, 1st ed.; Demus, D., Goodby, G., Gray, G.W., Spiess, H.-W., Vill, V., Eds.; Wiley-VCH: Weinheim, Germany; New York, NY, USA; Chichester, UK; Singapore, 1998; Volume 1, pp. 17–23. [Google Scholar]
- Usol’tseva, N.V.; Akopova, O.B.; Bykova, V.V.; Smirnova, A.I.; Pikin, S.A. Liquid Crystals: Discotic Mesogens, 1st ed.; Usol’tseva, N.V., Ed.; Ivanovo State University: Ivanovo, Russia, 2004; p. 546. ISBN 5-7807-0458-9. (In Russian) [Google Scholar]
- Usol’tseva, N.V. Lyotropic Liquid Crystals: Chemical and Supramolecular Structure; Ivanovo State University: Ivanovo, Russia, 1994; p. 220. ISBN 5-230-02212-4. (In Russian) [Google Scholar]
- Volkmar, V.; Galewski, Z. LiqCryst 5.0. Database of Liquid Crystals; LCI Publisher: Hamburg, Germany, 2010. [Google Scholar]
- Cognard, J. Lubrication with Liquid Crystals. In Tribology and the Liquid Crystalline State; ACS Symposium Series; Biresow, G., Ed.; American Chemical Society: Washington, DC, USA, 1990; Volume 441, pp. 1–47. [Google Scholar]
- Mori, S.; Iwata, H. Relationship between tribological performance of liquid crystals and their molecular structure. Tribol. Intern. 1996, 29, 35–39. [Google Scholar] [CrossRef]
- Nakano, K. Tribology of Liquid Crystals. Chem. Ind. 2004, 55, 460–465. [Google Scholar]
- Amann, T.; Kailer, A. Relationship between ultralow friction of mesogenic-like fluids and their lateral chain length. Tribol. Lett. 2011, 41, 121–129. [Google Scholar] [CrossRef]
- Wažyńska, D.; Okowiak, J.A. Tribological properties of nematic and smectic liquid crystalline mixtures used as lubricants. Tribol. Lett. 2006, 24, 1–5. [Google Scholar] [CrossRef]
- Ermakov, S.F.; Rodnenkov, V.G.; Beloenko, E.D.; Kupchinov, B.I. Liquid Crystals in Engineering and Medicine; Asap: Minsk, Belarus; ChePo: Moscow, Russia, 2002; p. 411. ISBN 985-6572-66-5, 5-88711-175-5. (In Russian) [Google Scholar]
- Demus, D.; Demus, H.; Zaschke, H. Flussige Kristalle in Tabellen; VEB Deut.: Leipzig, Germany, 1974. [Google Scholar]
- Syrbu, S.A.; Novikov, V.V.; Burchenkov, K.S.; Fedorov, M.S. New Functional Nanostructured Materials for Tribosystems Based on Nematogen Systems. In Organic and Hybrid Nanomaterials: Production, Research, Application: Monograph; Razumov, V.F., Kluev, M.V., Eds.; Ivanovo State University: Ivanovo, Russia, 2019; pp. 307–337. (In Russian) [Google Scholar]
- Buyanovsky, A.I.; Zacharov, S.M. Lubrication. In Friction, Wear and Lubrication (Tribology and Tribotechnology); Chichivadze, A.V., Ed.; Mashinostroenie: Moscow, Russia, 2003; pp. 184–248. (In Russian) [Google Scholar]
- Kupchinov, B.I.; Ermakov, S.F.; Parkalov, V.P.; Rodnenkov, V.G. Study of the influence of mesogenic lubricant additives on the kinetics of the friction coefficient. Dokl. Natl. Acad. Sci. Belarus 1989, 33, 340–343. (In Russian) [Google Scholar]
- Bermúdez, M.D.; Martinez-Nicolás, G.; Carrion-Vilches, F.J. Tribological properties of liquid crystals as lubricant additives. Wear 1997, 212, 188–194. [Google Scholar] [CrossRef]
- Vektris, V.; Mokshin, V. Tribological research of industrial oil with liquid-crystal additives. Mater. Sci. 2008, 44, 730–734. [Google Scholar] [CrossRef]
- Latyshev, V.N.; Syrbu, S.A.; Novikov, V.V.; Kolbashov, M.A. Reological Properties of Lubricating Oils with the Additives of Cholesteric Liquid Crystals. Liq. Cryst. Appl. 2008, 3, 52–59. [Google Scholar]
- Berezina, E.V.; Korsakov, M.N.; Pavlov, A.S.; Bykova, V.V.; Usol’tseva, N.V. Curve currents of viscous lubricants with liquid crystal additives. Liq. Cryst. Appl. 2010, 2, 85–96. [Google Scholar]
- Berezina, E.V.; Korsakov, M.N.; Pavlov, A.S.; Usol’tseva, N.V. Viscometry of plastic lubricants with mesogenic additives. Fiz. Him. Mekhanika Tribosistem 2009, 8, 171–173. (In Russian) [Google Scholar]
- Rebinder, P.A. Surface Phenomena in Disperse Systems. Physicochemical Mechanics; Nauka: Moscow, Russia, 1979; p. 428. (In Russian) [Google Scholar]
- Ermakov, S.F.; Myshkin, N.K. Liquid-Crystal Nanomaterials. Tribology and Applications; Springer Intern Publishing AG: Basel, Switzerland, 2018; Volume 267. [Google Scholar]
- Popova, M.N.; Zharova, M.A.; Usol’tseva, N.V.; Smirnova, A.I.; Bogdanov, V.S. Rheological and tribological properties of industrial oil with mesogenic additives and carbon nanotubes. Liq. Cryst. Appl. 2014, 14, 52–61. [Google Scholar]
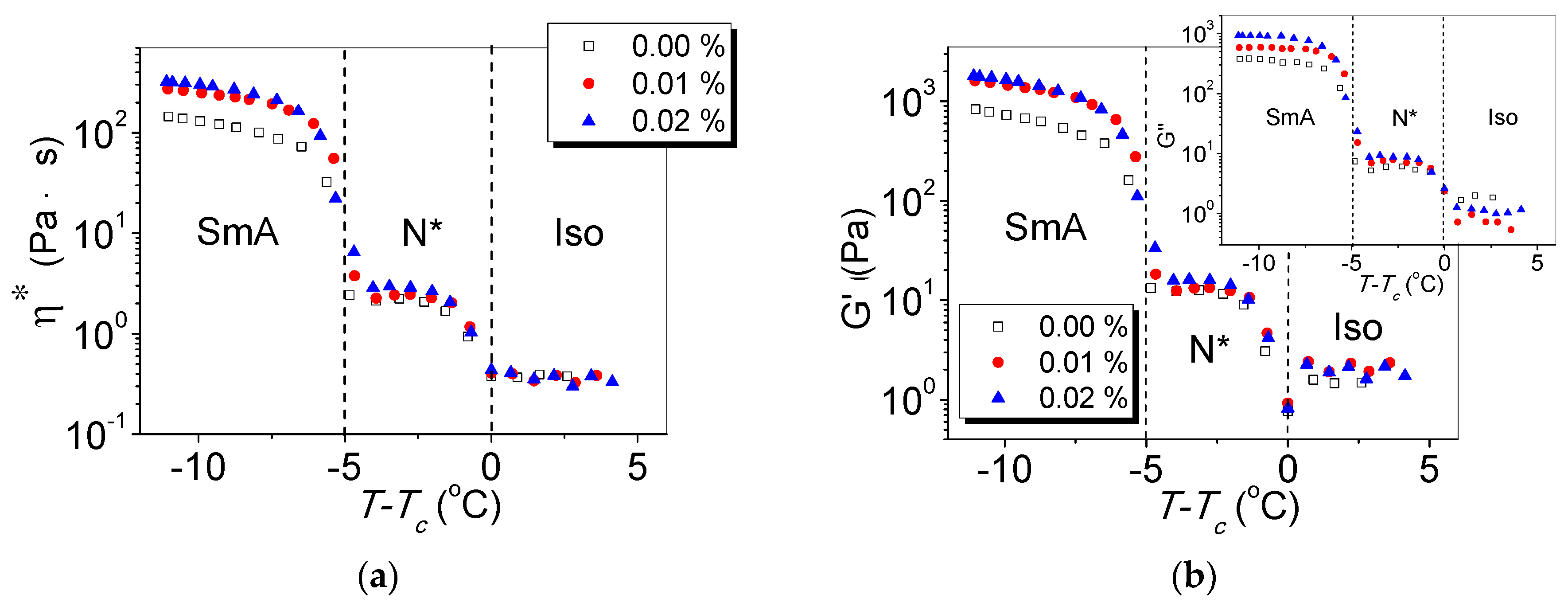
- Usol’tseva, N.V.; Smirnova, M.V.; Kazak, A.V.; Smirnova, A.I.; Bumbina, N.V.; Il’in, S.O.; Rozhkova, N.N. Reological Characteristics of Different Carbon Nanoparticles in Cholesteric Mesogen Dispersions as Lubricant Coolant Additives. J. Frict. Wear 2015, 36, 380–385. [Google Scholar] [CrossRef]
- Usol’tseva, N.V.; Yakemseva, M.V. Mesogens and Polymers in Systems with Carbon Nanoparticles. In Organic and Hybrid Nanomaterials: Trends and Prospects: Monograph; Razumov, V.F., Kluev, M.V., Eds.; Ivanovo State University: Ivanovo, Russia, 2013; pp. 228–280. (In Russian) [Google Scholar]
- De Jeu, W.H. Physical Properties of Liquid Crystalline Materials; Gordon and Breach Science Publishers: New York, NY, USA; London, UK; Paris, France, 1980; 133p. [Google Scholar]
- Yakemseva, M.V.; Usol’tseva, N.V. Viscoelastic properties of cholesteric liquid crystal—Multiwall carbon nanotubes composite. Liq. Cryst. Appl. 2013, 2, 90–94. [Google Scholar]
- Usol’tseva, N.V.; Smirnova, M.V.; Sotsky, V.V.; Smirnova, A.I. Physical properties of cholesteric liquid crystals—Carbon nanotube dispersions. J. Phys. Conf. Ser. 2014, 558, 012003. [Google Scholar] [CrossRef]
- Ali, I.; Bashneer, A.A.; Kucherova, A.; Memetov, N.; Pasko, T.; Ovchinnikov, K.; Pershin, V.; Kuznetsov, D.; Galunin, E.; Grachev, V.; et al. Advances in carbon nanomaterials as lubricants modifiers. J. Mol. Liq. 2019, 279, 251–266. [Google Scholar] [CrossRef]
- Usol’tseva, N.V.; Akopova, O.B. Molecular Structure of Organic Compounds and Their Thermotropic Mesomorphism. In Liquid Crystals: Discotic Mesogens, 1st ed.; Usol’tseva, N.V., Ed.; Ivanovo State University: Ivanovo, Russia, 2004; pp. 10–96. ISBN 5-7807-0458-9. (In Russian) [Google Scholar]
- Eidenschink, R. Mechanical Component. Patent No 3821855.0 FRG, 29 June 1988. [Google Scholar]
- Akopova, O.B.; Bobrov, V.I.; Tuneva, G.A. Anti-friction and Antiwear Lubricant Additive. SU No. 1664818, 22 March 1991. [Google Scholar]
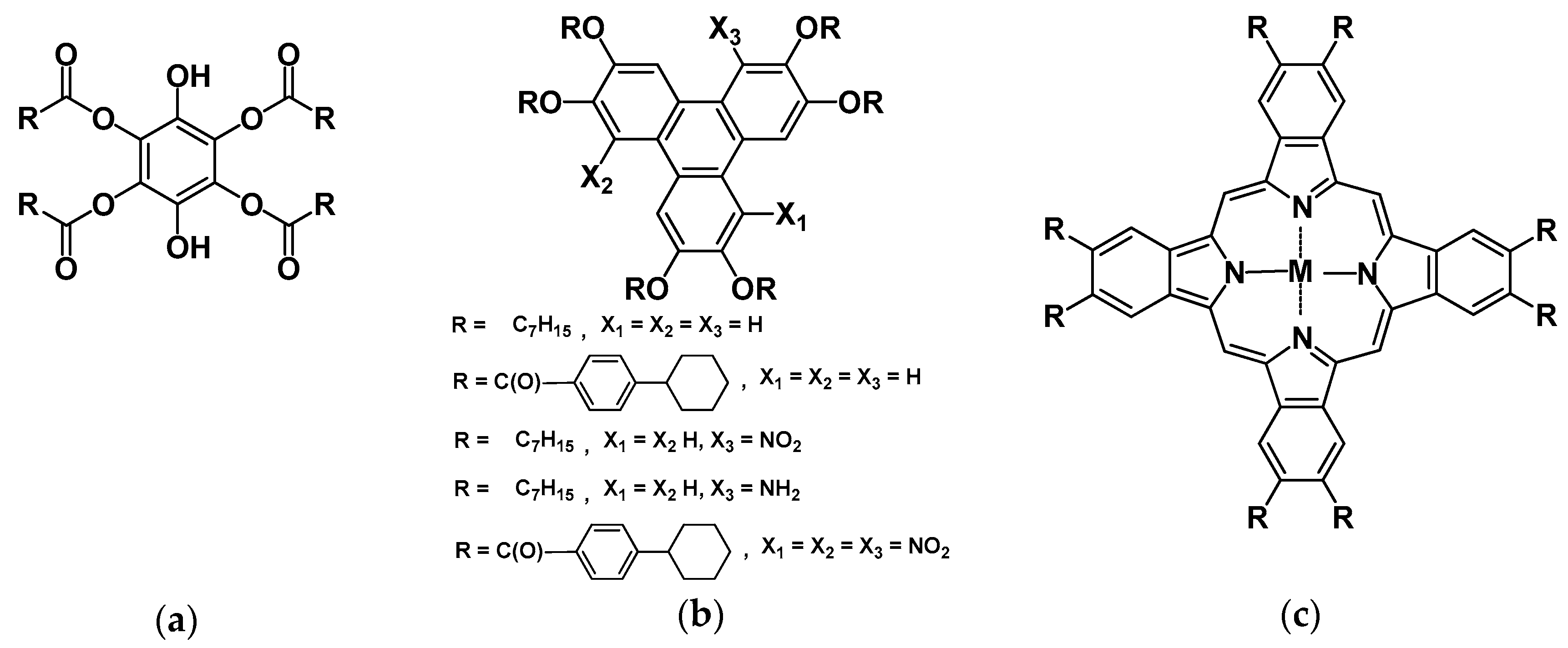
- Usol’tseva, N.V.; Akopova, O.B.; Bykova, V.V. Physico-Chemical Properties of Discotic Mesogens that Determine Their Use. In Liquid Crystals: Discotic Mesogens, 1st ed.; Usol’tseva, N.V., Ed.; Ivanovo State University: Ivanovo, Russia, 2004; pp. 490–538. ISBN 5-7807-0458-9. (In Russian) [Google Scholar]
- Akopova, O.B.; Zemtsova, O.V.; Kotovich, L.N.; Lapshin, V.B. Tribological Characteristics of Triphenylene Derivatives with Columnar and ND Mesophases. In Vestnik IvGU; Ivanovo State University: Ivanovo, Russia, 2001; pp. 71–75. (In Russian) [Google Scholar]
- Akopova, O.B.; Gribova, L.K.; Zemtsova, O.V.; Savchenko, V.E.; Usol’tseva, N.V. Application of quartz resonators for research of apolar and polar hexaalkoxytriphenylene derivatives phase transitions. In Proceedings of the XIV Conference on Liquid Crystals, Zakopane, Poland, 1 June 2001; p. 23. [Google Scholar]
- Zemtsova, O.V.; Akopova, O.B.; Usol’tseva, N.V.; Erdelen, C.H. Columnar mesomorphism of the mixed substituted apolar triphenylenes. Prognosis and experimental data. Mol. Cryst. Liq. Cryst. 2001, 364, 625–634. [Google Scholar] [CrossRef]
- Akopova, O.B.; Logacheva, N.M.; Baulin, V.E.; Tsivadze, A.Y.; Terentiev, V.V.; Subbotin, K.V. Investigation of tribological properties of discotic mesogens—Derivatives of phthalocyanine containing crown ether fragments. In Proceedings of the VII International Scientific Conference “Lyotropic Liquid Crystals and Nanomaterials” Together with the Symposium “Success in the Study of Thermotropic Liquid Crystals” (the Vth Chistyakov’s Readings), Ivanovo, Russia, 16–18 December 2009; p. 93. (In Russian). [Google Scholar]
- Latyshev, V.N.; Terentiev, V.V.; Akopova, O.B.; Subbotin, K.V. The use of copper carboxylates as additives for solidol. In Proceedings of the International Scientific Conference “Actual Problems and Prospects for the Development of Agriculture”, Ivanovo, Russia, 26 August 2009; Volume 2, pp. 95–96. (In Russian). [Google Scholar]
- Berezina, E.V.; Godlevskiy, V.A. Mesogenic Compounds as Triboactive Additives. In Achivements of Liquid Crystals Research, 1st ed.; Usol’tseva, N.V., Ed.; Ivanovo State University: Ivanovo, Russia, 2007; pp. 80–94. (In Russian) [Google Scholar]
- Akopova, O.B.; Usol’tseva, N.V. Discotic Mesogens: From Monomers to Polymers and Dendrimers; Ivanovo State University: Ivanovo, Russia, 2010; p. 112. (In Russian) [Google Scholar]
- Usol’tseva, N.V.; Bykova, V.V.; Akopova, O.B. Achivements of Liquid Crystals Research, 1st ed.; Usol’tseva, N.V., Ed.; Ivanovo State University: Ivanovo, Russia, 2007; 100p, ISBN 5-7807-0655-7. (In Russian) [Google Scholar]
- Usol’tseva, N.V. Chemical characterization, biological and medical significance of lyotropic liquid crystals. Zhurnal Vsesoyuznogo Him. Obs. Mendeleeva 1983, 28, 36–45. (In Russian) [Google Scholar]
- Ekwall, P. Composition, Properties of Liquid Crystalline Phases in Systems of Amphiphilic Compounds. In Advances in Liquid Crystals; Brown, G.H., Ed.; Academic Press: New York, NY, USA; San Francisco, CA, USA; London, UK, 1975; Volume 1, pp. 1–142. [Google Scholar]
- Sułek, M.W.; Ogorzałek, M.; Wasilewski, T.; Klimaszewska, E. Alkyl Polyglucosides as components of water based lubricants. J. Surfactants Deterg. 2013, 16, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.S.; Li, G.Z.; Shen, Q.A. Study of lyotropic liquid crystal in lubrication on aluminium alloy surfaces. J. Dispers. Sci. Technol. 1999, 20, 1025–1030. [Google Scholar] [CrossRef]
- Boschkova, K.; Elvesjo, J.; Kronberg, B. Frictional properties of lyotropic liquid crystalline mesophases at surfaces. Colloid Surf. A Physicochem. Eng. Asp. 2000, 166, 67–77. [Google Scholar] [CrossRef]
- Latyshev, V.N.; Lazuk, Y.N.; Usol’tseva, N.V. Compositions based on lyotropic mesogens and their practical application in tribology. In Proceedings of the Republic Conference “Liquid Crystals and Their Application”; Union of Soviet Socialist Republics: Baku, USSR, 1990; pp. 37–38. (In Russian) [Google Scholar]
- Bobrov, V.I.; Krasikov, N.N. Birefringence resulting from friction in thin surfactant layers in a solid state. Trenie Isnos 1981, 2, 545–548. (In Russian) [Google Scholar]
- Levchenko, V.A. Epitropic liquid crystals—A new liquid phase. J. Mol. Liq. 2000, 85, 197–210. [Google Scholar] [CrossRef]
- Frieberg, S.E.; Ward, A.J.; Gunsel, S.; Lockwood, F.E. Lyotropic Liquid Crystals in Lubrication. In Tribology and the Liquid-Crystalline State; ACS Symposium Series; Bireslaw, G., Ed.; American Chemical Society: Washington, DC, USA, 1990; Volume 441, pp. 101–110. [Google Scholar]
- Pennzoil Products Company. Pennzoil Products Company. Non-Aqueous Lamellar Liquid Crystalline Lubricants. U.S. Patent No. 4,999,122, 30 December 1988. [Google Scholar]
- Sułek, M.W.; Bąk-Sowinska, A. New Ecological Lubricants on the Basis of Lyotropic Liquid Crystals Formed by Solutions of Maracuja Oil Ethoxylate. Ind. Eng. Chem. Res. 2013, 52, 16169–16174. [Google Scholar] [CrossRef]
- Shilov, M.A. Investigation of self-organization mechanism of non-ionic surface-active substances and their combinations with ionic ones in water-lubricating friction junctions. Liq. Cryst. Appl. 2011, 1, 57–64. [Google Scholar]
- Berezina, E.V.; Shilov, M.A. Structured gels as lubricant and coolant mixtures in drilling. Russ. Eng. Res. 2011, 31, 91–93. [Google Scholar] [CrossRef]
- Araos, M.U.; Warr, G.G. Self-Assembly of Nonionic Surfactants into Lyotropic Liquid Crystals in Ethylammonium Nitrate, a Room-Temperature Ionic Liquid. J. Phys. Chem. B 2005, 109, 14275–14277. [Google Scholar] [CrossRef] [PubMed]
- Berezina, E.V. Improving the Workability of Steels and Alloys Using Synthetic Aqueous Lubricant-Cooling Agent with New Triboactive Additives. Ph.D. Thesis, Ivanovo State University, Ivanovo, Russia, 1992. [Google Scholar]
- Berezina, E.V.; Godlevskiy, V.A. On the use of aqueous solutions of phthalocyanine as triboactive additives to technological media for metal cutting. Bull. Acad. Sci. 1991, 55, 1757–1759. (In Russian) [Google Scholar]
- Berezina, E.V.; Godlevskiy, V.A.; Usol’tseva, N.V.; Chrunov, A.A. The study of the viscosity concentration dependences of aqueous solutions of dyes. Liq. Cryst. Appl. 2004, 3, 63–71. [Google Scholar]
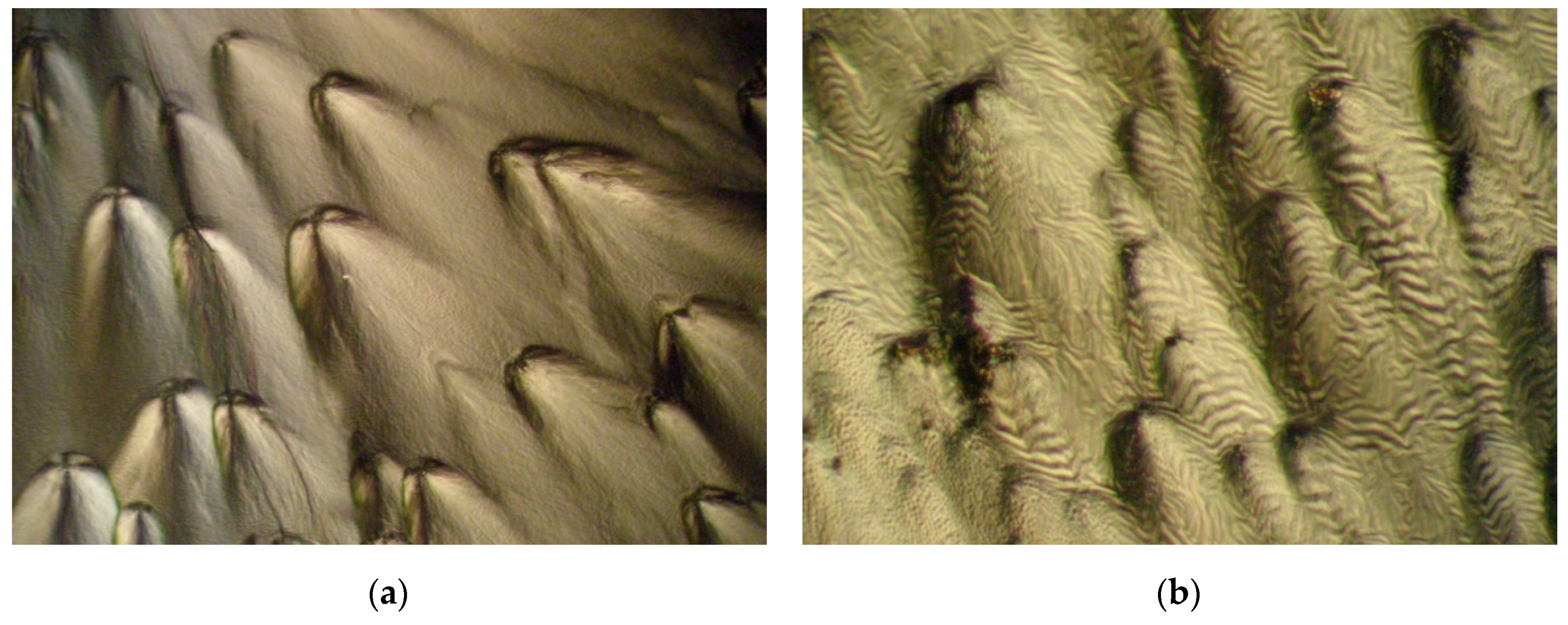
- Usol’tseva, N.V.; Latyshev, V.N.; Bobrov, V.I.; Pischasova, G.K. On the Liquid Crystalline State of Saliva as a Lubricant Fluid. In Frictional Interaction of Solids Taking into Account the Medium; Ivanovo State University: Ivanovo, Russia, 1982; pp. 148–151. (In Russian) [Google Scholar]
- Barchan, G.P. Liquid crystalline state of interfacial layers at sliding. Doklady Natl. Acad. Sci. Belarus 1981, 258, 86–88. (In Russian) [Google Scholar]
- Kupchinov, B.I.; Ermakov, S.F.; Rodnenkov, V.G.; Bobrysheva, S.N.; Beloenko, E.D.; Kestelman, V.N. Role of liquid crystals in the lubrication of living joints. Smart Mater. Struct. 1993, 2, 7–12. [Google Scholar] [CrossRef]
- Kupchinov, B.I. Tribological aspects of joint functioning. Trenie Isnos 1989, 10, 1013–1018. (In Russian) [Google Scholar]
- Kupchinov, B.I.; Ermakov, S.F.; Beloenko, E.D. Biotribology in Synovial Joints; NANB–MZRB: Minsk, Belarus, 1997. [Google Scholar]
- Szwajczak, E.; Kucaba-Pietal, A. Liquid crystalline properties of synovial fluid. Eng. Trans. 2001, 49, 315–358. [Google Scholar]
- Dowson, D. Biotribology of Natural and Replacement Synovial Joints. In Biomechanics of Diarthrodial Joints; Mow, V.C., Woo, S.L.-Y., Ratcliffe, A., Eds.; Spribger: Berlin, Germany, 1990; Volume 2, pp. 305–345. [Google Scholar]
- Ghosh, S.; Choudhury, D.; Roy, T.; Moradi, A.; Masjuki, H.H.; Pingguan-Murphy, B. Tribological performance of the biological components of synovial fluid in artificial joint implants. Sci. Technol. Adv. Mater. 2015, 16, 045002. [Google Scholar] [CrossRef]
- Ruiz-Fernández, A.R.; Tejos, R.; Ahumada-Gutierrez, H.; Muñoz-Gacitúa, D.; Martínez-Cifuentes, M.; Araya-Maturana, R.; Weiss-López, B.E. Characterisation of a new nematic lyotropic liquid crystal with natural lipids from soybean. Mol. Phys. 2018, 117, 158–166. [Google Scholar] [CrossRef]
- Papkov, S.P.; Kulichikhin, V.G. The Liquid Crystalline State of Polymers; Chimia: Moscow, Russia, 1977; p. 240. (In Russian) [Google Scholar]
- Usol’tseva, N.V.; Bobrov, V.I.; Shabishev, L.S.; Pischasova, G.K. Polarization-Microscopic Analysis of Biological Fluid Containing Friction-Oriented Glycoproteins. In Abstract Book of the 1st All-Union Symposium on the Liquid Crystalline State of Polymers; USSR Academy of Sciences, Scientific Council on Macromolecular Compounds: Suzdal, Russia, 1982; pp. 96–97. (In Russian) [Google Scholar]
- Usol’tseva, N.V. Molecular and Supramolecular Structure of Lyomesogens and Properties of Lyomesophases. Ph.D. Thesis, Leningrad State Univerisity, Leningrad, Russia, 1990. (In Russian). [Google Scholar]
- Vazina, A.A.; Eliakova, L.A.; Skopinov, S.A.; Chernoborodova, S.V.; Yakovleva, S.V. Lyotropic Mesomorphism of Glucanes. In Abstract Book of the VIth All-Union Conference on Liquid Crystals and Their Application; Chernihiv National Pedagogical University named after T.G. Shevchenko: Chernigov, Russia, 1988; Volume 3, p. 427. (In Russian) [Google Scholar]
- Lazuk, Y.N.; Latyshev, V.N.; Usol’tseva, N.V.; Kolesnikov, N.D. Lubricant-Cooling Agent. SU No 1692814A1, 11 October 1989. (In Russian). [Google Scholar]

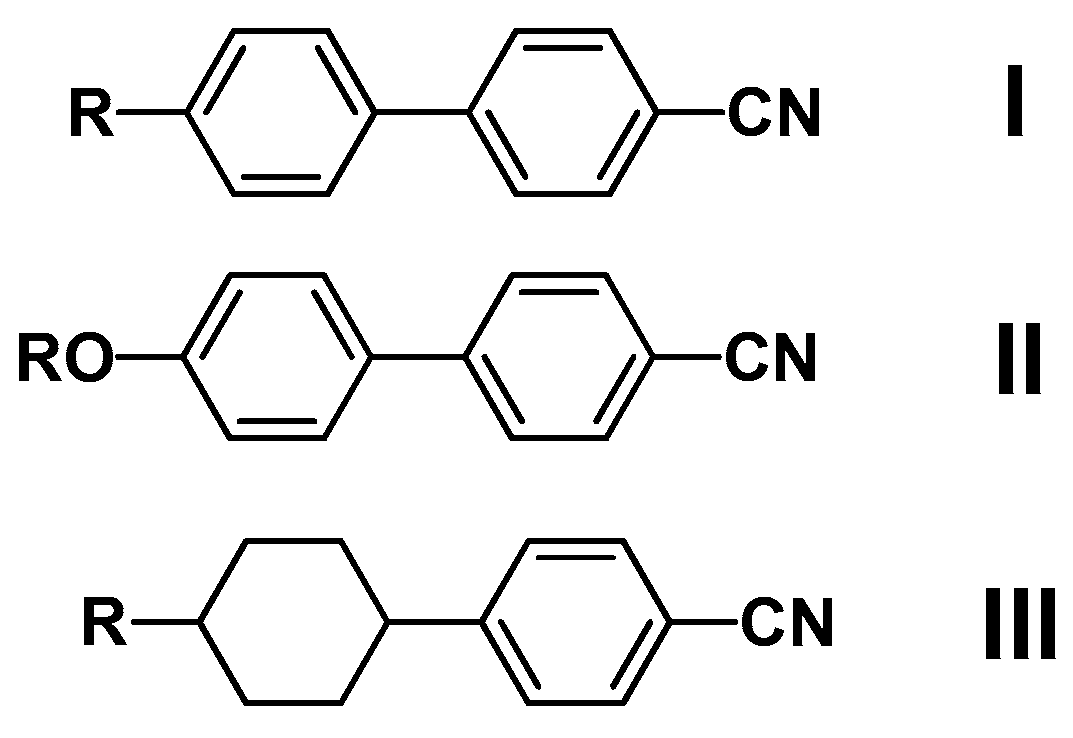
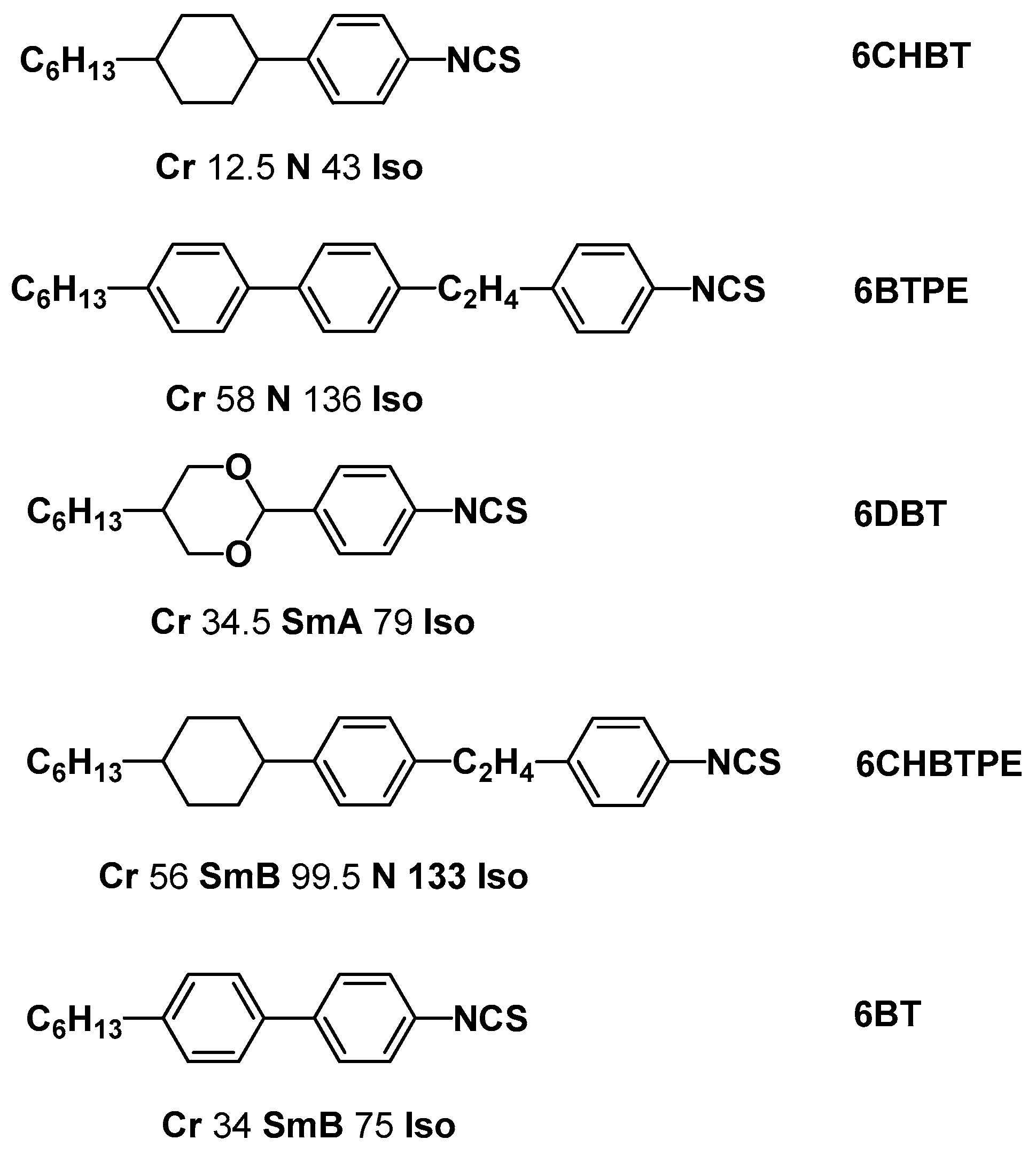
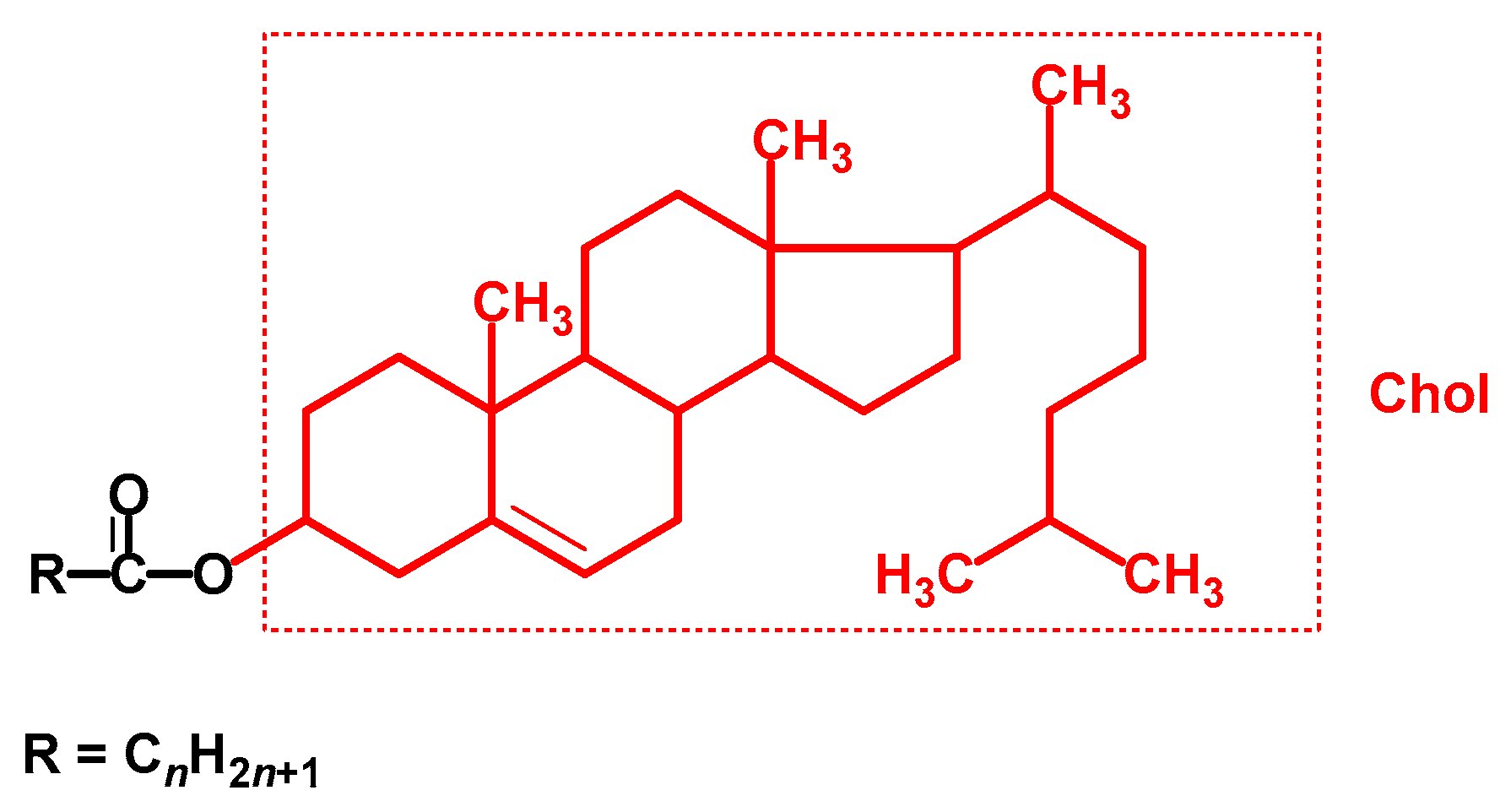
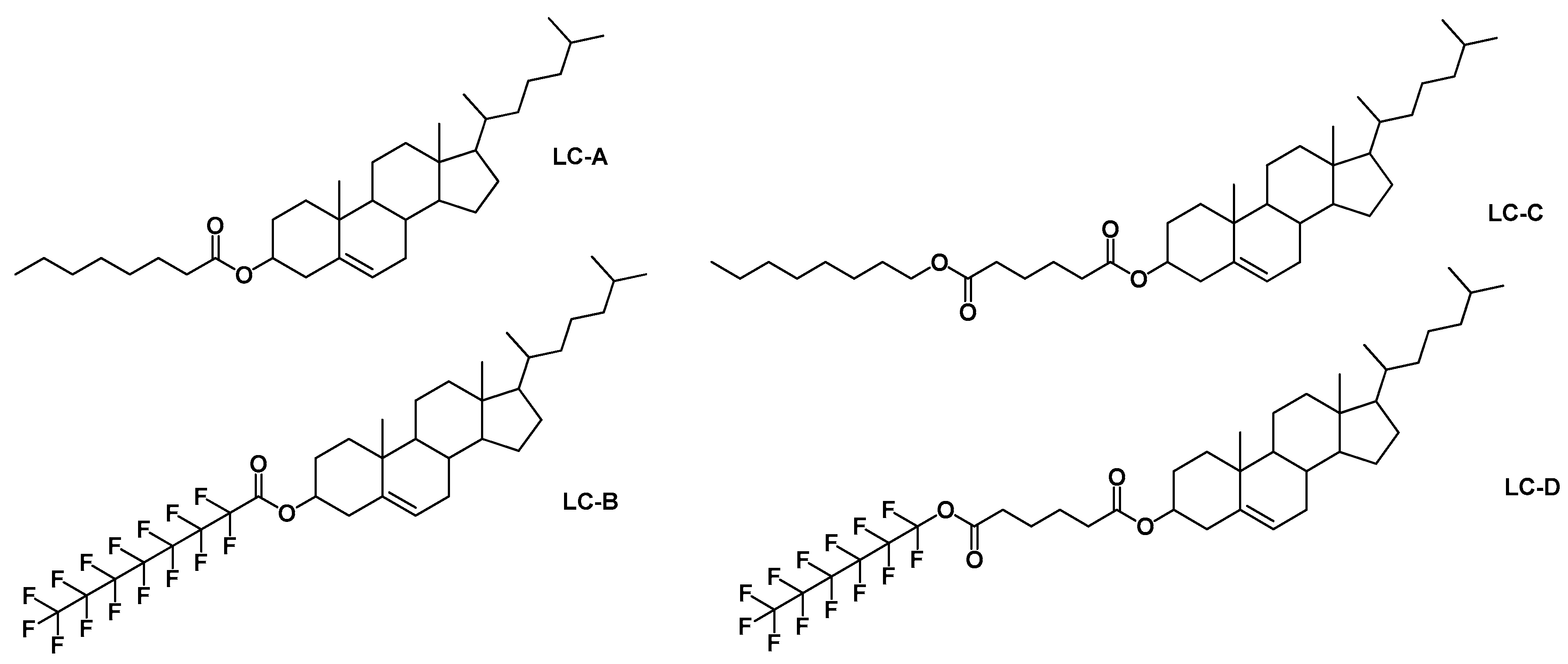

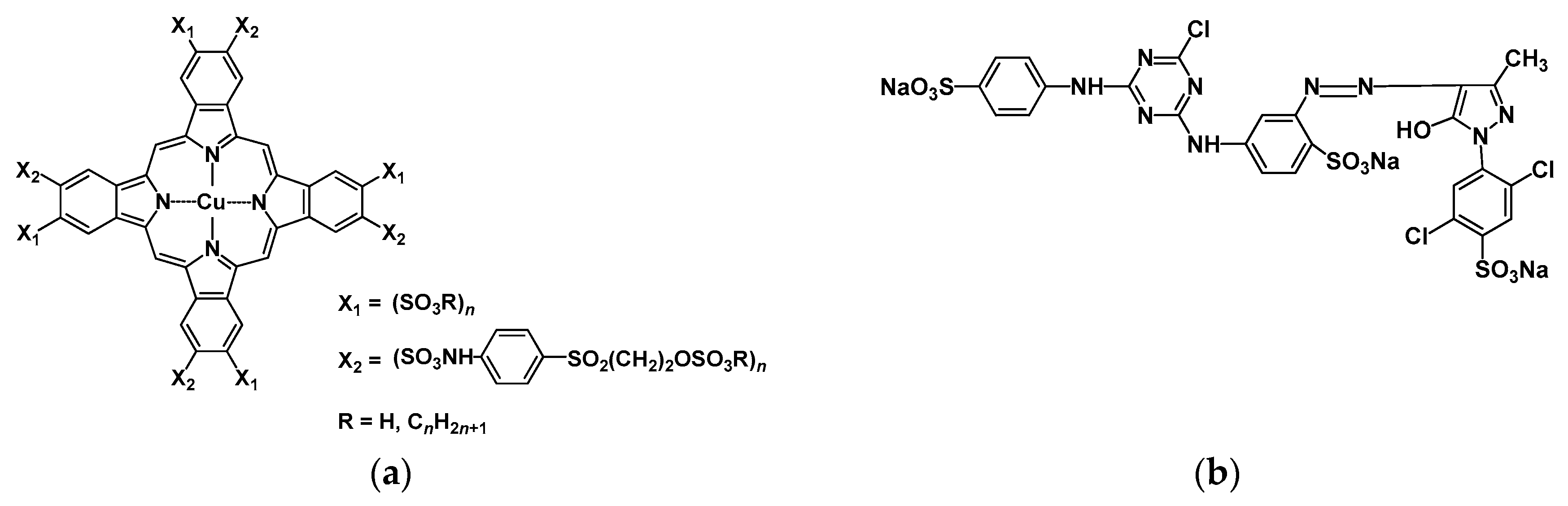



| Notation | Chemical Structure | Notation | Chemical Structure |
|---|---|---|---|
| MBBA | 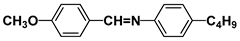 | PEPN4 | 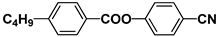 |
| EBBA |  | PEPN5 | 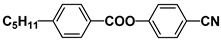 |
| PAA | 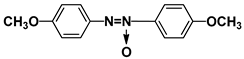 | PEPN6 | 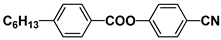 |
| PAP | 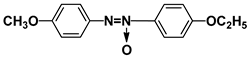 | PEPN7 | 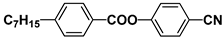 |
| S3 | 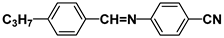 | HPEH23 | 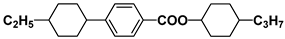 |
| S6 | 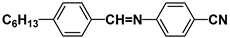 | HPEH33 | 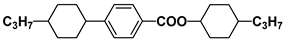 |
| K15 | 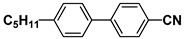 | HPEH43 | 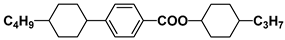 |
| K21 | 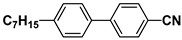 | PCH3 | 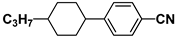 |
| M9 | 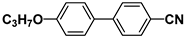 | PCH5 | 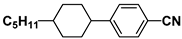 |
| M15 | 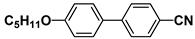 | PCH7 | 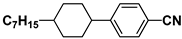 |
| M21 |  | BCH5 | 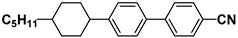 |
| M24 | 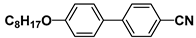 | P35 | 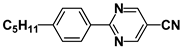 |
| M27 | 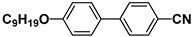 | P37 | 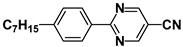 |
| CB15 | 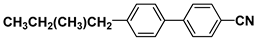 | TP34 | 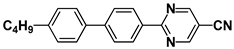 |
| T15 | 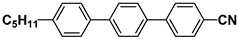 | 3HPPH3 | 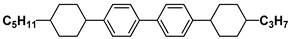 |
| Notation | Compound | Molecular Weight | Phase Transitions (°C) |
|---|---|---|---|
| X-19 | Chol–COOH | 414 | Cr 97.5 Iso |
| X-26 | Chol–Cl | 405 | Cr 97.0 Iso |
| X-4 | Chol–COOC4H9 | 470 | Cr 93 Ch 101.5 Iso |
| X-6 | Chol–COOC7H15 | 512 | Cr 110 Iso |
| X-5 | Chol–COOC9H19 | 540 | Cr 85.5 Ch 92.5 Iso |
| X-18 | Chol–COOC10H21 | 554 | Cr 92.5 Iso |
| X-20 | Chol–COOC12H25 | 582 | Cr 63.5 Sm 78.8 Ch 84.8 Iso |
| X-28 | Chol–COOC14H29 | 610 | Cr 70 Sm 78.3 Ch 82.9 Iso |
| X-37 | Chol–COO–(C6H4)–C8H17 | 602 | Cr 138 Sm 171.5 Ch 220.5 Iso |
| X-68 | Chol–COO–(C6H4)–C12H25 | 658 | Cr 128.5 Sm 179.5 Ch 200.5 Iso |
| Chol– | C27H45 | 369 |
| Two-Component Systems | Three-Component Systems |
|---|---|
| Industrial oil I-20A (base) with additives: | |
| X-7 (2%) | X-7 (2%) + 0.0001% of MWCNT |
| X-10 (2%) | X-10 (2%) + 0.0001% of MWCNT |
| X-16 (2%) | X-16 (2%) + 0.0001% of MWCNT |
| X-20 (2%) | X-20 (2%) + 0.0001% of MWCNT |
| Sample | Temperature (°C) | Wear of the Roller (mm) | Area of the Ball Wear Spot (mm) | Friction Coefficient (f) | |
|---|---|---|---|---|---|
| Start | End | ||||
| Industrial Oil I-20A | 19 | 50 | 0.35 | 0.110 | 0.051 |
| I-20A + X-7 | 22 | 55 | 0.30 | 0.106 | 0.088 |
| I-20A + X-7 + MWCNT | 19 | 51 | 0.525 | 0.280 | 0.046 |
| I-20A + X-20 | 30 | 61 | 0.275 | 0.102 | 0.064 |
| I-20A + X-20 + MWCNT | 19 | 49 | 0.50 | 0.165 | 0.046 |
| I-20A + X-10 | 19 | 48 | 0.625 | 0.298 | 0.051 |
| I-20A + X-10 + MWCNT | 21 | 56 | 0.60 | 0.306 | 0.088 |
| I-20A + X-16 | 24 | 54 | 0.60 | 0.158 | 0.088 |
| I-20A + X-16 + MWCNT | 24 | 54 | 0.575 | 0.108 | 0.076 |
| Notation | Surfactant | Structural Formula |
|---|---|---|
| Surf 1 | Neonol 9/6 | 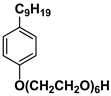 |
| Surf 2 | Neonol 9/10 | 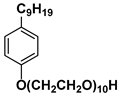 |
| Surf 3 | Syntanol | 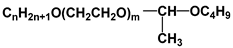 |
| Surf 4 | Phenoxol 9/10 | 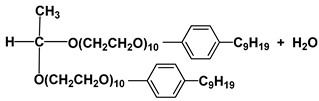 |
| Surf 5 | DSSA | 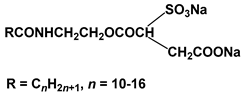 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usol’tseva, N.V.; Smirnova, A.I. Liquid Crystals as Lubricants. Lubricants 2019, 7, 111. https://doi.org/10.3390/lubricants7120111
Usol’tseva NV, Smirnova AI. Liquid Crystals as Lubricants. Lubricants. 2019; 7(12):111. https://doi.org/10.3390/lubricants7120111
Chicago/Turabian StyleUsol’tseva, Nadezhda V., and Antonina I. Smirnova. 2019. "Liquid Crystals as Lubricants" Lubricants 7, no. 12: 111. https://doi.org/10.3390/lubricants7120111
APA StyleUsol’tseva, N. V., & Smirnova, A. I. (2019). Liquid Crystals as Lubricants. Lubricants, 7(12), 111. https://doi.org/10.3390/lubricants7120111





